Abstract
Background
Guillain‐Barré syndrome (GBS) is a rare inflammatory peripheral nerve disorder with variable recovery. Evidence is lacking on experiences of people with GBS and measurement of these experiences.
Objective
We aimed to develop and validate an instrument to measure experiences of people with GBS.
Design
We used a cross‐sectional design and online self‐administered questionnaire survey. Question domains, based on a previous systematic review and qualitative study, covered experiences of GBS, symptom severity at each stage, healthcare and factors supporting or hindering recovery. Descriptive, exploratory factor and reliability analyses and multivariable regression analysis were used to investigate the relationships between variables of interest, explore questionnaire reliability and validity and identify factors predicting recovery.
Setting and Participants
People with a previous diagnosis of GBS were recruited through a social media advert.
Results
A total of 291 responders, of different sexes, and marital statuses, were included, with most diagnosed between 2015 and 2019. Factor analysis showed four scales: symptoms, information provided, factors affecting recovery and care received. Positive social interactions, physical activity including physiotherapy and movement, changes made at home and immunoglobulin treatment were important for recovery. Multivariable models showed that immunoglobulin and/or plasma exchange were significant predictors of recovery. Employment and recovery factors (positive interactions, work support and changes at work or home, physical activity and therapy), though associated with recovery, did not reach statistical significance.
Conclusion
The questionnaire demonstrated good internal reliability of scales and subscales and construct validity for people following GBS.
Patient Contribution
Patients were involved in developing and piloting the questionnaire.
Keywords: experiences, Guillain‐Barré syndrome, patients, patient‐reported experience measure, recovery
1. INTRODUCTION
Guillain‐Barré syndrome (GBS) is a rare inflammatory disorder, affecting peripheral nerves, with an incidence of 1–2/100,000 per year. 1 The disorder produces symmetrical weakness and numbness of the limbs, progressing proximally usually over 2–4 weeks, with symptom onset to nadir within 6 weeks. There are several atypical variants of GBS including Miller Fisher syndrome, which can affect cranial nerves (causing eye, facial or swallowing problems), balance and coordination and Bickerstaff brainstem encephalitis, which also affects the central nervous system, and although some investigations, such as nerve conduction studies and cerebrospinal fluid analysis are supportive, the diagnosis is largely made clinically. 2
The severity of GBS is variable, with patients with mild GBS experiencing little disability and recovering spontaneously, but in 20%–30% of cases, a more severe generalized form rapidly progresses to affect facial and respiratory muscles, and causes symptoms leading to more severe disability or even death. 3 Treatment, particularly for more severe cases, may involve life‐saving supportive therapy at the intensive care unit, administration of intravenous immunoglobulins or plasma exchange (PE), which significantly shortens the time to (but not the extent of) recovery, 4 , 5 followed by rehabilitation. 6
Large prospective studies such as the International GBS Outcome Study (IGOS) have shown wide variations in outcomes. 7 , 8 Many patients, particularly those with mild forms of GBS, recover completely within 1–2 years, but others will have residual or long‐lasting physical, psychological or social sequelae. Physical effects include pain, chronic fatigue and difficulty in walking. 3 , 9 Reported psychological symptoms include experiences of sleep disturbance, anxiety or posttraumatic stress disorder, which can affect a person's daily life activities, work or social function over years. 10
A recent systematic review and metasynthesis of qualitative studies of people with GBS showed the complexity of experience of the illness, its care and rehabilitation from illness onset to hospitalisation with acute symptoms, recovery and adjustment in the case of longer‐term problems. 11 This and other studies of patient‐reported experiences of neurological conditions have identified common factors associated with care quality. 12 A further qualitative study exploring those factors associated with recovery demonstrated the importance of early diagnosis, positive experiences of inpatient care, active support for recovery and good communication and information provision. 13
Quality in healthcare is widely considered to consist of three interrelated components: safety, effectiveness and experience. 14 , 15 Tools such as the Inflammatory Rasch‐built Overall Disability Scale, the Medical Research Council sum score and the Inflammatory Neuropathy Cause and Treatment disability score have been used to monitor the effectiveness of treatment and disease progression. 16 Healthcare experiences include ‘experiences of what health services and staff are like and do’ and experiences of how they feel services ‘enable [them] to be and do what [they] value being and doing within and beyond [their] healthcare encounters’. 17 Patient‐reported experience measures (PREMs) are widely used to assess patient experience as a key aspect of quality. 18 , 19 This is also relevant and important for patients' experiences of conditions such as GBS and its variants.
We aimed to develop and validate a questionnaire to quantify experiences of people with GBS.
