Abstract
Background:
Individuals with left ventricular hypertrophy (LVH) and elevated cardiac biomarkers in middle-age are at increased risk for the development of heart failure with preserved ejection fraction (HFpEF). Prolonged exercise training reverses the LV stiffening associated with healthy but sedentary aging; however, whether it can also normalize LV myocardial stiffness in patients at high risk for HFpEF is unknown. In a prospective, randomized-controlled trial, we hypothesized that 1-year prolonged exercise training would reduce LV myocardial stiffness in patients with LVH.
Methods:
Forty-six patients with LVH (LV septum >1.1 mm) and elevated cardiac biomarkers [NT-proBNP (>40 pg/ml) or hsTnT (>0.6pg/ml)] were randomly assigned to either 1 year of high-intensity exercise training (N=30) or attention control (N=16). Right-heart catheterization and 3D-echocardiography were performed while preload was manipulated using both lower body negative pressure and rapid saline infusion to the define LV end-diastolic pressure-volume relationship (EDPVR). A constant representing LV myocardial stiffness was calculated from: P = S * [Exp { a (V–V0) } – 1], where P is transmural pressure [pulmonary capillary wedge pressure – right atrial pressure], S is the pressure asymptote of the curve, V is LVEDV index, V0 is equilibrium volume, and “a” is the constant that characterizes LV myocardial stiffness.
Results:
Thirty-one participants [exercise group (N=20); 54±6 years; 65% male and controls (N=11): 51±6 years, 55% male] completed the study. One-year of exercise training increased V̇O2max by 21% (pre 26.0±5.3 to post 31.3±5.8 ml/min/kg, P<0.0001, interaction P=0.0004), whereas there was no significant change in V̇O2 max in controls (pre 24.6±3.4 to post 24.2±4.1 ml/min/kg, P=0.986). LV myocardial stiffness was reduced (right and downward shift in the EDPVR; pre LV myocardial stiffness 0.062±0.020 to post 0.031±0.009), whereas there was no significant change in controls (pre 0.061±0.033 to post 0.066±0.031, interaction P=0.001).
Conclusions:
In patients with LVH and elevated cardiac biomarkers (stage-B HFpEF), 1-year of exercise training reduced LV myocardial stiffness. Thus, exercise training may provide protection against the future risk of HFpEF in such patients.
Clinical Trial Registration:
Keywords: Left Ventricular Stiffness, Transmural Stiffness, Left Ventricular Hypertrophy, HFpEF, Diastolic Function, Pressure-Volume loop theory, End-diastolic pressure and volume relation (EDPVR)
Introduction
Despite the evolution of guideline-directed management for heart failure (HF), HF remains a devastating disease that affects 6.5 million Americans ≥20 years of age.1 About half of patients with HF have apparently “preserved” ejection fraction, also known as Heart Failure with preserved Ejection Fraction (HFpEF). The pathophysiology of HFpEF is associated with increased left ventricular (LV) stiffness and compromised ventricular-arterial coupling.2–5 Therapeutic strategies for HFpEF are limited with high re-hospitalization rates and mortality.6 To date, no compellingly effective therapy for HFpEF has been found. Therefore, clinical strategies that may prevent HFpEF are critical.7
Our group has documented that LV stiffening begins to occur in middle age, and becomes progressively stiffer over the course of sedentary aging, even in the absence of cardiovascular disease.8 This stiffening process can be prevented by life-long physical activity at the right dose (at least 4–5 days/week of endurance exercise).9 However when exercise training is initiated late in life, after age 65, in either healthy seniors10 or patients with HFpEF11 the cardiac atrophy and stiffening of sedentary aging cannot be reversed, though modest improvements in compliance have been demonstrated when exercise training is accompanied by a drug that can break advanced glycation end-products.12 Conversely, middle-aged hearts retain substantial plasticity and may respond to an adequate dose of training to restore youthful myocardial compliance.13
Identifying and targeting the patients most likely to benefit from such a preventive strategy may be problematic. Clearly, low physical fitness is a powerful risk factor for the future development of heart failure and HFpEF.14, 15 Moreover, in a representative, population-based sample of adults with no previous HF, individuals with LVH plus elevations in biomarkers reflecting subclinical myocardial injury (cardiac troponin T: cTnT) or neurohormonal activation due to hemodynamic stress (NT-proBNP) had a substantial increased risk of developing heart failure, a substantial fraction of which was HFpEF.16 Finally, we have reported recently that LV myocardial stiffness in patients with LVH and elevated biomarkers (AHA/ACC stage-B HFpEF) was greater than in age- and sex- matched controls, which appears to represent a transitional state from a normal healthy heart to HFpEF17 and could be the ideal population to target with behavioral modification.
Therefore, we hypothesized that committed exercise training, when implemented 4–5 times/week over a prolonged period of time in sedentary high-risk middle-aged men and women, aged 45–64, would improve LV compliance in patients with LVH and elevated biomarkers. To test this hypothesis, we performed comprehensive invasive and non-invasive assessments of cardiovascular structure and systolic/diastolic function in patients with LVH (as AHA/ACC stage-B HFpEF), before and after 1 year of a well periodized exercise program including high-intensity aerobic intervals, lower intensity endurance training, and strength training, compared with a control group.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participant Population and Study Design
This study was a prospective, parallel group, randomized controlled 1-year exercise training study. Middle-aged (45–64 years) participants with LVH were recruited from the Dallas Heart Study (DHS),18 enriched by review of hospital electrocardiography and echocardiography data bases to identify patients with asymptomatic LVH in whom NT-proBNP and hs-cTnT were measured subsequently. Subjects were stratified by sex and allocated to either exercise or yoga interventions using a stratified block randomization at a 2:1 exercise-to-control ratio (allowing for greater attrition for the exercise group). The randomization schema was programmed using SAS Proc Plan and performed by the study biostatistician. A yoga-based attention control allowed for equipoise for the volunteers by improving quality of life without affecting fitness.19, 20
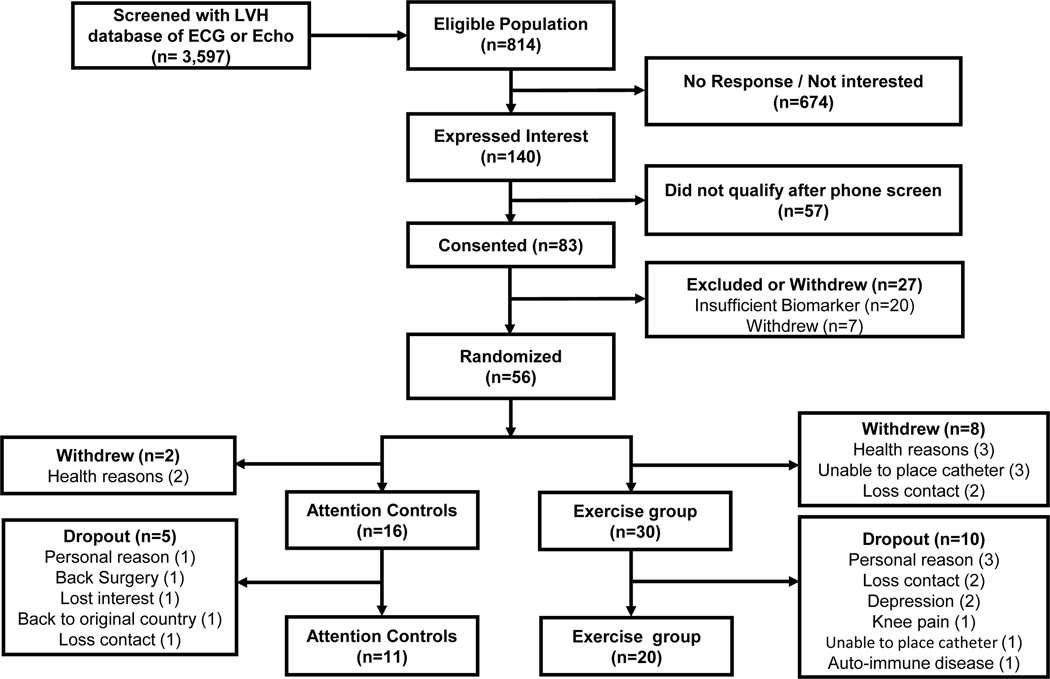
In total, 3,597 potential candidates were screened; 814 adults met the initial inclusion criteria for our study including an ejection fraction >50% and documented LVH by MRI (125g/m2) or echocardiography (left ventricular septum >11 mm) without exclusion criteria. One hundred and forty individuals who expressed an interest in the study underwent phone screening. Eighty-three subjects were tested for elevated biomarkers: either an elevated NT-proBNP (>40 pg/ml) or high sensitivity troponin (>0.6 pg/ml). 21 Participants were excluded if they had signs or symptoms of heart failure, hypertrophic cardiomyopathy, cardiac amyloidosis, ischemic heart disease, prior myocardial infarction or stroke, greater than moderate valvular heart disease, COPD, sleep apnea syndrome, exercised more than 3 days per week or were unable to exercise. Fifty-six participants with LVH and elevated biomarkers were enrolled (signed a consent form) and randomized, comprising 18 controls and 38 exercisers. Following randomization, but before completing all preliminary testing and beginning the intervention, 2 patients assigned to the control group, and 8 patients assigned to exercise withdrew, leaving a total of 46 patients who completed all baseline studies and started their assigned intervention (30 exercisers, and 16 controls). (Figure 1). Over the course of the 1-year intervention, 15 of these subjects dropped out prior to completing post intervention testing (5 in the attention controls, and 10 in the exercise group) leaving a total of N=11 attention controls and N=20 exercise subjects who ultimately completed the intervention and all post-intervention studies
Figure 1. Consort Diagram in the LVH Study.
