Abstract
Mycobacterium abscessus and Mycobacterium chelonae are two closely related species that are often not distinguished by clinical laboratories despite the fact they cause diseases requiring different treatment regimens. Multilocus enzyme electrophoresis, PCR-restriction fragment length polymorphism analysis of the 65-kDa heat shock protein gene, biochemical tests, and high-performance liquid chromatography of mycolic acids were used to identify 75 isolates as either M. abscessus or M. chelonae that were originally submitted for drug susceptibility testing. Only 36 of these isolates were submitted with an identification at the species level. Using the above methods, 46 of the isolates were found to be M. abscessus and 29 were identified as M. chelonae. Eight isolates originally submitted as M. chelonae were identified as M. abscessus, and one isolate submitted as M. abscessus was found to be M. chelonae. The four identification methods were in agreement in identifying 74 of the 75 isolates. In drug susceptibility testing, all isolates of M. abscessus exhibited resistance to tobramycin (MIC of 8 to ≥16 μg/ml), while all isolates of M. chelonae were susceptible to this drug (MIC of ≤4 μg/ml). The results suggest that once an identification method is selected, clinical laboratories should be able to easily identify isolates of M. abscessus and M. chelonae.
Mycobacterium abscessus and Mycobacterium chelonae are two species of rapidly growing mycobacteria frequently associated with nosocomial outbreaks and pseudo-outbreaks (1, 11, 24). Widespread outbreaks of postinjection abscesses have occurred due to medications contaminated with M. abscessus (6, 22). Contamination of hospital equipment and medications can generally be traced to the ubiquitous presence of these organisms in tap water and their resistance to commonly used disinfectants (24).
It is clinically important to correctly identify these organisms since they cause infections requiring different treatment regimens (R. J. Wallace, Jr., B. A. Brown, D. E. Griffith, Letter, Pediatr. Infect. Dis. J. 16:829, 1997). Prior to 1992, M. abscessus was considered a subspecies of M. chelonae. Though Kusunoki and Ezaki (10) firmly established through DNA hybridization that these organisms are separate species, they are nearly indistinguishable phenotypically. Only two biochemical tests, those for sodium chloride tolerance and utilization of citrate, are useful for identifying these organisms at the species level (17), but these tests may take up to 4 weeks to complete (5). Identification of rapidly growing species using high-performance liquid chromatography (HPLC) of mycolic acids has been limited with M. abscessus and M. chelonae because they produce very similar mycolic acid patterns (2). Recently, PCR-based methods targeting polymorphic regions of the 65-kDa heat shock protein (HSP) gene have been used successfully to identify isolates of M. abscessus and M. chelonae (8, 15; A. R. Lakshmy, N. Siddiqi, M. Shamim, M. Deb, G. Mehta, and S. E. Hasnain, Letter, Emerg. Infect. Dis. 6:561–562, 2000). Multilocus enzyme electrophoresis (MEE) can also be a useful tool for clustering mycobacterial strains at the species level (25, 27) and has been used successfully in studies with rapidly growing mycobacteria (1, 6, 23).
The purpose of this study was to evaluate three recognized identification methods for their ability to separate M. abscessus and M. chelonae isolates in order to help individual clinical laboratories select an identification regimen. The methods compared were (i) biochemical tests consisting of sodium chloride and citrate utilization (17), (ii) a PCR-restriction fragment length polymorphism (RFLP)-based method targeting a 439-bp segment of the 65-kDa HSP gene (8), and (iii) HPLC of mycolic acids (3, 4). MEE (25, 27) was also used to confirm the identification of each isolate and to categorize isolates of each species by electrophoretic type (ET). Once identified, isolates were also submitted for drug susceptibility testing (12) to determine if antimicrobial agents could be used to distinguish each species.
MATERIALS AND METHODS
Isolates of mycobacteria.
A total of 77 isolates were included in our study (Table 1). Of these, 75 were submitted to our laboratory for drug susceptibility testing from January 1999 through January 2000. Reference strains for M. abscessus (ATCC 23007) and M. chelonae (ATCC 35752) were included in all tests.
TABLE 1.
