Abstract
Background.
During the outbreak of Zika virus (ZIKV) disease in Puerto Rico in 2016, nonpregnant women aged 20–39 years were disproportionately identified with ZIKV disease. We used household-based cluster investigations to determine whether this disparity was associated with age- or sex-dependent differences in the rate of ZIKV infection or reported symptoms.
Methods.
Participation was offered to residents of households within a 100-m radius of the residences of a convenience sample of 19 laboratory-confirmed ZIKV disease cases. Participants answered a questionnaire and provided specimens for diagnostic testing by reverse transcription-polymerase chain reaction (RT-PCR) and enzyme-linked immunosorbent assay (ELISA).
Results.
Among 367 study participants, 114 (31.1%) were laboratory positive for ZIKV infection, of whom 30% reported a recent illness (defined as self-reported rash or arthralgia) attributable to ZIKV infection. Age and sex were not associated with ZIKV infection. Female sex (adjusted prevalence ratio [aPR], 2.28; 95% confidence interval [CI], 1.40, 3.67), age <40 years (aPR, 2.39; 95% CI, 1.55, 3.70), and asthma (aPR, 1.63; 95% CI, 1.12, 2.37) were independently associated with symptomatic infection.
Conclusions.
Although neither female sex nor age were associated with an increased prevalence of ZIKV infection, both were associated with symptomatic infection. Further investigation to identify a potential mechanism of age- and sex-dependent differences in reporting symptomatic ZIKV infection is warranted.
Keywords: Zika virus, cluster investigation, surveillance, Puerto Rico, Aedes aegypti, flavivirus
Zika virus (ZIKV) was first detected in the Americas during May 2015 in Brazil [1]. As of 2 February 2017, 48 countries and territories in the Americas had confirmed local transmission of ZIKV [2]. Previous reports suggested that 18%-57% of ZIKV infections are symptomatic [3, 4]. Those who experience symptomatic infection typically develop rash, arthralgia, fever, myalgia, or conjunctivitis, which self-resolves within a week [5]. The first case of locally acquired ZIKV infection in Puerto Rico occurred in November 2015, and the peak incidence of ZIKV disease cases reported to the Puerto Rico Department of Health occurred during mid-August 2016. In total, >38000 laboratory-positive cases were reported through the end of 2016 [6, 7].
Puerto Rico, Yap Island, Brazil, and El Salvador have reported disproportionately higher rates of ZIKV disease among females and persons aged 10–39 years [4, 8, 9]. When comparing the rates of ZIKV disease among men and women in Puerto Rico, the disparity was most prominent among women aged 20–39 years [9]. Although sexual transmission has been proposed to explain these differences [10], such an association cannot be made without comparing rates of both infection and symptomatic infection among age and sex groups. Moreover, as has been documented for dengue virus (DENV) and chikungunya virus (CHIKV) infections throughout the tropics [11], many more symptomatic ZIKV infections likely occurred than were reported. Underidentification of cases by surveillance systems may be affected by patients’ care-seeking behavior and clinicians’ reporting practices, both of which may be influenced by patients’ age or sex [12, 13]. Household-based cluster investigations have been used to identify risk factors for infection with DENV or CHIKV and reporting symptomatic infection, and to estimate the frequency of underreporting of clinically apparent cases [14,15].
ZIKV, CHIKV, and the 4 DENVs are transmitted by Aedes mosquitoes, most commonly Aedes aegypti [5]. Because A. aegypti use humans as a source of both blood meals and breeding habitats (eg, discarded trash, tires), A. aegypti mosquito densities typically correlate with human population density [16]. Moreover, A. aegypti mosquitoes typically have a flight range of about 100 m, beyond which the primary mode of DENV dissemination is human movement [17]. Consequently, DENV and CHIKV transmission often occur in clusters around households [14, 16, 18–21].
During September-October 2016, we conducted household-based cluster investigations in Puerto Rico to (1) estimate the prevalence of ZIKV infection in households around confirmed cases, (2) estimate the proportion of ZIKV infections that are symptomatic, (3) identify factors associated with ZIKV infection and symptomatic infection, and (4) identify individual characteristics associated with seeking care, accurate clinical diagnosis, and case reporting.
METHODS
Ethics Statement
The protocol for this investigation was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board (protocol 6901). Adults (defined as individuals aged ≥14 years who live outside their parents’ home, have children, or are married or individuals aged ≥21 years) gave written informed consent for themselves and children for whom they were responsible. All minors provided assent. Consent, assent, and questionnaires were conducted in Spanish.
Study Setting
In 2015, Puerto Rico had an estimated population of 3 474 182 [22]. Both DENV and CHIKV are endemic in Puerto Rico [23]. Municipalities in the San Juan metropolitan area have densely populated areas that are mostly flat, as well as more-sparsely populated mountainous areas.
Study Design
The study was conducted during September 16-October 27 2016 in 5 municipalities in the San Juan metropolitan area: Bayamon, Carolina, Guaynabo, Toa Alta, and Toa Baja. All patients with ZIKV infection confirmed by reverse transcription-polymerase chain reaction (RT-PCR) analysis (hereafter, “index cases”) were identified by weekly queries of the PRDH Passive Arboviral Diseases Surveillance System (PADSS) database for residents of these 5 municipalities. Index cases or their parent or guardian were contacted by telephone within 30 days of the patient’s reported date of illness onset, and the study was explained. A home visit was scheduled for respondents interested in participating.
Prior to each household visit, all residences within a 100-m radius of each index case’s residence (hereafter, “clusters”) were identified and were mapped using ArcGIS, version 10.4.1 (ESRI, Redlands, CA). On the day of the scheduled visit, the study team approached all households within the cluster and classified them as vacant or occupied. Occupied houses were approached, and, if the head of household was present, the study team provided an overview of the study and offered participation. Households were not revisited if the head of household was not home.
In participating households, all household members who were residents of Puerto Rico were invited to participate. A questionnaire addressing household characteristics was administered to the head of household, and an individual questionnaire was administered to each participant, addressing demographic characteristics, contact with mosquitoes, medical history, recent illnesses, healthcare-seeking behavior, and self-reported clinical diagnoses (Supplementary Materials). Parents or guardians answered questionnaires by proxy for participants aged <8 years.
Entomological Investigation
Mosquitoes were collected using a portable electromechanical aspirator (Prokopack, 12 V DC 18 amp-hours) and a hand net [24]. Mosquitoes were separated by collection from bedrooms, other indoor areas, and outdoors. Mosquito pools of up to 20 female A. aegypti or Aedes mediovittatus per house were prepared, stored at −70°C, macerated, and tested by Trioplex RT-PCR [25], which detects ZIKV, DENV, and CHIKV nucleic acid.
Diagnostic Testing
Serum, whole blood, and urine specimens were collected from all participants, stored at 4°C, and transported to the laboratory the same day they were collected. All specimens were tested at the CDC Dengue Branch in San Juan, Puerto Rico, by Trioplex RT-PCR, and serum specimens were tested by immunoglobulin M (IgM) capture enzyme-linked immunosorbent assay (MACELISA) for ZIKV, DENV, and CHIKV antibodies [26, 27], Viral loads were measured using an RNA standard curve generated from the Trioplex RT-PCR assay target amplicons.
