Abstract
Most work on COVID-19 vaccine hesitancy has focused on its attitudinal and demographic correlates among individuals, but the characteristics of vaccines themselves also appear to be important. People are more willing to take vaccines with higher reported levels of efficacy and safety. Has this dynamic sparked comparative hesitancy towards specific COVID-19 vaccines? We conduct a series of cross-sectional survey experiments to test for brand-based differences in perceived effectiveness, perceived safety, and vaccination intention. Examining more than 6,200 individuals in a series of cross-sectional surveys, we find considerably more reluctance to take the AstraZeneca and Johnson & Johnson vaccines compared to those from Pfizer and Moderna if offered, despite all vaccines being approved and deemed safe and effective by a federal regulator. Comparative hesitancy towards these vaccines grew over the course of fielding as controversy arose over their link to extremely rare, but serious side effects. Comparative vaccine-specific hesitancy is strongest among people who are usually most open to mass vaccination efforts. Its effects are substantial: most respondents reported a willingness to wait months for their preferred vaccine rather than receive either the AstraZeneca or Johnson & Johnson vaccine immediately. Our findings call for additional research on the determinants and consequences of COVID-19 vaccine-specific hesitancy and communication strategies to minimize this challenge.
Keywords: Vaccine hesitancy, Vaccine skepticism, Vaccine confidence, Science communication, Health communication, COVID-19
1. Introduction
A globally-scaled mass vaccination campaign is integral to containing the COVID-19 pandemic. As new variants emerge and as protection from initial vaccines wane, it will also be necessary to distribute follow-up “booster” shots. Encouraging continued uptake of COVID-19 vaccines by members of the public is a vital and urgent public health issue.
A growing body of research has been dedicated to understanding the sources and dynamics of COVID-19 vaccine hesitancy, and how it complicates efforts at mass vaccination. Most of this research, however, aims to understand hesitancy towards COVID-19 vaccines in general. It is also likely, however, that people have preferences over currently available vaccine options and exhibit considerable hesitancy towards specific vaccines that are influenced by objective features of competing vaccines or communications surrounding them. Depending on the availability of various vaccines in different national contexts, this could be deeply problematic for mass vaccination efforts.
This paper uses Canada as a case study of these dynamics. As of January 2022, the national regulator, Health Canada, had approved four COVID-19 vaccines for distribution: Pfizer, Moderna, AstraZeneca, and Johnson & Johnson, each with different recommended usage guidelines, levels of availability, and safety and efficacy profiles. We ask three principal research questions: 1) to what degree do people exhibit hesitancy towards specific vaccines? 2) How does this hesitancy vary over time in response to the communication environment? And 3) which groups of citizens are more likely to exhibit this hesitancy?
We answer these questions with a study included in repeated cross-sectional surveys of adult Canadian citizens, fielded between February and May 2021 as Canada’s mass vaccination campaign began a large-scale roll-out. Importantly, the initially limited supply of available vaccines meant that individuals were sometimes unable to choose a particular brand, though brand choice eventually became possible, depending on an individual’s province of residence and other individual-level factors. The study features an experiment asking respondents to rate the effectiveness and safety of a randomly assigned vaccine, and indicate their willingness to take the vaccine if offered, conducted over three waves as controversy emerged over the AstraZeneca and Johnson & Johnson vaccines. We rely on comparisons between individuals to infer vaccine-specific hesitancy.
We find substantial vaccine-specific hesitancy in the Canadian context. Respondents reported comparatively more hesitancy towards the AstraZeneca and Johnson & Johnson vaccines, and this hesitancy grew over the course of fielding as controversy related to these vaccines arose. Second, we show that people’s beliefs about the safety of vaccines became an increasingly important influence on their willingness to take them if offered, especially among respondents who were randomly assigned AstraZeneca or Johnson & Johnson. We also show that comparative aversion to the AstraZeneca and Johnson & Johnson vaccines is stronger for those who are typically most supportive of vaccines. It is also intense: a sizable segment of the population is willing to wait months to receive their preferred vaccine over those from AstraZeneca or Johnson & Johnson.
2. COVID-19 vaccine hesitancy
Ensuring continued widespread uptake of vaccines is vitally important for controlling the COVID-19 pandemic. However, we know from history that vaccination efforts are often met with resistance in some quarters of society, despite being one of the most important public health advances in history [25]. A large body of scholarly work has sought to identify the factors that make people more vaccine hesitant – a reluctance or refusal of an individual to receive a vaccine for themselves or their children – or prone to endorsing vaccine misinformation and anti-vaccine policy attitudes.
Several important lessons have emerged from this line of research. Ideological conservatives appear to be more vaccine hesitant than liberals in the United States [3], which may not have been true in the past. Republican supporters are also less likely to hold accurate beliefs about vaccines, with possible downstream consequences for vaccine policy preferences [10], [18]. Support for specific vaccines can also decrease in response to politicization of a given vaccine in the news media [7]. Lower knowledge levels are also associated with greater confidence that one knows more than vaccine experts, which is in turn associated with anti-vaccine policy attitudes – a Dunning-Kruger effect [19]. Distrust in scientific authorities is also heavily connected to vaccine misinformation endorsement, as is social media use [22], consistent with a wide range of work documenting vaccine misinformation online [6], [9], [12], [21], [23].
