Summary
Background
Transgender women (TW) in Peru are disproportionately affected by HIV. The role that cisgender men who have sex with TW (MSTW) and their sexual networks play in TW's risk of acquiring HIV is not well understood. We used HIV sequences from TW, MSTW, and cisgender men who have sex with men (MSM) to examine transmission dynamics between these groups.
Methods
We used HIV-1 pol sequences and epidemiologic data collected through three Lima-based studies from 2013 to 2018 (n = 139 TW, n = 25 MSTW, n = 303 MSM). We identified molecular clusters based on pairwise genetic distance and used structured coalescent phylodynamic modeling to estimate transmission patterns between groups.
Findings
Among 200 participants (43%) found in 62 clusters, the probability of clustering did not differ by group. Both MSM and TW were more likely to cluster with members of their own group than would be expected based on random mixing. Phylodynamic modeling estimated that there was frequent transmission from MSTW to TW (67·9% of transmission from MSTW; 95%CI = 52·8–83·2%) and from TW to MSTW (76·5% of transmissions from TW; 95%CI = 65·5–90·3%). HIV transmission between MSM and TW was estimated to comprise a small proportion of overall transmissions (4·9% of transmissions from MSM, and 11·8% of transmissions from TW), as were transmissions between MSM and MSTW (7·2% of transmissions from MSM, and 32·0% of transmissions from MSTW).
Interpretation
These results provide quantitative evidence that MSTW play an important role in TW's HIV vulnerability and that MSTW have an HIV transmission network that is largely distinct from MSM.
Funding
National Institutes of Health.
Research in context.
Evidence before this study
On November 20, 2020, we searched PubMed for molecular epidemiologic and phylodynamic research including transgender women and/or their partners using the terms “molecular epidemiology”, “phylogenetic”, or “phylodynamic” and “transgender”. Molecular epidemiology using HIV viral genetic sequences is now commonly used to identify HIV clusters and understand patterns of transmission. In approaches based on pairwise genetic similarity, sequences that cluster together are inferred to have epidemiological links, either between the individuals identified or through additional unidentified intermediaries. Analysing cluster membership can provide inference about sexual networks and potential factors associated with HIV transmission in local epidemics. In contrast, phylodynamic approaches aim to make inferences about epidemic dynamics from the topology of viral phylogenetic trees, which represent the evolutionary relationships between the sampled sequences. Both molecular epidemiology and phylodynamic analyses can be combined with epidemiologic data to identify patterns in HIV transmission that may not be evident using traditional epidemiologic methods alone. Despite a high burden of HIV among transgender women in many settings globally, only two studies to date, both in California, United States, have used molecular epidemiology to better understand transmission dynamics among transgender women and their sexual networks. Large scale reconstruction of HIV transmission networks is possible in some high-income settings using public health surveillance data from HIV drug resistance testing. In low- or middle-income countries where drug resistance testing is not routinely performed, sample collection and HIV sequencing can be difficult and costly.
Added value of this study
We used HIV sequences and epidemiologic data collected from transgender women, cisgender men who have sex with transgender women (MSTW), and cisgender men who have sex with men (MSM) in three research studies in Lima, Peru, to examine patterns of HIV transmission. This study was the first to include self-identified MSTW in molecular epidemiologic analyses. We examined patterns of clustering and transmission flows within and between groups to quantify the relationship between the HIV transmission networks of MSTW, transgender women, and MSM. We found that MSTW and transgender women clustered with MSM less frequently than would be expected given random mixing. Further, phylodynamic modeling estimated that HIV transmission between MSTW or transgender women and MSM accounted for a relatively small proportion of transmission events, with transgender women most frequently acquiring HIV from MSTW. Our findings suggest that transgender women and their sexual partners are a largely distinct sexual network. Some overlap with MSM sexual networks was evident in this analysis but may have been overestimated due to biased sampling and inclusion criteria for our study population, which preferentially selected MSTW with cisgender male partners.
Implications of all the available evidence
Few HIV interventions have been implemented specifically for transgender people and their sexual partners to prevent or treat HIV, despite high prevalence. Understanding the transmission dynamics of key populations is critical to intervention efforts, including transmission modeling, targeted recruitment, and implementation. Available evidence suggests that cisgender MSTW are a population that may not be adequately measured or reached by current HIV research and public health practice.
Alt-text: Unlabelled box
Introduction
Transgender women are disproportionally impacted by HIV, with an estimated global prevalence of 19%.1 A robust body of literature documents the structural factors rooted in stigma and discrimination that increase transgender women's vulnerability to HIV.2,3 However, little is known about the men who have sex with transgender women (MSTW) and their role in transgender women's HIV acquisition risk. Prior research suggests that in the Americas, the majority of MSTW are cisgender (i.e. non-transgender) men who identify as heterosexual or bisexual.4, 5, 6, 7, 8 Few studies provide data on sexual behavior among MSTW, but these studies report a high HIV prevalence and few cisgender male partners among MSTW.5,7 These studies also highlight how HIV-related syndemic factors among cisgender men with transgender partners—including stigma, sexual practices (condomless sex and concurrent partnerships), substance use, and mental health-may increase transgender women's risk of HIV acquisition.7, 8, 9 However, these findings may not be generalizable because data on MSTW originate from studies conducted among transgender women reporting on male partners,7 or from small qualitative studies.4 In addition, MSTW are frequently aggregated with men who have sex with men (MSM) and thus the limited data collected directly from MSTW come from convenience samples of cisgender MSM.5,6
Appropriately defining sub-populations who are impacted by HIV and understanding transmission within and between these populations is critical for the development of targeted and combination HIV interventions (e.g., PrEP, HIV testing and linkage to care, and treatment as prevention) as well as mathematical models to evaluate them. Molecular epidemiology and phylodynamic methods using HIV viral genetic sequences can serve as tools to better understand transmission dynamics between populations.10 Measuring genetic distance between HIV viral sequences allows for the identification of clusters of sequences that are closely related, which are presumed to represent relatively recent chains of HIV transmission. Analysing cluster membership can provide inference about factors potentially associated with transmission in local epidemics.11 Phylodynamic methods can also be used to make inferences about epidemic dynamics based on the topology of phylogenetic trees, which represent the evolutionary relationship between HIV lineages. In particular, recently developed phylodynamic approaches can be used to estimate HIV transmission rates and transmission flows between sub-populations, which are requisite inputs for mathematical models of HIV transmission.12
The objective of this analysis was to use molecular epidemiology and phylodynamics to better understand HIV transmission dynamics among transgender women, MSTW, and MSM in Lima, Peru. We used HIV sequences from blood samples collected in research settings to examine if transgender women and cisgender men (both MSM and MSTW) are found in the same viral clusters, and to identify characteristics associated with clustering. Further, we estimated transmission rates and the probability of transmission between subgroups, to better understand the role sexual networks may play in transgender women's vulnerability to HIV acquisition.
