Abstract
Objective
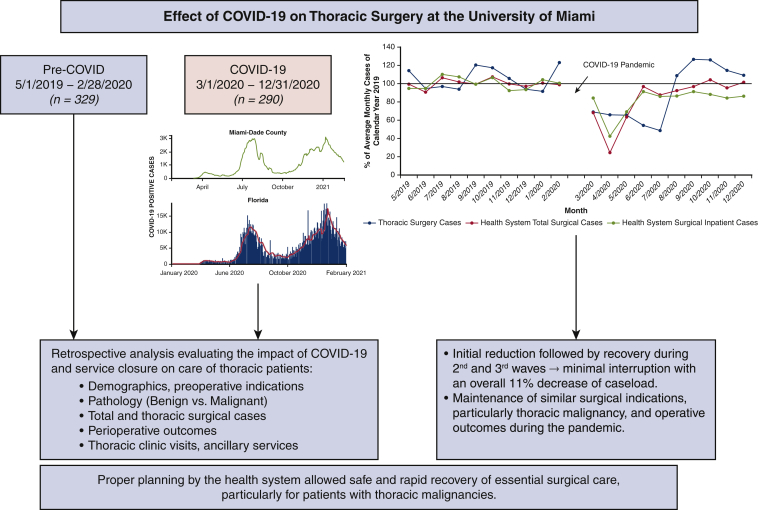
In this study we aimed to determine the effect of the COVID-19 pandemic on the delivery of care for thoracic surgical patients at an urban medical center.
Methods
A retrospective analysis of all thoracic surgical cases from May 1, 2019, to December 31, 2020, was conducted. Demographic characteristics, preoperative surgical indications, procedures, final pathologic diagnoses, and perioperative outcomes were recorded. A census of operative cases, relevant ancillary services, and outpatient thoracic clinics were obtained from our institutional database.
Results
Six hundred nineteen cases were included in this study (329 pre-COVID-19 and 290 COVID-19, representing an 11.8% reduction). There were no differences in type of thoracic procedures or perioperative outcomes among the 2 cohorts. Prolonged reduction of thoracic surgical cases (50% of baseline) during the first half of the COVID-19 period was followed by a resurgence of surgical volumes to 110% of baseline in the second half. A similar incidence of cases were performed for oncologic indications during the first half whereas more benign cases were performed in the second half, coinciding with the launch of our robotic foregut surgery program. After undergoing surgery during the pandemic, none of our patients reported COVID-19 symptoms within 14 days of discharge.
Conclusions
During the initial surge of COVID-19, while there was temporary closure of operative services, our health care system continued to provide safe care for thoracic surgery patients, particularly those with oncologic indications. Since phased reopening, we have experienced a rebound of surgical volume and case mix, ultimately mitigating the initial negative effect of the pandemic on delivery of thoracic surgical care.
Key Words: severe acute respiratory syndrome coronavirus 2, COVID-19, thoracic surgery, lung cancer
Abbreviations and Acronyms: CT, computed tomography; PFT, pulmonary function test; PPE, personal protective equipment; RT-PCR, reverse transcription polymerase chain reaction; R-VATS, robotic thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery
Graphical abstract
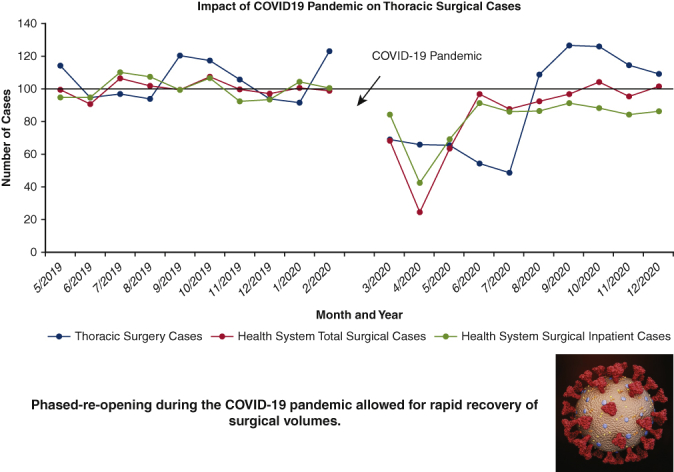
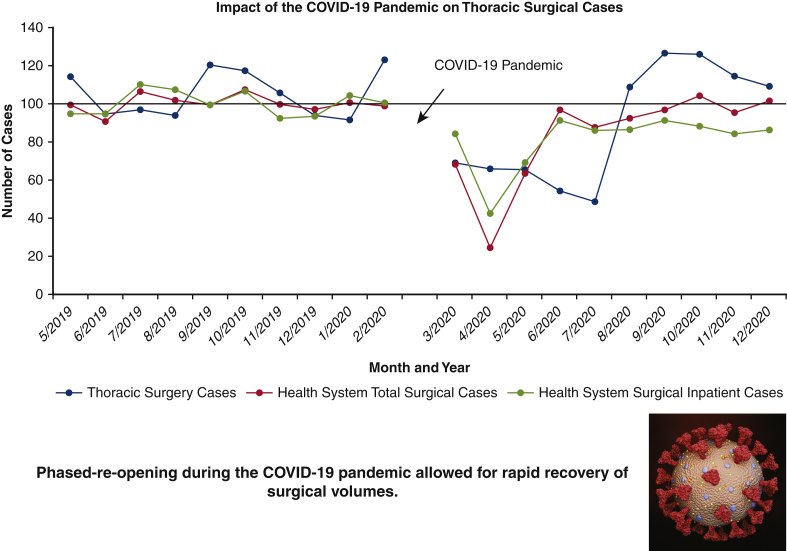
Phased reopening in the COVID-19 pandemic allowed for rapid recovery of surgical volume at the University of Miami Medical Center.
Central Message.
Conforming to guidelines established by surgical societies and the health system allowed for safe delivery of care for thoracic surgical patients during the COVID-19 pandemic with minimal disruption.
Perspective.
This study demonstrates expedient preparation and deployment of protocols that aimed to reallocate resources and minimize the risk of SARS-CoV-2 transmission enabled the continuity of high-quality and safe delivery of care, at reduced capacity. Phased reopening of surgical care, corresponding with the regional COVID-19 burden and available health system resources, mitigated the initial adverse effects of the pandemic.
See Commentary on page 469.
