Abstract
Small interfering RNA (siRNA) therapeutic is considered to be a promising modality for the treatment of hyperlipidemia. Establishment of a thermostable clinically applicable delivery system remains a most challenging issue for siRNA drug development. Here, a series of ionizable lipid-like materials were rationally designed; 4 panels of lipid formulations were fabricated and evaluated on the basis of four representative structures. The lead lipid (A1-D1-5) was stable at 40°C, and the optimized formulation (iLAND) showed dose and time dual-dependent gene silencing pattern with median effective dose of 0.18 mg/kg. In addition, potent and durable reduction of serum cholesterol and triglyceride were achieved by administering siRNAs targeting angiopoietin-like 3 or apolipoprotein C3 (APOC3) in high-fat diet–fed mice, db/db mice, and human APOC3 transgenic mice, respectively, accompanied by displaying ideal safety profiles. Therefore, siRNA@iLAND prepared with thermostable A1-D1-5 demonstrates substantial value for siRNA delivery, hyperlipidemia therapy, and prevention of subsequent metabolic diseases.
Gene silencing nanomedicine empowered potent and durable “bad” lipid reduction in multiple hyperlipidemia animal models.
INTRODUCTION
Hyperlipidemia encompasses various genetic and acquired disorders that describe elevated levels of lipids or lipoproteins within the body, which is recognized as one substantial risk factor of fatal diseases such as cardiovascular diseases (CVDs), type 2 diabetes mellitus, pancreatitis, and even certain types of cancer (1–4). Regardless of the availability of established antihyperlipidemic agents, the healthy lipid level in most high-risk patients is still not achieved (5). Statins, the representative class of small-molecule drugs, can significantly decrease intracellular cholesterol (CHO) by selectively competing with β-hydroxy-β-methylglutaryl coenzyme A reductase (6), leading to satisfactory treatment outcomes. Despite the therapeutic advantages in both primary and secondary prevention, the statins have been widely criticized for their safety concerns, especially the potential harmful effects in both muscles and liver. Moreover, other concerns have been reported with the use of statins, especially the risk of new-onset diabetes mellitus, cognitive impairment, and hemorrhagic stroke as well as the risk of achieving very low levels of low-density lipoprotein (LDL) CHO (LDL-C) (7). With further development, it is accepted that the onset of hyperlipidemia is usually accompanied by the abnormal expression of certain genes, such as proprotein convertase subtilisin/kexin type 9 (PCSK9) (8), angiopoietin-like 3 (ANGPTL3) (9), and apolipoprotein C3 (ApoC3) (10). Specific suppression of these genes can greatly cure disease and reduce the occurrence of side effects. One good example is the remarkable efficacy of PSCK9 inhibitors, such as alirocumab and evolocumab. As reported, treatment with alirocumab achieved further reduction in LDL-C levels compared to statins (11). Application of PSCK9 inhibitor evolocumab also provides evidence of large improvements in lipid levels (12). More impressively, the PSCK9 inhibitors showed better safety profile than statins. However, these drugs only effect on LDL-C reduction but barely effect on triglyceride (Tg). It was reported that elevated plasma TG levels are independent risk factors for coronary artery disease (13). Meanwhile, alirocumab and evolocumab may still induce some adverse effects such as high blood sugar or allergic reactions. In addition, PCSK9 antibodies, as proteins, have to undergo complex preparation and purification processes, and these procedures surely increase the cost of drugs.
Small interfering RNAs (siRNAs) can specifically down-regulate target mRNA in a sequence-specific recognition way (14, 15), showing bright future in the treatment of diseases that appeared to be untargetable or undruggable for small-molecule and antibody drugs (16). Because the function of siRNAs relies only on right base pairing, this functional mechanism theoretically endows them with the huge advantage of suppressing any gene of interest (17). Thus, siRNA therapeutics are regarded as a next-generation modality of gene regulator and have attracted researchers to devote themselves to development of this field. Unfortunately, the innate defects such as instability, unsatisfied pharmacokinetic profiles, poor cellular internalization, and cytosolic release limit the broad application of siRNA therapeutics. To address these issues, numerous delivery systems were developed (18–27). Among them, lipid nanoparticles (LNPs) are one of the most successful delivery systems because of their mature industrial manufacture technology, high cellular uptake, low cell toxicity, and low immunogenicity (18, 28–33). However, the arsenal of clinically applicable LNPs is not yet well established, especially because thermostable lipid is extremely lacking in this field. The process of structural design and component optimization are still challenging and complicated. Any changes to the content will certainly affect the performance of the system such as morphology, particle size (34), circulation time, cellular uptake, and endosomal escape (35).
Here, we rationally designed a series of novel ionizable lipid-like materials and synthesized four of them as the key lipid of ionizable LNP (iLNP), also used 1,2-dioctadecanoyl-sn-glycero-3-phophocholine (DSPC), CHO, and 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (DMG-PEG2000) as constitutive lipids. An optimized formulation was selected by introducing rational design and screening process. The optimized LNP, named ionizable lipid assisted nucleic acid delivery system (iLAND, also means intelligent LANDing technology), showed good physicochemical properties, thermostability, and excellent siRNA transportation efficiency. The positive surface charge at acidic pH facilitated the iLAND to efficiently encapsulate negative-charged siRNA. In addition, the iLAND successfully delivered antihyperlipidemia siRNAs with a median effective dose (ED50) at around 0.18 mg/kg and a 3-week reduction of serum lipids after a single dose. Efficacy studies in three disease models including high-fat diet (HFD)–fed mice, db/db mice, and hApoC3-Tg mice showed that the low dose of siRNA achieved desired therapeutic effects. All results suggested that the iLAND constitutes a promising platform for nucleic acid delivery, while the siRNA@iLAND represents a potential therapeutic agent for the treatment of hyperlipidemia and the prevention of subsequent metabolic diseases, such as CVDs.
RESULTS
Novel lipid design and synthesis
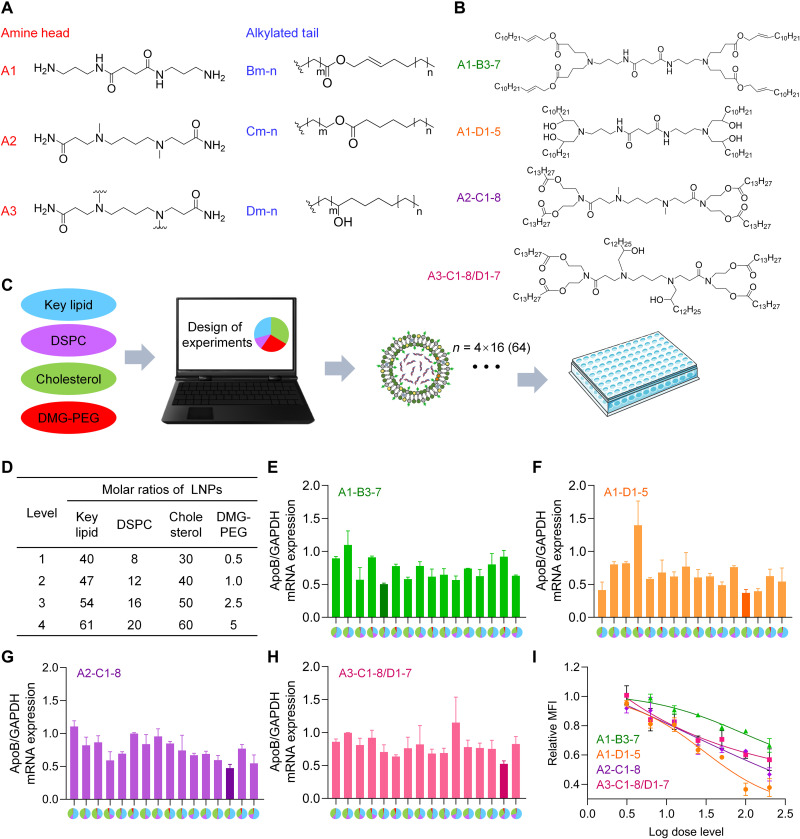
In this study, a library of novel lipids was rationally designed on the basis of three different hydrophilic heads and three hydrophobic tails (Fig. 1A). Then, four representative ionizable lipids, A1-B3-7, A1-D1-5, A2-C1-8, and A3-C1-8/D1-7, were generated and synthesized (Fig. 1B). Their synthesis processes were described in Materials and Methods and shown in the supporting information (figs. S1 to S4). The confirmation of the lipid structures was examined by 1H nuclear magnetic resonance (NMR) and 13C NMR (figs. S5 to S8).
Fig. 1. Screening and component optimization of novel iLNPs.
(A) The chemical structures of lipid building blocks, including three amine heads and three alkylated tails. (B) Chemical structures of four representative novel ionizable lipids evaluated in this study. (C) Optimization scheme of iLNPs. DOE was used to minimize the number of formulation candidates based on each lipid from 256 to 16. As a result, 64 formulations in total were prepared and evaluated in this study on the basis of four novel ionizable lipids, with 16 formulations for each ionizable lipid. (D) Levels of each component of iLNP calculated by molar ratio. (E to H) In vitro gene silencing efficiencies of 4 panels (64 in total) of formulations prepared with A1-B3-7 (E), A1-D1-5 (F) A2-C1-8 (G), and A3-C1-8/D1-7 (H), respectively. Transfection concentration of siRNA was 50 nM. Four colors in pie charts of the x axis represent four lipids used in LNP formulations. Blue, proposed ionizable lipid; purple, DSPC; green, CHO; red, DMG-PEG2000. The area percentage of each color in the pie charts represents the molar percentage of the lipid in the formulation. (I) Transfection efficiencies of four leading formulations selected from panels of (E), (F), (G), and (H), respectively. The transfections concentrations nM, respectively. GAPDH, glyceraldehyde phosphate dehydrogenase; MFI, mean fluorescence intensity.
In vitro formulation screening
It was reported that phospholipid, CHO, and PEGylated lipid can improve intracellular delivery efficiency of LNPs and the stability of nanoparticles (18, 36–38). DSPC and DMG-PEG2000, together with CHO and proposed novel ionizable lipids, were used for siRNA delivery in this study. The molar ratio of the four components is of great importance for achieving effective nucleic acid delivery and ideal safety profile (39). Inspired by previous literature (39), the relative molar ratios of lipids in the LNPs were designed as follows in this study: lipid (40 to 61%), DSPC (8 to 20%), CHO (30 to 60%), and DMG-PEG2000 (0.5 to 5%). For each lipid, formulations were generated by using design of experiment (DOE) to minimize the candidates/samples from 256 to 16 (Fig. 1, C and D, and tables S1 to S10). The molar ratios of each component in iLNP formulations tested in this study were shown in table S1 (in this case, the sum of molar ratios of four lipids might not be 100). The molar percentages of each component in iLNP formulations were shown in table S2 (in this case, the sum of molar ratios of four lipids was 100 for each formulation). In addition, the mass percentages of four lipids in all 64 LNPs were shown in tables S3, S4, S5, and S6, respectively. Because the molecular weights of four tested lipids were different from each other, the mass percentages in all 64 LNPs also varied from each other.
Physiochemical characterizations of siRNA-loaded iLNPs based on A1-B3-7, A1-D1-5, A2-C1-8, and A3-C1-8/D1-7, including the particle size, polydispersity index (PDI), encapsulation efficiency (also called entrapment efficiency) and loading efficiency, were shown in tables S7, S8, S9, and S10, respectively. According to the data, the particle size of all 64 formulations were basically less than 200 nm, while, the particle size of iLNPs prepared with A1-D1-5 were generally less than the iLNPs prepared with A1-B3-7, A2-C1-8, and A3-C1-8/D1-7 (tables S7 to S10). The PDIs of all 64 iLNPs were basically lower than 0.2, and the encapsulation efficiency of each iLNPs was higher than 90%. The loading efficiency varied among all 64 formulations; each of the formulation showed a loading efficiency of higher than 60%. Moreover, a loading efficiency of 90%, could be achieved in an optimized formulation. iLNPs prepared with A3-C1-8/D1-7 showed relatively poor performances regarding the loading efficiency, compared with iLNPs prepared with A1-B3-7, A1-D1-5, and A2-C1-8 (tables S7 to S10).
Subsequently, gel retardation assay was performed to visually evaluate the loading efficiency of proposed iLNPs. Here, ionizable lipid of A1-D1-5-1 was used to prepare a series of iLNPs by changing the mass ratios between the LNP and siRNA. As shown in fig. S9, there was small amount of siRNA leakage at a mass ratio of 2:1, while siRNA could be completely entrapped by iLNP when the mass ratio was higher 15:1. Therefore, iLNP were mixed with siRNA at the mass ratio of 15:1 in following studies.
Next, 64 formulation candidates were prepared by encapsulating apolipoprotein B (ApoB)–against siRNA (siApoB) to form siApoB/LNP complexes. Then, all complexes were transfected into HepG2 cells to give a final concentration of siApoB at 50 nM. After the determination of ApoB gene relative expression by real-time quantitative polymerase chain reaction (RT-qPCR), formulations of A1-B3-7-5 (Fig. 1E), A1-D1-5-13 (Fig. 1F), A2-C1-8-14 (Fig. 1G), and A3-C1-8/D1-7-15 (Fig. 1H) showed the best gene silencing activity based on each key lipid, respectively, and which were selected for further evaluation. Then, we evaluated the transfection efficiency of these four formulations at different transfection concentrations in vitro, which indicated that A1-D1-5-13 formulation is the best among all to deliver siRNA in cells (Fig. 1I). Therefore, A1-D1-5-13 was lastly selected as the leading one and termed as iLAND. iLAND also means an intelligent LANDing technology for delivering nucleic acid modalities to the targeted tissue and cell.