Bacterial isolates submitted to CDC and identified as either M. chelonae or M. abscessus
| Isolate no. | Submitter identification | Submitter identification method(s)a | CDC identification | ET |
|---|---|---|---|---|
| CDC 99-6175 | M. chelonae/M. abscessus | HPLC | M. abscessus | 1 |
| CDC 99-6202 | M. abscessus | Biochemicals | M. abscessus | 2 |
| CDC 99-6029 | M. chelonae | Biochemicals | M. abscessus | 3 |
| CDC 99-6044 | M. chelonae | HPLC | M. abscessus | 4 |
| CDC 99-6126 | M. chelonae/M. abscessus | Biochemicals | M. abscessus | 5 |
| CDC 00-6000 | M. chelonae/M. abscessus | HPLC, biochemicals | M. abscessus | 5 |
| CDC 99-6165 | M. chelonae | Biochemicals | M. abscessus | 5 |
| CDC 99-6067 | M. chelonae complex | Biochemicals | M. abscessus | 5 |
| CDC 99-6066 | M. chelonae complex | HPLC | M. abscessus | 5 |
| CDC 99-6052 | M. abscessus | Biochemicals | M. abscessus | 5 |
| CDC 99-6049 | M. abscessus | HPLC | M. abscessus | 5 |
| CDC 99-6143 | M. chelonae | Biochemicals | M. abscessus | 5 |
| CDC 99-6144 | M. chelonae | Biochemicals | M. abscessus | 5 |
| CDC 99-6168 | Rapid grower | HPLC | M. abscessus | 5 |
| CDC 99-6149 | M. chelonae/M. abscessus | Biochemicals | M. abscessus | 5 |
| CDC 99-6028 | M. abscessus | Biochemicals | M. abscessus | 6 |
| CDC 99-6102 | M. chelonae/M. abscessus | HPLC | M. abscessus | 6 |
| CDC 99-6081 | M. abscessus | HPLC, biochemicals | M. abscessus | 6 |
| CDC 99-6078 | M. abscessus | HPLC | M. abscessus | 6 |
| CDC 99-6046 | M. chelonae/M. abscessus | Biochemicals | M. abscessus | 6 |
| CDC 99-6036 | M. chelonae | HPLC | M. abscessus | 6 |
| CDC 99-6027 | M. abscessus | Biochemicals | M. abscessus | 6 |
| CDC 99-6034 | M. chelonae group | HPLC | M. abscessus | 6 |
| CDC 99-6151 | M. chelonae/M. abscessus | HPLC | M. abscessus | 7 |
| CDC 99-6006 | M. chelonae/M. abscessus | HPLC | M. abscessus | 8 |
| CDC 99-6047 | M. chelonae/M. abscessus | HPLC | M. abscessus | 8 |
| CDC 99-6088 | M. chelonae | HPLC | M. abscessus | 9 |
| CDC 99-6051 | M. chelonae/M. abscessus | Biochemicals | M. abscessus | 10 |
| CDC 99-6139 | M. chelonae/M. abscessus | HPLC | M. abscessus | 11 |
| CDC 99-6127 | M. chelonae/M. abscessus | HPLC | M. abscessus | 11 |
| CDC 99-6117 | M. chelonae/M. abscessus | HPLC | M. abscessus | 11 |
| CDC 99-6099 | M. chelonae/M. abscessus | HPLC | M. abscessus | 11 |
| ATCC 35752 | M. abscessus reference strain | M. abscessus | 11 | |
| CDC 99-6033 | M. chelonae | HPLC | M. abscessus | 12 |
| CDC 99-6026 | M. chelonae/M. abscessus | HPLC | M. abscessus | 13 |
| CDC 99-6137 | M. abscessus | Biochemicals | M. abscessus | 14 |
| CDC 99-6176 | M. abscessus | HPLC, biochemicals | M. abscessus | 14 |
| CDC 99-6131 | M. chelonae/M. abscessus | HPLC | M. abscessus | 14 |
| CDC 99-6145 | M. abscessus | Biochemicals | M. abscessus | 14 |
| CDC 99-6068 | M. chelonae/M. abscessus | HPLC | M. abscessus | 15 |
| CDC 99-6069 | M. chelonae/M. abscessus | HPLC | M. abscessus | 15 |
| CDC 99-6015 | M. chelonae/M. abscessus | HPLC | M. abscessus | 16 |
| CDC 99-6073 | M. chelonae/M. abscessus | HPLC | M. abscessus | 17 |
| CDC 99-6050 | M. abscessus | HPLC | M. abscessus | 18 |
| CDC 99-6174 | Unknown | Biochemicals | M. abscessus | 18 |
| CDC 99-6017 | M. chelonae/M. abscessus | HPLC | M. abscessus | 19 |
| CDC 99-6025 | M. chelonae complex | HPLC | M. abscessus | 20 |
| CDC 99-6180 | Rapid grower | Biochemicals | M. chelonae | 21 |
| CDC 99-6010 | M. chelonae | Biochemicals | M. chelonae | 22 |
| CDC 99-6109 | M. chelonae/M. abscessus | HPLC, biochemicals | M. chelonae | 22 |
| CDC 99-6108 | M. chelonae | Biochemicals | M. chelonae | 22 |
| CDC 00-6005 | M. chelonae/M. abscessus | HPLC | M. chelonae | 22 |
| CDC 99-6141 | M. chelonae | HPLC, biochemicals | M. chelonae | 22 |
| CDC 99-6083 | M. chelonae | HPLC | M. chelonae | 22 |
| CDC 99-6064 | M. chelonae group | HPLC | M. chelonae | 22 |
| CDC 99-6054 | M. abscessus | HPLC, biochemicals | M. chelonae | 22 |
| CDC 99-6146 | M. chelonae/M. abscessus | HPLC | M. chelonae | 22 |
| CDC 99-6199 | Mycobacterium | Biochemicals | M. chelonae | 22 |
| CDC 99-6162 | Unknown | Biochemicals | M. chelonae | 22 |
| CDC 99-6163 | Unknown | Biochemicals | M. chelonae | 22 |
| CDC 99-6048 | M. chelonae | Biochemicals | M. chelonae | 23 |
| CDC 99-6021 | M. chelonae | HPLC, biochemicals | M. chelonae | 23 |
| CDC 99-6090 | M. chelonae | HPLC, biochemicals | M. chelonae | 23 |
| CDC 99-6082 | M. chelonae | HPLC, biochemicals | M. chelonae | 23 |
| CDC 99-6206 | M. chelonae | HPLC, biochemicals | M. chelonae | 23 |
| CDC 99-6164 | M. chelonae | Biochemicals | M. chelonae | 23 |
| ATCC 35752 | M. chelonae reference strain | M. chelonae | 23 | |
| CDC 99-6132 | M. chelonae/M. abscessus | HPLC | M. chelonae | 24 |
| CDC 99-6016 | M. chelonae/M. abscessus | HPLC | M. chelonae | 24 |
| CDC 99-6041 | M. chelonae | HPLC, biochemicals | M. chelonae | 24 |
| CDC 99-6008 | M. chelonae/M. abscessus | HPLC | M. chelonae | 25 |
| CDC 99-6142 | Runyon group IV | Biochemicals | M. chelonae | 25 |
| CDC 99-6007 | M. chelonae | Biochemicals | M. chelonae | 26 |
| CDC 99-6079 | M. chelonae | HPLC, biochemicals | M. chelonae | 27 |
| CDC 99-6110 | M. chelonae | Biochemicals | M. chelonae | 28 |
| CDC 99-6075 | M. chelonae | Biochemicals | M. chelonae | 29 |
| CDC 99-6076 | M. chelonae | Biochemicals | M. chelonae | 29 |
Biochemicals, biochemical tests.