The rapid emergence and spread of SARS-CoV-2, which causes COVID-19, overwhelmed entire health care systems in the United States and worldwide in the beginning of 2020. On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic.1 The spectrum of clinical manifestations of this airborne infection range from no symptoms to rapidly progressive severe respiratory failure with high morbidity and mortality.2,3 The first line of defense included limiting spread via social distancing and personal protection using face masks. Health care workers used the N95 respirator.4 Initially, personal protective equipment (PPE) was in short supply, and rapid, accurate diagnostic tests were being developed with sparse testing center capabilities.5 Because of the many unknowns and the profound, devastating effect of SARS-CoV-2 to the Northeast United States, drastic actions were rapidly implemented nationwide to reallocate medical resources to prepare for the inevitable future waves of COVID-19.
Hospital systems developed plans and new polices to limit admissions and elective surgical procedures to maintain inpatient bed availability for COVID-19 patients, to ensure a safe work environment for staff, and to preserve precious, limited PPE.6,7 Leading professional surgical organizations provided guidelines to assist medical centers and surgeons in triaging surgical cases.8, 9, 10 Surgical care was rationed and nonemergent cases were postponed, with a focus on life-saving operations, particularly those for neoplasms.8,11 At baseline, the lung cancer population is highly vulnerable, with an increased risk for morbidity and mortality from COVID- 19 because of their innate characteristics, including cardiovascular and pulmonary comorbidities, and immunocompromised state secondary to cancer treatment.12, 13, 14, 15, 16 Moreover, surgical procedures of the aerodigestive tract, notably head and neck cancer cases, esophagectomies, and pulmonary resections impart an additional risk for health care providers, particularly for anesthesiologists and the surgical team.16, 17, 18
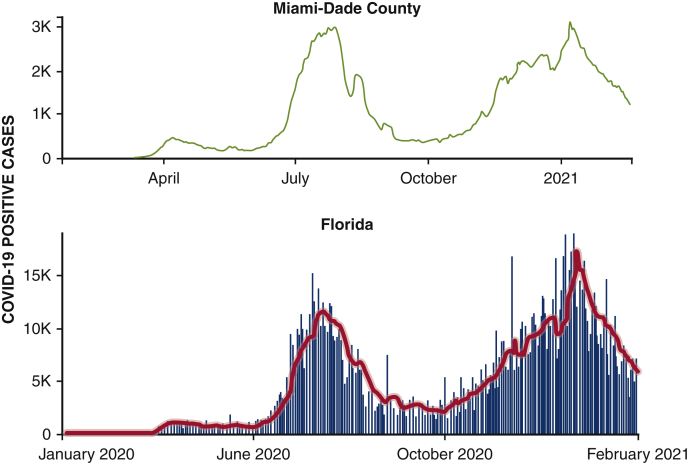
The University of Miami Health System is an academic tertiary care center serving large counties of South Florida, including Miami-Dade, Broward, and the Florida Keys. In conjunction with the state health department, we implemented a systematic closure of elective, nonessential surgical procedures and ancillary services in mid-March of 2020 in preparation for the first wave of SARS-CoV-2 projected to reach South Florida in April and May 2020.19 Patients scheduled to undergo surgical procedures were required to have a negative SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) nasopharyngeal swab within 48 hours of the procedure. We started a deliberate phased reopening starting in June 2020, as described,20,21 allowing properly triaged elective surgical cases to proceed and remain fully operational while weathering the second and third surges of SARS-CoV-2, which began in July and October 2020, respectively (Figure 1).22
Figure 1.
Daily trends of confirmed COVID-19 positive cases in Miami-Dade County and the State of Florida. The red line represents the 7-day moving average. Data from the CDC Data COVID-19 Tracker (https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases). COVID-19, Coronavirus disease-19.
We sought to determine the effect of the COVID-19 pandemic, including the closure of surgical services to ration resources, on the delivery of thoracic surgical care. We aimed to survey the effect of the pandemic on our surgical volume and case composition, and ultimately determine if such restrictions of care affected surgical outcomes.
Methods
Data Collection and Outcome Metric Measurements
We performed a retrospective review of our prospectively maintained database and the electronic medical record, Epic Systems Corporation, for patients who received thoracic operations at the University of Miami Health System from May 1, 2019, to December 31, 2020. An institutional review board approval with a waiver of patient consent requirement was obtained (number 20210242; date of approval: March 17, 2021). The following information was acquired for each patient: demographic characteristics (age, gender), thoracic surgical procedure approach (sternotomy, thoracotomy, video-assisted thoracoscopic surgery [VATS], robotic thoracoscopic surgery [R-VATS]), surgical procedure performed (lung resections [ie, pneumonectomy, lobectomy, segmentectomy, wedge resection]; mediastinal procedures [ie, resection of mediastinal tumors, thymectomy, neurogenic tumors, pericardial window, diaphragmatic plication]; pleural procedures [ie, pleurodesis, pleurectomy]; benign foregut procedures [ie, for achalasia, gastroesophageal reflux disease, hiatal hernia]), preoperative indications for the operation (benign vs malignant), pathologic diagnosis (benign vs malignant), in-hospital and 30-day postoperative morbidity/mortality (Clavien–Dindo classification), and length of hospital stay. The number of inpatient, outpatient, and overnight admission operations, as well as outpatient clinic visits (new, follow-up, postoperative), diagnostic radiology procedures such as positron emission tomography/computed tomography (CT) scan and chest CT, and pulmonary function test (PFT) were derived from the health care system central database. Data collected from March 1, 2020, to December 31, 2020 formed the COVID-19 group, which was further subdivided into two 5-month periods (March 1, 2020, to July 31, 2020, and August 1, 2020, to December 31, 2020), representing the first wave and the subsequent second and third waves, respectively. Clinical data collected from the preceding 10 months (May 1, 2019, to February 28, 2020) served as the historic control. Surgical cases, procedures, and thoracic clinic visits for each month throughout the study period were calculated as percentages of the respective average of monthly numbers of the 2019 calendar year. Within a month of the first pandemic surge, our outpatient thoracic clinic transitioned to telemedicine encounters (including new patient, follow-up, and postoperative visits). All patients in the COVID-19 group who underwent a procedure were required to reply to a questionnaire on postdischarge COVID-19 symptoms, which included fever, cough, shortness of breath, and loss of taste or smell, or confirmed COVID-19 infection at subsequent clinical encounters, including for telehealth visits, for up to 1 month postprocedure.23 Any postprocedure COVID-19 testing result in our health system was obtained.