A growing body of work examining the correlates of COVID-19 vaccine hesitancy has arrived at very similar conclusions. Hesitancy is found among ideological conservatives, at least in the American context, as well as those with low trust in experts. It is also higher among racialized minorities and women, those with more hesitancy towards childhood vaccines, and those who are skeptical of the seriousness of the COVID-19 pandemic [5], [16]. These findings also line up with research examining the correlates of compliance with other public health guidelines like mask wearing and physical distancing [2], [8], [14], [20].
The case of COVID-19 vaccines, however, is unique in one other way: there are multiple vaccines on offer each with different characteristics and safety and efficacy profiles. The availability of these vaccines will vary over time and across national contexts. The above work examines attitudinal and demographic correlates of hesitancy towards COVID-19 vaccines as an aggregate category, but the characteristics of vaccines may also matter for hesitancy towards specific vaccines.
There are good reasons to expect people may have hesitancy towards specific COVID-19 vaccines. Trial data for COVID-19 vaccines by AstraZeneca and Johnston & Johnston indicated they were less effective at preventing symptomatic infection than those by Pfizer and Moderna, though they were reported to be similarly effective at preventing severe disease and death [1], [24]. More recently, public health agencies in some countries raised alarms about a link between the AstraZeneca and Johnson & Johnson vaccines and rare, but severe adverse events, which was widely reported in the news media. These dynamics may have induced vaccine-specific hesitancy. In short, people may have formed preferences over the range of available COVID-19 vaccines and developed comparative hesitancy towards some of them based on discourse surrounding the safety and efficacy characteristics of these vaccines.
There has been some work exploring how the characteristics of hypothetical COVID-19 vaccines affect hesitancy. People are less willing to take foreign-manufactured vaccines, as well as those with weaker safety and efficacy profiles [11], [13], [17]. But these studies were done before it was clear which COVID-19 vaccines were going to be on offer. Merkley & Loewen [15] focus on identifying communication strategies to improve willingness to take the AstraZeneca and Johnson & Johnson vaccines, but do not identify how hesitancy towards these vaccines compares to those from Pfizer or Moderna, how that has changed over time, nor who exactly is most likely to differentiate by brand. This is our focus in this article.
3. Materials and methods
Our research was approved by the University of Toronto Social Sciences, Humanities and Education Research Ethics Board (protocol no. 38251). All respondents included in the following analyses provided their informed consent. Our research was fielded in three cross-sectional waves – February 23-March 1, 2021 (N = 2,495), March 17–23, 2021 (N = 2,511) and April 15–20, 2021 (N = 1,455) – conducted on non-probability samples of adult Canadian citizens from the online panel provider Dynata. Quotas in each wave were set on age (i.e., 18/34, 35/54, and 55+), gender (i.e., male, female), region (i.e., Atlantic, Quebec, Ontario, and West), and language (i.e., English, French) to match population benchmarks in the 2016 Canadian census. Table S1 of the Supplementary Materials provides a breakdown of the demographics in each of the three samples along with the population benchmark.1
We conducted a four condition between-subjects experiment. Respondents were randomly assigned into four groups: Pfizer, Moderna, AstraZeneca, and Johnson & Johnson.2 They received the following three questions where the brand of the vaccine was piped into the text based on their assigned condition: 1) If you were offered the [insert brand] coronavirus vaccine, how likely would you be to take it? (very likely, somewhat likely, not very likely, not at all likely); 2) How would you rate the effectiveness of the [insert brand] coronavirus vaccine? (very effective, somewhat effective, not very effective, not at all effective); and 3) How would you rate the safety of the [insert brand] coronavirus vaccine? (very safe, somewhat safe, not very safe, not at all safe). All of our outcomes are rescaled from 0 to 1 where 1 indicates the most positive vaccine evaluations.
We pre-registered expectations that intention (H1), perceived effectiveness (H2), and perceived safety (H3) would be lower for respondents in the AstraZeneca and Johnson & Johnson treatment conditions compared to those in the Moderna and Pfizer conditions.3 Information on the comparatively lower efficacy of the AstraZeneca and Johnson & Johnson vaccines likely weakened confidence in their effectiveness, while media coverage of a link between these vaccines and serious side effects reduced perceptions of their safety. These changing perceptions, in turn, reduced willingness to receive these vaccines.4
We test our hypotheses with an independent samples t-test (1 = AstraZeneca/Johnson & Johnson, 0 = Pfizer/Moderna). We also present the model predictions for the following equation with survey wave fixed effects (X) estimated using Ordinary Least Squares regression to illustrate variation across our three outcomes for each vaccine:
| (1) |
All significance tests are two-tailed. We use a p-value threshold of 0.05 to determine statistical significance. We use HC2-robust standard errors. All analyses are conducted using STATA version 16.