Growth conditions and purification of isolates.
Isolates were initially transferred from the solid media on which they were submitted to 5 ml of Middlebrook-Cohn 7H9 (Remel Co., Lenexa, Kans.) liquid medium and incubated for 7 days at 30°C. The broth culture was then streaked onto Middlebrook-Cohn 7H10 medium (Remel) to isolate individual colonies. Single colony picks were transferred to 5 ml of Middlebrook-Cohn 7H9 and incubated again for 7 days at 30°C. This broth culture was then used as the inoculum in all subsequent tests. All tests were performed without identifiers.
Biochemical tests.
Utilization of sodium citrate was determined as described by Silcox et al. (17). A basal medium was prepared by dissolving 2.4 g of (NH4)2SO4 (Sigma, St. Louis, Mo.), 0.5 g of KH2PO4 (Fisher Scientific Co., Pittsburgh, Pa.), and 0.5 g of MgSO4 · 7H2O (Fisher) in 950 ml of distilled water. The pH of the solution was adjusted to 7.0, 20 g of Noble agar (Difco Laboratories, Detroit, Mich.) was added, and the medium was autoclaved. The substrate solution was prepared by dissolving 5.6 g of sodium citrate (Fisher) in 50 ml of distilled water and then filter sterilizing the solution. The substrate solution was added aseptically to the cooled basal medium, and slants were made by dispensing the medium into 8-ml screw-cap tubes. Lowenstein-Jensen (L-J) slants with 5% NaCl (Remel) were used to evaluate sodium chloride tolerance. All slants were inoculated with 0.1 ml of a 1:10 dilution of a 7-day culture in Middlebrook-Cohn 7H9 broth (Remel) and incubated at 30°C for 3 weeks. Inoculated L-J slants (Remel) served as positive growth controls. Growth on the test medium was considered a positive test result, and lack of growth was considered a negative test result.
PCR-RFLP analysis.
Template DNA was extracted, amplified, and digested according to methods previously described (8). Briefly, a 439-bp segment of the 65-kDa HSP gene (13) was targeted for amplification using primers TB11 and TB12 (19). Reaction mixtures were amplified for 35 cycles (30 s at 96°C, 30 s at 61°C, 30 s at 72°C), followed by a 10-min incubation at 72°C in a model 9600 thermocycler (Perkin-Elmer Applied Biosystems, Inc., Foster City, Calif.). Amplimers were divided and digested separately with HaeIII (37°C for 1 h) and BstEII (60°C for 1 h). Restriction products were separated on 10% polyacrylamide gel electrophoresis gels for 2.5 h at 150 V using a Tris-borate-EDTA buffer system at 13°C. DNA was visualized on a UV transilluminator after staining for 10 min in 0.5 μg of ethidium bromide per ml and destaining for 10 min.
HPLC.
Inoculated L-J slants serving as positive growth controls for the biochemical testing procedure were used for HPLC analysis. Mycolic acids were extracted from whole cells and analyzed using previously described methods (3, 4). Mycolic acids were separated and analyzed using an HPLC, model System Gold (Beckman Instruments, Inc., Fullerton, Calif.), with a Beckman C18 reverse-phase ultrasphere-XL cartridge column and a Beckman model 166 UV detector. Retention times and peak heights were recorded with an HPLC model 450 data system controller (Beckman). Peaks were identified by their relative retention times (RRTs) and numbered in order based on their emergence from the column. Isolates of M. chelonae and M. abscessus were distinguished from each other by comparing peak height ratios for corresponding peaks with the same RRT as previously described (2).
MEE.
Enzyme extracts were prepared as previously described (27). Enzymes were separated on 11% starch gels using a Tris-citrate buffer system (pH 8.0) and stained by methods described by Selander et al. (16). Five enzyme systems which exhibited differences for M. abscessus and M. chelonae isolates were used for identification. The enzymes detected were 6-phosphogluconate dehydrogenase, glutamate oxalacetic transaminase, adenylate kinase, phosphoglucose mutase, and esterase. Variations in the mobility of an enzyme were recorded by assigning ascending allele numbers to enzyme bands based on increasing migration towards the anode. An absence of activity for an enzyme was recorded as 0 and considered a null allele. Each isolate with a unique profile of allele numbers was assigned to an ET. Genetic relationships among ETs were demonstrated by a dendrogram generated by the average-linkage method of clustering from a matrix of coefficients of weighted distance (16, 18) based on five enzymes by using SAS/GRAPH software developed by Jacobs (9).
Drug susceptibility testing.
