Abstract
Background
The newly emerged SARS-CoV-2 variant of concern (VOC) Omicron is spreading quickly worldwide, which manifests an urgent need of simple and rapid assay to detect and diagnose Omicron infection and track its spread.
Methods
To design allele-specific CRISPR RNAs (crRNAs) targeting the signature mutations in the spike protein of Omicron variant, and to develop a CRISPR-Cas12a-based assay to specifically detect Omicron variant.
Results
Our system showed a low limit of detection of 2 copies per reaction for the plasmid DNA of Omicron variant, and could readily detect Omicron variant in 5 laboratory-confirmed clinical samples and distinguish them from 57 SARS-CoV-2 positive clinical samples (4 virus isolates and 53 oropharyngeal swab specimens) infected with wild-type (N = 8) and the variants of Alpha (N = 17), Beta (N = 17) and Delta (N = 15). The testing results could be measured by fluorescent detector or judged by naked eyes. In addition, no cross-reaction was observed when detecting 16 clinical samples infected with 9 common respiratory pathogens.
Conclusions
The rapid assay could be easily set up in laboratories already conducting SARS-CoV-2 nucleic acid amplification tests and implemented routinely in resource-limited settings to monitor and track the spread of Omicron variant.
Keywords: SARS-CoV-2, Variants of concern, Omicron, Variant genotyping, CRISPR-Cas12a system
1. Introduction
Continuing spread and evolution of SARS-CoV-2 have resulted in the emergence of various variants that have infected and killed millions of people (Zhu et al., 2020; Dong et al., 2021). The newly emerged fifth variant of concern (VOC) Omicron was firstly reported in South Africa on November 24, 2021 and has been detected in many countries (Abdel Latif et al., 2021). Omicron variant contains more than 32 amino acid mutations in the spike protein, including multiple vital amino acid mutations (K417N, T478K, E484A, N501Y, and D614G) that have been already detected in other VOCs of SARS-CoV-2 and proved to be associated with enhanced transmissibility, virulence, and greater resistance to the immune protection induced by COVID-19 vaccines (Harvey et al., 2021). The new features of Omicron manifested the importance of tracking its spread.
Reverse transcription polymerase chain reaction (RT-PCR) has been widely used for diagnosing SARS-CoV-2 infection and genotyping SARS-CoV-2 variants (Zelyas et al., 2021; Wang et al., 2021). Both Alpha and Omicron variant contain a specific deletion mutation in the amino acid 69–70 of spike protein (69-70del), which yields a negative reaction or spike gene target failure (SGTF) in RT-PCR tests when use SARS-CoV-2 S gene as testing target (Bal et al., 2021). Therefore, SGTF caused by the 69-70del mutation was previously used to distinguish Alpha variant (Bal et al., 2021) and recently proposed as a proxy by Scott et al. to track Omicron variant (Scott et al., 2021). However, the 69-70del mutation is not exclusive for Omicron variant. Furthermore, Omicron lineage (B.1.1.529) has now evolved and divided into three sub-lineages BA.1, BA.2, and BA.3. Unfortunately, BA.2 variant does not carry the 69-70del mutation (Supplementary Fig. S1); therefore, the proposal raised by Scott et al. may not be suitable for detecting and distinguishing all Omicron variants from other SARS-CoV-2 variants.
We have previously successfully developed a CRISPR-Cas12-based multiplex allele-specific assay for SARS-CoV-2 variant genotyping with high sensitivity and specificity (Liang et al., 2021). The new system is the combination of RT-PCR and CRISPR-Cas12 cleavage, and can be easily implemented in laboratories already conducting SARS-CoV-2 testing to screen SARS-CoV-2 variants in resource-limited settings (Liang et al., 2021). Here, we refined this system for detection of Omicron variant by designing new crRNAs specific for Omicron variant.
