Abstract
We characterized the prevalence, correlates, and prognosis of hypertension-mediated organ damage (HMOD) in the community-based Framingham Study. 7898 participants (mean age 51.6 years, 54% women) underwent assessment for the following HMOD: electrocardiographic and echocardiographic left ventricular hypertrophy (LVH), abnormal brain imaging findings consistent with vascular injury, increased carotid intima-media thickness (CIMT), elevated carotid-femoral pulse wave velocity (CFPWV), reduced kidney function, microalbuminuria, and low ankle-brachial index (ABI). We characterized HMOD prevalence according to blood pressure (BP) categories defined by four international BP guidelines. Participants were followed up for incidence of cardiovascular disease (CVD).
The prevalence of HMOD varied positively with systolic BP and pulse pressure but negatively with diastolic BP; it increased with age, was similar in both sexes, and varied across BP guidelines based on their thresholds defining hypertension. Among participants with hypertension, elevated CFPWV was the most prevalent HMOD (40–60%), whereas low ABI was the least prevalent (<5%). LVH, reduced kidney function, microalbuminuria, increased CIMT, and abnormal brain imaging findings had an intermediate prevalence (20–40%). HMOD frequently clustered within individuals.
On follow-up (median 14.1 years), there were 384 CVD events among 5865 participants with concurrent assessment of LV mass, CFPWV, kidney function, and microalbuminuria. For every BP category above optimal (referent group), the presence of HMOD increased CVD risk compared to its absence.
The prevalence of HMOD varies across international BP guidelines based on their different thresholds for defining hypertension. The presence of HMOD confers incremental prognostic information regarding CVD risk at every BP category.
Keywords: hypertension, epidemiology, cardiovascular disease, target organ damage, cohort studies, blood pressure
Background
National and international blood pressure (BP)/hypertension (HTN) guidelines emphasize the importance of screening individuals with elevated BP for the presence of end-organ damage, referred to as hypertension-mediated organ damage (HMOD).1–5 Previously termed ‘target organ damage,’ HMOD more accurately describes HTN-related structural or functional changes in major organ systems (i.e., the heart, the brain, the retina, the kidneys, and the vasculature).5
The presence of HMOD plays a central role in the management of people with HTN at the time of their diagnosis and during follow-up.5 Thus, detecting HMOD may upgrade an individual’s CVD risk (e.g., from low to moderate risk), indicate a need for instituting BP-lowering treatment in younger individuals with mildly elevated BP (who may otherwise not be treated), guide the timing of initiation of drug therapy in individual patients, or suggest the need for home/ambulatory BP monitoring when masked HTN, or white-coat HTN are suspected.5 Further, the presence of multiple HMOD in the same person can raise a suspicion of a secondary cause for HTN and further escalate CVD risk.6–9 Therefore, characterizing the epidemiology of HMOD in the community is of public health importance, given the high global burden of elevated BP.10,11
Numerous studies have investigated the prevalence of HMOD in patients with hypertension. These studies suggest that HMOD is common in severe or long-standing HTN but is also prevalent in less severe HTN, including asymptomatic people with elevated BP.12,13 A majority of prior investigations evaluated referral samples, and few assessed a set of HMOD concomitantly. Data are limited regarding the relative prevalence of different HMOD in unselected community-based samples, the co-clustering of HMOD, and the incremental prognostic significance of HMOD on the CVD risk associated with BP stages (grades) defined by international guidelines.
We hypothesized that the prevalence of HMOD increases with age and BP, varies by sex and that multiple HMOD frequently cluster in individuals. We postulated that the prevalence of HMOD would vary across BP categories defined by various HTN guidelines based on their choice of cutpoints for partitioning BP. We posited that the presence of HMOD (and its clustering) increases CVD risk associated with any degree of BP elevation. We tested these hypotheses in a large community-based sample that has undergone comprehensive phenotypic characterization for different HMOD. Accordingly, we evaluated the prevalence, the clinical correlates, and the prognostic significance of HMOD in the Framingham Heart Study (FHS) cohorts. Specifically, we investigated how the prevalence of HMOD varied in people with elevated BP and HTN stages based on major national and international BP guidelines and how the presence of HMOD influences CVD risk in the presence of varying degrees of BP elevation.
Methods
All data and materials have been made publicly available at the NHLBI data repository BioLINCC and can be accessed at https://biolincc.nhlbi.nih.gov/studies/framoffspring/
Study Sample
The design and selection criteria of the Framingham Offspring Study (FOS) and the FHS Third Generation (Gen3) cohorts and their companion multi-ethnic Omni-1 and Omni-2 cohorts have been described previously.14–18 There were 7927 FHS participants (see Figure S1 for study sample derivation) who attended the eighth examination cycle of the FOS, the third examination cycle of the Omni-1 (2005–2008), and the first examination of the Gen3 (2002–2005) and Omni-2 (2003–2005) cohorts who were eligible for inclusion; these examination cycles served as ‘baseline’ for the present investigation. Participants at these examinations underwent an assessment of HMOD (see below). We excluded participants with missing or incomplete blood pressure data (n = 23) or no data on any HMOD (n=6), yielding a base sample of 7898 participants for cross-sectional analyses. Of this base sample, we excluded participants for analyses of individual HMOD prevalence if a specific measure of HMOD was missing (Figure S1). For the incident CVD analyses, we restricted the sample to those who underwent assessment for LVH, reduced kidney function, microalbuminuria, and CFPWV and excluded people with prevalent CVD at baseline (n=390) yielding a sample of 5,865 participants.
The present study complies with the Declaration of Helsinki, and the Boston University Medical Center Institutional Review Board approved the study protocol. All study participants provided written informed consent.
Measurement of Clinical Covariates
FHS participants underwent assessment of their medical history and a cardiovascular-focused physical examination at each examination cycle, including standardized measurements of resting systolic blood pressure (SBP) and diastolic blood pressure (DBP), and laboratory assays of vascular risk factors, including fasting blood total and HDL cholesterol, and glucose. Participants were classified as having diabetes mellitus if their fasting blood glucose concentrations were ≥126 mg/dL or if they were treated with anti-diabetic medications. Current smoking was defined as regular cigarette smoking in the year before the baseline examination.
