Case Description:
A 58-year-old man with history of obesity, hyperlipidemia, and anxiety was referred to cerebrovascular clinic with intermittent dizziness when turning his head to the right. These symptoms started at least a year prior to presentation but were becoming more frequent and severe. He further described the dizziness as feeling like a “head rush” without a room spinning sensation. It occurred while standing, sitting, or lying, but always in the setting of turning his head to the right. He also described mild bilateral hearing loss and tinnitus over the last ten years. The patient denied diplopia, nausea, vomiting, or headache. He had no history of falls or gait instability. He was previously evaluated in otolaryngology clinic and was told his symptoms were inconsistent with peripheral vertigo.
The patient’s initial neurologic examination was significant for normal mental status, normal cranial nerves except for mild decreased hearing bilaterally, normal strength without drift, intact sensation to light touch, and normal reflexes. There was no frank ataxia, but there was instability with tandem gait. Both Romberg and Dix-Hallpike tests were negative. The symptoms could be intermittently induced with right head turn.
Head and neck computed tomography angiography revealed a C5-C6 osteophyte in proximity to the right vertebral artery (Figure 1). Cervical vertebral artery dynamic ultrasonography revealed increased peak systolic flow velocity increasing from 50 to 159 cm/s with right head turn (Figure 2A–B). There was no effect with left head turn (Figure 2C). Intracranial vertebral artery dynamic transcranial Doppler demonstrated reduced peak systolic flow velocity from 24 to 15 cm/s with right head turn (Figure 2D). Digital subtraction angiography revealed the right vertebral artery was unremarkable in the standard position but turning the head to the right elicited a focal region of stenosis (Figure 3A–B). The left vertebral artery was congenitally hypoplastic (Figure 3C). Furthermore, there was a chronic-appearing mid-basilar occlusion, and the bilateral posterior cerebral, bilateral superior cerebellar, and superior basilar arteries were supplied by the anterior circulation through the right posterior communicating artery (Figure 3D–E).
Figure 1.
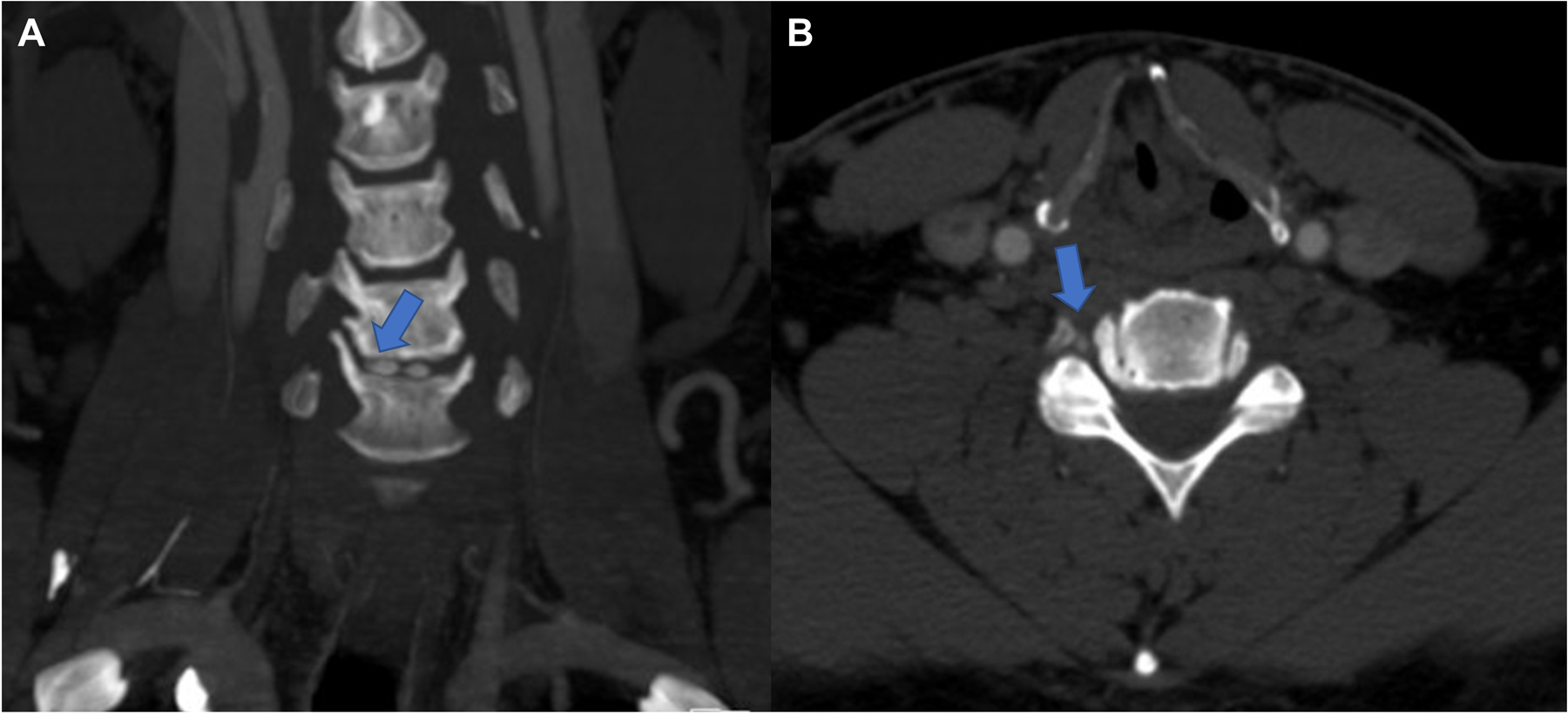
Head and neck computed tomography angiography revealed a C5-C6 osteophyte (arrow in panel A) in proximity to the right vertebral artery (arrow in panel B).
Figure 2.
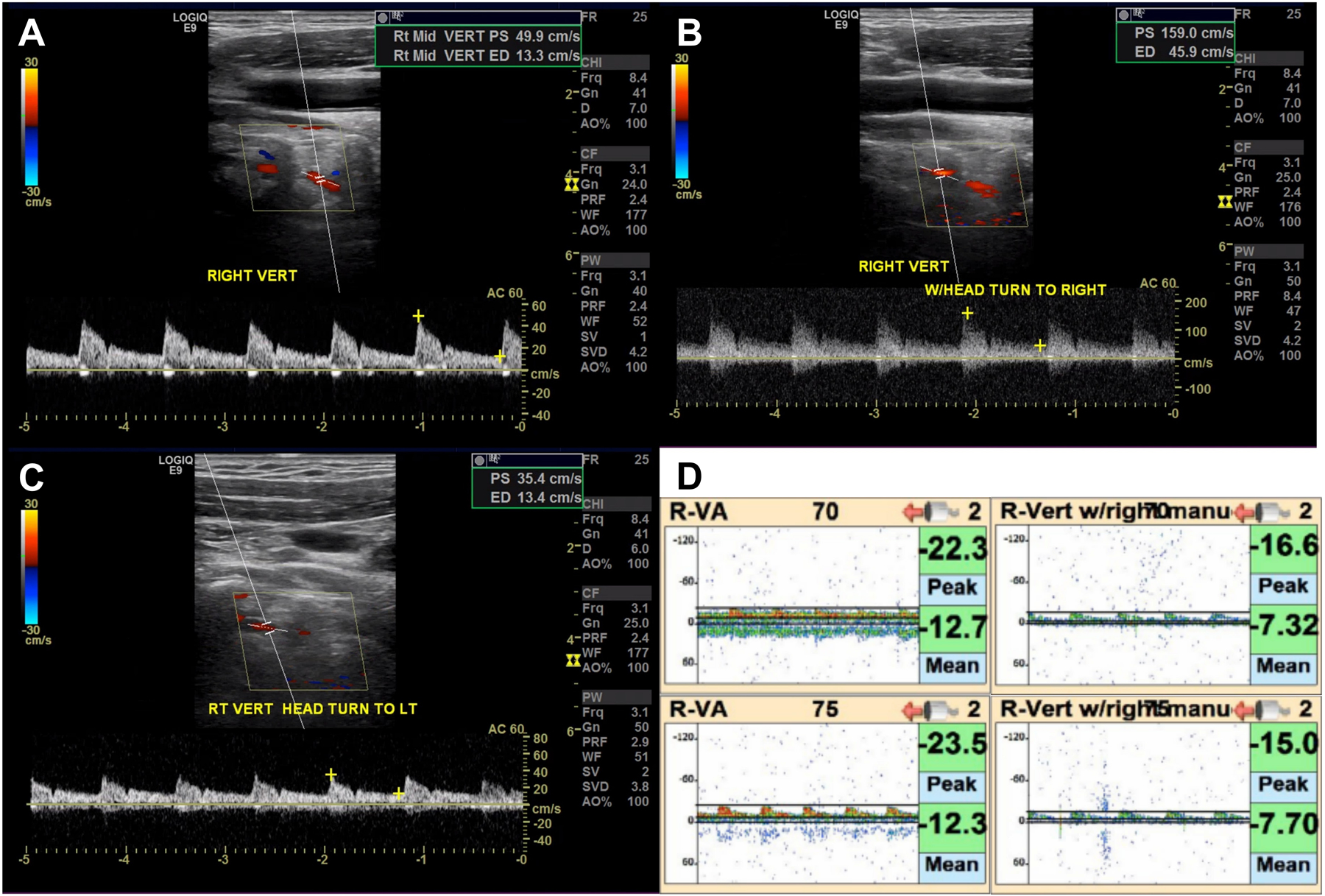
A-B. Cervical vertebral artery dynamic ultrasonography revealed increased peak systolic flow velocity with right head turn. C. There was no effect of left head turn. D. Intracranial vertebral artery dynamic transcranial Doppler revealed reduced peak systolic flow velocity with right head turn.
