Abstract
Background:
Although heart failure (HF) risk and cardiac structure/function have been reported to differ by race and gender, limited data exist in late-life when HF risk is highest.
Objectives:
To study race/gender-based differences in HF risk factors, cardiac structure/function, and incident HF in late-life.
Methods:
We studied 5,149 HF-free participants in the Atherosclerosis Risk in Communities (ARIC) study, a prospective epidemiologic cohort study, who attended Visit 5 (2011–2013) and underwent echocardiography. Participants were subsequently followed for a median 5.5 years for incident HF/death.
Results:
Mean age was 75±5 years, 59% were women, and 20% were Black race. Male gender and Black race were associated with lower mean LVEF. Black race was also associated with greater LV wall thickness and concentricity, differences that persisted after adjusting for cardiovascular comorbidities. After adjusting for cardiovascular comorbidities, men were at higher risk for HF and HFrEF in Black compared to White participants (HF: HR 2.36 [95% CI 1.37–4.08] vs. 1.16 [0.89–1.51], interaction P = 0.016; HFrEF: 3.70 [1.72–7.95] vs. 1.55 [1.01–2.37] respectively, interaction P = 0.039). Black race was associated with higher incidence of HF overall and HFrEF in men only (HF: 1.65 [1.07–2.53] vs. 0.77 [0.49–1.17]; HFrEF: 2.55 [1.46–4.44] vs. 0.91 [0.46–1.83]). No race/gender-based differences were observed in risk of incident HFpEF.
Conclusions:
Among older persons free of HF, men and Black participants demonstrate worse systolic performance and are at heightened risk for HFrEF, while the risk of HFpEF is similar across gender and race groups.
Keywords: heart failure, elderly, race, gender, echocardiography
Condensed abstract:
We aimed to study race/gender-based differences in HF risk factors, cardiac structure/function, and incident HF in late-life. Male gender and Black race were associated with lower mean LVEF. Black race was also associated with greater LV wall thickness and concentricity, differences that persisted after adjusting for cardiovascular comorbidities. After adjusting for cardiovascular comorbidities, men were at higher risk for HF and HFrEF in Black compared to White participants. Black race was associated with higher incidence of HF overall and HFrEF in men only. No race/gender-based differences were observed in risk of incident HFpEF.
Introduction
Heart failure (HF) incidence and prevalence are increasing in the United States.(1,2) Important age and gender differences in HF epidemiology have been reported. In early adulthood and mid-life, Black Americans demonstrate higher HF incidence and worse prognosis after HF develops,(3–6) while White women demonstrate lower HF incidence compared to Black women and to men (of either race).(7) Race and gender-based differences have also been observed in risk of HF with reduced (HFrEF) compared to HF with preserved ejection fraction (HFpEF). In a recent analysis of persons >45 years of age in 2 large cohort studies, the lifetime risk for HFrEF was higher in men compared to women and did not differ by race.(8) In contrast, the lifetime risk for HFpEF did not differ by gender but was higher in non-Black compared to Black participants.(8) Community-based surveillance of HF hospitalizations also demonstrated greater HFrEF-related hospitalization in men compared to women without differences by race, while hospitalization for HFpEF was more common in White women compared to Black women and to men (of either race).(9) Race and gender-based differences also exist in HF risk factors and associated alterations in cardiac structure and function. For example, diabetes and hypertension appear more strongly associated with HF incidence in women compared to men.(10,11) Furthermore, in early adulthood and mid-life, Black men demonstrate greater left ventricular (LV) size, mass, and concentricity, and worse systolic and diastolic function when compared to other groups.(12,13)
HF incidence and prevalence are highest in late life, with ≥80% of HF hospitalizations occurring in persons age ≥65 years.(14) Most of community-based persons in this age range have HF risk factors (American Heart Association/American College of Cardiology Stage A HF) or asymptomatic cardiac dysfunction (Stage B HF), and are at elevated risk for developing symptomatic HF.(15,16) However, despite important influences in early adulthood and mid-life, race- and gender-based differences in HF risk factors, cardiac structure and function, and incident HF (HFrEF and HFpEF) in late life remain understudied.(17–20) We aimed to address these knowledge gaps by studying community-based participants in the Atherosclerosis Risk in Communities (ARIC) longitudinal cohort study who were ≥65 years of age and free of HF at the fifth study visit (2011–2013).
Methods
Study Population
ARIC is an ongoing prospective epidemiologic cohort study that enrolled 15,792 participants between age 45–64 years in 1987–1989 at 4 U.S. centers: Forsyth County, NC; Jackson, MS; suburban Minneapolis, MN; and Washington County, MD. The design and rationale of the study have been previously described.(21) For this analysis, we evaluated 6,538 participants who attended the fifth study visit (Visit 5, 2011–2013) at which time comprehensive echocardiography was performed.(22) Gender and race were self-reported at Visit 1. We excluded participants without echocardiographic data (n=420), with race that was neither Black nor White (n=16), and with prevalent HF at Visit 5 (n=953). Prevalent heart failure was defined as any of the following: physician-adjudicated HF for hospitalizations that occurred after 2005,(23) International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) 428 code in any position for hospitalization prior to 2005,(7) or self-reported HF at Visits 3–5 or on annual follow-up phone calls. A total of 5,149 participants were included in this analysis. The ARIC study protocol was approved by institutional review boards at all 4 field centers. All participants provided written informed consent.
Ascertainment of Clinical Risk Factors at Visit 5
Since study inception (1987–1989), ARIC participants have been followed for hospitalizations for coronary heart disease (CHD) events (definite/probable myocardial infarction, or coronary revascularization), which undergo chart abstraction and committee adjudication as previously described.(24) CHD was defined as self-report of CHD at Visit 1 or an adjudicated incident CHD event between Visits 1 and 5. Atrial fibrillation was ascertained based on electrocardiograms obtained during study visits and hospital discharge codes.(25) Hypertension was defined based on blood pressure ≥140/90 mmHg at any study visit or self-report of medication use. Diabetes mellitus was defined based on fasting glucose ≥126 mg/dL or non-fasting glucose ≥200 mg/dL, self-report of a physician diagnosis, or anti-diabetic medication use at any study visit.
Echocardiography Measurements at Visit 5
Detailed echocardiographic imaging and analysis protocol at ARIC Visit 5, including reproducibility metrics, have been previously described.(22) Briefly, trained sonographers at each Field Center performed transthoracic echocardiogram by a study specific imaging protocol and using uniform dedicated Philips IE33 machines (Andover, MA). Quantitative analysis was performed in a dedicated reading center (Brigham and Women’s Hospital, Boston, MA) according to American Society of Echocardiography guidelines(26–28) by expert technicians and certified echocardiographers who were blinded to clinical characteristics. Based on ARIC reference limits, LV hypertrophy (LVH) was defined as LV mass indexed to height2.7 (LVMi): >41.5 g/m2.7 in women and >45 g/m2.7 in men.(15) Pattern of LVH was defined as concentric if relative wall thickness (RWT) ≥0.42 and eccentric if <0.42, while concentric remodeling was defined RWT ≥0.42 in the absence of LVH. LV enlargement was defined by LV end-diastolic volume (LVEDV) indexed to body surface area per ARIC reference limits: >60.2 mL/m2 in men and >51.9 mL/m2 in women. Abnormal LV structure was defined by the presence of LV enlargement or LVH. ARIC reference limits were previously established based on gender-specific 95th percentile limits derived from a subgroup of 413 healthy participants without prevalent cardiovascular disease or risk factors(15); abnormal LVEF was defined as <59.0% in men and <57.4% in women, and abnormal global longitudinal strain (GLS) was defined as >−14.7% in men and >−15.2% in women. Abnormal systolic function was defined by reduced LVEF or GLS. ARIC-based reference limits for diastolic function parameters were:(29) septal e’ <4.6 cm/s for men and <4.5 cm/s for women; septal E/e’ >13.3 for men, >15.1 for women; and left atrial volume index, >31 mL/m2 for men, >30 mL/m2 for women. Abnormal diastolic function was defined by abnormality in at least one of the following measurements: septal e’, septal E/e’, and left atrial volume index.
Clinical Outcomes
Participants were followed for a median of 5.5 [interquartile interval 5.0–6.0] years post-Visit 5 for incident HF or death. Incident HF events were ascertained through ARIC surveillance for hospitalizations with HF-related ICD discharge codes, with subsequent chart abstractions and physician adjudication as previously described.(23) Chart abstraction included LVEF during hospitalization when available. LVEF during the incident HF hospitalization was not available in 41 participants. Incident HFrEF was defined as an adjudicated HF hospitalization with LVEF <50% at the time of hospitalization, while HFpEF was defined as an adjudicated HF hospitalization with an LVEF ≥50% at the time of hospitalization. Death was ascertained by ARIC surveillance or National Death Index.
