Summary
Gestational diabetes mellitus (GDM), which has traditionally been defined as glucose intolerance of varying severity with first onset in pregnancy, is rising in prevalence with maternal hyperglycemia currently affecting one in every six pregnancies worldwide. Although often perceived as a medical complication of pregnancy, GDM is actually a chronic cardiometabolic disorder that identifies women who have an elevated lifetime risk of ultimately developing type 2 diabetes and cardiovascular disease. In identifying high-risk women early in the natural history of these conditions, the diagnosis of GDM raises the tantalizing possibility of early intervention and risk modification. However, before such promise can be realized in practice, a series of clinical challenges/obstacles (reviewed herein) must be overcome. Ultimately, the coupling of this life course perspective of GDM with concerted efforts to overcome these challenges may enable fulfilment of this unique opportunity for the primary prevention of diabetes and heart disease in women.
Keywords: Gestational diabetes, Type 2 diabetes, Cardiovascular disease, Prevention, Women's health
Introduction
Gestational diabetes mellitus (GDM) has traditionally been defined as any degree of glucose intolerance with onset or first recognition during pregnancy, though it is now recognized that this definition does not appropriately differentiate between women with pre-existing diabetes that was not identified before pregnancy (i.e. diabetes in pregnancy (DIP)) and those with hyperglycemia detected on routine antepartum testing (typically in late 2nd trimester) that does not meet the diagnostic criteria for DIP (i.e. GDM).1 Importantly, the International Diabetes Federation estimated that maternal hyperglycemia affected one in every six pregnancies globally in 2019.2 Largely reflecting the rising prevalence of GDM, this trend is being driven by multiple factors including both rising rates of maternal overweight/obesity and greater appreciation of the clinical importance of screening pregnant women for hyperglycemia.3 Indeed, though protocols and diagnostic criteria vary between jurisdictions and even between centres within a jurisdiction, the screening of pregnant women for GDM represents the only situation in current clinical practice in which population testing for diabetes is performed. While the optimal approach to this screening remains a topic of ongoing debate (as discussed later in this review), the importance of identifying GDM is widely accepted, owing to the immediate obstetrical and neonatal implications of the diagnosis.3 Moreover, although it is considered a medical complication of pregnancy, the diagnosis of GDM also carries long-term implications for both mother and child that extend well beyond gestation.4 Notably, since GDM identifies women who have an elevated lifetime risk of developing type 2 diabetes (T2DM) and cardiovascular disease,5, 6, 7 this diagnosis provides a potential opportunity for primary prevention of these conditions early in their natural history. In this review, we will consider current understanding of this unique opportunity and the clinical challenges that will need to be overcome before its potential benefit can be fully realized in practice.
Immediate implications of GDM in pregnancy
Human pregnancy is characterized by a progressive decline in maternal insulin sensitivity from mid-gestation onwards, which partly serves to support nutrient supply to the fetus. In response to this insulin resistance of the latter half of gestation, the pancreatic beta-cells must increase their secretion of insulin for glucose homeostasis to be maintained. Women who develop GDM have a chronic defect in beta-cell function that typically first comes to clinical attention through the maternal hyperglycemia that arises because of their inability to fully compensate for the challenge posed by the insulin resistance of late pregnancy (Figure 1).8,9 Screening for GDM has thus become a standard component of obstetrical care because of the clinical implications of maternal hyperglycemia. Specifically, maternal hyperglycemia leads to fetal hyperglycemia which, in turn, stimulates fetal insulin secretion. Since insulin has anabolic effects in addition to its metabolic activity, fetal hyperinsulinemia can promote excessive growth. The resultant fetal overgrowth can contribute to a host of adverse neonatal outcomes including macrosomia, shoulder dystocia, birth injury, prematurity, perinatal mortality, and need for Caesarean section.3 Notably, the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study showed that there are continuous associations between maternal glycemia and both (i) adverse outcomes (including need for primary Caesarean delivery, premature delivery, shoulder dystocia or birth injury, pre-eclampsia, neonatal hypoglycemia, neonatal hyperbilirubinemia, and need for neonatal intensive care) and (ii) clinical consequences of maternal glycemia that contribute to these associations – namely fetal overgrowth (i.e. birthweight above the 90th percentile) and fetal hyperinsulinemia (as evidenced by cord-blood serum C-peptide above the 90th percentile).10 These data support the rationale for glucose-lowering therapy as a focus of clinical management in women who are diagnosed with GDM. Indeed, controlling maternal glycemia has been shown to reduce fetal overgrowth and the incidence of adverse obstetrical/neonatal outcomes in women with GDM.11,12 Thus, current clinical management of GDM focuses on glucose-lowering therapy consisting of lifestyle modification (targeting diet and physical activity) followed by pharmacotherapy (typically exogenous insulin), if needed.
