Abstract
Mycobacterium kansasii is a nontuberculous mycobacterium that causes pulmonary symptoms, commonly associated with underlying conditions, including malignancy, prior transplant, and HIV. However, rarely does Mycobacterium kansasii present with pleural effusion. We present a case of a 56-year-old female who presented with dyspnea and chest pain, and sputum culture was positive for acid-fast bacilli. A CT scan revealed a left-sided pleural effusion. Based on a thorough review of the literature using Embase and PubMed, we found that only 22 cases of a Mycobacterium kansasii pleural effusion have been reported. We provide a discussion on maintaining a broad differential in the treatment of immunocompromised individuals with Mycobacterium infection.
Keywords: rare cause of pleural effusion, non-tuberculous pleural effusion, non-tuberculous mycobacterium, mycobacterium kansasii pleural effusion, mycobacterium kansasii
Introduction
Mycobacterium kansasii is a nontuberculous mycobacterium (NTM) that was first described in 1953 by Buhler and Pollack, who identified this bacterium as a human pathogen in patients with a pulmonary disease resembling tuberculosis [1]. This bacterium is a slow-growing, atypical acid-fast bacillus that is ubiquitously found in the environment, namely tap water [1,2]. Therefore, this pathogen was commonly considered contamination or colonization. We now know that this pathogen can cause a wide range of diseases including lung disease, lymphadenopathy, skin, and soft tissue infection, osteomyelitis, and disseminated disease [2]. These presentations occur more commonly in immunocompromised patients.
M. kansasii is the second most common cause of lung disease secondary to an NTM following Mycobacterium avium complex (MAC) in the United States [3,4]. Bronchopulmonary disease is the most frequent presentation of infection with M. kansasii and commonly occurs in patients with underlying lung disease [3,5]. Presenting symptoms may include dyspnea, cough, hemoptysis, weight loss, fever, and night sweats [5]. The course of the disease can be indolent, lasting several months to years; however, it may occasionally be rapidly progressive [6,7]. Infection due to M. kansasii can be clinically indistinguishable from Mycobacterium tuberculosis. Infection is usually diagnosed via bronchoscopy, tissue biopsy, thoracentesis, or pericardiocentesis using culture findings, biochemical testing, or high-performance liquid chromatography. While pleural effusions can be seen with pulmonary disease due to mycobacterium, around 5% of cases are due to M. tuberculosis, they are very rarely seen with M. kansasii [6].
We present a case of M. kansasii pleural effusion and review an additional 22 cases in the English-language literature regarding risk factors for disease, clinical presentation, treatment, and outcome for this unusual infection. This case was previously presented as a meeting abstract at the 2021 Ohio/Air Force Scientific Meeting on October 28, 2021.
Case presentation
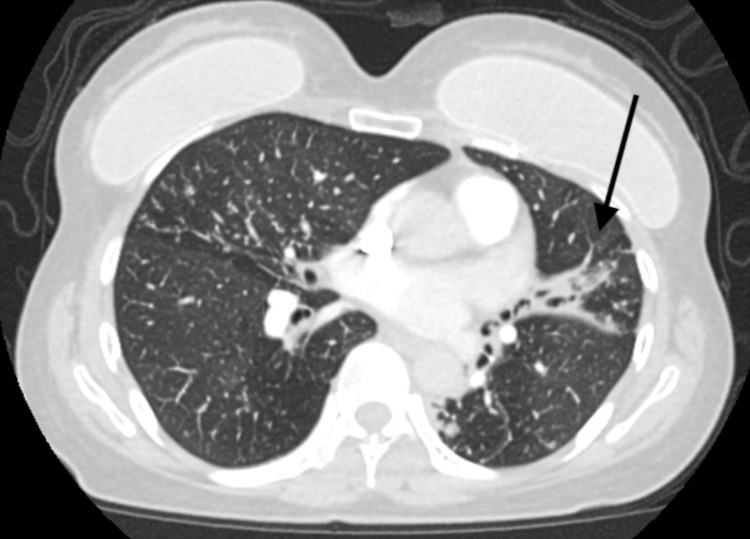
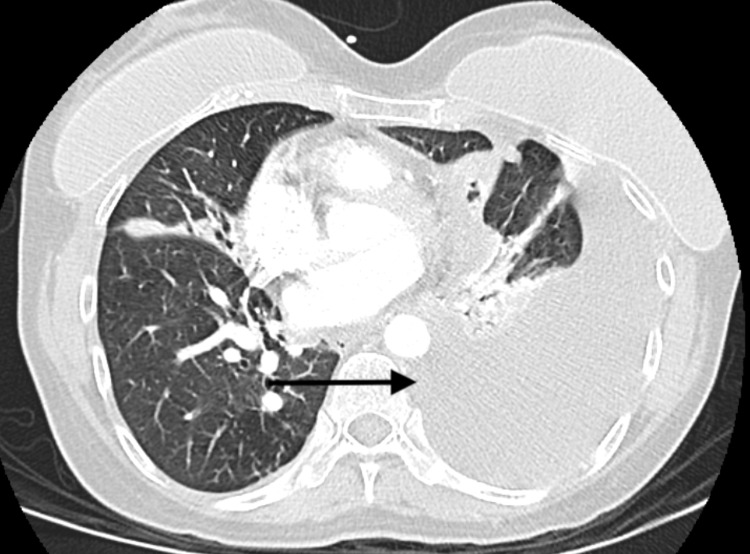