The broth microdilution method for susceptibility testing of rapidly growing mycobacteria (12) was used for isolates in this study. Commercially available plates (Trek Diagnostics, Westlake, Ohio) containing test antibiotics were used to determine MICs. Isolates were tested for susceptibility against twofold dilutions of clarithromycin (0.06 to 64 μg/ml), imipenem (1 to 64 μg/ml), cefoxitin (2 to 256 μg/ml), amikacin (1 to 128 μg/ml), sulfamethoxazole (1 to 64 μg/ml), doxycycline (0.25 to 32 μg/ml), tobramycin (1 to 32 μg/ml), and ciprofloxacin (0.125 to 16 μg/ml). Isolates were grown in Middlebrook-Cohn 7H9 broth, and the cell turbidity was adjusted until it matched a 0.5 standard on the McFarland scale by visual examination. Each plate well was inoculated according to the manufacturer's instructions, with a resulting organism density of approximately 5 × 105 CFU/ml. The plates were sealed and incubated for 96 h at 30°C. The MIC was recorded as the lowest concentration of antibiotic that inhibited visible growth for all drugs except sulfamethoxazole. For sulfamethoxazole, the MIC was defined as the concentration of the drug in the well with approximately 80% inhibition of growth compared to the growth in the control well with no drug. The isolates were classified as either susceptible, intermediate, or resistant to a particular antibiotic according to breakpoints recommended by NCCLS (12) listed in Table 2.
TABLE 2.
Antimicrobial agents used in this study
| Drug | MIC (μg/ml)a
|
||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Clarithromycin | ≤2 | 4 | ≥8 |
| Imipenem | ≤4 | 8 | ≥16 |
| Cefoxitin | ≤16 | 32–64 | ≥128 |
| Amikacin | ≤16 | 32 | ≥64 |
| Sulfamethoxazole | ≤32 | ≥64 | |
| Doxycycline | ≤1 | 2–8 | ≥128 |
| Tobramycin | ≤4 | 8 | ≥16 |
| Ciprofloxacin | ≤1 | 2 | ≥4 |
Based on interpretive criteria from NCCLS guidelines (12).
RESULTS
The 77 isolates used in this study are listed in Table 1 in ascending order by ET as determined by MEE results (Table 3) and by a dendrogram generated on the basis of electrophoretic profiles (Fig. 1). Isolates were submitted from 20 state labs and from Canada. Among the submitting laboratories, nine used biochemicals to identify rapid growers (29 isolates), eight used HPLC (34 isolates), and four used a combination of HPLC and biochemicals (12 isolates). Twenty-four isolates were submitted as M. chelonae, and 12 were received as M. abscessus. The remaining isolates were submitted as M. chelonae/M. abscessus (27 isolates), M. chelonae complex (3 isolates), M. chelonae group (2 isolates), rapid grower (2 isolates), Runyon group IV (1 isolate), mycobacterium (1 isolate), and unknown (3 isolates). Five laboratories using only HPLC for identification did not to attempt to separate each species; this accounted for 22 of the 27 isolates submitted as M. chelonae/M. abscessus. Based on our biochemical tests, PCR-RFLP analysis, and HPLC of mycolic acids, a final identification was assigned to each isolate. The three methods described above were in agreement in identifying 74 of the 75 submitted isolates. A final identification of M. abscessus was assigned to 46 isolates, and the remaining 29 isolates were identified as M. chelonae. Of the 24 isolates submitted to our laboratory as M. chelonae, 8 were found to be M. abscessus by our testing methods. Among the 12 isolates submitted as M. abscessus, 1 was identified by us as M. chelonae.
TABLE 3.
Enzyme profiles for 47 isolates of M. abscessus and 30 isolates of M. chelonae
| ET | No. of isolates | Species identified | Allele no. at locus for corresponding enzymea
|
||||
|---|---|---|---|---|---|---|---|
| 6PD | GOT | ADK | PGM | EST | |||
| 1 | 1 | M. abscessus | 3 | 1 | 3 | 3 | 2 |
| 2 | 1 | M. abscessus | 3 | 1 | 3 | 2 | 2 |
| 3 | 1 | M. abscessus | 3 | 2 | 2 | 1 | 1 |
| 4 | 1 | M. abscessus | 1 | 1 | 2 | 0 | 2 |
| 5 | 11 | M. abscessus | 1 | 1 | 2 | 2 | 1 |
| 6 | 8 | M. abscessus | 1 | 1 | 2 | 1 | 1 |
| 7 | 1 | M. abscessus | 2 | 1 | 2 | 2 | 1 |
| 8 | 2 | M. abscessus | 2 | 1 | 2 | 1 | 1 |
| 9 | 1 | M. abscessus | 3 | 1 | 2 | 2 | 8 |
| 10 | 1 | M. abscessus | 3 | 1 | 2 | 2 | 5 |
| 11 | 5 | M. abscessus | 3 | 1 | 2 | 2 | 1 |
| 12 | 1 | M. abscessus | 3 | 1 | 2 | 1 | 2 |
| 13 | 1 | M. abscessus | 3 | 1 | 2 | 4 | 2 |
| 14 | 4 | M. abscessus | 3 | 1 | 2 | 2 | 2 |
| 15 | 2 | M. abscessus | 3 | 1 | 2 | 3 | 2 |
| 16 | 1 | M. abscessus | 2 | 1 | 2 | 2 | 3 |
| 17 | 1 | M. abscessus | 2 | 1 | 2 | 2 | 2 |
| 18 | 2 | M. abscessus | 2 | 1 | 2 | 3 | 1 |
| 19 | 1 | M. abscessus | 2 | 1 | 2 | 1 | 4 |
| 20 | 1 | M. abscessus | 2 | 1 | 2 | 0 | 4 |
| 21 | 1 | M. chelonae | 4 | 2 | 1 | 3 | 7 |
| 22 | 12 | M. chelonae | 4 | 2 | 1 | 4 | 5 |
| 23 | 7 | M. chelonae | 4 | 2 | 1 | 3 | 5 |
| 24 | 3 | M. chelonae | 4 | 2 | 1 | 0 | 5 |
| 25 | 2 | M. chelonae | 4 | 2 | 1 | 4 | 6 |
| 26 | 1 | M. chelonae | 4 | 1 | 1 | 5 | 5 |
| 27 | 1 | M. chelonae | 5 | 1 | 1 | 3 | 5 |
| 28 | 1 | M. chelonae | 4 | 1 | 1 | 0 | 3 |
| 29 | 2 | M. chelonae | 4 | 1 | 1 | 3 | 6 |
6PD, 6-phosphogluconate dehydrogenase; GOT, glutamate oxalacetic transaminase; ADK, adenylate kinase; PGM, phosphoglucomutase; EST, esterase.