2. Materials and methods
2.1. Plasmid construction and virus RNA of SARS-CoV-2
The full-length genomic fragments of SARS-CoV-2 spike protein were synthesized based on the wild-type strain of SARS-CoV-2 isolated in Wuhan, China (nt 21,563–25,384 of NCBI accession number MN908947) and the Omicron variant from Belgium (nt 21,497–25,309 of NCBI accession number OL672836), respectively by Sangon Biotech Co., Ltd. (Shanghai, China) and inserted into the vector pUC57. The gene fragment of Omicron spike protein includes mutations of A67V, 69/70del, T95I, 142/144del, Y145D, 211del, L212I, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F. The plasmid DNAs were quantified and diluted in nuclease-free water (Accurate Biotechnology, Hunan, China) to prepare a series of diluted templates. Plasmid copy number was calculated using the following formula: Plasmid copy number (copies/μL) = {[6.02 × 1023 × plasmid concentration (ng/μL) × 10−9]}/[Plasmid length × 660].
Four SARS-CoV-2 virus isolates, including wild-type strain (19A) isolated from COVID-19 patient in Wuhan, China, and variant Alpha (B1.1.7), Beta (B.1.351) and Delta (B1.617.2) isolated from imported COVID-19 patients, were grown in Vero cells. Viral RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA), aliquoted and stored at −80 °C until use. Furthermore, five oropharyngeal swab specimens were collected from Omicron variant positive COVID-19 patients who were confirmed by sequencing and kept in virus transportation medium (VTM). Additionally, 53 SARS-CoV-2 positive oropharyngeal swab specimens infected with wild-type (N = 7) and the variants of Alpha (N = 16), Beta (N = 16), Delta (N = 14) were collected and analyzed in the study (Supplementary Table S1). Moreover, 16 clinical samples that are negative for SARS-CoV-2 and positive for 9 common respiratory pathogens including common human coronavirus (HCoV) 229E, OC43, and HKU1 as well as rhinovirus (HRV), adenovirus (AdVs), respiratory syncytial virus (RSV) A and B, human bocavirus (HBoV), human metapneumovirus (HMPV), and human parainfluenza virus one (HPIV-1) and four (HPIV-4) were used as negative controls to validate the assay specificity. Written informed consents were obtained from all subjects enrolled in this study. Research protocols were in accordance with the Declaration of Helsinki. Personal information and samples were de-identified and analyzed anonymously.
2.2. Design and synthesis of allele-specific CRISPR RNAs and primers
Two Omicron-specific crRNAs were designed according to the working principle of CRISPR-Cas12a system (Zetsche et al., 2015). To prepare crRNAs, DNA oligonucleotides containing T7 promoter, conserved stem-loop sequences, and guide sequences and the complementary single stranded DNAs were synthesized and annealed at 95 °C for 10 min and then reduce to 25 °C at the reduction speed of 2 °C every minute. Afterward, 1 μg purified dsDNA was transcribed at 37 °C for 4 h using HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs, Massachusetts, USA). The transcription product was treated with 4 unites of DNase I (New England Biolabs, Massachusetts, USA) at 37 °C for 40 min and then purified using miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany). The concentration of crRNAs was quantified using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Massachusetts, USA). The primers used in our study were designed to target the conserved sequences in the spike gene of SARS-CoV-2 and validated to be specific for SARS-CoV-2 using NCBI Primer-BLAST tool (Ye et al., 2012).