Methods
Genetic data source
Data were collected from HIV-seropositive participants enrolled in three research studies conducted in Lima, Peru from 2013 to 2018. The Sabes study, which accounts for 78% of participants in this analysis, has been previously described.13 Briefly, Sabes enrolled cisgender MSM and transgender women who did not know their HIV serostatus and were at elevated risk for HIV due to participation in sex work, having an HIV-positive sex partner, or having a sexually transmitted infection in the past six months. This analysis includes 106 participants who were HIV-seropositive at baseline and 261 participants with incident HIV during study follow up. This analysis also included 40 participants (n = 37 HIV-seropositive at baseline, n = 3 seroconverted during follow up) from the Feminas study, which tested an integrated care package combining HIV services, social support, and provision of feminizing hormones among transgender women in Lima.14 Finally, 63 HIV-seropositive MSM, transgender women, and MSTW are included from the Microepidemics study, which sought to identify hotspots for incident HIV in Lima using geospatial and phylogenetic data and HIV point-of-care testing at social venues (i.e., bars, saunas). Male sex assigned at birth and reporting sex with a cisgender man or transgender woman were inclusion criteria for all studies.
Sabes and Microepidemics obtained informed consent for study samples to be used for phylogenetic analyses, and were both approved by the institutional review boards (IRB) at la Asociación Civil Impacta Salud y Educación (IMPACTA; Lima) and the Fred Hutchinson Cancer Research Center (Seattle). Feminas obtained informed consent for the use of study samples for future research and was approved by the IMPACTA IRB.
This analysis includes plasma samples collected ≤6 months after HIV acquisition (“early diagnoses” defined through incident infection during study follow-up) and from prevalent cases (samples collected >6 months after presumed HIV acquisition or for which no data were available about date of HIV acquisition; see Supplemental Content for further details). If a study collected multiple samples, or a participant enrolled in more than one study (n = 8), the first available sample was included in the analysis.
Epidemiologic data source
Viral sequences were linked to baseline epidemiologic data in the Sabes and Feminas studies (questionnaire data were not collected in Microepidemics). Both studies administered baseline questionnaires assessing demographics, gender and sexual identity, sexual behavior, and substance use using questions with nearly or fully identical wording between the two studies.
Groups of interest for this analysis include transgender women, cisgender MSM, and cisgender MSTW. Definitions used for gender identities differed slightly in the three studies; for this analysis, all participants were categorized based on their identity in their respective parent study. In Sabes and Microepidemics, transgender women self-identified as transgender on a single gender-identity question. Feminas used a two-step method to assess both sex assigned at birth and current gender identity.15 MSM in Sabes were defined as eligible participants who did not identify as transgender and did not report sex with transgender women. In Microepidemics, MSM were defined as cisgender men who reported cisgender male partners. Sabes defined MSTW as participants who reported a transgender woman as one of three most recent sexual partners in the last 3 months. Microepidemics defined MSTW as anyone who self-reported having a transgender woman partner.
Molecular cluster analyses
Derivation of the viral genome sequences used for these analyses were described previously.16 The MAFFT algorithm was used for sequence alignment using the HXB2 sequence as reference (length = ∼700 nucleotides; from amino acid positions 87–99 of protease and 1–220 of reverse transcriptase, relative to HXB2).17 The alignment also included all available South American HIV pol sequences from the Los Alamos National Laboratory (LANL) HIV Sequence Database (n = 552; available from www.hiv.lanl.gov). Molecular clusters were defined as ≥ 2 sequences with Tamura-Nei (TN93) pairwise distance below a genetic distance threshold of 1·5%. Sensitivity analyses were conducted using a less conservative threshold of 3% (Supplemental Content).
Data from all three cohorts were used to assess the relationship between clustering and diagnosis year, study, demographic group, and early/prevalent HIV. Analyses of correlates were conducted using data from Sabes and Feminas, while predictors of clustering with transgender women were assessed using Sabes data only. We used univariate Poisson regression with robust standard errors to calculate unadjusted prevalence ratios with 95% confidence intervals (CI) and p-values (α = 0·05) associated with being in a cluster. Co-clustering by the demographic group was assessed by comparing observed clustering patterns to an expected null distribution based on the relative representation of each group in the study sample. Groups that are better represented in the sample (i.e., MSM) are more likely to be found in clusters because they comprise a large proportion of the overall sample. We generated null distributions assuming random mixing by randomly permuting group labels (N = 10,000 iterations) and estimating p-values based on the resulting empirical distribution curves.
Phylodynamic analyses
To infer transmission rates and flows among transgender women, MSTW, and MSM we applied a structured coalescent modeling approach,18 a model-based approach to phylodynamic inference that uses a time-calibrated phylogeny to estimate the parameters of standard deterministic mathematical models that describe population-level HIV transmission patterns. Our epidemic model included the three demographic groups of interest and a source compartment of South American LANL sequences representing the regional reservoir of HIV. Due to the relatively small sample size and limited data on CD4 counts at sampling, our model only includes one stage of disease progression. We modeled transmission between groups as:
Where , and are HIV transmission rates per person-year for transgender women, MSTW, and MSM, respectively (Supplemental Content). The proportion of overall transmissions between demographic groups is parameterized by the proportion of transmissions from one group to another, for example, the proportion of transmission from transgender women to MSM (We constrained the proportion of transmissions to equal one, and therefore do not explicitly estimate within-group probabilities (i.e., , and ). We chose this parameterization because we were primarily interested in between-group transmission patterns. Using previously described methodology,12 we modeled a regional reservoir as having a constant effective population size.
