In the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, patients with hematologic diseases have an increased risk for severe disease courses.1 Several studies reported that patients with plasma cell malignancies and in particular those who received systemic treatment are at high risk of death after COVID-19 infection.2 The management of COVID-19 infections in patients with systemic light chain (AL) amyloidosis might be even more challenging given the impaired immune system and the multisystemic involvement resulting in organ dysfunction.3
COVID-19 vaccines have been proven to be highly efficacious in preventing COVID-19 infections in immunocompetent individuals.4–6 However, there is emerging evidence that the antibody and cellular responses after COVID-19 vaccination in patients with plasma/B-cell malignancies are lower than in healthy individuals.7–10 A recent study on a cohort of 59 AL amyloidosis patients reported a lower antibody response rate compared with healthy controls after the first COVID-19 vaccination.11 In this study, we retrospectively analyzed the anti-SARS-CoV-2-S1 antibody response rate after the second COVID-19 vaccination in 117 patients with AL amyloidosis.
All patients eligible for this retrospective study were diagnosed with an AL amyloidosis and an underlying plasma cell dyscrasia, had received two doses of COVID-19 vaccinations, and had a measurement of antibodies against SARS-CoV-2 spike protein (anti-SARS-CoV-2-S1), routinely performed at our institution. Patients with a known history of COVID-19 infection or detected response against the nucleocapsid protein (anti-SARS-CoV-2-N) were excluded from this study. Baseline patient characteristics, treatment details, vaccination details, and the results of the routinely performed anti-SARS-CoV-2-S1 and anti-SARS-CoV-2-N antibody monitoring were collected by chart review. The measurement of anti-SARS-CoV-2-S1 and anti-SARS-CoV-2-N antibodies was performed as previously described.12 The retrospective analysis was approved by the Ethics Committee of the University of Heidelberg and was conducted in accordance with the principles of the Helsinki declaration. All participants gave written informed consent to retrospective analysis of their clinical data.
The majority of patients included in this study (80%) received BNT162b2/BioNTech, whereas smaller proportions of patients received two vaccinations of AZD122/AstraZeneca (13%) or mRNA-1273/Moderna (3%). Only 4 of the 117 patients (3%) had a heterologous vaccine regimen. Patient and treatment characteristics are shown in Table 1 and Suppl. Table S1. Sixty-four percent of patients (75/117) were diagnosed with an underlying smoldering myeloma and 29% (34/117) with a monoclonal gammopathy of clinical significance. Seven percent (8/117) had an underlying multiple myeloma at the time of vaccination. There was a wide span between the first diagnosis of AL amyloidosis and the vaccination, which ranges from 4 days to 22.2 years. Cardiac and renal involvement were present in 74% (86/117) and 62% (72/117) of patients, respectively. Patients had received a median of two treatment lines (range 0–8) and 46% (54/117) were on active treatment at time of vaccination or had received their last treatment within 3 months before vaccination. Sixty-two percent of patients (73/117) were in a complete response or very good partial response at the time of vaccination. Based on convenient sampling, the median time from the second vaccination to serology testing was 35 days (IQR: 22–59 days).
Table 1.
Patient Baseline Characteristics at Time of Vaccination
| Overall (N = 117) | |
|---|---|
| Age, y | 64 [44, 84] |
| Male sex | 66 (56 %) |
| Underlying clonal disease | |
| MGCS | 34 (29 %) |
| Smoldering myeloma | 75 (64 %) |
| Multiple myeloma | 8 (7 %) |
| >2 organs involved | 57 (49 %) |
| Treatment naive | 5 (4 %) |
| Lines of treatment | 2 [0, 8] |
| Interval between last treatment and first vaccination (patients with current or prior treatment), days | |
| <3 months | 54 (46 %) |
| >3 and <12 months | 16 (14 %) |
| >12 months | 42 (36 %) |
| Disease in hematologic remission (CR or VGPR) | 73 (62 %) |
| Vaccines | |
| AZD122 (AstraZeneca) | 15 (13 %) |
| BNT162b2 (BioNTech) | 94 (80 %) |
| Heterologous vaccines | 4 (3 %) |
| mRNA-1273 (Moderna) | 4 (3 %) |
Data are number of patients (%) or median [range].