2.3. Detection of SARS-CoV-2 variants
Viral RNA was extracted from oropharyngeal swab samples by using QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). As shown in Fig. 1 , target nucleic acid was amplified by polymerase chain reaction (PCR) for DNA templates or by reverse transcription PCR (RT-PCR) for RNA templates. Viral RNA was reverse transcribed into cDNA using Oligo (dT) and random primer according to the manufacturer's instructions (Roche Diagnostics, Indianapolis, USA) and amplified using SARS-CoV-2 specific primers through PCR. Briefly, 26 μL High-Fidelity Master Mix (Tsingke Biotechnology Co., Ltd., Beijing, China), 1 μL of forward and reverse primers (10 μM, Forward primer: 5′-GCTGATTATTCTGTCCTTTATA-3’; Reverse primer: 5′-CTGACACTACTGATGCTGTCC GTGATCCACAGAC-3′), and 2 μL of target template were mixed. The reaction was run at 98 °C for 2 min followed by 40 cycles of 98 °C for 10 s, 55 °C for 15 s, 72 °C for 40 s. Afterward, 150 nM AsCas12a (made in house) was pre-incubated with 1000 nM crRNA in 1 × NEB Buffer 2.1 at 37 °C for 10 min to form crRNA-Cas12a complex followed by addition of 1 μL amplified product of target DNA and 400 nM probe reporter (5′-6-FAM-TTATT-BHQ-1-3′, synthesized in Sangon Biotech (Shanghai, China) and incubation at 37 °C for 30 min. Finally, the fluorescence signal was measured by using fluorescent detector (Qitian, Jiangsu, China) or judged by naked eyes.
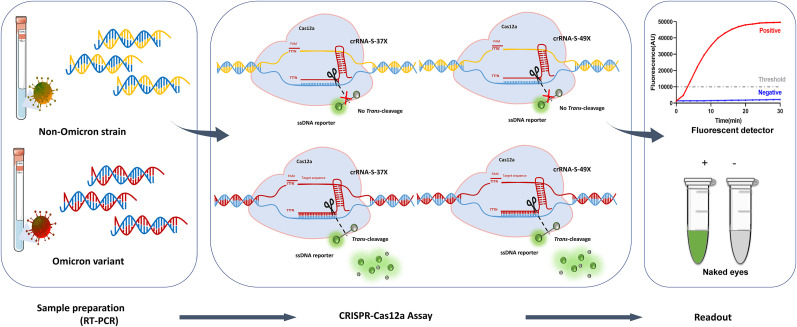
Fig. 1.
Workflow of RT-PCR/CRISPR-Cas12a-mediated assay for detection of SARS-CoV-2 Omicron variant. Viral RNA was extracted from oropharyngeal swab samples infected with Omicron variant or non-Omicron strain, reverse-transcribed into cDNA by using Oligo (dT) and random primer, and amplified using SARS-CoV-2 specific primers through PCR. After amplification, Cas12a was pre-incubated with Omicron-specific crRNA-S-37X and crRNA-S-49X to form crRNA-Cas12a complex followed by addition of the amplified product and ssDNA reporter (5′-6-FAM-TTATT-BHQ-1-3′). For the Omicron template, the ssDNA reporter was trans-cleaved by the activated Cas12a to release fluorescence signal while no fluorescence signal was released in the reaction of non-Omicron strain. Finally, the fluorescence signal was measured by using fluorescent detector or judged by naked eyes. The detection results were presented as positive (red line or green tube) and negative (blue line or grey tube), respectively.
2.4. Statistical analysis
Data were analyzed using R software, version 4.1.0 (R Foundation for Statistical Computing). Two-tailed Student t-test was used to analyzed the fluorescence difference between on-target and off-target template detected by CRISPR-Cas12a-based assay. P < 0.05 was considered statistically significant.