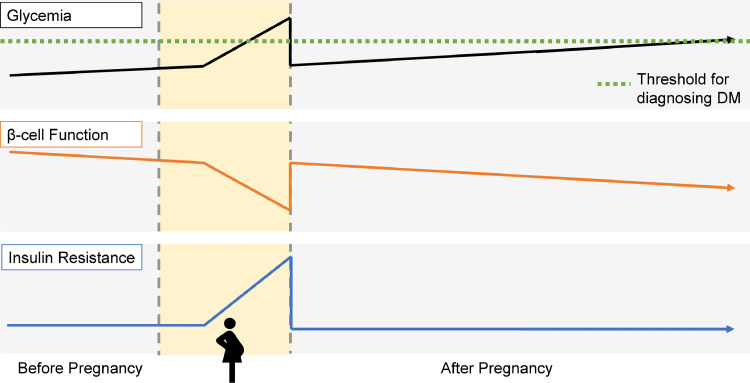
Figure 1.
Schematic showing the life course perspective of insulin resistance, beta-cell function, and glycemia in a woman with GDM. Specifically, women with GDM have a chronic beta-cell defect that leads to rising glycemia even before pregnancy. When pregnant, insufficient beta-cell compensation for the insulin resistance of the latter half of gestation yields the hyperglycemia by which GDM is identified. Upon delivery and abatement of the insulin resistance of pregnancy, glycemia initially improves. However, the ongoing deterioration of beta-cell function leads to rising glycemia over time that may ultimately reach the diagnostic threshold for diabetes.
Future health implications of GDM after pregnancy
While antepartum glucose-lowering therapy focuses on reducing immediate obstetrical and neonatal risks associated with GDM, there are also long-term implications to consider for both child and mother. Notably, the offspring of GDM pregnancies have an increased prevalence of overweight/obesity, dysglycemia, dyslipidemia and metabolic syndrome in childhood.13, 14, 15 This propensity for metabolic dysfunction that links mother and child may be the consequence of multiple elements including (i) shared genetic factors, (ii) the home environment and associated lifestyle, and (iii) fetal exposure to the altered intrauterine environment of the GDM pregnancy, which potentially may program adverse developmental pathways as per the Developmental Origins of Health and Disease (DOHaD) paradigm.16, 17, 18, 19, 20 Moreover, evidence to date suggests that current antepartum treatment of GDM does not reduce these future risks in the offspring21,22 and it remains uncertain whether non-insulin pharmacologic therapy for GDM (such as glyburide or metformin) could even have deleterious effects on the offspring that might emerge during childhood.23
After delivery, the insulin resistance of pregnancy abates such that blood glucose levels typically return to the normal range in women who had GDM (Figure 1), thereby obviating the need for ongoing glucose-lowering therapy. While the observed transient hyperglycemia that is limited to gestation may support perception of GDM as strictly a medical complication of pregnancy, the glycemic implications of this diagnosis actually extend well beyond gestation. Specifically, the beta-cell defect that yields insufficient compensation for the insulin resistance of pregnancy is both chronic and progressive in nature. Accordingly, women who develop GDM typically experience progressive worsening of beta-cell function in the years after the index pregnancy, resulting in rising glycemia over time that can lead to pre-diabetes and T2DM (Figure 1).24, 25, 26, 27 This deterioration of beta-cell function, which may be further exacerbated by the secretory demands placed by chronic insulin resistance, is the pathophysiologic basis for the elevated lifetime risk of T2DM in women with a history of GDM. Indeed, compared to their peers, women who develop GDM have a 7- to 10-fold higher risk of progressing to T2DM in the years thereafter.5,6 This striking potency of GDM as a predictor of future T2DM reflects the shared pathophysiology (beta-cell dysfunction) underlying both conditions. Similarly, since any degree of beta-cell dysfunction may compromise appropriate compensation for the insulin resistance of pregnancy, even mild degrees of gestational glycemia predict future risk of T2DM.27, 28, 29 Women with such mild dysglycemia in pregnancy include those with an abnormal screening glucose challenge test (GCT) but a normal oral glucose tolerance test (OGTT) and those with mild abnormalities on the OGTT that do not meet the thresholds of certain GDM diagnostic criteria.27, 28, 29 Thus, any degree of beta-cell dysfunction and resultant dysglycemia in pregnancy identifies future risk of T2DM.