Statistical Analysis
Statistical analysis of categorical variables, expressed as percentages and frequencies, were analyzed using Fisher exact test. Nonparametric continuous variables, expressed as median and interquartile range, were compared using the Mann–Whitney U test. Statistical analysis was performed with GraphPad software.
Results
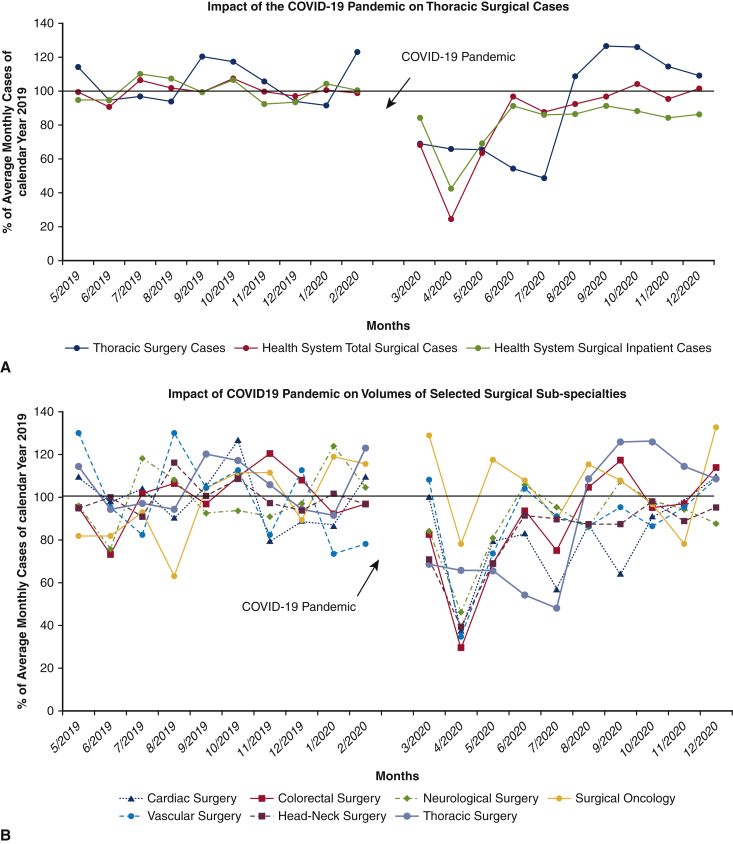
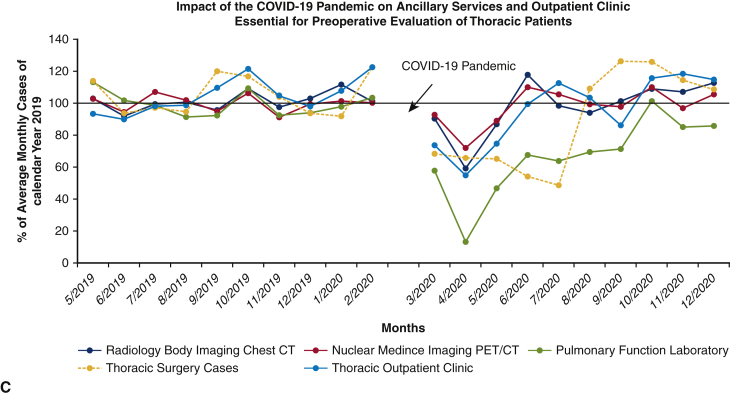
A total of 619 thoracic surgical cases (610 patients) were performed over the 20-month study period; 329 procedures in the pre-COVID-19 control group (May 1, 2019, to February 28, 2020), and 290 procedures in the COVID-19 group (March 1, 2020, to December 31, 2020) representing a cumulative 11.8% reduction in case volume (Table 1). To date, of the patients admitted for thoracic surgical operations, none reported SARS-CoV-2 symptoms or infection within 14 days of discharge from the hospital. Figure 2, A shows the effect of the pandemic and hospital closure on thoracic surgical cases, inpatient cases, and all surgical procedures over the 10-month period with the preceding 10 months serving as a historical control. There was a dramatic but rapid recovery of total and inpatient cases performed, with the nadir in April 2020, of approximately 22% of historic control. Although total cases performed returned to baseline values within 2 months, inpatient surgical volumes only reached 85% to 90% of expected control. Thoracic surgery volume, which reflects inpatient-only cases, was decreased to 50% to 60% of baseline during the first wave (March 2020 to July 2020) but rebounded to be consistently 110% to 120% of baseline volume during the second and third waves (August 2020 to December 2020). The overall reduction in thoracic cases for the entire 10-month period during the pandemic was 11.8%. Figure 2, B provides a granular analysis of the pandemic's effect on surgical volumes of 6 representative surgical service lines in addition to thoracic surgery. All surgical services, except for surgical oncology, experienced a sharp decline of same-day-admit surgical cases in April 2020, and returned to baseline in June 2020; full recovery of thoracic surgical volumes occurred only in August of 2020. Figure 2, C shows the effect of the COVID-19 pandemic on outpatient thoracic clinics, diagnostic radiology imaging services, and PFT volumes. Initially, outpatient thoracic clinics experienced a decline in the total number of postoperative visits in the COVID-19 cohort, however with telehealth encounters, there was a rapid rebound to baseline values. Imaging services and outpatient clinic activities returned to baseline after a 3-month delay whereas the pulmonary function laboratory experienced a longer reduction of services for nearly 7 months.
Table 1.
Total thoracic surgical cases
| Pre-COVID-19 (May 1, 2019 to February 28, 2020) | COVID-19 (March 1, 2020 to December 31, 2020) | % Change | |
|---|---|---|---|
| Total cases | 329 | 290 | −11.80 |
| Thoracotomy | 55 | 35 | −36.4 |
| VATS | 62 | 55 | −11.3 |
| Robotic VATS | 212 | 200 | −5.6 |
| % Preoperative oncologic indication | 78.70 | 75.50 | P = .47 |
| COVID-19 symptoms noted on postoperative visit | N/A | 0 |
COVID-19, Coronavirus disease-19; VATS, video-assisted thoracoscopic surgery; N/A, not applicable.
Figure 2.