Study enrollment, randomization, and retention of study participants randomly assigned to the exercise training or control group. LVH, left ventricular hypertrophy; ECG, electrocardiography
The experimental procedures were explained to all participants, with informed consent obtained as approved by the institutional review boards of University of Texas Southwestern Medical Center at Dallas and Texas Health Presbyterian Hospital Dallas. All procedures conformed to the standards set by the Declaration of Helsinki. The trial was registered prospectively on ClinicalTrials.gov (NCT03476785).
Intervention
Exercise Training
For the exercise group, a training program was developed individually for each subject with the goal of increasing duration and intensity consistent with modern training techniques.22–24 A day-by-day training calendar was provided to the subjects. Workouts varied with respect to mode (walk, cycle, swim), duration (30–60 minutes), and intensity (base, interval, recovery) to optimize the training response. Each subject was assigned a personal trainer and a HR monitor to ensure that every session was carefully tracked and recorded. For high intensity interval training, we used “aerobic intervals” which have been shown recently to be highly effective at improving V̇O2max and cardiovascular function.25–28. To individualize training intensity, the maximal steady state (MSS) zone was first determined from the ventilatory and lactate thresholds measured during the maximal exercise test as previously described.13 Based on the MSS HR and peak HR (HRpeak), 4 training zones were established for each participant: (1) MSS; (2) base pace (1–20 beats below MSS); (3) interval (>95% HR peak); and recovery (<base pace). The early training phase (month 1–2) focused on establishing an endurance base and regular exercise routine with participants performing 3, 30-minute base pace sessions per week. As participants acclimated to the training, MSS sessions were added starting with 2 sessions per month during the second month and increasing to 3 sessions in month 3. In the third month, aerobic intervals consisting 4×4 interval sessions (4 minutes of exercise at 95% peak HR followed by 3 minutes of active recovery at 60%–75% peak HR, repeated 4 times) were incorporated. The exercise program goal was gradually increased to two aerobic interval training sessions per week over the first 7 months, and then included at least two interval session per week for the duration as maintenance. Subjects also performed strength training 1–2 days/week.29 All studies were repeated after 1 year of training.
Control Intervention
Attention controls were prescribed a combination of yoga, balance, and strength training using light weights 3 times per week for 1 year. Participants attended group yoga or stretching classes or completed online or video classes at home. This prescription allowed for a similar level of interaction with research staff between both groups. To that end, each participant (exercise group and attention control group) was assigned an exercise physiologist who monitored their training compliance throughout the 1-year intervention. An exercise log and heart rate monitor (Polar) were used to monitor training compliance.
Cardiopulmonary exercise testing
Maximal oxygen uptake (V̇O2 max) was measured using a modified Astrand-Saltin treadmill protocol and the Douglas bag technique; gas fractions were analyzed by mass spectrometry, and ventilatory volumes were analyzed by a Tissot spirometer, as previously reported.30 V̇O2 max was defined as the highest oxygen uptake measured from at least a 30-second Douglas bag.
Hemodynamics
All hemodynamic experiments were conducted in the morning, in a quiet environmentally controlled laboratory with an ambient temperature of 25°C. All participants had a light breakfast at least two hours before experiments commenced and were asked to refrain from heavy exercise and caffeinated or alcoholic beverages for at least 24 hours prior to the day. A 6-F balloon-tipped, fluid-filled catheter (Swan-Ganz catheter, Baxter, Deerfield, IL) was placed through an antecubital vein into the pulmonary artery using fluoroscopic guidance. Intravascular pressures were referenced to atmospheric pressure, with the pressure transducer (Transpac IV, Abbott, Chicago, IL) zero reference point set at 5.0 cm below the sternal angle. After at least 20-minutes of quiet supine rest, baseline data were collected. Analog waveforms were sampled at 250 Hz and the digital waveforms were analyzed offline using customized software (Biopac Systems Inc., Santa Barbara, CA). The mean pulmonary capillary wedge pressure (PCWP) and right atrial pressure (RAP) were measured using three separate measurements during a quiet, held end expiration (~ 5 seconds), excluding the V waves. Because external constraint influences ventricular volumes and pressure, LV end-diastolic transmural pressure-volume relationships were constructed using estimated transmural pressure (TMP= PCWP–RAP).31
Cardiac filling and thus left ventricular end-diastolic pressure (LVEDP) was decreased by 2-sequential levels of lower body negative pressure (LBNP) of −15 mmHg and −30 mmHg as previously described.13, 32 Five minutes into each level of cardiac unloading, 3 separate measurements of mean PCWP and RAP were obtained at end-expiration. After release of the LBNP, subjects were given a small break. A resting baseline was then repeated to ensure return to hemodynamic steady-state and to provide and additional, “pre-saline” measurement point before cardiac filling was increased by 2-sequential levels with a rapid infusion of 15 and 30 ml/kg warm (37°C) isotonic saline at 200 ml/min. Hemodynamic measurements were obtained as previously described.17
Echocardiography
The LV was imaged by 3-dimensional echocardiography (iE33; Phillips Medical System) at all loading conditions during the study. LV end-diastolic volume (LVEDV) was analyzed offline (Qlab 9.0; Phillips Medical System) by an experienced cardiologist who was blinded to filling pressures. The typical error of the LV volume measurement by echocardiography in our laboratory, expressed as a coefficient of variation, is 10% (95% confidence interval, 8%–12%).
Analysis of Hemodynamic Data
Cardiac output (Qc) was measured with the C2H2 rebreathing method during exercise testing and during manipulation of preload.33 Heart rate was monitored continuously via an electrocardiograph (ECG), and stroke volume (SV) was calculated from cardiac output divided by heart rate. Blood pressure was measured at the brachial artery during cardiac output measurements. Arm cuff systolic and diastolic blood pressures (sBP and dBP) were measured by electrosphygmomanometry, with a microphone placed over the brachial artery to detect Korotkoff sounds. Lean body mass was measured by dual energy X-ray absorptiometry (DEXA). The body surface area was used to scale all chamber volume measurements.17 A constant for LV chamber and myocardial stiffness was modeled using commercially available software (SigmaPlot version 12.0, Systat Software Inc, San Jose, CA), which uses an iterative technique to solve the following exponential equation: P = S [ Exp {a (V-V0) −1}],34 where P is PCWP (for chamber stiffness calculations) or transmural pressure (for myocardial stiffness calculations), S is pressure asymptote of the curve, V is LVEDV index, V0 is the equilibrium volume at which transmural P is assumed to be 0 mm Hg, and a is the constant that characterizes chamber stiffness. Individual LV myocardial stiffness constants for each participant was averaged within each group and reported as individual stiffness.17 PCWP and SV data were used to construct Frank-Starling curves. The SV, mean arterial pressure (MAP), and 3-dimensional LVEDV data were used to calculate preload recruitable stroke work relationships ( PRSW = [ SV × MAP ] / LVEDV ),35 where the slope was used as an index of global systolic function. The effective arterial elastance (Ea), representing afterload was calculated using 0.9 x systolic blood pressure divided by SV.36
Statistical Analysis
Continuous variables are expressed as mean ± SD, and categorical variables are expressed as n (%). The primary analysis included all participants who completed the 1-year follow-up. Continuous variables were compared between groups by using mixed-effects model repeated-measures ANOVA analysis. The repeated-measures models included the intervention group factor (attention controls versus exercise group), a repeated factor for study visits (baseline [pre] and 1-year later [post]), and a group×visit interaction; the study participant was modeled as a random effect. Pairwise comparisons were made using the least square contrasts derived from these mixed-effects models. We performed post hoc analyses to explore the impact of V̇O2 max, LVEDV, and LV stiffness. Random effects linear regression models with quadratic terms were used to model the relationships in the PCWP and transmural pressure–volume curves and Frank-Starling curves, as well as to compare group responses with tests of interactions between group and independent variables. The covariance structure for mixed-effects models was selected based on Akaike Information Criteria and model parsimony. P-value of < 0.05 was considered statistically significant. Statistical analysis was performed using JMP ver. 11.0 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics
Eighty-three candidate participants were consented and assessed for eligibility to participate in this study. Of these, 56 participants were randomly assigned and 10 (2 participants in the controls and 8 in the exercise group) withdrew prior to completing baseline assessments and beginning their assigned intervention (Figure 1). The baseline characteristics of the 46 subjects who completed all baseline studies and began the study intervention are shown in Table 1. The two groups were comparable in age, sex, race, blood pressure, and maximal oxygen uptake.
Table 1.