Statistical Analysis
Participants were categorized by gender and race. Clinical characteristics, laboratory variables, and echocardiographic measures of LV structure, systolic function, and diastolic function were described by each race-gender category (White men, Black men, White women, and Black women). Continuous variables are shown as mean ± standard deviation or mean ± standard error in adjusted models. Categorical variables are shown as percentages. Association of race with clinical risk factors and echocardiographic measures was performed stratified by gender using t-test for continuous variables and chi-square test for categorical variables. Comparisons of echocardiographic measures between groups were adjusted for age using multivariable linear and logistic regression. Comparisons were then additionally adjusted for the following HF risk factors that differed significantly between groups: hypertension, body mass index (BMI), diabetes, history of CHD, atrial fibrillation, and education level. Multiplicative interaction terms were used to test for effect modification of gender on the association of race with clinical HF risk factors and echocardiographic measures.
Kaplan-Meier method was used to plot survival curves that were stratified by race-gender group. Multivariable Cox proportional hazards models were used to assess the relationship of race and gender with the following clinical outcomes post-Visit 5: incident HF, HFpEF, HFrEF; and HF hospitalization/death. For incident HFrEF as an outcome, participants developing HFrEF or HF with unknown LVEF were censored at the date of HF. Similarly, for incident HFrEF, participants developing HFpEF or HF with unknown LVEF were censored at the date of HF. Two multivariable models were created: Model 1 adjusted for age; and Model 2 additionally adjusted for variables that differed significantly by races in Table 1: hypertension, BMI, diabetes, CHD, atrial fibrillation, and education level. To assess to potential impact of HF events with unknown LVEF, additional sensitivity analyses were performed assigning incident HF with unknown LVEF events as either HFpEF or HFrEF. To account for the potential impact of non-random Visit 5 non-attendance, additional sensitivity analyses were performed incorporating inverse-probability-of-attrition weighting (IPAW).(30) Visit 5 nonattendance was modeled among participants alive at the beginning of visit 5 using the following Visit 1 covariates: age, gender, race, study center, systolic and diastolic blood pressure, heart rate, BMI, smoking and drinking status, diabetes, hypertension, and chronic kidney disease (defined as estimated glomerular filtration <60 mL/min/1.73 m2). The resulting calculated weights were incorporated into multivariable models for the associations of race and gender with cardiac structure and function.
Table 1.
Clinical characteristics of participants free of prevalent heart failure by gender and race
| Men | Women | P race-gender interaction* | |||||
|---|---|---|---|---|---|---|---|
| White (n = 1764) | Black (n = 337) | P value* | White (n = 2377) | Black (n = 671) | P value* | ||
| Age (years) | 76 ± 5 | 74 ± 5 | <0.001 | 75 ± 5 | 74 ± 5 | <0.001 | 0.39 |
| Study center | <0.001 | <0.001 | 0.46 | ||||
| Forsyth | 28% | 10% | 27% | 7% | |||
| Jackson | 0% | 87% | 0% | 92% | |||
| Minneapolis | 39% | 1% | 38% | 0% | |||
| Washington | 33% | 2% | 35% | 1% | |||
| CHD | 19% | 6% | <0.001 | 6% | 2% | 0.002 | 0.33 |
| Atrial fibrillation | 7% | 2% | 0.002 | 4% | 2% | 0.014 | 0.42 |
| Hypertension | 80% | 89% | <0.001 | 77% | 95% | <0.001 | <0.001 |
| Systolic BP (mmHg) | 128 ± 16 | 133 ± 18 | <0.001 | 130 ± 18 | 135 ± 19 | <0.001 | 0.93 |
| Heart rate (beats per min) | 60 ± 10 | 64 ± 11 | <0.001 | 63 ± 10 | 64 ± 11 | 0.017 | <0.001 |
| Diabetes mellitus | 36% | 45% | 0.003 | 29% | 42% | <0.001 | 0.24 |
| BMI (kg/m2) | 28 ± 4 | 29 ± 5 | 0.87 | 28 ± 6 | 31 ± 7 | <0.001 | <0.001 |
| BMI ≥30 | 31% | 35% | 0.6 | 29% | 48% | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 70 ± 16 | 74 ± 19 | 0.005 | 70 ± 15 | 74 ± 20 | <0.001 | 0.50 |
| eGFR <60 | 27% | 23% | 0.81 | 25% | 24% | 0.77 | 0.62 |
| NT-proBNP (pg/mL) | 111 [59, 233] | 69 [31, 150] | <0.001 | 152 [81,264] | 84 [46, 157] | <0.001 | 0.13 |
| hs-troponin T (ng/L) | 1.3 [0.9, 1.8] | 1.4 [1.0, 2.1] | <0.001 | 0.8 [0.6, 1.2] | 1.0 [0.7, 1.3] | <0.001 | 0.08 |
| Current smoking | 5% | 8% | 0.12 | 6% | 6% | 0.94 | 0.26 |
| Current alcohol use | 64% | 32% | <0.001 | 55% | 17% | <0.001 | 0.025 |
| Education level | <0.001 | 0.001 | 0.009 | ||||
| Did not complete HS | 8% | 27% | 9% | 24% | |||
| Graduated high/vocational school | 39% | 27% | 50% | 32% | |||
| Attended at least college | 53% | 46% | 42% | 44% | |||
Values represent mean ± standard deviation or percentages.
For all variables except age, P values are adjusted for age. NT-proBNP, hs-CRP, and hs-troponin T were log-transformed for the multivariable modeling.
Abbreviations: CHD – coronary heart disease; BP – blood pressure; BMI – body mass index; eGFR – estimated glomerular filtration rate; NT-proBNP – N-terminal pro-brain natriuretic peptide; hs-troponin – high-sensitivity troponin T
Two-sided P values <0.05 were considered significant. Analyses were performed using Stata version 14 (College Station, TX).
Results
Gender, HF risk factors, and cardiac structure and function
Among the 5,149 ARIC participants free of prevalent HF at Visit 5, the mean age was 75±5 years, 59% were women, and 20% were Black race. CHD was present in 10% and atrial fibrillation in 5%. Hypertension (81%), and diabetes (34%) were common. CHD was more common in men compared to women (5% vs. 17% respectively, P<0.001), while atrial fibrillation and diabetes were less frequent in men compared to women (atrial fibrillation: 4% vs. 6% respectively, P<0.001; diabetes: 32% vs. 38%, P<0.001). N-terminal pro-brain natriuretic peptide (NT-proBNP) values were higher in women compared to men (P<0.001 for both), whereas high-sensitivity troponin T (hs-TnT) values were higher in men (P<0.001).
The absolute values for LV end-diastolic diameter (4.2 ± 0.4 vs. 4.6 ± 0.5, P<0.001) and mean wall thickness (0.95 ± 0.12 vs. 1.02 ± 0.14, P<0.001) were greater in men compared to women; however, using gender-specific cut-points, women had higher prevalence of LVH (26% vs. 20%, P<0.001), concentric hypertrophy (13% vs. 11%, P=0.033), and eccentric hypertrophy (12% vs. 8%, P<0.001). In models adjusted for age, men had lower adjusted mean LVEF (64% ± 0.1 vs. 67% ± 0.1, P<0.001) and lower absolute GLS (17.6 ± 0.0 vs. 18.4 ± 0.0, P<0.001) compared to women. Men had higher TDI e’ (5.8 cm/s ± 0.0 vs. 5.7 cm/s ± 0.0, P<0.001) and lower E/e’ ratio (11.4 ± 0.1 vs. 12.7 ± 0.1, P<0.001), but larger LA volume index (26.7 mL/m2 ± 0.2 vs. 24.6 mL/m2 ± 0.2, P<0.001), compared to women.
Race, HF risk factors, and cardiac structure and function
Black race was associated with a higher prevalence of diabetes and hypertension, and a lower prevalence of CHD and atrial fibrillation (Table 1). Log hs-TnT was higher in Black participants compared to White participants regardless of gender (P<0.001 for both), whereas log NT-proBNP was higher in White participants compared to Black in both men and women (P<0.001 for both). Race-based differences in hypertension prevalence were more prominent in women compared to men. Black race was associated with a higher prevalence of obesity only in women. Consistent with known differences in socio-economic status, Black race was associated with higher prevalence of not completing high school.
Black race was associated with smaller LV end-diastolic dimension, higher RWT, and higher prevalence of concentric remodeling, after adjusting for age, hypertension, body mass index, diabetes, history of coronary heart disease, atrial fibrillation, and education level (Table 2). Black race was associated with greater LV wall thickness in women but not men, resulting in an association with lower LV mass index in men but not women. Abnormal LV structure, defined as the presence of LVH, LV enlargement, or moderate/greater valvular disease was present in 29% of participants overall, and was lowest among Black men (21%; Figure 1). Among both men and women, Black race was associated with worse LVEF and global longitudinal strain (Table 2). Abnormal systolic function, based on reduced LVEF or GLS, was present in 17% of the study participants overall, and the prevalence was highest among Black men (30%; Figure 1). Consistent race-based differences in diastolic measures were not observed. Black race was associated with lower e’ but not E/e’ ratio, and with larger LAVi in women but smaller LAVi in men (interaction P<0.001). These findings largely persisted in unadjusted models (Supplemental Table 1). Similar findings were also observed in sensitivity analyses incorporating inverse–probability-of-attrition weighting to account for potential attendance bias (Supplemental Table 2).