A 56-year-old female with no significant past medical history presented to the emergency department with shortness of breath and pain in her left chest. Approximately three months prior to her presentation, she was seen in the outpatient setting due to hemoptysis. At that time, she had no associated symptoms and denied fever, chills, night sweats, cough, and nausea but a CT scan of her chest (Figure 1) revealed multifocal nodular opacities in a tree-in-bud distribution, suggestive of atypical mycobacterial or fungal infection. An expectorated sputum sample and follow-up bronchoscopy were positive for acid-fast bacilli and subsequently grew M. kansasii. Prior to the start of azithromycin, ethambutol, and rifampin, she presented to the emergency department with shortness of breath and chest pain. A follow-up CT demonstrated a new loculated left pleural effusion at the left lung base with tracking along the left lateral pleural surface (Figure 2). A left-sided chest tube was placed and 1.5 liters of serosanguinous fluid was drained in the first 24 hours. Analysis of the fluid revealed an exudate with LDH of 128U/L, total protein of 5.9g/dL and white blood cell count of 2938 cells/uL with lymphocytic predominance. The fluid was AFB positive on acid-fast smear, however, cultures remained negative. She was ultimately discharged on ethambutol, azithromycin, and rifampin and her clinical condition continues to improve at this time.
Figure 1. Multifocal nodular opacities in a tree-in-bud distribution.
Figure 2. Left pleural effusion at the lung base with tracking along the lateral pleural surface .
Discussion
Methods
Case reports of Mycobacterium kansasii empyema were identified through a computer-generated search using Embase and PubMed with subsequent review of noted references. Search terms included “mycobacterium kansasii” and “pleural effusion”. Only cases from the English language literature were included. A case was defined by documented M. kansasii infection with the recovery of this organism from a culture of either sputum, bronchoscopy, gastric aspirate, or other normally sterile fluid such as blood or cerebrospinal fluid in the presence of empyema without other etiology between 1980 and 2020. Cases of empyema were defined using the standard definition of collection of infected exudate within the pleural cavity. Cases were included in the analysis if enough demographic information was available to allow the identification of individual patients. Demographic information included age and gender.
Results
Demographics
A total of 22 cases of M. kansasii pleural effusion were reported between 1980 and 2020 [2-4,6-21]. These included 13 men (59.0 %) and nine women (40.9%). Ages ranged from 24 to 87 years with a mean age of 52.9 ± 19.5.
Underlying Conditions
Over two-thirds of the patients reviewed were noted to have underlying comorbidities with the majority of these causing a weakened immune system (Table 1). The most common conditions were malignancies and receipt of an organ transplant. Nine patients (39.2%) were taking immunosuppressive medications [4,6,9,12-13,16,18-21].
Table 1. Underlying conditions of 23 patients with Mycobacterium kansasii pleural effusion.
