Background:
The perspective of the patient in measuring the outcome of their hand treatment is of key importance. We developed a hand-specific patient-reported outcome measure to provide a means to measure outcomes and experiences of care from the patient perspective, that is, HAND-Q.
Methods:
Data were collected from people with a broad range of hand conditions in hand clinics in six countries between April 2018 and January 2021. Rasch measurement theory analysis was used to perform item reduction and to examine reliability and validity of each HAND-Q scale.
Results:
A sample of 1277 patients was recruited. Participants ranged in age from 16 to 89 years, 54% were women, and a broad range of congenital and acquired hand conditions were represented. Rasch measurement theory analysis led to the refinement of 14 independently functioning scales that measure hand appearance, health-related quality of life, experience of care, and treatment outcome. Each scale evidenced reliability and validity. Examination of differential item functioning by age, gender, language, and type of hand condition (ie, nontraumatic versus traumatic) confirmed that a common scoring algorithm for each scale could be implemented.
Conclusions:
The HAND-Q was developed following robust psychometric methods to provide a comprehensive modular independently functioning set of scales. HAND-Q scales can be used to assess and compare evidence-based outcomes in patients with any type of hand condition.
Takeaways
Question: Will the HAND-Q prove to be reliable and valid in an international field-test study?
Findings: In total, 1277 people with a broad range of hand conditions from six countries completed the HAND-Q. A modern psychometric analysis provided evidence to support the use of 14 scales that measure hand appearance, health-related quality of life, experience of care, and treatment.
Meaning: This study provides researchers and clinicians with a new questionnaire measuring outcomes that matter to people with hand conditions.
INTRODUCTION
Strength, sensibility, and range of motion are commonly used as metrics for gauging the success of hand surgery or other treatments. Less common to routine clinical practice is the incorporation of the patient perspective, which is measurable using patient-reported outcome measures (PROMs). There are currently a range of hand-specific PROMs that can be divided into regional anatomical PROMs designed for the upper limb or hand, and PROMs designed for specific hand conditions. The development and psychometric properties of current available instruments for hand conditions are the subject of a growing number of systematic reviews.1–4 The findings show that few current PROMs fulfill international development and validation guidelines.1–4 For example, the Disabilities of the Arm, Shoulder and Hand (DASH)5 was developed without the input of patients with hand conditions, which is a necessity in the development of instruments measuring the perspective of the patient.4 Furthermore, most PROMs have been developed using psychometric methods that are now outdated, resulting in ordinal measurement that results in unequal distances between scores of the scale, like a ruler without consistency between measurement points. As a result, these PROMs are not ideal for measuring change over time in research studies or with individual patients, since the application of scores over the full breadth of the scale is not equal.4,6,7 PROMs developed using Rasch measurement theory (RMT) analysis,8 or item response theory,9 produce interval data with equal distances between scores on a scale. Such instruments are mathematically sound and provide a means to accurately measure change over time in research studies and in patient care.
Of the PROMs commonly used in hand research, the DASH,5 QuickDASH (qDASH),10 and Michigan Hand Outcomes Questionnaire11 stand out as having the most published psychometric properties. But even these PROMs have incomplete evidence to support their use in hand surgery research and clinical practice based on contemporary PROM standards, such as put forward by the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN).12,13
Hand conditions are extremely common and have a major impact on patients’ lives. Since patients are the best source of information on how they function and feel, it is vital to include their perspective in the assessment of outcomes. A comprehensive PROM for hand conditions developed using a modern psychometric approach is required. Such a PROM needs to address the varied impacts of hand conditions on an individual, with scales designed to measure unidimensional concepts in order to allow for ease of interpretation of scores.
To address the need for a comprehensive hand-specific PROM, our team recently developed the HAND-Q. We interviewed 62 people with a range of conditions, including carpal tunnel syndrome, Dupuytren disease, trigger finger, osteoarthritis, rheumatoid arthritis, and injuries of various kinds. Analysis of the qualitative data led to the development of a conceptual framework covering important outcomes and care experiences. Key concepts were included in scales that were refined through cognitive debriefing interviews with 20 patients, and a survey of 25 healthcare professions with expertise in treating hand conditions. Findings from phase 1 are published elsewhere.14 Following guidelines,15 the scales were translated and culturally adapted into French and Finnish for inclusion in a phase 2 multinational field-test study.
The aim of this paper was to describe the psychometric findings from the phase 2 field-test study that used a modern psychometric method (ie, RMT analysis8) to examine each HAND-Q scale, and to remove items and scales that were redundant or exhibited poor psychometric performance. We conducted additional tests of construct validity, including correlations between scores on HAND-Q scales, and examination of relationships between HAND-Q scores and clinical variables, that is, visibility and severity of the hand condition, and need for surgery.