A, There was a 5-month reduction in thoracic surgical volume at the beginning of the COVID-19 pandemic in south Florida, with monthly caseloads approximately 50% of the pre-COVID-19 period volume. This was followed by a robust recovery of monthly surgical volume averaging 110% of pre-COVID-19, starting in August 2021. This is in contrast with the profound (averaging 30% of pre-COVID-19 monthly volume) but very brief (2 months) reduction of overall surgical caseload of the entire health system. B, Detailed analysis of the effect of operative service closures on 6 representative surgical subspecialties (colorectal surgery, head-neck oncology surgery, surgical oncology, neurological surgery, vascular surgery, cardiac surgery) in addition to thoracic surgery. Similar to thoracic surgery, the cardiac surgery service had a rather protracted course to full recovery whereas other service lines fully recovered by June 2020. C, The effect of COVID-19 on University of Miami Health System thoracic surgery cases compared with thoracic surgery outpatient clinics, diagnostic radiology, nuclear medicine, and pulmonary function laboratory for pre-COVID-19 and COVID-19 cohorts. Surgical cases, procedures, and thoracic clinic visits for each month throughout the study period were calculated as percentages of the respective average of monthly numbers of the 2019 calendar year. COVID-19, Coronavirus disease-19; CT, computed tomography; PET, positron emission tomography.
Detailed analysis of the effect of the pandemic on thoracic surgery surgical volumes and case composition are shown in Tables 2 and 3. Although fewer thoracotomies were performed during the pandemic, the 2 cohorts were comparable in terms of preoperative oncologic indications, proportion of cases for lung cancer and other intrathoracic malignancies, postoperative outcomes, and hospital length of stay (Table 2). For non–small cell lung cancers with curative-intent thoracotomy, there was no difference in tumor sizes or stage 1A to 2B; there were more stage 3 and 4 patients in the COVID-19 cohort (46.7% vs 29.4% of the pre-COVID-19 group; P < .01). There were more anatomic resections, largely lobectomies, performed during the pandemic (57% vs 34.5%; P = .05). There were 2 mortalities after thoracotomy pulmonary resections for locally advanced primary lung cancer during the pandemic cohort, compared with zero in the historical control. These mortalities were unrelated to the COVID-19 pandemic and were not attributed to lack of intensive care resources. One patient suffered irreversible left ventricular outflow tract obstruction due to known idiopathic hypertrophic subaortic stenosis. The second mortality was due to multiorgan failure after a lobectomy and chest wall resection in a patient with a previous, remote contralateral lung lobectomy for a metachronous non–small cell lung cancer. There was no meaningful difference in the in-hospital and 30-day complication rates and mortality among the 2 cohorts; there were few grade 1 to 2 complications noted within 30 days of the operation that occurred after discharge from the hospital. Fewer VATS cases were performed during the pandemic (55 vs 62 cases) representing an 11.3% decrease. The indications for VATS cases were similar among the 2 cohorts, with a preoperative oncologic indication of 62% versus 64.5% for the COVID-19 and control groups, respectively.
Table 2.
Total thoracotomy cases before and during COVID-19 pandemic
| Pre-COVID-19 (n = 55) | During COVID-19 (n = 35) | P value | |||
|---|---|---|---|---|---|
| Age (range), years | 60 (47-66) | 62 (43-67) | |||
| Male/female | 35/20 | 24/11 | |||
| Preoperative oncologic indication, n (% of total) | 43 (78.2) | 29 (82.8) | .32 | ||
| Pathologic diagnosis of malignancy, n (% of total) | 43 | 29 | |||
| Thoracic malignancies, n | 43 | 29 | |||
| Primary lung cancer (n, % malignant cases) | 16 (37.2) | 15 (51.7) | .22 | ||
| T, n (%) | |||||
| T1 | 2 (12.5) | 2 (13.3) | |||
| T2 | 6 (37.5) | 5 (33.3) | |||
| T3 | 6 (37.5) | 4 (26.6) | |||
| T4 | 2 (12.5) | 4 (26.6) | |||
| Pathologic stage, n (%) | |||||
| 0-1 | 4 (25.0) | 3 (20.0) | .74 | ||
| 2 A/B | 7 (43.7) | 5 (33.3) | .19 | ||
| 3-4 | 5 (29.4) | 7 (46.7) | .01 | ||
| Secondary lung cancer and other neoplasms | 26 (60.5) | 14 (48.3) | |||
| Anatomic lung resections, n (% of total) | 19 (34.5) | 20 (57.1) | .05 | ||
| LOS (range), days | 7 (3.5-20.5) | 4 (3.5-7.5) | .39 | ||
| Complications (Clavien–Dindo), n | In-hospital | 30-Day | In-hospital | 30-Day | |
| 0 | 12 | 10 | 13 | 12 | 1.00 |
| 1-2 | 3 | 5 | 4 | 5 | |
| 3-4 | 4 | 4 | 1 | 1 | |
| 5 | 0 | 0 | 2 | 2 | |
| Wedge resection, mediastinal-pleural procedures, n (% of total) | 36 (65.4) | 15 (42.8) | |||
| LOS (range), days | 8 (3-13) | 5 (4-8.7) | .81 | ||
| 30-Day complications (Clavien–Dindo), n | In-hospital | 30-Day | In-hospital | 30-Day | |
| 0 | 25 | 23 | 10 | 9 | 1.00 |
| 1-2 | 7 | 8 | 1 | 2 | |
| 3-4 | 3 | 4 | 3 | 3 | |
| 5 | 1 | 1 | 1 | 1 | |
Data in bold reflect a P value <.05.
COVID-19, Coronavirus disease-19; LOS, length of stay.
Table 3.