*Some patients had more than one underlying condition † Rheumatoid arthritis (2), pneumothorax (1), prior severe viral infection (1), coronary artery disease (1), COPD(1)
| Condition or risk-associated status | No. (%) of patients* |
| HIV | 2 (8.7) |
| Diabetes mellitus | 2 (8.7) |
| Hypertension | 2 (8.7) |
| Transplant recipient | 3 (13.0) |
| Hematologic malignancy | 5 (21.7) |
| Solid organ malignancy | 1 (4.3) |
| Other † | 7 (30.4) |
| None | 6 (26.1) |
Pleural Effusion
The laterality of effusion was identified in 14 cases (60.9%). Seven (50%) of these patients had a right-sided effusion [4,7,9-11], three (21.4%), including our case, had a left-sided effusion [13] and four (28.9%) had bilateral effusions [8,13-14,21]. Two left pleural effusions were reported as large [4,8] and two right pleural effusions were reported as small [7,11]. Otherwise, size was not reported. The pleural effusions were found to be exudative in five (21.7%) cases [6-8,14]; the other 18 cases did not report pleural fluid studies to be able to determine this information. Of the cases, 15 were isolated pleural effusions, while eight showed pulmonary signs of pneumothorax [7,8], cavitation [4,12], abscess [4], infiltrate [10,13,14], and lung nodules [15].
Diagnosis of pleural effusion involved computed tomography (CT) scan in 12 (52.2%) cases [7-11,13-15,18,20-21], and chest radiograph in nine (39.1%) [5,7-9,11,12,14,18,20]. Seven cases had both imaging studies performed [7-9,11,14,18,20].
Cultures of the pleural fluid were reported in all cases. All cases were noted to have cultures positive for M. kansasii except for one where cultures were negative. In this case, the patient was noted to have a positive culture previously [14].
Symptoms and Signs
Fifteen (65.2%) of the cases described reported signs and symptoms of the patient upon presentation and throughout the hospital stay (Table 2). The most commonly reported symptoms included fever, pulmonary symptoms including chest pain, cough, and dyspnea as well as constitutional symptoms. Cases with suspicion of disseminated disease presented atypically. The primary complaints of these patients included gastrointestinal and cardiac symptoms [4,12,15].
Table 2. Signs and symptoms of 15 patients with Mycobacterium kansasii pleural effusion.
| Symptom | Number of Patients (%) |
| Fever | 11 (73.3) |
| Chills | 2 (13.3) |
| Constitutional symptoms (weight loss, headache, fatigue, night sweats, and malaise) | 7 (46.7) |
| Pulmonary symptoms | 13 (86.7) |
| Chest pain | 6 (40.0) |
| Dry cough | 5 (33.3) |
| Productive cough | 1 (6.7) |
| Hemoptysis | 1 (6.7) |
| Dyspnea | 7 (46.7) |
| Gastrointestinal symptoms | 1 (6.7) |
| Cardiac symptoms (cardiopulmonary arrest, QT prolongation, asystole) | 2 (13.3) |
Treatment
Definitive therapy was reported for 19 of the 23 cases (82.6%). Most patients initially received some variation of RIPE therapy consisting of rifampin (RFP) and isoniazid (INH) with either pyrazinamide (PZA) or ethambutol (EB) or, in some cases, both PZA and EB (Table 3). In all the cases that mentioned therapy, the patients were found to have received combination therapy. The most common combination included RFP, INH, and EB only. Of the cases reporting the use of RIPE, only one mentioned additional therapy with prednisone; it is noted that this patient did survive [14]. One case reported the use of open-window thoracostomy in management [9]. The duration of treatment was reported for only six patients: two for ≤6 months, two between 6-12 months, and two for >12 months. Susceptibility of the bacterial isolates was reported in three cases [10,14-15].
Table 3. Medication regiment and outcome of 19 patients with Mycobacterium kansasii pleural effusion.