Phylodynamic analyses were conducted using the phydynR package in R, which was used to calculate the likelihood of a parameter set given the phylogeny and the model compartment to which each sequence belonged. We first estimated a time-calibrated phylogeny using the date of sequence sampling and the treedater algorithm assuming a strict molecular clock.19 We then fit the mathematical model to the time-calibrated phylogeny using a Bayesian approach using the BayesianTools package in R. Model parameters were estimated using a differential evolution Markov Chain Monte Carlo (MCMC) zs sampler (N = 100,000 iterations). We report the maximum aposteriori value (MAP)—-or mode-and the 95% credible interval (CI) of the resulting posterior distribution of each model parameter. Prior distributions based on a review of the literature were used to constrain the parameters (Supplementary Table 6), and we conducted sensitivity analyses using non-informative priors (Supplemental Content). Model convergence was evaluated based on effective sample size, Gelman-Rubin's diagnostic for convergence using potential scale reduction factors, and trace plots. All analyses were conducted in R v3·6·2 and Stata v15·1 (College Station, TX, USA, 2017).
Role of the funding source
The study funders had no role in study design, data collection, analysis, interpretation, or manuscript preparation. The corresponding authors had full access to all study data and final responsibility for the decision to submit for publication.
Results
Cluster membership
This analysis included 139 transgender women, 25 cisgender MSTW, and 303 cisgender MSM (N = 467). Overall, 200 (42·8%) participants clustered into 62 genetic clusters, which ranged in size from 2 to 27 study participants. Among the clusters identified, 48% included transgender women, 79% contained cisgender MSM, and 15% contained cisgender MSTW. The likelihood of appearing in a cluster did not differ by demographic group (Table 1). We found no differences in the likelihood of clustering by study, diagnosis year, or whether the HIV diagnosis was early or presumed prevalent. In analysing possible demographic or behavioral predictors of being found in a cluster, no observed characteristics were associated with cluster membership (Table 1).
Table 1.
Univariate predictors of cluster membership (N = 467).
| Characteristica | N | Clustered | |||
|---|---|---|---|---|---|
| n (%) | PR | 95% CI | p-value | ||
| Diagnosis year | 467 | 200 (42·8) | 0·98 | (0·90, 1·06) | 0·555 |
| Study | |||||
| Sabes | 364 | 157 (43·1) | ref | ||
| Feminas | 40 | 19 (47·5) | 1·10 | (0·78, 1·56) | 0·586 |
| Microepidemics | 63 | 24 (38·1) | 0·88 | (0·63, 1·23) | 0·470 |
| Group | |||||
| Cisgender MSM | 303 | 134 (44·2) | ref | ||
| Transgender Women | 139 | 56 (40·3) | 0·91 | (0·72, 1·16) | 0·444 |
| Cisgender MSTW | 25 | 10 (40·0) | 0·90 | (0·55, 1·49) | 0·692 |
| HIV Diagnosis | |||||
| Prevalent | 165 | 67 (40·6) | ref | ||
| Early (< 6 months) | 302 | 133 (44·0) | 1·08 | (0·87, 1·36) | 0·478 |
| Cityb | |||||
| Lima | 342 | 151 (44·2) | ref | ||
| Callao | 56 | 22 (39·3) | 0·89 | (0·63, 1·26) | 0·510 |
| Age categoryc: | |||||
| < 25 | 196 | 89 (45·4) | ref | ||
| 25–34 | 155 | 66 (42·6) | 0·94 | (0·74, 1·19) | 0·598 |
| ≥ 35 | 38 | 15 (39.5) | 0·87 | (0·57, 1·33) | 0·516 |
| Any post-secondary schoold | |||||
| No | 158 | 68 (43.0) | ref | ||
| Yes | 245 | 107 (43.7) | 1·01 | (0·81, 1·28) | 0·900 |
| Sexual Orientatione | |||||
| Gay | 193 | 92 (47·7) | ref | ||
| Bisexual | 91 | 38 (41·8) | 0·88 | (0·66, 1·16) | 0·362 |
| Heterosexual | 10 | 6 (60·0) | 1·26 | (0·74, 2·13) | 0·393 |
| Housing statusf | |||||
| Own place/alone | 88 | 37 (42·1) | ref | ||
| With sexual partner | 44 | 20 (45·5) | 1·08 | (0·72, 1·62) | 0·707 |
| With parent or family | 222 | 98 (44·1) | 1·05 | (0·79, 1·40) | 0·739 |
| With friend | 30 | 11 (36·7) | 0·87 | (0·51, 1·48) | 0·613 |
| Sexual roleg | |||||
| Insertive | 53 | 22 (41·5) | ref | ||
| Receptive | 161 | 68 (42·2) | 1·02 | (0·70, 1·47) | 0·926 |
| Versatile | 185 | 82 (44·3) | 1·07 | (0·75, 1·53) | 0·720 |
| Sex workerh | |||||
| No | 308 | 132 (42·9) | ref | ||
| Yes | 79 | 32 (40·5) | 0·95 | (0·70, 1·27) | 0·710 |
| Gender of partners reportedi | |||||
| Cisgender man | 231 | 198 (42·4) | 0·98 | (0·80, 1·21) | 0·862 |
| Transgender woman | 16 | 7 (43·8) | 1·02 | (0·58, 1·80) | 0·939 |
| Cisgender woman | 7 | 0 (0·0) | N/A | ||
| Reported partnership typei | |||||
| Stable/spouse | 151 | 68 (45·0) | 1·05 | (0·84, 1·32) | 0·644 |
| Casual | 66 | 28 (42·4) | 0·97 | (0·71, 1·32) | 0·840 |
| One time/anonymous | 102 | 42 (41·2) | 0·93 | (0·71, 1·21) | 0·580 |
| Sold/clientj | 19 | 6 (31·6) | 0·72 | (0·37, 1·40) | 0·328 |
| Purchasedj | 9 | 4 (44·4) | 1·02 | (0·49, 2·14) | 0·957 |
PR: prevalence ratio; CI: confidence interval; MSM: men who have sex with men; TW: transgender women; MSTW: partners of transgender women.