Table 2.
Cardiac structure and function among participants free of prevalent heart failure by gender and race
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| White (n = 1764) | Black (n = 337) | P value | White (n = 2377) | Black (n = 671) | P value | P race-gender interaction | |
| LV structure | |||||||
| LVEDD (cm) | 4.63 ± 0.01 | 4.46 ± 0.03 | <0.001 | 4.25 ± 0.01 | 4.12 ± 0.02 | <0.001 | 0.08 |
| Mean WT (cm) | 1.02 ± 0.00 | 1.03 ± 0.01 | 0.27 | 0.94 ± 0.00 | 0.97 ± 0.00 | <0.001 | 0.14 |
| LVMi (g/m2.7) | 38 ± 0 | 36 ± 1 | <0.001 | 37 ± 0 | 35 ± 0 | <0.001 | 0.80 |
| LVH | 20% | 14 % | 0.01 | 28% | 20% | <0.001 | 0.58 |
| RWT | 0.42 ± 0.00 | 0.44 ± 0.00 | <0.001 | 0.42 ± 0.00 | 0.44 ± 0.00 | <0.001 | 0.79 |
| RWT >0.42 | 42% | 53% | <0.001 | 44% | 54% | <0.001 | 0.68 |
| LV geometry | |||||||
| Normal | 49% | 42% | 0.029 | 43% | 38% | 0.02 | 0.90 |
| Concentric remodeling | 30% | 43% | <0.001 | 29% | 43% | <0.001 | 0.81 |
| Concentric LVH | 12% | 10% | 0.48 | 14% | 11% | 0.031 | 0.65 |
| Eccentric LVH | 9% | 4% | 0.004 | 14% | 9% | 0.001 | 0.07 |
| LVEDVi (mL/m2) | 48.4 ± 0.3 | 48.2 ± 0.6 | 0.69 | 39.5 ± 0.2 | 40.6 ± 0.3 | 0.007 | 0.031 |
| Abnormal LVEDVi† | 13% | 9% | 0.10 | 8% | 8% | 0.98 | 0.16 |
| Moderate or greater valvular disease* | 3% | 3% | 0.88 | 4% | 2% | 0.06 | 0.19 |
| LV systolic function | |||||||
| LVEF (%) | 64.5 ± 0.1 | 63.1 ± 0.3 | <0.001 | 66.9 ± 0.1 | 66.0 ± 0.2 | <0.001 | 0.18 |
| Abnormal LVEF† | 14 % | 20% | 0.003 | 4% | 6% | 0.16 | 0.47 |
| LVEF <50%* | 2% | 5% | <0.001 | 1% | 1% | 0.27 | 0.34 |
| GLS | −17.7 ± 0.1 | −17.0 ± 0.1 | <0.001 | −18.4 ± 0.0 | −18.1 ± 0.1 | <0.001 | 0.034 |
| Abnormal GLS† | 10% | 20% | <0.001 | 9% | 13% | 0.006 | 0.12 |
| LV diastolic function | |||||||
| TDI e’ (cm/s) | 5.9 ± 0.0 | 5.6 ± 0.1 | 0.015 | 5.7 ± 0.0 | 5.6 ± 0.1 | 0.027 | 0.78 |
| Abnormal e’† | 11% | 16% | 0.041 | 10% | 14% | 0.003 | 0.58 |
| E/e’ ratio | 11.4 ± 0.1 | 11.8 ± 0.2 | 0.14 | 12.6 ± 0.1 | 12.7 ± 0.2 | 0.66 | 0.42 |
| Abnormal E/e’† | 15% | 16% | 0.75 | 11% | 12% | 0.39 | 0.94 |
| LAVi (mL/m2) | 27.0 ± 0.2 | 25.6 ± 0.5 | 0.023 | 24.4 ± 0.2 | 25.2 ± 0.3 | 0.013 | <0.001 |
| Abnormal LAVi† | 16% | 12% | 0.14 | 12% | 17% | 0.003 | 0.005 |
Values represent mean ± standard error or percentages. All P values were adjusted for age, hypertension, body mass index, diabetes, history of coronary heart disease, atrial fibrillation, and education level
except for moderate or greater valvular disease and LVEF <50% variables which were only adjusted for age to avoid overfitting.
indicates values worse than the ARIC-based reference ranges.
Abbreviations: LV – left ventricle; LVEDD – LV end diastolic diameter; WT – wall thickness; LVMi – LV mass index; LVH – LV hypertrophy; RWT – relative wall thickness; LVEDVi – LV end diastolic volume index; LVEF – LV ejection fraction; GLS – global longitudinal strain; TDI e’ – tissue Doppler imaging septal e’; LAVi – left atrial volume index
Figure 1. Cardiac structural and functional abnormalities in participants without heart failure.

Prevalence of cardiac structural abnormalities and left ventricular systolic and diastolic abnormalities among participants free of prevalent heart failure in subgroups defined according to gender and race.
Differences in HF incidence among race-gender groups
Over a median follow-up of 5.5 years [interquartile interval 5.0–6.0], 303 participants developed incident HF (135 HFpEF, 127 HFrEF, 41 with unknown LVEF) and 490 participants died. Of those with LVEF recorded, 20 had LVEF between 40–50%. Age-adjusted incidence of HF was highest among Black men (19.7 per 1000 person-years, 95% CI 12.6 – 26.8; Figure 2), followed by White men (13.6 per 1000 person-years, 95% CI 11.2 – 16.0). Male gender was associated with higher incidence of HF overall (HR 1.33, 95% 1.05 – 1.69) and HFrEF (HR 1.94, 95% CI 1.34 – 2.81), but not HFpEF (HR 0.90, 95% CI 0.63 – 1.30), in fully adjusted models (Model 2). Male gender was associated with heightened risk of HFrEF in both race groups (Table 3, Central Illustration), but this association was more pronounced in Black compared to White participants (HR 3.70 [95% CI 1.72 – 7.95] vs 1.55 [1.01 – 2.37] respectively, interaction P=0.039; Table 3B). Conversely, Black race was associated with higher incidence of HF overall and HFrEF in men but not women in fully adjusted models (Table 3, Central Illustration). There was no significant association between race or gender and risk of incident HFpEF. Similar findings were observed and in analyses using the composite endpoints of HF or death (Supplemental Table 3, Supplemental Figure 1), and in sensitivity analyses classifying HF events with unknown LVEF as HFrEF (Supplemental Table 4A) or as HFrEF (Supplemental Table 4B). Similar findings were also observed in models were adjusting for body mass index (BMI) as a categorical variable as opposed to a continuous variable (Supplemental Table 5), and in analyses censoring participants with intercurrent MI prior to HF event (Supplemental Table 6).
Figure 2. Association of race-gender groups with incident HF in late-life.