METHODS
Research Ethics
Research ethics board approval was obtained for the phase 2 field-test study from seven sites in six countries, namely Canada, Australia, the United Kingdom, the United States, France and Finland (see figure, Supplemental Digital Content 1, which shows the name of the ethics board to approve the study in each collaborating site, http://links.lww.com/PRSGO/B894). The first board to grant approval was the Southern Adelaide Clinical Human Research Ethics Committee, Adelaide.
Sample and Recruitment
The phase 2 field-test study took place between April 2018 and January 2021. Participants were aged 16 years and older with a hand condition for which they attended an outpatient clinic. Recruitment took place in clinics using either paper-based forms or electronic data capture via a tablet device. All data were entered into a REDCap survey16 hosted at Flinders University, Adelaide, South Australia, Australia.
Survey
The REDCap survey asked demographic (age, gender, education, and occupation) and clinical questions, including the side of the hand condition, hand dominance, type of condition and its severity (mild, moderate, and severe), how long the participant has had the condition, and whether surgery was needed (yes, no, and not sure). Table 1 shows scale characteristics for the 20 HAND-Q scales that were field-tested, and the branching logic used in the REDCap survey to ensure that each scale was completed by participants for whom it was relevant.
Table 1.
HAND-Q Scales Tested in Field-test Study, including Branching Logic
| Domain | Scale | Response Options | Recall Period | Items | Branching Logic |
|---|---|---|---|---|---|
| Appearance | Appearance | Satisfaction | Now | 30 | None |
| HRQL | Acceptance | Agreement | None | 7 | Hand problem lasting ≥ 6 mo |
| Function | Difficulty | Past week | 35 | None | |
| Life impact | Severity | Past week | 11 | None | |
| Psychological | Frequency | Past week | 19 | None | |
| Sexual | Bothered | None | 9 | Hand problem affects sex life | |
| Sleep | Frequency | Past week | 8 | Interfered with ≥1 night in past week | |
| Social | Agreement | Past week | 13 | None | |
| Symptoms | Severity | Past week | 22 | None | |
| Work | Agreement | None | 11 | Worked in job in past 3 mo | |
| Experience | Anesthesia | Bothered | None | 14 | Had surgery ≤7 mo ago |
| Anesthesia symptoms (post) | Severity | None | 13 | Had surgery ≤7 mo ago | |
| Awake procedure | Satisfaction | None | 17 | Had surgery ≤7 mo ago and had local anesthesia | |
| Hand clinic | Agreement | Recent appointments | 13 | Saw ≥1 in past 3 mo and not first appointment | |
| Hand therapist | Agreement | Recent appointments | 19 | Saw ≥1 in past 3 mo and not first appointment | |
| Information | Satisfaction | None | 20 | Had surgery ≤7 mo ago | |
| Office staff | Agreement | Recent appointments | 14 | Saw ≥1 in past 3 mo and not first appointment | |
| Surgeon | Agreement | Recent visit | 25 | Saw ≥1 in past 3 mo and not first appointment | |
| Treatment | Outcome | Agreement | Recent treatment | 9 | Saw ≥1 in past 3 mo and not first appointment |
| Splint | Satisfaction | Recent splint | 12 | Saw ≥1 and used splint/brace in past 3 mo |
Analysis
Data for each language version were merged in SPSS Version 26.0 (IBM Corporation, Armonk, N.Y., for Windows/Apple Mac). RMT analysis was performed using RUMM2030 software (RUMM version 2030, RUMM Laboratory Pty Ltd, Duncraig, Western Australia, 1998–2021) and the unrestricted Rasch model for polytomous data. Scales that comply to this model have a series of items that measure an amount of a single concept.8,17 A range of statistical and graphical tests were used to determine how well the data fit the Rasch model; specifically, the following tests were performed:
Thresholds for Item Response Options
Item response options were investigated to check whether item thresholds were ordered appropriately.18
Item Fit Statistics
Various fit indicators (item characteristic curve), log residuals (item–person interaction), and chi-square (item–trait interaction) were considered in conjunction with each other. Fit residual indicates if the item under or overdiscriminates compared with other items, and provide information about redundancy. Ideal fit residuals are between −2.5 and +2.5, with a nonsignificant chi-squared value after Bonferroni adjustment.18 The sample was adjusted to 500 for tests of item fit.17
Targeting
Ideally, items of a scale should reflect all levels of the measured concept experienced by the sample.17 Both graphical (person-item threshold distribution) and statistical (proportion of sample not captured by the range of the scale) tests were examined to establish if the items were adequately spread for each scale.