Baseline Characteristics
| Control group (N=16) | Exercise group (N=30) | |
|---|---|---|
|
| ||
| Age, years | 53±7 | 53±5 |
| Sex, M/F | 9/7 | 17/13 |
| Body Height, cm | 172±9 | 172±12 |
| Body Weight, kg | 96±14 | 87±16 |
| Body Surface Area (m2) | 2.14±0.20 | 2.03±0.24 |
| Body Mass Index (kg/m2) | 32.2±2.6 | 29.1±4.1 |
| Lean Body Mass, kg | 59±12 | 55±11 |
| Race, n (%) | ||
| White | 9 (56) | 15 (50) |
| Black | 6 (38) | 13 (43) |
| Hispanic | 1 (6) | 2 (7) |
| Risk factors, n (%) | ||
| Hypertension | 11 (69) | 21 (70) |
| Diabetes mellites | 1 (6) | 3 (10) |
| Chronic Kidney Disease | 0 (0) | 0 (0) |
| Smoking | 0 (0) | 1 (3) |
| Medication, n (%) | ||
| ACE inhibitor/ARB | 8 (50) | 19 (63) |
| Ca2+ channel blocker | 1 (6) | 7 (23) |
| Beta blocker | 2 (13) | 4 (13) |
| Diuretics | 5 (31) | 5 (17) |
| 24-hour ABPM sBP, mmHg | 131±13 | 134±15 |
| 24-hour ABPM dBP, mmHg | 77±6 | 81±9 |
| 24-hour ABPM mBP, mmHg | 95±8 | 97±14 |
| 24-hour ABPM HR, bpm | 74±8 | 74±13 |
| RER | 1.15±0.07 | 1.13±0.08 |
| V̇O2 max, L/min | 2.38±0.71 | 2.16±0.56 |
| V̇O2 max, mL/min/kg | 24.5±5.3 | 24.7±5.2 |
M, male; F, Female; ACE inhibitor, angiotensin comberting enzyme inhibitor; ARB, angiotensin II receptor blocker; ABPM, ambulatory blood pressure monitoring; sBP, dBP, and mBP, systolic, diastolic and mean blood pressure; RER, respiratory exchange ratio; V̇O2, oxygen consumption.
In total, 31 participants completed the 1-year study, 20 participants in the exercise group and 11 in the control group. Figure 1 includes the reasons for withdrawal. The main reason for withdrawal and dropout from this study was related to personal reasons (n=5) or lost contact (n=5). Participants in the exercise group had favorable exercise compliance with the 1-year exercise training program (mean compliance rate=67%), though somewhat lower than we have reported with healthy controls of this demographic.13 Baseline characteristics, exercise, and hemodynamic parameters in subjects who completed all baseline assessments but dropped out during the intervention, compared to those who completed the whole intervention, are shown in the Table I in the Supplement.
Effect of Exercise Training on Oxygen Uptake and LVEDV
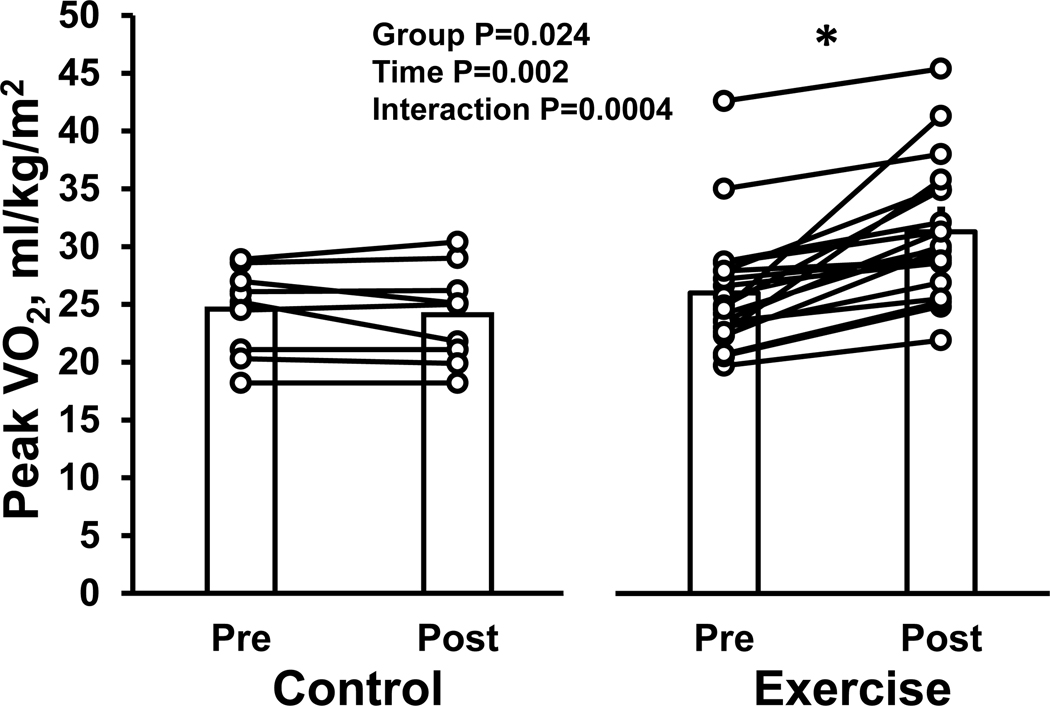
The exercise training program resulted in a significant increase in V̇O2 max in the exercise group (from 26.0±5.3 to 31.3±5.8 mL/min/kg, P<0.0001). In contrast, there was no significant change in the control group (from 24.6±3.4 to 24.1±4.1 mL/min/kg, P=0.986) (interaction P value=0.0004) (Table 2 and Figure 2).
Table 2.
Cardiopulmonary Exercise Test
| Control group (N=11) | Exercise group (N=20) | Group × Time interaction P value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Pre | Post | Pre | Post | ||
|
| |||||
| Rest | |||||
| Heart rate, bpm | 81±12 | 78±12 | 77±13 | 69±11 | 0.254 |
| Systolic blood pressure, mmHg | 128±16 | 127±22 | 129±13 | 134±15 | 0.470 |
| Diastolic blood pressure, mmHg | 83±8 | 80±14 | 89±7 | 88±7 | 0.450 |
| Mean blood pressure, mmHg | 98±9 | 96±16 | 102±7 | 104±8 | 0.357 |
| Cardiac output, L/min | 5.4±0.9 | 4.9±1.0 | 4.7±1.0 | 4.8±0.8 | 0.110 |
| V̇O2, L/min | 0.33±0.06 | 0.30±0.07 | 0.30±0.06 | 0.29±0.05 | 0.424 |
| V̇O2, mL/min/kg | 3.4±0.4 | 3.2±0.3 | 3.6±0.9 | 3.5±0.5 | 0.647 |
| Peak | |||||
| RER | 1.14±0.06 | 1.13±0.04 | 1.13±0.08 | 1.13±0.06 | 0.446 |
| Heart rate, bpm | 168±12 | 163±17 | 166±21 | 165±22 | 0.103 |
| Systolic blood pressure, mmHg | 200±12 | 205±31 | 200±25 | 218±23 | 0.297 |
| Diastolic blood pressure, mmHg | 91±13 | 80±8 | 88±16 | 91±19 | 0.075 |
| Mean blood pressure, mmHg | 127±8 | 122±14 | 125±14 | 133±16 | 0.080 |
| Cardiac output, L/min | 17.3±4.2 | 16.2±3.9 | 17.6±4.1 | 18.1±3.4 | 0.220 |
| V̇O2 max, L/min | 2.4±0.6 | 2.3±0.6 | 2.3±0.6 | 2.6±0.6 * | 0.0003 |
| V̇O2 max, mL/min/kg | 24.6±3.4 | 24.1±4.1 | 26.0±5.3 | 31.3±5.8 *#$ | 0.0004 |
| peak AV-O2 difference | 14.3±2.0 | 14.0±1.7 | 13.1±2.3 | 14.3±2.1 | 0.068 |
| peak Lactate, mmol/L | 7.5±1.5 | 6.4±1.1 | 7.1±2.5 | 7.9±1.6 | 0.002 |
V̇O2, oxygen consumption; AV-O2 difference, arteriovenous oxygen difference; RER, respiratory exchange ratio.
: P<0.05 denotes significantly different from pre in the same group.
: P<0.05 denotes significantly different from control group at pre.
: P<0.05 denotes significantly different from control group at post.
Figure 2. Effect of high-intensity exercise training on peak oxygen consumption in LVH patients.
The individual change and group mean response for peak oxygen uptake are shown for the control and exercise group. *P<0.05 denotes significantly different from pre.
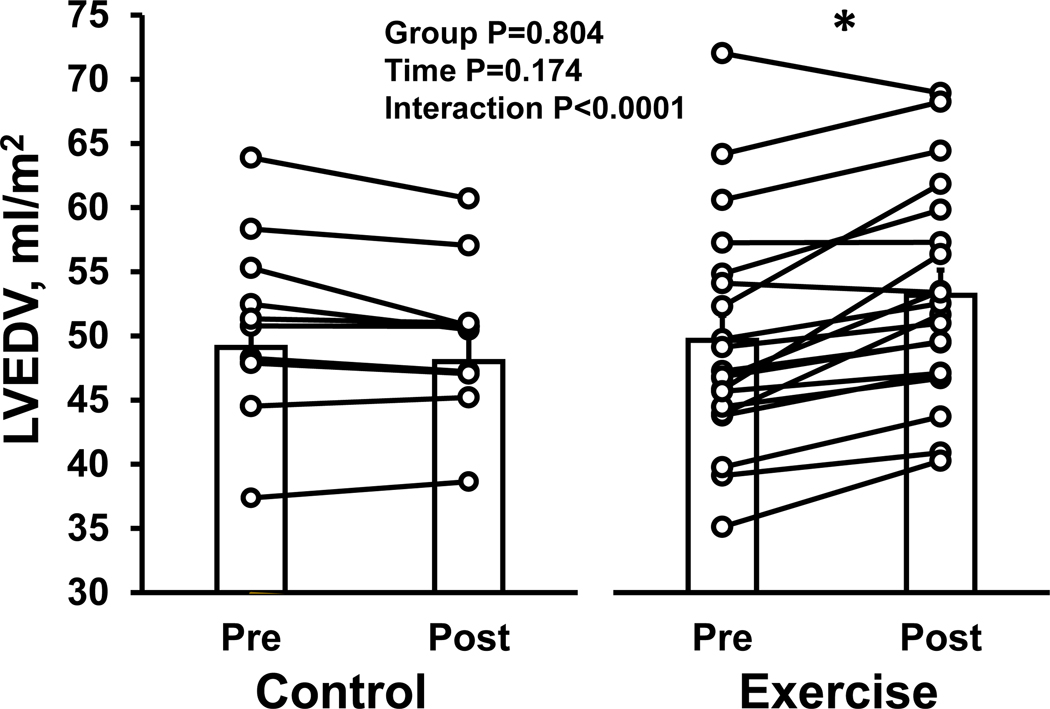
We observed a similar pattern between changes in LVEDV in the two groups (interaction P value<0.0001) (Figure 3). In the exercise group, LVEDV increased significantly after 1-year exercise training (P<0.0001). There was no significant change in LVEDV in the control group (P=0.175).