Measurement and Categorization of Blood Pressure
A physician measured BP twice on seated participants (who had rested for at least five minutes) using a mercury column sphygmomanometer, an appropriately-sized cuff, and a standardized protocol. The average of these two BP readings was used to categorize participants. Table S1 shows the categorization of BP according to the following four BP guidelines: the Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7),1 the 2017 American College of Cardiology/American Heart Association/American (ACC-AHA) BP guidelines,4 the 2018 European Society of Cardiology/European Society of Hypertension (ESC-ESH) guidelines,5 and the 2020 International Society of Hypertension (ISH) Global Hypertension Practice guidelines.3
Measurements of HMOD
We assessed the prevalence of eight types of HMOD across four organ systems, i.e., heart (electrocardiographic left ventricular hypertrophy [LVH]; echocardiographic LVH7), kidneys (reduction in function as measured using the estimated glomerular filtration rate [eGFR];19 the presence of microalbuminuria20), the vasculature (large artery stiffness as indicated by increased carotid-femoral pulse wave velocity [CFPWV];21 carotid intima-media thickness (CIMT);22 and a low ankle-brachial index [ABI]23) and the brain (suggested by the presence of lacunes, microbleeds, or large white matter hyperintensities on magnetic resonance imaging [MRI]24,25). Details of acquisition of each HMOD are provided under Supplementary Methods,19,26–38 and their definitions are listed in Table S2.7,21–25,39,40
Definition of Outcome Events
All FHS participants undergo longitudinal surveillance for the occurrence of CVD events and death. For the present investigation, CVD was defined as a composite of coronary heart disease (myocardial infarction, coronary insufficiency, and angina pectoris), stroke or transient ischemic attack, peripheral arterial disease (intermittent claudication), heart failure or CVD-related mortality, consistent with prior Framingham publications.41 A panel of three physicians adjudicated all CVD events using a standardized protocol as detailed elsewhere.
Statistical Analyses
Prevalence of HMOD across the BP range and in stages defined by each of the four HTN guidelines
We evaluated the prevalence of HMOD by organ system in our sample. We assessed the variation in the prevalence of HMOD (by organ system) across the BP range using SBP categories <120, 120–129, 130–139, 140–149, 150–159, ≥160 mm Hg, and DBP categories <80, 80–84, 85–89, ≥90 mm Hg. For individuals on antihypertensive medications, we imputed SBP/DBP using a method that accounts for the number of BP-lowering agents, drug class, and participants’ race.42,43 We also investigated the prevalence of HMOD by quartile of pulse pressure (PP), i.e., <38, 38–45, 46–56, ≥57 mm Hg.
We assessed the prevalence of each HMOD across the BP categories defined by each of the four BP international guidelines; analyses were performed for pooled sexes, by sex, and by age groups (dichotomized at the median age of 50 years). We also evaluated the co-clustering of HMOD (defined as the presence of ≥1 HMOD and ≥2 HMOD; separate analyses for each outcome) within individuals by BP category in each BP guideline.
We conducted secondary analyses assessing the prevalence of HMOD in each BP stage (using the AHA/ACC 2017 guidelines), stratifying the stage into two groups (BP stage duration <5 versus ≥5 years) based on a subset of Gen 2 and Omni-1 cohort participants who attended their previous examination.
Cross-Sectional Correlates of HMOD
We assessed the cross-sectional correlates of HMOD using generalized linear models (logistic option) to relate clinical correlates (independent variables) to the presence of each HMOD (binary dependent variable) adjusting for cohort type. The candidate clinical correlates included age, sex, SBP, DBP, smoking, diabetes, and the ratio of total to HDL cholesterol; these variables have been associated with target organ damage in the context of elevated BP in the published literature.13,44 Analyses were repeated to assess the co-clustering of HMOD, defined as the presence of ≥1 HMOD and ≥2 HMOD (separate analyses for each outcome) in a subset with all measures of HMOD (N=2874), except CIMT (which was available in a much smaller subset). We repeated the analyses substituting PP for SBP and DBP and further including mean arterial pressure (MAP) to account conjointly for the pulsatile (PP) and steady-state (MAP) components of vascular load.44
Relations of BP categories to CVD incidence according to presence vs. absence of HMOD
We cross-classified each BP category defined according to the 2017 ACC-AHA and ESC-ESH guidelines (separate analyses for each) with the presence versus absence of any HMOD in 5865 individuals who were free of prevalent CVD at the baseline examination and had available measures of electrocardiographic and echocardiographic LVH, eGFR, microalbuminuria, and CFPWV. We chose these four HMOD to permit adequate statistical power because using more than four HMOD would result in a smaller sample size for these prospective analyses. We chose the ACC-AHA and ESC-ESH guidelines because they represent major US and European BP guidelines, respectively. For the ESH guidelines, we combined HTN grades 1–3 into a single category (HTN grade 1 or more). This cross-classification resulted in the primary exposures of eight groups (combinations of 4 BP categories by presence or absence of HMOD).
We used multivariable Cox proportional hazards regression to relate the cross-classified BP-HMOD categories to CVD incidence adjusting for standard risk factors, after verifying the proportionality of hazards assumption (by testing the interaction of log of follow-up time*BP-HMOD category). The group with normal BP (ACC/AHA) or optimal BP (ESC/ESH) and no HMOD served as the referent. Models were adjusted initially for age and sex (Model 1), and then for age, sex, smoking, diabetes, the ratio of total to HDL cholesterol, antihypertensive medication, and cohort type (Offspring vs. Third Generation, Offspring vs. Omni-1; Model 2). We stratified the baseline hazard by median age (<50 vs. ≥50) for both models. We tested for statistical interaction between HMOD and the BP category by incorporating a cross-product term HMOD*BP category in the multivariable models. A two-sided value of p<0.05 was considered statistically significant for all models. All analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC).
Results
The baseline characteristics of the different samples used in the present investigation are shown in Table 1. The samples comprised middle-aged adults with a risk factor profile representative of ambulatory adults in the general population.
Table 1.