Differential Item Function
Sample characteristics were examined for differential item function (DIF), including age group (16–39, 40–59, and ≥60 years), gender (men versus women), type of hand condition [nontraumatic (ie, elective) versus traumatic] and language (English versus other). DIF testing examines whether there is possible response bias between subgroups. Scales with a minimum data set of 150 responses were examined for DIF. A random sample of equal sized groups was used for the analysis, which was repeated three times. If DIF was found for any item in any of the three random sample analyses, the items with DIF were split on the sample characteristic. A Pearson correlation was then performed on the original and the new person locations to examine any impact of DIF on the scoring of the scale.18
Reliability
Person separation index (PSI) and Cronbach alpha values were used to examine reliability of each scale.20 The values range between 0 and 1, with a higher value indicating higher reliability. Coefficients of 0.70 or greater were considered adequate.21
Local Independence
Items were examined for residual correlations over 0.30, which were taken to indicate that answers for an item may depend on answers to another item.22–24 Subtesting was undertaken to determine any change in the PSI for scales with residual correlations above 0.30.19
Unidimensionality
The final set of items for each scale underwent principal component analysis in SPSS, version 26.0. It was hypothesized that items of each scale would relate to a single factor with loadings greater than 0.70.25
Construct Validity
Rasch logits were used to transform participant scores for each scale from 0 (worst) to 100 (best). Normality was assessed using Kurtosis and Skewness, and nonparametric statistics were used if distributions were nonnormal (ie, outside of −2 to 2).25 The scores were used to test four hypotheses to examine construct validity as follows:
HAND-Q scale scores within domains [eg, health-related quality of life (HRQL)] would correlate more strongly with each other than with scale scores from other domains.
HAND-Q scores for the scales measuring hand appearance would be lower for participants with a visible (ie, Dupuytren contracture, rheumatoid arthritis, and oesteoarthritis) versus an invisible (ie, carpal tunnel syndrome) hand condition.
HAND-Q scores for scales in the HRQL and treatment outcome domains would be incrementally lower for increasing severity (ie, mild, moderate, and severe) of the hand condition.
HAND-Q scores would be lower on the HRQL and treatment outcome scales for participants who needed versus did not need further surgery.
RESULTS
Demographics
Characteristics of the 1277 participants are shown in Table 2. Recruitment was highest in Australia and the United States, with smaller contributions from the United Kingdom, Canada, Finland, and France. The mean age of the sample was 50 (SD = 17) and ranged from 16 to 89 years. Most data were collected using the English version of the HAND-Q with other contributions in Finnish and French. The most common hand conditions were carpal tunnel syndrome, soft-tissue injury, and fractures. Nontraumatic conditions were more common than traumatic conditions. Most participants (N = 812, 63.6%) had a condition that affected their dominant hand. A total of 776 (61%) participants worked at a job in the past 3 months, and most of those who worked (N = 548, 71%) did so full time.
Table 2.
Characteristics of the 1277 Field-test Study Participants
| N | % | ||
|---|---|---|---|
| Country | Australia | 446 | 35 |
| Canada | 85 | 7 | |
| Finland | 184 | 14 | |
| France | 82 | 6 | |
| UK | 98 | 8 | |
| USA | 382 | 30 | |
| Language | English | 1011 | 79 |
| Other | 266 | 21 | |
| Gender | Male | 574 | 45 |
| Female | 680 | 53 | |
| Other | 6 | 1 | |
| Missing | 17 | 1 | |
| Age, y | 16–39 | 360 | 28 |
| 40–59 | 507 | 40 | |
| 60 + | 392 | 31 | |
| Missing | 18 | 1 | |
| Education | Primary school | 65 | 5 |
| High school | 564 | 44 | |
| Further education (college, university, or similar) | 572 | 45 | |
| Other | 54 | 4 | |
| Missing | 22 | 2 | |
| Hand condition | Carpal tunnel syndrome | 183 | 14 |
| Dupuytren’s disease | 36 | 3 | |
| Trigger finger | 70 | 5 | |
| Osteoarth | 62 | 5 | |
| Rheumatoid arthritis | 13 | 1 | |
| Other nerve compression | 23 | 2 | |
| Ganglion | 18 | 1 | |
| Trauma (injury and fracture) | 519 | 38 | |
| Multiple nontraumatic conditions | 117 | 9 | |
| Mixed nontraumatic and traumatic conditions | 65 | 5 | |
| Other | 171 | 13 | |
| Type of condition | Nontraumatic | 687 | 54 |
| Traumatic | 525 | 41 | |
| Both | 65 | 5 | |
| Need further surgery | Yes | 95 | 7 |
| No | 1170 | 92 | |
| Missing | 12 | 1 | |
| Severity of condition | Mild | 202 | 16 |
| Moderate | 521 | 41 | |
| Severe | 521 | 41 | |
| Missing | 33 | 3 | |
RMT Analysis
In the RMT analysis, data collected for seven scales (ie, acceptance, sleep, social, work, anesthesia, anesthesia symptoms, and awake procedure) were found to be incompatible with RMT analysis and were dropped. These scales had multiple items with disordered thresholds. After we rescored each scale’s items to reduce by one threshold, and dropped items with poor fit, scale reliability was low (PSI values < 0.70).