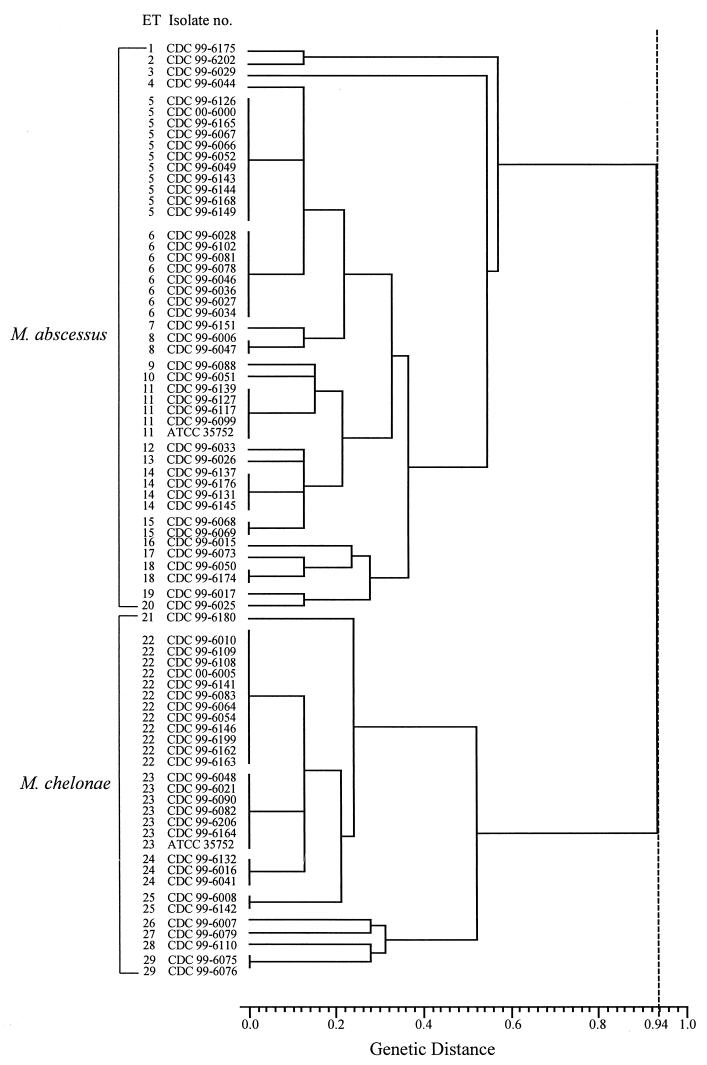
FIG. 1.
Dendrogram showing relationships among 29 ETs for isolates of M. abscessus and M. chelonae. M. abscessus isolates were separated from M. chelonae isolates by a genetic distance of 0.94 (broken line).
With one exception, biochemical tests conducted in our laboratory divided the isolates into two groups corresponding to the patterns for M. abscessus and M. chelonae reference strains. Isolates of M. abscessus grew on L-J medium with 5% NaCl but failed to grow on sodium citrate medium. In contrast, all isolates of M. chelonae grew on sodium citrate medium but not on L-J medium with 5% NaCl. One isolate, CDC 99-6025, produced biochemical results that were inconclusive since the isolate grew on both 5% NaCl and sodium citrate slants. Based on the results from PCR-RFLP, HPLC, and MEE, isolate CDC 99-6025 was identified as M. abscessus.
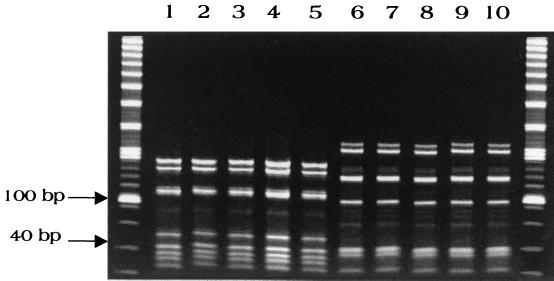
PCR-RFLP conducted on the test isolates gave two distinct patterns, as shown in Fig. 2. One pattern corresponded to the M. abscessus type strain, and the other corresponded to the M. chelonae type strain. Thus, these two patterns were used to identify isolates of each species.
FIG. 2.
PCR-RFLP patterns for isolates of M. abscessus and M. chelonae. Separate DNA digests were combined using HaeIII and BstEII. Lanes 1 to 5, M. abscessus isolates CDC 99-6126, CDC 99-6127, CDC 99-6139, CDC 99-6165, and CDC 99-6117; lanes 6 to 10, M. chelonae isolates CDC 99-6079, CDC 99-6108, CDC 99-6109, CDC 99-6110, and CDC 99-6111. Outside lanes contain combined 20- and 100-bp ladders.