Characterizations of iLAND
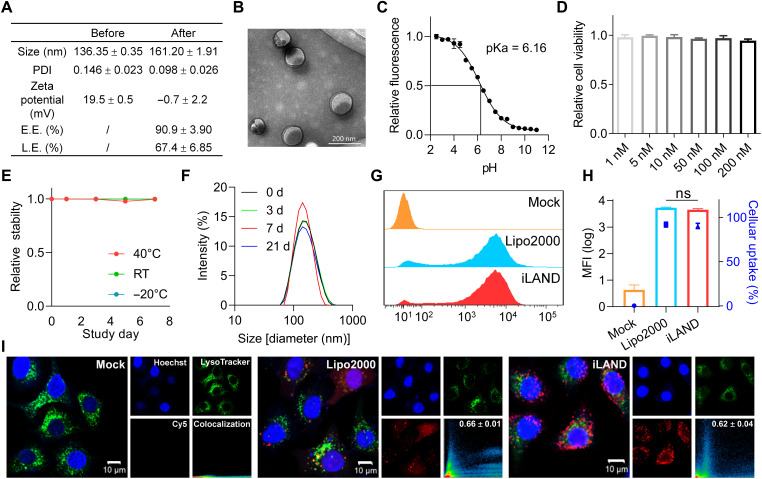
After screening and selection of the leading lipid structure (A1-D1-5) and formulation composition (A1-D1-5-13, or iLAND), the physicochemical properties including hydrodynamic size, PDI, and zeta potential of iLAND were first investigated by dynamic light scattering (DLS), which were around 140 nm, 0.15, and 20 mV, respectively (Fig. 2A). With the encapsulation of siRNA, the size of the nanoparticles was slightly larger and uniformed. Moreover, the zeta potential of iLNP was also sharply decreased to nearly 0 mV at neutral pH, indicating successful binding between iLNP and siRNA. In addition, encapsulation efficiency and loading efficiency were recorded as higher than 90 and 65%, respectively (Fig. 2A). Subsequently, the morphology of iLAND loaded with siRNA was observed by transmission electron microscopy (TEM). Lipid bilayer structures were clearly observed, and all particles displayed uniform size of less than 200 nm (Fig. 2B).
Fig. 2. Physicochemical property characterization of iLAND and its cellular uptake behavior.
(A) Physicochemical property characterization of iLAND before and after siRNA encapsulation, which includes the size, PDI, zeta potential, encapsulation efficiency (E.E.), and loading efficiency (L.E.). (B) TEM image of siRNA@iLAND. (C) TNS was used for pKa determination, and the pKa of iLAND was approximately 6.16. (D) Cell viabilities of siRNA@iLAND were evaluated by using HepG2 cells. n = 6. (E) Chemical stability of A1-D1-5 and (F) formulation stability of siRNA@iLAND. RT, room temperature. (G) Cellular uptake and (H) the MFI of siRNA@iLAND, comparison with siRNA@Lipo. (I) Subcellular localization of siRNA@iLAND or siRNA@Lipo2000. ns, not significant.
It was reported that the iLNP can robustly deliver nucleic acid payloads to targeting cells by achieving effective endosomal escape through interacting with anionic lipids of endosomal membrane such as phosphatidylserine in acidic environment. pKa is the determinant factor of such escape behavior of LNP in cells, and it must meet or exceed 5.5 (38). Hence, we determined the pKa of iLAND by using 2-(p-toluidino)-6-naphthalene sulfonic acid (TNS). The fluorescence intensities of iLAND in different pH, ranging from 2.5 to 11, were recorded with a luminescence spectrophotometer. A sigmoidal best fit analysis was applied to obtained data, and the pKa of iLAND recorded was approximately 6.16 (Fig. 2C).
In addition, the cell viabilities were evaluated in HepG2 cells. The viability of siNC@iLAND-treated cells (200 nM) was comparable to that of phosphate-buffered saline (PBS)–treated cells (94.7% versus 100%) (Fig. 2D), indicating that siRNA@iLAND complex has a good biocompatibility in vitro. Furthermore, the stability of A1-D1-5 and siRNA@iLAND was also evaluated. The results indicated that both A1-D1-5 and siRNA@iLAND have excellent stability. A1-D1-5 did not degrade at 40°C, room temperature (around 20°C), and −20°C for at least 1 week (Fig. 2E), and the LNP was also stable for at least 3 weeks at 4°C (Fig. 2F). This was critical to the development of RNA interference (RNAi) (or mRNA) therapies that can be stored and transported at refrigeration temperatures or even room temperature.
Internalization behavior of siRNA@iLAND and mechanism exploration
Achieving efficient cellular entry and endosomal escape is of great importance for siRNA delivery and drug development. To investigate the transfection behavior of iLAND, we encapsulated the Cy5-labeled siRNA (Cy5-siRNA) into the iLAND or Lipofectamine 2000 (Lipo2000). This siRNA targets the X gene of hepatitis B virus (HBV); therefore, there is no target transcript sequence in normal tissues and non-HBV–derived tumors. It can be considered as a negative control (NC) siRNA in this study. HepG2 cells were treated with Cy5-siRNA@iLAND or Cy5-siRNA@Lipo2000 for 4 hours. After that, the cellular uptake efficiency and intracellular distribution were determined. iLAND showed high internalization and endosomal escape efficiency. The cellular uptake efficiency (Fig. 2G), mean fluorescence intensity (MFI) (Fig. 2H), and the colocalization of siRNA and endosome/lysosome (Fig. 2I) were almost identical to commercial Lipo2000. It is well known that Lipo2000 is a gold standard for in vitro nucleic acid (siRNA) transfection; however, it can be hardly used for in vivo studies.
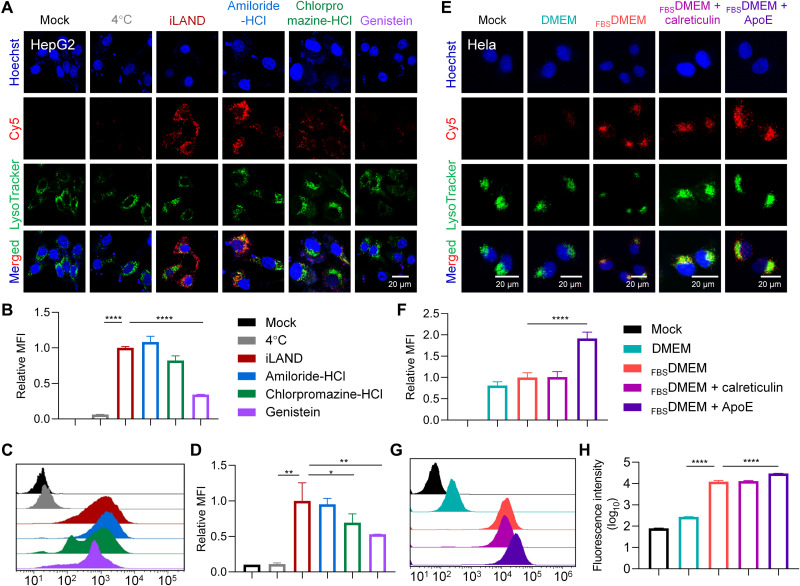
Furthermore, we have continued to identify the internalization pathway by which the iLAND entered the cells. It was reported that the uptake pathways of extracellular macromolecules are mainly macropinocytosis, caveolae-mediated endocytosis, clathrin-mediated endocytosis, and caveolae/clathrin dual-independent endocytosis (40). Accordingly, amiloride-HCl, genistein, and chlorpromazine-HCl were used to inhibit the first three types of process, respectively. After pretreatment with the inhibitors, 50 nM Cy5-siRNA@iLAND was added into the cells, followed by culturing for 4 hours at 37°C. Then, the confocal observation was performed to analyze the internalization behavior of siRNA@iLAND. Compared with iLAND transfected without the addition of inhibitor, the uptake efficiency of cells pretreated with different inhibitors varied greatly. Amiloride-HCl barely affected the uptake of siRNA@iLAND by HepG2 cells, while genistein and chlorpromazine-HCl significantly repressed the cellular entry of siRNA@iLAND (Fig. 3A). The quantitative analysis results of the confocal images (Fig. 3B) also confirmed these observations. Flow cytometry was further performed to analyze the internalization pathways of siRNA@iLAND (Fig. 3, C and D). Compared to cells without using inhibitor, the Cy5 fluorescence intensities of cell pretreated with genistein, chlorpromazine-HCl, and amiloride-HCl were decreased by 47, 31, and 5%, respectively (Fig. 3D). Hence, the internalization process of iLAND was mainly mediated by caveolae without the dependence of macropinocytosis. Furthermore, the cells transfected at 4°C displayed very low fluorescence intensity, suggesting that internalization of iLAND was strictly energy dependent (Fig. 3, C and D).
Fig. 3. Mechanism exploration for iLAND-mediated siRNA transfection.
(A) Subcellular localization of Cy5-siRNA@iLAND in HepG2 cells receiving various treatments. (B) Relative MFI of Cy5-siRNA recorded in (A). (C) Cellular uptake of Cy5-siRNA@iLAND in HepG2 cells as recorded by fluorescence-activated cell sorting (FACS). (D) Quantitative analysis of (C). (E) Confocal observation of the internalization of Cy5-siRNA@iLAND in HeLa cells. ApoE and calreticulin were used to determine whether they bound with iLAND and facilitated the cellular entry of Cy5-siRNA@iLAND. DMEM, Dulbecco’s modified Eagle’s medium; FBSDMEM, DMEM containing 10% fetal bovine serum. (F) Quantitative analysis of the fluorescence intensity of siRNA as recorded in (E). (G and H) Cellular uptake of Cy5-siRNA@iLAND in HeLa cells as recorded by FACS. siRNA was transfected at a final concentration of 50 nM. Scale bars, 20 μm.
When LNPs enter blood circulation, biomolecules in blood may absorb onto them to form “protein corona” (41), which potentially will decide the fate of LNPs in vivo. It was reported that iLNPs, but not cationic LNPs (cLNPs), may accumulate in liver via an apolipoprotein E (ApoE)–mediated active-targeting mechanism. It is because the ApoE binds onto iLNPs in circulation taking them to the liver and interact with its receptor (such as LDL receptor) and lastly achieve a ligand-receptor–meditated transportation process (42, 43). Therefore, we further investigated whether ApoE also increases the delivery efficiency of iLAND in vivo. HeLa cell line was used in this study because it expressed the receptor of ApoE without expressing ApoE (42). The cells were treated with Cy5-siRNA@iLAND complexes and cultured in Dulbecco’s modified Eagle’s medium (DMEM) or FBSDMEM [DMEM containing 10% fetal bovine serum (FBS)]. Meanwhile, Cy5-siRNA@iLAND was also preincubated with ApoE or calreticulin and then added into the FBSDMEM. Calreticulin resides primarily in the endoplasmic reticulum (ER) and is involved in a variety of cellular processes such as cell adhesion, protein folding quality control, and calcium homeostasis. Because calreticulin is usually only transported from the ER to the surface of the cell membrane under some physiological conditions, such as immunogenic cell death of cancer cells (44), it is generally assumed that calreticulin does not involved in the endocytosis of exogenous payloads. Therefore, calreticulin was used as a NC protein in this assay. In addition, cationic DOTAP [1,2-dioleoyloxy-3-(trimethylammonium) propane] LNP was introduced to verify whether ApoE specifically promoted iLNP internalization. In line with previous observations, addition of ApoE markedly enhanced cellular uptake of iLAND in HeLa cells, and calreticulin showed no benefit to internalization of iLAND, as recorded by confocal imaging (Fig. 3, E and F). However, either ApoE or calreticulin did not promote the cellular uptake of DOTAP cLNP in HeLa cells (fig. S10). In addition, fluorescence-activated cell sorting (FACS) results also demonstrated that a significantly higher number of siRNAs were transfected into HeLa cells when ApoE was used (Fig. 3, G and H). The MFI in cells treated with Cy5-siRNA@iLAND plus ApoE was approximately 1.9 times higher than that in cells treated with LNP without ApoE incubation. Collectively, ApoE is crucial for iLAND-mediated siRNA delivery to hepatocytes.
Endosomal escape of siRNA@iLAND
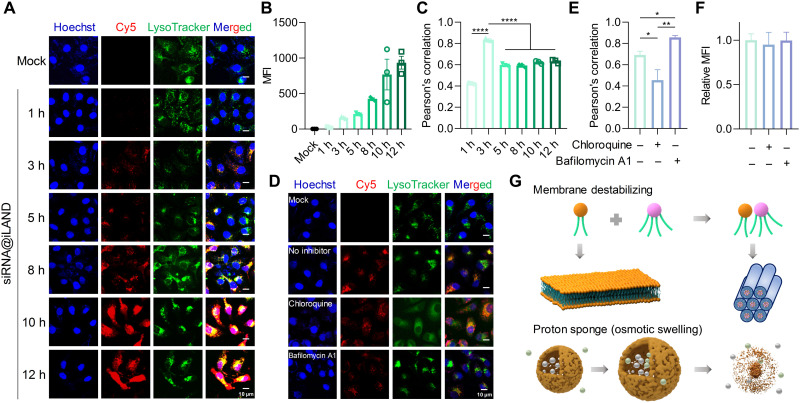
Previous assays proved that siRNA@iLAND entered cells mainly via caveolae-dependent endocytosis facilitated by ApoE. In this case, siRNA will be entrapped in the endosome and lysosome. Rapid and efficient escape of siRNA from the endosome and lysosome to reach RNA-induced silencing complex in the cytosol is of importance for gene silencing. Therefore, we investigated the intracellular trafficking process of siRNA@iLAND. As shown in Fig. 4 (A and B), the fluorescence intensity of Cy5-siRNA in cells increased alone with the extension of transfection time, suggesting that siRNA@iLAND were continuously internalized by cells. By analyzing the signals from siRNA and endosome/lysosome, we found that the highest level of colocalization between siRNA and endosome/lysosome occurred around 3 hours after transfection (Fig. 4C). This observation suggested that the amount of siRNA@iLAND endocytosed by cells and localized in the endosome/lysosome was higher than the amount of siRNA escaped to cytoplasm within the first 3 hours after transfection; after that, it was observed that the release speed became faster than the endocytosis speed.