Baseline characteristics of study samples.
| Cross-sectional analyses | Sample for incident CVD analyses | ||
|---|---|---|---|
| Characteristic | Largest sample with at least one HMOD | Sample to assess co-clustering | |
| N | 7898 | 2874 | 5865 |
| Age, y | 51.6 ± 15.9 | 54.0 ± 13.3 | 49.1 ± 14.7 |
| Women, n (%) | 4300 (54) | 1540 (54) | 3206 (55) |
| Systolic blood pressure, mm Hg | 122 ± 17 | 122 ± 16 | 120 ± 16 |
| Diastolic blood pressure, mm Hg | 74 ± 10 | 75 ± 10 | 75 ± 10 |
| Pulse pressure, mm Hg | 47 ± 15 | 47 ± 14 | 46 ± 13 |
| Antihypertensive medication, n (%) | 2092 (26) | 723 (25) | 1182 (20) |
| Body mass index, kg/m2 | 27.5 ± 5.6 | 27.0 ± 4.8 | 26.8 ± 5.0 |
| Prevalent CVD, n (%) | 656 (8.3) | 178 (6.2) | - |
| Smoking, n (%) | 945 (12) | 281 (9.8) | 743 (13) |
| Diabetes, n (%) | 641 (8.3) | 177 (6.2) | 309 (5.3) |
| Total/HDL cholesterol ratio | 3.7 ± 1.3 | 3.6 ± 1.2 | 3.6 ± 1.3 |
| Lipid-lowering medication, n (%) | 1758 (22) | 651 (23) | 973 (17) |
| Cohort, n (%) | |||
| Offspring | 3110 (39) | 1376 (48) | 1985 (34) |
| Third Generation | 4082 (52) | 1498 (52) | 3656 (62) |
| Omni 1 or 2 | 706 (9) | - | 224 (4) |
| ECG Left ventricular hypertrophy, n (%)* | 609 (7.8) | 222 (7.7) | 453 (7.7) |
| Echo Left ventricular hypertrophy ASE, n (%)* | 824 (11) | 330 (11) | 528 (9.0) |
| eGFR <60, n (%)* | 462 (6.0) | 155 (5.4) | 224 (3.8) |
| Urine albumin to creatinine ratio ≥30, n (%)* | 445 (6.2) | 136 (4.7) | 246 (4.2) |
| Carotid-femoral pulse wave velocity >10 m/sec, n (%)* | 1470 (20) | 592 (21) | 928 (16) |
| Ankle brachial index <0.9, n (%)* | 157 (2.7) | 50 (1.7) | 62 (1.4) |
| Brain MRI abnormality, n (%)* | 842 (20) | 612 (21) | 628 (19) |
| Increased CIMT, n (%)* | 481 (20) | 197 (16) | 255 (16) |
Subsample with available measurements. CIMT= carotid intimal-medial thickness. ECG= electrocardiogram. Echo= echocardiographic. eGFR= estimated glomerular filtration rate.
Prevalence of HMOD across the BP range
The prevalence of different HMOD according to cross-tabulated SBP and DBP categories is shown in Table S3. The prevalence of HMOD increased across SBP categories. At any level of SBP above 120 mm Hg, DBP was negatively related to the prevalence of HMOD, except for electrocardiographic LVH. Groups with DBP <80 mm Hg had the highest prevalence of HMOD. Prevalence of HMOD increased with rising pulse pressure; Figure S2 displays the co-clustering of HMOD across PP quartiles.
Prevalence of HMOD by BP category using four BP guidelines
The prevalence of each HMOD and co-clustering of HMOD according to each of the four international BP guidelines are shown in Figure 1 (for the ACC-AHA and ESC-ESH guidelines), and Figure S3 (for JNC-7 and ISH guidelines). Prevalence of HMOD varied across BP categories across the guidelines due to varying BP thresholds defining these categories.
Figure 1.

Prevalence of HMOD (by type) by different BP guidelines. Left Panel: ACC-AHA 2017 guidelines; Right Panel: ESC-ESH guidelines. ABI= ankle-brachial index, ASE= American society of Echocardiography, CIMT= carotid intimal-medial thickness. ECG= electrocardiogram. Echo= echocardiographic. eGFR= estimated glomerular filtration rate, LVH= left ventricular hypertrophy, MRI= magnetic resonance imaging, PWV= carotid-femoral pulse wave velocity, UACR= urine albumin to creatinine ratio.
Among individuals without HTN, the group with normal (JNC-7, ACC-AHA, and ISH guidelines) or optimal BP (ESC-ESH guidelines) had the lowest prevalence of HMOD. In this group, abnormal brain MRI findings were the most prevalent (15–16%), followed by increased CIMT (7–9%) and then LVH and increased CFPWV (3–6%). Reduced eGFR, microalbuminuria, and low ABI were relatively infrequent (1–3%). Almost a third of individuals in this group had more than one form of HMOD.
Among individuals with HTN, increased CFPWV was the most common form of HMOD (prevalence 40–60%), whereas low ABI was the least prevalent (<5%). LVH, reduced kidney function, microalbuminuria, increased CIMT, and brain imaging findings had an intermediate prevalence (20–40%). HMOD frequently co-clustered in this group, with over half (ACC-AHA guidelines) to over three quarters (other guidelines) having more than one form of HMOD.
When analyzed by sex, the prevalence of HMOD was generally similar in men and women, except for electrocardiographic LVH, which was more prevalent in men (Table S4, A–D), a finding consistent across BP guidelines. When analyzed by median age (<50 vs. ≥50 years), the prevalence of HMOD was much higher in those aged ≥50 years (Table S5, A–D), except for electrocardiographic LVH, which was more prevalent in those aged <50 years. Increased CFPWV, increased CIMT, microalbuminuria, reduced eGFR, and low ABI were rare in younger people (<50 years).
In a subset of Gen 2 and Omni-1 cohort participants who attended their previous examinations (1998–2001), groups with elevated BP or Stage 1 or higher BP (per AHA/ACC 2017 guidelines) of ≥5 years duration had a higher prevalence of HMOD (in 2005–2008) compared to their counterparts with the same BP stage of <5 years duration (Table S6). This finding was consistent across different types of HMOD. Conversely, individuals with normal BP ≥5 years had a lower prevalence of HMOD compared to their counterparts with the normal BP <5 years duration (Table S6).
Cross-Sectional Clinical Correlates of HMOD
Figure 2 shows the multivariable-adjusted odds ratio (95% CI) of having HMOD (separate models for each) with one standard deviation (SD) increment in SBP and DBP (Panel A), and PP (Panel B). SBP and PP were directly associated with HMOD, except for reduced eGFR, which was not associated with either BP component. Conversely, DBP was negatively associated with HMOD, except for reduced eGFR (positive association) and abnormal findings on brain MRI (no association).
Figure 2.