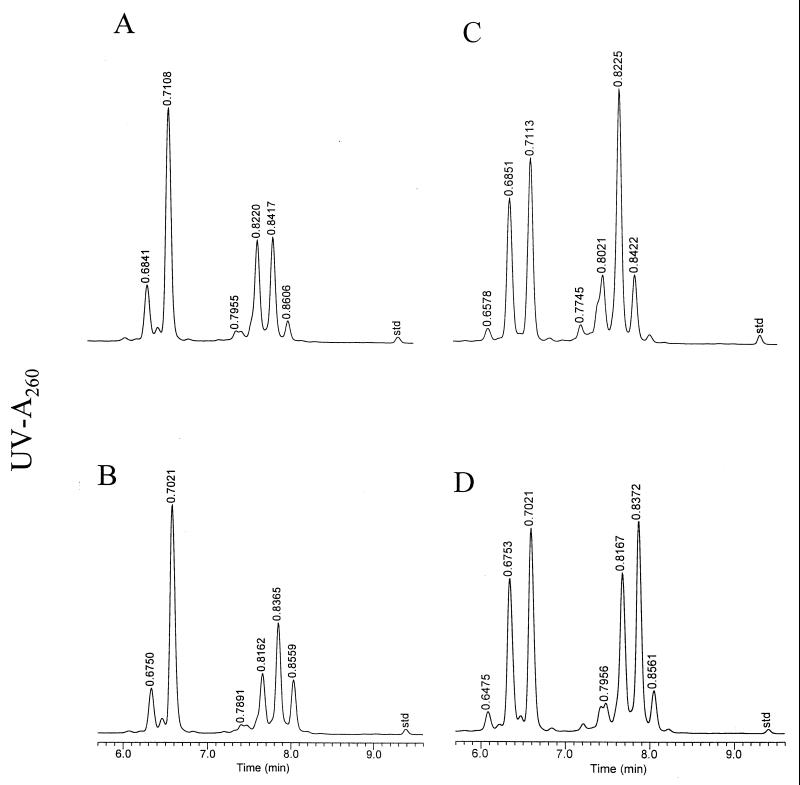
Isolates of M. abscessus were also distinguished from isolates of M. chelonae by patterns produced by HPLC of their mycolic acids. Isolates for each species produced two major patterns (Fig. 3). The peak height ratios of the two major peaks in the first cluster at RRTs of 0.68 min and 0.71 min were 0.20 for the M. abscessus patterns and 0.75 for the M. chelonae patterns. In addition, after normalization of the mycolic acid peaks, the contributions of the major peak in the first cluster at an RRT of 0.71 min were ∼43% for M. abscessus and ∼24% for M. chelonae. The above criteria were consistently applied to identify each isolate to the species level.
FIG. 3.
Representative mycolic acid patterns for isolates of M. abscessus and M. chelonae. (A) M. abscessus reference strain ATCC 23007; (B) M. abscessus patient isolate CDC 99-6202; (C) M. chelonae reference strain ATCC 35752; and (D) M. chelonae patient isolate CDC 99-6164.
Based on the MEE results (Table 3), the 47 isolates of M. abscessus were separated into 20 ETs (ETs 1 through 20), while the 30 isolates of M. chelonae separated into 9 ETs (ETs 21 through 29). Using the electrophoretic profiles from the analysis of only five enzymes to generate a dendrogram (Fig 1.), the M. abscessus isolates clustered together at a genetic distance of 0.58 while the M. chelonae isolates clustered together at a genetic distance of 0.52. M. abscessus isolates were very distinguishable from the M. chelonae ones, with the clusters for these species being separated by a genetic distance of 0.94.
The drug susceptibility results for 45 isolates of M. abscessus and 30 isolates of M. chelonae are listed in Table 4. The only drug that appeared useful for separating the two species was tobramycin. All isolates of M. abscessus were either intermediately or fully resistant to tobramycin, whereas isolates of M. chelonae were susceptible to this drug. Of 30 M. chelonae isolates, 29 were resistant to cefoxitin; however, 6 isolates of M. abscessus (13.3%) also appeared resistant to this drug. While 29 of the 30 M. chelonae isolates were sensitive to clarithromycin (96.7%), 16 M. abscessus isolates were resistant to this drug (35.6%) and 7 (16.6%) appeared to have intermediate resistance. When tested against amikacin, 41 out of 45 M. abscessus isolates were susceptible (91%), while only 12 of 30 (40%) M. chelonae isolates were susceptible to this antibiotic.
TABLE 4.
Drug susceptibility results for isolates identified as either M. abscessus or M. chelonae
| Drugb | No. of isolates with indicated susceptibility
|
|||||
|---|---|---|---|---|---|---|
|
M. abscessusa
|
M. chelonae
|
|||||
| Susceptible | Intermediate | Resistant | Susceptible | Intermediate | Resistant | |
| Clarithromycin | 22 | 7 | 16 | 29 | 0 | 1 |
| Imipenemc | 5 | 18 | 22 | 9 | 13 | 8 |
| Cefoxitin | 1 | 38 | 6 | 1 | 0 | 29 |
| Amikacin | 41 | 4 | 0 | 12 | 17 | 1 |
| Sulfamethoxazole | 2 | 43 | 0 | 30 | ||
| Doxycycline | 0 | 0 | 45 | 8 | 1 | 21 |
| Tobramycind | 0 | 23 | 22 | 30 | 0 | 0 |
| Ciprofloxacin | 1 | 0 | 44 | 4 | 2 | 24 |
Susceptibilities could not be determined for two isolates due to insufficient growth.
See Table 2 for MIC interpretations.