Figure 3.
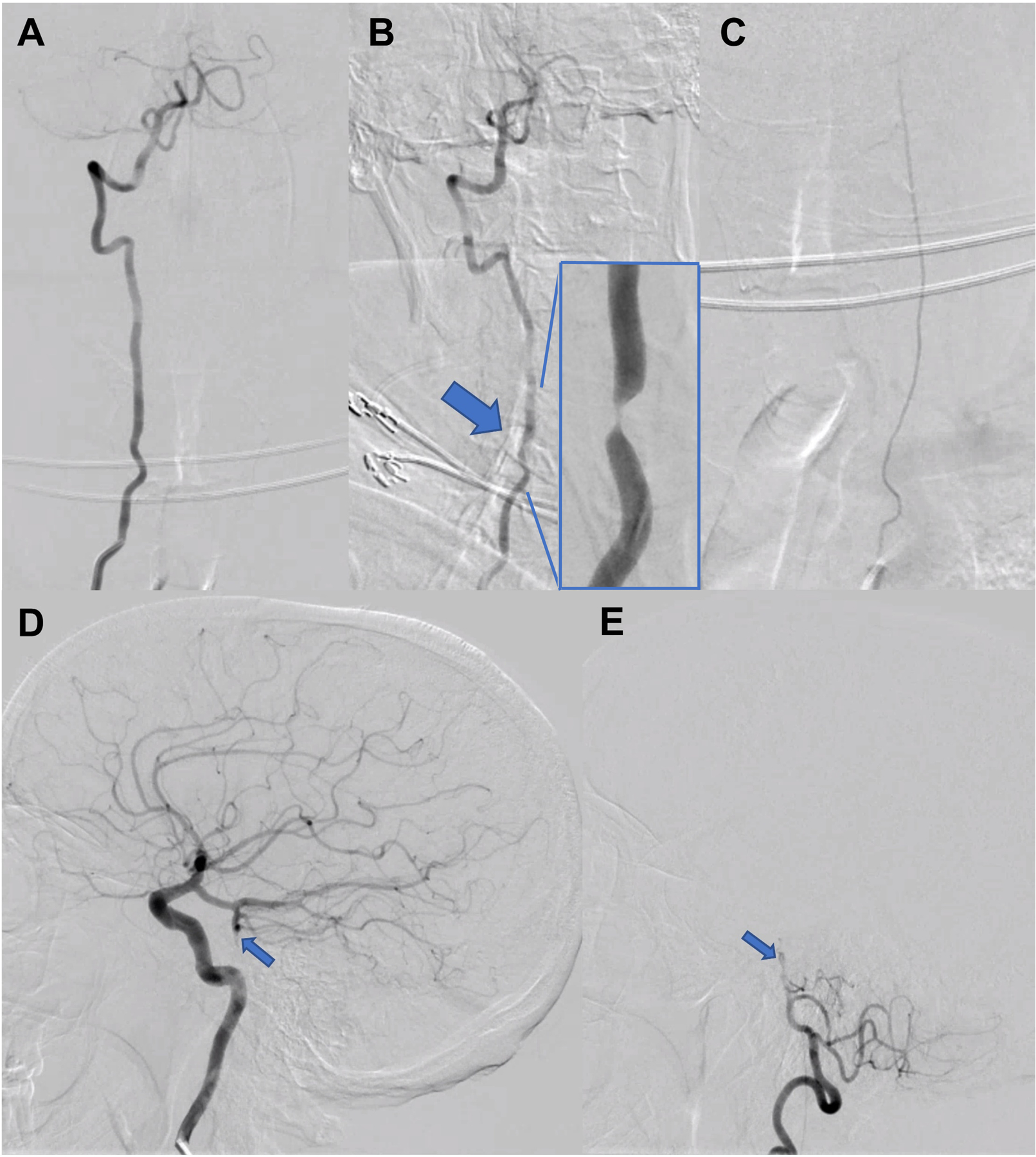
A-B. Digital subtraction angiography revealed the right vertebral artery was unremarkable in the standard AP position (A) but turning the head to the right elicited a focal region of stenosis (B, large arrow, magnified view is inset). C. The left vertebral artery was congenitally hypoplastic. AP view with left vertebral artery injection. D-E. There was a chronic-appearing mid-basilar occlusion (small arrows), and the bilateral posterior cerebral, bilateral superior cerebellar, and superior basilar arteries were supplied by the anterior circulation through a robust right posterior communicating artery. Both are lateral views; D is right internal carotid artery injection; E is right vertebral artery injection.
Given the risks associated with surgical intervention for the osteophyte near the right vertebral artery, particularly in the setting of a hypoplastic left vertebral artery, the patient and care team pursued conservative treatment with behavioral modification to minimize right head turning. He was also treated with aspirin 325 mg and atorvastatin 80 mg. The vertebral arteries will be monitored yearly with ultrasonography.
Discussion:
Bow hunter’s syndrome occurs when there is vertebral artery stenosis or occlusion during head rotation that results in vertebrobasilar insufficiency. It was initially described in 1978 in a bow hunter who developed symptoms during archery practice.1 Indeed, the stance when bow hunting forces near maximal rotation of the head in one direction to stabilize the arrow while aiming at a target. In addition to this stance, there is frequently also stabilization of a nocked arrow with the bow hunter’s thumb upon the occiput due to the high force used.1
In most cases the pathophysiology involves dynamic vertebral artery stenosis or occlusion by mechanical compression from a bony structure.2 Primary etiologies include osteophytes, herniated discs, spondylosis, tendinous bands, or tumors. Acquired etiologies are also possible, such as complications from cervical spine surgery and neck trauma.3 Patients with bow hunter’s syndrome also commonly have vascular risk factors, including hypertension, hyperlipidemia, diabetes, smoking, and coronary artery disease.4 Furthermore, some occupations that require extreme neck rotation, such as auto mechanic or construction worker, can exacerbate symptoms.
The resulting stenosis or occlusion with head rotation can result in downstream hypoperfusion of brain tissue or less likely thromboembolism if there is vessel injury.5 The vertebral artery enters the transverse foramina at the C6 level, courses cranially, and exits at the C1 level. Mechanical compression of the artery can occur anywhere along this course, but the C1-C2 level may be the most common location. The syndrome is often exacerbated by a stenotic or hypoplastic contralateral vertebral artery that is unable to provide adequate collateral flow and by limited anterior circulation collateral flow across the posterior communicating arteries, both demonstrated in the case presented.6