Definitions
Participants were household members who answered the individual questionnaire and provided a serum specimen. Current ZIKV infection was defined as detection of ZIKV nucleic acid in any specimen, and recent ZIKV infection was defined as detection of anti-ZIKV IgM antibody in serum (detailed definitions are in Supplementary Table 1) [28]. ZIKV positivity was defined as having either current or recent ZIKV infection. Recent flavivirus infection was defined as detection of both anti-ZIKV IgM and anti-DENV IgM antibody by ELISA in a serum specimen, in the absence of ZIKV or DENV nucleic acid detection, and is a subset of recent ZIKV infection. For participants with current ZIKV infection who reported no symptoms of recent illness, development of symptoms within 30 days after the household visit was determined by follow-up telephone call. For analyses, children were defined as participants aged <18 years.
Exclusion of participants with recent flavivirus infection from the ZIKV-positive group did not appreciably affect the statistical significance of analyses; hence, these participants were categorized as ZIKV positive. Inclusion of DENY and CHIKV diagnostic test results did not appreciably affect the statistical significance of analyses; hence, these were not included.
Following exploratory analyses of symptoms associated with ZIKV infection, a sensitivity analysis was performed to assess case definitions of symptomatic ZIKV infection (Supplementary Figure 1). Based on this analysis, symptomatic ZIKV infection was defined as the presence of rash or arthralgia. The attributable symptomatic infection rate was calculated by subtracting the proportion of ZIKV-negative participants reporting an acute illness with rash or arthralgia from the proportion of ZIKV-positive participants reporting an acute illness with rash or arthralgia.
Statistical Analysis
Generalized estimating equation analyses assuming an exchangeable correlation matrix (equal correlations among dependent observations) with a Poisson distribution (logarithm link) were used to model associations (prevalence ratios) among individual and household characteristics, entomological factors, and binary outcomes of ZIKV infection, healthcare-seeking behaviors, clinical diagnosis, and symptomatic ZIKV infection. The generalized estimating equation method accounts for correlations in data of members from the same household and cluster that might otherwise bias variance estimates. Confidence intervals (CIs) were constructed using the robust estimates for standard errors. We conducted exploratory bivariate analyses and subsequent multivariable analyses. Because specific a priori hypotheses were not being tested, variable selection for multivariable models was based on significance in bivariate analyses. The cluster investigation database was matched using Link Plus, version 2.0, with the PADSS database. Hierarchical cluster analysis was used to analyze patterns of symptoms among participants reporting a recent acute illness. Clusters were linked in an agglomerative (ie, bottom-up) fashion, using the Manhattan distance and the Ward method for agglomeration. Index cases were excluded from the statistical analysis, to reduce bias toward identification of symptomatic ZIKV infection. Data cleaning and analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) and R, version 3.3.0. Key R packages included gee and ggplot2 [29–31].
RESULTS
Identification of ZIKV-Infected Participants and Mosquitoes
Household investigations were conducted around the residences of 19 index cases (Figure 1). Of 1029 structures within all clusters combined, 106 (10.3%) were vacant or not houses. Of the 923 occupied households, 446 (48.3%) heads of household were present, and 244 (54.7%) accepted enrollment. Of the 645 residents of all enrolled households, 383 (59.4%) participated in the study. Twelve residents were index cases and were excluded from further analysis, and 4 had missing or insufficient specimens, resulting in 367 participants. More than half (58.6%) of participants were female, and more than one third (37.3%) were aged ≥65 years, which differs significantly from the general population of Puerto Rico (52.3% female [P = .01] and 18.0% aged ≥65 years [P <.001]) [22].
Figure 1.
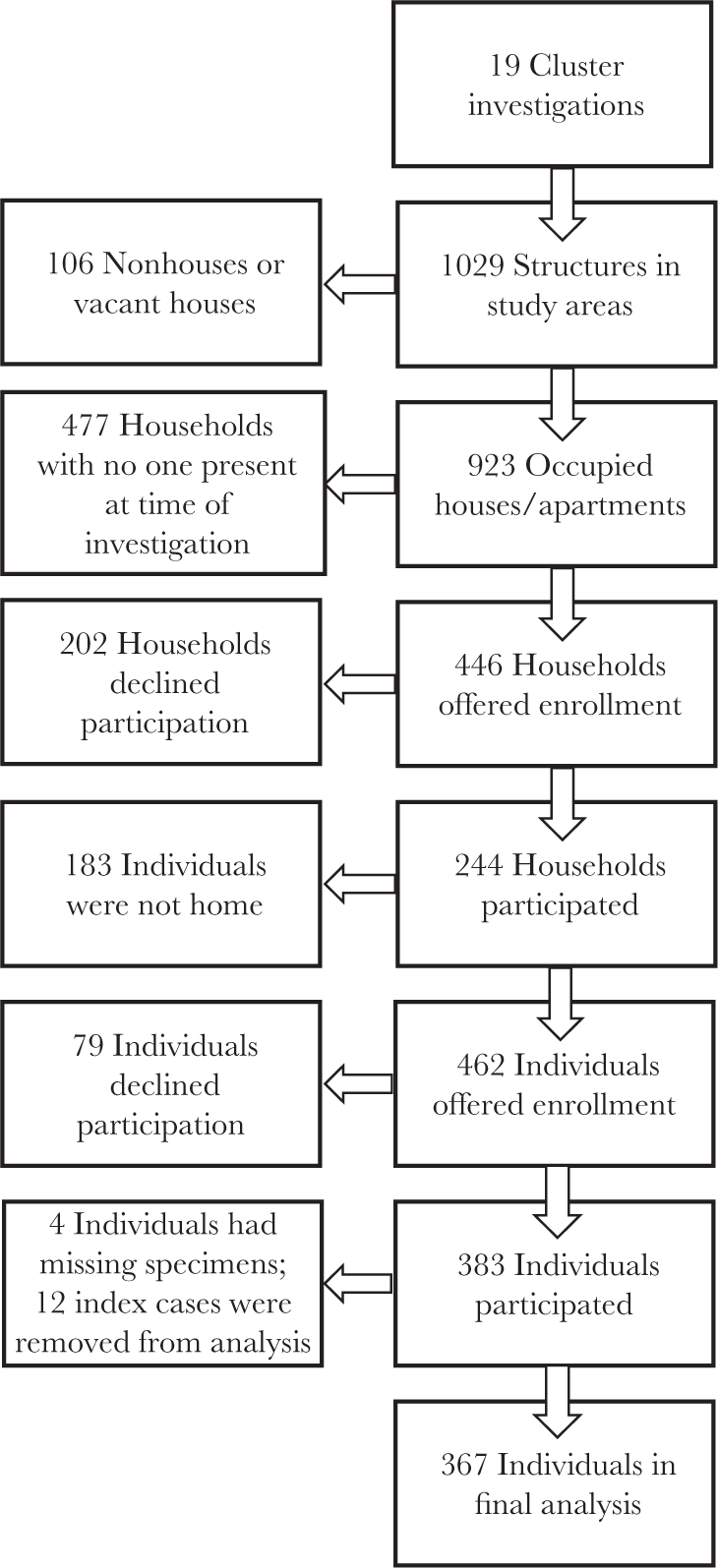
Flow diagram of households and participants enrolled in household-based cluster investigations—Puerto Rico, 16 September-27 October 2016.