Figure 3. Effect of high-intensity exercise training on left ventricular end-diastolic volume.
The individual change and group mean response for left ventricular end-diastolic volume are shown for the control and exercise group. *P<0.05 denotes significantly different from pre. LVEDV, left ventricular end-diastolic volume.
Supine hemodynamic parameters
The impact of the exercise training on the hemodynamic variables is summarized in Table 3. Resting blood pressure was unchanged in both groups. Resting stroke volume in the exercise group increased while it decreased in the yoga group (interaction P value=0.056). Heart rate in the exercise group decreased from 68±11 to 64±11 bpm, with no significant change in the yoga group though this difference was more variable (interaction P value=0.226). There were no significant changes in resting supine cardiac output, central venous pressure, pulmonary capillary wedge pressure, and transmural pressure in either group (Table 3).
Table 3.
Supine Hemodynamics and Cardiovascular Function
| Control group (N=11) | Exercise group (N=20) | Group × Time interaction P value | |||
|---|---|---|---|---|---|
|
|
|
||||
| Pre | Post | Pre | Post | ||
|
| |||||
| Heart rate, bpm | 69±7 | 69±9 | 68±11 | 64±11 | 0.226 |
| Systolic blood pressure, mmHg | 119±9 | 122±14 | 124±10 | 126±13 | 0.891 |
| Diastolic blood pressure, mmHg | 73±9 | 70±8 | 77±8 | 77±9 | 0.411 |
| Mean blood pressure, mmHg | 88±9 | 87±9 | 92±7 | 93±9 | 0.676 |
| Cardiac output, L/min | 6.2±1.0 | 5.7±1.0 | 5.5±1.1 | 5.3±1.0 | 0.250 |
| Cardiac index, L/min/m2 | 2.65±0.44 | 2.41±0.42 | 2.33±0.49 | 2.26±0.44 | 0.251 |
| Stroke volume, mL | 91±18 | 83±17 | 82±18 | 86±17 | 0.056 |
| Stroke volume index, mL/m2 | 39±8 | 35±7 | 35±7 | 36±7 | 0.062 |
| Systemic vascular resistance, dyne/sec/cm5 | 1154±152 | 1270±289 | 1409±310 | 1464±357 | 0.511 |
| Pulmonary capillary wedge pressure, mmHg | 14.1±2.5 | 12.9±2.4 | 13.1±2.6 | 12.3±2.8 | 0.783 |
| Central venous pressure, mmHg | 10.8±2.0 | 9.1±2.1 | 9.6±2.5 | 8.7±1.9 | 0.418 |
| Transmural pressure, mmHg | 3.2±1.0 | 3.7±1.4 | 3.5±1.1 | 3.6±1.2 | 0.480 |
| Interventricular septum, mm | 13.4±1.6 | 13.5±1.6 | 12.8±1.4 | 12.6±1.2 | 0.154 |
| Posterior wall, mm | 10.6±1.3 | 10.9±1.0 | 10.0±1.5 | 9.9±1.2 | 0.110 |
| Body weight, kg | 99±14 | 97±16 | 86±18 | 84±14 | 0.982 |
| Visceral fat, kg | 2.1±0.9 | 1.7±0.9 | 1.5±1.0 | 1.3±0.8 | 0.520 |
| Lean body mass, kg | 59±12 | 58±14 | 56±12 | 56±12 | 0.621 |
| Body mass index, kg/m2 | 33±2 | 32±2 | 28±4 | 28±3 | 0.638 |
LV pressure-volume relationship
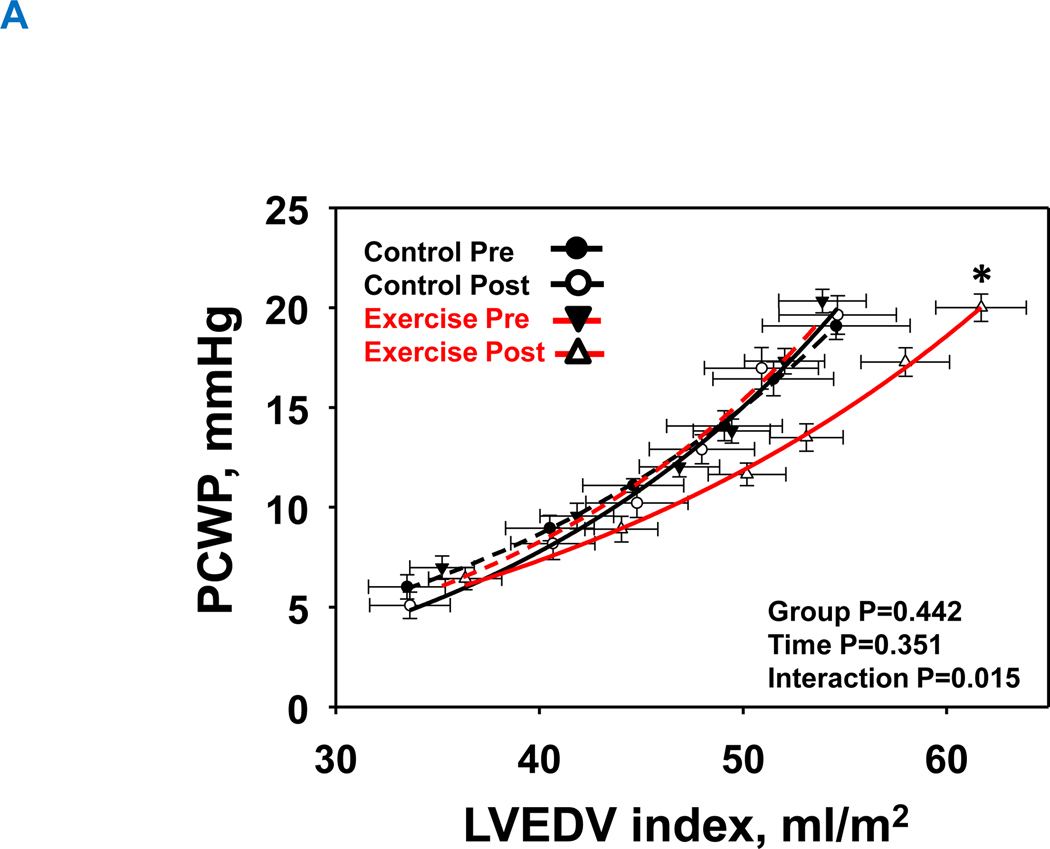
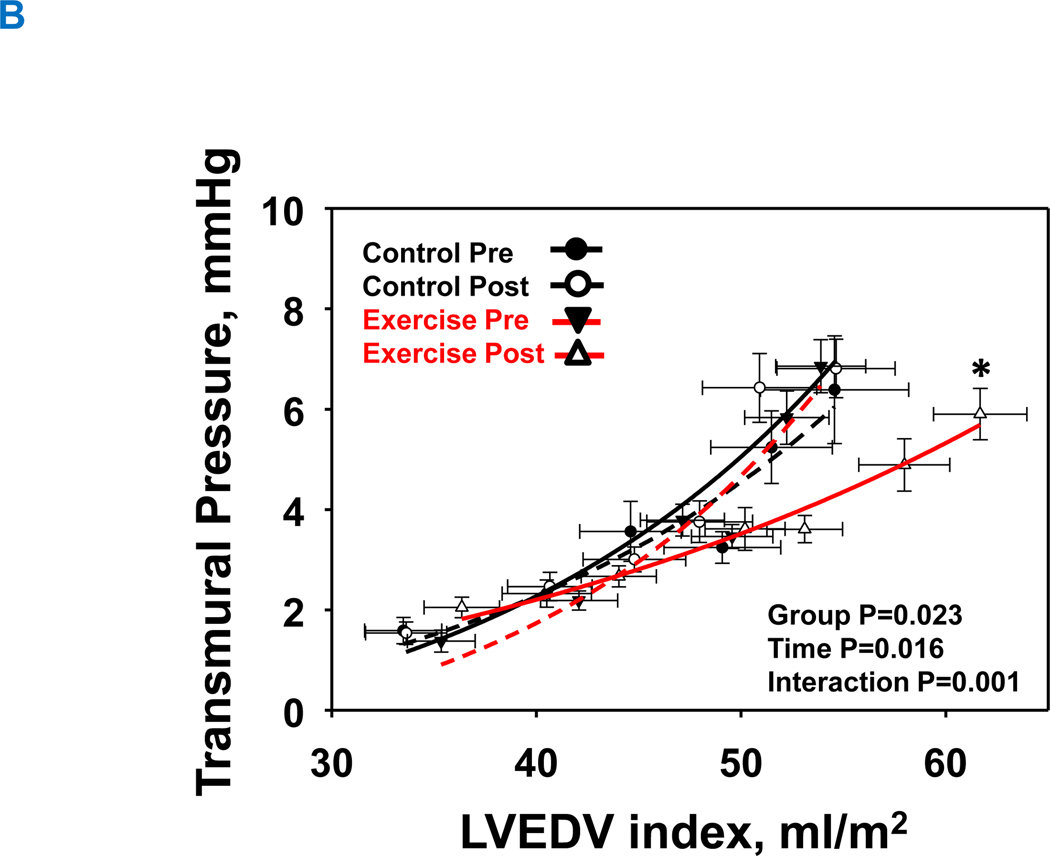
Both LV chamber and myocardial stiffness constants at baseline were comparable between the two groups (P=0.198 and P=0.997, respectively). LV pressure-volume relationships are shown as LV chamber stiffness (Figure 4A) and LV myocardial stiffness (Figure 4B). 1-year of exercise training significantly reduced LV chamber and myocardial stiffness constants (LV chamber stiffness: from 0.060±0.031 to 0.042±0.025; LV myocardial stiffness: from 0.062±0.020 to 0.031±0.009), with no significant changes in the control group (LV chamber stiffness: from 0.041±0.016 to 0.049±0.020; LV myocardial stiffness: from 0.061±0.033 to 0.066±0.031; Figure 4A: interaction P value=0.015 and Figure 4B: interaction P value=0.023).