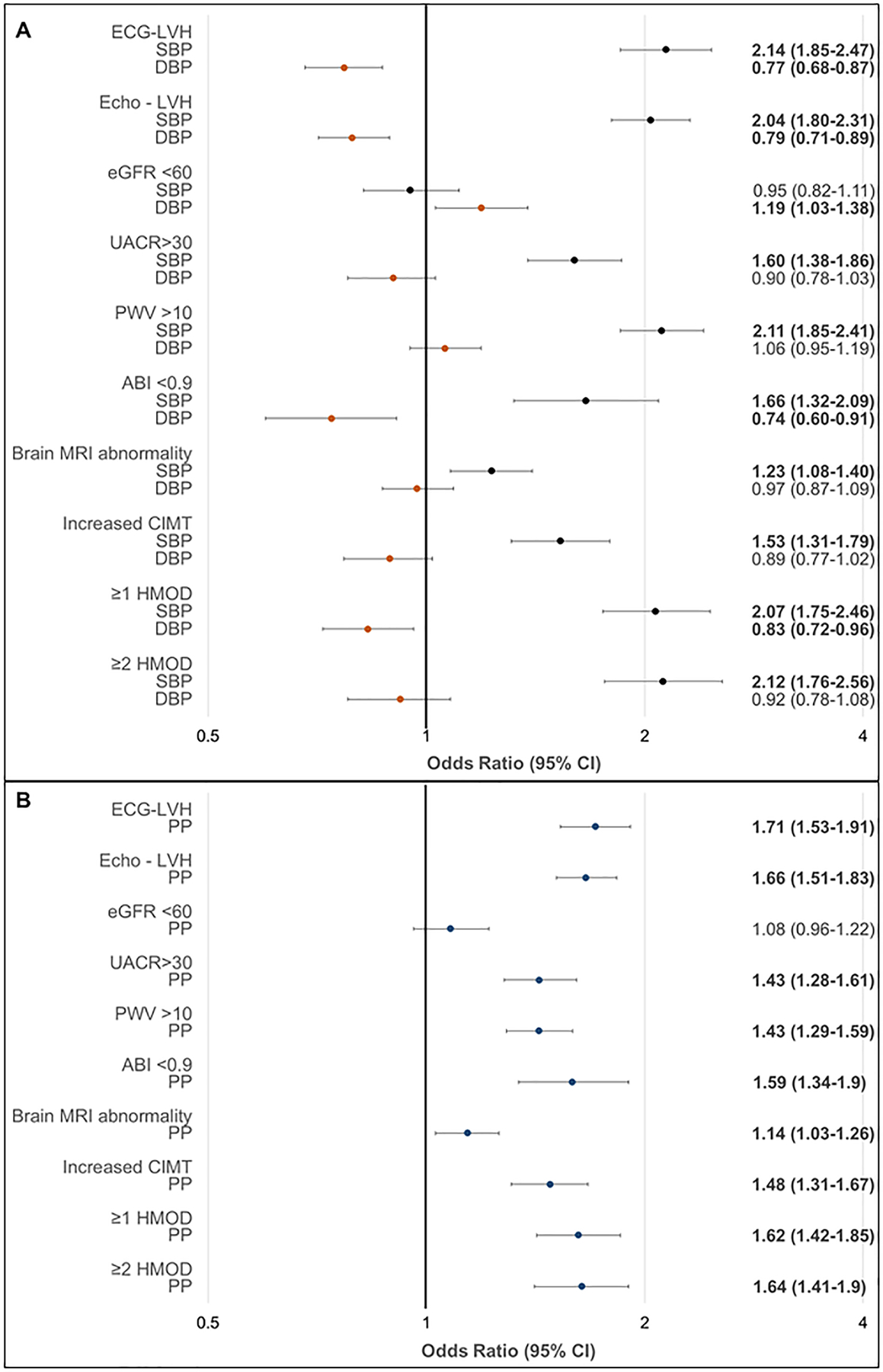
Association of blood pressure component with the prevalence of HMOD: logistic regression models adjusted for age, sex, total/HDL cholesterol ratio, smoking, and diabetes.
Panel A, results for systolic and diastolic BP (mutually adjusted). Panel B. results for PP (adjusted for mean arterial pressure).
For the multivariable-adjusted clinical correlates of each HMOD and co-clustering of HMOD, age was positively associated with each HMOD, except for electrocardiographic LVH, which was negatively related (Table S7). Female sex was negatively associated with electrocardiographic LVH and high CFPWV. Diabetes was associated with a 1.8-fold odds of high CFPWV and a greater than 3-fold odds of microalbuminuria. Smoking was associated with an almost 5-fold odds of having a reduced ABI. Higher SBP, older age, male sex, and prevalent diabetes were associated with the co-clustering of HMOD (Table S7).
Relations of BP-HMOD cross-classified groups to CVD Incidence according to BP categories defined by the ACC-AHA and ESC-ESH guidelines
On follow-up (median 14.1 years), there were 384 first CVD events (45% women) among 5865 participants. For both ACC-AHA and ESC-ESH guidelines, the BP categories with HMOD had a higher cumulative CVD incidence (approximately three-fold) compared to the same BP categories without HMOD (Figure 3).
Figure 3.