Of the 367 participants, 114 (31.1%) were ZIKV positive, including 27 (23.7%) with current ZIKV infection (14 in serum only, 4 in urine only, 4 in whole blood only, and 5 in multiple specimens), 79 (69.3%) with recent ZIKV infection, and 8 (7.0%) with recent flavivirus infection. No participants had current CHIKV infection, 5 (1.4%) had recent CHIKV infection (of whom 1 also had recent ZIKV infection, and another also had recent flavivirus infection), 3 (0.8%) had recent DENV infection, and none had current DENV infection. The median proportion of participants who were ZIKV positive among all clusters was 30.0% (range, 0.0%—57.1%; Figure 2).
Figure 2.
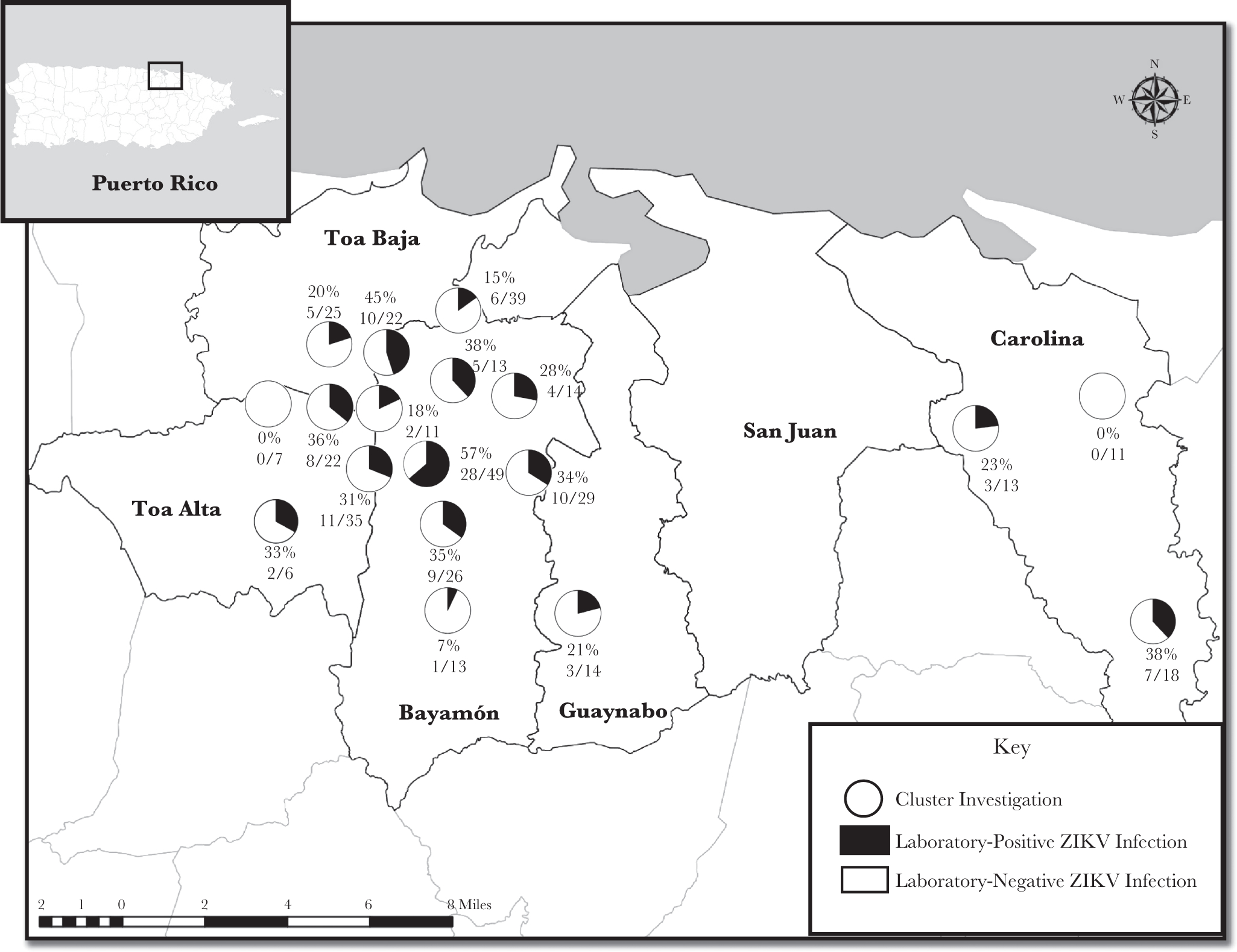
Cluster-specific rate of Zika virus infection among participants enrolled in household-based cluster investigations—Puerto Rico, 16 September-27 October 2016. (Note: One cluster had three participants, of whom one was an index case and the other two did not provide adequate specimens for analysis: hence, this cluster was not included on the map.)
A total of 211 houses (86.5%) were sampled for mosquitoes, in which 359 female A. aegypti were captured. At least 1 adult female A. aegypti was captured from 51.6% of sampled houses. Five or more mosquitoes were captured in 12.1% of sampled houses, and the maximum number of mosquitoes captured in a single household was 46. One female A. mediovittatus was captured. None of the 137 mosquito pools were positive for ZIKV, DENY, or CHIKV nucleic acid.
Factors Associated With ZIKV Infection
In bivariate analyses, age, sex, income, and education level were not significantly associated with ZIKV positivity (Table 1). The prevalence of ZIKV infection was 1.5 times higher among participants who reported being bitten by mosquitoes at least once per week. Among participants who reported ever being bitten, the prevalence of ZIKV infection was higher among those reporting being bitten in the morning and among participants who reported being bitten at home.
Table 1.