Unexpected events occurred during fielding that likely influenced vaccination intention and perceptions of their safety and efficacy above and beyond our baseline expectations derived from initial safety and efficacy trials. Wave 1 (February 23-March 1) was conducted before safety concerns arose with the AstraZeneca vaccine. Health Canada approved AstraZeneca for adults 18 and older on February 26 in the middle of fielding. Wave 2 (March 17–23) was conducted after considerable controversy arose with the AstraZeneca vaccine – specifically its link to rare, but serious blood clotting episodes. Denmark suspended administration of the AstraZeneca vaccine on March 11 and Germany and France followed suit on March 14. All told, a dozen European countries suspended AstraZeneca vaccine administration in between waves 1 and 2, while Health Canada maintained that the vaccine was safe and effective. Most of the skepticism towards the AstraZeneca vaccine expressed by public health authorities, to this point, was from public health agencies in other countries.
During the interval between waves 2 (March 17–23) and 3 (April 15–20), growing skepticism of the AstraZeneca vaccine was expressed by domestic health authorities. On March 29 the National Advisory Committee on Immunization (NACI) recommended suspending AstraZeneca administration for those under the age of 55 due to blood clotting concerns, and the provinces followed this recommendation immediately.5 Health Canada, however, continued to maintain that the benefits of the vaccine outweighed the risks – an announcement they made on April 14. In addition, on April 13 the U.S. Food and Drug Administration also recommended a pause in the administration of the Johnson & Johnson vaccine due to similar safety concerns. Our period of fielding allow us to observe potential dynamics in vaccine-specific hesitancy over this period.
We estimate a series of models where we regress our outcome variables on the brand conditions, the survey waves, and interactions between the brand conditions and survey waves as follows:
| (2) |
We display the marginal effects of each brand (reference = Pfizer) across each wave of our study to illustrate dynamics over the course of fielding.
We expect reductions in all three of our outcomes for the AstraZeneca and Johnson & Johnson vaccines as a result of communication surrounding these vaccines. But, this attention was primarily focused on rare side effects related to the AstraZeneca and Johnson & Johnson vaccines, rather than efficacy concerns. As a result, the communication environment may have primed safety perceptions. We may observe a growing association between safety perceptions and intention over the course of fielding. We estimate the following equation:
| (3) |
Increased communication around vaccine safety may have primed safety considerations across the board, or it may have increased the importance of these considerations only for the vaccines at the centre of the emerging controversy. So, we estimate the following equation, which allows us to evaluate this priming effect across treatment conditions (i.e., Pfizer/Moderna vs. AstraZeneca/Johnson & Johnson):
| (4) |
Finally, we conduct exploratory analyses to evaluate which groups of citizens exhibit the greatest comparative hesitancy towards the AstraZeneca or Johnson & Johnson vaccines. We focus on age, vaccination intention, anti-intellectualism or trust in experts, support for childhood vaccination, COVID-19 threat perceptions, and COVID-19 news exposure. We cannot make causal claims as to which specific trait moderates our treatment, but we can gain a general sense of the profile of individuals who are more likely to express comparative hesitancy towards the AstraZeneca or Johnson & Johnson vaccines.
We do not have clear theoretical expectations on the direction of the effects. On the one hand, it is possible we may observe stronger effects among those who are typically more supportive of vaccines. These individuals could be more responsive to communication from public health authorities and experts about the comparative efficacy and safety of COVID-19 vaccines, while vaccine skeptics could be generally unresponsive to communications disseminated by sources they do not trust.
On the other hand, we could observe something akin to a motivated reasoning process, where vaccine skeptics are persuaded by information about the comparatively higher risk and lower efficacy of the AstraZeneca and Johnson & Johnson vaccines because of its usefulness in bolstering their prior beliefs about the risks of vaccination, while vaccine supporters resist this information because of its perceived threat to their belief that COVID-19 vaccines are generally safe and effective.
We fit a series of linear models including an interaction between these demographic/attitudinal covariates and a binary indicator of AstraZeneca or Johnson & Johnson treatment (1 = AstraZeneca/Johnson & Johnson, 0 = Pfizer/Moderna) using OLS:
| (5) |
We describe the measurement of our covariates in Table S2 of the Supplementary Materials.
4. Results
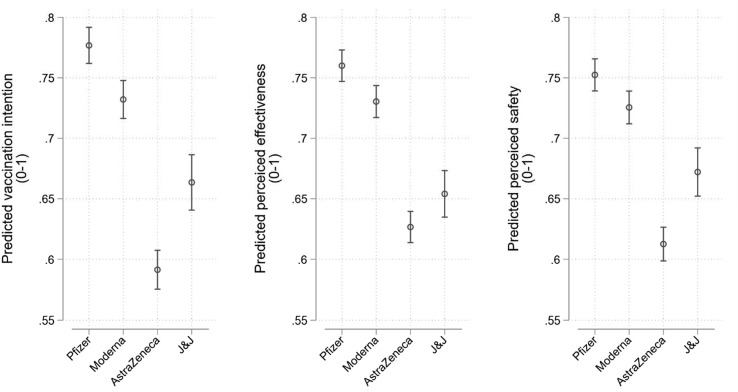
Our pre-registered expectations were met when pooling across our three survey waves. Intention is 0.14 points lower on a 0–1 scale in the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna, consistent with H1 (95% CI = -0.16, −0.12; p < 0.001). Perceived effectiveness is 0.11 points lower on a 0–1 scale in the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna, consistent with H2 (95% CI = -0.12, −0.09; p < 0.001). Perceived safety is also 0.11 points lower on a 0–1 scale in the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna, consistent with H3 (95% CI = -0.12, −0.09; p < 0.001). There are other, less sizable effects. Hesitancy towards the AstraZeneca vaccine appears to exceed that of Johnson & Johnson and skepticism towards the Moderna vaccine appears to be slightly higher than that of Pfizer. These differences are significant at the p < 0.001 level and are apparent in the model predictions from equation (1) that are presented in Fig. 1 .