Fig. 4. Intracellular trafficking and endosomal escape of siRNA@iLAND.
(A) Cellular uptake of Cy5-siRNA@iLAND in HepG2 cells at different transfection time points. (B) Intracellular fluorescence intensities of siRNA at different time points after transfection. (C) Colocalization between Cy5-siRNA and endosome or lysosome. The highest level occurred around 3 hours after transfection. (D) Influence of chloroquine and bafilomycin A1 on cellular entry and endosomal escape of siRNA@iLAND. (E and F) Colocalization analysis (E) and MFI of siRNA in cells (F) that recorded in (D). (G) Scheme of iLAND-mediated siRNA escape from endosome (or lysosome). siRNA was transfected at a final concentration of 50 nM. n = 6.
In addition, chloroquine and bafilomycin A1 were further used to explore the intracellular trafficking mechanism of siRNA@iLAND. Chloroquine can disrupt the membrane of endosome and lysosome, and bafilomycin A1 is a well-known proton pump inhibitor that selectively inhibits vacuolar H+–adenosine triphosphatase (V-ATPase). The subcellular observation and colocalization analysis (Fig. 4, D and E) proved that the chloroquine significantly enhanced the endosome escape efficiency, while bafilomycin A1 inhibited this process, because the Pearson’s correlations in cells treated with chloroquine and bafilomycin A1 were lower and higher, respectively, than that in cells without inhibitor treatment. Accordingly, the gene silencing activity of siApoB@iLAND was elevated and reduced by addition of chloroquine and bafilomycin A1, respectively (fig. S11). It was worth mentioning that these two inhibitors did not influence the cellular entry of siRNA@iLAND, as the MFI did not change when we used these agents (Fig. 4F).
It was reported that iLNP could achieve efficient endosomal escape mainly via a membrane destabilization mechanism (18, 29, 45, 46). Proton sponge effect and osmotic swelling may also contribute to this process (47). The pKa value of iLAND is approximately 6.16, which met the requirement of achieving aforementioned effects. When the endosome (or lysosome) and the ionizable lipid exist independently, the anionic lipids will maintain a columnar shape (Fig. 4G). After siRNA@iLAND accumulates in the endosome (or lysosome), it will be protonated because of the acceptance of protons by amine groups on the lipid. Thus, the charges on the nanoparticle will increase, leading to formation of ion pairs between ionizable lipids in iLNP and negatively charged lipid components in the endolysosomal membrane (such as phosphatidylserine). Then, the columnar shape of membrane changed to a conical structure, triggering siRNA release into the cytoplasm (Fig. 4G). In addition, because bafilomycin A1, an inhibitor of V-ATPase, significantly affected the release of siRNA@iLAND (Fig. 4, D and E), it was believed that proton sponge and osmotic swelling effects were also involved in this process (Fig. 4G).
In vivo biodistribution
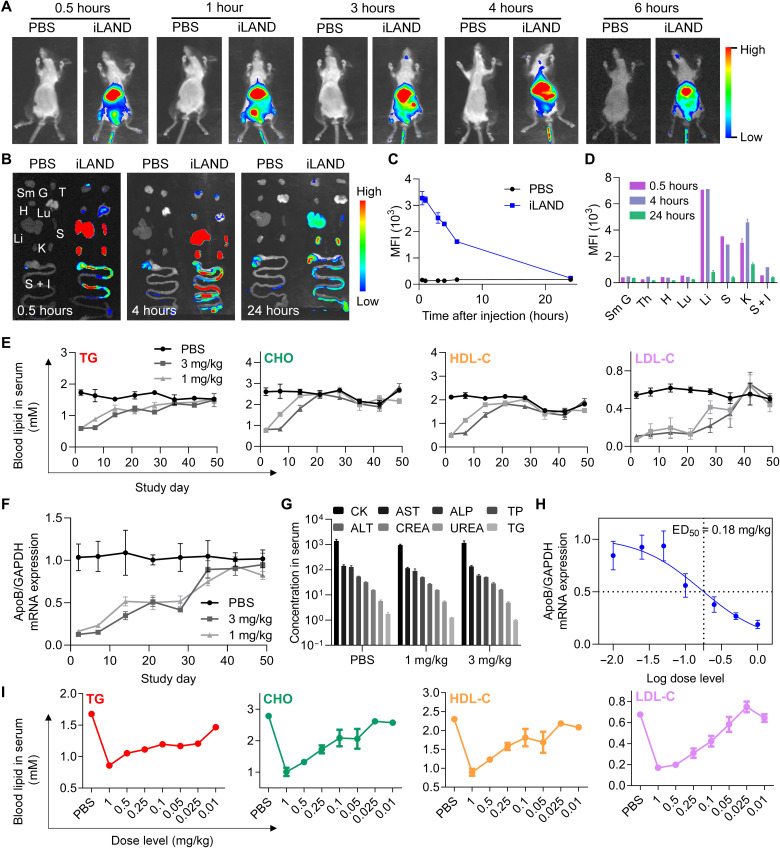
The in vivo biodistribution behavior of siRNA@iLAND were investigated by in vivo imaging. After intravenous injection, Cy5-siRNA@iLAND quickly and dominantly accumulated in the liver (Fig. 5, A to C, and fig. S12). To more accurately understand/analyze the biodistribution of siRNA@iLAND in each organ and tissue, one or two animals in each group was euthanized and dissected after 0.5, 4, and 24 hours after dose, respectively. As shown in Fig. 5B, a large portion of siRNA@iLAND accumulated in the liver, spleen, and kidneys, followed by the intestine. The distribution of nanoformulated siRNA accumulated in the intestine was in accordance with previous observations (48, 49). Furthermore, the relative signal ratio among all examined organs, including the submandibular gland, thymus gland, heart, lung, liver, spleen, kidney, stomach, and intestines, at different imaging times was analyzed by setting the signal ratio sum as 100%. As a result, the fluorescence signal recorded in the liver was 45, 41, and 20%, while 22, 17, and 10% in the spleen, whereas 19, 26, and 35% in the kidneys at 0.5, 4, and 24 hours after injection, respectively (Fig. 5D). The total amount of fluorescent signals reached the peak at approximately 4 hours after injection. While it was noted that the ratio of signal was decreased in the liver and spleen, it was increased in the kidney. In summary, these data suggested that siRNA@iLAND quickly and dominantly accumulated in the liver during absorption phase and distribution phase. According to the results, it was observed that the siRNA@iLAND was excreted mainly through kidneys and intestinal tract and barely accumulated in the heart, lungs, and the glands.
Fig. 5. In vivo biodistribution and activity evaluation of siRNA@iLAND.
(A) Whole-body imaging of the mice at indicated time points after intravenous injection of 1× PBS or Cy5-siRNA@iLAND (2 mg/kg). (B) Fluorescent imaging of isolated organs at 0.5, 4, and 24 hours after injection. Sm G, submandibular gland; T, thymus; H, heart; Lu, lung; Li, liver; S, spleen; K, kidney; S + I, stomach and intestines. (C) Quantitative analysis of the fluorescence signal in the liver as recorded in whole-body imaging. (D) Quantitative analysis of the fluorescence signal in isolated organs at indicated time points. (E) Concentrations of TG, CHO, HDL-C, and LDL-C in serum specimens collected at indicated time points from the mice receiving a single dose of 1× PBS, 1 mg/kg siApoB@iLAND, or 3 mg/kg siApoB@iLAND. (F) ApoB mRNA expression in liver tissues collected in the assay of (E). At each time point, the animals were euthanized and the livers were collected. Then, total RNA was extracted for determination of mRNA expression. (G) Serum biochemistry analysis. Samples were collected from CD-1 mice receiving 1× PBS, 1 mg/kg siNC@iLAND, or 3 mg/kg siNC@iLAND. Eight parameters including creatine kinase (CK; U/liter), aspartate aminotransferase (AST; U/liter), alkaline phosphatase (ALP; U/liter), total protein (TP; g/liter), alanine aminotransferase (ALT; U/liter), serum creatinine (CREA; μM), urea nitrogen (UREA; μM), and TG (mM) were recorded. (H) ApoB mRNA expression in the mice receiving different doses of siApoB@iLAND complexes. The doses ranged from 0.01 to 1 mg/kg. (I) Serum lipid changes in the dose-dependent assay.
In vivo performance of iLAND
To further understand in vivo performance of siRNA@iLAND, the ED50, duration of gene silencing, and safety were further evaluated. First, three groups of animals received a single dose of 1× PBS, 1 mg/kg siApoB@iLAND, and 3 mg/kg siApoB@iLAND, respectively. siApoB is an siRNA targeting ApoB, while, ApoB is a large protein that serves as the backbone of LDL and other lipoproteins. Mipomersen, an antisense oligonucleotide (ASO) therapeutic, is indicated for the treatment of homozygous familial hypercholesterolemia by repressing the expression of ApoB . After injection, the concentrations of total TG, total CHO, high-density lipoprotein CHO (HDL-C), and LDL-C were continuously analyzed. As shown in Fig. 5E, the reduction of these parameters was well maintained for 3 weeks. Then, the levels of CHO and HDL-C recovered to the baseline, while TG and LDL-C remained robustly inhibited until 5 weeks after administration. Organ coefficients of the liver recorded at the end of experiment showed that the values in animals receiving siApoB@iLAND treatment were dose-dependently elevated, indicating that lipid accumulation in the liver occurred (fig. S13A). Organ coefficients of the spleen of the animals receiving siApoB@iLAND treatment remained in a comparable level with that in the PBS group (fig. S13B). In addition, accumulation of hepatic fat was further analyzed. Data revealed that TG levels in the liver were dose-dependently elevated after treatment with siApoB@iLAND, consistent with observations in the preclinical and clinical investigation of Mipomersen (50) (fig. S14A). The CHO levels in the liver also slightly increased in the mice receiving relatively high doses (e.g., 1 and 0.5 mg/kg) of siApoB@iLAND (fig. S14B). In addition, the expression of ApoB mRNA was markedly and durably inhibited by siApoB@iLAND. As high as 50% down-regulation of ApoB mRNA was achieved even at day 28 after single injection (Fig. 5F).
Another three groups of animals were also treated with PBS, 1 mg/kg siNC@iLAND, and 3 mg/kg siNC@iLAND. siNC is a scramble siRNA without any target sequence in the transcripts of human beings, monkey, rat, and mouse. The animals were euthanized at 48 hours after injection, and the blood and organs were collected for safety evaluation. As shown in Fig. 5G and fig. S15, siNC@iLAND did not cause obvious side effects because the level of serum biochemistry parameters in siNC@iLAND-treated animals, including TG, alkaline phosphatase, creatine kinase, aspartate aminotransferase, total protein, alanine aminotransferase, serum creatinine, and urea nitrogen, all remained in normal range and showed no significant difference compared to PBS. No significant pathological change was observed for all three groups of animals (fig. S15).
Furthermore, dose-dependent assay was performed by using different doses of siApoB@iLAND, which ranged from 0.01 to 1 mg/kg. Two days later, animals were euthanized after a 6-hour food fast. Serum specimens and the livers were collected and analyzed. It was observed that the expression of ApoB mRNA and the reduction of serum lipids displayed strong dose-dependent patterns. As a result, the ED50 of siApoB@iLAND was calculated, and it was as low as 0.18 mg/kg (Fig. 5H). Meanwhile, on the basis of the assumptions that the highest dose of siApoB@iLAND triggers the highest reduction of serum lipid and that the reduction extent will not be further enhanced if we continue to enhance the dose of siApoB@iLAND, we can calculate the ED50 of siApoB@iLAND with the data of lipid concentration. Accordingly, ED50s of siApoB@iLAND, as calculated with the data of TG, CHO, LDL-C, and HDL-C, were 0.03, 0.25, 0.55, and 0.13 mg/kg, respectively (Fig. 5I). This is comparable with the ED50 determined with ApoB mRNA. Together, iLAND mediated robust mRNA repression in a time and dose dual-dependent manner. Long-lasting efficacy could be achieved when a relatively higher dose of siRNA@iLAND was applied.