Total robotic thoracoscopy cases before and during COVID-19 pandemic
| May 1, 2019 to February 28, 2020 (n = 212) | March 1, 2020 to December 31, 2020 (n = 200) | P value | March 1, 2020 to July 31, 2020; first COVID-19 wave (n = 65) | August 1, 2020 to December 31, 2020; second and third COVID-19 waves (n = 135) | P value first wave vs pre-COVID-19 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median age (IQR), years | 63 (54-70) | 63 (54-71) | 63 (55-73) | 63 (54-71) | ||||||
| Male/female, n | 82/130 | 81/119 | 34/31 | 47/88 | ||||||
| Preoperative oncologic indication, n (% of total) | 180 (86) | 144 (72) | <.01 | |||||||
| Pathologic diagnosis of malignancy, n (% of total) | 161 (75.9) | 124 (62) | <.01 | 44 (67.7) | 80 (59.2) | .19 | ||||
| Thoracic malignancies, n (% of total cases) | 161 (75.9) | 124 (62.0) | 44 (67.7) | 80 (59.2) | ||||||
| Primary lung cancer, n (% thoracic malignant cases) | 91 (56.5) | 75 (60.4) | .54 | 27 (61.5) | 48 (60.0) | |||||
| T, n (% of primary lung cancer) | ||||||||||
| T0 (post induction) | 0 | 3 (4.0) | 2 | 1 | ||||||
| T1a | 16 (17.6) | 12 (16.0) | .78 | 3 (0.07) | 9 (0.02) | |||||
| T1b | 21 (23.1) | 17 (22.7) | .98 | 3 (0.11) | 14 (0.19) | |||||
| T1c | 12 (13.2) | 11 (14.7) | .78 | 3 (0.11) | 8 (0.29) | |||||
| T2a | 11 (12.1) | 17 (22.7) | .07 | 8 (0.30) | 9 (0.19) | |||||
| Pathologic stage, n (%) | ||||||||||
| 0-1A/B | 63 (69.2) | 60 (80.0) | .11 | 19 (70.4) | 41 (85.4) | |||||
| 2A/B | 11 (12.1) | 6 (8.0) | .38 | 4 (14.8) | 2 (4.1) | |||||
| 3-4 | 17 (18.6) | 9 (12.0) | .24 | 4 (14.8) | 5 (10.4) | |||||
| Secondary lung cancer, n (% thoracic malignant cases) | 58 (36.0) | 36 (29.0) | .34 | 14 (31.8) | 22 (27.5) | |||||
| Mediastinal/pleural cancers, n (% of thoracic malignant cases) | 12 (7.4) | 13 (10.1) | 3 (6.8) | 10 (12.5) | ||||||
| Foregut procedures, n (% of total cases) | 2 (0.9) | 28 (14) | N/A | 3 (4.6) | 25 (18.5) | |||||
| Types of procedures | ||||||||||
| Lung resection, n (% of cases) | 159 (75) | 136 (68) | .12 | 50 (79) | 86 (63.7) | |||||
| Anatomic resection, n (% of lung resections) | 74 (46.5) | 76 (55.9) | .13 | 30 (60.0) | 46 (53.5) | .17 | ||||
| Median LOS (range), days | 2.5 (2-3) | 2 (2-3) | .03 | 2 (1-3) | 2 (1-3) | |||||
| Complications (Clavien–Dindo) | In-hospital | 30-Day | In-hospital | 30-Day | ||||||
| 0 | 70 | 68 | 70 | 69 | 1.00 | 29 | 42 | |||
| 1-2 | 2 | 2 | 5 | 6 | 1 | 2 | ||||
| 3-4 | 2 | 4 | 1 | 1 | 0 | 2 | ||||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Wedge resection, n (% of lung resections) | 85 (53.4) | 60 (44.1) | 20 (40.0) | 40 (46.5) | ||||||
| Median LOS (range), days | 1 (1-2) | 1 (1-2) | .70 | 1 (1-2) | 1 (1-2) | |||||
| Complications (Clavien–Dindo) | In-hospital | 30-d | In-hospital | 30-d | ||||||
| 0 | 79 | 77 | 55 | 55 | 1.0000 | 18 | 37 | |||
| 1-2 | 4 | 6 | 2 | 2 | 1 | 1 | ||||
| 3-4 | 2 | 2 | 3 | 3 | 1 | 2 | ||||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Mediastinal; pleural procedures, n (% of cases) | 51 (24.0) | 36 (18.0) | .15 | 12 (18.4) | 24 (17.8) | |||||
| Median LOS (range), days | 1 (1-3) | 1 (1-2) | .47 | 1 (1-2) | 1 (1-2.7) | |||||
| Complications (Clavien–Dindo) | In-hospital | 30-d | In-hospital | 30-d | ||||||
| 0 | 51 | 51 | 36 | 35 | 1.00 | 12 | 24 | |||
| 1-2 | 0 | 0 | 0 | 1 | 0 | 0 | ||||
| 3-4 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| Foregut procedures, n (% of cases) | 2 (0.9) | 28 (14) | N/A | 3 (4.6) | 25 (18.5) | |||||
| Median LOS (range), days | 1 (1-1.5) | 2 (2-2) | 1 (1-1) | |||||||
| Complications (Clavien–Dindo) | In-hospital | 30-d | In-hospital | 30-d | ||||||
| 0 | 2 | 27 | 27 | 3 | 24 | |||||
| 1-2 | 0 | 1 | 1 | 0 | 1 | |||||
| 3-4 | 0 | 0 | 0 | 0 | 0 | |||||
| 5 | 0 | 0 | 0 | 0 | 0 | |||||
Data in bold reflect a P value <.05.
COVID-19, Coronavirus disease-19; IQR, interquartile range; N/A, not applicable; LOS, length of stay.
A total of 412 R-VATS procedures were performed over the 20-month study period, 212 cases prepandemic with a monthly average of 21 cases, and 200 cases during the pandemic, representing a 5.6% decrease in volume. The most significant reduction occurred during the first 5 months, the first wave of the pandemic, with only 65 cases performed, averaging 12 cases per month. However, this downward trend was reversed when the University of Miami Health System went through a rapid phased reopening beginning in June to August 2020. There were 135 cases completed in second/third COVID-19 waves, averaging 27 cases per month, which represents a 28% increase above the monthly baseline of R-VATS in the prepandemic control cohort. Table 3 provides a detailed analysis of the case composition, indications for surgical interventions, final pathologic diagnosis, incidence of lung cancer, and other thoracic malignancies as well as postoperative outcomes of R-VATS cases. During the pandemic there was a lower incidence of preoperative oncologic indication (62% vs 86%; P < .01) and postoperative diagnosis of cancer (62% vs 75.9%; P < .01) in R-VATS cases performed. Further subgroup analysis of the pandemic cohort showed that in the first 5 months of the pandemic (March to July, 2020) the incidence of preoperative oncologic indication (85% vs 86%; P = .34) and postoperative diagnosis of malignancy (67.7% vs 75.9%; P = .19) were similar. In the second 5 months of the pandemic (August to December, 2020), fewer R-VATS were performed for oncologic indications and there were more foregut procedures (25 cases), coinciding with the launch of our robotic foregut surgery program. Furthermore, by excluding the 28 benign foregut cases in the COVID-19 cohort, there were 172 total intrathoracic cases, with the incidence of preoperative oncologic indication in 144 of 172 cases (83.7%) and the pathologic diagnosis of malignancy in 124 of 172 cases (72.1%), comparable with the pre-COVID-19 group. There was no difference in the T stages and the final pathologic stages of non–small lung cancer or in postoperative in-hospital and 30-day complications among the pre-COVID-19 and COVID-19 cohorts (Table 3).
