Dear Editor,
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA beta-coronavirus that causes coronavirus disease (COVID-19). COVID-19 may cause the development of a multisystemic disorder. Vaccinations are being developed and administered to reduce COVID-19-related morbidity and mortality and prevent its transmission. Among the various vaccines, the mRNA vaccine is known to prevent over 95% of infections with two doses [1]. SARS-CoV-2 infection is known to be a risk factor for herpetic ocular diseases, because it suppresses systemic immunity [2,3]. Recently, the inactivated COVID-19 vaccine has also been associated with reactivation of herpes zoster [4,5]. In this article, we present a unique case of relapsed herpetic stromal keratitis after vaccination with the mRNA COVID-19 vaccine.
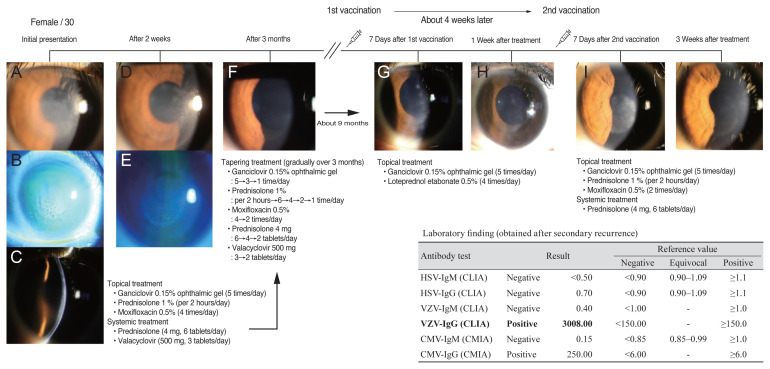
A 30-year-old female patient with no history of systemic disease, who was treated for herpetic stromal keratitis in her right eye about a year prior, and is undergoing follow-up, was revisited with decreased visual acuity in her right eye. At the time of presentation, the intraocular pressure was 13 mmHg and the best-corrected visual acuity (BCVA) in the right eye was 20/25. The patient was clinically diagnosed with recurrence of viral stromal keratitis that previously had appeared as round stromal infiltration and anterior chamber inflammation +1. Per medical history, the Pfizer-BioNTech SARS-CoV-2 mRNA vaccine (Pfizer, New York, NY, USA) was administered 7 days prior, and foreign body sensation and visual disturbance began to deteriorate in the right eye thereafter. At the first presentation 1 year prior, oral steroids and antiviral drugs were administered along with ganciclovir 0.15% ophthalmic gel (Virgan; Laboratoires Thea, Clermont-Ferrand, France) and topical prednisolone 1% (Pred-forte; Allergan, Irvine, CA, USA) eye drops by tapering for 3 months. However, in this relapse, the clinical symptoms were not severe, so only topical eye drops (ganciclovir 0.15% ophthalmic gel and loteprednol etabonate 0.5%) were used, and follow-up was observed. On follow-up 7 days later, the patient reported improved symptoms. Her BCVA was 20/20 without corneal infiltration, but only mild corneal haze. After that, the use of eye drops tapering was performed. After approximately 5 weeks of recurrence, the patient returned to the hospital with the same symptoms and signs 1 week after the second vaccination. We resumed topical treatment together with systemic treatment, and planned tapering (Fig. 1).
Fig. 1.
Timeline and sequential images of clinical presentation in a 30-year-old female patient. (A–C) Initial photographs of the herpetic stromal keratitis with corneal stromal, endothelial infiltration, and corneal edema. (D,E) Slit-lamp photographs of the right eye show improvement of corneal infiltration and edema. (F) Mild corneal opacity remains after initial topical and systemic treatment and tapering. (G) Slit-lamp photograph of the right eye shows relapsed corneal infiltration 7 days after the first coronavirus disease 2019 mRNA vaccination. (H) Relapsed corneal infiltration improved after topical treatments. (I) Second relapsed corneal infiltration 1 week after the second coronavirus disease 2019 mRNA vaccination. (J) Mild corneal opacity remained after topical and systemic treatment. HSV = herpes simplex virus; IgM = immunoglobulin M; CLIA = chemiluminescent immunoassay; IgG = immunoglobulin G; VZV = varicella-zoster virus; CMV = cytomegalovirus; CMIA = chemiluminescent microparticle immunoassay.
Recently Richardson-May et al. [4] reported that 82-year-old male patient with a history of herpes simplex keratitis 40 years previously, showed reactivation of herpes simplex keratitis following viral vector vaccine (Oxford/AstraZeneca COVID-19 vaccine; AstraZeneca, Cambridge, UK) for COVID-19. To the best of our knowledge, this is the first case of herpes stromal keratitis reactivation following COVID-19 mRNA vaccination. Herpetic stromal keratitis is a primary endotheliitis that results in both stromal and epithelial edema in a disciform infiltration with anterior chamber reaction. Stromal keratitis may be caused by both the herpes simplex virus (HSV) and varicella-zoster virus (VZV). HSV and VZV are neurotrophic viruses latent in the trigeminal ganglion following primary corneal infection. In this case, the HSV antibody was negative, and the VZV antibody was high titer, over 3008 for immunoglobulin G (IgG). High titer of VZV-IgG antibody index can be considered reactivation status. In this case, VZV-related keratitis is strongly suspected because high VZV-IgG antibody titers were obtained despite secondary recurrence. Reactivation of the latent virus occurs following activation by a trigger, such as trauma, fever, or immunocompromised status. A recent study indicated that COVID-19 infection is also known to produce an immunosuppressive state secondary to functional impairment and a quantitative decrease in T cells [2].
The mRNA vaccine responds to infection by a mechanism different from that of existing vaccines using other types of viral vectors. The mRNA vaccines teach our immune cells how to make a protein, including antibodies, that trigger an immune response. The pathophysiological mechanisms by which mRNA vaccination is associated with recurrence and exacerbation of herpes virus disease are not known. A latent viral disease may recur due to an unknown immunologic response to the mRNA vaccine, or relapse may be triggered due to immunocompromised status due to general systemic reactogenicity such as fever, fatigue, and muscular pain following vaccination.
In conclusion, COVID-19 is a pandemic, and worldwide vaccination has a high priority. However, it is important to be aware that the existing herpetic corneal disease may recur following mRNA vaccination, and informed consent related to viral keratitis recurrence in patients prior to vaccination should be considered.
Acknowledgements
None.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Funding
The authors received no financial support for this article.
References
- 1.Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–7. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 2.Majtanova N, Kriskova P, Keri P, et al. Herpes simplex keratitis in patients with SARS-CoV-2 infection: a series of five cases. Medicina (Kaunas) 2021;57:412. doi: 10.3390/medicina57050412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen L, Deng C, Chen X, et al. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98:e951–9. doi: 10.1111/aos.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson-May J, Rothwell A, Rashid M. Reactivation of herpes simplex keratitis following vaccination for COVID-19. BMJ Case Rep. 2021;14:e245792. doi: 10.1136/bcr-2021-245792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eid E, Abdullah L, Kurban M, Abbas O. Herpes zoster emergence following mRNA COVID-19 vaccine. J Med Virol. 2021;93:5231–2. doi: 10.1002/jmv.27036. [DOI] [PMC free article] [PubMed] [Google Scholar]