In addition, an activity comparison study by using iLAND, Dlin-MC3-DMA (MC3) LNP, and GalNAc-siRNA conjugate was performed in mice. Both MC3 LNP and GalNAc-siRNA conjugate have been used in Food and Drug Administration–approved siRNA therapeutics (14). The siANG@iLAND and siANG@MC3 LNP were intravenously administered to the mice, and GalNAc-siANG was either intravenously or subcutaneously administered to the animals. The doses were 0.2, 1, and 3 mg/kg, respectively. The results demonstrated that both iLAND and MC3 LNP mediated robust gene silencing in liver tissues. The reduction efficiencies were overall comparable although MC3 LNP displayed slightly higher activity at the lowest dose (0.2 mg/kg), which was in line with the ED50 determination data of these two LNPs (Fig. 5H and fig. S16, A to C) (45). Meanwhile, both iLAND and MC3 LNP resulted in higher gene down-regulation than GalNAc-siANG at all three doses (fig. S16, A to C). This was also consistent with the observations of Alnylam Pharmaceuticals and other companies such as Arrowhead Pharmaceuticals, Dicerna Pharmaceuticals, etc. Because the pharmacokinetics property of the GalNAc-siRNA conjugate was different from that of LNPs, the highest knockdown normally happened on days 7 to 14 after subcutaneous injection of GalNAc-siRNA (51). However, to directly compare the activity of these representative hepatic siRNA delivery platforms, the sample collection time was kept the same (72 hours after injection). In addition, the safety analysis indicated that all formulations were well tolerated by the animals for all tested doses (fig. S16, D to F).
Lipid-lowing effects of siRNA@iLAND in HFD-fed model
Encouraged by previous data regarding the in vivo distribution, dose-dependent assay, and duration test, we continued to investigate the treatment effects of siRNA@iLAND in three animal disease models. First, HFD was applied to establish hyperlipidemia mouse model. Then, siRNA targeting ANGPTL3 (siANG) was encapsulated with iLAND. ANGPTL3 predominantly is expressed by the liver and involved in regulation of lipoprotein metabolism. Reduction of plasma TG, LDL-C, and HDL-C levels were observed in humans carrying ANGPTL3 loss-of-function mutations. Inhibition of ANGPTL3 with antibody, ASO, or siRNA reduced TG, LDL-C, and HDL-C in mice, monkeys, and humans (3, 52). siANG@iLAND was dosed at 0.25 mg/kg. Data showed that the levels of CHO and TG in serum were significantly elevated after HFD inducement, while after treatment with siANG@iLAND, the levels of CHO and TG both recovered to comparable levels of them in normal C57BL/6 mice (fig. S17). In addition, the expression of ANGPTL3 was determined at the end of experiment, suggesting that siANG@iLAND robustly inhibited the expression of ANGPTL3 in the liver. This study preliminarily demonstrated that siANG@iLAND could effectively reduce the levels of TG and CHO in circulation in an HFD-induced hyperlipidemia animal model.
Efficacy investigation of siRNA@iLAND in db/db mouse model
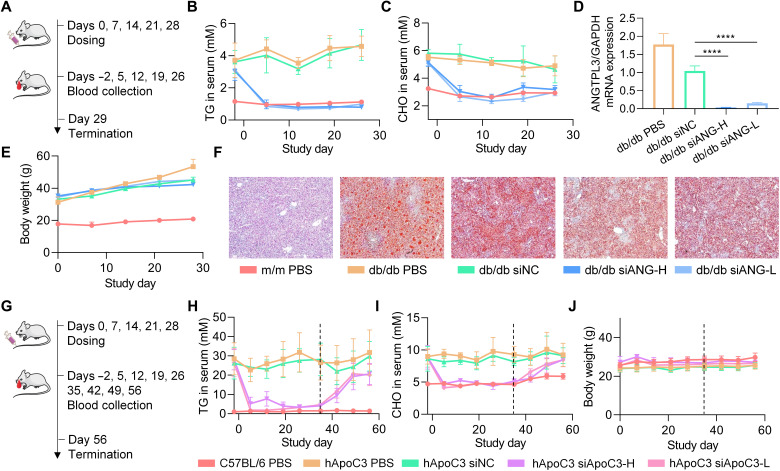
Furthermore, db/db mice differentiated by the spontaneous mutations in different sites of leptin receptors was used. The db/db mice are perfect animal models of type 2 diabetes displaying phenotypes of severe obesity, hyperlipidemia, hyperphagia, polydipsia, and polyuria. The m/m mice with the same genetic background were used as control. Five doses of siANG@iLAND or siNC@iLAND were administered once per week (Fig. 6A). The doses of siANG@iLAND were 0.5 mg/kg (siANG-H) and 0.25 mg/kg (siANG-L), respectively. The dose of siNC@iLAND was 0.5 mg/kg. The levels of TG and CHO and the body weight were monitored during the whole treatment course. Animals were then euthanized to collect liver samples for mRNA expression determination by qPCR at the end of the experiment. As a result, siANG@iLAND triggered significant reduction of TG and CHO in serum, even at a low dose of 0.25 mg/kg (Fig. 6, B and C). After administering the first dose, the serum TG and CHO levels in db/db mice reduced to comparable levels in normal m/m mice. The inhibition pattern was well maintained, and no fluctuation was observed during the whole treatment course. Data also showed that siANG delivered by iLAND markedly inhibited the mRNA expression of ANGPTL3. Compared with siNC (0.5 mg/kg), siANG-H suppressed 97% of ANGPTL3 expression, and the inhibition efficiency mediated by siANG-L was 85% (Fig. 6D). The body weights of animals in all groups grew slowly compared with the weights at the starting point (Fig. 6E). It was reported that the livers of db/db mice were featured by lipid deposition (53). According to the Oil red O staining results, the livers of db/db mice that received the treatment of siANG@iLAND showed less lipid droplet accumulation in cytoplasm (Fig. 6F). In addition, the organ coefficients of the liver and spleen were calculated, suggesting that the weights of the livers remained at normal range. The spleen coefficient in db/db mice receiving siANG treatment increased compared with that in db/db mice receiving PBS treatment, beginning to approach the values of normal m/m mice (fig. S18). The heart, lung, liver, spleen, and kidney were harvested for hematoxylin and eosin (H&E) staining at the end of the experiment. No obvious pathological change was observed in all tissue samples (fig. S19).
Fig. 6. Efficacy studies of siANG@iLAND and siApoC3@iLAND in disease models.
(A) Treatment schedule of siANG@iLAND in db/db mice. (B and C) Levels of serum TG (B) and serum CHO (C) recorded during the treatment course. (D) ANGPTL3 mRNA expression determined by RT-qPCR at the end of study. (E) Body weight changes during the study. Serum specimens were collected after a 6-hour food fasting for determining the TG and CHO concentrations, and the body weights were recorded before food fasting. (F) Oil red O staining of the liver sections of each group. (G) Treatment schedule of siApoC3@iLAND in hApoC3-Tg mice. (H and I) Levels of serum TG (H) and serum CHO (I) recorded during the treatment and recovering courses. (J) Body weight changes during treatment and recovery periods.
Efficacy investigation of siRNA@iLAND in human ApoC-III Tg mouse model
Apolipoprotein C-III (ApoC-III, or ApoC3), an 8.8-kDa glycoprotein associated with TG-rich lipoproteins, LDLs, and HDLs, is predominantly produced by the liver and, to a lesser extent, the intestine. Rare mutations that disrupt ApoC3 function are associated with lower levels of plasma TGs and ApoC3. Carriers of these mutations were found to have a reduced risk of coronary heart disease (54). Inactivating mutations affecting the expression of ApoC3 in humans led to reduced plasma TG levels and protection against CVDs (55). Therefore, human ApoC-III Tg mice [B6;CBA-Tg(APOC3)3707Bres/J (stock 006907), the Jackson Laboratory] were used to assess the therapeutic effects of siRNA@iLAND. ApoC3-against siRNA (siApoC3) was applied in this study. Five doses of siApoC3@iLAND was intravenously administered once per week at 0.5 mg/kg (siApoC3-H) or 0.25 mg/kg (siApoC3-L), respectively (Fig. 6G). siNC@iLAND (siNC; 0.5 mg/kg) was also included as NC. Levels of TG and CHO in serum were also continuously monitored during treatment and recovery period (Fig. 6G). Similar to the observations in the study of db/db mice, serum TG and CHO levels in hApoC3-Tg mice reduced to normal levels in siApoC3-treated groups (Fig. 6, H and I). In addition, a slow increase in lipid concentration was observed after stopping the administration of siRNA for both serum TG and serum CHO. Serum CHO level returned to the preadministration level at 4 weeks after the last injection. TG did not return to the baseline until the end of the study. Continuous body weight monitoring showed that the formulation has no obvious side effects (Fig. 6J). The safety was also confirmed by pathological analysis of the H&E-stained tissue sections (fig. S20). Evidenced by abovementioned three studies in three different disease models, siRNAs delivered by iLAND effectively alleviated hyperlipidemia conditions and potentially could be used to protect against CVDs and other metabolic diseases. iLAND delivery platform also displayed ideal safety profiles in vivo.
DISCUSSION
Hyperlipidemia, or CVDs, is the leading cause of death in the world and is predicted to become a prominent problem worldwide. In addition to building a healthy lifestyle, taking medication is an essential way to control the disease. Currently, the lipid-lowering therapeutic interventions are aimed at lowering LDL-C. However, patients with significantly reduced LDL-C still had residual cardiovascular risk that gradually increased with each additional feature of the metabolic syndrome (13). This residual risk has raised concerns about elevated plasma TG levels, which are independent risk factors for coronary artery disease. Therefore, it is of great significance to develop therapeutic agents that can reduce both CHO and TG for the treatment of hyperlipidemia and the prevention of CVDs.
The slow identification and optimization of the lead compound of small molecule and antibody brings inconvenience to drug development. On the contrary, because of the short development time, high specificity, properties that are highly predictable and build confidence in translation from animals to man (56), siRNAs are promising modalities for the development of next-generation of lipid-lowering drugs. LNPs are extensively used in siRNA delivery because they can significantly improve the pharmacokinetic and pharmacodynamics properties of siRNA, and they have successfully pushed for the first approval of RNAi therapy (16). Therefore, the core mission is to develop and optimize a novel LNP delivery system with high transportation efficiency and desirable safety profile in vivo.
Here, a rational design process of ionizable lipid-like materials is demonstrated, which requires minimal work load using four novel lipid structures. It is well known that the proportion of the helper lipids in the composition significantly affects the efficiency of LNPs. Lower PEG density may induce undesired adverse effects and higher PEG density may reduce the delivery efficiency (35). In addition, the content of DSPC and CHO may affect the morphology and size and thus affect the delivery efficiency (34). Therefore, a rational range of molar percentage for each component was elaborately designed in the first round of screening, which were as follows: ionizable lipid, 32.0 to 53.7%; DSPC, 6.6 to 17.6%; CHO, 26.4 to 48.6%; and DMG-PEG2000, 0.4 to 4.3%. In the pilot study, we observed that LNP-related toxicity was mainly caused by excessive accumulation of lipid molecules in major organs such as the liver, kidney, and lung. The evidence suggested that the proportion of lipid components should be minimized in the system without affecting siRNA delivery efficiency. Results of gel retardation assay demonstrated that siRNA could be completely entrapped into LNP when the weight ratio of LNP to siRNA was higher than 15:1, indicating that this was the proper ratio between LNP and siRNA. We prepared 64 formulations in total to determine the factors that may affect delivery and safety, such as particle size and surface charge, etc. Fortunately, all candidates showed proper and uniform particle size and almost zero surface charge, which was important to ensure the in vivo safety because positively charged LNP may trigger immune response in circulation. The whole process, including the design of reasonable component ratio, the determination of the weight ratio of LNP and siRNA, the characterization of physiochemical properties, and evaluation of delivery efficiency, has been comprehensively optimized. As a result, the formulation of A1-D1-5-13, termed iLAND, was selected as the leading one, which was used in the following assays and treatments.
Consequently, delivery capability of iLAND was investigated. iLAND showed high cellular uptake and endosomal escape efficiencies in vitro, which were comparable to Lipo2000, a widely used in vitro transfection agent. In addition, we also explored the behavior and mechanisms of the internalization and intracellular trafficking processes of iLAND. Data revealed that cellular entry of siRNA@iLAND was mainly mediated by caveolae-dependent endocytosis. ApoE also played a pivotal role in mediating uptake by hepatocytes. After internalization, iLAND triggered rapid and efficient endosomal escape of siRNA from the endosome (or lysosome) via destabilizing the endosomal membrane and attracting bulk of protons into endosome. In addition, biodistribution assay suggested that iLAND quickly and dominantly accumulated in the liver. Dose-dependent activity evaluation demonstrated that the ED50 of siApoB@iLAND was as low as 0.18 mg/kg, and no obvious toxicity was observed when the dose increased to 3 mg/kg, revealing a considerable therapeutic window.
In addition, efficacy investigation was performed in three hyperlipidemia mouse models, which included HFD-fed mice, db/db mice, and hApoC3-Tg mice. Here, siANG was used in former two models, and siApoC3 was applied in hApoC3-Tg mice. It is assumed that the employment of these two siRNAs can not only down-regulate the serum CHO level but also reduce the serum TG level, which is very beneficial for the treatment of hyperlipidemia and the prevention of CVDs. Data manifested that the siANG delivered by iLAND could reduce the serum CHO and TG levels in the HFD model and the db/db model to the normal range, compared with those in normal C57BL/6 mice or m/m mice, respectively. These therapeutic effects were well maintained at least for 3 weeks at the dose of 0.5 mg/kg, while siNC could not achieved these outcomes, and robust gene suppression were observed after administrations, which both indicated that the treatment effects were mediated by RNAi mechanism. siApoC3 encapsulated by iLAND also triggered potent and durable reduction of serum CHO and TG in human ApoC3 transgenic mice. Repeated dose of siRNA@iLAND did not induce clinically significant toxicity in vivo. Collectively, rationally designed and screened iLAND constitutes an excellent delivery platform for siRNA therapeutic development, and most importantly, therapeutic RNAi agents prepared in this study showed excellent treatment effects in three different hyperlipidemia animal models.