Year of diagnosis, study, group, and HIV diagnosis (early vs prevalent) are reported for all three studies. All other data is reported from Sabes and Feminas.
City data missing for n = 5 Feminas participants and n = 1 Sabes participant.
Age data missing for n = 1 Feminas participant and n = 14 Sabes participants.
Education data missing for n = 1 Feminas participant. Education defined as any post-secondary or vocational training.
Sexual orientation data was not collected among TW in the Sabes study (n = 82). In Feminas, n = 28 TW identified their sexual orientation as transgender and are counted as missing for this analysis.
Housing status data missing for n = 6 Feminas participants and n = 14 Sabes participants.
Sex role data missing for n = 5 Feminas participants.
Sex worker data missing for n = 15 Feminas participants and n = 2 Sabes participants.
Partnership data reported from the last three sexual partners, beginning with the most recent.
Purchasing and selling sex defined as exchange goods, services, a place to sleep, or money for sex. Sold/client refers to encounters in which the participant acquired goods, services, or money, while purchased refers to encounters in which the participant gave goods, services, or money.
Clustering between demographic groups
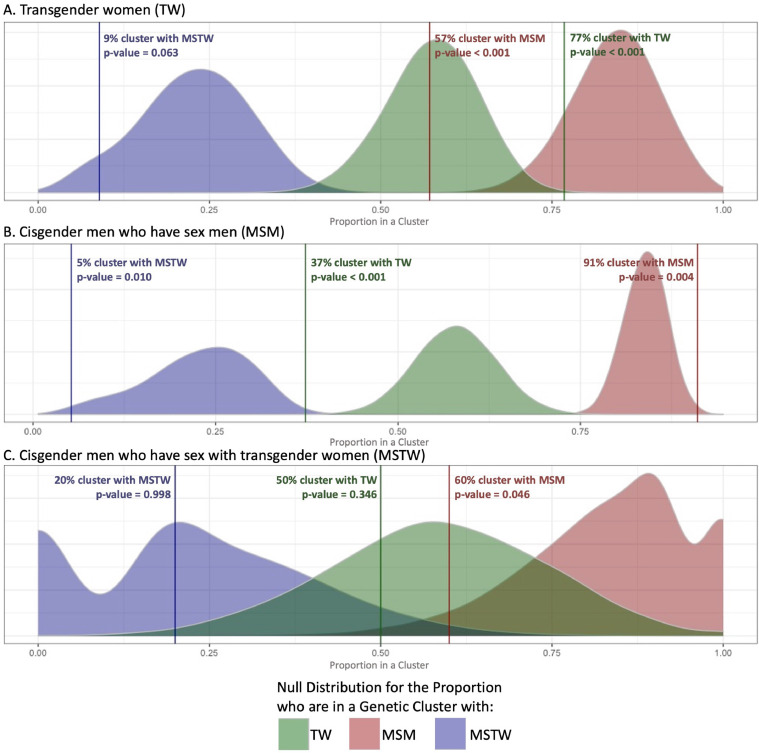
The likelihood of clustering with transgender women, MSM, and MSTW was assessed for each demographic group (Fig. 1). Among those found in clusters, 77% of transgender women clustered with other transgender women, while 57% clustered with MSM. Compared to a null distribution based on the relative representation of each group, the observed results suggest transgender women clustered with MSM significantly less often than expected (p < 0·001) and clustered with other transgender women more often than expected (p < 0·001) (Fig. 1A). Cisgender MSM, however, clustered with other MSM more often than expected (91% clustered with MSM, p = 0·004) and less often than expected with transgender women (37%, p < 0·001) and MSTW (5%, p = 0·010) (Fig. 1B). The likelihood that cisgender MSTW clustered with any group was difficult to ascertain due to the small sample size, but analyses suggested that MSTW clustered with MSM less often than expected (60%, p = 0·046) (Fig. 1C).
Fig. 1.
Null distribution and empirical p-values for the proportion who cluster with transgender women (TW), MSTW, and MSM. Panels compare the observed clustering patterns of each group to a null distribution of what would be expected under the assumption of random mixing. Curves demonstrate the null distribution, with purple curves representing expected clustering with MSTW, green curves representing expected clustering with MSM, and red curves representing the expected clustering with transgender women. Lines show the actual observed clustering with each respective group, with p-values estimated based on the resulting empirical distribution curves. Panel A demonstrates observed vs expected clustering patterns for transgender women. The observed clustering of transgender women with MSTW falls on the left tail of the expected curve, showing slightly less clustering than would be expected. Clustering with MSM however is much less likely than would be expected, with almost no overlap with the expected curve; similarly, clustering with other transgender woman was much more likely than would be expected. In Panel B, we see that MSM clustered with transgender women and MSTW less likely than would be expected, and clustered with other MSM more than would be expected. Due to small the small sample of MSTW, curves in Panel C are wider, but these data suggest that MSTW cluster with MSM less often than would be expected based on random mixing.
Predictors of clustering with transgender women
When examining characteristics among all cisgender men (MSM and MSTW) that may be associated with being in a cluster with one or more transgender women, we found that while the data suggests some trends, no statistically significant associations were evident (Table 2). The results did show some evidence that men who identify as bisexual were more likely to cluster with transgender women compared to those who identify as gay (PR = 1·52, 95% CI = 0·98–2·35). Results were similar in sensitivity analyses using a less conservative distance threshold (see Supplemental Content).
Table 2.