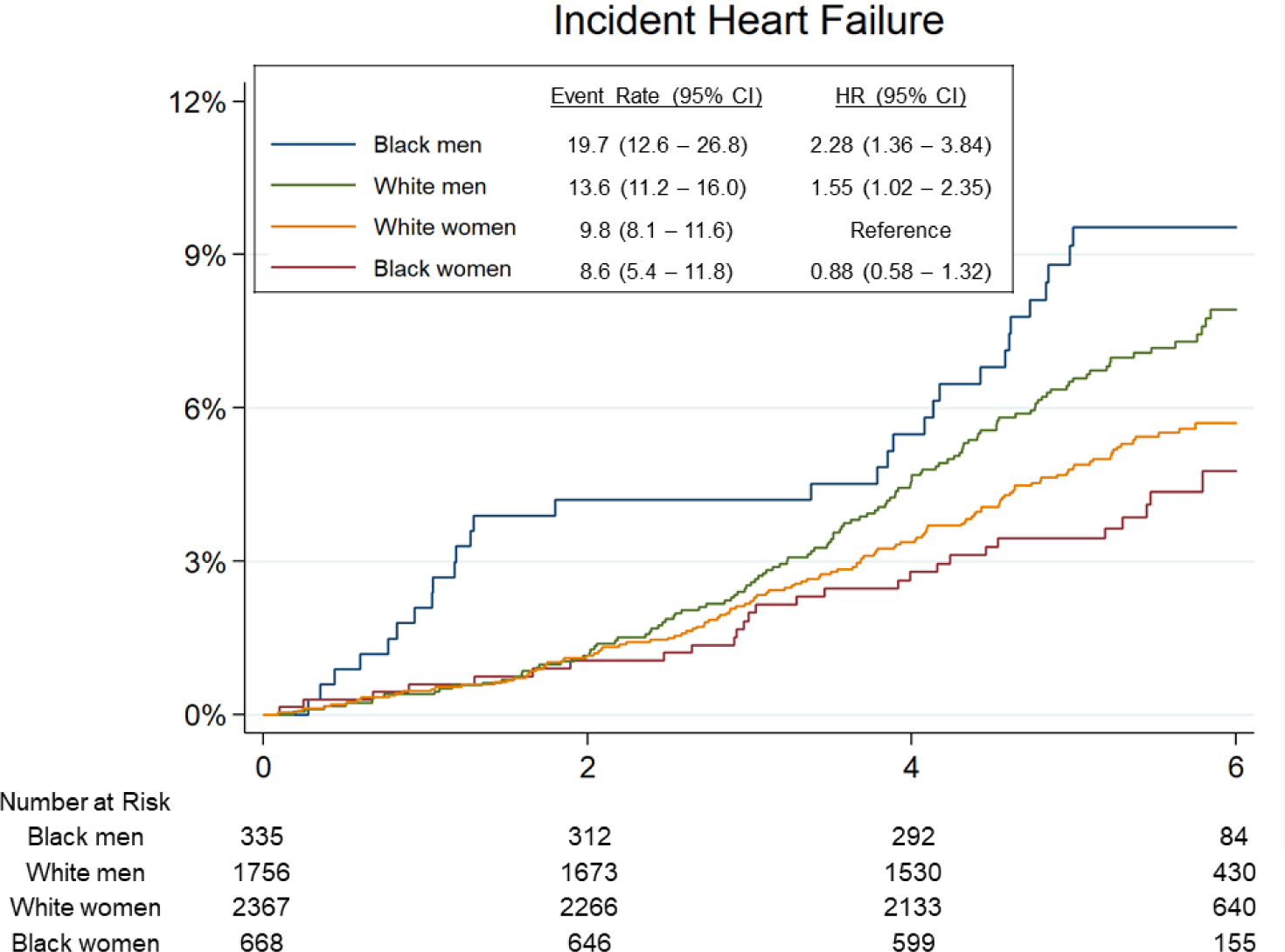
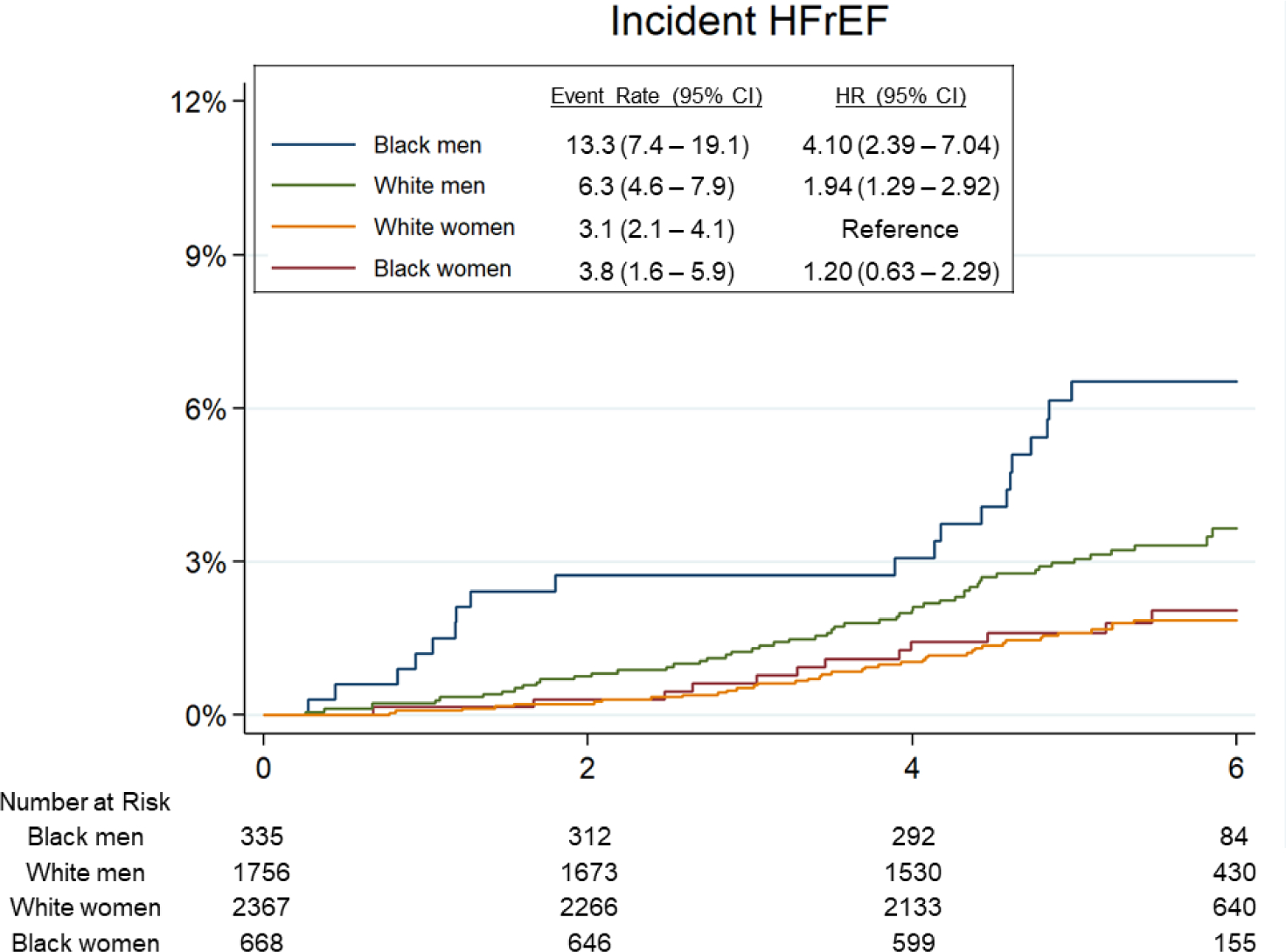
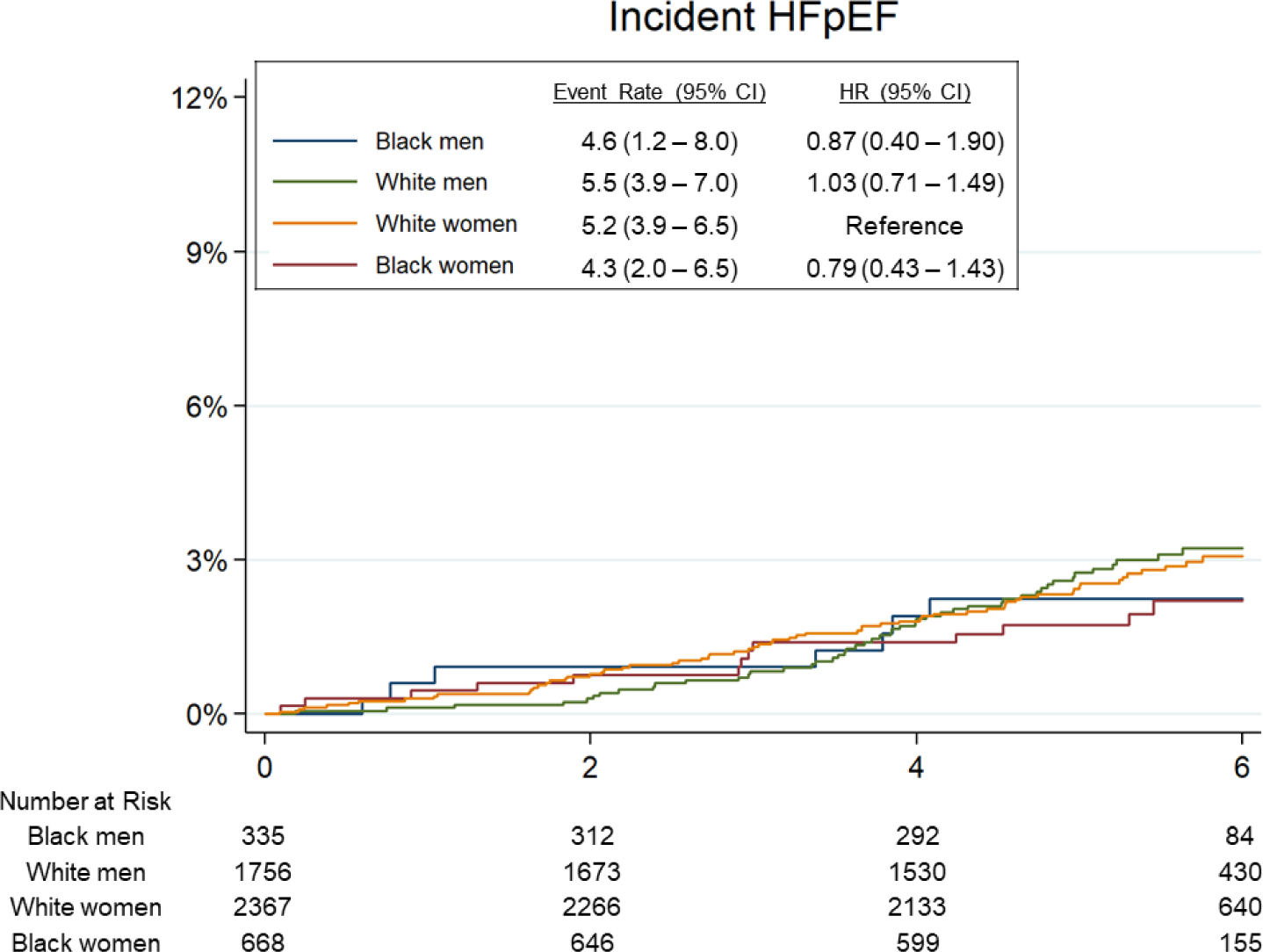
(A) Incident heart failure (HF) overall. (B) Incident heart failure with reduced ejection fraction (HFrEF). (C) Incident heart failure with pre-served ejection fraction (HFpEF). Median follow-up time for the end point was 5.5 years (interquartile interval: 5.0–6.0 years). Event rate is expressed per 1,000 person-years and is adjusted for age. HRs are adjusted for age.