3. Results
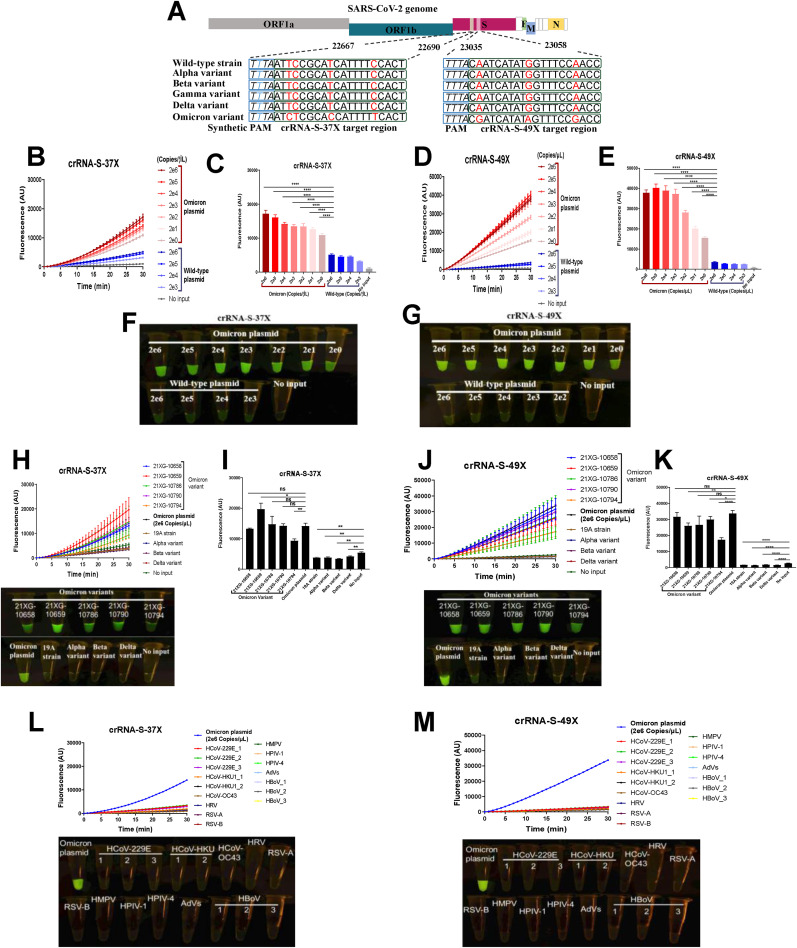
Different from the existing SARS-CoV-2 variants, Omicron has multiple mutations within the potential crRNA target sequences, which make it possible to use one or two crRNAs to specifically diagnose Omicron infection (Supplementary Fig. S1). In this study, we designed two Omicron-specific crRNAs, i.e., crRNA-S-37X (5′-UAAUUUCUACUAAGUGUAGAAUCUCGCACCAUUUUUCACU-3′) to cover S371L, S373P and S375F mutations, and crRNA-S-49X (5′-UAAUUUCUACUAAGUGUAGA CGAUCAUAUAGUUUCCGACC-3′) to cover Q493R, G496S and Q498R mutations, respectively (Fig. 2 A). In addition, a T-rich protospacer adjacent motif (PAM, 5′-TTTN-3′, where N refers to A/G/C) sequence was added at the 5′ terminus of crRNA-S-37X since the PAM sequence is necessary for the activity of Cas12a protein (Zetsche et al., 2015), but does not exist in the original Omicron sequences (Supplementary Fig. S1).
Fig. 2.
Detection of Omicron variant via CRISPR-Cas12a-mediated mutation-specific assay. (A) Schematic of the SARS-CoV-2 genome and the location and sequences of the two crRNAs used in our study. A series of 10-fold diluted SARS-CoV-2 plasmid DNAs of wild-type and Omicron variant of SARS-CoV-2 were used as the templates for PCR followed by detection of CRISPR-Cas12a-mediated assay. The low limit of detection was determined and quantitatively analyzed for crRNA-S-37X (B, D) and crRNA-S-49X (C, E), respectively. The testing results were visualized by naked eyes under blue light at 30 min after reaction (F, G). CRISPR-Cas12a-mediated assay using crRNA-S-37X (H, I) and crRNA-S-49X (J, K) could specifically detect the plasmid DNA of Omicron as positive control and five clinical samples of Omicron variant, but not 4 virus isolates including wild-type strain (N = 1) or Alpha (N = 1), Beta (N = 1), and Delta (N = 1) variant. The DNA plasmid of Omicron variant was used as positive control while SARS-CoV-2 negative clinical samples infected with common human coronavirus (HCoV) 229E, HCoV OC43, and HCoV HKU1 as well as various other respiratory pathogens including rhinovirus (HRV), respiratory syncytial virus (RSV) A and B, Human metapneumovirus (HMPV), human parainfluenza virus (HPIV-1 and HPIV-4), Human adenovirus (AdVs), and Human bocavirus (HBoV) were used as negative controls to validate the specificity of our assay (L, M). In all panels, error bars represent the mean ± standard deviation (SD) from 3 replicates of experiment. No input means negative control of no plasmid DNA. A two-tailed Student's t-test was used to analyze the fluorescence difference detected by CRISPR-Cas12a-based assay. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
The preliminary results indicated that the intensity of fluorescence signal is proportional to the concentration of Omicron template. Our system could readily detect two copies of Omicron plasmid DNA per reaction even in the presence of 2 × 106 copies of plasmid DNA of the wild-type strain by measuring the fluorescence signals (Fig. 2B–E). To estimate the detection limit of our RT-PCR/Cas12a-mediated assay in real virus, we took one clinical sample 21XG-10790 infected