Association of Individual Characteristics With Laboratory-Confirmed Zika Virus (ZIKV) Infection Status Among Participants of Household-Based Cluster Investigations—Puerto Rico, 16 September–27 October 2016
| Characteristic | ZIKV Negative (n = 253) | ZIKV Positive (n = 114) | PR (95% CI) | aPR (95% CI)a |
|---|---|---|---|---|
|
| ||||
| Demographic | ||||
| Female sex | 152 (60.1) | 63 (55.3) | 0.84 (.64–1.11) | ... |
| Pregnant | 2 (1.3) | 0 (0.0) | NA | ... |
| Age, y | ||||
| Overall | 58.0 (41.0–69.0) | 59.5 (38.2–72.0) | 1.01 (0.98, 1.05)b | ... |
| <40 | 62 (24.5) | 30 (26.3) | 1.09 (0.80, 1.49) | ... |
| Years lived in Puerto Rico | 52.0 (34.0–66.0) | 52.0 (28.2–66.0) | 1.00 (.99–1.01) | ... |
| Annual household income <$25 000 | 127 (59.9) | 57 (56.4) | 0.85 (0.59, 1.24) | ... |
| Education level | ||||
| Less than high school degree | 50 (19.9) | 31 (27.2) | Reference | ... |
| High school degree or equivalent | 48 (19.1) | 23 (20.2) | 0.87 (0.55, 1.38) | ... |
| Some college, associate’s degree, or technical school | 85 (33.9) | 34 (29.8) | 0.78 (0.52, 1.17) | ... |
| Bachelor’s degree or higher | 68 (27.1) | 26 (22.8) | 0.75 (0.47, 1.19) | ... |
| Medical | ||||
| Acute illness in the past 6 mo | 69 (27.4) | 58 (50.9) | 1.80 (1.36, 2.39) | ... |
| Conditions | ||||
| Diabetes | 49 (19.4) | 23 (20.2) | 1.04 (0.73, 1.49) | ... |
| Asthma | 47 (18.6) | 24 (21.1) | 1.15 (0.81, 1.63) | ... |
| Hypertension | 114 (45.1) | 55 (48.2) | 1.05 (0.78, 1.40) | ... |
| Heart disease | 37 (14.6) | 13 (11.4) | 0.80 (0.49, 1.28) | ... |
| Hypercholesterolemia | 75 (29.6) | 40 (35.1) | 1.18 (0.87, 1.60) | ... |
| Joint disease/arthritis | 82 (32.4) | 30 (26.3) | 0.83 (0.61, 1.12) | ... |
| Thyroid disease | 35 (13.8) | 17 (14.9) | 1.03 (0.70, 1.51) | ... |
| Cancer | 14 (5.5) | 10 (8.8) | 1.51 (0.93, 2.47) | ... |
| Otherb | 60 (23.7) | 23 (20.2) | 0.79 (0.53, 1.18) | ... |
| None | 62 (24.5) | 23 (20.2) | 0.83 (0.57, 1.19) | ... |
| Chronic | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.99 (0.92, 1.07) | ... |
| Daily medications | ||||
| NSAIDs | 51 (20.2) | 23 (20.2) | 1.01 (0.68, 1.50) | ... |
| Other | 138 (54.5) | 67 (58.8) | 1.08 (0.79, 1.48) | ... |
| None | 102 (40.3) | 42 (36.8) | 0.90 (0.64, 1.26) | ... |
| Behavioral | ||||
| Slept under mosquito net in past mo | 4 (1.6) | 2 (1.8) | 1.24 (0.53, 2.89) | ... |
| Time spent in community,c h/d | ||||
| <4 | 35 (14.0) | 9 (7.9) | Reference | ... |
| 5–8 | 80 (32.0) | 36 (31.6) | 1.35 (0.70, 2.62) | ... |
| 9–12 | 135 (54.0) | 69 (60.5) | 1.39 (0.77, 2.51) | ... |
| Frequency of mosquito-repellent use in past mo | ||||
| Daily | 53 (21.3) | 35 (31.0) | 1.43 (0.95, 2.16) | ... |
| Weekly | 81 (32.5) | 37 (32.7) | 1.12 (0.80, 1.58) | ... |
| Never | 115 (46.2) | 41 (36.3) | Reference | ... |
| Mosquito contact | ||||
| Bitten by mosquitoes at least once/wk | 95 (39.4) | 65 (61.9) | 1.49 (1.07, 2.07) | 1.65 (1.15, 2.39) |
| When mosquitoes bite | ||||
| In the morning | 37 (17.4) | 29 (29.6) | 1.48 (1.02, 2.15) | 1.47 (1.02, 2.12) |
| During the day | 42 (19.7) | 18 (18.4) | 0.86 (0.57, 1.29) | ... |
| In the evening | 71 (33.3) | 29 (29.6) | 0.92 (0.65, 1.30) | ... |
| At night | 95 (44.6) | 40 (40.8) | 0.91 (0.65, 1.28) | ... |
| Where mosquitoes bite | ||||
| Home | 165 (77.5) | 90 (91.8) | 2.40 (1.25, 4.60) | 1.04 (0.63, 1.71) |
| Work/school | 21 (9.9) | 6 (6.1) | 0.73 (0.39, 1.38) | ... |
| Other houses in the community | 11 (5.2) | 2 (2.0) | 0.43 (0.12, 1.55) | ... |
| Outside of the community | 25 (11.7) | 7 (7.1) | 0.80 (0.45, 1.44) | ... |
| Other place | 29 (13.6) | 8 (8.2) | 0.76 (0.44, 1.31) | ... |
Data are no. (%) of participants or median value (interquartile range), unless otherwise indicated.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; NA, not applicable; NSAID, nonsteroidal antiinflammatory drug; PR, prevalence ratio.
The adjusted model includes frequency bitten by mosquitoes, when mosquitoes bite, where mosquitoes bite, household member with acute illness in previous 3 months, number of vacant houses in cluster, never leaves doors and windows open, and at least 1 female Aedes aegypti mosquito captured indoors.
PR for 5-year age groups.
From 7:00 am through 7:00 pm.
Reporting never leaving windows and doors open was associated with a >2-fold reduction in the prevalence of ZIKV infection (Table 2). Participants reporting that a household member had been ill in the prior 3 months were significantly more likely to be ZIKV positive. A greater number of vacant homes in the cluster was significantly associated with being ZIKV-positive, as was capture of at least 1 female A. aegypti.
Table 2.
Association of Household, Community, and Entomologic Factors With Laboratory-Confirmed Zika Virus (ZIKV) Infection Status Among Participants of Household-Based Cluster Investigations—Puerto Rico, 16 September–27 October 2016
| Characteristic | ZIKV Negative (n = 253) | ZIKV Positive (n = 114) | PR (95% CI) | aPR (95% CI)a |
|---|---|---|---|---|
|
| ||||
| Household members participating. | 2.0 (1.0–2.0) | 2.0 (1.0–3.0) | 1.11 (0.92, 1.35) | ... |
| Household member with acute illness in past 3 mob | 118 (46.6) | 82 (71.9) | 2.19 (1.49, 3.22) | 1.70 (1.09, 2.63) |
| House within 50 m of index case residence | 100 (40.8) | 47 (41.2) | 0.98 (0.69, 1.40) | ... |
| Vacant houses in cluster, no. | 70 (4.0–9.0) | 8.0 (5.0–9.0) | 1.09 (1.03, 1.15)c | 1.09 (1.02, 1.16) |
| Occupied houses in cluster, no. | 58.0 (38.0–64.0) | 59.0 (48.0–65.0) | 1.01 (1.00, 1.02) | ... |
| Residence type | ||||
| 1-story house | 159 (62.8) | 78 (68.4) | Reference | ... |
| 2-story house | 83 (32.8) | 34 (29.8) | 0.84 (0.57, 1.24) | ... |
| Apartment/condominium | 11 (4.3) | 2 (1.8) | 0.51 (0.14, 1.87) | ... |
| Household annual income, $ | ||||
| ≤25 000 | 127 (59.9) | 57 (56.4) | Reference | ... |
| 26 000–50 000 | 64 (30.2) | 36 (35.6) | 1.22 (0.81, 1.81) | ... |
| 51 000–75 000 | 15 (7.1) | 5 (5.0) | 0.92 (0.38, 2.26) | ... |
| ≥76 000 | 6 (2.8) | 3 (3.0) | 1.23 (0.49, 3.10) | ... |
| Presence of screens on doors and windows | ||||
| Home has no screens | 42 (16.6) | 19 (16.7) | 0.95 (0.59, 1.55) | ... |
| Home has screened windows and doors in some rooms | 109 (43.1) | 49 (43.0) | 0.93 (0.63, 1.37) | ... |
| Home has intact screens in all doors and windows | 102 (40.3) | 46 (40.4) | Reference | ... |
| Air conditioner use | ||||
| Never uses or does not have | 68 (26.9) | 33 (28.9) | 1.02 (0.70, 1.48) | ... |
| Uses in home at least sometimes | 186 (73.1) | 81 (71.1) | Reference | ... |
| Practices with doors and windows | ||||
| Never leaves doors or windows open | 75 (29.6) | 15 (13.2) | 0.47 (0.27, 0.80) | 0.59 (0.32, 1.08) |
| Leave doors or windows open regularly | 178 (70.4) | 99 (86.8) | Reference | ... |
| Mosquito control activities | ||||
| Uses mosquito coils in house or patio | 60 (23.7) | 27 (23.7) | 1.04 (0.70, 1.57) | ... |
| Uses citronella candles in house or patio | 85 (33.6) | 32 (28.1) | 0.80 (0.53, 1.20) | ... |
| Uses mosquito traps in house or patio | 7 (2.8) | 7 (6.1) | 1.52 (0.79, 2.93) | ... |
| Female A. aegypti mosquitoes captured, no. | ||||
| Indoors | ||||
| Quantity | 0 (0.0–1.0) | 1.0 (0.0–2.8) | 1.03 (1.02, 1.04) | ... |
| At least 1 | 73 (38.6) | 47 (54.7) | 1.57 (1.06, 2.33) | 1.22 (0.84, 1.77) |
| Outdoors | ||||
| Quantity | 0 (0.0–1.0) | 0.0 (0.0–2.0) | 1.20 (1.04, 1.38) | ... |
| At least 1 | 33 (34.0) | 14 (40.0) | 1.18 (0.60, 2.33) | ... |
| Total | ||||
| Quantity | 0 (0.0–2.0) | 1.0 (0.0–3.0) | 1.03 (1.02, 1.04) | ... |
| At least 1 | 101 (47.9) | 57 (60.0) | 1.42 (0.97, 2.08) | ... |
Data are no. (%) of participants or median value (interquartile range).