RMT analysis provided evidence of reliability and validity for the remaining 14 scales, that is, seven HRQL scales, five experience of care scales, and two treatment outcome scales. The HRQL scales include two for hand appearance, that is, one measures hand appearance in general, and the other measures age-related hand appearance. The total number of items in the 14 scales was reduced from 238 to 133. The clinic scale had four items with disordered thresholds. After reducing response options by rescoring across the “definitely disagree” and “somewhat disagree” categories, item thresholds were ordered. The RMT analysis used the rescored data.
Figure, Supplemental Digital Content 2, shows the item fit and DIF statistics (http://links.lww.com/PRSGO/B895). All 133 items had nonsignificant X2 P values after Bonferroni adjustment. Item fit was within ±2.5 for 102 items. A total of 77 items from eight scales were tested for DIF. Of these, 40 items demonstrated DIF, including 12 when tested by gender, 15 when tested by age-group, 19 when tested by type of hand condition, and 21 when tested by language. Pearson correlations between person locations before and after splitting the items for DIF showed minimal impact on scoring (all correlations > 0.99).
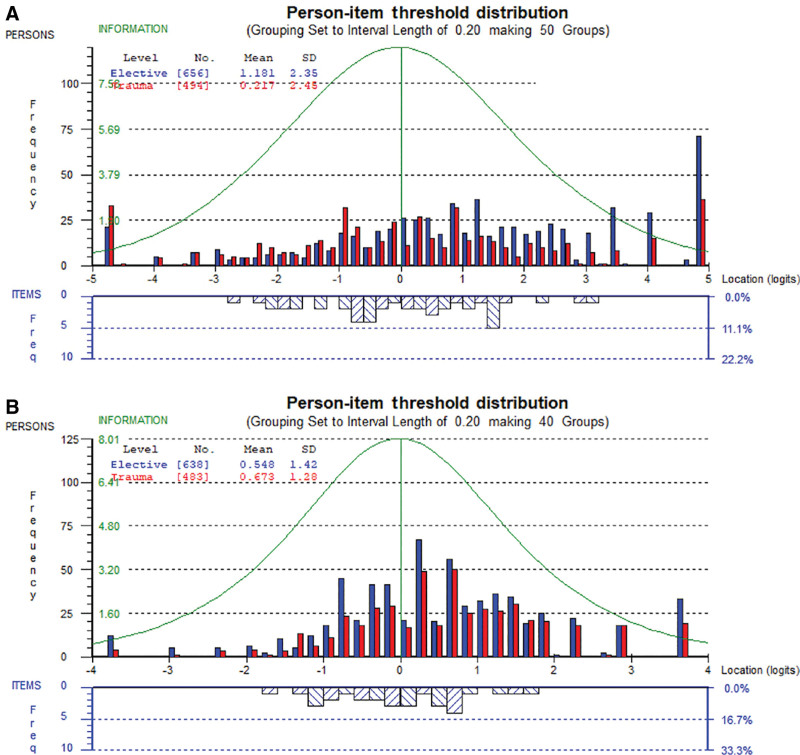
Scale-level findings are summarized in Table 3. Data fit the Rasch model for 11 scales, which had nonsignificant P values, with marginal misfit for the sexual, clinic, and splint scales. The proportion of participants to score within the range provided by each of the HRQL scales ranged from 94% (symptoms) to 76% (sexual). Targeting is further illustrated in Figure 1, which shows the findings for the two physical scales (ie, function and symptoms) as examples.
Table 3.