with Omicron variant in our study. The estimated virus titer of the sample 21XG-10790 is 104 copies/μL based on its cycle threshold (Ct) value of 20 and the standard curve between Ct value obtained from qRT-PCR assay and the corresponding viral loads provided by the manufacture Easydiagnosis Biomedicine Co., Ltd, in Wuhan, China (Supplementary Fig. S4 and Table S1). A series of 10-fold dilution of the sample 21XG-10790 were made and tested by our assay using crRNA-S-49X. The low detection limit of our assay in real virus was 100 copies/reaction (Supplementary Fig. S4). Moreover, the testing results can be easily detected under blue-light observed by naked eye (Fig. 2F and G). Furthermore, we evaluated the performance of our system in distinguishing Omicron variant from other SARS-CoV-2 strains including wild-type strain from Wuhan, China, and the variants of Alpha, Beta and Delta isolated from imported COVID-19 patients. Our assay could specifically detect Omicron variant in 5 clinical samples infected with Omicron and distinguish them from 57 samples (including 4 virus isolates and 53 oropharyngeal swab specimens) infected with wild-type or other variants of SARS-CoV-2 (Fig. 2H–K, Supplementary Fig. S2). Different efficiency of our CRISPR-Cas12a-based mutation-specific assay was observed when using crRNA-S-37X and crRNA-S-49X to detect clinical samples (Fig. 2H–K). Of note, stronger fluorescence signal was obtained for crRNA-S-49X than for crRNA-S-37X probably due to the absence of PAM sequence in the original sequences of crRNA-S-37X but not crRNA-S-49X, or the difference of the mismatches between crRNAs and the target sequences for crRNA-S-37X and crRNA-S-49X since the efficiency of crRNAs to trigger collateral cleavage capability of Cas proteins was affected by the mismatches between crRNAs and target sequences especially when the mismatches are in the PAM proximal regions (Chen et al., 2018). Furthermore, a relatively lower signal was observed for the Alpha, Beta and Delta variants than for the 'no input' negative control (Fig. 2H–K), but the fluorescence level was below the cutoff value, which in turn confirms the high specificity of the assay. There was no cross-reaction observed when detecting 16 SARS-CoV-2 negative clinical samples infected with 9 common respiratory pathogens including human coronavirus (HCoV) 229E, HCoV OC43, and HCoV HKU1 as well as rhinovirus (HRV), respiratory syncytial virus (RSV) A and B, Human metapneumovirus (HMPV), human parainfluenza virus (HPIV-1 and HPIV-4), Human adenovirus (AdVs), and Human bocavirus (HBoV) (Fig. 2L and M).
4. Discussion
The emerging and continuing spread of Omicron variant poses great threat and burden to public health system. It is important to timely diagnose Omicron variant and monitor its circulation. Viral whole-genome sequencing is still the gold-standard method for the identification of SARS-CoV-2 variants; however, it is cost-prohibitive and time-consuming and unsuitable for routine genotyping of SARS-CoV-2 variants (Chiara et al., 2021). Therefore, a rapid and cost-effective assay for screening Omicron variant is urgently needed.