MATERIALS AND METHODS
Materials
siRNAs were provided by Suzhou Ribo Life Science Co. Ltd. (Suzhou, China) or Suzhou Biosyntech Co. Ltd. (Suzhou, China). GalNAc-siANG was provided by Suzhou Ribo Life Science Co. Ltd. (Suzhou, China). The detailed sequence information of siRNAs and primer sets were available in tables S11 and S12. DSPC, CHO, and DMG-PEG2000 were purchased from A.V.T. (Shanghai) Pharmaceutical Co. Ltd. Lipo2000, DMEM, Opti-MEM, penicillin-streptomycin, trypsin, and Quant-iT RiboGreen RNA Assay kit were purchased from Thermo Fisher Scientific Inc. (USA). FBS was purchased from PAN Biotech (Germany), and 6× loading buffer was purchased from Takara Biotechnology (China). Fluorescent mounting medium with DAPI (4′,6-diamidino-2-phenylindole) was purchased from Zhongshan Golden Bridge Biotechnology Co. Ltd. RNA isolater was purchased from Vazyme (Nanjing, China). Chloroform and isopropanol were purchased from Beijing Chemical Works. Transcript One-Step gDNA Removal and cDNA Synthesis kits and gel stain were purchased from Transgen Biotech (Beijing, China). Hieff qPCR SYBR Green Mix was purchased from YEASEN (Shanghai, China). ApoE (CI02) was purchased from Novoprotein Scientific Inc. (Shanghai, China). Calreticulin (RPB486Ra01) was purchased from Cloud-Clone Corp (Wuhan, China).
Synthesis of A1-B3-7
The reaction was monitored by thin-layer chromatography (TLC) on a precoated silica gel GF254 plate with a potassium permanganate solution. Column chromatography was performed on silica gel (200e300 mesh). 1H and 13C NMR spectra were recorded using a Bruker Avance III 400 or 700 spectrometer at the Beijing Institute of Technology Analysis and Testing Center, and tetramethylsilane was used as internal standard. High-resolution mass spectrometry (HRMS) was obtained on an Agilent 6520 liquid chromatography–mass spectrometry–quadrupole–time-of-flight mass spectrometer at the Beijing Institute of Technology Analysis and Testing Center.
(E)-tridec-2-en-1-yl-bromopropanoate [A1-B3-7-(1)]
Twenty milliliters of dichloromethane, 2 g of trans-2-tridecene-1-ol, and 1.71 g of 3-bromopropionyl chloride were added into a reaction flask under the protection of argon. The reaction was carried out for 2 hours at room temperature and monitored by TLC detection. After the completion of reaction, dichloromethane was removed, and the light-yellow oily substance was purified by a silica gel column with a yield of 89%. 1H NMR (400 MHz, CDCl3) δ: 5.75 to 5.86 (1, m, CH═CH), 5.52 to 5.65 (1, m, CH═CH), 4.56 to 4.61 (2H, d, OCH2), 3.56 to 3.62 (2H, t, CH2), 2.89 to 2.97 (2H, t, CH2), 2.16 to 2.97 (2H, m, CH2), 1.22 to 1.45 (16H, m), and 0.90 (3H, t, CH3) parts per million (ppm).
Di((E)-tridec-2-en-1-yl)3,3′-((3-((tert-butoxycarbonyl)amino)propyl)azanediyl)dipropionate [A1-B3-7-(2)]
Ten milliliters of anhydrous N,N′-dimethylformamide (DMF) was first added into the reaction flask under the protection of argon, and then 280 mg of N-BoC-1,3-diaminopropane hydrochloride and 721.4 mg of diisopropylethylamine were added into the flask under stirring. After a 2-hour reaction at room temperature, 1 g of A1-B3-7-(1) was added and reacted for overnight at room temperature. At the end of the reaction, DMF was removed by decompression, and 163 mg of light-yellow oily substance [A1-B3-7-(2)] was obtained by silica gel column chromatography with the yield of 18%. 1H NMR (400 MHz, CDCl3) δ: 5.76 to 5.83 (2H, m, CH═CH), 5.54 to 5.62 (2, m, CH═CH), 4.53 to 4.57 (4H, d, OCH2), 2.71 to 2.78 (4H, t, CH2), 2.43 to 2.52 (6H, m, CH2), 2.04 to 2.18 (4H, m, CH2), 1.60 to 1.68 (2H, m), 1.43 to 1.50 (12H, m), 1.22 to 1.36 (32H, m), and 0.84 to 0.90 (6H, m, CH3) ppm.
Di((E)-tridec-2-en-1-yl)3,3′-((3-aminopropyl)azanediyl)dipropionate hydrochloride [A1-B3-7-(3)]
A1-B3-7-(2) (163 mg) was added to 5 ml of 4 M HCl ethyl acetate solution and stirred at room temperature. The completion of reaction was determined by TLC after 1 hour. The solvent was removed by vacuum concentration.
Di((E)-tridec-2-en-1-yl)9,12-dioxo-4,17-bis(3-oxo-3-(((E)-tridec-2-en-1-yl)oxy)propyl)-4,8,13,17-tetraazaicosanedioate (A1-B3-7)
The A1-B3-7-(3) was dissolved in 2 ml of dichloroethane, and then 45 mg of triethylamine was added into the reaction flask under the protection of argon. After a 30-min reaction at room temperature, 14 mg of succinyl chloride was added. Then, the mixture was stirred at room temperature for 1 hour. After the completion of the reaction, the solution was diluted with 10 ml of dichloromethane and then washed with 1 M hydrochloric acid, and the organic phase was collected. The organic phase was concentrated by decompression and then separated and purified by passing through a silica gel column with a developing solvent of DCM:MeOH = 60:1 with 0.5% ammonium hydroxide to collect A1-B3-7. The two-step yield was 37%. 1H NMR (400 MHz, CDCl3) δ 6.92 (s, 2H), 5.76 (dd, J = 14.5, 7.5 Hz, 4H), 5.54 (dt, J = 14.2, 6.6 Hz, 4H), 4.51 (d, J = 6.5 Hz, 8H), 3.22 (m, 4H), 2.79 (s, 8H), 2.52 (m, 16H), 2.04 (q, J = 7.0 Hz, 8H), 1.68 (s, 4H), 1.41 to 1.32 (m, 8H), 1.26 (m, 64H), 0.88 (t, J = 6.6 Hz, 12H);13C NMR (100 MHz, CDCl3) δ 173.75, 142.64, 128.10, 125.51, 124.76, 118.07, 111.05, 66.24, 65.39, 60.77, 59.85, 35.43, 35.20, 31.93, 29.67, 29.59, 29.37, 25.41, 25.33, 22.70, and 14.13. HRMS (EI+) mass/charge ratio (m/z) calculated for C78H142N4O10(M+) 1295.07, found 1295.9.
Synthesis of A1-D1-5
Tert-butyl (3-(bis(2-hydroxydodecyl) amino) propyl) carbamate [A1-D1-5-(1)]
The reaction was carried out at 50°C overnight in a three-necked flask containing tert-butyl (3-aminopropyl) carbamate (1.01 g, 5.70 mmol), 1,2-epoxydodecane (2.33 g, 12.6 mmol), and 12.0 ml of ethanol refluxed under nitrogen. The reaction was monitored by TLC and stopped after completion. After being concentrated under reduced pressure, the crude product was separated and purified by passing through a silica gel column using methylene chloride:methanol 30:1 as a developing agent. A1-D1-5-(1) (1.61 g, yield 78.1%) was obtained as a transparent liquid. 1H NMR (400 MHz, CDCl3) δ (ppm) 0.88 (t, J = 13.68 Hz, 6H), 1.26 to 1.44 (m, 45H), 1.67 (m, 2H), 2.38 to 2.70 (m, 6H), 3.18 (m, 2H), 3.70 (m, 2H); 13C NMR (100 MHz, CDCl3) δ (ppm) 156.22, 79.39, 69.58, 62.90, 52.70, 38.58, 38.53, 35.13, 34.98, 31.92, 29.62, 29.35, 28.44, 25.65, 22.70, and 14.13; HRMS (EI+) m/z calcd for C32H66N2O4 (M+) 543.5023, found 543.5108.
1,1′-((3-aminopropyl) azanediyl)bis(dodecan-2-ol) [A1-D1-5-(2)]
In a three-necked flask containing the compound A1-D1-5-(1) (1.04 g, 1.91 mmol) and 20.0 ml of dichloromethane, hydrochloric acid was added slowly while stirring. The reaction was carried out at room temperature overnight and stopped after completion was monitored by TLC plate detection. The reaction mixture was adjusted pH to 7 to 8 using 1 M NaOH aqueous solution, then extracted with H2O and ethyl acetate three times, and concentrated under reduced pressure. After that, the crude product was separated and purified by passing through a silica gel column with a developing solvent of dichloromethane:methanol 15:1. A1-D1-5-(2) (398.00 mg, yield 38.3%) was obtained as a clear liquid. 1H NMR (400 MHz, CDCl3) δ (ppm) 0.88 (t, J = 13.08 Hz, 6H), 1.26 (m, 32H), 1.43 (m, 4H), 1.96 (m, J = 14.5 Hz, 2H), 2.60 (m, J = 20.9 Hz, 4H), 3.01 (m, 2H), 3.25 (m, 2H), 3.80 (m, 2H); 13C NMR (100 MHz, CDCl3) δ (ppm) 70.41, 63.21, 58.29, 40.79, 36.15, 31.94, 29.91, 29.69, 29.39, 23.90, 22.70, and 14.11; HRMS (EI+) m/z calcd for C27H58N2O2(M+) 443.4498, found 443.4576.
N1, N4-bis(3-(bis(2-hydroxydodecyl)amino)propyl)succinimide (A1-D1-5)
A1-D1-5-(2) (2.20 g, 4.96 mmol) and succinic acid (263.3 mg, 2.30 mmol) was dissolved in 20.0 ml of dichloromethane in a three-necked flask. Catalyst 1-hydroxybenzotriazole (753.3 mg, 5.57 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (1.13 g, 5.89 mmol) were then added. The reaction was allowed to proceed overnight at room temperature under the protection of nitrogen. The reaction was stopped after completion. The crude product was washed twice with water and twice with saturated brine, and the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. The crude product was separated and purified by passing through a silica gel column using methylene chloride:methanol 30:1 as a developing agent. A1-D1-5 (0.10 g, yield 4.06%) was obtained as a light-yellow liquid. 1H NMR (700 MHz, CDCl3) δ 8.02 (s, 2H), 3.97 (s, 2H), 3.86 (s, 2H), 3.31 (m,8H), 3.04 to 2.41 (m, 16H), 1.86 (m, 4H), 1.54 to 1.10 (m, 56H), 0.87 (m, 16H); 13C NMR (175 MHz, CDCl3) δ 173.53, 68.16, 67.54, 66.48, 61.52, 60.19, 38.73, 35.21, 31.94, 31.76, 30.36, 29.60, 28.93, 25.60, 24.73, 23.74, 22.99, 22.71, 14.06, and 10.96; HRMS (EI+) m/z calcd for C58H118N4O6(M+) 967.9051, found 967.9137.
Synthesis of A2-C1-8
N,N-bis(2-hydroxyethyl)acrylamide [A2-C1-8-(1)]
A total of 1.0 g of 2,2′-azanediyl bis(ethan-1-ol) (1.0 eq) was dissolved in 15 ml of dichloromethane, and 0.97 g of triethylamine (1.01 eq) was then added into the solution. Then, 0.87 g of acryloyl chloride (1.01 eq) was dropped into the system under argon protection reaction. After a 2-hour reaction in an ice bath, the crude product, A2-C1-8-(1), was obtained.
(acryloylazanediyl)bis(ethane-2,1-diyl)ditetradecanoate [A2-C1-8-(2)]
A total of 1.92 g (2.1 eq) of triethylamine and 4.92 g of myristic chloride were injected into the reaction system of the previous step, and the reaction was continued under the protection of argon and ice bath. After 2 hours, the solution was washed three times with 1 M dilute hydrochloric acid and washed with water one time, and then the organic phase was collected. After drying with anhydrous sodium sulfate, the solvent was removed by decompressed concentration. The product was purified by a silica gel column (DCM/DCM:MeOH = 500:1), and 3.0 g of white solid, A2-C1-8-(2), was obtained. 1H NMR (400 MHz, CDCl3) δ:6.58 to 5.63 (1H, m, CH═CH), 6.32 to 5.42 (1H, d, CH═CH), 5.68 to 5.73 (1, d, CH═CH), 4.18 to 4.30 (4H, dt, NCH2), 3.64 to 3.74 (4H, t, CH2), 2.30 to 2.38 (4H, t, CH2), 1.58 to 1.68 (4H, m), 1.29 to 1.29 (42H, m), 0.85 to 0.90 (6H, m, CH3) ppm.
Di-tert-butyl propane-1,3-diyldicarbamate [A2-C1-8-(3)]
A total of 1.0 g (1.0 eq) of butanediamine was dissolved in 30 ml of dichloroethane, and 5.2 g (2.1 eq) Boc anhydride was dropped into the system under an ice bath. After a reaction of 6 hours, 1 M dilute hydrochloric acid and saturated NaHCO3 were successively added for washing, and the organic phase was collected. The product was concentrated by decompression as 2.2 g of white solid powder.