The exact prevalence and incidence of bow hunter’s syndrome is not well described given its relative rarity.2 It most often presents during the fifth to seventh decades of life, and there may be a male predominance. The hypoperfusion can cause a spectrum of presentations from transient ischemic attack to stroke. Therefore, symptoms range in severity from vertigo to posterior circulation stroke, and include nausea, dysarthria, dysphagia, vision changes, gait abnormalities, and headaches.7 One series of 36 cases described the following signs on neurologic examination: 5% visual field deficits, 8% cerebellar signs, 8% locked-in syndrome, 28% Wallenberg’s syndrome, 49% other brain stem findings, and 2% unclassified; among these patients long-term disability or mortality was observed in 28%.8 One review of published cases suggested that up to 50% of patients managed conservatively could suffer stroke or residual deficits.9 However, outcomes vary widely by report with another series of 19 conservatively managed patients showing none developed posterior circulation stroke and four had resolution of symptoms over median 37.5 months.3
Clinical presentation should prompt a thorough imaging work-up. Importantly, standard noninvasive studies do not always exclude the diagnosis and often dynamic imaging is necessary. Computed tomography and magnetic resonance imaging can identify the offending compressing structure. Ultrasonography and transcranial Doppler can be used to screen for this pathophysiology dynamically by performing the studies with head turns. The gold standard for diagnosis remains digital subtraction angiography with head turns, which may also be essential for surgical planning and assessing response to treatment.6
Treatments include conservative management with antithrombotic medications and behavioral modification to limit head rotation, surgical resection of the compressing structure, surgical C1-C2 fusion, surgical vertebral artery decompression, vertebral artery stenting, and rarely vessel sacrifice.2 A multidisciplinary discussion involving the patient should be pursued, weighing the risks versus benefits of these options. Different lesions may lend themselves to certain approaches, but surgery is often reserved for patients with stroke or disabling symptoms not responsive to conservative management. Intraoperative angiography can provide a real time assessment of the efficacy of decompression resection.10 Future studies to determine the best treatment are warranted but challenging given the relative rarity and heterogeneity of this entity.