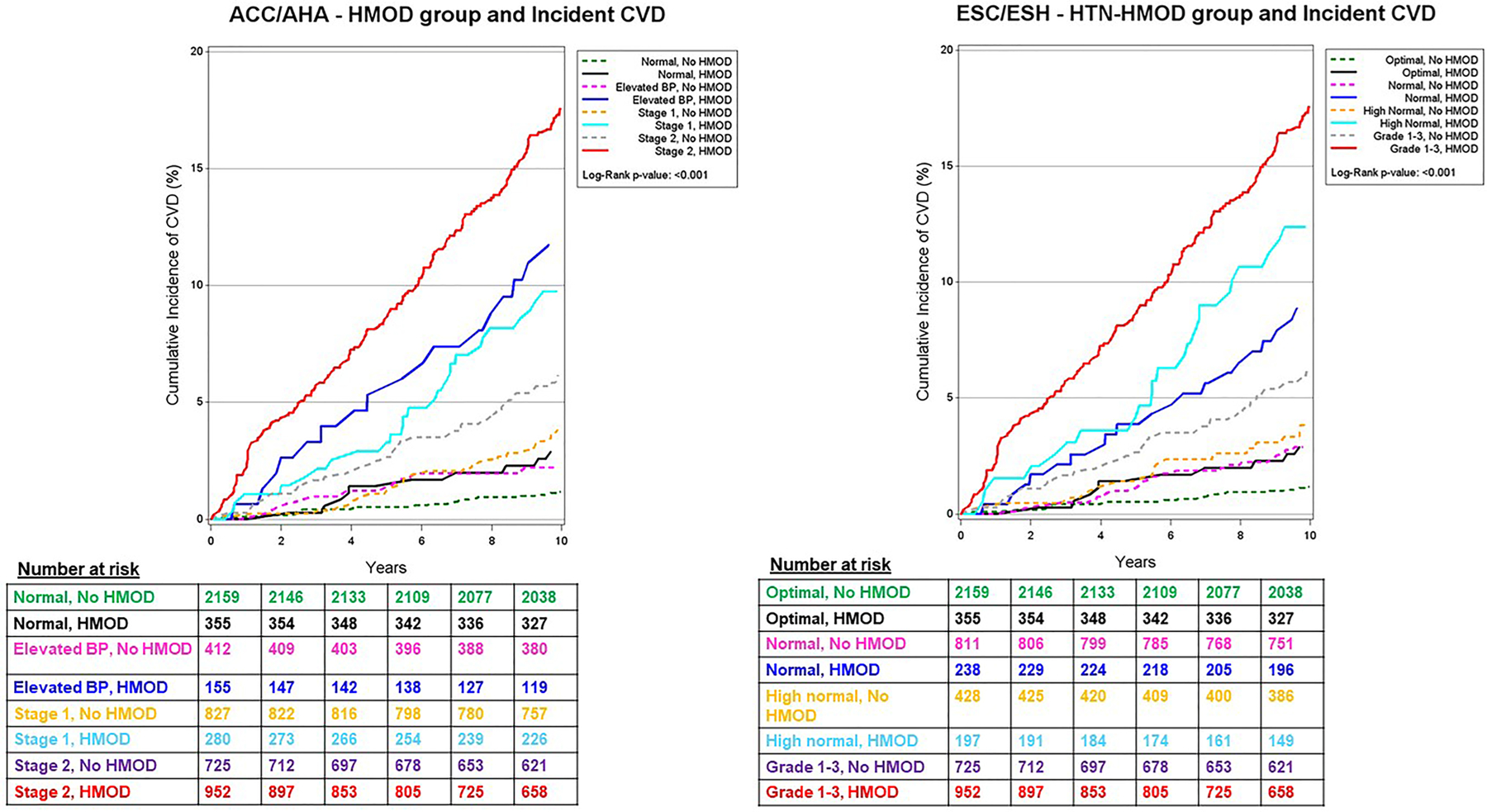
Cumulative incidence of CVD according to the 2017 ACC-AHA BP categories (Left Panel) and the 2018 ESC-ESH BP groupings (Right Panel) stratified by presence versus absence of HMOD.
At any given BP category above the normal (ACC-AHA) or optimal (ESC-ESH) referent group, the presence of HMOD was associated with a 2 to 3-fold increase in the risk of CVD compared to the referent group in multivariable-adjusted models (Table 2). Each BP category with HMOD was associated with higher CVD risk than the same BP category without HMOD (compared to the referent group). There was no statistically significant interaction of HMOD with BP category (p values for the cross-product term HMOD*BP category exceeded 0.85 for both guidelines).
Table 2.
Association of Cross-classified BP category (by the 2017 ACC-AHA and the 2018 ESC-ESH guidelines) and presence versus absence of HMOD with the incidence of cardiovascular disease
| BP category (systolic and diastolic BP range) | HMOD | No. events/ No. at-risk | Unadjusted Incidence Rate per 1000 Person-years | Age- sex-adjusted Model |
Multivariable-adjusted Model | ||
|---|---|---|---|---|---|---|---|
| Hazards Ratio (95% CI) | P-value | Hazards Ratio (95% CI) | P-value | ||||
| ACC-AHA Guidelines | |||||||
| Normal BP (systolic BP <120 and diastolic BP <80 mm Hg) | absent | 38/2159 | 1.24 | Referent | Referent | ||
| present | 15/355 | 3.11 | 1.50 (0.82–2.76) | 0.19 | 1.48 (0.81–2.72) | 0.21 | |
| Elevated BP (systolic BP 120–129 and diastolic <80 mm Hg) | absent | 17/412 | 3.04 | 1.64 (0.92–2.91) | 0.09 | 1.53 (0.86–2.72) | 0.15 |
| present | 17/155 | 9.50 | 2.44 (1.34–4.45) | 0.004 | 2.22 (1.21–4.08) | 0.01 | |
| Stage 1 HTN (systolic BP 120–139 or diastolic 80–89 mm Hg) | absent | 38/827 | 3.32 | 2.05 (1.30–3.22) | 0.002 | 1.95 (1.24–3.07) | 0.004 |
| present | 29/280 | 8.54 | 2.72 (1.64–4.50) | <0.001 | 2.38 (1.43–3.98) | 0.001 | |
| Stage 2 HTN (systolic BP ≥ 140 or diastolic BP ≥90 mm Hg) | absent | 50/725 | 5.50 | 2.05 (1.32–3.16) | 0.001 | 1.86 (1.14–3.05) | 0.01 |
| present | 180/952 | 17.95 | 3.42 (2.28–5.14) | <0.001 | 2.93 (1.85–4.66) | <0.001 | |
| ESC-ESH Guidelines | |||||||
| Optimal (systolic BP <120 and diastolic BP <80 mm Hg) | absent | 38/2159 | 1.24 | Referent | Referent | ||
| present | 15/355 | 3.11 | 1.51 (0.82–2.77) | 0.18 | 1.49 (0.81–2.73) | 0.20 | |
| Normal (systolic BP 120–129 or diastolic BP 80–89 mm Hg) | absent | 34/811 | 3.03 | 1.86 (1.17–2.95) | 0.009 | 1.77 (1.11–2.82) | 0.016 |
| present | 21/238 | 7.18 | 2.33 (1.34–4.06) | 0.003 | 2.14 (1.22–3.74) | 0.008 | |
| High Normal (systolic BP 130–139 or diastolic BP 85–89 mm Hg) | absent | 21/428 | 3.63 | 1.99 (1.16–3.40) | 0.012 | 1.85 (1.08–3.17) | 0.02 |
| present | 25/197 | 11.06 | 2.94 (1.73–4.99) | <0.001 | 2.54 (1.48–4.37) | 0.001 | |
| ≥Grade1 (systolic BP ≥140 or diastolic BP ≥90 mm Hg) | absent | 50/725 | 5.50 | 2.05 (1.33–3.18) | 0.001 | 1.87 (1.14–3.06) | 0.01 |
| present | 180/952 | 17.95 | 3.45 (2.30–5.18) | <0.001 | 2.95 (1.86–4.68) | <0.001 | |
Note: Models are adjusted for age, sex, body mass index, the ratio of total to HDL cholesterol, smoking status, prevalent diabetes, antihypertensive treatment, and cohort type. The baseline hazard was stratified by median age (<50 vs. ≥50)
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; ESC, European Society of Cardiology; ESH, European Society of Hypertension
Discussion
A comprehensive assessment of the prevalence of HMOD (across multiple organ systems) in the community is lacking in the published literature. Existing data on HMOD prevalence are typically based on the evaluation of patients in HTN clinics or referral samples using a limited set of indicators of HMOD. Also, the incremental prognostic significance of HMOD across the spectrum of BP stages/grades for CVD risk remains unclear; our investigation bridges these fundamental knowledge gaps.