In the past two decades, it has emerged that the diagnosis of GDM identifies a population of young women who are at future risk of other chronic non-communicable diseases (NCDs) besides T2DM. Notably, women with a history of GDM have elevated risks of developing renal dysfunction, serious liver disease and cardiovascular disease (CVD).30, 31, 32, 33 Indeed, a meta-analysis involving >5 million women revealed that those with a history of GDM have a 2-fold higher risk of CVD than their peers that begins to manifest within the first decade after the index pregnancy.7 Importantly, while their risks of severe liver disease and kidney disease appear to be dependent upon the inter-current development of T2DM,30,32 women with GDM have an elevated lifetime incidence of CVD even if they do not progress to diabetes.7 Moreover, as with the risk of T2DM, milder degrees of gestational dysglycemia that do not meet the diagnosis of GDM also predict an elevated lifetime risk of CVD.33,34 Indeed, even an elevated GCT in the absence of GDM predicts future CVD.34 Thus, the continuum of gestational glycemia provides insight into a woman's likelihood of developing metabolic and vascular disease well beyond pregnancy, with GDM representing the most extreme element along this glycemic spectrum.
In the past decade, converging lines of evidence have shaped the emerging perspective of GDM as a chronic cardiometabolic disorder (rather than one that is limited to pregnancy).18 First, even by as early as 3-months postpartum, women with recent GDM exhibit an adverse cardiovascular risk factor profile compared to that of their peers, as evidenced by higher rates of dsyglycemia, hypertension, dyslipidemia, and metabolic syndrome.28,35,36 Second, the measurement of cardiometabolic biomarkers in 1st trimester (such as glycemic and lipid measures, adiponectin, C-reactive protein, tissue plasminogen activator antigen, and insulin-like growth factor binding protein-2) can predict the subsequent development of GDM later in pregnancy.37 Third, it has been shown that the amniotic fluid of women who go on to develop GDM already shows metabolic changes in 1st trimester and fetal overgrowth can occur before the diagnosis of GDM.38,39 Finally, and most importantly, cardiometabolic differences between women who go on to develop GDM and those who do not are already present even before the pregnancy. These subtle differences include greater glycemia (higher A1c and fasting glucose) and a more adverse lipid profile (higher LDL cholesterol, higher triglycerides, lower HDL).40, 41, 42 While initially modest in magnitude, these differences become more pronounced over time, owing to divergent trajectories of these risk factors between women who develop GDM and their peers, both in the years prior to pregnancy and in the years thereafter.42,43 Accordingly, pregnancy can be viewed as a life event that is superimposed upon existing tracks of cardiometabolic risk and enables the identification of women are already on a high-risk track (i.e. those who develop GDM).18 From this life course perspective, GDM can be seen be as a chronic cardiometabolic disorder (Figure 2) that comes to clinical attention in pregnancy because antepartum glucose screening is performed in the setting of the stress test that gestation poses for the beta-cells. While the ultimate objective of this screening is to reduce the obstetrical/neonatal risks associated with GDM, the concomitant insight that may be gained into a woman's long-term risk of metabolic and vascular disease provides a unique opportunity for preventive care. Specifically, the recognition of GDM as a chronic cardiometabolic disorder presents a potential opportunity for early risk-modifying intervention aimed at the primary prevention of T2DM and CVD. However, before this promise can be realized in practice, there are a series of clinical challenges and hurdles that will need to be overcome.