In our previous study, detection of SARS-CoV-2 variants depends on several crRNAs that specifically detect individual signature mutations found in the variants of SARS-CoV-2, and a comprehensive interpretation of multiple reaction results since no single mutation or one crRNA could distinguish all the variants of SARS-CoV-2 (Liang et al., 2021). In the current study, we found that one crRNA that contains 3–4 mutations could readily diagnose and distinguish Omicron variant, which in turn make the diagnosis and tracking of Omicron much simpler and more feasible. We have recently confirmed that the detection sensitivity and specificity could be significantly improved by artificially introducing into crRNAs extra mutation around the target mutation site (unpublished data). Similar findings indicate that the number of mismatches in crRNAs adjacent to the target mutations or in the PAM proximal regions is critical for efficient collateral cleavage of CRISPR-Cas proteins (Huang et al., 2021). The new strategy to design and select crRNAs with multiple mutations could be one principle for CRISPR-based testing assay.
In our study, Omicron and wild-type targets were separately tested. We indeed spiked both Omicron and wild-type template of SARS-CoV-2 in the same reaction, and the signal was only observed in the presence of both the target and the crRNA specific for the target (Supplementary Fig. S3), indicating the high specificity of the allele-specific crRNAs and our CRISPR-Cas12a-based assay. Gootenberg et al. has reported a multiplexed nucleic acid detection platform by using different Cas proteins and multiple reporters labeled with different dyes (Gootenberg et al., 2018). Therefore, further refinement could make our CRISPR-based assay possible for simultaneous detection of more than two targets.
In conclusion, the performance of CRISPR-Cas12a-based mutation-specific assay was useful for detection of Omicron variant. The CRISPR-Cas12a-mediated genotyping assay can effectively improve the accessibility of genotyping for Omicron variant especially in resource-limited settings. It is a simple and useful tool to globally monitor and track the circulating Omicron variant and the dynamics of COVID-19 pandemic. However, we must emphasize that this is a preliminary evaluation of our CRISPR-Cas12a-mediated genotyping assay, and a large number of clinical samples are needed for further validation and optimization of this assay.
Ethical approval and consent to participate
Written informed consents were obtained from all subjects enrolled in this study. Research protocols were conducted in accordance with the Declaration of Helsinki. Personal information and samples were de-identified and analyzed anonymously.
Availability of data and materials
The data generated during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by the National Major Science and Technology Project of China (grant number 2018ZX10732-401-003-003).
CRediT authorship contribution statement
Yuanhao Liang: Conceptualization, Methodology, Investigation, Visualization, Writing – original draft, Writing – review & editing. Hongqing Lin: Methodology, Investigation, Visualization, Writing – original draft, Writing – review & editing. Lirong Zou: Investigation. Xiaoling Deng: Conceptualization, Project administration, Investigation, Supervision. Shixing Tang: Conceptualization, Methodology, Investigation, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bios.2022.114098.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Fig. S1. Multiple alignment of amino acid sequences of spike protein from different SARS-CoV-2 variants, and compared with wild-type strain of SARS-CoV-2 isolated from Wuhan, China (accession number MN908947). Multiple alignment was performed and visualized using MAFFT version 7 and ESPript 3.0, respectively. The accession number, pangolin lineage and WHO classification are shown on the left side of the sequences. The red boxes represent identical residues while similar residues are indicated by red letters.Fig. S2. The testing results of SARS-CoV-2 positive clinical samples were read out by naked eyes under blue light at 30 min after the CRISPR-Cas12a-mediated reaction. A total of 58 SARS-CoV-2 positive oropharyngeal swab specimens in a panel of 5 Omicron variants, 7 wild-type strains, 16 Alpha variants, 16 Beta variants, 14 Delta variants were detected in CRISPR-Cas12a assay using crRNA-S-37X or crRNA-S-49X. The genotyping results based on sequencing are labeled in the left of the pictures and the