Rasch Measurement Theory and Scale-level Statistics
| Domain | Scale | N Final Items | N Completed Scale | N in RMT | % Scored on Scale | χ2 | DF | P | PSI +ext | PSI –ext |
CA +ext | CA -ext | % Floor | % Ceiling | % Missing Data |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | Appearance | 10 | 1190 | 923 | 78 | 105.54 | 90 | 0.13 | 0.86 | 0.87 | 0.95 | 0.91 | 0.8 | 19.3 | 12.4 |
| Appearance: age-related | 10 | 1188 | 952 | 80 | 88.50 | 90 | 0.52 | 0.86 | 0.87 | 0.94 | 0.91 | 0.8 | 16.7 | 11.4 | |
| HRQL | Function | 15 | 1213 | 1031 | 85 | 161.34 | 135 | 0.06 | 0.92 | 0.93 | 0.97 | 0.96 | 4.3 | 9.2 | 12.9 |
| Life impact | 8 | 1174 | 1031 | 88 | 84.14 | 72 | 0.15 | 0.83 | 0.81 | 0.91 | 0.87 | 2.1 | 9.6 | 5.6 | |
| Psychological | 10 | 1192 | 1031 | 87 | 92.79 | 90 | 0.40 | 0.82 | 0.83 | 0.92 | 0.90 | 0.4 | 12.8 | 4.5 | |
| Sexual | 7 | 445 | 313 | 76 | 42.69 | 28 | 0.04 | 0.87 | 0.86 | 0.95 | 0.90 | 4.7 | 22.9 | 6.5 | |
| Symptoms | 10 | 1183 | 1111 | 94 | 55.79 | 80 | 0.98 | 0.84 | 0.83 | 0.90 | 0.87 | 1.4 | 4.3 | 8.9 | |
| Experience | Clinic | 10 | 500 | 258 | 52 | 50.57 | 30 | 0.01 | 0.67 | 0.77 | 0.93 | 0.84 | 0.4 | 44.4 | 10.0 |
| Doctor | 10 | 634 | 181 | 29 | 31.44 | 20 | 0.05 | 0.51 | 0.81 | 0.96 | 0.91 | 0.6 | 67.0 | 5.7 | |
| Hand therapist | 10 | 249 | 65 | 26 | 23.38 | 20 | 0.27 | 0.71 | 0.90 | 0.98 | 0.94 | 0.8 | 70.3 | 6.4 | |
| Information | 10 | 430 | 214 | 50 | 28.9 | 20 | 0.09 | 0.75 | 0.87 | 0.96 | 0.93 | 0.7 | 45.1 | 7.2 | |
| Office staff | 8 | 329 | 74 | 23 | 10.13 | 16 | 0.86 | 0.49 | 0.84 | 0.96 | 0.90 | 0.3 | 74.2 | 4.0 | |
| Treatment | Outcome | 7 | 582 | 323 | 56 | 32.94 | 28 | 0.24 | 0.80 | 0.84 | 0.95 | 0.90 | 1.4 | 39.3 | 10.5 |
| Splint | 8 | 432 | 366 | 85 | 52.41 | 32 | 0.01 | 0.82 | 0.81 | 0.90 | 0.85 | 0.5 | 13.2 | 12.5 |
χ2, Chi-square; CA, Cronbach alpha; DF, degrees of freedom; ext, extremes; N, number; PSI, Person Separation Index.
Fig. 1.
A, HAND-Q Function scale by type of hand condition. B, HAND-Q Symptom scale by type of hand condition.
The figures show the distribution of person measurement (top histograms) and item locations (lower histograms), with higher (better) scores to the right. The person measurements are shown separately by type of hand condition (nontraumatic versus traumatic). The findings provide evidence that each scale’s items mapped out a construct that was experienced by most participants in the sample (ie, they scored on the scale). Furthermore, each scale worked well to measure hand function and symptoms for people with nontraumatic and traumatic hand conditions. Table 3 shows other targeting statistics (floor and ceiling effects) as well as missing data, that is, the proportion of eligible participants who left one or more items blank in a scale.
PSI values for the HRQL and treatment outcome scales were 0.80 or greater (see Table 3). PSI values without extremes (≥0.77) for the experience of care scales were acceptable and higher than with extremes (≥0.49) due to the high ceiling effects for these scales. Cronbach alpha values for the 14 scales were 0.90 or greater (with extremes) to 0.84 or greater (without extremes). The item residual correlations were greater than 0.30 for 10 pairs of items in seven scales. Subtests provided evidence that the correlated items had marginal impact on scale reliability (ie, <0.05 drop in PSI values). The principal component analysis results for each scale provided broad support for single factors; factor loadings ranged from 0.64 to 0.95, with only six items in three scales (splint, symptoms, and psychological) below 0.70.
Construct validity was examined with the predetermined hypotheses. Table 4 shows the Spearman correlations between HAND-Q scales relating to hypothesis 1. All scales except for outcome correlated more strongly with scales within their domain (eg, HRQL), as hypothesized. The strongest correlations were found in scales that have commonality in the underlying construct, such as function and symptoms, or life impact and psychological.
Table 4.