Di-tert-butyl butane-1,4-diylbis(methylcarbamate) [A2-C1-8-(4)]
A total of 1.0 g (1.0 eq) of A2-C1-8-(3) was dissolved in 20 ml of dichloromethane, and 0.55 g (3.0 eq) NaH with a mass fraction of 60% was slowly added into the system under an ice bath. After 15 min, 1.0 g (2.0 eq) of CH3I was slowly dropped into the system by using a constant-pressure drop funnel. After the reaction at low temperature for 1 hour, 1 M dilute hydrochloric acid was added for quenching reaction, and DMF was removed by decompression. The product was dissolved by DCM, washed and extracted by DCM/H2O, and concentrated by decompression. Then, the product without solvent was purified via a silica gel column (polyethylene:ethyl acetate = 10:1). 1H NMR (400 MHz, CDCl3) δ: 3,24 (4H, m), 2.83 (6H, s, CH3), 1.46 to 1.51 (4H, m), 1.38 to 1.48 (18H, m) ppm.
N1,N4-dimethylbutane-1,4-diamine dihydrochloride [A2-C1-8-(5)]
A total of 2.5 g (1.0 eq) of A2-C1-8-(4) was dissolved in 30 ml of HCl ethyl acetate solution (4 M), the reaction was carried out at room temperature for 3 hours, and the production was then concentrated under reduced pressure to remove the solvent. 1H NMR (400 MHz, CDCl3) δ: 2.97 (4H, s), 2.57 (6H, s, CH3), 1.56 to 1.61 (4H, m) ppm.
7,12-dimethyl-20-methylene-4,15-dioxo-16-(2-(pentadec-1-en-2-yloxy)ethyl)-3-(2-(tetradecanoyloxy)ethyl)-19-oxa-3,7,12,16-tetraazatritriacontyl tetradecanoate (A2-C1-8)
One hundred thirty milligrams (1.0 eq) of A2-C1-8-(5) was dissolved in 15 ml of isopropanol, and 142.6 mg (2.05 eq) triethylamine was added into the system. After stirring at room temperature for 30 min, 817.1 mg (2.05 eq) of A2-C1-8-(2) was added and reacted at 90°C for 24 hours. Consequently, the production was concentrated by decompression and dissolved in DCM and then washed with 1 M dilute hydrochloric acid three times and H2O one time. The organic phase was collected and dried with anhydrous sodium sulfate, and the solvent was removed by vacuum concentration and purified by a silica gel column to obtain A2-C1-8 with a yield of 16%. 1H NMR (400 MHz, CDCl3) δ 4.21 (m, 8H), 3.81 to 3.57 (m, 8H), 3.27 to 2.99 (m, 8H), 2.82 (s, 6H), 2.33 (m, 8H), 2.11 (s, 4H), 1.77 (s, 4H), 1.59 (s, 8H), 1.25 (s, 80H), 0.87 (m, 12H). 13C NMR (100 MHz, CDCl3) δ 173.62, 169.34, 61.84, 61.64, 56.02, 52.42, 47.59, 46.26, 40.38, 34.18, 31.93, 29.65, 29.37, 28.36, 24.85, 22.70, 21.67, and 14.13; HRMS (EI+) m/z calcd for C76H146N4O10(M+) 1275.99, found 1276.0.
Synthesis of A3-C1-8/D1-7
1,1′-(butane-1,4-diylbis(azanediyl))bis(tetradecan-2-ol) [A3-C1-8/D1-7-(1)]
Two hundred milligrams (2.27 mmol) of 1,4-butanediamine was dissolved in 10 ml of isopropanol, and 1.1 g (4.76 mmol) of 2-dodecyloxirane was added, the system was heated to 70°C and stirred for 3 hours until white solid was precipitated. After that, the reaction was continuously carried out for 5 hours. Then, isopropanol was removed by decompression. Consequently, 20 ml of n-hexane was added and stirred at room temperature for 1 hour, A3-C1-8/D1-7-(1) was obtained by pumping and filtration with yield of 47%. 1H NMR (400 MHz, CDCl3) δ: 3.61 (2H, s), 2.58 to 2.65 (6H, m), 2.44 to 2.52 (2H, m), 1.47 to 1.59 (8H, m), 1.23 to 1.34 (40H, m), 0.73 to 0.88 (6H, t, CH3) ppm.
7,12-bis(2-hydroxytetradecyl)-20-methylene-4,15-dioxo-16-(2-(pentadec-1-en-2-yloxy)ethyl)-3-(2-(tetradecanoyloxy)ethyl)-19-oxa-3,7,12,16-tetraazatritriacontyl tetradecanoate (A3-C1-8/D1-7)
The A2-C1-8-(2) was dissolved in 5 ml of isopropanol, and then 57.49 mg (0.112 mmol) of A3-C1-8/D1-7-(1) was added. The reaction was carried out at 90°C for 10 hours to obtain A3-C1-8/D1-7 with yield 29%. 1H NMR (400 MHz, CDCl3) δ 4.19 (t, J = 5.5 Hz, 10H), 3.66 to 3.52 (m, 10H), 2.99 (s, 4H), 2.48 (m, 10H), 2.34 to 2.23 (m, 12H), 2.03 (s, 2H), 1.64 to 1.55 (m, 18H), 1.25 (m, 113H), 0.87 (t, J = 6.7 Hz, 18H); 13C NMR (100 MHz, CDCl3) δ 173.61, 173.48, 62.09, 61.66, 47.45, 45.78, 34.90, 34.11, 31.94, 31.81, 29.92, 29.70, 29.39, 29.21, 25.85, 24.86, 22.71, and 14.13. HRMS (EI+) m/z calcd for C102H198N4O12(M+) 1672.68, found 1673.6.
Preparation of LNPs
Sixteen formulations of each lipid were designed by software called Orthogonal Designing Assistant II V3.1. Lipid, DSPC, CHO, and DMG-PEG2000 were dissolved in ethanol at a given molar ratio as organic phase and added into a penicillin bottle. Citrate buffer (0.05 M, pH 4.0), three times the volume of the organic phase, was rapidly added to the organic phase under stirring to form LNPs. siRNAs were dissolved in 25% ethanol. Various weight ratios ranging from 1:1 to 30:1 (LNPs:siRNA) were mixed and then incubated for 20 min at 50°C. The formulations were then dialyzed with 1× PBS for at least 2 hours (formulation: 1× PBS < 1:1000, v/v).
Agarose gel electrophoresis assay
Lipid formulations containing 200 ng of siRNA were mixed with 6× loading buffer, and the mixture was then added into 1% agarose gel containing 0.01% gel stain. Electrophoresis was carried out at a voltage of 90 V for 30 min in 1× tris-acetate-EDTA running buffer. Result was recorded at 320-nm ultraviolet light wavelength using gel image analysis system (Tanon, China).
In vitro gene silencing
HepG2 cells were seeded into a 12-well plate for 1 × 105 cells per well. After 24 hours, formulations were incubated with siApoB at 50°C for 20 min. Consequently, formulations were dialyzed in PBS for 3 hours and added into corresponding wells for siApoB at a final concentration of 50 nM. After 4 hours, fresh DMEM (1 ml per well) was added, and cells were cultured for further 20 hours. After that, medium was removed, and cells were washed with PBS and harvested by using RNA isolater (1 ml per well). The cell lysates were transferred into 1.5-ml centrifuge vials, followed by the addition of chloroform (200 μl per vial) and held for 3 min. All the resultant samples were then centrifuged at 12,000 rpm for 10 min. The supernatant was absorbed into a new vial, and an equal volume of isopropanol was added, and the samples were centrifuged at 12,000 rpm for 10 min after a 10-min standing. Supernatant was discarded, and 1 ml of 75% ethanol was added for each sample. All samples were shaken and centrifuged for 10 min at 12,000 rpm and then dried at room temperature. Last, diethyl pyrocarbonate–treated water was added to the vials to dissolve the samples. A total of 1 μg of RNA was reversely transcripted to synthesize complementary DNA according to the protocol provided by the kit’s manufacturer. Gene silencing was determined by RT-qPCR, and relative expression of ApoB gene refers to glyceraldehyde phosphate dehydrogenase.
Activity comparison of four iLNPs in vitro
HepG2-luc cells stably expressing firefly luciferase were seeded into 24-well plates before the day of transfection. At the day of transfection, four iLNP formulations were freshly prepared with A1-B3-7, A1-D1-5, A2-C1-8, and A3-C1-8/D1-7, respectively. siRNA against firefly was encapsulated here. Then, the formulations were added into corresponding wells. The transfection concentrations were 200, 100, 50, 25, 12.5, 6.25, and 3.125 nM, respectively. After 4 hours, fresh DMEM (1 ml per well) was added, and cells were cultured for further 20 hours. The cells were then harvested using passive lysis buffer, and the lysis solution and luciferin were added into well. Last, their signals were determined/recorded immediately.
Physiochemical property characterization of LNPs
The particle size and zeta potential of LNPs before and after siRNA encapsulation were determined by DLS (Zetasizer 3000HS, Malvern) at a wavelength of 677 nm with a constant angle of 90° at room temperature. TEM was used to observe the structure of LNP. The Quant-iT RiboGreen RNA Assay kit was used to determine the concentration of siRNA encapsulated in LNP. Procedures were based on its standard protocol. Briefly, the dialyzed LNP was diluted into Tris-EDTA (TE) buffer in a 96-well plate. Meanwhile, the dialyzed LNP was also diluted into TE buffer with 2% Triton X-100 for 30 min. After that, RiboGreen working solution was added into corresponding wells, and then fluorescence intensity was recorded by a microplate reader to reflect the amount of free siRNA and the siRNA after dialysis. The concentration of siRNA was calculated by its standard curve. Encapsulation efficiency and loading efficiency were calculated according to the following formula
Determination of the pKa
To determine the pKa, iLAND was prepared and diluted in PBS at a concentration of around 2 mM. LNP was then diluted to 100 μM by a buffer containing 130 mM NaCl, 10 mM ammonium acetate, 10 mM Hepes, and 10 mM MES. After that, the iLAND solutions were divided into 19 equal parts. The pH of each solution was adjusted in the range of 2.5 to 11 by using NaOH or HCl. TNS was dissolved in distilled water to the concentration of 100 μM and added into pH-adjusted iLAND solutions to give a final concentration of 1 μM. The fluorescence intensity of each formulation was measured by a luminescence spectrophotometer at room temperature using excitation wavelengths of 321 nm and an emission of 445 nm. The pKa was defined as the pH rise to half-maximal fluorescence intensity.
Cell viability
HepG2 cells were seeded into a 96-well plate of 1.25 × 104 cells per well and cultured in DMEM with 10% FBS. After 24 hours, the cells in each well were treated with siNC@iLAND for siNC concentration ranging from 1 to 200 nM. At 4 hours after treatment, fresh complete medium (200 μl per well) was added for further 20 hours of culture. After that, the medium was replaced with 100 μl of medium containing 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-diphenytetrazoliumromide (MTT) (0.5 mg/ml) for 4-hour incubation. The MTT solution was then replaced with 50 μl of dimethyl sulfoxide for another 10-min incubation at 37°C. Last, the absorbance was measured at 540 nm with a reference wavelength of 650 nm using a microplate reader. PBS-treated cells were used as mock. All experiments were carried out with six replicates. The relative cell viability was calculated according the following equation, based on the optical density (OD)
Stability determination of A1-D1-5 and iLAND
For lipid stability determination, A1-D1-5 was dissolved in incubation buffer that contains 90% ethanol and 10% citrate buffer at a final concentration of 1 mg/ml and a final volume of 3 ml. At day 0, 100-μl formulation was obtained, and the remaining sample was divided into three parts and incubated at three different conditions as 40°C, room temperature (around 20°C), and −20°C. Sample collection was carried out at days 1, 3, 5, and 7, respectively. All samples were determined by high-performance liquid chromatography. For iLAND stability determination, iLAND was prepared following the aforementioned protocol and stored at 4°C. The stability was reflected by DLS at days 0, 3, 7, and 21.
Cellular uptake
HepG2 cells were seeded into six-well plates before the day of transfection. Adherent cells were treated with different treatments at the second day. Four hours later, cells were digested with trypsin (0.25%), washed with PBS three times, and then resuspended in 300 μl of 1× PBS. Determination was conducted by a flow cytometer (FACSverse, BD), and analysis was performed by using software FlowJo 7.6.
Subcellular localization
HepG2 cells (2 × 105) were seeded into each 35-mm dish at the day before transfection. Culture condition was the same as the cell viability experiment. On the second day, cells were treated with corresponding treatments and then cultured at 37°C. At the scheduled timing, the treated cells were washed with PBS three times and stained by using Lysotracker Green (1:3000 dilution) and Hoechst 33342 (0.1 mg/ml) or DAPI (1:1000 dilution) for 15 min at 37°C, and lastly, the images were taken by confocal microscopy. Colocalization analysis was performed by using software ImageJ.
Mechanism of internalization and intracellular trafficking
HepG2 cells were seeded into 35-mm dishes before the day of transfection. On the second day, adherent cells for transfection parts were treated with amiloride-HCl (100 μM), chlorpromazine-HCl (30 μM), genistein (1 mM), or fresh DMEM for 30 min, respectively. Then, Cy5-siRNA@iLAND was added into corresponding wells for siRNA final concentration at 50 nM, cells were cultured at 37° or 4°C for 4 hours. The following procedures of cellular uptake or subcellular localization analysis were the same as the aforementioned protocol.