Abbreviations: A. aegypti, Aedes aegypti; aPR, adjusted prevalence ratio; CI, confidence interval; PR, prevalence ratio.
The adjusted model includes frequency bitten by mosquitoes, when mosquitoes bite, where mosquitoes bite, household member with acute illness in previous 3 months, number of vacant houses in cluster, never leaves doors and windows open, and at least 1 female A. aegypti mosquito captured indoors.
Question was administered to the head of household.
PR for a 1-unit increase
Following multivariable analysis, recent illness in a household member, the number of vacant houses in the cluster, being bitten by a mosquito at least once weekly, and being bitten by mosquitoes in the morning remained significantly associated with being ZIKV-positive.
Signs and Symptoms Associated With ZIKV Infection
Of 127 participants who reported an acute illness within the past 6 months, 58 (45.7%) were ZIKV positive (Table 3). In bivariate analyses, rash, fever, arthralgia, and arthritis were significantly associated with being ZIKV-positive, and sore throat was significantly associated with being ZIKV-negative. Similarly, hierarchical cluster analysis indicated that headache, myalgia, arthralgia, rash, and fever clustered separately from sore throat, rhinorrhea, and cough (Figure 3). Symptomatic, ZIKV-negative participants had a significantly longer period of time between symptom onset and specimen collection than ZIKV-positive participants. Among participants that sought medical care, clinical diagnosis with Zika by the healthcare provider was twice as prevalent among those that were ZIKV-positive. ZIKV-negative participants who sought care were significantly more likely to have something other than an arboviral illness diagnosed.
Table 3.
Symptoms Associated With Laboratory-Confirmed Zika Virus (ZIKV) Infection Among Participants of a Household-Based Cluster Investigations Who Reported Acute Illness in the Past 6 Months—Puerto Rico, 16 September–27 October 2016
| Characteristic | ZIKV Negative (n = 69) | ZIKV Positive (n = 58) | PR (95% CI) |
|---|---|---|---|
|
| |||
| Time from illness onset to specimen collection, d | 41.5 (17–81.5) | 33 (19–59) | 0.99 (0.99, 0.99) |
| Illness duration, d | 7 (2–90) | 7 (2–45) | 0.99 (0.97, 1.01) |
| Febrile | |||
| Rash | 19 (27.5) | 37 (63.8) | 2.15 (1.40, 3.30) |
| Fever | 27 (39.1) | 36 (62.1) | 1.61 (1.09, 2.39) |
| Chills | 26 (37.7) | 17 (29.3) | 0.80 (0.52, 1.25) |
| Headache | 39 (56.5) | 39 (67.2) | 1.31 (0.87, 1.96) |
| Myalgia | 42 (60.9) | 36 (62.1) | 1.03 (0.67, 1.58) |
| Calf pain | 13 (18.8) | 15 (25.9) | 1.20 (0.84, 1.71) |
| Arthralgia | 27 (39.1) | 38 (65.5) | 1.62 (1.10, 2.37) |
| Arthritis | 14 (20.3) | 24 (41.4) | 1.47 (1.06, 2.05) |
| Conjunctivitis | 15 (21.7) | 21 (36.2) | 1.34 (0.91, 1.96) |
| Retro-orbital pain | 14 (20.3) | 10 (17.2) | 0.99 (0.61, 1.62) |
| Light bleeding | 2 (2.9) | 4 (6.9) | 1.34 (0.70, 2.56) |
| Respiratory | |||
| Nasal discharge | 41 (59.4) | 24 (41.4) | 0.75 (0.50, 1.12) |
| Sore throat | 44 (63.8) | 23 (39.7) | 0.62 (0.42, 0.91) |
| Cough | 37 (53.6) | 22 (37.9) | 0.74 (0.51, 1.09) |
| Gastrointestinal | |||
| Vomiting or nausea | 15 (21.7) | 13 (22.4) | 1.03 (0.61, 1.73) |
| Diarrhea | 16 (23.2) | 14 (24.1) | 1.11 (0.68, 1.82) |
| Sought medical care | 30 (43.5) | 34 (58.6) | 1.45 (0.97, 2.14) |
| Sought care more than once | 10 (14.5) | 16 (27.6) | 1.41 (0.97, 2.03) |
| Diagnosis | |||
| Zika | 1 (3.3) | 10 (29.4) | 2.01 (1.43, 2.81) |
| Chikungunya | 0 (0) | 1 (2.9) | ... |
| Dengue | 0 (0) | 1 (2.9) | ... |
| Viral syndrome | 13 (43.3) | 11 (32.4) | 0.78 (0.43, 1.41) |
| Other (unspecified) | 15 (50.0) | 8 (23.5) | 0.52 (0.28, 0.97) |
| Hospitalized because of illness | 1 (3.3) | 1 (2.9) | 0.95 (0.23, 3.86) |
| Hospitalization duration, d | 8 (NA) | 4 (NA) | ... |
Data are no. (%) of participants or median value (interquartile range).
Abbreviations: CI, confidence interval; NA, not applicable; PR, prevalence ratio.
Figure 3.