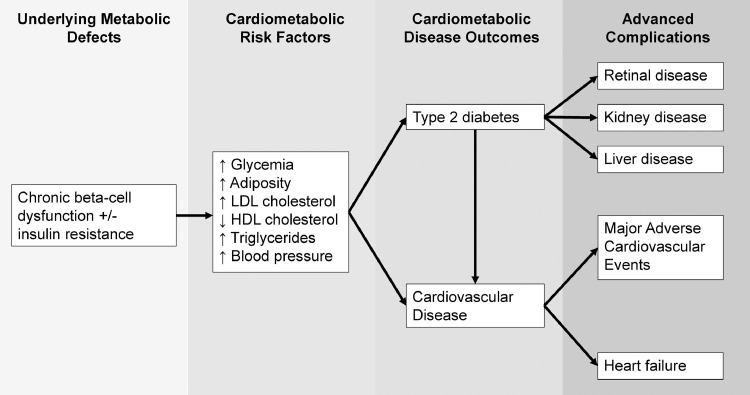
Figure 2.
The life course perspective of GDM encompasses underlying metabolic defects, an adverse cardiometabolic risk factor profile that worsens over time, and elevated lifetime risks of cardiometabolic disease outcomes and associated advanced complications.
Challenges facing this potential opportunity for primary prevention
(I) Identification of GDM
Since the initial description of GDM over 50 years ago, several different approaches to identify GDM have been proposed and debated in medical communities worldwide. To date, there is no consensus on the optimal approach, resulting in varying screening protocols and diagnostic criteria across jurisdictions and furthering uncertainty and frustration on the part of patients and healthcare providers. Moreover, pre-analytic factors can also impact the accuracy of glucose measurements and thereby further hamper the diagnosis of GDM.44
The initial diagnostic approach for GDM, proposed by O'Sullivan and Mahan in 1964, utilized a fasting 3 h 100 g oral glucose tolerance test (OGTT), with glucose measurements made from whole blood (Somogyi-Nelson method).45 Glycemic thresholds on the OGTT were established that predicted future risk of T2DM, with two or more elevated values (from fasting, 1 h, 2 h, and 3 h postprandial glucose measurements) required for diagnosis of GDM.45,46 In 1979, based on laboratory transition from venous whole blood to plasma glucose measurements, the National Diabetes Data Group (NDDG) proposed revised thresholds for diagnosing GDM based on 3 h 100 g OGTT, by applying a factor of 1.14 to each value.47 Carpenter and Coustan further modified the diagnostic thresholds in 1982 to account for improved specificity of enzymatic glucose assays (e.g. hexokinase and glucose oxidase) compared to the Somogyi-Nelson method, as the newer assays did not measure reducing substances other than glucose.48 At the same time, a 1 h 50 g oral glucose challenge test (GCT) was adapted by O'Sullivan et al.49 to screen all pregnant women after 24-weeks gestation and identify those at highest risk for GDM. The GCT is a non-fasting screening test, which can be easily incorporated into a routine antenatal visit with primary care provider or obstetrician, and is typically used as the initial step of a two-step diagnostic strategy. As laboratory assays improved, several different cut-offs for the 1 h post-challenge glucose threshold were also proposed (e.g. 130 mg/dL, 135 mg/dL, and 140 mg/dL), each with varying sensitivities and specificities.49 In GCT-based two-step protocols, women who are positive on the screening GCT then proceed to the OGTT for diagnosis of GDM. To date, the two-step approach (50 g GCT followed by 100 g OGTT by either the Carpenter and Coustan criteria or the NDDG criteria) remains the protocol to identify GDM endorsed by the American College of Obstetricians and Gynecologists (ACOG) and National Institutes of Health (NIH).50,51
In 2010, based on the findings of the HAPO Study, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) recommended a one-step universal screening strategy for GDM using the fasting 2 h 75 g OGTT, for which glycemic thresholds were developed based on odds ratios of 1.75 for birthweight >90th percentile, cord C-peptide >90th percentile and percent body fat >90th percentile in the study cohort.52 In contrast to Carpenter and Coustan criteria for the 100 g OGTT, the IADPSG criteria required only one elevated value on the 75 g OGTT for diagnosing GDM.46,52 Currently, the one-step IADPSG screening approach is endorsed by the American Diabetes Association (ADA), the World Health Organization (WHO), and the International Federation of Gynecology and Obstetrics (FIGO).1,53,54