*Reported based on final treatment (before discharge/death)
RFP: rifampin; INH: isoniazid; PZA: pyrazinamide; EB: ethambutol
| Medication Regiment* | Number of Patients (%) | Outcomes (%) |
| INH, RFP, EB | 11 (57.9) | Survived: 8 (72.7) |
| Died: 2 (18.2) | ||
| Unknown: 1 (9.1) | ||
| INH, RFP, EB, PZA (RIPE therapy) | 3 (15.8) | Survived: 3 (100) |
| Died: 0 | ||
| Unknown: 0 | ||
| EB, RFP + another class | 3 (15.8) | Survived: 3 (100) |
| Died: 0 | ||
| Unknown: 0 | ||
| Ofloxacin, Clofazimin, Azithromycin | 1 (5.3) | Survived: 0 |
| Died: 0 | ||
| Unknown: 1 (100) |
Complications and Outcomes.
The outcome was reported for 21 of the 23 cases (91.3%), with 14 surviving, five dying, and two being lost to follow-up. While the causes of death varied (Table 4), the most commonly reported was overwhelming sepsis while receiving treatment in the inpatient setting [13,15].
Table 4. Cause of death in five patients with Mycobacterium kansasii pleural effusion.
DIC: disseminated intravascular coagulation
| Cause of death | No. (%) of patients |
| Sepsis | 2 (40%) |
| Cardiopulmonary arrest | 1 (20%) |
| DIC/multi-organ failure | 1 (20%) |
| Unspecified | 1 (20%) |
Discussion
Mycobacterium kansasii is an NTM known to be a ubiquitous environmental pathogen, found mainly in tap water. It is the second most common NTM in AIDS patients, after MAC [4]. There are seven identified genotypes of M. kansasii with Types I and II being the most common clinical isolates. Type I is most likely responsible for infections in Europe, the United States, and Japan [22]. Infection usually occurs via aspiration or local environmental inoculation via the aerosol route and there is little evidence of person-to-person transmission.
The incidence of M. kansasii infection increased following the emergence of HIV. Longitudinal prevalence studies have been inconsistent, which is thought to be related to considerable regional variability [22]. In a survey prior to the HIV epidemic, the annual incidence was 0.5 per 100,000 in 44 states of the United States. However, the incidence of infection is estimated to be as high as 532 cases per 100,000 in populations with HIV [22]. Rates are relatively high in England, Wales, and South America with increasing incidence in Israel, Korea, Spain, Portugal, France, Brazil, and Japan.
In HIV-positive patients with advanced immunosuppression, the lung is the most common organ involved in M. kansasii infection [23,24]. It is reported that 20% of HIV-positive patients with M. kansasii infection will develop disseminated disease [23]. Of the two cases with HIV-positive individuals in our review, neither developed disseminated disease and both survived [10,11]. This review demonstrated fewer cases in patients who had HIV as an underlying condition than initially anticipated considering widely supported data about differential rates of transmission. Despite being statistically insignificant, it is noteworthy that four cases reported hematologic malignancy, including myelodysplastic syndrome, hairy cell leukemia, chronic myelogenous leukemia, and lung cancer.
The most common causes of pleural effusion are congestive heart failure, cancer, bacterial pneumonia, and pulmonary embolism [25]. Pleural effusions secondary to bacterial pneumonia are characterized by exudate with associated symptoms of cough, fever, and infiltrate. Signs and symptoms were not reported for all cases, and the effusions in only five cases were distinguished as exudate. Symptoms typically seen in pulmonary M. kansasii include cough, sputum production, weight loss, shortness of breath, hemoptysis, and fever and sweats [23]
Five of the cases presented patients who died during their hospital course. One case did not report the reason for death, but in case of the other four patients, deaths occurred in the hospital, with conditions not directly related to the M. kansasii pleural effusion (cardiopulmonary arrest, sepsis, multi-organ failure).
Despite the 22 cases reported in the literature, data was not consistently reported across the case reports. Without consistent data, large conclusions cannot be drawn. Reporting guidelines, such as the Case Reports (CARE) guidelines, should be implemented to ensure transparency and complete reporting [26]. The CARE guidelines include demographic information, main symptoms, medical history, physical exam, intervention, outcomes, and other items to be included in a case report. The inclusion of many of these items consistently across reviewed cases may have improved the conclusions of this paper.