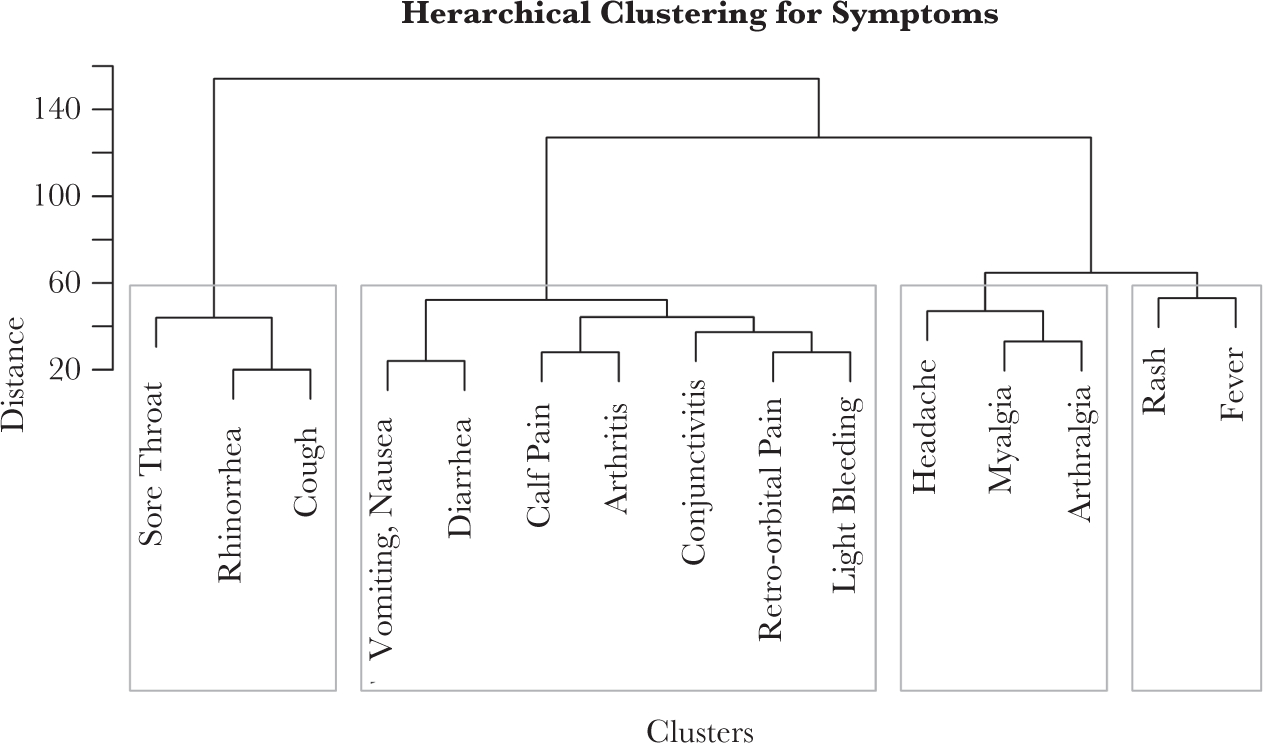
Hierarchical clustering for symptoms among Zika virus-positive participants of household-based cluster investigations—Puerto Rico, 16 September-27 October 2016 (n = 114).
Factors Associated With Symptomatic ZIKV Infection
Symptomatic ZIKV infection was identified in 49 ZIKV-positive participants (43.0%), while 34 ZIKV-negative participants (13.4%) reported the same symptoms, resulting in a ZIKVattributable symptomatic infection rate of 29.6%. Among participants positive for ZIKV nucleic acid in serum, viral loads were not significantly different between participants with symptomatic ZIKV infection (n = 5; median, 2018 genome copies/mL [range, 1187–130831 genome copies/mL]) and participants with asymptomatic ZIKV infection (n = 13; median, 3485 genome copies/mL [range, 624–1656939 genome copies/mL]; P = .43). Of the 15 participants with current ZIKV infection who reported no symptoms at the household visit, none (0%) reported developing symptoms during the 30 days after the household visit.
Bivariate analyses showed that female sex, younger age, asthma, and not taking any daily medications were significantly associated with having symptomatic ZIKV infection (Table 4). Specifically, 80% of participants aged <40 years had symptomatic ZIKV infection as compared to 29.8% of participants aged ≥40 years (prevalence ratio, 2.68; 95% CI, 1.85, 3.88). The corresponding trend was also noted when using categorized age. All participants with symptomatic ZIKV infection reported a household member having been ill in the prior 3 months, compared with half of participants with asymptomatic ZIKV infection. Following multivariable analysis, female sex, younger age, and asthma remained significantly associated with symptomatic ZIKV infection. Attributable symptomatic infection rates were higher for women than those for men (42.9% vs 13.6%), for participants aged <40 years than those for participants aged ≥40 years (62.3% vs 17.7%), and for persons with asthma than those for persons without asthma (47.5% vs 24.5%).
Table 4.
Associations of Patient Characteristics With Symptomatic Zika Virus (ZIKV) Infection Among Participants of Household-Based Cluster Investigations—Puerto Rico, 16 September–27 October 2016
| Characteristic | Asymptomatic (n = 65) | Symptomatic (n = 49) | PR (95% CI) | aPR (95% CI)b |
|---|---|---|---|---|
|
| ||||
| Sociodemographic | ||||
| Female sex | 26 (40.0) | 37 (75.5) | 2.31 (1.33, 4.02) | 2.28 (1.40, 3.69) |
| Age, y | ||||
| Overall | 67 (57–77) | 41 (24–62) | 0.90 (0.87, 0.93)d | ... |
| <18 | 1 (1.5) | 7 (14.3) | 1.13 (0.80, 1.59) | 1.67 (0.25, 11.02) |
| 18–39 | 5 (7.7) | 17 (34.7) | Reference | ... |
| 40–64 | 22 (33.8) | 16 (32.7) | 0.54 (0.36, 0.82) | 0.17 (0.04, 0.68) |
| ≥65 | 37 (56.9) | 9 (18.4) | 0.25 (0.13, 0.47) | 0.04 (0.01, 0.22) |
| Education level | ||||
| Less than high school degree | 14 (21.5) | 12 (24.5) | 1.35 (0.78, 2.34) | ... |
| High school degree or equivalent | 18 (27.7) | 5 (10.2) | 0.53 (0.23, 1.23) | ... |
| Some college, associate’s degree, or technical school | 15 (23.1) | 16 (32.7) | 1.13 (0.62, 2.05) | ... |
| Bachelor’s degree or higher | 18 (27.7) | 16 (32.7) | Reference | ... |
| Somebody in household sick within last 3 mo | 33 (50.8) | 49 (100) | ... | ... |
| Medical | ||||
| Conditions | ||||
| Diabetes | 18 (27.7) | 5 (10.2) | 0.52 (0.21, 1.29) | ... |
| Asthma | 8 (12.3) | 16 (32.7) | 2.30 (1.54, 3.45) | 1.63 (1.12–2.37) |
| Hypertension | 37 (56.9) | 18 (36.7) | 0.72 (0.43, 1.18) | ... |
| Heart disease | 6 (9.2) | 7 (14.3) | 1.47 (0.76, 2.86) | ... |
| Hypercholesterolemia | 27 (41.5) | 13 (26.5) | 0.77 (0.46, 1.27) | ... |
| Joint disease/arthritis | 20 (30.8) | 10 (20.4) | 0.74 (0.41, 1.35) | ... |
| Thyroid disease | 12 (18.5) | 5 (10.2) | 0.67 (0.29, 1.55) | ... |
| Cancer | 7 (10.8) | 3 (6.1) | 0.78 (0.29, 2.06) | ... |
| Otherc | 12 (18.5) | 11 (22.4) | 1.16 (0.68, 1.98) | ... |
| None | 11 (16.9) | 12 (24.5) | 1.06 (0.60, 1.85) | ... |
| Daily medications | ||||
| NSAIDs | 18 (27.7) | 5 (10.2) | 0.59 (0.27, 1.27) | ... |
| Other | 44 (67.7) | 23 (46.9) | 0.63 (0.40, 0.98) | ... |
| None | 17 (26.2) | 25 (51.0) | 1.71 (1.10, 2.66) | 1.06 (.69–1.64) |
ZIKV infection was considered symptomatic if rash or arthralgia were present within the past 6 months.