Correlates of clustering with TW among all cisgender men (n = 144).
| Characteristics | N | TW in clustera | PR | 95% CI | P-value | |
|---|---|---|---|---|---|---|
| n | (%) | |||||
| Reported TW partner | ||||||
| No | 134 | 50 | (37·3) | 1 | ||
| Yes | 10 | 5 | (50·0) | 1·34 | (0·69, 2·59) | 0·385 |
| Sexual Orientation | ||||||
| Gay | 88 | 29 | (33·0) | 1 | ||
| Bisexual | 38 | 19 | (50·0) | 1·52 | (0·98, 2·35) | 0·062 |
| Heterosexual | 3 | 1 | (33·3) | 1·01 | (0·20, 5·18) | 0·989 |
| HIV diagnosis | ||||||
| Presumed prevalent | 28 | 13 | (46·4) | 1 | ||
| Early (< 6 months) | 116 | 42 | (36·2) | 0·78 | (0·49, 1·24) | 0·297 |
| Age category | ||||||
| < 25 | 66 | 24 | (36·4) | 1 | ||
| ≥ 25 | 78 | 31 | (39·7) | 1·09 | (0·72, 1·67) | 0·679 |
| Any post-secondaryb education | ||||||
| No | 40 | 18 | (45·0) | 1 | ||
| Yes | 89 | 31 | (34·8) | 0·77 | (0·50, 1·21) | 0·261 |
| Sexual role | ||||||
| Insertive | 21 | 9 | (42·9) | 1 | ||
| Receptive | 34 | 12 | (35·3) | 0·82 | (0·42, 1·62) | 0·572 |
| Versatile | 74 | 28 | (37·8) | 0·88 | (0·50, 1·57) | 0·672 |
| Purchased sexc (6 months) | ||||||
| No | 109 | 42 | (38·5) | 1 | ||
| Yes | 18 | 7 | (38·9) | 1·01 | (0·54, 1·89) | 0·977 |
| Sold sexc (6 months) | ||||||
| No | 99 | 38 | (38·4) | 1 | ||
| Yes | 28 | 11 | (39·3) | 1·02 | (0·60, 1·73) | 0·931 |
TW: transgender women; PR: prevalence ratio; CI: confidence interval.
Data on reporting a TW partner and HIV diagnosis are from both Sabes (n = 129) and Microepidemics (n = 15) participants. All other variables include cisgender men from the Sabes study. TW found in the cluster could be from Sabes, Feminas, or Microepidemics.
Post secondary education defined as any school after secondary school, or vocational training.
Purchasing and selling sex defined as exchange goods, services, a place to sleep, or money for sex. Data on purchased and sold sex missing for n = 2 Sabes participants.
Estimated HIV transmission between groups
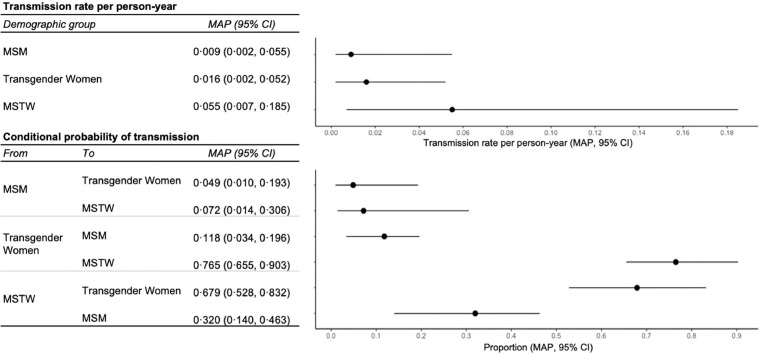
Phylodynamic modeling produced estimates for model parameters that describe the overall proportion of transmissions from one group to another. We estimated that there was frequent transmission between transgender women and MSTW and that the majority of transmissions from MSTW were to transgender women (67·9%; 95% CI = 52·8–83·2%; Fig. 2). Transgender women also had a higher probability of transmission to MSTW (76·5%; 95% CI = 65·5–90·3%) than MSM (11·8%; 95% CI = 3·4–19·6%). There was a low probability of transmission from MSM to transgender women (4·9%; 95% CI: 1·0–19·5%) or MSTW (7·2%; 95% CI = 1·4–30·6%). Approximately a third of transmissions from MSTW were to MSM (32·0%; 95% CI = 14·0–46·3%). We estimated that the HIV transmission rate varied slightly between groups, though confidence intervals largely overlapped (transgender women: 1·6, 95% CI = 0·2–5·2; MSM: 0·9, 95% CI = 0·2–5·5; and MSTW: 5·5, 95% CI = 0·7–18·5 per 100 person-years; Fig. 2). Sensitivity analyses using non-informative priors obtained similar estimates (see Supplemental Content).
Fig. 2.
Parameter estimates for the structured coalescent phylodynamic model of HIV transmission. This figure shows maximum aposteriori (MAP) and credible intervals (CI) for the Bayesian parameter estimates for the transmission rates per person-year as well as conditional probabilities of transmission across demographic groups: transgender women, cisgender men who have sex with transgender women (MSTW), and cisgender men who have sex with men (MSM).
Discussion
This analysis used complementary methods for analysing HIV sequences to provide insight into transmission dynamics among three distinct populations with high HIV prevalence in Peru. Cluster analyses suggested that both transgender women and cisgender MSTW cluster with cisgender MSM less frequently than would be expected based on random mixing, with evidence suggesting men with bisexual identities are more likely to cluster with transgender women compared to men who self-identify as gay. Phylodynamic modeling results similarly suggested that a small proportion of transmission events occurred between transgender women or MSTW and MSM, and that transgender women were more likely to transmit to cisgender MSTW (77%) than cisgender MSM (12%). Moreover, modeling suggested that 68% of transmissions from MSTW were acquired by transgender women, compared to only 5% of transmissions from MSM. We also observed less frequent transmission between MSTW and MSM. Notably, we obtained similar inferences from cluster-based and phylodynamic approaches with regards to transmission patterns among transgender women and MSTW.