CR = complete remission; MGCS = monoclonal gammopathy of clinical significance; VGPR = very good partial response
The overall seroconversion rate in our cohort after two vaccination doses, defined as an index of anti-SARS-CoV-2-S1 ≥1, was 87% (102/117). With a median antibody level of 36.6 among the seroconverted patients, the antibody level was remarkably lower than the level, which was reported in a large healthy vaccinated cohort using the same analysis pipeline (median reported antibody level in healthy cohort: 116.2).12 The observation of inferior antibody levels in patients with AL amyloidosis is quite similar to reported low-antibody levels in patients with multiple myeloma7 and underscores the immunosuppressive effect of the underlying plasma cell clone and the administered antiplasma cell treatments in AL amyloidosis.11,13
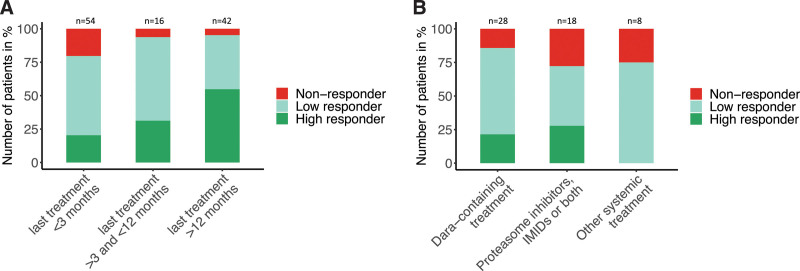
The majority of patients in this study (96%) were on active treatment or had received treatment before vaccination, which allowed us to further explore the impact of timing and type of treatment on anti-SARS-CoV-2-S1 antibody responses. Based on the large spectrum of antibody levels, we divided patients in 3 groups, which included high responders (anti-SARS-CoV-2-S1 ≥100), low responders (anti-SARS-CoV-2-S1 ≥1 and <100), and nonresponders (anti-SARS-CoV-2-S1 <1). The interval between the last treatment and first COVID-19 vaccination was positively associated with an increasing rate of antibody responders (low and high responders) and a decreasing rate of nonresponders (Figure 1A).
Figure 1.
Antibody responses after two doses of COVID-19 vaccination in patients with AL amyloidosis. (A) Antibody responses in patients with prior or current treatment (n = 112) according to the interval between the last systemic treatment and first COVID-19 vaccination (≤3 months: n = 54, >3 months and <12 months: n = 16, ≥12 months: n = 42). Based on the antibody levels after 2 doses of COVID-19 vaccination, patients were divided in 3 groups which included high responders (anti-SARS-CoV-2-S1 ≥100), low responders (anti-SARS-CoV-2-S1 ≥1 and <100), and nonresponders (anti-SARS-CoV-2-S1 <1). The distribution of all three response groups significantly differed between the 3 time intervals (Fisher’s exact test: P = 0.005). A higher proportion of nonresponders were found in the subgroup of patients who had received their last treatment (including patients on active treatment) within 3 months before vaccination (nonresponder according to last treatment: ≤3 months: 20%, >3 and <12 months: 6%, ≥12 months: 5%). Vice versa, more patients achieved high antibody responses in the subgroup of patients with a treatment-free interval of >3 months or even >12 months before vaccination (high responder according to last treatment: ≥12 months: 55%, >3 and <12 months: 31%, ≤3 months: 20%). (B) Antibody responses according to the treatment type in the subgroup of patients with AL amyloidosis who received their last treatment within 3 months before the first COVID-19 vaccination (n = 54). Treatment regimens were grouped in daratumumab-containing regimens (n = 28), regimens including proteasome inhibitors, immunomodulatory drugs or both (n = 18), and other systemic treatments (n = 8; including 3 patients with chemotherapy, 4 patients with venetoclax, and 1 patient with elotuzumab and dexamethasone). Dara = daratumumab; IMIDs = immunomodulatory drugs.