Principal Findings
Our principal findings are four-fold. First, HMOD is frequent in the community. The prevalence varies according to the type of HMOD, the degree and duration of BP elevation, and the age and sex of individuals. Of note, up to a third of the general population with normal levels of BP have some form of HMOD, with the prevalence being higher in older people. It is conceivable that the term HMOD in such individuals may be incorrect in this context. The frequent occurrence of HMOD in the absence of BP elevation may be because of the prevalence of additional risk factors for HMOD (such as diabetes, obesity, smoking, and advancing age), the statistical thresholds (percentiles) that we used to define select HMOD or the use of sensitive imaging techniques (brain MRI). It is also conceivable that clinic BP levels misclassify actual hypertension status in some people or that the presence of HMOD may be a marker of future hypertension risk. Future studies that relate BP stage by ambulatory BP monitoring to the prevalence of HMOD could investigate if misclassification of BP stage contributed to the high frequency of HMOD in people with normal clinic BP.
Second, SBP was directly associated with all forms of HMOD cross-sectionally, except for reduced eGFR, which was not associated with SBP in our sample. Conversely, DBP was negatively associated with most HMOD, except eGFR (positive association) and abnormal brain MRI findings (no association). Pulse pressure was directly associated with all types of HMOD except for reduced eGFR. When SBP and DBP categories were cross-classified, we observed direct associations of SBP with HMOD and negative associations of DBP with HMOD.
Third, the prevalence of HMOD in BP categories defined according to different BP guidelines varied based on the SBP and DBP thresholds used to define the BP categories and HTN. Thus, for example, the prevalence of HMOD in Stage 1 HTN defined by ACC-AHA criteria was lower than that for Stage 1 HTN defined by JNC-7, ESC, or ISH guidelines because the latter three used higher SBP and DBP thresholds to define hypertension. HMOD prevalence was similar in men and women, with the notable exceptions of ECG LVH and high CFPWV, which were more prevalent in men. For any given BP guideline, the prevalence of all HMOD (except for ECG LVH) increased in individuals over 50 years of age. Several forms of HMOD (high CFPWV or CIMT, microalbuminuria, low eGFR, and low ABI) were relatively infrequent in individuals below age 50, indicating a minimal diagnostic yield for these tests in younger individuals with HTN.
Among individuals with HTN, the prevalence of HMOD varied based on the stage/grade, the organ system evaluated, and the choice of the diagnostic test. Overall, the prevalence of high CFPWV was the highest (ranging from 40–60%), followed by echocardiographic LVH, abnormal brain MRI findings, and increased CIMT (20–40%), and then microalbuminuria and reduced eGFR (10–20%). Electrocardiographic LVH was infrequent (10–15%), and a low ABI was the least prevalent (2–6%).
Co-clustering of HMOD was frequent in people with elevated BP. Between a half (ACC-AHA guidelines) and three-quarters (all other BP guidelines) of individuals with high BP had at least one type of HMOD; between a third (ACC-AHA guidelines) and a half (all other BP guidelines) had two or more HMOD types.
Fourth, the presence of HMOD was a powerful predictor of CVD risk at any BP category exceeding normal (ACC-AHA) or optimal (ESC-ESH) groups. In people without evidence of HMOD, unadjusted CVD incidence rates were similar for elevated BP versus stage 1 HTN (ACC-AHA guidelines), and for those with normal versus high normal BP (ESC-ESH guidelines). The presence of HMOD increased both the absolute risk and the relative risk (compared to the referent group) of CVD for any given BP category.
Comparison with the published literature and clinical significance
As noted above, several investigations have assessed the prevalence and co-clustering of HMOD among people with HTN.7,8,12,13,45–49 It is challenging to compare the prevalence of HMOD in these studies with that in our investigation for reasons noted previously. Our investigation underscores the limited clinical utility of most tests to assess HMOD in individuals below age 50, except for electrocardiographic or echocardiographic LVH or abnormal findings on brain MRI. Assessment of electrocardiographic LVH may seem reasonable in this age group; its accessibility may counterbalance its limited sensitivity. Among individuals over age 50 years, assessment of CFPWV, echocardiographic LVH, renal function and microalbuminuria, brain MRI and carotid sonography have moderate diagnostic yield. We were unable to perform a detailed comparative analysis of the predictive value of these individual tests for predicting CVD events due to our overall moderate-sized sample. Future studies of larger samples could evaluate this premise more comprehensively.
Strengths and limitations
Our investigation has several strengths, including the large community-based sample, the conjoint phenotypic characterization of a comprehensive HMOD panel, and the assessment of HMOD prevalence based on BP thresholds defined by four major international BP guidelines. Additionally, the longitudinal surveillance facilitated an investigation of the prognostic impact of HMOD across the full range of BP distribution.
It is also essential to acknowledge several limitations of our investigation. We categorized the BP of our participants based on measurements on a single occasion, which may misclassify the BP category and their HTN status. Some HMOD measures were available on smaller subsamples, which limited our ability to assess their prognostic utility. Additionally, we did not evaluate HMOD reflected by retinal vascular changes. Lastly, most of our participants were middle-aged white adults of European-ancestry, although we did include the modest-sized, multi-ethnic FHS Omni cohorts. Therefore, the generalizability of our results may be limited. Additional studies are warranted to characterize the prevalence and prognosis of HMOD in diverse cohorts and across a broader age range.