Our findings have several important implications. First, they suggest that transgender women and their cisgender male partners (MSTW) comprise a largely separate HIV transmission network, with a small degree of overlap with that of cisgender MSM. Specifically, they indicate that transgender women are significantly more likely to acquire HIV from MSTW compared to MSM. Qualitative research has previously asserted that transgender women and cisgender MSM have distinct sexual networks due to differing structural factors that influence HIV vulnerability.20 Research conducted among MSTW in the Americas suggests that cisgender men represent a relatively small proportion of sexual partners of MSTW4,5,7,21 ranging from 21% in San Francisco7 to 7% in Lima.22 A Lima-based study reported that MSTW most frequently reported sexual attraction to transgender (84%) and cisgender (68%) women, and few reported attraction to cisgender men (7%).22 Similarly, in the Sabes study, MSTW reported primarily transgender and cisgender women partners, with fewer than 20% reporting male partners.21 Our results add quantitative evidence to this growing body of research and further suggest that there is little overlap in the HIV transmission networks of MSM and MSTW. These findings underscore that cisgender MSM constitute a small proportion of the sexual network of transgender women, and relationships between MSM and transgender women are unlikely to play a key role in HIV transmission in either sexual network. The clustering patterns between cisgender MSM and transgender women we observed align with recent research conducted in Los Angeles County using sequences collected through the Los Angeles Department of Public Health for drug resistance testing.23 That analysis found that transgender women were likely to cluster with MSM, but had lower odds of clustering with MSM than would be expected based on sample distribution. While data on MSTW were not included, they found that cisgender men not categorized as MSM were more likely to be found in a cluster with transgender women compared to MSM. A similar phylogenetic analysis in San Francisco that found a high likelihood of clustering between transgender women and MSM called into question the categorization of MSM in public health surveillance data, suggesting that men may inaccurately be labelled MSM for having sexual partnerships with transgender women.24 Disaggregation of MSTW from MSM, as we did in the present analysis, would provide clearer insight into prevention and treatment opportunities for these populations.
Second, these findings have important implications for modeling the impact and cost-effectiveness of HIV interventions in Peru. To date, only six mathematical models of HIV transmission have included transgender women, including five set in Lima (Supplemental Content). Two of these models do not disaggregate transgender women from cisgender men; three models separately consider MSM, men who have sex with women, and transgender women; and two exclusively model transmission between transgender women engaged in sex work and MSTW. Our findings suggest that cisgender MSTW should be included as a high-prevalence population separate from cisgender MSM and men who have sex with men and cisgender women, in addition to disaggregating transgender women from MSM. Failing to do so may obscure transmission patterns and potentially different impacts of interventions within these distinct populations.
Third, we found that the transgender women in our sample frequently appear in clusters with other transgender women (77%, p-value < 0·001). Although US-based studies have found that transgender people frequently have other transgender partners,25 few studies have documented this within Latin America. Previous analyses of the Sabes cohort show that only 2·1% of transgender women reported having sex with another transgender woman in the last 3 months, and 0·7% reported sex with a cisgender woman.21 A combined analysis of participants from Feminas and the TransPrEP study found that among 1167 partners reported by 389 transgender women, 91·7% (n = 1070) were cisgender men and 8·3% (n = 97) were another gender.26 Therefore, it is possible that our results can be partially explained by under-sampling of MSTW who may act as intermediaries in HIV transmission events between transgender women (i.e., transgender woman cluster together because they share the same, unsampled partners). Alternatively, the lack of data on the diversity of the gender of sexual partners of transgender women in existing studies may reflect researcher assumptions that transgender women only partner with cisgender men, or be a by-product of biased HIV study inclusion criteria, which only enroll participants who report having sex with cisgender men (e.g., the Sabes study).20,27 Further studies are necessary to provide context for these findings and explore transgender women partnerships, including those with other transgender women.
The cultural context in Peru is important to understand the patterns of HIV transmission revealed by this study. Stigma and discrimination against transgender communities make them disproportionately vulnerable to HIV due to lack of legal protections and access to medical care, reliance on sex work due to economic disempowerment, experiences of violence, power imbalances in sexual relationships, and substance use.2,3 Previous research among transgender women in Peru has found a 20–30% HIV prevalence28,29 while two-thirds reported exchanging sex for money or goods,29 and nearly 80% reported recent condomless receptive anal intercourse.28 Among MSTW, current research in Latin America suggest that while participation in sex work and reported condomless anal intercourse are high,21 both HIV risk perception and PrEP awareness are low,5,30,31 highlighting the need for targeted prevention strategies to reach these groups.
This study had several strengths. This is the first molecular epidemiologic study that disaggregates MSTW from MSM. The majority of samples were collected during early infection - before the virus had accumulated substantial genetic divergence – and, thus, were more likely to reveal recent transmission events.10 Additionally, there are valid concerns about the ethics of HIV molecular epidemiology, centered on issues of informed consent, stigma, and criminalization of HIV transmission.32 The majority of our viral sequence and linked behavioral and epidemiological data were obtained with explicit informed consent to conduct phylogenetic research.
Our results should be interpreted in light of several limitations. This sample included a small fraction of the target populations, which makes cluster detection difficult ,11 reduces power in statistical analysis, and limits inference, particularly relating to the role of MSTW. More research is needed with larger sampling fractions and more representative study populations, including MSTW not recruited from studies primarily focused on MSM, transgender men, non-binary people, and cisgender women. Second, clustering can be biased by time from infection to sampling and differing sampling fractions of subgroups.18 However, our analysis found no statistical difference in clustering by demographic group despite differences in subgroup sample sizes. Third, our analyses using linked epidemiologic data did not include data from participants in the Microepidemics study, includes only study baseline data, and did not capture syndemic factors that may be related to transgender women's risk of HIV acquisition (e.g., gender affirmation, stigma, intimate partner violence, harassment, and discrimination) or other likely correlates of clustering (e.g., concurrency), so we were unable to assess if these factors were associated with clustering. Fourth, structured coalescent modeling assumes random sampling of HIV sequences, although this method has been previously applied to study populations recruited through chain-referral.33 Thus, our findings may be less generalizable to other populations due to violations of this assumption in study recruitment. Data for MSTW and transgender women primarily came from Sabes, which recruited using outreach methods that targeted MSM. Therefore, our sample of MSTW and transgender women may be biased towards those closely identified with MSM social networks and with cisgender male partners, and we likely overestimated the proportion of transmission with MSM. Finally, the validity of our phylodynamic modeling results is conditional on the correct specification of the mathematical model; because our model did not include other gender groups (e.g., cisgender women, transgender men) and used a single stage of disease progression, our estimates should be interpreted with caution.