2. METHODS
2.1. Design
We used a cross‐sectional design using a self‐administered online questionnaire survey designed to explore symptoms, care experiences and recovery in people who previously had GBS.
2.2. Questionnaire development
Questionnaire domains and items were based on a systematic review and metasynthesis of qualitative studies 11 and an interview study of people with the condition. 13 The domains for people who previously had GBS included participant characteristics; severity of symptoms (physical, psychological and social) at each stage of illness; medical health‐seeking experience; treatment and care experiences; follow‐up and support; and social or work‐related experience.
The initial questionnaire was piloted with four people who had recovered from GBS, of whom two had taken part in an earlier interview study. 13 The questionnaire was also discussed with the Guillain‐Barré Syndrome and Associated Inflammatory Neuropathies (GAIN) charity, the Healthier Ageing Patient and Public Involvement group at the University of Lincoln and members of the research team. Comments and suggestions were used to revise some of the questions to ensure that they were appropriate for the intended population of GBS patients.
2.3. Participant recruitment and data collection
Ethical approval was obtained from the University of Lincoln Human Ethics Committee (2019‐Jul‐0738). A convenience sample of people with GBS living in the United Kingdom was recruited through a social media advertisement posted on Twitter and the UK GBS charity, GAIN website, Facebook page and member list. Information about the research (including consent and a link to the questionnaire) was posted at the GAIN and University of Lincoln Community and Health Research Unit websites (https://www.cahru.org.uk/) accessible to potential participants. Participants, who self‐identified with a diagnosis of GBS, consented and completed the questionnaire online. No financial incentives were given to responders. Participants were encouraged to contact a member of the research team (J. A.) if they needed further information or assistance to complete the questionnaire. The survey remained open for 2 months (August and September 2019), and once completed by participants, the questionnaire was retrieved and stored securely for analysis.
2.4. Data analysis
The internal consistency of the GBS questionnaire was assessed using Cronbach's α. 20 This test was used to establish the level of agreement between items belonging to the same scale. Four main scales were developed, which contained items scored on a 7‐point Likert scale including symptoms, care received, factors affecting recovery and information provided. Some of these scales were divided into further subscales: initial, in‐hospital, after‐hospital and current symptoms as well as care received in hospital and after discharge from hospital.
Factor analyses were run to identify questionnaire subscales. The scales included were suitable for this type of analysis as indicated by the Kaiser–Meyer–Olkin measure, which was higher than 0.7 for all of them. Retained factors were those with eigenvalues greater than 1 and items with loadings higher than 0.4. 21 As such, the scales included in the factor analysis (FA) were symptoms (initial, in hospital, residual and current), factors affecting recovery and information provided.
Multivariate linear regression models were used to identify the factors predicting recovery. Two regression models were run: The first one using the scales of the questionnaire as predictors and the second one using the subscales derived from factor analyses as the main predictors together with demographic characteristics that might have influenced the outcome. These demographic predictors included age and the binary variables: sex (female or male), employment status (employed or unemployed) and living with someone else or alone. The recovery score, which was used as the main outcome, was computed using the formula: recovery score = mean score of in hospital symptoms − mean score of present symptoms.
The assumption of normality was met as indicated by both histograms and P–P plots of residuals. Homoscedasticity was present as indicated by scatterplots. The assumption of no multicollinearity was also met for both models as indicated by Durbin Watson tests with values close to 2 (1.93 for the first model and 2.12 for the second model), tolerance values higher than 1 and Variance Inflation Factor values smaller than 10.
3. RESULTS
3.1. Responder characteristics
In total, 291 participants responded fully or partially to the questionnaire. Table 1 shows the demographic characteristics of the participants who responded. Of the responders, 123 (45.6%) were aged between 60 and 79 years; 140 (51.9%) were male and 130 (48.1.%) were female. Most participants were of White ethnicity (264, 97.8%) compared with the minority, who were either BAME (2, 0.7%) or mixed race (3, 1.1%) or other (1, 0.4%). 178 (65.9) were married or in civil partnership compared with 57 (21.1%) who were single or 35 (13.0%) who did not declare their marital status. At the time of the survey, most participants (252, 86.6%) resided in the United Kingdom compared with non‐UK residence (39, 13.4%). More participants were retired from work (89, 38%) compared with those in full‐time employment (55, 23.5%) or part‐time work (31, 13.2%) or those on disability and/or other benefits and not working (30, 12.8%). At the time of the study, the majority of participants (177, 65.6%) were living with their spouse compared with other family members (27, 10%) or alone (38, 14.1%) or other (27, 10%).
Table 1.