Figure 4. Effect of high-intensity exercise training on left ventricular chamber. (A) and transmural stiffness (B).
The group mean left ventricular pressure-volume relationships before and after 1-year of intervention. Control group at pre, black-dash line with closed black circle; Control group at post, black line with open circle; Exercise group at pre, red-dash line with closed triangle; Exercise group at post, red line with opened circle. In the exercise group, both the LV chamber (A) and LV transmural curves (B) were shifted rightward with a flattening slope demonstrating improved LV compliance and distensibility. Those curves in the control group were unchanged. *P<0.05 denotes significantly different from pre.
LVH, left ventricular hypertrophy group; PCWP, pulmonary capillary wedge pressure; LVEDV was scaled to body surface area, Transmural pressure = PCWP ─ right atrial pressure (RAP).
Starling mechanism and preload recruitable stroke work
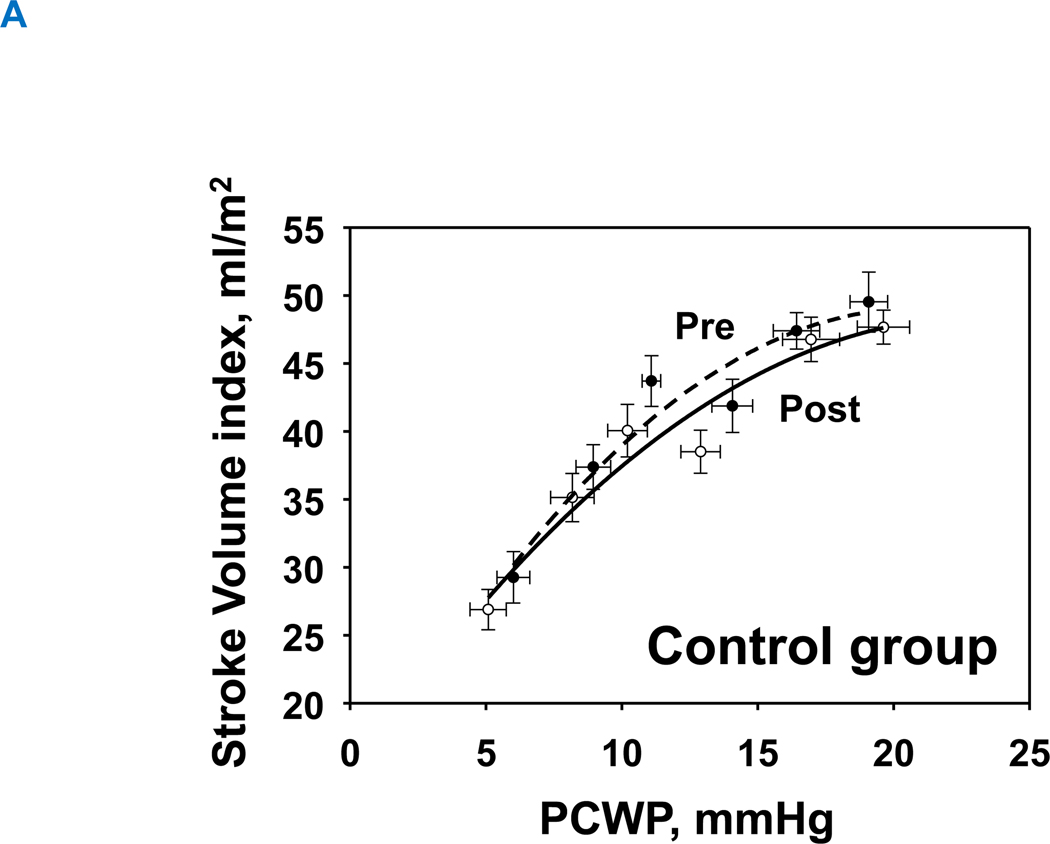
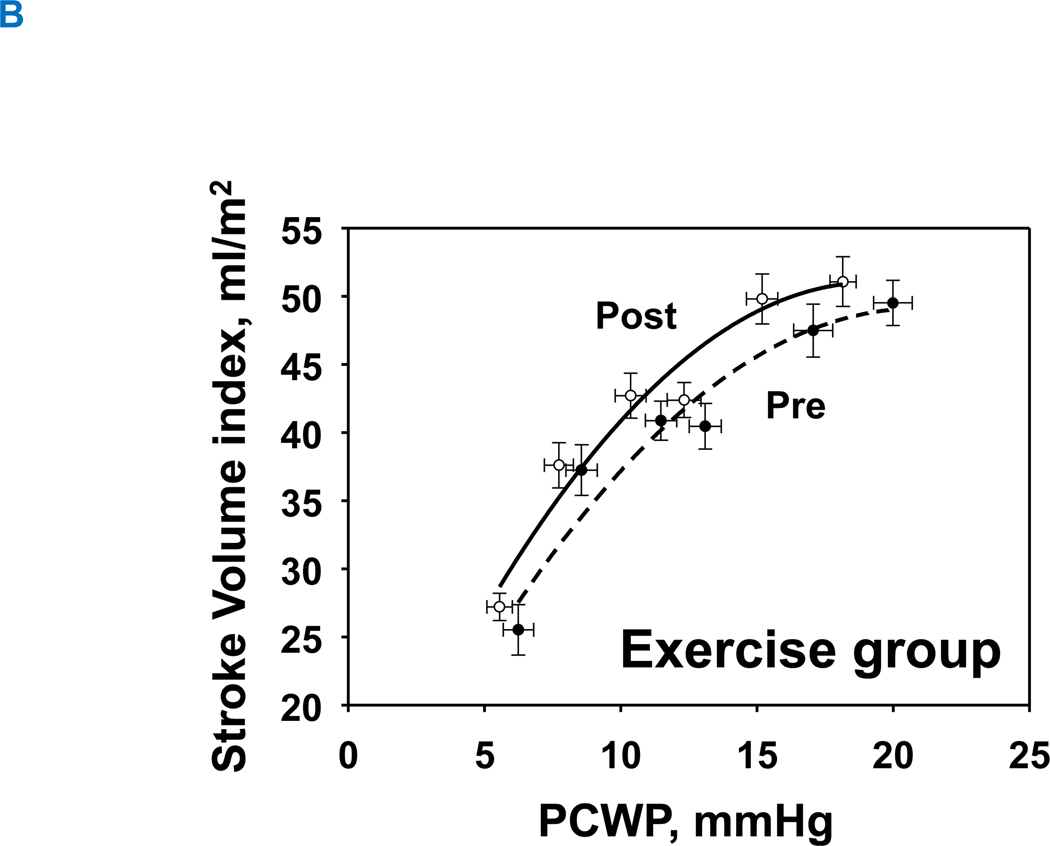
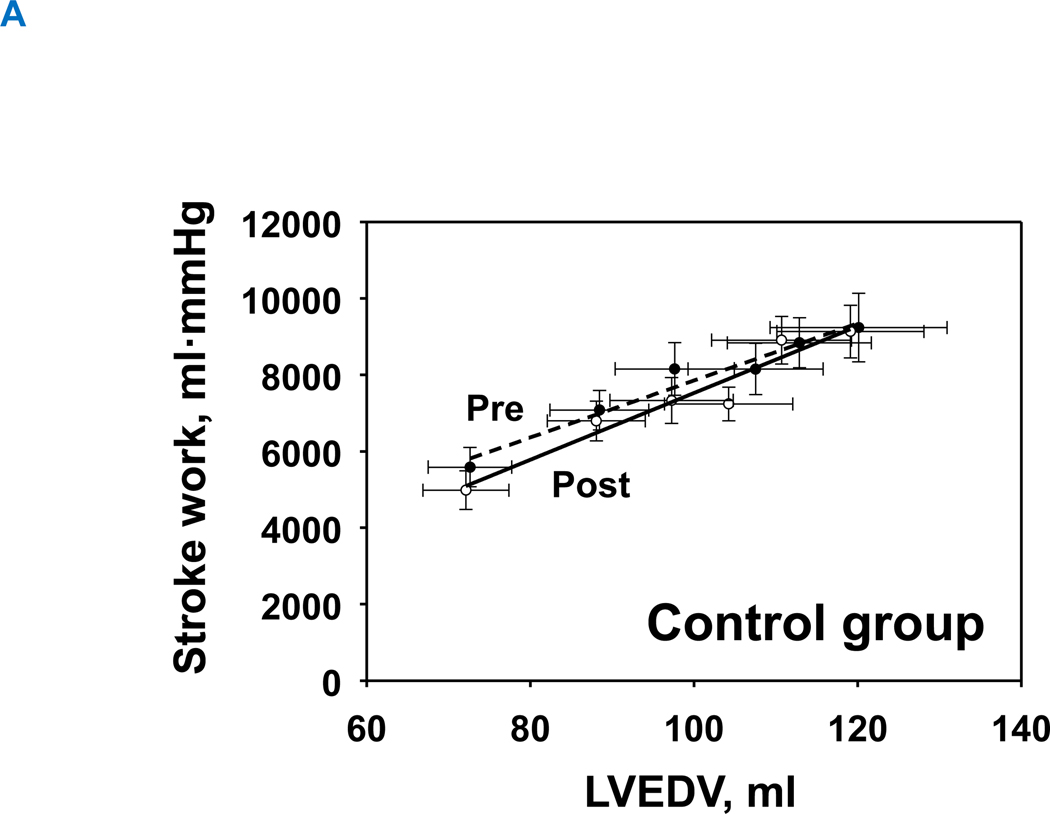
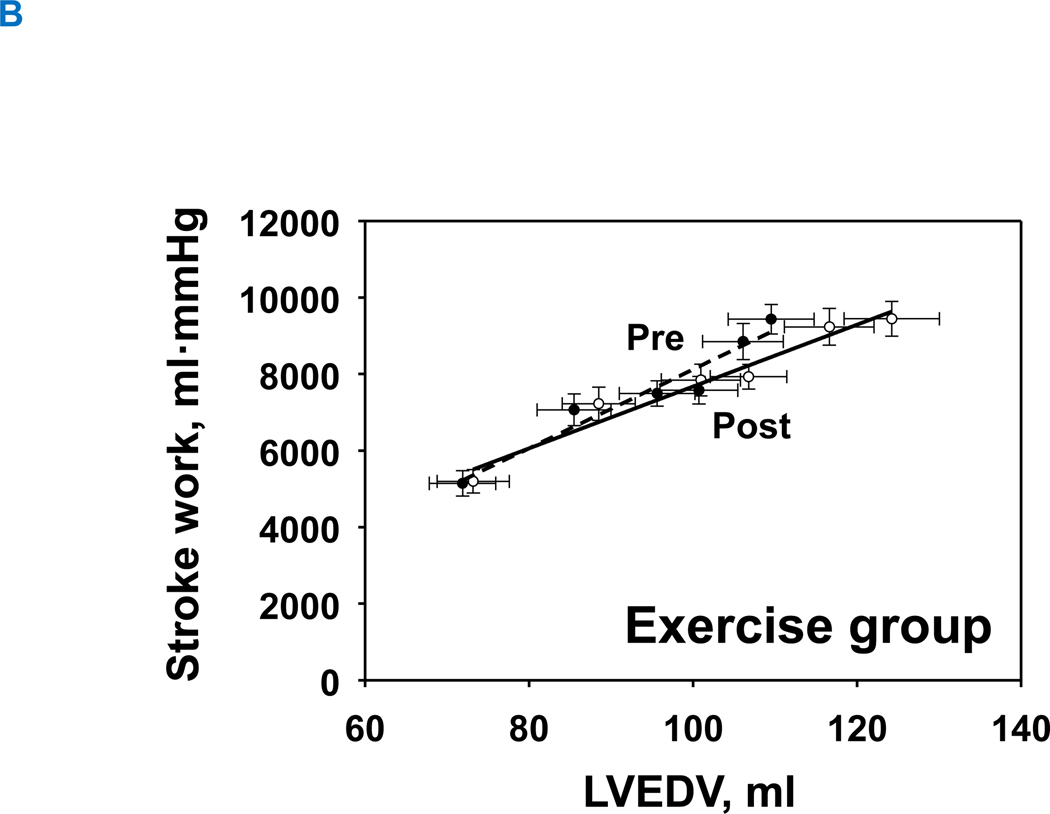
1-year of exercise training resulted in an upward-shift in the Starling curves in the exercise group, allowing for slightly greater stroke volume for any given LV filling pressure (Figure 5A). In contrast, the Starling curves in the control group were comparable between pre and post (Figure 5B). Neither exercise nor control groups changed global systolic function as assessed by the slope of the preload recruitable stroke work (Interaction P value=0.150) (Figure 6A and 6B).
Figure 5. Starling mechanism.
Change in Starling relationship. Pre, black-dash line with closed circle; Post, black line with opened circle. There was no significant change in the control group (A), whereas 1-year of training improved Starling curves (B), such that an increase in stroke volume index was observed compared to baseline for a given LV filling pressure.
LVH, left ventricular hypertrophy group; PCWP, pulmonary capillary wedge pressure, SV was scaled to body surface area.
Figure 6. Preload-recruitable stroke work.
(A) control group and (B) training group. Pre, black-dash line with closed circle; Post, black line with opened circle. There was no significant effect of exercise training or aging on preload recruitable stroke work. Interaction P value=0.150, group P value=0.940, and time P value=0.111.
LVH, left ventricular hypertrophy group; LVEDV, left ventricular end-diastolic volume.
Discussion
This study was a prospective randomized controlled trial to elucidate the effect of prolonged exercise training on LV end-diastolic pressure-volume relationships (LV-EDPVR) in patients with Stage-B HFpEF. The key new findings from this study are that 1-year of prolonged exercise training in this population can improve (a) physical fitness and V̇O2 max; (b) LV diastolic chamber and myocardial stiffness (right-downward-shift), and (c) the Starling mechanism (upward-shift) in patients with LVH and elevated cardiac biomarkers. These patients have already demonstrated LV stiffening17 and thus fit the AHA/ACC definition of stage-B HFpEF, specifically patients with structural heart disease but no current or prior symptoms of HF.37 We propose that targeting such patients at an early stage of their disease with a pathophysiologically directed life-style intervention may be an especially attractive strategy to protect against the full elaboration of the HFpEF syndrome which is so difficult to treat once established.
Cardiac Stiffening in HFpEF: A Target for Prevention