Spearman Correlations between HAND-Q Scales—Hypothesis 1
| Appearance | Appearance Age-related | Function | Life Impact | Psychological | Sexual | Symptoms | Clinic | Doctor | Hand Therapist | Information | Office Staff | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance age-related | 0.88* | ||||||||||||
| Function | 0.30* | 0.28* | |||||||||||
| Life impact | 0.33* | 0.34* | 0.62* | ||||||||||
| Psychological | 0.42* | 0.42* | 0.50* | 0.73* | |||||||||
| Sexual | 0.26* | 0.23* | 0.49* | 0.68* | 0.55* | ||||||||
| Symptoms | 0.41* | 0.40* | 0.61* | 0.65* | 0.63* | 0.49* | |||||||
| Clinic | 0.23* | 0.20* | 0.08 | 0.15* | 0.21* | 0.17† | 0.12† | ||||||
| Doctor | 0.19* | 0.17* | 0.04 | 0.16* | 0.24* | 0.18* | 0.16* | 0.58* | |||||
| Hand therapist | 0.23* | 0.26* | 0.06 | 0.16† | 0.22* | 0.19† | 0.18* | 0.45* | 0.54* | ||||
| Information | 0.33* | 0.33* | 0.16* | 0.33* | 0.39* | 0.36* | 0.30* | 0.62* | 0.65* | 0.51* | |||
| Office staff | 0.22* | 0.17* | 0.13† | 0.20* | 0.25* | 0.29* | 0.19* | 0.59* | 0.55* | 0.46* | 0.55* | ||
| Outcome | 0.34* | 0.32* | 0.13* | 0.22* | 0.32* | 0.23* | 0.28* | 0.48* | 0.49* | 0.45* | 0.54* | 0.33* | |
| Splint | 0.29* | 0.34* | 0.17* | 0.35* | 0.37* | 0.33* | 0.30* | 0.37* | 0.31* | 0.27* | 0.44* | 0.25* | 0.46* |
*Correlation is significant at the 0.01 level (two-tailed).
†Correlation is significant at the 0.05 level (two-tailed).
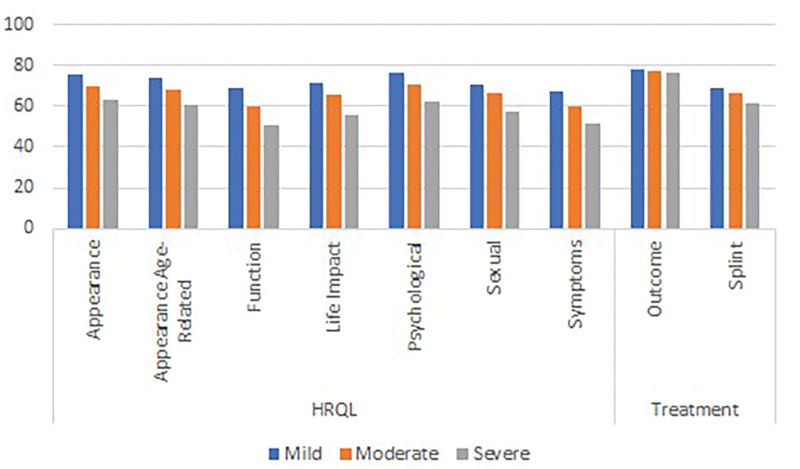
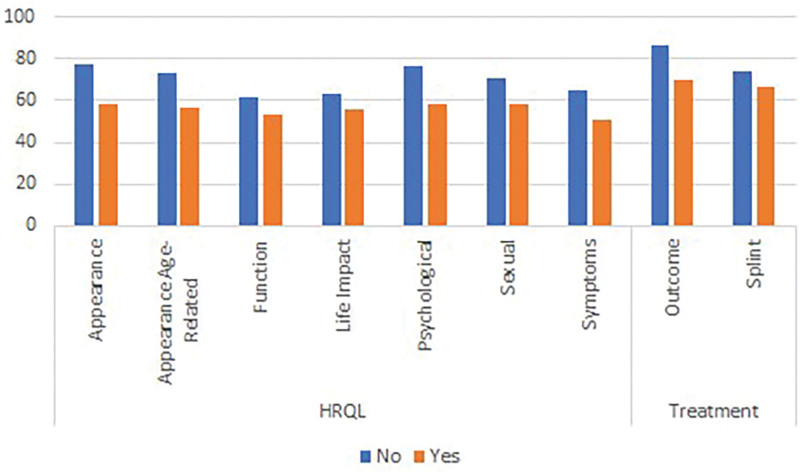
In regard to hypothesis 2, analysis confirmed that the mean scores were significantly lower (P < 0.001 on independent samples t-tests) on the appearance scale (62, SD = 24 versus 77, SD = 22) and age-related appearance scale (59, SD = 22 versus 72, SD = 22) for participants with conditions that can affect the appearance of the hands compared with participants with carpal tunnel. The results for hypothesis 3 are graphically demonstrated in Figure 2, which shows that the mean scores by severity of the hand condition were incrementally lower by increasing severity on the outcome and all HRQL scales (P ≤ 0.002 on ANOVA), as well as the splint scale (P = 0.018). Finally, for hypothesis 4, the mean scores on the HRQL and the outcome scales for the 95 participants in the sample who reported that they need more surgery were lower compared with the 249 who did not need more surgery (P < 0.022 on independent samples t test). Contrary to our hypothesis, no difference was found on the splint scale score by need for surgery (see Fig. 3). The characteristics of the subgroups for tests of construct validity can be found in figure, Supplemental Digital Content 3, http://links.lww.com/PRSGO/B896.