Conclusions
While our patient presented without the symptoms typically associated with Mycobacterium infections, including fever, chills, night sweats, and cough, a CT scan was indicative of atypical mycobacterial infection. Follow-up sputum sample and bronchoscopy guided the diagnosis of M. kansasii infection, rather than the more common M. tuberculosis. Through this case report, we hope to encourage others to keep a broad differential, especially in immunocompromised patient populations, as the treatments differ.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Human infection with atypical acid-fast organisms report of two cases with pathologic findings. Buhler V, Pollack A. Am J Clin Pathol. 1953;23:363–374. doi: 10.1093/ajcp/23.4.363. [DOI] [PubMed] [Google Scholar]
- 2.Clinical features and prognosis of nontuberculous mycobacterial pleuritis. Ando T, Kawashima M, Matsui H, et al. Respiration. 2018;96:507–513. doi: 10.1159/000490548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatal aortic pseudoaneurysm from disseminated Mycobacterium kansasii infection: case report. Ehsani L, Reddy SC, Mosunjac M, Kraft CS, Guarner J. Hum Pathol. 2015;46:467–470. doi: 10.1016/j.humpath.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Infection with Mycobacterium malmoense in Sweden: report of 221 cases. Henriques B, Hoffner SE, Petrini B, Juhlin I, Wåhlén P, Källenius G. Clin Infect Dis. 1994;18:596–600. doi: 10.1093/clinids/18.4.596. [DOI] [PubMed] [Google Scholar]
- 5.Clinical characteristics and treatment outcomes of pleural effusions in patients with nontuberculous mycobacterial disease. Park S, Jo KW, Lee SD, Kim WS, Shim TS. Respir Med. 2017;133:36–41. doi: 10.1016/j.rmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Successful treatment of non-tuberculous mycobacterial pleurisy with acute fever by antimycobacterial therapy. Yamoto M, Kawagishi Y, Murata A, Tsuji H. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical features of nontuberculous mycobacterial pleurisy: a review of 12 cases. Naito M, Maekura T, Kurahara Y, et al. Intern Med. 2018;57:13–16. doi: 10.2169/internalmedicine.9119-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilateral pleural effusions and right pneumothorax in a 25-year-old man. Olafsson EJ, Naum CC, Sarosi GA, Mastronarde JG. Chest. 2004;126:986–992. doi: 10.1378/chest.126.3.986. [DOI] [PubMed] [Google Scholar]
- 9.Mycobacterium kansasii empyema in a renal transplant recipient case report and review of the literature. Paull D, Decker G, Brown R. Transplantation. 2003;76:270–271. doi: 10.1097/01.TP.0000072018.06230.A1. [DOI] [PubMed] [Google Scholar]
- 10.Mycobacterium kansasii abscess of the chest wall after drainage of bacterial empyema. Tietz A, Tamm M, Battegay M. https://search.proquest.com/openview/686c94bc34821c60bf078d6d34415cb7/1?pq-origsite=gscholar&cbl=54107&casa_token=us4DU_htrZ8AAAAA:gTXqT4n9T9cZ3Eg4v9Gh_nUyLHAHi64AbJ6wxGRa9aiV_bj_isA11SeHlyUNLuiE41vpRIdN. Eur J Clin Microbiol and Infect Dis. 2000;19:72–73. doi: 10.1007/s100960050017. [DOI] [PubMed] [Google Scholar]
- 11.Mycobacterium kansasii osteomyelitis in a patient with AIDS on highly active antiretroviral therapy. Yano T, Okuda S, Kato K, Kato K, Kishimoto T. https://www.jstage.jst.go.jp/article/internalmedicine/43/11/43_11_1084/_article/-char/ja/ Intern Med. 2004;43:1084–1086. doi: 10.2169/internalmedicine.43.1084. [DOI] [PubMed] [Google Scholar]
- 12.Pulmonary infection with M. kansasii in a renal transplant patient. Cruz N, Ramírez-Muxó O, Bermúdez RH, Santiago-Delpín EA. Nephron. 1980;26:187–188. doi: 10.1159/000181978. [DOI] [PubMed] [Google Scholar]