Participants' demographic characteristics
| Characteristic | Number (N) | Percentage (%) | |
|---|---|---|---|
| Age (years) | |||
| Below 18 | 2 | 0.7 | |
| 19–39 | 40 | 14.8 | |
| 40–59 | 96 | 35.6 | |
| 60–79 | 123 | 45.6 | |
| 80+ | 9 | 3.3 | |
| Total | 270 | 100 | |
| Sex | |||
| Female | 130 | 48.1 | |
| Male | 140 | 51.9 | |
| Total | 270 | 100 | |
| Ethnicity | |||
| White | 264 | 97.8 | |
| BAME | 2 | 0.7 | |
| Mixed race | 3 | 1.1 | |
| Other | 1 | 0.4 | |
| Total | 270 | 100 | |
| Marital status | |||
| Married | 168 | 62.2 | |
| Civil partnership | 10 | 3.7 | |
| Single | 57 | 21.1 | |
| Other | 34 | 12.6 | |
| Prefer not to say | 1 | 0.4 | |
| Total | 270 | 100 | |
| Residence | |||
| United Kingdom | 252 | 86.6 | |
| Non‐United Kingdom | 39 | 13.4 | |
| Total | 291 | 100 | |
| Employment status | |||
| In full‐time time work | 55 | 23.5 | |
| In part‐time work | 31 | 13.2 | |
| In work with disability and/or other benefits | 6 | 2.6 | |
| On disability and/or other benefits and not working | 30 | 12.8 | |
| Unemployed | 3 | 1.3 | |
| Retired | 89 | 38 | |
| Other | 20 | 8.5 | |
| Total | 234 | 100 | |
| Household status | |||
| Spouse | 177 | 65.6 | |
| Other family member | 27 | 10 | |
| Alone | 38 | 14.1 | |
| Other | 27 | 10 | |
| Prefer not to say | 1 | 0.4 | |
| Total | 270 | 100 | |
Disease characteristics of the responders are shown in Table 2. Most responders had a diagnosis of GBS (202, 74.8%) compared with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (46, 17.0%) or a related condition (22, 8.1%). Most responders sought help within the first 3 days (158, 60.3%) of feeling unwell and help was most commonly sought from a general practitioner (GP) surgery (163, 62.2%) compared with the emergency department (67, 25.6%). Most responders (166, 64.6%) received a GBS diagnosis or its variant rather than a different diagnosis (91, 35.4%), 106 responders receiving it on their first visit.
Table 2.
Participants' disease characteristics
| Characteristic | Number (N) | Percentage (%) | |
|---|---|---|---|
| Diagnosis | |||
| GBS | 202 | 74.8 | |
| CIDP | 46 | 17.0 | |
| Related condition | 22 | 8.1 | |
| Total | 270 | 100 | |
| Help sought after (days) | |||
| 1–3 | 158 | 60.3 | |
| 4–6 | 40 | 15.3 | |
| 7–9 | 19 | 7.3 | |
| 10–14 | 14 | 5.3 | |
| 15–28 | 11 | 4.2 | |
| >28 | 20 | 7.6 | |
| Total | 262 | 100 | |
| Help sought from | |||
| General practitioner surgery | 163 | 62.2 | |
| Emergency department | 67 | 25.6 | |
| Other (please state) | 32 | 12.2 | |
| Total | 262 | 100.0 | |
| Delay in days after first visit | |||
| 1–7 days | 161 | 61.7 | |
| 8–14 days | 31 | 11.9 | |
| 15–28 days | 28 | 10.7 | |
| More than 4 weeks (please state how many weeks approximately) | 41 | 15.7 | |
| Total | |||
| Number of consultations before diagnosis | |||
| 1 | 106 | 44.9 | |
| 2 | 42 | 17.8 | |
| 3 | 38 | 16.1 | |
| 4 | 18 | 7.6 | |
| 5 | 13 | 5.5 | |
| ≥6 | 5 | 7.8 | |
| Too many to recall | 6 | 2.5 | |
| Total | 236 | 100.0 | |
| Another/other diagnosis | |||
| No | 166 | 64.6 | |
| Yes | 91 | 35.4 | |
| Place treatment | |||
| Intensive care unit (ICU) | 40 | 16.7 | |
| Hospital general ward | 36 | 15.1 | |
| Received | |||
| Hospital general ward and ICU | 76 | 31.8 | |
| Hospital general ward, outpatient | 16 | 6.7 | |
| Hospital general ward, Regional Neurological centre | 71 | 29.7 | |
| Total | 239 | 100 | |
| Year of diagnosis | |||
| Before 2000 | 49 | 18.1 | |
| 2000–2009 | 51 | 19.9 | |
| 2010–2019 | 170 | 63.0 | |
| Total | 270 | 100.0 | |
Abbreviations: CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; GBS, Guillain‐Barré syndrome.