HFpEF is a syndrome characterized by older age, multiple co-morbidities including diabetes and hypertension, and ultimately a stiff heart that relaxes slowly.32, 38 Symptoms in patients with HFpEF are dominated by exercise intolerance, particularly dyspnea on exertion. Although there are many potential mechanisms for this core symptom, it is closely associated with very high filling pressures during exercise.39 Hearts of patients with HFpEF evince steep (and stiff) LV pressure-volume curves with abnormal chamber and myocardial compliance2, 32 suggesting that increased myocardial stiffness underlies much of the rapid rise of pulmonary capillary wedge pressure as LV filling increases during exercise.
Slowed relaxation, increased pericardial constraint, and increases in passive myocardial stiffness may all contribute to a rise in filling pressure during exercise, and all have been demonstrated to some degree or another in patients with HFpEF.2, 5, 32, 39, 40 Increases in passive stiffness have been consistently demonstrated and have been attributed to alterations in both the collagen dependent connective tissue matrix, and the phosphorylation of titin, the large spring-like protein which determines much of the compliance of myocardial tissue.41, 42 Patients with HFpEF also have increased fibrosis and myocardial cell hypertrophy, though the absolute magnitude of the fibrosis is relatively mild in the majority of such patients.43
Patients with hypertension alone without HFpEF though appear to be less clearly affected with limited evidence of excessive stiffening or fibrosis.3, 41 Moreover, sedentary aging, even without co-morbidities leads to LV chamber stiffness that is not radically different from patients with HFpEF.32 Thus, sedentary aging by itself at least sets the stage for exacerbation and secondary remodeling from comorbidities such as obesity, diabetes, and hypertension which leads to the full expression of the HFpEF syndrome.44
Exercise Training Preserves or Increases Myocardial Compliance
In contrast to sedentary aging, high levels of physical activity throughout the life-span preserve youthful LV chamber and myocardial compliance30 as well as vascular compliance,45 though fitness effects on preserving active myocardial relaxation are less protective.46 Cross-sectional studies suggest that four to five days per week of committed exercise throughout the aging process are sufficient to achieve most of these effects9, 47 which is consistent with recent physical activity guidelines for optimal health.29
Prolonged exercise training in youth can recapitulate much of the essential cardiac phenotype of the athlete’s heart which is characterized by a large, compliant LV that can accommodate large volumes during exercise.24, 48 However, once the heart has stiffened in older age, improvements in cardiac or vascular compliance are much harder to obtain. For example, a year of prolonged and intensive exercise training in previously sedentary healthy senior men and women failed to change LV chamber and myocardial10 or vascular49 compliance. Training was similarly ineffective in changing cardiac compliance in patients with established HFpEF.11 Intriguingly, the addition of a drug that breaks advanced glycation end-products, when combined with exercise training induced modest improvements in myocardial compliance in sedentary seniors,12 suggesting that at least some of the changes in passive stiffness with sedentary aging that set the stage for HFpEF may accrue through changes in the connective tissue matrix.