Table 3.
Association of Black race and male gender with risk of incident heart failure
| A. Heart failure overall (303 events) | ||||
| Age-adjusted event rate per 1000 person-years (95% CI) | Black | White | Age-adjusted Black-to-White HR (95% CI) | Age- and RF- adjusted Black-to-White HR (95% CI) |
| Men | 19.7 (12.6 – 26.8) | 13.6 (11.2 – 16.0) | 1.45 (0.97 – 2.16) P=0.069 |
1.65 (1.07 – 2.53) P=0.023 |
| Women | 8.6 (5.4 – 11.8) | 9.8 (8.1 – 11.6) | 0.87 (0.58 – 1.31) P=0.51 |
0.76 (0.49 – 1.17) P=0.21 |
| Age- adjusted men-to-women HR (95% CI) | 2.20 (1.31 – 3.68) P=0.003 |
1.34 (1.04 – 1.72) P=0.023 |
P interaction of gender-race=0.09 | |
| Age- and RF- adjusted men-to-women HR (95% CI) | 2.36 (1.37 – 4.08) P=0.27 |
1.16 (0.89 – 1.51) P=0.27 |
P interaction of gender-race=0.016 | |
| B. Heart failure with reduced ejection fraction (127 events) | ||||
| Age-adjusted event rate per 1000 person-years (95% CI) | Black | White | Age-adjusted Black-to-White HR (95% CI) | Age- and RF- adjusted Black-to-White HR (95% CI) |
| Men | 13.3 (7.4 – 19.1) | 6.3 (4.6 – 7.9) | 2.12 (1.27 – 3.54) P=0.004 |
2.55 (1.46 – 4.44) P=0.001 |
| Women | 3.8 (1.6 – 5.9) | 3.1 (2.1 – 4.1) | 1.19 (0.62 – 2.28) P=0.60 |
0.91 (0.46 – 1.83) P=0.80 |
| Age- adjusted men-to-women HR (95% CI) | 3.41 (1.67 – 6.98) P=0.001 |
1.94 (1.29 – 2.92) P=0.001 |
P interaction of gender-race=0.18 | |
| Age- and RF- adjusted men-to-women HR (95% CI) | 3.70 (1.72 – 7.95) P=0.001 |
1.55 (1.01 – 2.37) P=0.045 |
P interaction of gender-race=0.039 | |
| C. Heart failure with preserved ejection fraction (135 events) | ||||
| Age-adjusted event rate per 1000 person-years (95% CI) | Black | White | Age-adjusted Black-to-White HR (95% CI) | Age- and RF- adjusted Black-to-White HR (95% CI) |
| Men | 4.6 (1.19 – 8.05) | 5.5 (3.94 – 7.01) | 0.84 (0.38 – 1.86) P=0.67 |
0.88 (0.37 – 2.11) P=0.77 |
| Women | 4.3 (2.03 – 6.53) | 5.2 (3.94 – 6.47) | 0.82 (0.46 – 1.47) P=0.51 |
0.75 (0.41 – 1.36) P=0.34 |
| Age- adjusted men-to-women HR (95% CI) | 1.03 (0.42 – 2.55) P=0.95 |
1.02 (0.70 – 1.47) P=0.93 |
P interaction of gender-race=0.99 | |
| Age- and RF- adjusted men-to-women HR (95% CI) | 0.97 (0.36 – 2.62) P=0.96 |
0.90 (0.61 – 1.33) P=0.60 |
P interaction of gender-race=0.83 | |
Abbreviations: CI – confidence intervals; HR – hazard ratio; RF – risk factor. Risk factors included age, hypertension, body mass index, diabetes, history of coronary heart disease, atrial fibrillation, and education level.
Central Illustration. Association of Black race and male gender with incident HF.
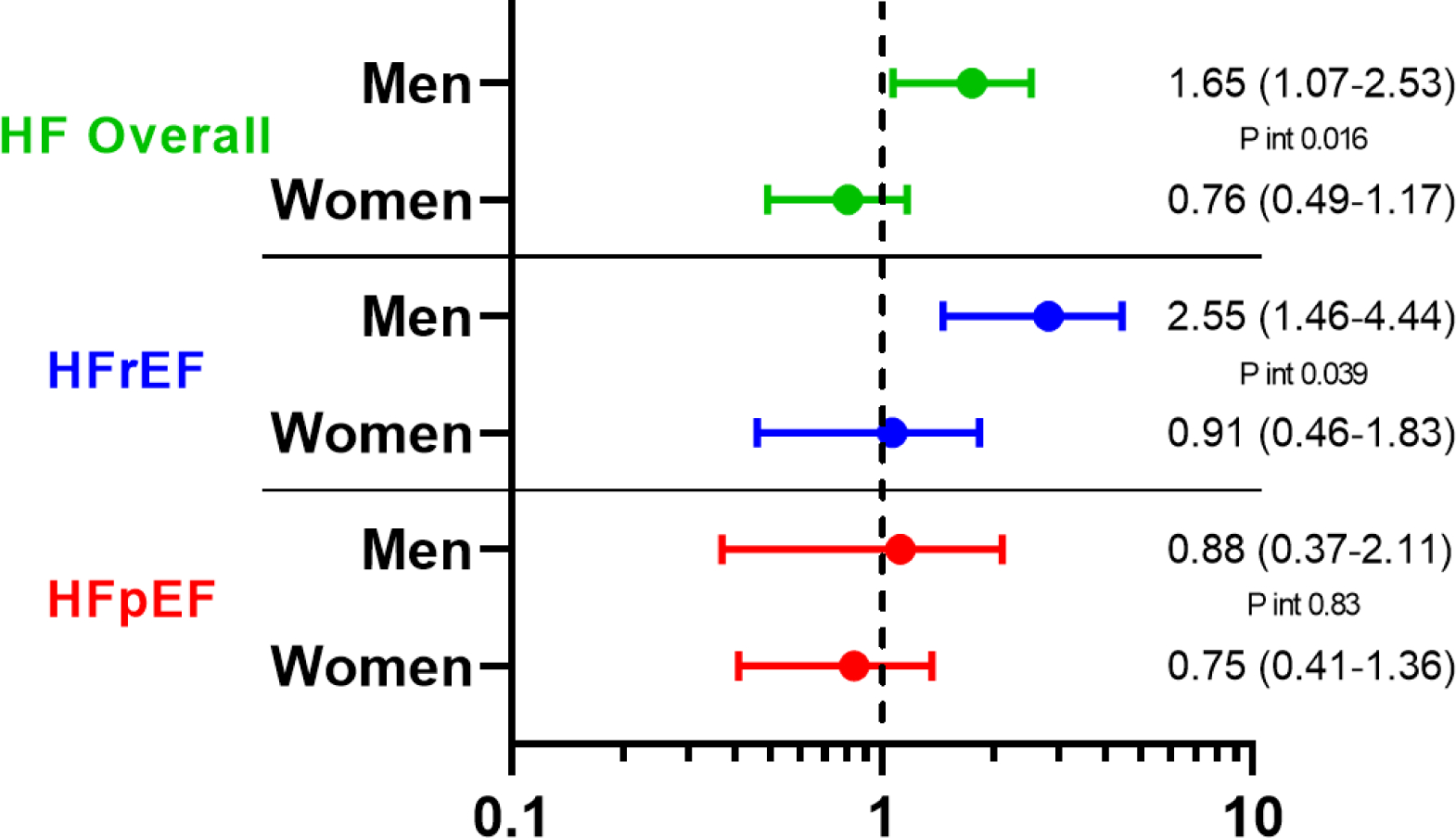
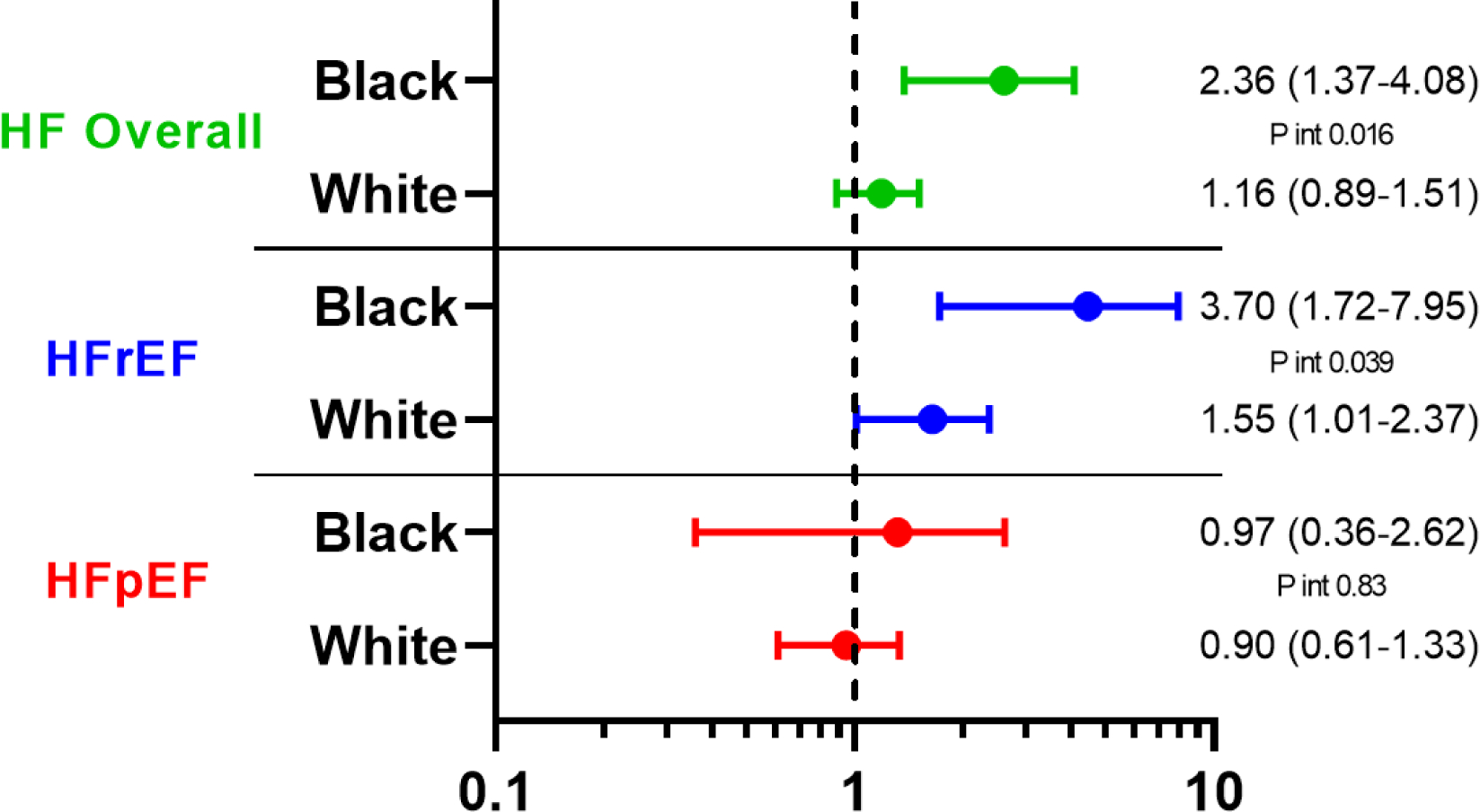
Hazard ratio for incident HF hospitalization associated with Black race (Panel A) and male gender (Panel B) among participants free of prevalent HF. Multivariable models are adjusted for age, study center, hypertension, body mass index, diabetes, history of coronary heart disease, atrial fibrillation, and education level.
Given the recognized differences in circulating NT-proBNP and hs-TnT levels by gender and race, we examined the association of NT-proBNP and hs-TnT with incident HF by race-gender groups and found no evidence of effect modification of race-gender on the association of NT-proBNP or hs-TnT with incident HF, HFrEF, or HFpEF (Supplemental Tables 7 and 8). We also performed additional analyses adjusting for NT-proBNP, hs-TnT, or both in models relating race-gender group to incident HF. For both incident HF overall and incident HfrEF, further adjustment for NT-proBNP accentuated the relative risk associated with Black race, without appreciable change in the race-sex interaction (Figure 3; Supplemental Tables 9 and 10). Adjustment for hs-TnT resulted in modest attenuation of the risk associated with Black race in men and associated with male gender in both races. As a result, models accounting for both NT-proBNP and hs-TnT resulted in similar findings to our primary analysis not including cardiac biomarkers.
Figure 3. Race and gender’s associations with incident HF adjusting for biomarkers.
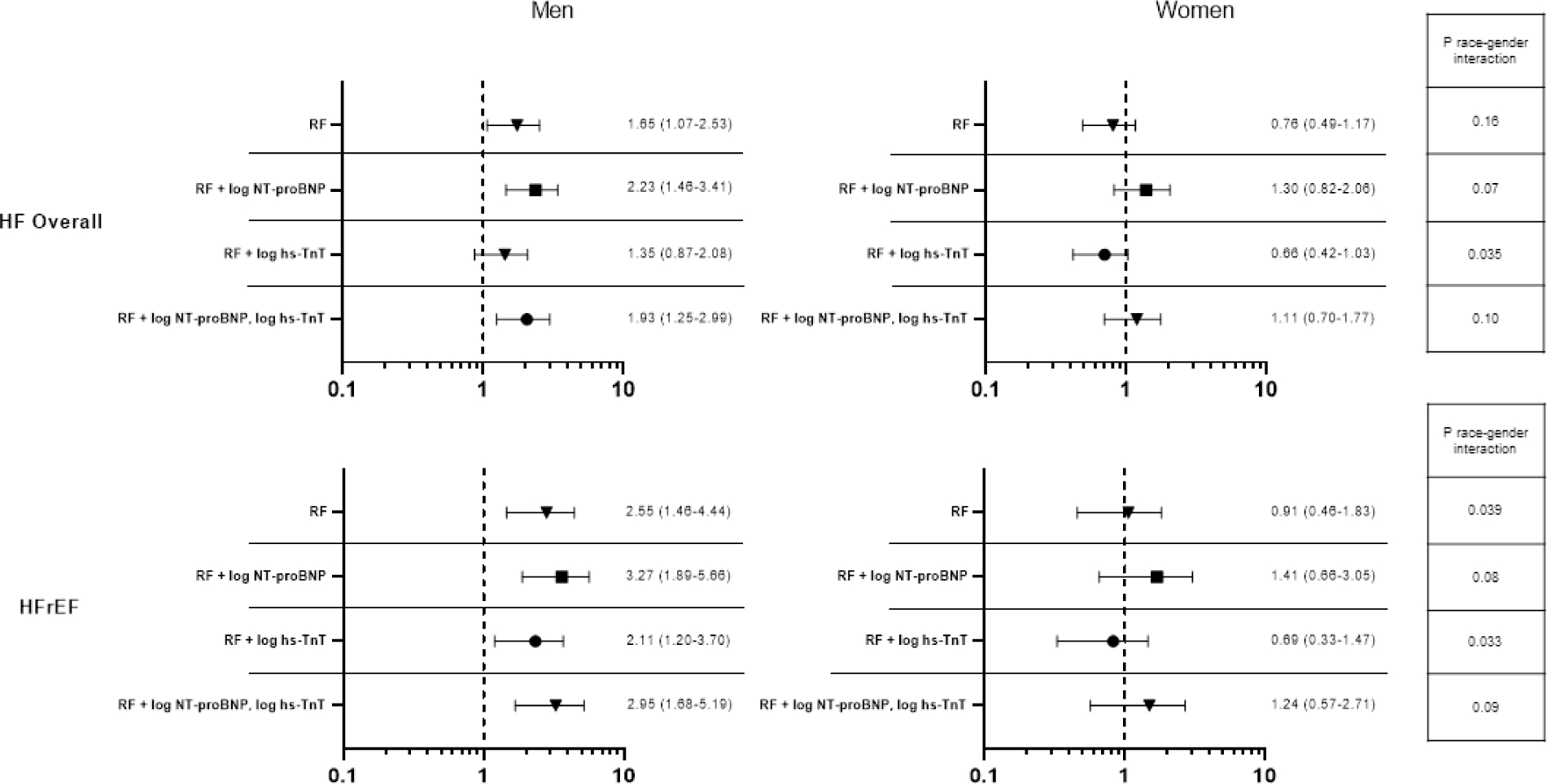