Fig. 1.
Mean vaccination intention (left), perceived effectiveness (centre), and perceived safety (right) across brand conditions. Results pooled across three waves. Model predictions from equation (1). Estimates can be found in Table S3 of the Supplementary Materials. 95% confidence intervals.
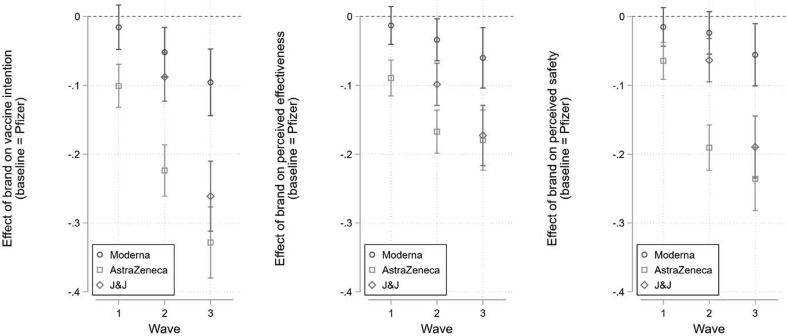
There is also important variation in comparative hesitancy towards available COVID-19 vaccines over the time of fielding. The estimated marginal effects are shown in Fig. 2 (from equation (2)). In wave 1 we observed only a 0.10 point difference in intention between the Pfizer and AstraZeneca conditions (95% CI = -0.13, −0.07; p < 0.001). This effect more than doubled to 0.22 points by wave 2 (95% CI = -0.26, −0.19; p < 0.001) and tripled to 0.33 points by wave 3 (95% CI = -0.38, −0.28; p < 0.001). We see a similar pattern with Johnson & Johnson after the FDA recommended a pause in its administration. Intention was initially 0.09 points lower in the Johnson & Johnson condition compared to Pfizer in wave 2 (95% CI = -0.12, −0.05; p < 0.001). This grew to 0.26 points lower by wave 3 after the FDA announcement (95% CI = -0.31, −0.21; p < 0.001).
Fig. 2.
Marginal effect of brand treatment on intention (left), perceived effectiveness (centre), and perceived safety (right) across waves. Reference = Pfizer. Wave 1 = February 23-March 1, 2021 (N = 2,495); Wave 2 = March 17–23, 2021 (N = 2,511); Wave 3 = April 15–20, 2021 (N = 1,455). 95% confidence intervals. Regression estimates from equation (2) can be found in Table S4.
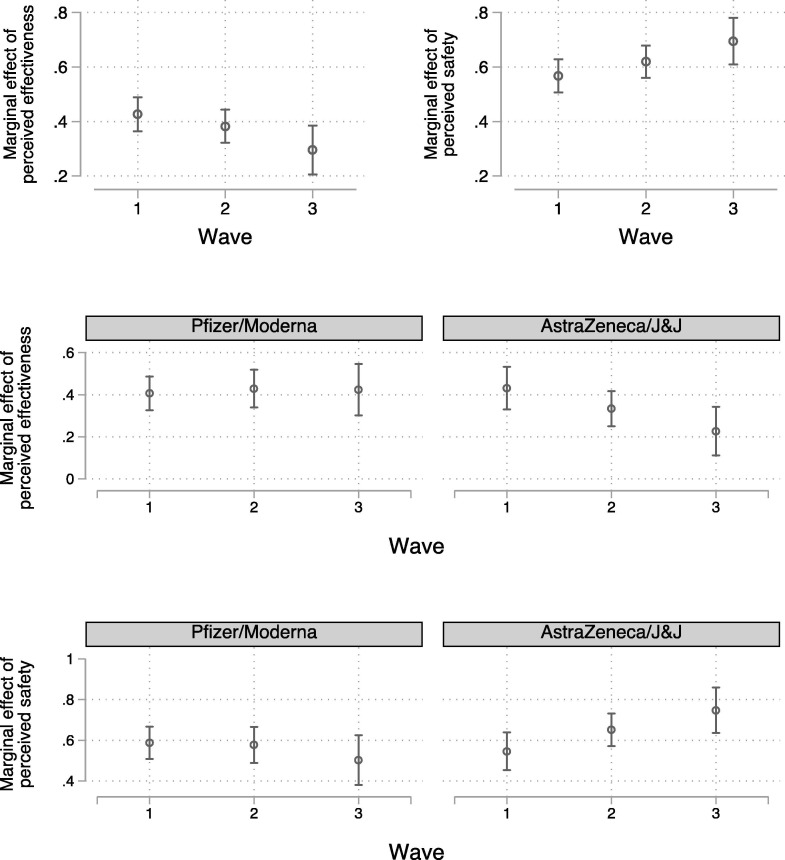
We also see evidence of priming effects: safety perceptions became a more important determinate of intention over the course of fielding. In the top two panels of Fig. 3 we display the marginal effects of perceived effectiveness (left) and safety (right) on intention across our three waves (from equation (3)). The marginal effect of perceived effectiveness on intention (both on 0–1 scales) dropped from 0.43 in wave 1 (95% CI = 0.36, 0.49, p < 0.001) to 0.30 in wave 3 (95% CI = 0.20, 0.38, p < 0.001). This difference is statistically significant (p = 0.019). At the same time, the marginal effect of perceived safety increased from 0.57 in wave 1 (95% CI = 0.51, 0.63, p < 0.001) to 0.69 in wave 3 (95% CI = 0.61, 0.78, p < 0.001). This difference is also statistically significant (p = 0.017).