Right Dose, Right Time, Right Patient Population
It is clear that once established, HFpEF is very difficult to treat with few effective therapies.50 Therefore strategies to prevent this widespread disorder are essential.7 Previous work from our group has shown that the heart begins to stiffen in late middle age8 which suggested that initiating an exercise training program earlier in the aging process might be more beneficial than once cardiac stiffening has become firmly established. Indeed a recent study showed that 2 years of exercise training in middle aged men and women, at the dose (frequency and intensity) that preserved cardiac and vascular compliance with aging (i.e, 4–5 days/week)9 was able to reverse the cardiac effects of sedentary aging and restore youthful chamber and myocardial compliance.13
Patients with hypertension and LVH form a major population at high risk for developing HFpEF and thus are an ideal target for interventions to prevent HFpEF. Indeed, recent data from our group showed that patients with LVH who also have elevated biomarkers, including hs-cTnT and NT-proBNP, representing ongoing cardiac injury and/or hemodynamic stress16 have a phenotype that is intermediate between healthy sedentary aging and HFpEF with increased myocardial stiffness (Stage-B HFpEF).17 Whether they could respond similarly to exercise training as healthy middle-aged individuals however was unknown and the focus of this study.
The presence of elevated biomarkers in patients with LVH is not only a clinical marker of high risk, but suggests the presence of pathologic remodeling, which involves distinct biologic pathways compared to the physiologic remodeling of exercise.51, 52 Pathologic growth is characterized by activation of fetal gene programs which include the induction of natriuretic peptides as well as other changes to the sarcomere.52 In contrast, such changes are not induced by exercise training which is mediated by completely different pathways.53 Indeed, activation of physiologic growth programs, involving Akt, P13K, and IGF-1 signaling,54 may directly antagonize the effects of pathologic growth.52 Numerous pathways mediating physiologic growth have been identified, modulated by post-transcriptional regulation by microRNAs which alter titin isoforms, matrix metalloproteinases, and collagen expression.53, 55, 56 These adaptations lead to increased length of cardiomyocytes 53 thus adding sarcomeres in series (reducing the force required to stretch the spring action of titin) and improving passive myocardial compliance with exercise training.
In the present study, we demonstrated that a year of training in patients at high risk for developing HFpEF can be quite effective. V̇O2 max was increased by 20% confirming the expected response to training in this population. LVEDV increased consistent with physiologic remodeling and was accompanied by a prominent increase in both LV chamber (including pericardial constraint) and myocardial compliance. Due to differences in body size, it is not possible to make a complete comparison of the LV myocardial stiffness constant. Indeed, although these patients with Stage-B HFpEF started with greater myocardial stiffness (constant 0.062±0.020) than our previously reported healthy, sedentary middle aged individuals (0.051±0.028)13, their myocardial stiffness constant after training was smaller (i.e., less stiff; 0.031±0.009) than the healthy, sedentary middle aged individuals at baseline, and equivalent to those subjects after 2 years of training (0.039±0.020)13.
Although the exact mechanism of this increased compliance cannot be determined from this study, this outcome is consistent with activation of physiologic growth pathways that antagonized the pathologic growth initiated by both sedentary behavior and the presence of LVH with elevated biomarkers. Whether these changes can be sustained over time, especially if patients revert to their previous sedentary habits, is unknown. Moreover 1 year is too short to determine whether HFpEF can actually be prevented with such an intervention. However, such adaptations as we observed do suggest that the underlying pathophysiology of these Stage-B patients can be altered. Finally, cross-sectional studies of patients with documented sustained high levels of physical activity demonstrate normal and youthful levels of cardiac compliance even into the 7th and 8th decade of life9 providing some support for the concept that sustained physical activity in high-risk patients may be able to forestall HFpEF.
Although the practice of exercise training to improve cardiovascular function is not new, the idea of “Exercise is Medicine” is paradigm shifting.13, 57 This concept refers to the global idea that daily physical activity has such profound and important benefits that it should be considered as a specific medical therapy. Considering exercise as a drug with a specific “dose” (frequency, intensity, duration) targeted for a well-defined, high risk population and a specific biologic outcome using evidence-based training strategies is especially relevant to diseases like HFpEF where prevention may be more effective than treatment.58–60
Study limitations
This study has several limitations. First, this study had a relatively high dropout rate and the training compliance in the exercise group was relatively low (67%) compared to our previous study in healthy middle-aged men and women.13 Moreover, the effective number of participants was small. Nevertheless, the outcomes for performance variables such as V̇O2 max was clear, and the changes in LVEDV, LV chamber and myocardial compliance were compelling, especially compared to a control group where no significant changes were observed. Moreover, there were no significant differences in baseline characteristics, cardiorespiratory fitness or hemodynamic parameters between those participants who completed the intervention, and those who withdrew. Compliance with exercise training, like any therapeutic intervention may be challenging, though there are many proposed strategies to sustain higher rates of physical activity in clinical populations.61, 62 Second, our study is limited to patients with Stage-B HFpEF with LVH and elevated biomarkers and may not be generalizable to other pre-HFpEF patient populations, such as patients with diabetes or marked obesity. In addition, we did not study patients who had LVH without elevated biomarkers. We suspect, but cannot prove, that it is unlikely that such patients would have stiffer LVs than patients with LVH with elevated biomarkers, especially given the lack apparent increased passive stiffness in biopsies from patients with hypertension but not HFpEF taken during cardiac surgery.41 Thus, although other groups of patients might also demonstrate an intermediate stage-B phenotype, we strongly believe that the specific patients studied here represent a particularly high-risk group and should be considered for targeted preventative therapies.
Conclusion
In patients with LVH and elevated cardiac biomarkers (stage-B HFpEF), 1-year of committed exercise training can improve fitness and reverse LV myocardial stiffening. Vigorous exercise training, implemented 4–5 times/week in high-risk middle-aged men and women over a prolonged period, holds promise as a potential intervention to protect against the future risk of HFpEF in such patients.
Supplementary Material
Clinical Perspective.
1). What is new?
In middle aged patients with LVH and elevated cardiac biomarkers (AHA/ACC stage-B HFpEF), 1 year of prolonged exercise training reversed LV chamber and myocardial stiffening. Exercise training may provide protection against the future risk of HFpEF in such patients.
2). What are the clinical implications?
Sustained, adequately dosed aerobic exercise training to improve left ventricular compliance may prevent the full manifestation of the HFpEF syndrome in these high-risk individuals.
Identifying such high-risk patients early in the evolution of HFpEF, and focusing on life-style change with adoption of life-long exercise training may be an effective strategy against this difficult to treat syndrome.
Acknowledgements
We gratefully acknowledge the participants in this study for their time and patience, and the work of past and present members of our laboratory. We would also like to thank Cyrus Oufi, and Ramanathan Murugappan for their technical support in performing the experiments.
Sources of Funding
This study was supported by National Institute of Health grant (AG017479), and by the American Heart Association Strategically Focused Research Network (14SFRN20600009-03). Dr. Hieda was also supported by American Heart Association post-doctoral fellowship grant (18POST33960092) and the Harry S. Moss Heart Trust. Dr. Hearon was supported by National Institute of Health grant (F32HL137285 and K99HL153777-01).