Abbreviations: aPR, adjusted prevalence ratio; CI, confidence interval; NA, not applicable; NSAID, nonsteroidal antiinflammatory drug; PR, prevalence ratio.
The adjusted model includes sex, age, asthma, and daily medications.
Includes lung disease, liver disease, renal disease, stroke, lupus, and any other condition mentioned by the participant.
PR for 5-year age groups.
Factors Associated With Seeking Medical Care, Clinical Diagnosis, and Case Reporting
Among the 49 participants with symptomatic ZIKV infection, 27 (55.1%) sought medical care. Whereas all children with symptomatic ZIKV infection sought medical care, less than half (47.6%) of adults did so (P < .01). History of diabetes or heart disease were associated with seeking medical care, as was increasing number of underlying medical conditions (Supplementary Table 2). Of the 27 participants with symptomatic ZIKV infection who sought medical care, 10 (37.0%) reported a clinical diagnosis of Zika (Supplementary Table 3). The prevalence of Zika diagnosis among female participants was about half that among men, while participants reporting conjunctivitis had a prevalence of Zika diagnosis >3 times that among participants without conjunctivitis. Participants with multiple comorbidities were less likely to receive a diagnosis of Zika. Five of 27 participants (18.5%) with symptomatic ZIKV infection who sought medical care were confirmed to have been reported to public health authorities via the PADSS (Supplementary Figure 2). Reporting abdominal pain or a chronic medical condition other than those listed were associated with being reported to the PADSS (Supplementary Table 4).
DISCUSSION
By conducting household-based cluster investigations soon after the peak of the ZIKV outbreak in Puerto Rico, we were able to identify factors associated with infection, describe the spectrum of ZIKV disease, and identify characteristics associated with seeking healthcare, receiving a diagnosis of Zika, and being reported to public health authorities. Most prominently, while neither female sex nor age of <40 years were associated with an increased prevalence of ZIKV infection, both were associated with an increased likelihood of reporting symptomatic ZIKV infection. These observations assist in elucidating reasons for observed age- and sex-specific differences in identification of ZIKV disease cases reported to public health authorities.
In a 2014 cluster investigation during the CHIKV outbreak in Puerto Rico, 30% of participants had CHIKV-positive laboratory findings, which is similar to the rate of ZIKV infection observed in this investigation (31%) [14]. This suggests a similar regional attack rate of A. aegypti-transmitted viruses after introduction into an immunologically naive populations. ZIKV seroprevalence in other studies has varied from 50% (95% CI, 43%-56%) in a serosurvey conducted during the 2013–2014 ZIKV outbreak in French Polynesia [32] to 73% (95% CI, 68%-77%) in a household survey conducted on Yap Island after the 2007 ZIKV outbreak [4]. Factors such as the timing of the studies, population density, human geographic movement, and mosquito abundance may explain the different rates of infection.
The factors associated with ZIKV infection underscore the importance of mosquito avoidance and control. Keeping doors and windows closed helps prevent mosquitoes from entering a house, where they can potentially infect residents [33]. Although other studies have identified air conditioning and door and window screens as factors that protect against infection with viruses transmitted by A. aegypti [14, 34–36], these variables were not associated with ZIKV infection in our analyses. The association between the number of vacant houses and an increased odds of ZIKV infection may reflect vacant houses as areas that can harbor abundant mosquito breeding sites [37–39]. The association between ZIKV infection and reporting a recently ill household member provides evidence that ZIKV may cluster within families, similar to CHIKV and DENV [14, 40]. The lack of appreciable differences in the prevalence of ZIKV infection with respect to age or sex suggests that differential rates of infection (as from sexual transmission) may not significantly contribute to the observed age- and sex-dependent disparities in ZIKV disease cases in Puerto Rico [4, 8, 9].
ZIKV-positive females, individuals aged <40 years, and those with asthma were significantly more likely to report symptoms of ZIKV disease. These associations have not been observed among individuals with DENV or CHIKV infection. While asthma is associated with more-severe outcomes of respiratory infections (eg, influenza), we are not aware of an obvious explanation for this finding and cannot rule out the possibility that this finding may be a chance observation. However, the association of younger age with increased prevalence of symptomatic ZIKV infection is consistent with findings from French Polynesia, where 71% of schoolchildren reported symptomatic infection, compared with 57% of adults [3]. Anti-DENV IgG antibody may provide a degree of cross-protection from symptomatic ZIKV infection in regions such as Puerto Rico, where adults have often experienced multiple DENV infections [41]. To our knowledge, the association between reporting symptomatic ZIKV infection and being female has not been previously reported. Possible explanations include sex-specific differences in immune response to ZIKV infection, recall bias among women owing to increased concern about the consequences of ZIKV infection, and female sex-specific hormonal effects on development of symptomatic ZIKV infection [42–44]. Our findings of increased symptomatic ZIKV infection among women and those aged <40 years may explain the higher prevalence of ZIKV disease cases observed among women in Puerto Rico aged 20–39 years [9].
Clinical diagnosis of ZIKV disease among participants with symptomatic ZIKV infection who sought medical care was higher in the present study than clinical diagnosis of chikungunya in household-based cluster investigations conducted in Puerto Rico in 2014 (37% vs 23%) [14]. Similarly, 19% of participants with symptomatic ZIKV infection who sought medical care had been reported to public health authorities, compared with 9% of participants with chikungunya in 2014. Such findings were unexpected because ZIKV disease is often considered to be a mild, nonspecific illness as compared to chikungunya. The observed trends may be attributable to increased public and community outreach efforts conducted by the PRDH and CDC, which emphasized the need for individuals with Zika-like illness to seek care and for clinicians to report suspected ZIKV disease cases to public health authorities [45].
Although this study benefited from active case finding among >200 homes, coupled with comprehensive diagnostic testing for ZIKV infection, the findings have several limitations. First, the study was limited to 5 municipalities in the San Juan metropolitan area in regions where ZIKV was likely to be circulating. Also, women and older individuals were more likely to participate in the study, and recently ill individuals may have been more likely to participate. In addition, household and individual participation rates (26.4% and 59.4%, respectively) were low. These aspects demonstrate that the observed prevalence of ZIKV infection may not be representative of all residents of Puerto Rico and may have been higher than the island-wide prevalence of ZIKV infection. Second, because IgM antibody generated in response to infection with a flavivirus (eg, ZIKV) may cross-react with other flaviviruses (eg, DENV), including the possibility of original antigenic sin [46], some DENV or ZIKV infections defined by detection of IgM antibody may have been misclassified. Third, we could not always decipher whether the household member who had been ill in the past 3 months was the participant or another household member, potentially resulting in misclassification. However, a sensitivity analysis of the multivariable model that included only heads of household did not change the findings of these analyses. Last, some participants with a long interval between illness onset and specimen collection may have been misclassified as ZIKV negative because of loss of detectable anti-ZIKV IgM antibody.