Fig. 2.
Mean score for HAND-Q HRQL and splint scales by self-reported condition severity.
Fig. 3.
Mean scores for HAND-Q HRQL and splint scales by need for more surgery.
DISCUSSION
The strength of the HAND-Q stems from its robust content validity, which results from the input from a broad cross-section of participants from six countries who have experience of a hand condition and its treatment, as well as expert input from professionals who treat hand conditions.14 Participants described in detail their experiences of living with a hand condition, the impact their hand condition had on their day-to-day life, and their interactions with the healthcare environment. Based on this rich qualitative data set, we created 20 field-test scales that covered outcomes of interest and the experiences of care. A further strength of our study is the large-scale field test that incorporated multiple languages and enabled vigorous psychometric analysis of the preliminary scales. By using the RUMM2030 software to perform the RMT analysis, we were able to identify that a number of the preliminary scales did not meet stringent expectations for psychometric function. These scales were not included in the final version of the HAND-Q. The remaining 14 scales were found to have strong reliability and validity indicators. These scales measure a broad range of concepts that patients identified as important. The fact that the DIF that was identified did not impact scoring supports international application of the HAND-Q scales for research, clinical practice, and quality improvement applications.
The HAND-Q is a novel hand-specific PROM with a modular structure that provides a comprehensive set of scales, each of which is independently functioning. This attribute allows for tailoring of the scales used to a specific application, thereby minimizing respondent burden by only measuring the concepts of interest. It was observed that the HRQL and treatment outcome scales demonstrated good targeting and distribution of scores, whereas the patient experience scales had larger ceiling effects. Generally, patients report being satisfied with the care that is provided to them and therefore it can be difficult to develop scales that accurately measure patient satisfaction with healthcare services. These experience scales were designed for use in a clinical setting rather than research applications. As such, the ceiling effects are thought to be acceptable; the scales can be used to identify those patients who are not satisfied with the services provided, and to assist with pinpointing the issues to allow for these to be addressed and improved upon.
This study has some limitations. There are numerous conditions that can affect the hands, including congenital, degenerative, and traumatic etiologies. Although our sample has included a wide variety of pathology, it was not possible to evaluate the experience of patients with all types of hand conditions. Rare conditions, such as congenital hand differences and hand transplant, were not included in the sample. Further research into the use of the HAND-Q scales in these conditions would be necessary to support the content validity. Although we exceeded our projected sample of 1000, and were able to include patients from six countries, an important limitation of our sample is that all participants were from high resource countries. The HAND-Q was translated into Urdu, Tamil, Bengali, and Hindi in preparation to include patients from India and Pakistan, but recruitment in these countries was impacted by the COVID-19 pandemic, and we were not able to include field-test results from these countries. We were, however, able to use feedback about items that were difficult to translate into consideration during the item reduction processes. Future studies to examine the psychometric performance of the HAND-Q in other countries are warranted. Finally, some psychometric properties (responsiveness and test–retest reliability) were not examined in this study and should be examined in future research.
CONCLUSIONS
The HAND-Q was designed for patients with all types of hand conditions, including those presenting for elective surgery and or those associated with trauma. This new modular PROM measures hand function, symptoms, life impact, psychological impact, sexual impact, satisfaction with outcome, and experience of care. This work compliments the existing Q-Portfolio of condition-specific PROMs for surgery patients that includes the BREAST-Q, FACE-Q, BODY-Q, CLEFT-Q, SCAR-Q and WOUND-Q (see www.qportfolio.org). The HAND-Q has been designed from a strong foundation that included extensive involvement of patients and experts in the field, a large multinational field-test and the use of RMT analysis to produce unidimensional scales with interval-level measurement properties. The HAND-Q is a valuable new measurement tool to incorporate the patient perspective into evidence-based clinical care, quality assurance and regulatory decisions in the care of patients with hand conditions.
ACKNOWLEDGMENTS
Development of the HAND-Q has involved hundreds of people with hand conditions and the collaboration of numerous healthcare professionals and researchers around the world. We are truly grateful for their dedication and help with our research.