Several concerns have been raised that have hindered adoption of the IADPSG approach in clinical practice. When IADPSG criteria were applied to the HAPO cohort, the prevalence of GDM was ∼18%, representing an approximately three-fold increase from rates observed in other cohorts in which GDM was diagnosed by historical approaches.49 With the increase in prevalence comes the concern of over-diagnosis and resulting economic impacts on the healthcare system and psychosocial impacts on women who are labelled as having GDM.49 Observational studies comparing IADPSG criteria to historical controls also found that, despite the increase in GDM prevalence, the treatment of women who otherwise would not have been labelled as GDM did not consistently reduce adverse pregnancy outcomes in the overall population.55 In light of these concerns, the NIH Consensus Panel in 2013 cautioned against adoption of the IADPSG approach and recommended that a randomized controlled trial (RCT) be conducted to compare these approaches with respect to clinically important outcomes.49,55
Five RCTs (n = 25,772) have been conducted comparing IADPSG versus Carpenter and Coustan criteria for GDM screening, according to a recent systematic review by the US Preventative Task Force (USPTF).56 The largest of these studies was the ScreenR2GDM trial (n = 23,792) by Hillier et al. in 2021, in which 23,792 women treated in the Kaiser Permanente system were randomized to either one-step screening by 75 g OGTT with IADPSG criteria or two-step screening by 50 g GCT followed by 100 g OGTT if the GCT were positive.57 One-step screening versus two-step screening was associated with a significantly higher prevalence of GDM in 16.5% vs. 8.5% of participants; however no significant difference were detected between the two groups in any pregnancy or fetal outcome (including gestational hypertension or preeclampsia, primary Caesarean section, large for gestational age infants, or a perinatal composite outcome of stillbirth, neonatal death, shoulder dystocia, bone fracture or nerve palsy related to birth injury).57 Potential limitations of the study have been raised, including questions pertaining to the adequacy of the sample size calculation and the lack of long-term data on maternal or fetal benefits.46 Of note, 27% of women in the one-step group crossed over to two-step screening. In addition, 1.4% of women in the two-step group were treated for fasting hyperglycemia despite no diagnosis of GDM, possibly increasing the apparent efficacy of the two-step approach. Accordingly, at present, the debate on the optimal approach to GDM diagnosis is ongoing.
(II) Postpartum Follow-up
Given their well-established risk of postpartum progression to T2DM, it is widely recommended that women with GDM undergo glucose tolerance testing by 75 g OGTT within the first 6 months after delivery.58 Despite its broad endorsement by authoritative bodies (such as the Endocrine Society, American Diabetes Association, and American College of Obstetricians and Gynecologists) and inclusion in clinical practice guidelines, the rates of postpartum glucose tolerance screening have been consistently suboptimal across jurisdictions, ranging from 19 to 73% according to a recent systematic review.59 Both provider and patient factors have been identified as predictors of the likelihood of postpartum testing.