Overall, 116 (of 291 responders, i.e., 43%) were diagnosed between 2015 and 2019, and time to diagnosis was usually 1–7 days (161, 61.7%) compared with later (100, 38.3%). Responders were generally treated in a hospital general ward or an intensive care unit (76, 31.8%) and a hospital general ward or a regional neurological unit (71, 29.7%).
3.2. Reliability of scales and subscales
The reliability (internal consistency) of the main scales was excellent for symptoms (α > .9), good for information provided and for factors affecting recovery (α > .8) and acceptable for care received (α ≥ .7). Importantly, none of the scales had poor reliability (α ≤ .6). 20 Overall, these results presented in Table 3 indicate that the questionnaire was a reliable measure, with good internal consistency.
Table 3.
Reliability of each scale and subscale of the GBS questionnaire
| Cronbach's α | Items | Observations | |
|---|---|---|---|
| Scales | |||
| Symptoms | 0.94 | 56 | 60 |
| Care received | 0.70 | 9 | 175 |
| Factors affecting recovery | 0.80 | 28 | 47 |
| Information provided | 0.88 | 11 | 208 |
| Subscales | |||
| Symptoms | |||
| Initial symptoms | 0.89 | 13 | 158 |
| Hospital symptoms | 0.88 | 13 | 156 |
| Residual symptoms | 0.86 | 13 | 163 |
| Current symptoms | 0.89 | 13 | 172 |
| Care received | |||
| In hospital | 0.65 | 4 | 232 |
| After | 0.56 | 4 | 181 |
Abbreviation: GBS, Guillain‐Barré syndrome.
Further subscales were identified following FA. Symptoms included the following subscales: peripheral nerve symptoms, cranial nerve and respiratory symptoms and psychological symptoms. Factors affecting recovery included positive interactions, work support, changes at work, changes at home, physical activity, therapy and other subscales. Because the subscale, ‘other’, had a very low reliability, the items of this subscale were introduced separately in the regression models. These two items were immunoglobulin treatment and caring responsibilities. The information provided was divided into two further subscales: provided by specialists including physiotherapists, occupational therapists and by nonspecialists including nurses, junior doctors and GPs. A detailed account of each subscale and the items in these can be seen in Table S1. The internal reliability of the new subscales is presented in Table 4.
Table 4.
Internal consistency of subscales identified following factor analysis
| Cronbach's α | Items | Observations | |
|---|---|---|---|
| Subscales | |||
| Prompted symptoms | |||
| Peripheral nerve | 0.85 | 7 | 174 |
| Cranial nerve/respiratory | 0.86 | 3 | 178 |
| Psychological | 0.78 | 3 | 180 |
| Hospital symptoms | |||
| Peripheral nerve | 0.86 | 7 | 187 |
| Cranial nerve/respiratory | 0.84 | 3 | 181 |
| Psychological | 0.75 | 3 | 182 |
| Residual symptoms | |||
| Peripheral nerve | 0.85 | 7 | 187 |
| Cranial nerve/respiratory | 0.59 | 3 | 179 |
| Psychological | 0.79 | 3 | 185 |
| Current symptoms | |||
| Peripheral nerve | 0.91 | 6 | 194 |
| Cranial nerve/respiratory | 0.67 | 3 | 191 |
| Psychological | 0.78 | 4 | 185 |
| Factors affecting recovery | |||
| Positive interactions | 0.84 | 6 | 213 |
| Work support | 0.85 | 4 | 191 |
| Changes at work | 0.65 | 5 | 184 |
| Changes at home | 0.66 | 4 | 202 |
| Physical activity | 0.59 | 3 | 210 |
| Therapy | 0.52 | 2 | 209 |
| Other | 0.20 | 3 | 190 |
| Information provided | |||
| Nonprofessionals | 0.72 | 4 | 219 |
| Professionals | 0.67 | 3 | 223 |
The reported severity of symptoms for each subscale at different time points (initial before admission to hospital, in hospital, residual and current, i.e., when responders were completing the questionnaire) indicated that symptoms were most severe when responders were in hospital and those affecting the peripheral nervous system were most prominent (Figure 1).
Figure 1.
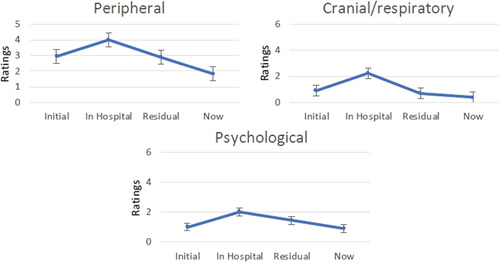
Severity of symptoms for each subscale over time
Responders were more satisfied with the information provided by specialists rather than nonspecialists (Figure 2 and Table S2).