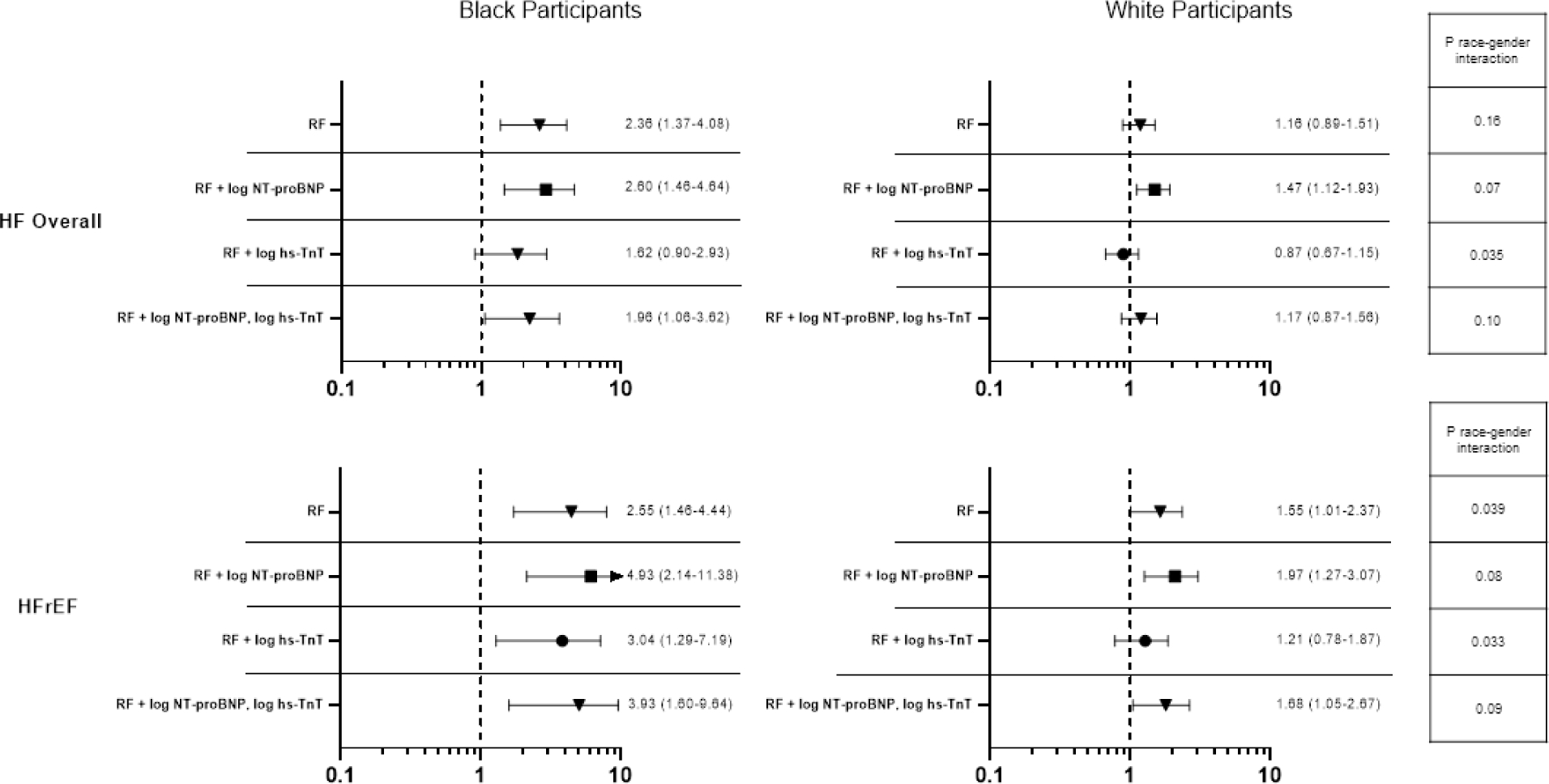
HR (95% CI) for incident HF hospitalization associated with Black race (A) and male gender (B) among participants free of prevalent HF. Risk factors included age, hypertension, body mass index, diabetes, history of coronary heart disease, atrial fibrillation, and education level. NT-proBNP ¼ N-terminal pro–B-type natriuretic peptide; hs-TnT ¼ high-sensitivity troponin T; RF ¼ risk factor; other abbreviations as in Figure 2.
Discussion
Our analysis of gender- and race-based differences in cardiac structure and function and risk of incident HF in the community-based ARIC cohort in late life (age 66–90 years) has 3 major findings. First, men demonstrated worse measures of LV systolic function and were at higher risk of incident HFrEF compared to women, particularly among Black participants. Second, Black race is associated with greater LV concentric remodeling and worse LV systolic function in both genders, and a heightened risk of HFrEF among men. Third, consistent gender- and race-based differences in diastolic measures were not observed, and the risk of incident HFpEF was similar across gender and race groups. The persistence of these differences in HF risk – and HFrEF risk in particular – by race-gender group in analyses adjusting for traditional cardiovascular risk factors suggest important residual risk related to race and gender that persists into late life.
Differences in the prevalence of HF risk factors have been observed by race and gender. Consistent with prior studies, Black men and Black women had higher prevalence of diabetes and hypertension(31) and lower prevalence of atrial fibrillation(32,33) and non-fatal CHD(34) compared to White men and women in our study. Black women have previously been reported to have the highest rates of obesity in the U.S., followed by Black men.(35) In our study of persons in late life, we also found that Black women had a significantly higher prevalence of obesity compared to White women, but did not observe a difference in obesity prevalence between Black and White men. Given that Black participants demonstrate higher incidence and worse prognosis of HF in younger age groups, thought to be caused by higher risk factor burden,(3,4) this discrepancy could be explained by the lower life expectancy of Black men(36) causing survival bias in our late-life cohort.
Important gender- and race-based differences have also been described in cardiovascular changes with age and in response to hemodynamic stress.(40,41) Black Americans more frequently develop HF in the context of hypertension, and based on studies in mid-life, appear to develop worse diastolic dysfunction,(37) concentric remodeling,(38) and LVH(39) for a set degree of hypertension. In studies of persons in early adulthood and mid-life, Black men demonstrate greater LV size, mass, RWT, and worse LV systolic and diastolic function compared to White men, White women, and Black women.(12,13) Our findings extend this association of Black race with higher RWT and worse systolic function to persons in late-life. However, greater RWT among Black participants was related primarily to smaller LV cavity size among Black compared to White participants. In contrast, Black race was only associated with greater LV wall thickness among women but not men. This finding is consistent with the steeper increase in LV mass associated with aging in women.(40) Furthermore, hypertension prevalence was particularly high in Black women, and women have previously been shown to demonstrate an exaggerated hypertrophic response to pressure overload compared to men.(41–43) We also observed worse LVEF and GLS noted among men and Black participants independent of traditional HF risk factors and education level. These findings extend to late-life previous studies demonstrating lower LVEF in men regardless of age and other cardiovascular risk factors,(12,26) and lowest GLS among Black men among gender-race groups.(12,44) In contrast, while Black men demonstrate worse LV diastolic function (higher E/A ratio and lower TDI e’) compared to White men and women and Black women in mid-life,(12) we did not observe consistent gender- or race-based differences in LV diastolic measures in late-life consistent with one other study in an older population.(45)