Fig. 3.
Marginal effect of perceived effectiveness on intention (top-left). Marginal effect of perceived safety on intention (top-right). Marginal effect of perceived effectiveness on Pfizer or Moderna intention (centre-left) and AstraZeneca or Johnson & Johnson intention (centre-right). Marginal effect of perceived safety on Pfizer or Moderna intention (bottom-left) and AstraZeneca or Johnson & Johnson intention (bottom-right). Note: 95% confidence intervals. J&J = Johnson & Johnson. Regression estimates derived from equations (3), (4) can be found in Table S5.
It does not appear, however, that this is true irrespective of the vaccine in question. The centre and bottom panels of Fig. 3 plot the marginal effects of perceived effectiveness and perceived safety, respectively, on intention (from equation (4)) for those in the Pfizer/Moderna conditions (left) and those in the AstraZeneca/Johnson & Johnson conditions (right). We only observe a growing (weakening) link between safety (effectiveness) perceptions and intention in the AstraZeneca/Johnson & Johnson conditions.6
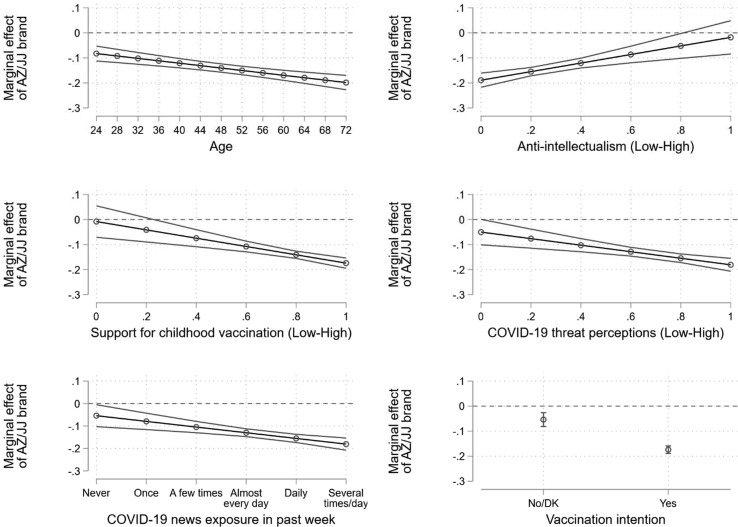
Our results raise the question of who is most likely to exhibit comparative hesitancy towards the AstraZeneca and Johnson & Johnson vaccines. The marginal effects of the model estimates from equation (5) are shown in Fig. 4 . Broadly speaking, comparative hesitancy towards the AstraZeneca and Johnson & Johnson vaccines is not strongest among the typically vaccine hesitant. First, we see stronger effects among older respondents. Intention is estimated to be 0.08 points lower in the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna for those aged 24 (95% CI = -0.11, −0.05; p < 0.001; top-left panel). This difference grows to 0.20 points for those aged 72 (95% CI = -0.23, −0.17; p < 0.001; top-left panel).
Fig. 4.
Marginal effect of AstraZeneca or Johnson & Johnson brand, compared to Pfizer and Moderna, across age (top-left), anti-intellectualism (top-right), support for childhood vaccination (centre-left), COVID-19 threat perceptions (centre-right), COVID-19 news exposure (bottom-left), and previously reported vaccination intention (bottom-right). Note: 95% confidence intervals. DK = Don’t know. AZ = AstraZeneca, JJ = Johnson & Johnson. Regression estimates from equation (5) can be found in Table S6.
Second, we see stronger effects among people with predispositions typically sympathetic to vaccination. Among those who are most trusting of experts, we expect a 0.19 point difference in intention between the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna (95% CI = -0.22, −0.16; p < 0.001; top-right panel). In contrast, we expect a small and not statistically significant 0.02 point difference for those with the highest levels of anti-intellectual sentiment (95% CI = -0.08, 0.05; p = 0.595; top-right panel). For those who are most hostile to childhood vaccinations, we expect only a small and not statistically significant difference in intention between the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna (-0.01; 95% CI = -0.07, 0.05; p = 0.798; centre-left panel). Among those who are most supportive of childhood vaccination, the expected difference grows to 0.17 points (95% CI = -0.19, −0.15; p < 0.001; centre-left panel).