NCCLS (12) recommends that imipenem results should not be reported for M. chelonae and M. abscessus due to reproducibility and interpretation problems.
NCCLS (12) recommends that tobramycin results should be reported only for M. chelonae because this drug is therapeutically superior to amikacin for treatment of M. chelonae infections.
DISCUSSION
Although M. abscessus and M. chelonae have been recognized as distinct species since 1992 (9), many clinical laboratories either fail to distinguish these species or incorrectly identify them. In this study, only 12 of 46 isolates (26.1%) were correctly submitted to the Centers for Disease Control and Prevention (CDC) as M. abscessus. For M. chelonae, only 16 of 29 isolates (55.2%) were submitted with the correct identification. Eight isolates were submitted to the CDC as M. chelonae but were identified as M. abscessus, and one isolate was submitted as M. abscessus but was identified as M. chelonae. Ringuet et al. (15) reported that the most common error in databases for rapidly growing mycobacteria was misidentification of M. abscessus sequences as those of M. chelonae.
Our laboratory easily identified and separated isolates of M. abscessus from M. chelonae using either MEE, PCR-RFLP, biochemical tests, or HPLC. Except for MEE, any of these methods could be easily incorporated for routine identification of these species by either a reference or clinical laboratory.
The genetic distances generated by MEE clearly demonstrated that M. abscessus is taxonomically distinct from M. chelonae. MEE was extremely useful in this study for supporting the identification results from PCR-RFLP, biochemical tests, and HPLC. Though MEE is a very useful typing technique for epidemiological studies (1, 6, 23, 27), it is too labor-intensive for routine identification purposes.
PCR-RFLP of polymorphic regions of the hsp65 gene has the greatest potential for being widely used as a quick method for identifying all mycobacteria (8, 14, 15, 19, 20, 21, 26). A small number of cells can be used for detection and identification. Numerical algorithms can be used to eliminate subjectivity from pattern interpretation (8). In this study, isolates of M. abscessus and M. chelonae were easily identified since isolates of each species produced a single unique pattern.
For laboratories that routinely use biochemical tests for identification, our results suggest that properly interpreted tests for sodium citrate utilization and 5% NaCl tolerance can be used to distinguish M. abscessus from M. chelonae. Results from a previous study indicated that the 5% NaCl tolerance test is unreliable unless the inoculum is equal to a 1 McFarland standard and slants are incubated at 35°C for 4 weeks (5). In contrast, we were able to record final results for both biochemical tests 3 weeks after inoculating slants with a 1:10 dilution of a 7-day culture and incubation at 30°C. Obviously, variations in how individuals conduct and interpret these tests along with the incubation time required for a final reading are all drawbacks for using biochemicals to identify species.
When isolates are grown under standardized conditions, our results indicate that HPLC can also be used to separate M. abscessus from M. chelonae. Using HPLC and pattern recognition software, Glickman et al. (7) reported 94% specificity for identifying 17 isolates of M. chelonae and 31 isolates of M. abscessus. However, reference laboratories using HPLC (ours included) do not routinely attempt to distinguish these two species and report results as being an M. abscessus/M. chelonae pattern. Though the mycolic acid patterns are very similar, the appropriately chosen peak height ratios can be used to differentiate these two rapid growers.
Using the methods discussed above, we were able to establish a well-defined set of isolates representing each species. When these isolates were submitted to us for drug susceptibility testing, it also became obvious that susceptibility to tobramycin could assist in identification. All isolates identified as M. chelonae were susceptible to this drug, while isolates identified as M. abscessus were either resistant or intermediately resistant. Disturbingly, more than 50% of the M. abscessus isolates tested against clarithromycin in this study were either resistant or intermediately resistant to this antimicrobial. Clarithromycin is often the drug of choice for treating infections with rapid growers. This level of resistance is not representative of our overall experience with these organisms. Approximately 20% of clinical isolates submitted to our laboratory for susceptibility testing between 1999 and 2000 have shown resistance to clarithromycin (B. Metchock, unpublished data). Others have found that nearly all isolates of M. abscessus submitted for susceptibility testing between 1990 and 1995 were susceptible to clarithromycin (B. A. Brown-Elliot and R. J. Wallace, Jr., Letter, J. Clin. Microbiol. 39:2745–2746, 2001). In addition, we report in this study that 97% of M. abscessus isolates were either resistant or intermediately resistant to cefoxitin. A combination of clarithromycin and cefoxitin is a common regimen for treating M. abscessus otitis media (Wallace et al., letter). In our in vitro analysis, the only drug that all M. abscessus isolates were susceptible to was amikacin.
Several methods are effective for separating M. abscessus from M. chelonae. Techniques such as biochemical testing are inexpensive to initiate, and we found the results from our procedure easy to interpret. Reference laboratories typically have access to PCR equipment that can potentially be used for identification of mycobacteria by PCR-RFLP methods. Laboratories performing HPLC can use the criteria described in this study to identify M. abscessus and M. chelonae by their mycolic acid patterns. Therefore, reference laboratories should be able to routinely identify these Mycobacterium species. Drug susceptibility results are needed to provide appropriate therapy and may also aid in identification of these species. However, drug results should be used only to support another identification method and should not be the sole criterion.