Figure 2.
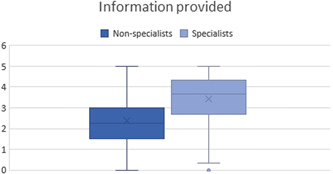
Boxplot showing satisfaction with information provided by specialists compared with nonspecialists
A combination of physical, psychological and social factors was associated with recovery; these factors were identified following the FA and an average score was calculated for each factor; details can be seen in Table S3. The factors considered by responders to be most important for recovery were positive social interactions, physical activity including physiotherapy and movement, changes made at home and immunoglobulin treatment (Figure 3).
Figure 3.
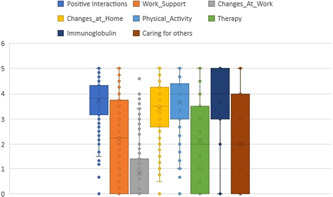
Boxplot of the main factors affecting recovery illustrating the distribution of average scores for each factor, three representing the median
3.3. Prediction models
Multivariable regression models were fitted to the data. The predictors used were gender, age, employment status and living alone or with someone, since these variables had been previously shown to influence recovery. 11 , 13 In the first regression model, the main scales of the questionnaire (shown in Table 5) were included as predictors. In the second regression model, the main subscales of the questionnaire (shown in Table 6) were included in the model. The results indicated that immunoglobulin and/or PE treatment were significant predictors of recovery.
Table 5.
Questionnaire scales predicting the recovery score
| Predictors | Recovery score | |||
|---|---|---|---|---|
| B | β | 95% CI of B | p‐Value | |
| Gender | −0.33 | −.31 | −0.83, 0.20 | .23 |
| Age | −0.29 | −.15 | −0.71, 0.14 | .19 |
| Employment | 0.50 | .20 | −0.07, 1.07 | .09 |
| Household status | 0.49 | .16 | −0.19, 1.18 | .16 |
| Care received | 0.04 | .03 | −0.37, 0.44 | .86 |
| Factors affecting recovery | 0.36 | .24 | −0.03, 0.74 | .07 |
| Information provided | 0.22 | .20 | −0.10, 0.54 | .17 |
| R 2 = .32, F (7,64) = 4.24, p = .001 | ||||
Abbreviation: CI, confidence interval.
Table 6.
Questionnaire subscales predicting the recovery score
| Predictors | Recovery score | |||
|---|---|---|---|---|
| B | β | 95% CI of B | p‐Value | |
| Gender | 0.18 | .09 | −0.73, 1.08 | .69 |
| Age | −0.30 | −.19 | −0.94, 0.33 | .33 |
| Employment | 0.57 | .27 | −0.51, 1.65 | .29 |
| Household status | −0.35 | −.12 | −1.54, 0.84 | .54 |
| Care received in hospital | −0.18 | −.17 | −0.83, 0.47 | .57 |
| Care received after | −0.09 | −.10 | −0.48, 0.31 | .66 |
| Information provided by nonspecialists | 0.09 | .12 | −0.26, 0.44 | .59 |
| Information provided by specialists | 0.24 | .29 | −0.25, 0.73 | .33 |
| Positive social interactions | 0.36 | .37 | −0.21, 0.92 | .21 |
| Work support | −0.06 | −.11 | −0.33, 0.21 | .67 |
| Changes at work | −0.04 | −.06 | −0.35, 0.26 | .78 |
| Changes at home | 0.04 | .05 | −0.42, 0.51 | .85 |
| Physical activity | −0.21 | −.26 | −0.73, 0.30 | .40 |
| Therapy | 0.10 | .16 | −0.17, 0.37 | .44 |
| Immunoglobulin and/or plasmapheresis | 0.23 | .38 | 0.01, 0.45 | .04* |
| Caring responsibilities | 0.01 | .02 | −0.21, 0.24 | .91 |
| R 2 = .55, F(16,22) = 1.68, p = .13 | ||||
Abbreviation: CI, confidence interval.
p < .05.
4. DISCUSSION
4.1. Main findings
The high completion rate and low rates of missing data for most questions supported the content and face validity of the questionnaire. The questionnaire showed reliability as excellent for symptoms, good for information provided and factors affecting recovery and acceptable for care received and symptom subscales. Physical, psychological and social factors were associated with recovery, and concordance with recent studies 11 , 13 supports construct validity. Factors considered by responders to be most important for recovery were positive social interactions, physical activity, changes made at home and immunoglobulin treatment. Responders were more satisfied with information provided by specialists rather than nonspecialists. Multivariable models showed that immunoglobulin and/or PE treatment were significant predictors of recovery. 4 Being in employment and recovery factors in combination (positive social interactions, support and changes at work support, changes at home, physical activity and counselling or occupation therapy) were positively associated with recovery, but this did not reach statistical significance.