Third, we see stronger effects among people who perceive the most risk from COVID-19 and among those who are most attentive to COVID-19 news. The difference in intention between the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna is 0.18 points for those who perceive the threat from COVID-19 to be most serious (95% CI = -0.21, −0.16; p < 0.001; centre-right panel). This difference weakens to 0.05 points among those who perceive the least amount of threat, which is not quite statistically significant (95% CI = -0.05, 0.00; p = 0.051; centre-right panel). Similarly, the difference in intention between the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna is larger among those who pay attention to COVID-19 news several times a day (-0.18; 95% CI = -0.21, −0.15; p < 0.001; bottom-left panel) than those who do not pay any attention at all (-0.05; 95% CI = -0.10, −0.01; p = 0.030; bottom-left panel).
Finally, the difference in intention between the AstraZeneca and Johnson & Johnson conditions compared to Pfizer and Moderna is more than three times as strong among those who reported that they would take a COVID-19 vaccine when offered (-0.17; 95% CI = -0.19, −0.16; p < 0.001; bottom-right panel) − a question that was asked earlier in the survey − than those who said no or expressed uncertainty on this question (-0.05; 95% CI = -0.08, −0.03; p < 0.001; bottom-right panel). Comparative hesitancy towards AstraZeneca and Johnson & Johnson is strongest among those who are most amenable to vaccination.
5. Discussion
Mass COVID-19 vaccination campaigns have to wrestle with the challenges posed by vaccine hesitancy. One dimension of this challenge is quite different from what threatens campaigns for other more established vaccines: there are multiple vaccines with different safety and efficacy profiles with levels of availability and accessibility that vary over time and across national contexts. People may habour strong preferences for, or aversion to, specific vaccines. This can pose a problem for public health agencies relying on less-preferred vaccines.
Our analyses provide three central contributions. First, we show that there is a significant level of aversion to COVID-19 vaccines from AstraZeneca and Johnson & Johnson in the Canadian context, consistent with our pre-registered expectations. Descriptively, it appears that our brand treatments mostly influenced willingness to take the randomly assigned vaccine indirectly through safety and efficacy perceptions (see Supplementary Materials).
Second, we illustrate important dynamics in this process by leveraging the time period of our analysis. Hesitancy towards the AstraZeneca and Johnson & Johnson vaccines intensified greatly as public health agencies in Canada, the U.S., and Europe issued warnings and restrictions on their usage and news media covered these developments. These communications also appear to have shifted the fundamental drivers of people’s willingness to take certain vaccines. Perceptions of vaccine safety grew in importance in explaining our respondents’ willingness to take a given vaccine if offered, particularly among heavy news consumers in the AstraZeneca and Johnson & Johnson treatment conditions. As the communication environment highlighted safety concerns around these vaccines, attentive citizens responded accordingly.
Importantly, hesitancy towards the AstraZeneca and Johnson & Johnson vaccines did not emerge solely – or even primarily – due to the actions of domestic health officials. For example, although Johnson & Johnson had been approved by Health Canada, it had not been administered to Canadians and Canadian public health agencies had not changed their usage recommendations. We observe increased aversion towards Johnson & Johnson entirely due to decisions made by foreign regulatory agencies and related news coverage.
Third, we show that it is not the case that vaccine hesitant groups are more likely to be repelled by the AstraZeneca and Johnson & Johnson vaccines, as we might expect if people engaged in motivated reasoning – accepting and rejecting communication related to the safety and efficacy of these vaccines based on its convenience for their prior beliefs about COVID-19 vaccines more generally. Instead, we find that individuals who are more trusting of experts, more supportive of childhood vaccines, and more worried about the threat posed by COVID-19 are more inclined to differentiate by brand at the expense of AstraZeneca and Johnson & Johnson.7 We likely cannot tackle vaccine-specific hesitancy with communication strategies aimed at those skeptical of vaccines and scientific expertise generally.
Our results occur in a national context (Canada) where several vaccines have been approved, but are not equally available to all citizens. It is an open question whether comparative hesitancy towards certain vaccines would exist absent the potential of receiving another vaccine, or if it is endogenous to the supply of more than one vaccine. It is also an open question, given skepticism of specific vaccines, whether countries may actually slow down vaccination rates by procuring multiple types of vaccine, only some of which citizens will be willing to take if the potential of waiting for another vaccine exists. Importantly, we find little evidence that overall intention to vaccinate declined as controversy swirled around the AstraZeneca and Johnson & Johnson vaccines. The share of citizens reporting that they intended to vaccinate (or already had) increased 4 points by wave 2 (95% CI = 0.01, 0.06, p = 0.003) and 9 points by wave 3 (95% CI = 0.06, 0.12, p < 0.001).
Nevertheless these findings are still important. It is clear that people are not solely responsive to information from domestic health authorities. Communications and recommendations by foreign health agencies – at least when paired with substantial news coverage – are also highly influential. There is a risk that attentive individuals in countries with limited vaccine supply and a less diverse portfolio of vaccines may turn against the few vaccines that happen to be available depending on these dynamics. This problem may be most acute with the AstraZeneca vaccine, which is logistically much more attractive to developing countries than competing mRNA vaccines. More cross-national research is needed to observe whether these dynamics hold in contexts with less volume and diversity in vaccine supply.