Supplementary Material
Footnotes
Published online 31 January 2022.
The HAND-Q study has been funded by research grants from the Australasian Foundation for Plastic Surgery—Foundation Plastic Surgery and Reconstructive Surgical (PRS) Research Grant, and the Royal Australasian College Surgeons Small Project Grant. No other sources of funds supported this work. No products, devices or drugs were used in this study.
Disclosure: Drs. Sierakowski, Dean, Pusic, and Klassen are co-developers of the HAND-Q and will receive a share of any license revenue on the inventor sharing policies of the institutions where the HAND-Q was developed. Dr Klassen is owner of EVENTUM Research which provides consulting services to the pharmaceutical industry. The other authors have no financial interest to declare.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Wormald JCR, Geoghegan L, Sierakowski K, et al. Site-specific patient-reported outcome measures for hand conditions: systematic review of development and psychometric properties. Plast Reconstr Surg Glob Open. 2019;7:e2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dacombe PJ, Amirfeyz R, Davis T. Patient-reported outcome measures for hand and wrist trauma: is there sufficient evidence of reliability, validity, and responsiveness? Hand (N Y). 2016;11:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Hughes H, Geoghegan L, Rodrigues J, et al. Systematic review of the use of patient reported outcome measures in studies of electively-managed hand conditions. J Hand Surg Asian Pac Vol. 2019;24:329–341. [DOI] [PubMed] [Google Scholar]
- 4.Sierakowski K, Evans Sanchez KA, Damarell RA, et al. Measuring quality of life and patient satisfaction in hand conditions: a systematic review of currently available patient reported outcome instruments. Australas J Plast Surg. 2018;1:58–99. [Google Scholar]
- 5.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med. 1996;29:602–608. [DOI] [PubMed] [Google Scholar]
- 6.Franchignoni F, Giordano A, Sartorio F, et al. Suggestions for refinement of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH): a factor analysis and Rasch validation study. Arch Phys Med Rehabil. 2010;91:1370–1377. [DOI] [PubMed] [Google Scholar]
- 7.Cano SJ, Klassen A, Pusic AL. The science behind quality-of-life measurement: a primer for plastic surgeons. Plast Reconstr Surg. 2009;123:98e–106e. [DOI] [PubMed] [Google Scholar]
- 8.Rasch G. Probabilistic Models for Some Intelligence and Attainment Tests. Chicago: University of Chicago Press, 1980. [Google Scholar]
- 9.Chang CH, Reeve BB. Item response theory and its applications to patient-reported outcomes measurement. Eval Health Prof. 2005;28:264–282. [DOI] [PubMed] [Google Scholar]
- 10.Beaton DE, Wright JG, Katz JN; Upper Extremity Collaborative Group. Development of the QuickDASH: comparison of three item-reduction approaches. J Bone Joint Surg Am. 2005;87:1038–1046. [DOI] [PubMed] [Google Scholar]
- 11.Chung KC, Pillsbury MS, Walters MR, et al. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am. 1998;23:575–587. [DOI] [PubMed] [Google Scholar]
- 12.Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: a clarification of its content. BMC Med Res Methodol. 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prinsen CAC, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sierakowski KL, Kaur MN, Sanchez KE, et al. A qualitative study informing the development and content validity of the HAND-Q: a modular patient-reported outcome measure for hand conditions. BMJ Open (accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild D, Grove A, Martin M, et al. ; ISPOR Task Force for Translation and Cultural Adaptation. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health. 2005;8:94–104. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hobart J, Cano S. Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods. Health Technol Assess. 2009;13:iii, ix,–iiix, 1. [DOI] [PubMed] [Google Scholar]
- 18.Andrich D. Rasch Models for Measurement. Beverley Hills, CA: Sage Publications; 1988. [Google Scholar]
- 19.Wright BD, Masters GN. Rating Scale Analysis. Chicago: MESA Press; 1982. [Google Scholar]
- 20.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. [Google Scholar]
- 21.Nunnally JC,, Bernstein IH.Psychometric theory. New York: McGraw-Hill; 1994. [Google Scholar]
- 22.Christensen KB, Makransky G, Horton M. Critical values for Yen’s Q3: identification of local dependence in the rasch model using residual correlations. Appl Psychol Meas. 2017;41:178–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrich D. An elaboration of Guttman scaling with Rasch models for measurement. Sociol Methodol. 1985;15:33–80. [Google Scholar]
- 24.Gaskin CJ, Happell B. On exploratory factor analysis: a review of recent evidence, an assessment of current practice, and recommendations for future use. Int J Nurs Stud. 2014;51:511–521. [DOI] [PubMed] [Google Scholar]
- 25.Kim HY. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38:52–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.