To investigate whether ApoE contributes to the targeting and endocytosis of iLAND, HepG2 cells were seeded in a six-well plate (for FACS) or 35-mm dishes (for subcellular localization analysis) at the day before transfection. Cy5-siRNA@iLAND was prepared according to the aforementioned protocol. To prepare Cy5-siRNA@DOTAP LNP, DOTAP lipid was rapidly added to citrate buffer (0.05 M, pH 4.0) to form LNPs. Cy5-siRNA entrapment was performed in 50°C for 20 min. The formulations were then dialyzed with 1× PBS for at least 2 hours before transfection. ApoE and calreticulin were incubated with iLAND or DOTAP LNP for 5 min at 37°C, respectively. Then, the samples of Cy5-siRNA@iLAND plus ApoE or calreticulin, as well as the samples of Cy5-siRNA@DOTAP LNP plus ApoE or calreticulin, were added into corresponding wells or dishes. The final transfection concentration of ApoE or calreticulin was 23.7 nM. The medium used in this assay was “FBSDMEM” (DMEM containing 10% FBS), while DMEM without addition of FBS was included as control. In addition, equivalent iLAND or DOTAP LNP without incubation with ApoE or calreticulin was also transfected to cells cultured with DMEM or FBSDMEM. After 4 hours of transfection, cellular uptake was determined by FACS, and subcellular localization was observed under a confocal microscope.
To determine the effects of chloroquine and bafilomycin A1 on endosomal escape of iLAND, HepG2 cells were seeded in 35-mm dishes and treated with chloroquine (100 μM) or bafilomycin A1 (200 nM) for 1 hour. After that, Cy5-siRNA@iLAND was added into the dishes for a 4-hour transfection, and changes of cellular uptake and colocalization between siRNA and endosome/lysosome were determined by confocal microscopy and analyzed with supported software.
Animal studies
C57BL/6 mice and CD-1 mice aged 6 to 8 weeks were purchased from Vital River (Beijing, China). db/db (C57BLKS/JGpt- Leprem2Cd/Gpt) mice aged of 6 to 8 weeks and m/m (C57BLKS/JGpt) were purchased form GemPharmatech (Nanjing, China). B6;CBA-Tg(APOC3)3707Bres/J (human ApoC-III Tg) mice were provided by Suzhou Ribo Life Science Co. Ltd. (Suzhou, Jiangsu, China). All protocols were approved by the Guide of Care and Use of Laboratory Animals. Mice were housed in a temperature-controlled, ventilated room and illuminated by artificial light for 12-hour daylight and 12-hour darkness. Animals had free access to food (normal rodent chow) and water.
In vivo biodistribution
C57BL/6 mice were divided into two groups and were treated with PBS or Cy5-siRNA@iLAND (2 mg/kg) via tail vein injection, respectively. The Cy5 fluorescence signals throughout body were detected using the Kodak in vivo imaging system FX Pro (Carestream Health, USA) at given time points. One or two animals of each group were euthanized and dissected after the living imaging of 0.5-, 4-, and 24-hour postdose, tissues including the heart, lungs, liver, spleen, kidneys, stomach, intestine, submandibular gland, and thymus were isolated and examined using the Kodak in vivo imaging system FX Pro.
ED50 determination
Thirty-two C57BL/6 mice were divided into eight groups with four animals per group. PBS or siApoB@iLAND (0.01 to 1 mg/kg) was administered via tail vein injection. siApoB is a siRNA targeting ApoB gene. After 42 hours, all animals were food-fasted for 6 hours and then euthanized. The whole blood was collected, the serum was isolated for blood lipid test, and liver samples were isolated and kept in RNAlater for further mRNA expression test. Protocols are same as in vitro gene silencing.
Determination of lipid accumulation in the liver
Thirty milligrams of each liver sample was weighted precisely with analytical balance (Mettler-Toledo International Inc., USA) and transferred into a vial. One milliliter of extract solution containing 67% trichloromethane and 33% methanol (v/v) was added into each vial, followed by homogenizing the samples with a homogenizer (Superfine Homogenizers, Fluko, Germany) and incubating at 4°C overnight. Then, 300 μl of deionized water was added to the homogenized samples, and the solutions were then centrifuged for 10 min at 8000 rpm at 4°C (Eppendorf Centrifuge 5417R, Eppendorf, Germany). The organic phase (down layer) was collected into a new vial and volatilized with centrifugation to obtain dried samples (Concentrator 5301, Eppendorf, Germany). The samples were then added with 600 μl of PBS containing 5% (w/w) Triton X-100, followed by incubating in water bath at 60°C for 30 min and ultrasonically treating for 30 min alternatively three times to solve the lipids. Last, the levels of TG and CHO were determined with commercial kits (Biosino Bio-Technology and Science Incorporation, China) according to standard protocols.
Duration study
Ninety-six C57BL/6 mice were randomly divided into three groups with 32 animals per group and administered with PBS, 3 mg/kg siApoB@iLAND, or 1 mg/kg siApoB@iLAND, respectively. After the administration, four animals of each group were euthanized at days 2, 7, 14, 21, 28, 35, 42, and 49, respectively. Following tests are same as ED50 determination.
Pilot safety evaluation in vivo
Twelve CD-1 mice received 1× PBS, 3 mg/kg siNC@iLAND, or 1 mg/kg siNC@iLAND, respectively. Forty-eight hours later, animals were euthanized to obtain blood and organs including the heart, lungs, liver, spleen, and kidneys for serum biochemistry analysis and pathology determination, respectively.
Lipid-lowering test in HFD-fed animals
Twenty-four C57BL/6 mice were fed with HFD (Research Diets, D12451) from study days −51 to −1 to establish an HFD-induced hyperlipidemia model. Eight C57BL/6 mice were fed with standard chow diet and set as control. During the treating period, the serum CHO and TG levels were monitored every week. On study day −2, animals were divided into four groups and treated with following formulations, respectively: (i) C57BL/6 PBS, (ii) HFD PBS, (iii) HFD siNC, and (iv) HFD siANG. Here, siNC and siANG means negative siRNA and ANGPTL3-against siRNA that were encapsulated by iLAND, respectively. siNC@iLAND and siANG@iLAND were dosed at 0.25 mg/kg. Then, all animals were received four injections (one injection per week). Levels of serum CHO and TG were determined at 48 hours after the first dose, and relative ANGPTL3 mRNA expression was determined by qPCR at the end of the study. HFD-induced animals were fed with HFD, and animals in control group were fed with standard chow diet during the whole study.
Efficacy study in db/db mice
Twenty-four db/db mice and 6 m/m mice were assigned into five groups as follows: (i) m/m PBS, n = 6; (ii) db/db PBS, n = 6; (iii) db/db siNC (siNC, 0.5 mg/kg), n = 6; (iv) db/db siANG-H (siANGPTL3, 0.5 mg/kg), n = 6; and (v) db/db siANG-L (siANGPTL3, 0.25 mg/kg), n = 6. siNC and siANG also means negative siRNA and ANGPTL3-against siRNA that encapsulated by iLAND, respectively. Formulations were intravenously dosed weekly for five continuous weeks. On 5 days after each administration, animals were food-fasted for 6 hours. Blood samples were collected from eye sockets of each animal to monitor the changes in blood lipids. On day 1 after the last dose, all animals were euthanized for organ collection. Part of liver tissue of each animal were preserved properly for further determination of mRNA expression. Meanwhile, one animal in each group was used for additional pathological test. Tissues including the heart, lungs, liver, spleen, and kidneys were fixed in 4% formalin for H&E staining. Liver samples were also dyed by Oil red O to determinate lipid accumulation.
Efficacy study on hApoC3 mice
Twenty-four animals were randomly assigned into four groups with six animals per group. Six C57BL/6 mice were used as control. The animals were intravenously administered with PBS, siNC@iLAND (0.5 mg/kg), siApoC3@iLAND (0.5 mg/kg), or siApoC3@iLAND (0.25 mg/kg), respectively. Animals were treated with five injections and recovered for additional 4 weeks. During the treatment course, blood samples were collected from eye sockets to monitor lipid changes. After that, animals were euthanized, followed by sampling and testing according to the same protocol as described above.
Statistical analysis
Statistical analysis for comparing two experimental groups was performed using two-sided Student’s t tests. In experiments with multiple groups, one- or two-way analysis of variance (ANOVA) with a Tukey correction was used to calculate differences among multiple populations. Analyses were performed with Prism 7 (GraphPad Software). Differences are labeled ns for not significant, * for P ≤ 0.05, ** for P ≤ 0.01, *** for P ≤ 0.001, and **** for P ≤ 0.0001. Each bar represents the means ± SEM.
Acknowledgments
We acknowledge the Beijing Institute of Technology Research Fund Program for Young Scholars. We thank Biological & Medical Engineering Core Facilities (Beijing Institute of Technology) for providing advanced equipment.
Funding: This work was supported by the National Natural Science Foundation of China (31871003, 31901053, and 32001008), the Beijing Nova Program from Beijing Municipal Science & Technology Commission (Z201100006820005), the Beijing-Tianjin-Hebei Basic Research Cooperation Project (19JCZDJC64100), the National Key R&D Program of China (2021YFE0106900), and the Natural Science Foundation of Guangdong Province (2019A1515010776).
Author contributions: B.H. performed most of the experiments. B.L. established the synthesis rout of A1-D1-5 and was involved in writing the original draft. K.L., Y.L., T.Z., Q.L., and X.D. were involved in lipid synthesis. L.Z. participated in activity comparison of the four panels of iLNPs. C.L., M.Z., T.Y., and S.G. participated in animal studies. A.H. was involved in discussion and polished the language. Y.W., L.P., Y.Z., and X.-J.L. analyzed the data and was involved in discussion. X.-J.L. provided insightful suggestions and comments. Y.H. conceived the project, designed research, supervised experiments, and wrote and critically reviewed the manuscript.