These findings are also important moving forward. As variants of COVID-19 continue to emerge, governments will need to distribute additional booster shots. This puts added stress on vaccine supply. People may be less able to get their preferred vaccines immediately and could respond by holding out until their preferred vaccine becomes available. It appears that Canadians were willing to wait a considerable amount of time to avoid taking the AstraZeneca or Johnson & Johnson vaccines at the time of our study. In the Supplementary Materials we provide evidence from two additional surveys that asked respondents for their preferred vaccine and how long they would be willing to wait for it, rather than an immediately available, randomly assigned alternative (like AstraZeneca). Only 23% of respondents reported a willingness to take AstraZeneca or Johnson & Johnson immediately, 41% were willing to wait 12 months or more for their preferred vaccine, while 36% were willing to tolerate some intermediate delay.
It is also possible that the set of available vaccines will change in Canada and elsewhere in the future. Variants may emerge that successfully evade the protection of currently available vaccines and require new products to be developed. And, of course, COVID-19 will not be the last pandemic we encounter. In either case, we may again see competing vaccines with important variation in their safety and efficacy profiles, as well as their availability, that together produce vaccine-specific hesitancy in different national contexts. We need more attentiveness to these dynamics. They may prove to be immensely consequential in situations where there is some degree of vaccine choice, but relatively limited supply that prevents people from getting the vaccines they prefer.
There are some important limitations to our study. We evaluate behavioural intention and attitudes rather than observed behaviour. We cannot make strong claims that self-reported aversion to AstraZeneca or Johnson & Johnson is indicative of respondents’ behaviour and this is especially true considering the limited distribution of these vaccines in Canada. Further research should be conducted in countries more dependent on these vaccines for the success of their mass vaccination campaigns. Notwithstanding our inability to link attitudes and behavioural intention to observed behaviour, our results consistently demonstrate the existence of vaccine-specific hesitancy and a particular aversion to those offered by AstraZeneca.
Acknowledging the existence of vaccine-specific hesitancy raises questions of how to combat it. Our findings suggest we cannot rely on communication strategies that target those who are generally skeptical of vaccines. We encourage future research on alternative communication strategies that can enhance uptake of less-preferred vaccines.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Peter Loewen reports financial support was provided by 19toZero. Peter Loewen reports financial support was provided by Department of Canadian Heritage. Peter Loewen reports a relationship with 19toZero that includes: consulting or advisory and funding grants. Eric Merkley reports a relationship with 19toZero that includes: consulting or advisory and funding grants.
Acknowledgements
We thank members of the Policy, Elections and Representation lab at the Munk School of Public Policy and Global Affairs for their helpful feedback on this project. We also thank the University of Toronto, 19toZero, and the Department of Canadian Heritage for funding the surveys in which these experiments were included.
Footnotes
The mass vaccination campaign in Canada began to roll out after our second wave. Consequently, we began tracking the number of people who reported their first COVID-19 vaccine dose as of wave 3. In one departure from pre-registration, we remove 213 respondents from our third wave who self-reported being vaccinated. The results from the following analyses, however, remain virtually identical when including these respondents.
In the first wave Johnson & Johnson was excluded because it was not yet approved by Health Canada.
The pre-registration for our studies can be found here: (https://osf.io/74e2x). The first wave was conducted as a pilot study prior to registration.
We cannot directly shed light on the causal mechanism linking COVID-19 vaccine brand to intention with these design (see Bullock et al. [4] for challenges of causal inference with mediation analysis). That being said, we include a path analysis in the Supplementary Materials that descriptively shows the direct and indirect effects of our COVID-19 vaccine brand treatment on intention to vaccinate. These analysis show that 75% of the effect of the brand treatment on intention runs indirectly through changes in perceived effectiveness and safety of the vaccine. More details on this analysis and its limitations can be found in the Supplementary Materials.
Importantly, NACI operates as an advisory board and not a regulator. It existed before the COVID-19 pandemic, and previously focussed principally on childhood vaccinations.
It also appears that this priming effect occurs exclusively among respondents with higher levels of news consumption. These results can be found in Figures S1 and S2 of the Supplementary Materials.
A more mechanical explanation for this result could be floor effects, where vaccine hesitant groups are so resistant to COVID-19 vaccines that there is little room for the values of outcome variables to fall further in response to random assignment. In Table S7 we provide the baseline values for vaccination intention for high and low values of each of our examined covariates and by vaccine condition. Although we cannot rule out floor effects, it appears unlikely to be a major factor.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.033.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Advisory Committee on Immunization Practices. (2021). Overview of Janssen’s Single-Dose COVID-19 Vaccine, Ad26.COV2.S. US Centers for Disease Control and Prevention. February 28. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/02-COVID-Douoguih.pdf.