4.2. Comparison with the existing literature
Although many people with GBS are told that they will recover and some do so completely, many are still affected in the longer term. Early results from the largest ongoing prospective study, the IGOS, 7 have shown that 8% could not walk and 7% had died at 1 year, with wide international variations in outcome. 8 Previous studies have also shown long‐term neurological deficits in most patients after a year or beyond. 22 , 23 Furthermore, a third had changed work or were affected in their functional ability and half had altered their leisure activities. 22 Psychological 24 and social dysfunction 25 often persist longer term, affecting health‐related quality of life. 26
Previous research has suggested a wide variation in positive and negative experiences at various stages of treatment and recovery from GBS. 11 , 13 We also found wide variations in experiences of care from different healthcare professionals during the illness journey, with the most positive experiences of care in hospital, from consultants, followed by nurses and therapists. Consultants, followed by physiotherapists were also rated highly for care at follow‐up, and although in this study physical, psychological and social support were (nonsignificantly) associated with improvement in symptoms, experiences of care and psychosocial support remain important aspects of quality of care.
Rehabilitation studies, involving careful follow‐up, show positive benefits of rehabilitation on function 27 and mortality 28 before discharge from hospital, but intensive physiotherapy beyond 6 months was also found to improve functional outcomes. 6 Responders in our study valued physiotherapy and perceived this to improve their recovery, but shortfalls in provision for both inpatient and outpatient rehabilitation have been found in previous studies. 29 , 30
Positive social interactions and changes at home were also associated with recovery in this study. Positive social interactions include family or peer support. 13 A systematic review found that peer support as a potential intervention for recovery in critical care populations reduced psychologic morbidity and improved self‐efficacy, although the quality of included studies was low. 31 Finally, complementary therapies such as acupuncture, vitamins and hyperbaric oxygen have been used as an adjunct to conventional treatment, but the only nonrandomized study was deemed of low quality. 32
4.3. Strengths and limitations
The number of questionnaires returned was sufficient for the planned analysis, and most participants who began completed the questionnaire. The sample was not intended to be representative of GBS patients as our main aim was to explore the reliability and validity of the questionnaire for measuring responders' experience of GBS and its care. As such, the sample comprised mostly participants over 40 years old, while patients with more severe sequelae were less likely to respond. The diagnosis of GBS or a variant was based on responder self‐identification and we were unable to confirm this from medical records. However, we considered it unlikely that people with conditions other than GBS would identify themselves as having GBS and then go on to complete an extensive questionnaire of their experiences. Some participant characteristics such as year of and time since diagnosis, time to seek help, number of consultations and delay before diagnosis, place of treatment and length of hospital stay may have been subject to recall bias.
4.4. Implications for practice and research
The responses to the survey confirmed recent studies suggesting that various physical, psychological and social factors were associated with recovery. 11 , 13 Because the survey showed good evidence of face and construct validity and internal consistency, it could be used to assess patient experience and how experience of care and support could be improved in a larger population of people with GBS.
Further research needs to be done to develop patient‐reported outcome measures 33 and PREMs for GBS beyond traditional disability measures such as the GBS Disability Scale. 34 The experience scales developed in this survey could be used to develop and evaluate the effect of interventions designed to improve experiences at various stages of treatment and recovery including in the longer term, including better access to rehabilitation and innovative social interventions such as peer or employer support.
5. CONCLUSION
Our findings showed that the GBS patient experience survey showed characteristics of a good measure, with evidence of internal consistency and construct validity. The GBS patient experience questionnaire should be tested more widely to seek further evidence of reliability, construct validity and sensitivity to differences in care and setting.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Aloysius N. Siriwardena had the original idea for the study. The study was designed by Aloysius N. Siriwardena and Joseph N. A. Akanuwe, supported by Zahid B. Asghar, Vanessa Botan, Despina Laparidou, Ffion Curtis, Jennifer Jackson and Timothy L. Hodgson. Fieldwork was conducted by Joseph N. A. Akanuwe and analysis by Vanessa Botan, supported by Aloysius N. Siriwardena, Zahid B. Asghar, Despina Laparidou, Ffion Curtis and Jennifer Jackson. Aloysius N. Siriwardena wrote the first draft of the paper and all the authors edited and approved the paper.