Discussion
The consequences of the COVID-19 pandemic worldwide have been immense, with uncertain long-term implications, especially for oncology patients, and a varying degree of ramifications for years to come.24, 25, 26 Although our thoracic service did experience a decrease in the number of procedures performed during the pandemic, the overall reduction in case volume was only 11%, with no real effect on the delivery of care for patients with thoracic malignancies. The initial surge of COVID-19 transiently affected our surgical services in general and with a slightly longer delay for thoracic surgery. We were able to rapidly recover, and even to overperform during the subsequent waves of the pandemic.
Much has been written from centers around the world on the effect of the COVID-19 pandemic on the delivery of surgical care, with particular attention on the effect of treatment delay for patients with primary cancers of the lung.24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 A delay of more than 8 weeks, from the initial diagnosis of cancer to definitive treatment, has been associated with worse outcomes for primary lung cancer.35 Certainly, the effect of surgical treatment delay is more critical for locally advanced primary cancer patients receiving multimodal therapy, whereas postponing immediate resection is considered more acceptable for T1 partial solid ground-glass opacity tumors.35 We observed that there was a small difference in the T status (slightly more T2a in the COVID-19 robotic cohort; P = .07) and pathologic stage of primary lung cancer undergoing robotic resections. Of note, there were more advanced, stage 3 to 4, patients who underwent thoracotomies during the COVID-19 pandemic (46% vs 29%; P < .01 according to χ2 test). This might serve as a surrogate indicator that there was no delay in the care of early-stage lung cancer patients and expedited use of thoracotomies during the pandemic for locally advanced lung cancer. A more comprehensive investigation using a larger patient cohort such as the Society of Thoracic Surgeons General Thoracic Surgery Database or the National Cancer Database over a longer period of time would provide more definitive answers regarding the effect of COVID-19 on lung cancer care and outcomes. We did not change our practice managing fluorodeoxyglucose-avid peripherally located solid or part solid lesions that are amenable to intraoperative wedge resection for intraoperative tissue diagnosis and immediate therapeutic pulmonary resection if proven malignant. We do not routinely obtain preresection tissue diagnosis using transthoracic needle aspirate/biopsy or using navigational bronchoscopy and transbronchial biopsy. The false positive rates were not meaningfully different between the 2 cohorts (19 of 180 cases—10.6% of the pre-COVID-19 group, and 20 of 144 cases—13.8% of the COVID-19 group; P = .37) and these values are slightly lower than the false positive rate of 16% as previously reported.38 Fewer thoracotomies were performed in the COVID-19 period, attributed to a self-imposed suspension of radical lung-sparing pleurectomy for early-stage mesothelioma and pulmonary decortications for complex pleural effusions unless empyema with lung trapping was present. Our thoracic surgery staff (attending surgeons, trainees, and advanced nurse practitioners) were not redeployed to other essential “front-line” services (intensive care unit, emergency department, COVID-19 unit); thus, there was minimal disruption of patient care.
Early in the pandemic, the focus was aimed to preserve and reallocate essential resources in anticipation for the first wave of COVID-19 reaching the Southeastern United States. Care components, such as outpatient clinic visits, were not heavily affected by the pandemic with the rapid implementation of telemedicine. Other ancillary services such as diagnostic radiology, which provided facilities for CT or positron emission tomography/CT, reopened with proper social distancing and basic PPE practice. Meanwhile, the total volume of PFT and invasive pulmonary diagnostic procedures such as endobronchial ultrasound and bronchoscopy, were severely affected; the “aerosolized” nature of the procedures required a negative SARS-CoV-2 RT-PCR nasopharyngeal swab before access to these services. Initially there was limited availability and accuracy of COVID-19 tests, which only became readily available at our institution in July 2020, thus explaining the prolonged reduction of PFT capability.39 Phased reopening of operative services began in late May 2020, guided by the implementation of predictive models to estimate COVID-19 cases in upcoming weeks.20 Using these models, the following practices were implemented: adaptive alignment of resources commensurate with real-time COVID-19 and non-COVID-19 clinical needs, segregation of COVID-19 cases from the rest of the general patient population (hospital-in-a-hospital model), stringent preventive measures (PPE utilization, social distancing, and preadmission RT-PCR nasopharyngeal swabs for all patients requiring admission), coordination of care delivery by observing directives from state and county public health departments, and regular updates of hospital staff regarding hospital resources and COVID-19 volumes. This adaptive realignment of resources via the predictive model projections of COVID-19 cases allowed supply chain teams to adjust PPE allocation/procurement in real time and anticipate the number of beds, particularly negative-pressure rooms to accommodate COVID-19 admissions. Bed reallocations affected the hospital's overall capacity to carry out elective surgical cases. The predictive model allowed estimation of operating room capacity and the type of case -mix to avoid last-minute cancellations. Thus, the operating room capacity was constantly adjusted on the basis of our functional predictive model to maximize utilization and minimize last-minute postponements. This data-driven management of hospital function allowed us to acutely deal with COVID-19 admissions and simultaneously provide necessary care to our patients with urgent needs, and allow safe phased reopening of all surgical specialties. Elective overnight admissions and outpatient procedures were the first to be reinstituted, followed by complex, in-hospital procedures when hospital resources and SARS-CoV-2 testing capability became readily available. This allowed for a faster recovery of overall surgical volume with inpatient procedures and total surgical cases approaching 80% to 100% of baseline by June 2020. This trend, however, was not observed for thoracic cases. Possible causes for the prolonged reduction in thoracic case volume during the first wave of the pandemic (March 2020 to July 2020) include: reduction of ancillary services (eg, our pulmonary function laboratory), diagnostic tests (interventional radiology transthoracic biopsy or interventional bronchoscopy), patient reluctance to be admitted, postponed nonurgent cases, and a temporary pause of thoracic case referrals.