Multiple studies have reported higher HF incidence among Black Americans compared to White Americans in young adulthood and mid-life.(3,4) A previous analysis of the ARIC cohort at younger age (mean age 57 ± 5 years) demonstrated an association between Black race and higher incidence of HF in both genders, although this association was no longer significant after adjustment for other HF risk factors.(7) In our analysis of the ARIC cohort in late-life, Black race was associated with incident HF only in men, and this association persisted after adjustment for traditional HF risk factors and education level. Previous studies have demonstrated a higher short-term and life-time risk of HFrEF in men compared to women, but a similar risk of HFpEF in both genders.(9,46,47) Gender-based differences in HFrEF incidence may be related to earlier onset and higher burden of CHD in men when compared to women. We now extend these findings to late life, such that male gender was associated with a higher incidence HFrEF, but there was no difference in incident HFpEF. Notably, Black race was also associated with higher incidence of HFrEF but only in men, concordant with a prior cross-sectional study from ARIC community surveillance showing the highest proportion of acute HFrEF events among Black men.(48) To our knowledge, our study is one of the first to report these differences in incident HF based on gender and race in an elderly population free of prevalent HF. These differences matched well with the observation of worse systolic function among men and Black participants independent of traditional HF risk factors and education level.
Differences in circulating NT-proBNP and hs-TnT levels by gender and race are well recognized, with Black race and male gender associated with lower NT-proBNP and higher hs-TnT.(49,50) The mechanisms underlying these differences are unclear but may relate to several comorbidity severity, health behaviors, and social determinants of health not captured or accounted for most analyses, or to genetic ancestry. Consistent with prior studies, we found no evidence of effect modification of race-gender group on the association of these cardiac biomarkers with HF risk. Furthermore, differences in HF risk by race-gender group persisted after further adjusting for these biomarkers. Together, these findings indicate that differences in circulating NT-proBNP and hs-TnT levels by gender and race do not account for the disparities in HF risk in late life.
Several limitations of this analysis should be noted. Of ARIC participants alive at Visit 5, 62% chose to attend, which may introduce attendance bias and limit the generalizability of our findings. However, we performed sensitivity analysis using inverse–probability-of-attrition weighting which showed similar results as the primary analysis. Due to study design which designated LVEF <50% as HFrEF, we could not change the LVEF cut-off for HFrEF to 40% without losing a significant number of events. Notably, of those with LVEF recorded, only 23 had LVEF between 40–50%. Because the large majority of our Black participants were from one study center, we cannot disentangle race/ethnicity effects from study center effects. Furthermore, our findings may not generalize to Black Americans from other geographic regions. Our analysis cannot address the mechanisms responsible for the gender- and race-based differences observed in this analysis. Indeed, both gender and race are cultural constructs as opposed to simply biologic variables. As such, they correlate with several social determinants of health, health behaviors, and comorbidity severity not captured or accounted for in this analysis. Although we attempted to mitigate this by accounting for measures of education level in multivariable models, we acknowledge this is an incomplete surrogate of these effects.