Competing interests: B.H. and Y.H. are inventors on a patent application related to this work filed by Beijing Institute of Technology (no. PCT/CN2021/094937, filed 20 May 2021). The authors declare that they have no other competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S20
Tables S1 to S12
REFERENCES AND NOTES
- 1.Yao Y. S., Li T. D., Zeng Z. H., Mechanisms underlying direct actions of hyperlipidemia on myocardium: An updated review. Lipids Health Dis. 19, 23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudet D., Alexander V. J., Baker B. F., Brisson D., Tremblay K., Singleton W., Geary R. S., Hughes S. G., Viney N. J., Graham M. J., Crooke R. M., Witztum J. L., Brunzell J. D., Kastelein J. J., Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 373, 438–447 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Graham M. J., Lee R. G., Brandt T. A., Tai L. J., Fu W., Peralta R., Yu R., Hurh E., Paz E., McEvoy B. W., Baker B. F., Pham N. C., Digenio A., Hughes S. G., Geary R. S., Witztum J. L., Crooke R. M., Tsimikas S., Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 377, 222–232 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Koene R. J., Prizment A. E., Blaes A., Konety S. H., Shared risk factors in cardiovascular disease and cancer. Circulation 133, 1104–1114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macchi C., Sirtori C. R., Corsini A., Santos R. D., Watts G. F., Ruscica M., A new dawn for managing dyslipidemias: The era of rna-based therapies. Pharmacol. Res. 150, 104413 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Ray K. K., Corral P., Morales E., Nicholls S. J., Pharmacological lipid-modification therapies for prevention of ischaemic heart disease: Current and future options. Lancet 394, 697–708 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Adhyaru B. B., Jacobson T. A., Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 15, 757–769 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Stein E. A., Gipe D., Bergeron J., Gaudet D., Weiss R., Dufour R., Wu R., Pordy R., Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: A phase 2 randomised controlled trial. Lancet 380, 29–36 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Gaudet D., Gipe D. A., Pordy R., Ahmad Z., Cuchel M., Shah P. K., Chyu K. Y., Sasiela W. J., Chan K. C., Brisson D., Khoury E., Banerjee P., Gusarova V., Gromada J., Stahl N., Yancopoulos G. D., Hovingh G. K., ANGPTL3 inhibition in homozygous familial hypercholesterolemia. N. Engl. J. Med. 377, 296–297 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Gaudet D., Brisson D., Tremblay K., Alexander V. J., Singleton W., Hughes S. G., Geary R. S., Baker B. F., Graham M. J., Crooke R. M., Witztum J. L., Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 371, 2200–2206 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Schwartz G. G., Steg P. G., Szarek M., Bhatt D. L., Bittner V. A., Diaz R., Edelberg J. M., Goodman S. G., Hanotin C., Harrington R. A., Jukema J. W., Lecorps G., Mahaffey K. W., Moryusef A., Pordy R., Quintero K., Roe M. T., Sasiela W. J., Tamby J. F., Tricoci P., White H. D., Zeiher A. M.; ODYSSEY OUTCOMES Committees and Investigators , Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107 (2018). [DOI] [PubMed] [Google Scholar]
- 12.McDonagh M., Peterson K., Holzhammer B., Fazio S., A systematic review of PCSK9 inhibitors alirocumab and evolocumab. J. Manag. Care Spec. Pharm. 22, 641–653q (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordts P. L., Nock R., Son N. H., Ramms B., Lew I., Gonzales J. C., Thacker B. E., Basu D., Lee R. G., Mullick A. E., Graham M. J., Goldberg I. J., Crooke R. M., Witztum J. L., Esko J. D., ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J. Clin. Invest. 126, 2855–2866 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu B., Zhong L., Weng Y., Peng L., Huang Y., Zhao Y., Liang X. J., Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 5, 101 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittrup A., Lieberman J., Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 16, 543–552 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weng Y., Xiao H., Zhang J., Liang X. J., Huang Y., RNAi therapeutic and its innovative biotechnological evolution. Biotechnol. Adv. 37, 801–825 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Hu B., Weng Y., Xia X.-H., Liang X.-J., Huang Y., Clinical advances of siRNA therapeutics. J. Gene Med. 21, e3097 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Semple S. C., Akinc A., Chen J., Sandhu A. P., Mui B. L., Cho C. K., Sah D. W., Stebbing D., Crosley E. J., Yaworski E., Hafez I. M., Dorkin J. R., Qin J., Lam K., Rajeev K. G., Wong K. F., Jeffs L. B., Nechev L., Eisenhardt M. L., Jayaraman M., Kazem M., Maier M. A., Srinivasulu M., Weinstein M. J., Chen Q., Alvarez R., Barros S. A., De S., Klimuk S. K., Borland T., Kosovrasti V., Cantley W. L., Tam Y. K., Manoharan M., Ciufolini M. A., Tracy M. A., de Fougerolles A., MacLachlan I., Cullis P. R., Madden T. D., Hope M. J., Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 28, 172–176 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Zorde Khvalevsky E., Gabai R., Rachmut I. H., Horwitz E., Brunschwig Z., Orbach A., Shemi A., Golan T., Domb A. J., Yavin E., Giladi H., Rivkin L., Simerzin A., Eliakim R., Khalaileh A., Hubert A., Lahav M., Kopelman Y., Goldin E., Dancour A., Hants Y., Arbel-Alon S., Abramovitch R., Shemi A., Galun E., Mutant KRAS is a druggable target for pancreatic cancer. Proc. Natl. Acad. Sci. U.S.A. 110, 20723–20728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellert-Miklaszewska A., Ochocka N., Maleszewska M., Ding L., Laurini E., Jiang Y., Roura A. J., Giorgio S., Gielniewski B., Pricl S., Peng L., Kaminska B., Efficient and innocuous delivery of small interfering RNA to microglia using an amphiphilic dendrimer nanovector. Nanomedicine (Lond.) 14, 2441–2459 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Kamerkar S., LeBleu V. S., Sugimoto H., Yang S., Ruivo C. F., Melo S. A., Lee J. J., Kalluri R., Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu C., Han H. H., Sun J., Zhang H. T., Wei W., Cui S. H., Chen X., Wang J. C., Zhang Q., Regulating intracellular fate of siRNA by endoplasmic reticulum membrane-decorated hybrid nanoplexes. Nat. Commun. 10, 2702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Y., Wang X., Huang W., Cheng Q., Zheng S., Guo S., Cao H., Liang X. J., Du Q., Liang Z., Systemic administration of siRNA via cRGD-containing peptide. Sci. Rep. 5, 12458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin D., Cheng Q., Jiang Q., Huang Y., Yang Z., Han S., Zhao Y., Guo S., Liang Z., Dong A., Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient siRNA delivery in vitro and in vivo. Nanoscale 5, 4291–4301 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Sato Y., Hatakeyama H., Sakurai Y., Hyodo M., Akita H., Harashima H., A pH-sensitive cationic lipid facilitates the delivery of liposomal siRNA and gene silencing activity in vitro and in vivo. J. Control. Release 163, 267–276 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Rozema D. B., Lewis D. L., Wakefield D. H., Wong S. C., Klein J. J., Roesch P. L., Bertin S. L., Reppen T. W., Chu Q., Blokhin A. V., Hagstrom J. E., Wolff J. A., Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 104, 12982–12987 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou K., Nguyen L. H., Miller J. B., Yan Y., Kos P., Xiong H., Li L., Hao J., Minnig J. T., Zhu H., Siegwart D. J., Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc. Natl. Acad. Sci. U.S.A. 113, 520–525 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rietwyk S., Peer D., Next-generation lipids in RNA interference therapeutics. ACS Nano 11, 7572–7586 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Zheng S., Wang X., Weng Y. H., Jin X., Ji J. L., Guo L., Hu B., Liu N., Cheng Q., Zhang J., Bai H., Yang T., Xia X. H., Zhang H. Y., Gao S., Huang Y., siRNA knockdown of RRM2 effectively suppressed pancreatic tumor growth alone or synergistically with doxorubicin. Mol. Ther. Nucleic Acids 12, 805–816 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C., Yang T., Weng Y., Zhang M., Zhao D., Guo S., Hu B., Shao W., Wang X., Hussain A., Liang X.-J., Huang Y., Ionizable lipid-assisted efficient hepatic delivery of gene editing elements for oncotherapy. Bioact Mater 9, 590–601 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahay G., Querbes W., Alabi C., Eltoukhy A., Sarkar S., Zurenko C., Karagiannis E., Love K., Chen D., Zoncu R., Buganim Y., Schroeder A., Langer R., Anderson D. G., Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat. Biotechnol. 31, 653–658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittrup A., Ai A., Liu X., Hamar P., Trifonova R., Charisse K., Manoharan M., Kirchhausen T., Lieberman J., Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 33, 870–876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo S., Li K., Hu B., Li C., Zhang M., Hussain A., Wang X., Cheng Q., Yang F., Ge K., Zhang J., Chang J., Liang X.-J., Weng Y., Huang Y., Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exp. Dermatol. 1, 35–49 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni J. A., Witzigmann D., Leung J., Tam Y. Y. C., Cullis P. R., On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale 11, 21733–21739 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Kumar V., Qin J., Jiang Y., Duncan R. G., Brigham B., Fishman S., Nair J. K., Akinc A., Barros S. A., Kasperkovitz P. V., Shielding of lipid nanoparticles for siRNA delivery: Impact on physicochemical properties, cytokine induction, and efficacy. Mol Ther Nucleic Acids 3, e210 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin P. J., Tam Y. Y., Hafez I., Sandhu A., Chen S., Ciufolini M. A., Nabi I. R., Cullis P. R., Influence of cationic lipid composition on uptake and intracellular processing of lipid nanoparticle formulations of siRNA. Nanomedicine 9, 233–246 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Mui B. L., Tam Y. K., Jayaraman M., Ansell S. M., Du X., Tam Y. Y., Lin P. J., Chen S., Narayanannair J. K., Rajeev K. G., Manoharan M., Akinc A., Maier M. A., Cullis P., Madden T. D., Hope M. J., Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2, e139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead K. A., Dorkin J. R., Vegas A. J., Chang P. H., Veiseh O., Matthews J., Fenton O. S., Zhang Y., Olejnik K. T., Yesilyurt V., Chen D., Barros S., Klebanov B., Novobrantseva T., Langer R., Anderson D. G., Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 5, 4277 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weng Y., Li C., Yang T., Hu B., Zhang M., Guo S., Xiao H., Liang X. J., Huang Y., The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 40, 107534 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Sahay G., Alakhova D. Y., Kabanov A. V., Endocytosis of nanomedicines. J. Control. Release 145, 182–195 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen D., Parayath N., Ganesh S., Wang W., Amiji M., The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for in vivo targeted delivery and transfection of oligonucleotides in murine tumor models. Nanoscale 11, 18806–18824 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K. N., Jayaraman M., Rajeev K. G., Cantley W. L., Dorkin J. R., Butler J. S., Qin L., Racie T., Sprague A., Fava E., Zeigerer A., Hope M. J., Zerial M., Sah D. W., Fitzgerald K., Tracy M. A., Manoharan M., Koteliansky V., Fougerolles A., Maier M. A., Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Y., Love K. T., Dorkin J. R., Sirirungruang S., Zhang Y., Chen D., Bogorad R. L., Yin H., Chen Y., Vegas A. J., Alabi C. A., Sahay G., Olejnik K. T., Wang W., Schroeder A., Lytton-Jean A. K., Siegwart D. J., Akinc A., Barnes C., Barros S. A., Carioto M., Fitzgerald K., Hettinger J., Kumar V., Novobrantseva T. I., Qin J., Querbes W., Koteliansky V., Langer R., Anderson D. G., Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl. Acad. Sci. U.S.A. 111, 3955–3960 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fucikova J., Spisek R., Kroemer G., Galluzzi L., Calreticulin and cancer. Cell Res. 31, 5–16 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jayaraman M., Ansell S. M., Mui B. L., Tam Y. K., Chen J., Du X., Butler D., Eltepu L., Matsuda S., Narayanannair J. K., Rajeev K. G., Hafez I. M., Akinc A., Maier M. A., Tracy M. A., Cullis P. R., Madden T. D., Manoharan M., Hope M. J., Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 51, 8529–8533 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang T., Li C., Wang X., Zhao D., Zhang M., Cao H., Liang Z., Xiao H., Liang X. J., Weng Y., Huang Y., Efficient hepatic delivery and protein expression enabled by optimized mRNA and ionizable lipid nanoparticle. Bioact. Mater. 5, 1053–1061 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel P., Ibrahim N. M., Cheng K., The importance of apparent pKa in the development of nanoparticles encapsulating siRNA and mRNA. Trends Pharmacol. Sci. 42, 448–460 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Y., Hong J., Zheng S., Ding Y., Guo S., Zhang H., Zhang X., Du Q., Liang Z., Elimination pathways of systemically delivered siRNA. Mol. Ther. 19, 381–385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Y., Cheng Q., Ji J. L., Zheng S., Du L., Meng L., Wu Y., Zhao D., Wang X., Lai L., Cao H., Xiao K., Gao S., Liang Z., Pharmacokinetic behaviors of intravenously administered siRNA in glandular tissues. Theranostics 6, 1528–1541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashemi N., Odze R. D., McGowan M. P., Santos R. D., Stroes E. S. G., Cohen D. E., Liver histology during Mipomersen therapy for severe hypercholesterolemia. J. Clin. Lipidol. 8, 606–611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair J. K., Attarwala H., Sehgal A., Wang Q., Aluri K., Zhang X., Gao M., Liu J., Indrakanti R., Schofield S., Kretschmer P., Brown C. R., Gupta S., Willoughby J. L. S., Boshar J. A., Jadhav V., Charisse K., Zimmermann T., Fitzgerald K., Manoharan M., Rajeev K. G., Akinc A., Hutabarat R., Maier M. A., Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc-siRNA conjugates. Nucleic Acids Res. 45, 10969–10977 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Butler A. A., Graham J. L., Stanhope K. L., Wong S., King S., Bremer A. A., Krauss R. M., Hamilton J., Havel P. J., Role of angiopoietin-like protein 3 in sugar-induced dyslipidemia in rhesus macaques: Suppression by fish oil or RNAi. J. Lipid Res. 61, 376–386 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X., Zhen M., Zhou C., Deng R., Yu T., Wu Y., Shu C., Wang C., Bai C., Gadofullerene nanoparticles reverse dysfunctions of pancreas and improve hepatic insulin resistance for type 2 diabetes mellitus treatment. ACS Nano 13, 8597–8608 (2019). [DOI] [PubMed] [Google Scholar]
- 54.TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute, Crosby J., Peloso G. M., Auer P. L., Crosslin D. R., Stitziel N. O., Lange L. A., Lu Y., Tang Z. Z., Zhang H., Hindy G., Masca N., Stirrups K., Kanoni S., Do R., Jun G., Hu Y., Kang H. M., Xue C., Goel A., Farrall M., Duga S., Merlini P. A., Asselta R., Girelli D., Olivieri O., Martinelli N., Yin W., Reilly D., Speliotes E., Fox C. S., Hveem K., Holmen O. L., Nikpay M., Farlow D. N., Assimes T. L., Franceschini N., Robinson J., North K. E., Martin L. W., DePristo M., Gupta N., Escher S. A., Jansson J. H., Van Zuydam N., Palmer C. N., Wareham N., Koch W., Meitinger T., Peters A., Lieb W., Erbel R., Konig I. R., Kruppa J., Degenhardt F., Gottesman O., Bottinger E. P., O’Donnell C. J., Psaty B. M., Ballantyne C. M., Abecasis G., Ordovas J. M., Melander O., Watkins H., Orho-Melander M., Ardissino D., Loos R. J., McPherson R., Willer C. J., Erdmann J., Hall A. S., Samani N. J., Deloukas P., Schunkert H., Wilson J. G., Kooperberg C., Rich S. S., Tracy R. P., Lin D. Y., Altshuler D., Gabriel S., Nickerson D. A., Jarvik G. P., Cupples L. A., Reiner A. P., Boerwinkle E., Kathiresan S., Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371, 22–31 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jorgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., Tybjaerg-Hansen A., Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 371, 32–41 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Chong S., Agarwal S., Agarwal S., Aluri K. C., Arciprete M., Brown C., Charisse K., Cichocki J., Fitzgerald K., Goel V., Gu Y., Guenther D., Habtemariam B., Jadhav V., Janas M., Jayaraman M., Kurz J., Li J., Liou S., Liu J., Liu X., Maclauchlin C., Maier M., Manoharan M., McDougall R., Nair J., Ramsden D., Robbie G., Schmidt K., Smith P., Theile C., Vaishnaw A., Waldron S., Wu J. T., Xu Y., Zhang X., Zlatev I., Castellanos-Rizaldos E., The nonclinical disposition and PK/PD properties of GalNAc-conjugated siRNA are highly predictable and build confidence in translation to man. Drug Metab. Dispos. 10.1124/dmd.121.000428, (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S20
Tables S1 to S12