During the second and third waves (August 2020 to December 2020), our thoracic surgical service functioned at, and even above, pre-COVID-19 volumes, with a larger proportion of benign pathologies in the case composition (Figure 3). This reflects improved resource management and allocation of care and a potential backlog of patients with benign thoracic conditions who were deemed nonemergent in the first wave. Furthermore, the increased surgical performance of the thoracic service also coincided with the launch of our robotic benign foregut surgery program in Fall 2020. Not only was our thoracic surgery service able to recover to levels above our pre-COVID-19 baseline, despite a much larger second wave volume of COVID-19 patients, we were able to expand to address local demand and manage benign foregut diseases. Strict enforcement of precautionary measures enabled us to provide care without an increased risk to patients or providers; throughout the entire study period duration, none of the patients reported COVID-19 symptoms within 14 days of discharge and no thoracic surgical team members tested positive for SARS-CoV-2 according to RT-PCR testing.
Figure 3.
Effect of COVID-19 pandemic on the thoracic surgical service at the University of Miami Medical Center. COVID-19, Coronavirus disease-19.
Only careful surveillance and systematic population-based epidemiologic studies in the future will reveal the short-and long-term effect of the COVID-19 pandemic on cancer care.15 Lung cancer is the second most common cancer and is the most common cause of cancer-related death in the United States.40 The long-term adverse effect of the pandemic on lung cancer detection and treatment outcomes is conceivably significant. Early-stage lung cancers are mainly detected in thoracic imaging studies (ie, lung cancer screening or an incidental finding).27 The delay in imaging ancillary services, coupled with deferred new patient enrollment and postponed repeat annual lung cancer screening might contribute to a rift in the cancer diagnosis balance, with some institutions reporting a decrease in primary cancer diagnoses whereas others report an increase in lung cancer mortality.24,25,27, 28, 29 This was further exacerbated by fear of contracting COVID-19, leading to fewer symptomatic patients seeking medical treatment and increased cancelation and no-show rates for appointments and procedures.27
There are several limitations to consider. We have reported the results of our retrospective, observational study from a single institutional experience, which might prevent the generalizability of our findings. Analysis was performed using a historical control (the preceding 10 months before the COVID-19 pandemic), which might not be directly comparable, and the observed difference might be because of an unmeasurable or unknown variable not related to the pandemic. We report in-hospital and 30-day morbidity and mortality. Long-term follow-up was not the main objective of this observational study intended to document the immediate effect of hospital and operative service closure on surgical volume and short-term postoperative outcomes. Finally, patients might have presented to outside facilities, not reported to our service care providers during in-person or virtual clinic visits, which is not included in the data analysis.
Conclusions
In summary, one can safely operate on thoracic surgical patients during a pandemic by following appropriate protocols incorporating public health measures and applying predictive models to estimate COVID-19 admission and resource availability, leading to phased-reopening of surgical services (Figure 4). We discuss the relevance of our study in the broader societal context (Video 1). Although the negative consequences of the COVID-19 pandemic are immense and the future has yet to reveal its entire effect and long-term outcomes, the lessons we learned at the University of Miami, as a society, and as a health care system are extremely valuable. In the face of potential future pandemic surges or other global health system crises, we are prepared to respond more efficiently and rapidly with policies in place, to guide systematic resource allocation, workplace safety, and prioritization of care for the most vulnerable patient populations. For our thoracic surgery service, although COVID-19 might have initially slowed our case volume, the overall effect of COVID-19 was minimal, and we were able to quickly respond, adjust, adapt, and safely provide high-level quality care, for benign and malignant thoracic pathologies.
Figure 4.
One can safely operate on thoracic surgical patients during a pandemic by following appropriate protocols, incorporating public health measures, and applying predictive models to estimate COVID-19 admission and resource availability, which leads to phased reopening of surgical services. COVID-19, Coronavirus disease-19.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/21%20AM/AM21_TH07/AM21_TH07_1.mp4.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Supplementary Data
Author discussion of study and clinical relevance in the context of the COVID-19 pandemic. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00064-X/fulltext.
References
- 1.World Health Organization Rolling updates on coronavirus disease (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen Accessed March 2021.
- 2.Kenny G., Mallon P.W. COVID19- clinical presentation and therapeutic considerations. Biochem Biophys Res Commun. 2021;538:125–131. doi: 10.1016/j.bbrc.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancarevic I., Tathineni P., Malik B.H. Coronavirus disease 2019 (COVID-19) in cancer patients. Cureus. 2020;12:e7835. doi: 10.7759/cureus.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H., Chen X., Su C., Liu Y., Wang H., Sun C., et al. Challenges and countermeasures of thoracic oncology in the epidemic of COVID-19. Transl Lung Cancer Res. 2020;9:337–347. doi: 10.21037/tlcr.2020.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jazieh A.R., Akbulut H., Curigliano G., Rogado A., Alsharm A.A., Razis E.D., et al. International Research Network on COVID-19 Impact on Cancer Care Impact of the COVID-19 pandemic on cancer care: a Global Collaborative Study. JCO Glob Oncol. 2020;6:1428–1438. doi: 10.1200/GO.20.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arrieta O., Cardona A.F., Lara-Mejía L., Heredia D., Barrón F., Zatarain-Barrón Z.L., et al. Recommendations for detection, prioritization, and treatment of thoracic oncology patients during the COVID-19 pandemic: the THOCOoP cooperative group. Crit Rev Oncol Hematol. 2020;153:103033. doi: 10.1016/j.critrevonc.2020.103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passaro A., Addeo A., Von Garnier C., Blackhall F., Planchard D., Felip E., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. ESMO Open. 2020;5(Suppl 3):e000820. doi: 10.1136/esmoopen-2020-000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American College of Surgeons COVID-19 guidelines for triage of cancer surgery patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/cancer-surgery Accessed March 2021.
- 9.American College of Surgeons COVID-19 guidelines for triage of thoracic patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/thoracic-cancer Accessed March 2021.