- 13.Mycobacterium kansasii in a patient with hairy cell leukaemia. Green L, Coumbe A, Sawicka E, De Lord C. https://www.researchgate.net/profile/Laura-Green-28/publication/23413770_Mycobacterium_kansasii_in_a_patient_with_hairy_cell_leukaemia/links/5b55bf9aaca27217ffb3b72d/Mycobacterium-kansasii-in-a-patient-with-hairy-cell-leukaemia.pdf. Br J Haematol. 2009;144:2. doi: 10.1111/j.1365-2141.2008.07312.x. [DOI] [PubMed] [Google Scholar]
- 14.Organizing pneumonia and non-necrotizing granulomata on transbronchial biopsy: coexistence or bronchiolitis obliterans organizing pneumonia secondary to Mycobacterium kansasii disease. Starobin D, Guller V, Gurevich A, Fink G, Huszar M, Tal S. Respir Care. 2011;56:1959–1961. doi: 10.4187/respcare.01239. [DOI] [PubMed] [Google Scholar]
- 15.Fatal disseminated Mycobacterium kansasii infection in a renal transplant patient. Madan R, Onyeacholem I, Chandra S, Kanagarajan K. Chest. 2009;136:59. [Google Scholar]
- 16.A non-HIV case with disseminated Mycobacterium kansasii disease associated with strong neutralizing autoantibody to interferon-γ. Nei T, Okabe M, Mikami I, et al. https://www.sciencedirect.com/science/article/pii/S221300711200041X. Respir Med Case Rep. 2013;8:10–13. doi: 10.1016/j.rmcr.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Non-tuberculous mycobacterial pleurisy: an 8-year single-centre experience in Taiwan. Shu C, Lee LN, Wang JT, Chien YT, Wang JY, Yu CJ. https://www.ingentaconnect.com/content/iuatld/ijtld/2010/00000014/00000005/art00019. Int J Tuberc Lung Dis. 2010;14:635–641. [PubMed] [Google Scholar]
- 18.Disseminated Mycobacterium kansasii in an HIV-negative patient. Tabarsi P, Baghaei P, Kashani BS, Adimi P, Novin A, Mansouri D. https://www.sciencedirect.com/science/article/pii/S221255311200009X. Int J Mycobacteriol. 2012;1:51–52. doi: 10.1016/j.ijmyco.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Clinical relevance and characteristics of nontuberculous Mycobacterial pleuritis. Wen P, Wei M, Xu YR, Dong L. Jpn J Infect Dis. 2020;73:282–287. doi: 10.7883/yoken.JJID.2019.314. [DOI] [PubMed] [Google Scholar]
- 20.Case report: disseminated Mycobacterium kansasii disease in a patient with anti-interferon-gamma antibody. Kashihara E, Fujita K, Uchida N, Yamamoto Y, Mio T, Koyama H. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6838586/ Am J Trop Med Hyg. 2019;101:1066–1069. doi: 10.4269/ajtmh.19-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mycobacterium kansasii infection diagnosed by pleural fluid cytology: a case report. Domfeh AB, Nodit L, Gradowski JF, Bastacky S. Acta Cytol. 2007;51:627–630. doi: 10.1159/000325813. [DOI] [PubMed] [Google Scholar]
- 22.Johnston JC, Chiang L, Elwood K. Tuberculosis and Nontuberculous Mycobacterial Infections, Seventh Edition. Hoboken, New Jersey: John Wiley & Sons, Inc; 2017. Mycibacterium kansasii; pp. 725–735. [Google Scholar]
- 23.Medscape: Mycobacterium kansasii clinical presentation. [ Nov; 2021 ];Koirala J. https://emedicine.medscape.com/article/223230-clinical 2020
- 24.The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS) Horsburgh CR Jr, Selik RM. Am Rev Respir Dis. 1989;139:4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- 25.Pleural effusion in adults—etiology, diagnosis, and treatment. Jany B, Welte T. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6647819/ Dtsch Arztebl Int. 2019;116:377–386. doi: 10.3238/arztebl.2019.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CARE guidelines for case reports: explanation and elaboration document. Riley DS, Barber MS, Kienle GS, et al. J Clin Epidemiol. 2017;89:218–235. doi: 10.1016/j.jclinepi.2017.04.026. [DOI] [PubMed] [Google Scholar]