ETHICS STATEMENT
This study was approved by the Lincoln University Ethics Committee. All interviewees provided informed consent to participate. This study was performed in accordance with the Declaration of Helsinki.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
This study was funded by the Guillain‐Barré and Associated Inflammatory Neuropathies (GAIN) charity. The views expressed are those of the authors and not necessarily those of the funder. The authors thank the study participants, the GAIN charity, members of the Community and Health Research Unit and the Healthier Ageing Patient and Public Involvement group who reviewed and advised on the conduct of study.
Siriwardena AN, Akanuwe JNA, Botan V, et al. Patient‐reported symptoms and experience following Guillain‐Barré syndrome and related conditions: Questionnaire development and validation. Health Expect. 2022;25:223‐231. 10.1111/hex.13367
DATA AVAILABILITY STATEMENT
The data analysed during this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain‐Barre syndrome: a systematic review and meta‐analysis. Neuroepidemiology. 2011;36(2):123‐133. 10.1159/000324710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van der Meché FG, Van Doorn PA, Meulstee J, Jennekens FG, GBS‐Consensus Group of the Dutch Neuromuscular Research Support Centre . Diagnostic and classification criteria for the Guillain‐Barre syndrome. Eur Neurol. 2001;45(3):133‐139. 10.1159/000052111 [DOI] [PubMed] [Google Scholar]
- 3. Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barre syndrome. Lancet. 2016;388(10045):717‐727. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 4. Hughes RA, Swan AV, Raphaël JC, Annane D, van Koningsveld R, van Doorn PA. Immunotherapy for Guillain‐Barre syndrome: a systematic review. Brain. 2007;130(Pt 9):2245‐2257. 10.1093/brain/awm004 [DOI] [PubMed] [Google Scholar]
- 5. Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain‐Barre syndrome. Cochrane Database Syst Rev. 2014;9:CD002063. 10.1002/14651858.CD002063.pub6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prada V, Massa F, Salerno A, et al. Importance of intensive and prolonged rehabilitative treatment on the Guillain‐Barre syndrome long‐term outcome: a retrospective study. Neurol Sci. 2020;41(2):321‐327. 10.1007/s10072-019-04077-x. [DOI] [PubMed] [Google Scholar]
- 7. Jacobs BC, van den Berg B, Verboon C, et al. International Guillain‐Barre syndrome outcome study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain‐Barre syndrome. J Peripher Nerv Syst. 2017;22(2):68‐76. 10.1111/jns.12209 [DOI] [PubMed] [Google Scholar]
- 8. Hughes RAC. Guillain‐Barre syndrome: looking back and forward. J Neurol Neurosurg Psychiatry. 2020;91(2):111‐112. 10.1136/jnnp-2019-322361 [DOI] [PubMed] [Google Scholar]
- 9. Yuki N, Hartung HP. Guillain‐Barre syndrome. N Engl J Med. 2012;366(24):2294‐2304. 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 10. Rajabally YA, Seri S, Cavanna AE. Neuropsychiatric manifestations in inflammatory neuropathies: a systematic review. Muscle Nerve. 2016;54(1):1‐8. 10.1002/mus.25112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laparidou D, Curtis F, Akanuwe J, Jackson J, Hodgson TL, Siriwardena AN. Patients' experiences and perceptions of Guillain‐Barre syndrome: a systematic review and meta‐synthesis of qualitative research. PLoS One. 2021;16(2):e0245826. 10.1371/journal.pone.0245826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackson K, Hamilton S, Jones S, Barr S. Patient reported experiences of using community rehabilitation and/or support services whilst living with a long‐term neurological condition: a qualitative systematic review and meta‐aggregation. Disabil Rehabil. 2019;41(23):2731‐2749. 10.1080/09638288.2018.1473508 [DOI] [PubMed] [Google Scholar]
- 13. Akanuwe JNA, Laparidou D, Curtis F, Jackson J, Hodgson TL, Siriwardena AN. Exploring the experiences of having Guillain‐Barre Syndrome: a qualitative interview study. Health Expect. 2020;23(5):1338‐1349. 10.1111/hex.13116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darzi A. High Quality Care For All: NHS Next Stage Review Final Report. TSO; 2008. [Google Scholar]
- 15. Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open. 2013;3(1). e001570. 10.1136/bmjopen-2012-001570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vanhoutte EK, Draak TH, Gorson KC, et al. Impairment measures versus inflammatory RODS in GBS and CIDP: a responsiveness comparison. J Peripher Nerv Syst. 2015;20(3):289‐295. 10.1111/jns.12118 [DOI] [PubMed] [Google Scholar]
- 17. Entwistle V, Firnigl D, Ryan M, Francis J, Kinghorn P. Which experiences of health care delivery matter to service users and why? A critical interpretive synthesis and conceptual map. J Health Serv Res Policy. 2012;17(2):70‐78. 10.1258/jhsrp.2011.011029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beattie M, Murphy DJ, Atherton I, Lauder W. Instruments to measure patient experience of healthcare quality in hospitals: a systematic review. Syst Rev. 2015;4:97. 10.1186/s13643-015-0089-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujisawa R, Klazinga NS . Measuring patient experiences (PREMS): progress made by the OECD and its member countries between 2006 and 2016 Organisation for Economic Co‐operation and Development (OECD Health Working Papers No. 102), Paris, France; 2017.