- 10.Thoracic Surgery Outcomes Research Network, Inc, Antonoff M., Backhus L., Boffa D.J., Broderick S.R., Brown L.M., et al. COVID-19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. Ann Thorac Surg. 2020;110:692–696. doi: 10.1016/j.athoracsur.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan A., Siglin J., Khan A. Comprehensive review of implications of COVID-19 on clinical outcomes of cancer patients and management of solid tumors during the pandemic. Cancer Med. 2020;9:9205–9218. doi: 10.1002/cam4.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha Z., Chang K., Mi J., Liang Z., Hu L., Long F., et al. The impact of the COVID-19 pandemic on lung cancer patients. Ann Palliat Med. 2020;9:3373–3378. doi: 10.21037/apm-20-1662. [DOI] [PubMed] [Google Scholar]
- 13.Luo J., Rizvi H., Preeshagul I.R., Egger J.V., Hoyos D., Bandlamudi C., et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–1396. doi: 10.1016/j.annonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K., Sheng Y., Huang C., Jin Y., Xiong N., Jiang K., et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garassino M.C., Whisenant J.G., Huang L.C., Trama A., Torri V., Agustoni F., et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverman D.A., Lin C., Tamaki A., Puram S.V., Carrau R.L., Seim N.B., et al. Respiratory and pulmonary complications in head and neck cancer patients: evidence-based review for the COVID-19 era. Head Neck. 2020;42:1218–1226. doi: 10.1002/hed.26217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addeo A., Friedlaender A. Cancer and COVID-19: unmasking their ties. Cancer Treat Rev. 2020;88:102041. doi: 10.1016/j.ctrv.2020.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johns Hopkins University Impact of opening and closing decisions by state: Florida. https://coronavirus.jhu.edu/data/state-timeline/new-confirmed-cases/florida Accessed March 2021.
- 20.Warde P.R., Patel S., Ferreira T., Gershengorn H., Bhatia M.C., Parekh D., et al. Linking prediction models to government ordinances to support hospital operations during the COVID-19 pandemic. BMJ Health Care Inform. 2021;28:e100248. doi: 10.1136/bmjhci-2020-100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K., Smith C.R., Lembcke B.T., Ferreira T.B.D. Elective surgery during the Covid-19 pandemic. N Engl J Med. 2020;383:1787–1790. doi: 10.1056/NEJMclde2028735. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention COVID data tracker. Trends in number of COVID-19 cases and deaths in the US reported by CDC, by state/territory. https://covid.cdc.gov/covid-data-tracker/#trends_dailytrendscases Accessed March 2021.
- 23.Szewczyk J.B., Nguyen D.M., Warde P.R., Shukla B., Ferreira T., Gershengorn H.B., et al. Incidence of postprocedural coronavirus disease 2019 (COVID-19) at an urban academic medical center. Infect Control Hosp Epidemiol. 2021;42:1279–1281. doi: 10.1017/ice.2021.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinmohamed A.G., Visser O., Verhoeven R.H.A., Louwman M.W.J., van Nederveen F.H., Willems S.M., et al. Fewer cancer diagnoses during the COVID-19 epidemic in The Netherlands. Lancet Oncol. 2020;21:750–751. doi: 10.1016/S1470-2045(20)30265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maringe C., Spicer J., Morris M., Purushotham A., Nolte E., Sullivan R., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21:1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVIDSurg Collaborative Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107:1440–1449. doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzone P.J., Gould M.K., Arenberg D.A., Chen A.C., Choi H.K., Detterbeck F.C., et al. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST Expert Panel Report. J Am Coll Radiol. 2020;17:845–854. doi: 10.1016/j.jacr.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Haren R.M., Delman A.M., Turner K.M., Waits B., Hemingway M., Shah S.A., et al. Impact of the COVID-19 pandemic on lung cancer screening program and subsequent lung cancer. J Am Coll Surg. 2021;232:600–605. doi: 10.1016/j.jamcollsurg.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patt D., Gordan L., Diaz M., Okon T., Grady L., Harmison M., et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059–1071. doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banna G., Curioni-Fontecedro A., Friedlaender A., Addeo A. How we treat patients with lung cancer during the SARS-CoV-2 pandemic: primum non nocere. ESMO Open. 2020;5:e000765. doi: 10.1136/esmoopen-2020-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casaluce F., Gridelli C. Narrative review of lung cancer treatment at the time of COVID-19 pandemia: pitfall and issues. Transl Lung Cancer Res. 2021;10:475–482. doi: 10.21037/tlcr-20-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu R., Wu L., Zhang C., Chu Q., Hu J., Lin G., et al. Real-world scenario of patients with lung cancer amid the coronavirus disease 2019 pandemic in the People's Republic of China. JTO Clin Res Rep. 2020;1:100053. doi: 10.1016/j.jtocrr.2020.100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glasbey J.C., Nepogodiev D., Simoes J.F.F., Omar O., Li E., Venn M.L., et al. Elective cancer surgery in COVID-19-free surgical pathways during the SARS-CoV-2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39:66–78. doi: 10.1200/JCO.20.01933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fligor S.C., Tsikis S.T., Wang S., Ore A.S., Allar B.G., Whitlock A.E., et al. Time to surgery in thoracic cancers and prioritization during COVID-19: a systematic review. J Thorac Dis. 2020;12:6640–6654. doi: 10.21037/jtd-20-2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurizi G., Rendina E.A. A high-volume Thoracic Surgery Division into the storm of the COVID-19 pandemic. Ann Thorac Surg. 2020;110:353–354. doi: 10.1016/j.athoracsur.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jheon S., Ahmed A.D., Fang V.W., Jung W., Khan A.Z., Lee J. General thoracic surgery services across Asia during the 2020 COVID-19 pandemic. Asian Cardiovasc Thorac Ann. 2020;28:243–249. doi: 10.1177/0218492320926886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohman L.J., Gu L., Altorki N., Scalzetti E., Veit L.J., Wallen J.M., et al. Biopsy first: lessons learned from Cancer and Leukemia Group B (CALGB) 140503. Thorac Cardiovasc Surg. 2017;153:1592–1597. doi: 10.1016/j.jtcvs.2016.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilzenrat R.A., Deen S.A., Yee J., Grant K.A., Ashrafi A.S., Coughlin S., et al. Thoracic surgeon impressions of the impact of the COVID-19 pandemic on lung cancer care-lessons from the first wave in Canada. Curr Oncol. 2021;28:940–949. doi: 10.3390/curroncol28010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Author discussion of study and clinical relevance in the context of the COVID-19 pandemic. Video available at: https://www.jtcvs.org/article/S2666-2736(22)00064-X/fulltext.