In summary, these results build on the growing use of molecular epidemiology to study transmission dynamics within and between subpopulations. Our findings provide unique insight on the HIV epidemic among cisgender men with transgender women partners and further highlights the need for more data on this population. Understanding transmission patterns that include MSTW is crucial for modeling, designing, and implementing effective HIV prevention and treatment interventions.
CRediT authorship contribution statement
Jessica E. Long: Visualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Diana M. Tordoff: Visualization, Methodology, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. Sari L. Reisner: Conceptualization, Data curation, Writing – review & editing. Sayan Dasgupta: Formal analysis, Data curation, Writing – review & editing. James I. Mullins: . Javier R. Lama: Visualization, Methodology, Conceptualization, Data curation, Writing – review & editing. Joshua T. Herbeck: Visualization, Methodology, Formal analysis, Data curation, Conceptualization, Writing – original draft, Writing – review & editing. Ann Duerr: Visualization, Methodology, Conceptualization, Data curation, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest. AD, JEL, and JTH received funding from the National Institutes of Health that supported this work.
Acknowledgments
Acknowledgments
We would like to thank the participants in this study, and acknowledge the contribution of the staff at Impacta, Epicentro, and Féminas Perú.
Funding
This work was funded by the NIH National Institute on Drug Abuse, through a Sexual and Gender Minorities Administrative Supplementary (3R01DA040532-03S2) to Dr Ann Duerr's R01 (R01 Grant DA032106), by NIH Research Training Grant #D43 TW009345 awarded to the Northern Pacific Global Health Fellows Program by the Fogarty International Center (Fellow: Jessica Long), by the Boeing International Fellowship awarded to Jessica Long, and by an NIH National Institute of Allergy and Infectious Disease grant awarded to Joshua Herbeck and Roxanne Kerani (R01AI127232).
Data sharing
All HIV genetic sequences have been deposited into GenBank (accession number OK647960 - OK648429). Demographic and Epidemiologic data were linked to sequences through a secure dataset managed by the HIV Outcomes, Prevention and Epidemiology (HOPE) Group at the Fred Hutchinson Cancer Research Center. De-identified data could be made available upon request at the discretion of the authors; requests can be sent to the corresponding author. Code for the structured coalescent modeling can be found at https://github.com/dianatordoff/LimaCoalescent.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lana.2021.100121.
Contributor Information
Jessica E. Long, Email: jesslong@uw.edu.
Diana M. Tordoff, Email: dtordoff@uw.edu.
Appendix. Supplementary materials
References
- 1.Baral S.D., Poteat T., Strömdahl S., Wirtz A.L., Guadamuz T.E., Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):214–222. doi: 10.1016/S1473-3099(12)70315-8. Mar. [DOI] [PubMed] [Google Scholar]
- 2.Poteat T., Reisner S.L., Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS. 2014;9(2):168–173. doi: 10.1097/COH.0000000000000030. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wesp L.M., Malcoe L.H., Elliott A., Poteat T. Intersectionality research for transgender health justice: a theory-driven conceptual framework for structural analysis of transgender health inequities. Transgend Health. 2019;4(1):287–296. doi: 10.1089/trgh.2019.0039. Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Operario D., Burton J., Underhill K., Sevelius J. Men who have sex with transgender women: challenges to category-based HIV prevention. AIDS Behav. 2008;12(1):18–26. doi: 10.1007/s10461-007-9303-y. Jan. [DOI] [PubMed] [Google Scholar]
- 5.Reisner S.L., Perez-Brumer A., Oldenburg C.E., et al. Characterizing HIV risk among cisgender men in Latin America who report transgender women as sexual partners: HIV risk in Latin America men. Int J STD AIDS. 2019;30(4):378–385. doi: 10.1177/0956462418802687. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockting W., Miner M., Rosser B.R.S. Latino men's sexual behavior with transgender persons. Arch Sex Behav. 2007;36(6):778–786. doi: 10.1007/s10508-006-9133-4. Dec. [DOI] [PubMed] [Google Scholar]
- 7.Operario D., Nemoto T., Iwamoto M., Moore T. Risk for HIV and unprotected sexual behavior in male primary partners of transgender women. Arch Sex Behav. 2011;40(6):1255–1261. doi: 10.1007/s10508-011-9781-x. Dec. [DOI] [PubMed] [Google Scholar]
- 8.Poteat T., Malik M., Wirtz A.L., Cooney E.E., Reisner S. Understanding HIV risk and vulnerability among cisgender men with transgender partners. Lancet HIV. 2020 Mar;7(3):e201–e208. doi: 10.1016/S2352-3018(19)30346-7. [DOI] [PubMed] [Google Scholar]
- 9.Noor S.W.B., Wilkerson J.M., Schick V., Iantaffi A. Non-monosexual partnerships: information, motivation and self-efficacy among methamphetamine-using men who have sex with men who also have sex with women or transgender persons. Int J Sex Health. 2016;28(3):205–215. doi: 10.1080/19317611.2016.1168903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown A.E., Gifford R.J., Clewley J.P., et al. Phylogenetic reconstruction of transmission events from individuals with acute HIV infection: toward more-rigorous epidemiological definitions. J Infect Dis. 2009;199(3):427–431. doi: 10.1086/596049. Feb 1. [DOI] [PubMed] [Google Scholar]