- 2.Allcott H., Boxell L., Conway J., Gentzkow M., Thaler M., Yang D. Polarization and public health: Partisan differences in social distancing during the coronavirus pandemic. J Public Econ. 2020;191:104254. doi: 10.1016/j.jpubeco.2020.104254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgaertner B., Carlisle J.E., Justwan F., Rabinowitz M. The influence of political ideology and trust on willingness to vaccinate. PLoS ONE. 2018;13(1):e0191728. doi: 10.1371/journal.pone.0191728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock J.G., Green D.P., Ha S.E. Yes, but what’s the mechanism? (Don’t expect an easy answer) J Pers Soc Psychol. 2010;98(4):550–558. doi: 10.1037/a0018933. [DOI] [PubMed] [Google Scholar]
- 5.Callaghan T., Moghtaderi A., Lueck J.A., Hotez P., Strych U., Dor A., et al. Correlates and disparities of intention to vaccinate against COVID-19. Soc Sci Med. 2021;272:113638. doi: 10.1016/j.socscimed.2020.113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkin L.E., Pullon S.R.H., Stubbe M.H. Should I vaccinate my child? Comparing the displayed stances of vaccine information retrieved from Google, Facebook and YouTube. Vaccine. 2020;38(13):2771–2778. doi: 10.1016/j.vaccine.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Fowler E.F., Gollust S.E. The content and effect of politicized health controversies. Ann Am Acad Political and Social Sci. 2015;658(1):155–171. [Google Scholar]
- 8.Gadarian S.K., Goodman S.W., Pepinsky T.B., Lupu N. Partisanship, health behavior, and policy attitudes in the early stages of the COVID-19 pandemic. PLoS ONE. 2021;16(4):e0249596. doi: 10.1371/journal.pone.0249596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamison A.M., Broniatowski D.A., Dredze M., Wood-Doughty Z., Khan D., Quinn S.C. Vaccine-related advertising in the Facebook ad archive. Vaccine. 2020;38(3):512–520. doi: 10.1016/j.vaccine.2019.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joslyn M.R., Sylvester S.M. The determinants and consequences of accurate beliefs about childhood vaccinations. Am Politics Res. 2019;47(3):628–649. [Google Scholar]
- 11.Kaplan R.M., Milstein A. Influence of a COVID-19 vaccine’s effectiveness and safety profile on vaccination acceptance. Proc Natl Acad Sci. 2021;118(10) doi: 10.1073/pnas.2021726118. e2021726118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keelan J., Pavri-Garcia V., Tomlinson G., Wilson K. YouTube as a source of information on immunization: a content analysis. J Am Med Assoc. 2007;298(21):2482–2484. doi: 10.1001/jama.298.21.2482. [DOI] [PubMed] [Google Scholar]
- 13.Kreps S., Prasad S., Brownstein J.S., Hswen Y., Garibaldi B.T., Zhang B., et al. Factors associated with us adults' likelihood of accepting COVID-19 vaccination. JAMA Network Open. 2020;3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merkley E., Loewen P.J. Anti-intellectualism and the mass public’s response to the COVID-19 pandemic. Nat Hum Behav. 2021;5(6):706–715. doi: 10.1038/s41562-021-01112-w. [DOI] [PubMed] [Google Scholar]
- 15.Merkley E, Loewen PJ. Assessment of Communication Strategies for Mitigating COVID-19 Vaccine-Specific Hesitancy in Canada. JAMA Network Open, In press [DOI] [PMC free article] [PubMed]
- 16.Motta M. President Trump promised a COVID vaccine by Election Day: That politicized vaccination intentions. In Jackson D, Coombs DS, Trevisan F, Lilleker D, Thorse E, editors. U.S. election analysis 2020: Media, voters and the campaign (pp. 18-19). Poole, U.K.: The Centre for Comparative Politics and Media Research, Bournemouth University; 2020.
- 17.Motta M. Can a COVID-19 vaccine live up to Americans’ expectations? A conjoint analysis of how vaccine characteristics influence vaccination intentions. Soc Sci Med. 2021;272 doi: 10.1016/j.socscimed.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motta M. Republicans, not Democrats, are more likely to endorse anti-vaccine misinformation. Am Politics Res. 2021;49(5):428–438. doi: 10.1177/1532673X211022639. [DOI] [Google Scholar]
- 19.Motta M., Callaghan T., Sylvester S. Knowing less but presuming more: Dunning-Kruger effects and the endorsement of anti-vaccine policy attitudes. Soc Sci Med. 2018;211:274–281. doi: 10.1016/j.socscimed.2018.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Pennycook G., McPhetres J., Bago B., Rand D.G. Beliefs about COVID-19 in Canada, the United Kingdom, and the United States: A novel test of political polarization and motivated reasoning. Pers Soc Psychol Bull. 2021 doi: 10.31234/osf.io/zhjkp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah Z., Surian D., Dyda A., Coiera E., Mandl K.D., Dunn A.G. Automatically appraising the credibility of vaccine-related web pages shared on social media: a Twitter surveillance study. J Med Int Res. 2019;21(11):e14007. doi: 10.2196/14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stecula D.A., Kuru O., Jamieson K.H. How trust in experts and media use affect acceptance of common anti-vaccination claims. Havard Kennedy School Misinformation Rev. 2020 doi: 10.37016/mr-2020-007. [DOI] [Google Scholar]
- 23.Tang L., Fujimoto K., Amith M., Cunningham R., Costantini R.A., York F., et al. Down the rabbit hole of vaccine misinformation on YouTube: Network exposure study. J Med Int Res. 2021;23(1):e23262. doi: 10.2196/23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teo S.P. Review of COVID-19 vaccines and their evidence in older adults. Ann Geriatric Med Res. 2021;25(1):4–9. doi: 10.4235/agmr.21.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe R.M., Sharpe L.K. Anti-vaccinationists past and present. Br Med J. 2002;325:430–432. doi: 10.1136/bmj.325.7361.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.