ABBREVIATIONS
- LV
left ventricular
- LVH
left ventricular hypertrophy
- HFpEF
heart failure with preserved ejection fraction
- PCWP
pulmonary capillary wedge pressure
- RAP
right atrial pressure
- LVEDP
left ventricular end-diastolic pressure
- LVEDV
left ventricular end-diastolic volume
- EDPVR
end-diastolic pressure and volume relationship
- LBNP
lower body negative pressure
- NS
rapid normal saline infusion
- SV
stroke volume
Footnotes
Disclosures
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zile MR, Baicu CF and Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. The New England journal of medicine. 2004;350:1953–1959. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi M, Hay I, Fetics B and Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. [DOI] [PubMed] [Google Scholar]
- 4.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA and Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma K and Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circulation research. 2014;115:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. [DOI] [PubMed] [Google Scholar]
- 7.Shah SJ and Gheorghiade M. Heart failure with preserved ejection fraction: treat now by treating comorbidities. JAMA : the journal of the American Medical Association. 2008;300:431–433. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D and Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. The Journal of physiology. 2012;590:1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhella PS, Hastings JL, Fujimoto N, Shibata S, Carrick-Ranson G, Palmer MD, Boyd KN, Adams-Huet B and Levine BD. Impact of lifelong exercise “dose” on left ventricular compliance and distensibility. Journal of the American College of Cardiology. 2014;64:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D and Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2011;122:1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto N, Prasad A, Hastings JL, Bhella PS, Shibata S, Palmer D and Levine BD. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. American heart journal. 2012;164:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto N, Hastings JL, Carrick-Ranson G, Shafer KM, Shibata S, Bhella PS, Abdullah SM, Barkley KW, Adams-Huet B, Boyd KN, Livingston SA, Palmer D and Levine BD. Cardiovascular effects of 1 year of alagebrium and endurance exercise training in healthy older individuals. Circulation Heart failure. 2013;6:1155–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams-Huet B and Levine BD. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation. 2018;137:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry JD, Pandey A, Gao A, Leonard D, Farzaneh-Far R, Ayers C, DeFina L and Willis B. Physical fitness and risk for heart failure and coronary artery disease. Circulation Heart failure. 2013;6:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey A, Patel KV, Vaduganathan M, Sarma S, Haykowsky MJ, Berry JD and Lavie CJ. Physical Activity, Fitness, and Obesity in Heart Failure With Preserved Ejection Fraction. JACC Heart failure. 2018;6:975–982. [DOI] [PubMed] [Google Scholar]
- 16.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T and de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. Journal of the American College of Cardiology. 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hieda M, Sarma S, Hearon CM Jr., Dias KA, Martinez J, Samels M, Everding B, Palmer D, Livingston S, Morris M, Howden E and Levine BD. Increased Myocardial Stiffness in Patients With High-Risk Left Ventricular Hypertrophy: The Hallmark of Stage-B Heart Failure With Preserved Ejection Fraction. Circulation. 2020;141:115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S, de Lemos JA, Matulevicius SA, Ayers C, Pandey A, Neeland IJ, Berry JD, McColl R, Maroules C, Peshock RM and Drazner MH. Association of Concentric Left Ventricular Hypertrophy With Subsequent Change in Left Ventricular End-Diastolic Volume: The Dallas Heart Study. Circulation Heart failure. 2017;10:e003959.. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, McKee DC, Reasoner J and Williams RS. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. J Gerontol. 1989;44:M147–157. [DOI] [PubMed] [Google Scholar]
- 20.Yeh GY, McCarthy EP, Wayne PM, Stevenson LW, Wood MJ, Forman D, Davis RB and Phillips RS. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Archives of internal medicine. 2011;171:750–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T and de Lemos JA. Biomarkers of Chronic Cardiac Injury and Hemodynamic Stress Identify a Malignant Phenotype of Left Ventricular Hypertrophy in the General Population. Journal of the American College of Cardiology. 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine BD and Stray-Gundersen J. “Living high-training low”: effect of moderate-altitude acclimatization with low-altitude training on performance. Journal of applied physiology. 1997;83:102–112. [DOI] [PubMed] [Google Scholar]
- 23.Okazaki K, Iwasaki K, Prasad A, Palmer MD, Martini ER, Fu Q, Arbab-Zadeh A, Zhang R and Levine BD. Dose-response relationship of endurance training for autonomic circulatory control in healthy seniors. J Appl Physiol. 2005;99:1041–1049. [DOI] [PubMed] [Google Scholar]
- 24.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ and Levine BD. Cardiac Remodeling in Response to 1 Year of Intensive Endurance Training. Circulation. 2014;9:2152–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisloff U, Nilsen TI, Droyvold WB, Morkved S, Slordahl SA and Vatten LJ. A single weekly bout of exercise may reduce cardiovascular mortality: how little pain for cardiac gain? ‘The HUNT study, Norway’. European journal of cardiovascular prevention and rehabilitation : official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2006;13:798–804. [DOI] [PubMed] [Google Scholar]
- 26.Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R and Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Medicine and science in sports and exercise. 2007;39:665–671. [DOI] [PubMed] [Google Scholar]
- 27.Molmen-Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB and Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;19:151–160. [DOI] [PubMed] [Google Scholar]
- 28.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O and Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. [DOI] [PubMed] [Google Scholar]
- 29.Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM and Olson RD. The Physical Activity Guidelines for Americans. JAMA : the journal of the American Medical Association. 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arbab-Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D and Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. [DOI] [PubMed] [Google Scholar]
- 31.Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA and Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation. 1986;73:428–432. [DOI] [PubMed] [Google Scholar]
- 32.Prasad A, Hastings JL, Shibata S, Popovic ZB, Arbab-Zadeh A, Bhella PS, Okazaki K, Fu Q, Berk M, Palmer D, Greenberg NL, Garcia MJ, Thomas JD and Levine BD. Characterization of static and dynamic left ventricular diastolic function in patients with heart failure with a preserved ejection fraction. Circulation Heart failure. 2010;3:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardin EA, Stoller D, Lawley J, Howden EJ, Hieda M, Pawelczyk J, Jarvis S, Prisk K, Sarma S and Levine BD. Noninvasive Assessment of Cardiac Output: Accuracy and Precision of the Closed-Circuit Acetylene Rebreathing Technique for Cardiac Output Measurement. Journal of the American Heart Association. 2020;9:e015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirsky I. Assessment of diastolic function: suggested methods and future considerations. Circulation. 1984;69:836–841. [DOI] [PubMed] [Google Scholar]
- 35.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC and Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71:994–1009. [DOI] [PubMed] [Google Scholar]
- 36.Kelly RP, Ting CT, Yang TM, Liu CP, Maughan WL, Chang MS and Kass DA. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg LR and Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–2860. [DOI] [PubMed] [Google Scholar]
- 38.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nature reviews Cardiology. 2014;11:507–515. [DOI] [PubMed] [Google Scholar]
- 39.Borlaug BA. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction. Circulation journal : official journal of the Japanese Circulation Society. 2014;78:20–32. [DOI] [PubMed] [Google Scholar]
- 40.Borlaug BA and Kass DA. Mechanisms of diastolic dysfunction in heart failure. Trends in cardiovascular medicine. 2006;16:273–279. [DOI] [PubMed] [Google Scholar]
- 41.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL and LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamdani N, Franssen C, Lourenco A, Falcao-Pires I, Fontoura D, Leite S, Plettig L, Lopez B, Ottenheijm CA, Becher PM, Gonzalez A, Tschope C, Diez J, Linke WA, Leite-Moreira AF and Paulus WJ. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circulation Heart failure. 2013;6:1239–1249. [DOI] [PubMed] [Google Scholar]
- 43.Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C Jr., Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA and Sharma K. Endomyocardial Biopsy Characterization of Heart Failure With Preserved Ejection Fraction and Prevalence of Cardiac Amyloidosis. JACC Heart failure. 2020;8:712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paulus WJ and Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62:263–271. [DOI] [PubMed] [Google Scholar]
- 45.Shibata S and BD L. Biologic Aortic Age Derived from the Arterial Pressure Waveform. J Appl Phsiol. 2011;110:981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prasad A, Popovic ZB, Arbab-Zadeh A, Fu Q, Palmer D, Dijk E, Greenberg NL, Garcia MJ, Thomas JD and Levine BD. The effects of aging and physical activity on Doppler measures of diastolic function. The American journal of cardiology. 2007;99:1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM Jr. and Levine BD. The effect of lifelong exercise frequency on arterial stiffness. The Journal of physiology. 2018;596:2783–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levine BD, Lane LD, Buckey JC, Friedman DB and Blomqvist CG. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation. 1991;84:1016–1023. [DOI] [PubMed] [Google Scholar]
- 49.Shibata S and Levine BD. No Improvement in Biologic Aortic Age in Healthy Seniors Aged Over 65 Years Even After One Year of Endurance Exercise Training. Am J Physiol-Heart and Circulatory Physiology. 2012; 302: 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddy YN and Borlaug BA. Heart Failure With Preserved Ejection Fraction. Current problems in cardiology. 2016;41:145–188. [DOI] [PubMed] [Google Scholar]
- 51.Hill JA and Olson EN. Cardiac plasticity. The New England journal of medicine. 2008;358:1370–80. [DOI] [PubMed] [Google Scholar]
- 52.Vega RB, Konhilas JP, Kelly DP and Leinwand LA. Molecular Mechanisms Underlying Cardiac Adaptation to Exercise. Cell metabolism. 2017;25:1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM and Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuttler D, Clauss S, Weckbach LT and Brunner S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells. 2019;8:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaturvedi P, Kalani A, Medina I, Familtseva A and Tyagi SC. Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. Journal of cellular and molecular medicine. 2015;19:2153–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soci UP, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, Irigoyen MC, Phillips MI and Oliveira EM. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiological genomics. 2011;43:665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sallis RE. Exercise is medicine and physicians need to prescribe it! British journal of sports medicine. 2009;43:3–4. [DOI] [PubMed] [Google Scholar]
- 58.Blair SN. Physical inactivity: the biggest public health problem of the 21st century. British journal of sports medicine. 2009;43:1–2. [PubMed] [Google Scholar]
- 59.Levine BD and Stray-Gundersen J. Dose-response of altitude training: how much altitude is enough? Adv Exp Med Biol. 2006;588:233–247. [DOI] [PubMed] [Google Scholar]
- 60.Kraus WE and Levine BD. Exercise training for diabetes: the “strength” of the evidence. Annals of internal medicine. 2007;147:423–424. [DOI] [PubMed] [Google Scholar]
- 61.Simons-Morton DG, Hogan P, Dunn AL, Pruitt L, King AC, Levine BD and Miller ST. Characteristics of inactive primary care patients: baseline data from the activity counseling trial. For the Activity Counseling Trial Research Group. Prev Med. 2000;31:513–21. [DOI] [PubMed] [Google Scholar]
- 62.Stonerock GL and Blumenthal JA. Role of Counseling to Promote Adherence in Healthy Lifestyle Medicine: Strategies to Improve Exercise Adherence and Enhance Physical Activity. Progress in cardiovascular diseases. 2017;59:455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.