Conclusions
Among elders in the community free of HF, men demonstrated worse measures of LV systolic function regardless of race and were at higher risk of incident HFrEF compared to women. Black race was associated with greater LV concentric remodeling and worse LV systolic function in both genders and a heightened risk of HFrEF among men. The risk of incident HFpEF was similar across gender and race groups. Further studies are necessary to better understand the factors contributing to these gender- and race-based differences in cardiac performance and HF risk in late-life.
Supplementary Material
Clinical Perspectives.
Competency in Medical Knowledge:
Male gender and Black race are associated with worse left ventricular systolic function and heightened risk of heart failure with reduced ejection fraction late in life.
Translational Outlook:
Future studies should investigate the impact of more intensive prevention strategies in this population.
Acknowledgements:
The authors thank ARIC participants and staff for their important contributions.
Funding:
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I).
Dr. Shah was supported by NIH/NHLBI grants R01HL135008, R01HL143224, R01HL150342, R01HL148218 and K24HL152008. Dr. Chandra was supported by National Institutes of Health grant 5T32HL094301-08.
Disclosures:
Dr Shah reports receiving research support from Novartis and Philips Ultrasound through the Brigham and Women’s Hospital and consulting fees from Philips Ultrasound and Edwards Lifesciences.
Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi-Sankyo, Gilead, GSK, Ironwood, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Dinaqor, Tremeau.
Abbreviations:
- HF
heart failure
- ARIC
Atherosclerosis Risk in Communities
- LVEF
left ventricular ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HFpEF
heart failure with preserved ejection fraction
- CHD
coronary heart disease
- BMI
body mass index
- LVH
left ventricular hypertrophy
- RWT
relative wall thickness
- GLS
global longitudinal strain
Footnotes
All other authors declared no relevant disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Croft JB, Giles WH, Pollard RA, Keenan NL, Casper ML, Anda RF. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med. 1999;159:505–510. [DOI] [PubMed] [Google Scholar]
- 2.Tighe D, Brest AN. Congestive heart failure in the elderly. CardiovascClin 1992;22:127–38. [PubMed] [Google Scholar]
- 3.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017. Nov 21;136(21):e393–e423. [DOI] [PubMed] [Google Scholar]
- 6.Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013. Apr 9;61(14):1510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008. Apr 1;101(7):1016–22. [DOI] [PubMed] [Google Scholar]
- 8.Pandey A, Omar W, Ayers C, et al. Sex and Race Differences in Lifetime Risk of Heart Failure With Preserved Ejection Fraction and Heart Failure With Reduced Ejection Fraction. Circulation. 2018. Apr 24;137(17):1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang PP, Wruck LM, Shahar E, et al. Trends in Hospitalizations and Survival of Acute Decompensated Heart Failure in Four US Communities (2005–2014): ARIC Study Community Surveillance. Circulation. 2018. Jul 3;138(1):12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lundberg G, Walsh MN, Mehta LS. Gender-Specific Differences in Risk Factors for Development of Heart Failure in Women. Heart Fail Clin. 2019. Jan;15(1):1–8. [DOI] [PubMed] [Google Scholar]
- 11.Bibbins-Domingo K, Lin F, Vittinghoff E, Barrett-Connor E, Hulley SB, Grady D, Shlipak MG. Predictors of heart failure among women with coronary disease. Circulation. 2004. Sep 14;110(11):1424–30. [DOI] [PubMed] [Google Scholar]
- 12.Kishi S, Reis JP, Venkatesh BA, et al. Race-ethnic and gender differences in left ventricular structure and function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2015. Mar 13;4(3):e001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004. Jun;43(6):1182–8. [DOI] [PubMed] [Google Scholar]
- 14.Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008. Aug 5;52(6):428–34. [DOI] [PubMed] [Google Scholar]
- 15.Shah AM, Claggett B, Loehr LR, et al. Heart Failure Stages Among Older Adults in the Community: The Atherosclerosis Risk in Communities Study. Circulation. 2017. Jan 17;135(3):224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. [DOI] [PubMed] [Google Scholar]
- 17.Hsich EM, Pina IL. Heart failure in women: a need for prospective data. J Am Coll Cardiol. 2009;54:491–8. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW. Heart failure in African Americans: pathophysiology and treatment. J Card Fail. 2003;9:s210–5. [DOI] [PubMed] [Google Scholar]
- 19.Parashar S, Katz R, Smith NL, et al. Race, gender, and mortality in adults > or =65 years of age with incident heart failure (from the Cardiovascular Health Study). Am J Cardiol. 2009. Apr 15;103(8):1120–7. doi: 10.1016/j.amjcard.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000. May;35(6):1628–37. [DOI] [PubMed] [Google Scholar]
- 21.The atherosclerosis risk in communities (aric) study: Design and objectives. The aric investigators. American journal of epidemiology. 1989; 129:687–702. [PubMed] [Google Scholar]
- 22.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: The atherosclerosis risk in communities study. Circ Cardiovasc Imaging. 2014; 7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996. Feb;49(2):223–33. [DOI] [PubMed] [Google Scholar]
- 25.Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015. Jan;28(1):1–39.e14. [DOI] [PubMed] [Google Scholar]
- 27.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016. Apr;29(4):277–314. [DOI] [PubMed] [Google Scholar]
- 28.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010. Jul;23(7):685–713; quiz 786–8. [DOI] [PubMed] [Google Scholar]
- 29.Shah AM, Claggett B, Kitzman D, et al. Contemporary Assessment of Left Ventricular Diastolic Function in Older Adults: The Atherosclerosis Risk in Communities Study. Circulation. 2017. Jan 31;135(5):426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179(8):956–966. d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dyke M, Greer S, Odom E, et al. Heart Disease Death Rates Among Blacks and Whites Aged ≥35 Years - United States, 1968–2015. MMWR Surveill Summ. 2018. Mar 30;67(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128(23):2470–2477. [DOI] [PubMed] [Google Scholar]
- 33.Thomas KL, Piccini JP, Liang L, et al. Get With the Guidelines Steering Committee and Hospitals. Racial differences in the prevalence and outcomes of atrial fibrillation among patients hospitalized with heart failure. J Am Heart Assoc. 2013;2(5):e000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colantonio LD, Gamboa CM, Richman JS, et al. Black-White Differences in Incident Fatal, Nonfatal, and Total Coronary Heart Disease. Circulation. 2017. Jul 11;136(2):152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Byrd AS, Toth AT, Stanford FC. Racial Disparities in Obesity Treatment. Curr Obes Rep. 2018. Jun;7(2):130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bond MJ, Herman AA. Lagging Life Expectancy for Black Men: A Public Health Imperative. Am J Public Health. 2016. Jul;106(7):1167–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008;52:1015–21. [DOI] [PubMed] [Google Scholar]
- 38.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43;1182–8. [DOI] [PubMed] [Google Scholar]
- 39.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–9. [DOI] [PubMed] [Google Scholar]
- 40.Hees PS, Fleg JL, Lakatta EG, Shapiro EP. Left ventricular remodeling with age in normal men versus women: novel insights using three-dimensional magnetic resonance imaging. Am J Cardiol. 2002. Dec 1;90(11):1231–6. [DOI] [PubMed] [Google Scholar]
- 41.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol 1993;72:310–3. [DOI] [PubMed] [Google Scholar]
- 42.Garavaglia GE, Messerli FH, Schmieder RE, Nunez BD, Oren S. Sex differences in cardiac adaptation to essential hypertension. Eur Heart J 1989;10:1110–4. [DOI] [PubMed] [Google Scholar]
- 43.Aurigemma GP, Silver KH, McLaughlin M, Mauser J, Gaasch WH. Impact of chamber geometry and gender on left ventricular systolic function in patients >60 years of age with aortic stenosis. Am J Cardiol 1994;74:794–8. [DOI] [PubMed] [Google Scholar]
- 44.Fernandes VR, Cheng S, Cheng YJ, et al. Racial and ethnic differences in subclinical myocardial function: the Multi-Ethnic Study of Atherosclerosis. Heart. 2011; 97:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Russo C, Jin Z, Homma S, et al. Race/ethnic disparities in left ventricular diastolic function in a triethnic community cohort. Am Heart J. 2010; 160:152–158. doi: 10.1016/j.ahj.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho JE, Enserro D, Brouwers FP, et al. Predicting Heart Failure With Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2014;113:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta DK, Claggett B, Wells Q, et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc. 2015;4(5):e001831. Published 2015 May 21. doi: 10.1161/JAHA.115.001831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123(13):1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