Studies in multiple myeloma have shown that anti-CD38 treatment such as daratumumab significantly impair the anti-SARS-CoV-2 antibody response.7,10 Among the patients who were on active treatment or had received treatment with daratumumab within 3 months before vaccination (n = 28), we observed a relevant proportion of patients with high (n = 6) or at least detectable (n = 18) anti-SARS-CoV-2-S1 antibody levels (Figure 1B). Similar to others,13 our study did not show that the use of daratumumab significantly impairs the antibody response compared with other active treatments in amyloidosis. Considering that only 7% of the AL amyloidosis patients in this study were diagnosed with an underlying multiple myeloma, the immunosuppressive effect conferred by the smaller underlying plasma cell clone might be less stronger than in multiple myeloma treated with daratumumab. In the subgroup of patients who were treated with proteasome inhibitors, immunomodulatory drugs or both within the last 3 months before vaccination (n = 18), we observed a nearly similar distribution of high, low, and nonresponders compared with the daratumumab subgroup.
The majority of patients in our study were vaccinated with a homologous mRNA-based vaccination regimen (98/117). High-antibody levels (index ≥100) were observed in 35 out of 94 patients after homologous vaccination with BNT162b2, 2 out of 4 patients after homologous vaccination with mRNA-1273, and 4 out of 4 patients after a heterologous vaccination regimen. In contrast, in the small cohort of 15 patients who were vaccinated with two doses of AZD122 we observed only low responses (index <100) or a failed seroconversion (Suppl. Table S2). This finding reflects what is known from COVID-19 vaccination studies in healthy populations, in which mRNA-based vaccination and also the heterologous vaccine regimen mounted higher antibody levels than homologous vaccinations with AZD122.12,14
To investigate the association between patient characteristics and a failed seroconversion after two doses of COVID-19 vaccines, we performed a multivariate logistic regression (univariate analysis: Suppl. Table S3, multivariate analysis: Suppl. Table S4). This complete case analysis (n = 108) was adjusted for potential confounders including age, dosing interval between both COVID-19 vaccinations, and vaccination regimen (mRNA-based homologous/heterologous vaccination versus vector-based homologous vaccination). A time of <3 months between last treatment and vaccination was confirmed as relevant predictor of a failed antibody response after COVID-19 vaccination (OR, 6.5; 95% CI, 1.3-53; P = 0.04). A lower serum albumin level was an additional independent predictor for a failed antibody response (per decrease of 1 g/L; OR, 1.3; 95% CI, 1.1-1.4; P < 0.001). Due to collinearity, the level of daily proteinuria, the total serum protein level, and the IgG level were not included in this analysis and analyzed in separate multivariate models. Herein, a daily proteinuria (OR, 1.3; 95% CI, 1.04-1.6; P = 0.02) and a decreased total serum protein level (OR, 1.2; 95% CI, 1.1-1.3; P < 0.001) were further predictors of a failed seroconversion after vaccination. In addition, we regressed patients’ specific variables on log-transformed anti-SARS-CoV-2-S1 antibody levels as a continuous variable to identify variables, which predict high-antibody responses (Suppl. Table S5). Taking the upper detection cutoff of 150 into account, we used a multivariate linear censored model, which was adjusted for the same confounders which were already used in the logistic regression model. Nontreatment within 3 months prior vaccination, an increasing serum albumin level, and a GFR >60 mL/min were associated with higher anti-SARS-CoV-2-S1 antibody levels.
Due to the retrospective design of this study, some limitations should be considered: the rather small number of patients, the heterogeneous vaccination regimens, the lack of a healthy reference cohort and a predefined longitudinal sample collection after COVID-19 vaccination, and the arbitrary cutoffs in the absence of measured neutralizing antibodies.
In conclusion, we found a seroconversion rate of 87% after the second vaccination in patients with AL amyloidosis but the median antibody level was remarkably lower compared with published healthy controls. In the absence of measured neutralizing antibodies, it remains unclear to what extent the anti-SARS-CoV-2-S1 antibody response confers clinical protection to COVID-19 infections. However, the level of anti-SARS-CoV-2-S1 antibodies and the level of neutralizing antibodies were previously shown to be highly positive correlated.15 In the majority of our patients with AL amyloidosis, we were able to detect anti-SARS-CoV-2-S1 antibodies and it is likely that they will at least partially benefit from two COVID-19 vaccinations. This suggests that all patients with AL amyloidosis should be vaccinated independently of their treatment status. Patients who have risk factors of a failed or lower antibody response including active or recent treatment at the time of vaccination, reduced serum albumin/protein levels, proteinuria, and impaired kidney function might be candidates for a serological monitoring after COVID-19 vaccination. Future studies, which investigate the efficacy of an additional vaccination dose and analyze different immunization strategies in patients with low or lacking antibody responses after the second vaccination, will improve recommendation strategies for this vulnerable patient cohort.