REFERENCES
- 1.Ashford D A, Kellerman S, Yakrus M, Brim S, Good R C, Fineli L, Jarvis W R, McNeil M M. Pseudo-outbreak of septicemia due to rapidly growing mycobacteria associated with extrinsic contamination of culture supplement. J Clin Microbiol. 1997;35:2040–2042. doi: 10.1128/jcm.35.8.2040-2042.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler W R, Kilburn J O. High-performance liquid chromatography patterns of mycolic acids as criteria for identification of Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium smegmatis. J Clin Microbiol. 1990;28:2094–2098. doi: 10.1128/jcm.28.9.2094-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler W R, Jost K C, Jr, Kilburn J O. Identification of mycobacteria by high-performance liquid chromatography. J Clin Microbiol. 1991;29:2468–2472. doi: 10.1128/jcm.29.11.2468-2472.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler W R, Thibert L, Kilburn J O. Identification of Mycobacterium avium complex strains and some similar species by high-performance liquid chromatography. J Clin Microbiol. 1992;30:2698–2704. doi: 10.1128/jcm.30.10.2698-2704.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conville P S, Witebsky F G. Variables affecting results of sodium chloride tolerance test for identification of rapidly growing mycobacteria. J Clin Microbiol. 1998;36:1555–1559. doi: 10.1128/jcm.36.6.1555-1559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galil K, Miller L A, Yakrus M A, Wallace R J, Mosley D G, England B, Huitt G, McNeil M M, Perkins B A. Abscesses due to Mycobacterium abscessus linked to injection of unapproved alternative medication. Emerg Infect Dis. 1999;5:681–687. doi: 10.3201/eid0505.990509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glickman S E, Kilburn J O, Butler W R, Ramos L S. Rapid identification of mycolic acid patterns of mycobacteria by high-performance liquid chromatography using pattern recognition software and a Mycobacterium library. J Clin Microbiol. 1994;32:740–745. doi: 10.1128/jcm.32.3.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez M S, Morlock G P, Butler W R, Crawford J T, Cooksey R C. Identification of Mycobacterium species by PCR-restriction fragment length polymorphism analyses using fluorescence capillary electrophoresis. J Clin Microbiol. 1999;37:3688–3692. doi: 10.1128/jcm.37.11.3688-3692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs D. Proceedings of the 15th Annual SAS/User's Group International Conference. Cary, N.C: SAS Institute, Inc.; 1990. SAS/GRAPH software and numerical taxonomy; pp. 1413–1418. [Google Scholar]
- 10.Kusunoki S, Ezaki T. Proposal of Mycobacterium peregrinum sp. nov., nom. rev., and elevation of Mycobacterium chelonae subsp. abscessus (Kubica et al.) to species status: Mycobacterium abscessus comb. nov. Int J Syst Bacteriol. 1992;42:240–245. doi: 10.1099/00207713-42-2-240. [DOI] [PubMed] [Google Scholar]
- 11.Lai K K, Brown B A, Westerling J A, Fontecchio S A, Zhang Y, Wallace R J., Jr Long-term laboratory contamination by Mycobacterium abscessus resulting in two pseudo-outbreaks: recognition with the use of random amplified polymorphic DNA (RAPD) polymerase chain reaction. Clin Infect Dis. 1998;27:169–175. doi: 10.1086/514635. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes. Tentative standard M24–T2. 2nd ed. Wayne, Pa: National Committee for Clinical Laboratory Standards; 2000. [PubMed] [Google Scholar]
- 13.Plikaytis B B, Gelber R H, Shinnick T M. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990;28:1913–1917. doi: 10.1128/jcm.28.9.1913-1917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plikaytis B B, Plikaytis B D, Yakrus M A, Butler W R, Woodley C L, Silcox V A, Shinnick T M. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, Gaillard J L, Pierre-Audigier C. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol. 1999;37:852–857. doi: 10.1128/jcm.37.3.852-857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silcox V A, Good R C, Floyd M M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981;14:686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sneath P H A, Sokal R R. Numerical taxonomy. San Francisco, Calif: W. H. Freeman & Co.; 1973. pp. 230–234. [Google Scholar]
- 19.Steingrube V A, Gibson J L, Brown B A, Zhang Y, Wilson R W, Rajagopalan M, Wallace R J., Jr PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:149–153. doi: 10.1128/jcm.33.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telenti A, Marchesi F, Balz M, Bally F, Böttger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villanueva A, Calderon R V, Vargas B A, Ruiz F, Aguero S, Zhang Y, Brown B A, Wallace R J., Jr Report on an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24:1147–1153. doi: 10.1086/513656. [DOI] [PubMed] [Google Scholar]
- 23.Wallace R J, Jr, Musser J M, Hull S I, Silcox V A, Steele L C, Forrester G D, Labidi A, Selander R K. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac surgery. J Infect Dis. 1989;159:708–716. doi: 10.1093/infdis/159.4.708. [DOI] [PubMed] [Google Scholar]
- 24.Wallace R J, Jr, Brown B A, Griffith D E. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol. 1998;52:453–490. doi: 10.1146/annurev.micro.52.1.453. [DOI] [PubMed] [Google Scholar]
- 25.Wasem C F, McCarthy C M, Murray L W. Multilocus enzyme electrophoresis analysis of the Mycobacterium avium complex and other mycobacteria. J Clin Microbiol. 1991;29:264–271. doi: 10.1128/jcm.29.2.264-271.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson R W, Steingrube V A, Brown B A, Wallace R J., Jr Clinical application of PCR-restriction enzyme analysis for rapid identification of aerobic actinomycete isolates. J Clin Microbiol. 1998;36:148–152. doi: 10.1128/jcm.36.1.148-152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yakrus M A, Reeves M W, Hunter S B. Characterization of isolates of Mycobacterium avium serotypes 4 and 8 from patients with AIDS by multilocus enzyme electrophoresis. J Clin Microbiol. 1992;30:1474–1478. doi: 10.1128/jcm.30.6.1474-1478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]