- 20. Cronbach LJ. Essentials of Psychological Testing. 5th ed. HarperCollins; 1990. [Google Scholar]
- 21. Field AP. Discovering Statistics Using IBM SPSS Statistics: And Sex and Drugs and Rock 'n' Roll. 4th ed. SAGE; 2013. [Google Scholar]
- 22. Bernsen RA, de Jager AE, van der Meché FG, Suurmeijer TP. How Guillain‐Barre patients experience their functioning after 1 year. Acta Neurol Scand. 2005;112(1):51‐56. 10.1111/j.1600-0404.2005.00429.x [DOI] [PubMed] [Google Scholar]
- 23. Koeppen S, Kraywinkel K, Wessendorf TE, et al. Long‐term outcome of Guillain‐Barre syndrome. Neurocrit Care. 2006;5(3):235‐242. 10.1385/NCC:5:3:235 [DOI] [PubMed] [Google Scholar]
- 24. Bernsen RA, de Jager AE, Kuijer W, van der Meché FG, Suurmeijer TP. Psychosocial dysfunction in the first year after Guillain‐Barre syndrome. Muscle Nerve. 2010;41(4):533‐539. 10.1002/mus.21536 [DOI] [PubMed] [Google Scholar]
- 25. Bernsen RA, Jacobs HM, de Jager AE, van der Meché FG. Residual health status after Guillain‐Barre syndrome. J Neurol Neurosurg Psychiatry. 1997;62(6):637‐40. 10.1136/jnnp.62.6.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Darweesh SK, Polinder S, Mulder MJ, et al. Health‐related quality of life in Guillain‐Barre syndrome patients: a systematic review. J Peripher Nerv Syst. 2014;19(1):24‐35. 10.1111/jns5.12051 [DOI] [PubMed] [Google Scholar]
- 27. Alexandrescu R, Siegert RJ, Turner‐Stokes L. Functional outcomes and efficiency of rehabilitation in a national cohort of patients with Guillain‐Barre syndrome and other inflammatory polyneuropathies. PLoS One. 2014;9(11). e110532. 10.1371/journal.pone.0110532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inokuchi H, Yasunaga H, Nakahara Y, et al. Effect of rehabilitation on mortality of patients with Guillain‐Barre syndrome: a propensity‐matched analysis using nationwide database. Eur J Phys Rehabil Med. 2014;50(4):439‐446. [PubMed] [Google Scholar]
- 29. Davidson I, Wilson C, Walton T, Brissenden S. Physiotherapy and Guillain‐Barre syndrome: results of a national survey. Physiotherapy. 2009;95(3):157‐163. 10.1016/j.physio.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 30. Carroll A, McDonnell G, Barnes M. A review of the management of Guillain‐Barre syndrome in a regional neurological rehabilitation unit. Int J Rehabil Res. 2003;26(4):297‐302. 10.1097/00004356-200312000-00007 [DOI] [PubMed] [Google Scholar]
- 31. Haines KJ, Beesley SJ, Hopkins RO, et al. Peer support in critical care: a systematic review. Crit Care Med. 2018;46(9):1522‐1531. 10.1097/CCM.0000000000003293 [DOI] [PubMed] [Google Scholar]
- 32. Doets AY, Hughes RA, Brassington R, Hadden RD, Pritchard J. Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain‐Barre syndrome. Cochrane Database Syst Rev. 2020;1:CD008630. 10.1002/14651858.CD008630.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Djordjevic G, Stojanov A, Bozovic I, et al. Six‐month prospective study of quality of life in Guillain‐Barre syndrome. Acta Neurol Scand. 2020;141(3):236‐241. 10.1111/ane.13195 [DOI] [PubMed] [Google Scholar]
- 34. van Koningsveld R, Steyerberg EW, Hughes RA, Swan AV, van Doorn PA, Jacobs BC. A clinical prognostic scoring system for Guillain‐Barre syndrome. Lancet Neurol. 2007;6(7):589‐594. 10.1016/S1474-4422(07)70130-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data analysed during this study are available from the corresponding author on reasonable request.