Perspectives
HMOD was frequent in our large community-based sample, with the prevalence varying depending on the organ system screened, the diagnostic test used, and the age of individuals. HMOD was infrequent in people below age 50 years (except for electrocardiographic LVH), suggesting the low yield of other tests in this age group. Additionally, the varying prevalence of different forms of HMOD may guide the choice of clinical tests for detecting HMOD in older people with elevated BP, a vital area for future research. Notably, HMOD was prevalent in the group with normal BP, underscoring the role of other non-BP correlates and raising the possibility of masked hypertension in such individuals. Furthermore, HMOD prevalence varied directly with systolic BP and pulse pressure but negatively with diastolic BP. These associations raise the importance of pulsatile load in target organ damage and the need to target this component of BP to mitigate the adverse consequences of HMOD. The presence of HMOD conferred clinically crucial prognostic information regarding CVD risk at any given level of BP elevation. These observations support the importance of considering HMOD to guide treatment decisions in patients with elevated BP. Future investigations must compare the relative cost-effectiveness of screening people with hypertension with specific tests for HMOD (e.g., CFPWV versus echocardiographic LVH) and their comparative yield in clinical decision-making.
Supplementary Material
Novelty and Significance.
What is new?
Prevalence of HMOD varies across international BP guidelines and according to the age of individuals and the choice of the screening test.
Elevated CFPWV was the most prevalent HMOD overall, followed by echocardiographic LVH and abnormal brain imaging findings.
What is relevant?
HMOD presence elevates the absolute and the relative risk of CVD at any level of BP, a finding consistent across BP guidelines.
The direct relation of HMOD and pulse pressure underscores the need to target pulsatile load to mitigate vascular consequences.
Summary
Assessment of HMOD may be an important adjunct in the management of elevated BP.
Sources of Funding
This work is supported by Contracts NO1-HC-25195, HHSN268201500001I, 75N92019D00031 and grants NIH grants HL080124, HL071039, HL077447, HL107385, HL126136, HL107385, HL60040, HL70100, HL131532, and HL134168.from the National Heart, Lung, and Blood Institute.
Dr. Vasan is supported by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine.
The funding agency (National Heart, Lung and Blood Institute) has no role in the design and conduct of this report; the analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest and Financial Disclosures
Gary F. Mitchell is the owner of Cardiovascular Engineering, Inc., which designs and manufactures devices that measure vascular stiffness. The company uses these devices in clinical trials that evaluate the effects of diseases and interventions on vascular stiffness. He also reports receiving grants from the National Institutes of Health and Novartis and consulting fees from Novartis, Bayer, Merck, and Servier. The remaining authors declare no conflicts.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr., et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–52 [DOI] [PubMed] [Google Scholar]
- 2.Jones NR, McCormack T, Constanti M and McManus RJ. Diagnosis and management of hypertension in adults: NICE guideline update 2019. British Journal of General Practice 2020;70:90–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020;75:1334–1357 [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018;71:e127–e248 [DOI] [PubMed] [Google Scholar]
- 5.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104 [DOI] [PubMed] [Google Scholar]
- 6.Olesen TB, Pareek M, Stidsen JV, Blicher MK, Rasmussen S, Vishram-Nielsen JKK, Maagaard L and M HO. Association between antecedent blood pressure, hypertension-mediated organ damage and cardiovascular outcome. Blood pressure 2020;29:232–240 [DOI] [PubMed] [Google Scholar]
- 7.Perrone-Filardi P, Coca A, Galderisi M, Paolillo S, Alpendurada F, de Simone G, Donal E, Kahan T, Mancia G and Redon J. Non-invasive cardiovascular imaging for evaluating subclinical target organ damage in hypertensive patients: a consensus paper from the European Association of Cardiovascular Imaging (EACVI), the European Society of Cardiology Council on Hypertension, and the European Society of Hypertension (ESH). European Heart Journal-Cardiovascular Imaging 2017;18:945–960 [DOI] [PubMed] [Google Scholar]
- 8.Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Petersen C, Hildebrandt P and Olsen MH. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. European heart journal 2010;31:883–891 [DOI] [PubMed] [Google Scholar]
- 9.Pontremoli R, Ravera M, Bezante GP, Viazzi F, Nicolella C, Berruti V, Leoncini G, Del Sette M, Brunelli C and Tomolillo C. Left ventricular geometry and function in patients with essential hypertension and microalbuminuria. Journal of hypertension 1999;17:993–1000 [DOI] [PubMed] [Google Scholar]
- 10.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Abate KH and Akinyemiju TF. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. Jama 2017;317:165–182 [DOI] [PubMed] [Google Scholar]
- 11.Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, Gregg EW, Bennett JE, Solomon B and Singleton RK. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet 2021;398:957–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuspidi C, Ambrosioni E, Mancia G, Pessina AC, Trimarco B, Zanchetti A and Investigators A. Role of echocardiography and carotid ultrasonography in stratifying risk in patients with essential hypertension: the Assessment of Prognostic Risk Observational Survey. Journal of hypertension 2002;20:1307–1314 [DOI] [PubMed] [Google Scholar]
- 13.Cuspidi C, Valerio C, Negri F, Sala C, Masaidi M, Giudici V, Zanchetti A and Mancia G. Prevalence and correlates of multiple organ damage in referred treated hypertensives: data from the ETODH study. Journal of human hypertension 2008;22:801–803 [DOI] [PubMed] [Google Scholar]
- 14.Dawber TR, Meadors GF and Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. American journal of public health and the nation’s health 1951;41:279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ and Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol 1979;110:281–90 [DOI] [PubMed] [Google Scholar]
- 16.Quan SF, Howard BV, Iber C, Kiley JP, Nieto FJ, O’Connor GT, Rapoport DM, Redline S, Robbins J and Samet JM. The sleep heart health study: design, rationale, and methods. Sleep 1997;20:1077–1085 [PubMed] [Google Scholar]
- 17.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB Sr., Fox CS, Larson MG, Murabito JM, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–35 [DOI] [PubMed] [Google Scholar]
- 18.Tsao CW and Vasan RS. Cohort Profile: The Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol 2015;44:1800–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F and Greene T. A new equation to estimate glomerular filtration rate. Annals of internal medicine 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A and Joyce C. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. Jama 2001;286:421–426 [DOI] [PubMed] [Google Scholar]