In summary, this study identified both individual and environmental factors associated with ZIKV infection and observed that younger age and female sex were associated with symptomatic infection. Further investigation is needed to explore whether and potentially how hormonal and immunologic factors affect one’s likelihood of developing disease following ZIKV infection.
Supplementary Material
Acknowledgments.
We thank Arnaldo Nieves, for generation of maps; and the team of phlebotomists and interviewers from the Puerto Rico Department of Health and Centers for Disease Control and Prevention, for collecting blood specimens and performing interviews.
Financial support.
This work was supported by the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1.Faria NR, Azevedo RDSDS, Kraemer MUG, et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 2016; 352:345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan American Health Organization. Zika Epidemiological Update, 17 November. Washington, D.C.: Pan American Health Organization / World Health Organization, 2016. [Google Scholar]
- 3.Aubry M, Teissier A, Huart M, et al. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis 2017; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Zika Strategic Response Plan, Revised for July 2016 - December 2017. Geneva, Switzerland: World Health Organization, 2016. [Google Scholar]
- 6.Puerto Rico Department of Health. Arboviral Diseases Weekly Report - Week 9. http://www.salud.gov.pr/Estadisticas-Registros-y-Publicaciones/Informes%20Arbovirales/Reporte%20ArboV%20semana%209–2017.pdf. Accessed 22 March 2017.
- 7.Thomas DL, Sharp TM, Torres J, et al. Local transmission of Zika virus-Puerto Rico, November 23, 2015-January 28, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:154–8. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos T, Rodriguez A, Almiron M, et al. Zika virus and the Guillain-Barré Syndrome - case series from seven countries. N Engl J Med 2016; 375:1598–601. [DOI] [PubMed] [Google Scholar]
- 9.Lozier M, Adams L, Febo MF, et al. Incidence of Zika virus disease by age and sex - Puerto Rico, November 1, 2015-October 20, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1219–23. [DOI] [PubMed] [Google Scholar]
- 10.Coelho FC, Durovni B, Saraceni V, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis 2016; 51:128–32. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarker AR, Sultana M, Mahumud RA, Sheikh N, Van Der Meer R, Morton A. Prevalence and Health Care-Seeking Behavior for Childhood Diarrheal Disease in Bangladesh. Glob Pediatr Health 2016; 3:2333794X16680901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson AE, Anisimowicz Y, Miedema B, Hogg W, Wodchis WP, Aubrey-Bassler K. The influence of gender and other patient characteristics on health care-seeking behaviour: a QUALICOPC study. BMC Fam Pract 2016; 17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch D, Roth NM, Caraballo EV, et al. Use of household cluster investigations to identify factors associated with Chikungunya virus infection and frequency of case reporting in Puerto Rico. PLoS Negl Trop Dis 2016; 10:e0005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dechant EJ, Rigau-Perez JG. Hospitalizations for suspected dengue in Puerto Rico, 1991–1995: estimation by capture-recapture methods. The Puerto Rico Association of Epidemiologists. Am J Trop Med Hyg 1999; 61:574–8. [DOI] [PubMed] [Google Scholar]
- 16.Stoddard ST, Forshey BM, Morrison AC, et al. House-to-house human movement drives dengue virus transmission. Proc Natl Acad Sci U S A 2013; 110:994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington LC, Scott TW, Lerdthusnee K, et al. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 2005; 72:209–20. [PubMed] [Google Scholar]
- 18.Mammen MP, Pimgate C, Koenraadt CJ, et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med 2008; 5:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salje H, Cauchemez S, Alera MT, et al. Reconstruction of 60 Years of Chikungunya epidemiology in the Philippines demonstrates episodic and focal transmission. J Infect Dis 2016; 213:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp TM, Moreira R, Soares MJ, et al. Underrecognition of Dengue during 2013 Epidemic in Luanda, Angola. Emerg Infect Dis 2015;21:1311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nsoesie EO, Ricketts RP, Brown PIE, et al. Spatial and Temporal Clustering of Chikungunya Virus Transmission in Dominica. PLoS Negl Trop Dis 2015; 9:e0003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.United States Census Bureau. American Fact Finder, www.factfinder.census.gov. Accessed 11 January 2017.
- 23.Sharp I' M, Ryff KR, Alvarado L, et al. Surveillance for Chikungunya and Dengue during the first year of Chikungunya virus circulation in Puerto Rico. J Infect Dis 2016; 214:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez-Prokopec CM, Galvin WA, Kelly R, Kitron U. A new, cost-effective, battery-powered aspirator for adult mosquito collections. J Med Entomol 2009; 46:1256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Trioplex Real-time RT-PCR Assay, https://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM491592.pdf. Accessed 28 February 2017.
- 26.Centers for Disease Control and Prevention. Zika MACELISA. https://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM488044.pdf. Accessed 28 February 2017.
- 27.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 2000; 38:1823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabe IB, Staples JE, Villanueva J, et al. MTS. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb Mortal Wkly Rep 2016; 65:543–6. [DOI] [PubMed] [Google Scholar]
- 29.Wickham H Ggplot2: elegant graphics for data analysis. New York: Springer, 2009. [Google Scholar]
- 30.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 31.Carey VJ. Package “gee.” Generalized estimation equation solver. R package, version 4.13–19. https://cran.r-project.org/web/packages/gee/gee.pdf. Accessed 20 December 2017. [Google Scholar]
- 32.Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016; 387:2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis EM, Neatherlin JC, Delorey M, et al. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis 2015; 9:e0003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demanou M, Pouillot R, Grandadam M, et al. Evidence of dengue virus transmission and factors associated with the presence of anti-dengue virus antibodies in humans in three major towns in Cameroon. PLoS Negl Trop Dis 2014; 8:e2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiter P, Lathrop S, Bunning M, et al. Texas lifestyle limits transmission of dengue virus. Emerg Infect Dis 2003; 9:86–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waterman SH, Novak RJ, Sather GE, Bailey RE, Rios J, Gubler DJ. Dengue transmission in two Puerto Rican communities in 1982. Am J Trop Med Hyg 1985; 34:625–32. [DOI] [PubMed] [Google Scholar]
- 37.Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol 2006; 43:484–92. [DOI] [PubMed] [Google Scholar]
- 38.Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol 2008; 22:62–9. [DOI] [PubMed] [Google Scholar]
- 39.Barrera R, Amador M, MacKay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis 2011; 5:el378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anders KL, Nga le H, Thuy NT, et al. Households as foci for dengue transmission in highly urban Vietnam. PLoS Negl Trop Dis 2015; 9:e0003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharp TM, Hunsperger E, Santiago GA, et al. Virus-specific differences in rates of disease during the 2010 Dengue epidemic in Puerto Rico. PLoS Negl Trop Dis 2013; 7:e2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrows N], Campos RK, Powell ST, et al. A Screen of FDAApproved Drugs for Inhibitors of Zika Virus Infection. Cell Host Microbe 2016; 20:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharp TM, Hunsperger E, Munoz-Jordan JL, Margolis HS, Tomashek KM. Sequential episodes of dengue—Puerto Rico, 2005–2010. Am J Trop Med Hyg 2014; 91:235–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang WW, Young MP, Mamidi A, Regla-Nava JA, Kim K, Shresta S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep 2016; 17:3091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. When to test for Zika virus, https://www.cdc.gov/zika/pdfs/when-totest-zika.pdf. Accessed 26 May 2017. 2016
- 46.Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