ACKNOWLEDGMENTS
The authors thank the data manager and the laboratory staff of the Department of Infectious Diseases, Virology, Heidelberg University Hospital. NL was supported by a Heidelberg School of Oncology (HSO2) fellowship from the National Center for Tumor Diseases (NCT) Heidelberg. CS was funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine. SD was supported by a grant from the Hairy Cell Leukemia Foundation, Heidelberg Research Centre for Molecular Medicine and an e:med BMBF junior group grant and DFG through the SFB873 (project B7). CMT was supported by the BMBF: RECOVER 01KI20152. UH and SoS were partially supported by DFG through Research Unit FOR 2969, projects HE 8472/1-1, SCHO 1364/2-1.
AUTHOR CONTRIBUTIONS
NL and SD initiated and designed the study. NL, SOS, UH collected clinical data. PS performed the serologic antibody testing. NL, DE and SD analyzed data and performed statistical analysis. NL and SD wrote the paper. NL, SOS, CS, DE, PS, HK, CMT, UH and SD edited the manuscript.
DISCLOSURES
NL received honoraria from Takeda and Roche. PS received consultancy from Janssen-Cilag and MSD Sharp & Dohme. CM-T received membership on an entity’s Board of Directors or advisory committees and research funding from Pfizer; membership on an entity’s Board of Directors or advisory committees from Janssen-Cilag Gmbh; and research funding from Deutsche Krebshilfe, BMBF, Deutsche Forschungsgemeinschaft, Wilhelm-Sander-Stiftung, Jose-Carreras-Siftung, Bayer AG, Daiichi Sankyo, and BiolineRx. SD received membership on an entity’s Board of Directors or advisory committees from Roche; membership on an entity’s Board of Directors or advisory committees and research funding from Janssen; membership on an entity’s Board of Directors or advisory committees from Celgene; and membership on an entity’s Board of Directors or advisory committees from KITE. UH received honoraria and travel support to meetings from and membership on the advisory Board from Janssen; travel support to meetings from and membership on the advisory Board from Prothena; honoraria from and membership on the advisory Board of Pfizer; honoraria from Alnylam and Akcea. SOS received honoraria, travel support to meetings and research funding from Janssen, Prothena, Jazz and Takeda.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Vijenthira A, Gong IY, Fox TA, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook G, John Ashcroft A, Pratt G, et al. Real-world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID-19 disease) in patients with multiple myeloma receiving systemic anti-cancer therapy. Br J Haematol. 2020;190:e83–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kastritis E, Wechalekar A, Schönland S, et al. Challenges in the management of patients with systemic light chain (AL) amyloidosis during the COVID-19 pandemic. Br J Haematol. 2020;190:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J, Gray G, Vandebosch A, et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384:2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpos E, Gavriatopoulou M, Ntanasis-Stathopoulos I, et al. The neutralizing antibody response post COVID-19 vaccination in patients with myeloma is highly dependent on the type of anti-myeloma treatment. Blood Cancer J. 2021;11:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aleman A, Upadhyaya B, Tuballes K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell. 2021;39:1442–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebers N, Speer C, Benning L, et al. Humoral and cellular responses after COVID-19 vaccination in anti-CD20-treated lymphoma patients. Blood. 2022;139:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henriquez S, Zerbit J, Bruel T, et al. Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against Alpha and Delta variants in Multiple Myeloma patients. Blood. 2021. Dec 14. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastritis E, Terpos E, Sklirou A, et al. Antibody response after initial vaccination for SARS-CoV-2 in patients with amyloidosis. HemaSphere. 2021;5:e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benning L, Töllner M, Hidmark A, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines (Basel). 2021;9:857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastritis E, Terpos E, Evangelakou Z, et al. Kinetics of anti-SARS-CoV-2 neutralizing antibodies development after BNT162b2 vaccination in patients with amyloidosis and the impact of therapy. Am J Hematol. 2022;97:E27–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shrotri M, Navaratnam AMD, Nguyen V, et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.