Sample ID are indicated in the top of each reaction tube.Fig. S3. The specificity of CRISPR-Cas12a assay. Both plasmid DNA or SARS-CoV-2 positive clinical samples of Omicron variant or wild-type strain were detected by our CRISPR-Cas12a assay by using allele-specific crRNAs. The results were read out by naked eye. The signal was only observed in the presence of Omicron gene fragment and the crRNA-S-37X or crRNA-S-49X that are specific for Omicron, or in the presence of wild-type gene fragment and the wild-type-specific crRNA-S-501N. The detailed information including copy numbers of the synthesized wild-type (WT) or Omicron plasmid DNA and the clinical sample numbers were indicated in the corresponding reaction tubes. Sample 1 and 2 were infected with Omicron variant while sample 3 was infected with wild-type strain of SARS-CoV-2. No input represents negative control without SARS-CoV-2 plasmid DNA or SARS-CoV-2 positive clinical samples.Fig. S4. The low limit of detection (LOD) of the CRISPR-Cas12a assay in clinical sample. (A) Standard curve shows the liner relationship between SARS-CoV-2 viral load and the corresponding cycle threshold (Ct) values of qRT-PCR obtained when detecting patient samples. Ct values were derived from clinical samples targeting Orf1b/nucleocapsid (NP) genes sequence of SARS-CoV-2. (B) A series of 10-fold dilutions of one clinical sample infected with Omicron variant were made to determine the lower detection limit of our assay. 100-fold diluted sample was readily detected by our assay using crRNA-S-49X, indicating an LOD of 100 copies/reaction.
References
- Abdel Latif Alaa, Mullen Julia L., Alkuzweny Manar, et al. SARS-CoV-2 Omicron variant report. https://outbreak.info/situation-reports/omicron?loc=ZAF&loc=GBR&loc=USA&selected=ZAF Available at.
- Bal A, Destras G, Gaymard A, et al. Two-step strategy for the identification of SARS-CoV-2 variant of concern 202012/01 and other variants with spike deletion H69-V70, France, August to December 2020. Euro Surveill. : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 2021; 26(3). [DOI] [PMC free article] [PubMed]
- Chen J.S., Ma E., Harrington L.B., et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science. 2018;360(6387):436–439. doi: 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiara M., D'Erchia A.M., Gissi C., et al. Next generation sequencing of SARS-CoV-2 genomes: challenges, applications and opportunities. Briefings Bioinf. 2021;22(2):616–630. doi: 10.1093/bib/bbaa297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. https://coronavirus.jhu.edu/map.html Available at. [DOI] [PMC free article] [PubMed]
- Gootenberg J.S., Abudayyeh O.O., Kellner M.J., Joung J., Collins J.J., Zhang F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science. 2018;360(6387):439–444. doi: 10.1126/science.aaq0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey W.T., Carabelli A.M., Jackson B., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Zhang F., Zhu K., Lin W., Ma W. dsmCRISPR: dual synthetic mismatches CRISPR/Cas12a-based detection of SARS-CoV-2 D614G mutation. Virus Res. 2021;304:198530. doi: 10.1016/j.virusres.2021.198530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Lin H, Zou L, et al. CRISPR-Cas12a-Based detection for the major SARS-CoV-2 variants of concern. Microbiol. Spectr. 2021: e0101721. [DOI] [PMC free article] [PubMed]
- Scott L., Hsiao N.Y., Moyo S., et al. Science; New York, NY: 2021. Track Omicron's Spread with Molecular Data. [DOI] [PubMed] [Google Scholar]
- Wang H., Jean S., Eltringham R., et al. Mutation-specific SARS-CoV-2 PCR screen: rapid and accurate detection of variants of concern and the identification of a newly emerging variant with spike L452R mutation. J. Clin. Microbiol. 2021;59(8) doi: 10.1128/JCM.00926-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T.L. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinf. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelyas N., Pabbaraju K., Croxen M.A., et al. Precision response to the rise of the SARS-CoV-2 B.1.1.7 variant of concern by combining novel PCR assays and genome sequencing for rapid variant detection and surveillance. Microbiol. Spectr. 2021;9(1) doi: 10.1128/spectrum.00315-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche B., Gootenberg J.S., Abudayyeh O.O., et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 2015;163(3):759–771. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during the current study are available from the corresponding author on reasonable request.