- 11.Grabowski M.K., Herbeck J.T., Poon A.F.Y. Genetic cluster analysis for HIV prevention. Curr HIV AIDS Rep. 2018;15(2):182–189. doi: 10.1007/s11904-018-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento F.F., Baral S., Geidelberg L., et al. Phylodynamic analysis of HIV-1 subtypes B, C and CRF 02_AG in Senegal. Epidemics. 2020;30 doi: 10.1016/j.epidem.2019.100376. Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lama J.R., Brezak A., Dobbins J.G., et al. Design strategy of the sabes study: diagnosis and treatment of early HIV infection among men who have sex with men and transgender women in Lima, Peru, 2013–2017. Am J Epidemiol. 2018;187(8):1577–1585. doi: 10.1093/aje/kwy030. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lama J.R., Mayer K.H., Perez-Brumer A.G., et al. Integration of gender-affirming primary care and peer navigation with hiv prevention and treatment services to improve the health of transgender women: protocol for a prospective longitudinal cohort study. JMIR Res Protoc. 2019;8(6):e14091. doi: 10.2196/14091. Jun 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reisner S.L., Biello K., Rosenberger J.G., et al. Using a two-step method to measure transgender identity in Latin America/the Caribbean, Portugal, and Spain. Arch Sex Behav. 2014;43(8):1503–1514. doi: 10.1007/s10508-014-0314-2. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trebelcock W.L., Lama J.R., Duerr A., et al. HIV pretreatment drug resistance among cisgender MSM and transgender women from Lima, Peru. J Int AIDS Soc. 2019;22(11):e25411. doi: 10.1002/jia2.25411. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi: 10.1093/molbev/mst010. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volz E.M. Complex population dynamics and the coalescent under neutrality. Genetics. 2012;190(1):187–201. doi: 10.1534/genetics.111.134627. Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volz E.M., Frost S.D.W. Scalable relaxed clock phylogenetic dating. Virus Evol. 2017;3(2):vex025. Jul 1. [Google Scholar]
- 20.Sevelius J.M., Keatley J., Calma N., Arnold E. I am not a man”: trans-specific barriers and facilitators to PrEP acceptability among transgender women. Glob Public Health. 2016;11(7–8):1060–1075. doi: 10.1080/17441692.2016.1154085. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long J.E., Ulrich A., White E., et al. Characterizing men who have sex with transgender women in Lima, Peru: sexual behavior and partnership profiles. AIDS Behav. 2020;24(3):914–924. doi: 10.1007/s10461-019-02590-w. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long J.E., Sanchez H., Dasgupta S., Huerta L., Calderón Garcia D., Lama J.R., Duerr A. Exploring HIV risk behavior and sexual/gender identities among transgender women and their sexual partners in Peru using respondent-driven sampling. AIDS Care. 2021:1–9. doi: 10.1080/09540121.2021.1967855. Aug 23Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ragonnet-Cronin M., Hu Y.W., Morris S.R., Sheng Z., Poortinga K., Wertheim J.O. HIV transmission networks among transgender women in Los Angeles County, CA, USA: a phylogenetic analysis of surveillance data. Lancet HIV. 2019;6(3):e164–e172. doi: 10.1016/S2352-3018(18)30359-X. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truong H.H.M., et al. Proceedings of the 9th international AIDS society conference on HIV science. 2021. How are transwomen acquiring HIV? Insights from phylogenetic transmission clusters [Internet] 2017 Jul [cited 2017 Sep 21]; ParisAvailable from: http://programme.ias2017.org/Abstract/Abstract/3380. [Google Scholar]
- 25.Reisner S., Herman J., Bockting W., Krueger E., Meyer I. Measures of sexual orientation inclusive of transgender people: new tools for social epidemiological research. National Transgender Health; Summit. Oakland, CA: 2019. [Google Scholar]
- 26.Murphy E.C., Segura E.R., Lake J.E., et al. Intimate Partner violence against transgender women: prevalence and correlates in Lima, Peru (2016–2018) AIDS Behav. 2020;24(6):1743–1751. doi: 10.1007/s10461-019-02728-w. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poteat T., German D., Flynn C. The conflation of gender and sex: gaps and opportunities in HIV data among transgender women and MSM. Glob Public Health. 2016;11(7–8):835–848. doi: 10.1080/17441692.2015.1134615. Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long J.E., Montaño M., Cabello R., Sanchez H., Lama J.R., Duerr A. Brief report: comparing sexual risk behavior in a high-risk group of men who have sex with men and transgender women in Lima, Peru. J Acquir Immune Defic Syndr. 2019;80(5):522–526. doi: 10.1097/QAI.0000000000001966. Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva-Santisteban A., Raymond H.F., Salazar X., Villayzan J., Leon S., McFarland W., et al. Understanding the HIV/AIDS epidemic in transgender women of Lima, Peru: results from a sero-epidemiologic study using respondent driven sampling. AIDS Behav. 2012;16(4):872–881. doi: 10.1007/s10461-011-0053-5. May. [DOI] [PubMed] [Google Scholar]
- 30.Degtyar A., George P.E., Mallma P., et al. Sexual risk, behavior, and HIV testing and status among male and transgender women sex workers and their clients in Lima, Peru. Int J Sex Health. 2018;30(1):81–91. doi: 10.1080/19317611.2018.1429514. Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long J.E., Montaño M., Sanchez H., et al. Self-identity, beliefs, and behavior among men who have sex with transgender women: implications for HIV research and interventions. Arch Sex Behav. 2021;50(7):3287–3295. doi: 10.1007/s10508-021-02019-3. Oct 6[Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Molldrem S., Smith A.K.J. Reassessing the ethics of molecular HIV surveillance in the era of cluster detection and response: toward HIV data justice. Am J Bioeth. 2020;20(10):10–23. doi: 10.1080/15265161.2020.1806373. Oct 2. [DOI] [PubMed] [Google Scholar]
- 33.Volz E.M., Ndembi N., Nowak R., Kijak G.H., Idoko J., Dakum P., et al. Phylodynamic analysis to inform prevention efforts in mixed HIV epidemics. Virus Evol. 2017;3(2):vex014. doi: 10.1093/ve/vex014. Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.