Teaching Points:
Bow hunter’s syndrome occurs when there is vertebral artery stenosis or occlusion from compression during head rotation that results in vertebrobasilar insufficiency.
Symptoms can range in severity from transient vertigo to posterior circulation stroke.
Diagnosis requires dynamic imaging during head rotation, and the gold standard is digital subtraction angiography.
Management is often conservative with antithrombotic medications and behavioral modification to limit head rotation, but sometimes surgical resection of the compressing structure is necessary.
Funding:
NINDS R25NS065743
Footnotes
Disclosures: None relevant
References:
- 1.Sorensen BF. Bow hunter’s stroke. Neurosurgery 1978;2:259–261. [DOI] [PubMed] [Google Scholar]
- 2.Duan G, Xu J, Shi J, Cao Y. Advances in the Pathogenesis, Diagnosis and Treatment of Bow Hunter’s Syndrome: A Comprehensive Review of the Literature. Interv. Neurol 2016;5:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi KD, Choi JH, Kim JS, Kim HJ, Kim MJ, Lee TH, Lee H, Moon IS, Oh HJ, Kim J Il. Rotational vertebral artery occlusion: Mechanisms and long-term outcome. Stroke 2013;44:1817–1824. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi V, Rawls A, Moore O, Victorica B, Khan S, Saravanapavan P, Midivelli S, Raviraj P, Khanna A, Bidari S, et al. Rare Etiology of Bow Hunter’s Syndrome and Systematic Review of Literature. J. Vasc. Interv. Neurol 2015;8:7–16. [PMC free article] [PubMed] [Google Scholar]
- 5.Regenhardt RW, Das AS, Stapleton CJ, Chandra RV, Rabinov JD, Patel AB, Hirsch JA, Leslie-Mazwi TM. Blood pressure and penumbral sustenance in stroke from large vessel occlusion. Front. Neurol 2017;8:317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montano M, Alman K, Smith MJ, Boghosian G, Enochs WS. Bow Hunter’s Syndrome: A rare cause of vertebrobasilar insufficiency. Radiol. Case Reports 2021;16:867–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaidi HA, Albuquerque FC, Chowdhry SA, Zabramski JM, Ducruet AF, Spetzler RF. Diagnosis and management of bow hunter’s syndrome: 15-year experience at barrow neurological institute. World Neurosurg 2014;82:733–738. [DOI] [PubMed] [Google Scholar]
- 8.Frisoni GB, Anzola GP. Vertebrobasilar ischemia after neck motion. Stroke 1991;22:1452–1460. [DOI] [PubMed] [Google Scholar]
- 9.Kuether TA, Nesbit GM, Clark WM, Barnwell SL. Rotational Vertebral Artery Occlusion: A mechanism of vertebrobasilar insufficiency. Neurosurgery 1997;41:427–433. [DOI] [PubMed] [Google Scholar]
- 10.Ng S, Boetto J, Favier V, Thouvenot E, Costalat V, Lonjon N. Bow Hunter’s Syndrome: Surgical Vertebral Artery Decompression Guided by Dynamic Intraoperative Angiography. World Neurosurg 2018;118:290–295. [DOI] [PubMed] [Google Scholar]