- 21.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank J, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU and Protogerou AD. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. Journal of hypertension 2012;30:445–448 [DOI] [PubMed] [Google Scholar]
- 22.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U and Laurent S. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015;241:507–532 [DOI] [PubMed] [Google Scholar]
- 23.Feringa HH, Bax JJ, van Waning VH, Boersma E, Elhendy A, Schouten O, Tangelder MJ, van Sambeek MH, van den Meiracker AH and Poldermans D. The long-term prognostic value of the resting and postexercise ankle-brachial index. Archives of Internal Medicine 2006;166:529–535 [DOI] [PubMed] [Google Scholar]
- 24.Longstreth W, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D and Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people: the Cardiovascular Health Study. Stroke 1996;27:1274–1282 [DOI] [PubMed] [Google Scholar]
- 25.Vermeer SE, Longstreth WT Jr and Koudstaal PJ. Silent brain infarcts: a systematic review. The Lancet Neurology 2007;6:611–619 [DOI] [PubMed] [Google Scholar]
- 26.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F and Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. American Journal of Kidney Diseases 2002;39:920–929 [DOI] [PubMed] [Google Scholar]
- 27.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R and Wolf PA. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging 2005;26:491–510 [DOI] [PubMed] [Google Scholar]
- 28.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA and Kuznetsova T. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal-Cardiovascular Imaging 2015;16:233–271 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Coresh J, Bolton K, Culleton B, Harvey KS, Ikizler TA, Johnson CA, Kausz A, Kimmel PL and Kusek J. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. American Journal of Kidney Diseases 2002;39:i–ii+ S1-S266 [Google Scholar]
- 30.Marcon D, Tagetti A and Fava C. Subclinical Organ Damage in Children and Adolescents with Hypertension: Current Guidelines and Beyond. High blood pressure & cardiovascular prevention : the official journal of the Italian Society of Hypertension 2019;26:361–373 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell GF, Hwang S-J, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D and Benjamin EJ. Arterial Stiffness and Cardiovascular Events. Circulation 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS and Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension 2004;43:1239–1245 [DOI] [PubMed] [Google Scholar]
- 33.Nathan DM, Rosenbaum C and Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes care 1987;10:414–418 [DOI] [PubMed] [Google Scholar]
- 34.Pikula A, Beiser AS, DeCarli C, Himali JJ, Debette S, Au R, Selhub J, Toffler GH, Wang TJ, Meigs JB, et al. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation 2012;125:2100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, Benjamin EJ, Fontes J, Au R, Pikula A, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke 2012;43:3091–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundstrom J, Sullivan L, Selhub J, Benjamin EJ, D’Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PW and Vasan RS. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J 2004;25:523–30 [DOI] [PubMed] [Google Scholar]
- 37.Murabito JM, Evans JC, Nieto K, Larson MG, Levy D and Wilson PW. Prevalence and clinical correlates of peripheral arterial disease in the Framingham Offspring Study. Am Heart J 2002;143:961–965 [DOI] [PubMed] [Google Scholar]
- 38.Polak JF, Pencina MJ, Meisner A, Pencina KM, Brown LS, Wolf PA and D’Agostino RB Sr. Associations of carotid artery intima-media thickness (IMT) with risk factors and prevalent cardiovascular disease: comparison of mean common carotid artery IMT with maximum internal carotid artery IMT. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 2010;29:1759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schillaci G, Battista F and Pucci G. A review of the role of electrocardiography in the diagnosis of left ventricular hypertrophy in hypertension. Journal of electrocardiology 2012;45:617–23 [DOI] [PubMed] [Google Scholar]
- 40.Toto RD. Microalbuminuria: definition, detection, and clinical significance. The journal of clinical hypertension 2004;6:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kannel WB, Wolf PA and Garrison RJ. Section 34: Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. Framingham Heart Study, 30 Year Follow-Up Bethesda, MD: US Department of Health and Human Services; 1987. [Google Scholar]
- 42.Rana BK, Dhamija A, Panizzon MS, Spoon KM, Vasilopoulos T, Franz CE, Grant MD, Jacobson KC, Kim K and Lyons MJ. Imputing observed blood pressure for antihypertensive treatment: impact on population and genetic analyses. American journal of hypertension 2014;27:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, et al. A summary of the effects of antihypertensive medications on measured blood pressure. American journal of hypertension 2005;18:935–42 [DOI] [PubMed] [Google Scholar]
- 44.Vasan RS, Short MI, Niiranen TJ, Xanthakis V, DeCarli C, Cheng S, Seshadri S and Mitchell GF. Interrelations Between Arterial Stiffness, Target Organ Damage, and Cardiovascular Disease Outcomes. Journal of the American Heart Association 2019;8:e012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuspidi C, Sala C, Tadic M, Gherbesi E, Grassi G and Mancia G. Pre-hypertension and subclinical cardiac damage: A meta-analysis of echocardiographic studies. International Journal of Cardiology. 2018;270:302–308 [DOI] [PubMed] [Google Scholar]
- 46.Fuchs A, Kühl JT, Sigvardsen PE, Knudsen AD, Nilsson EJP, Stisen ZR, Jeppesen JL, Nordestgaard BG, Køber LV and Kofoed KF. Arterial hypertension and morphologic abnormalities of cardiac chambers: results from the Copenhagen General Population Study. Journal of hypertension 2021;39:703–710 [DOI] [PubMed] [Google Scholar]
- 47.Lehtonen AO, Puukka P, Varis J, Porthan K, Tikkanen JT, Nieminen MS, Huikuri HV, Anttila I, Nikus K and Kähönen M. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. Journal of hypertension 2016;34:959–966 [DOI] [PubMed] [Google Scholar]
- 48.Oh JS, Lee CH, Park JI, Park HK and Hwang JK. Hypertension-Mediated Organ Damage and Long-term Cardiovascular Outcomes in Asian Hypertensive Patients without Prior Cardiovascular Disease. Journal of Korean medical science 2020;35:e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papazafiropoulou A, Skliros E, Sotiropoulos A, Papafragos C, Gikas A, Apostolou O, Kaliora H and Tountas C. Prevalence of target organ damage in hypertensive subjects attending primary care: C.V.P.C. study (epidemiological cardio-vascular study in primary care). BMC family practice 2011;12:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