The frequency of ordering of the postpartum OGTT by healthcare providers has been identified as one contributing factor. One study reported that 48.9% of OGTT non-completion was attributable to provider noncompliance (i.e. test was never ordered), while 51.1% was due to lack of adherence by patients.60 Barriers identified from the healthcare provider perspective include lack of patient follow-up, inadequate communication between healthcare providers, inconsistent guidelines, lack of familiarity with screening protocols, and patients not perceving testing as necessary or affordable.59 Indeed, although the necessity of postpartum screening is clear from clinical practice guidelines, there is no clear direction as to which of the patient's care providers (primary care provider, obstetrician, or endocrinologist) bear responsibility for this task. Different jurisdictions also have differences in practice patterns. While Stuebe and colleagues found that primary care providers were most likely to order a postpartum screening test in Massachusetts, US,61 Shah and colleagues found that internists/endocrinologists ordered the majority of these tests in Ontario, Canada.62
A variety of patient factors have also been identified as predictors of the likelihood of adherence with postpartum follow-up. In a qualitative study by Bennett et al., themes of barriers that were identified included recent delivery experience/newborn health issues, adjustment to new baby (e.g. lack of time, burden of childcare, emotional stress), concerns about postpartum and future health (e.g. feeling healthy and not in need of care, fear of receiving bad news), dissatisfaction with care and logistics of accessing care.63 Additionally, in a systematic review by Nielson et al., patients were found to be more likely to undergo screening if they had GDM in a previous pregnancy, diagnosis of GDM at earlier gestational age, older maternal age, higher education level and income, and lower parity.59
Several interventions have been studied to address the poor adherence rates to postpartum screening among women with recent GDM pregnancies.58 Interventions have included verbal and written counselling, postal reminders, telephone calls, SMS reminders, or advanced order sets built into electronic medical records, and all have shown varying degrees of improvement in adherence upon implementation.58 In practice, however, rates of postpartum testing remain suboptimal. Ultimately, before the potential for primary prevention offered by GDM can be fully realized, the challenge of suboptimal postpartum follow-up will need be resolved.
(III) Appropriate Postpartum Intervention
Besides the challenges of determining an optimal approach to GDM identification and optimizing adherence to postpartum screening, the appropriate intervention for modifying the risk of developing T2DM is also a topic of ongoing research. In this context, both lifestyle and pharmacologic interventions have shown varying effectiveness at preventing postpartum diabetes.
Lifestyle interventions (i.e. diet, physical activity) have been shown to reduce postpartum weight, BMI, and waist circumference in women with previous GDM.64 These observations provide a mechanistic basis for reducing the risk of T2DM, since the reduction of insulin resistance secondary to weight loss should lower the secretory demands placed on the beta-cells and thereby potentially may mitigate their functional deterioration over time.18 Indeed, a recent meta-analysis of 10 RCTs of lifestyle interventions within 3 years of GDM pregnancy found that such intervention reduced the risk of postpartum diabetes as compared to controls (pooled RR 0.57, 95%CI 0.42–0.78).65 Table 1 lists selected trials of lifestyle intervention following GDM. These findings suggest that all women with a history of GDM should receive lifestyle counselling and intervention early after delivery. However, several barriers have been identified that may compromise the introduction of healthy lifestyle practices in postpartum months following GDM.59 These practical barriers have included lack of time and energy, limited childcare and social supports, emotional stress, lack of motivation, financial barriers, insufficient knowledge or understanding about GDM, body image concerns, and the need to maintain caloric intake for breastfeeding.59 It is also unclear if ethno-cultural differences may be relevant to the appropriate lifestyle recommendations in different populations.
Table 1.
Selected studies of lifestyle interventions for reducing the risk of progression to type 2 diabetes in women with a history of GDM.
| Study | Population analyzed | Intervention | Follow-up duration | Key findings |
|---|---|---|---|---|
| Diabetes Prevention Program (DPP)67 | Subset of DPP participants with pre-diabetes and previous GDM (n = 350) | Randomized to intensive lifestyle, metformin or placebo | 3 years | Both lifestyle and metformin reduced incident diabetes by ∼50% compared to placebo |
| Perez-Ferre et al.78 | Women with previous GDM, excluding those with impaired fasting glucose at first postpartum evaluation (n = 260) | Randomized to intervention group (Mediterranean diet and monitored physical activity) or control (usual care) | 36 months | Lifestyle intervention reduced incidence of glucose disorders compared to control (42.8% vs. 56.75%). |
| Shek et al.79 | Women with previous GDM and impaired glucose tolerance postpartum (n = 450) | Randomized to intervention group (advice on diet and exercise, reinforced at follow-up visits) or control (usual care) | 36 months | Trend towards reduced incident diabetes in intervention group vs. control (15% vs. 19%); did not reach statistical significance. |
| Hu et al.80 | Women with previous GDM (n = 1180) | Randomized to lifestyle intervention (dietician visits, physical activity counselling) or usual care | 12 months | Lifestyle intervention led to weight loss, improved cardiometabolic risk factors and reduced insulin resistance compared to usual care. |
| Wein et al.81 | Women with previous GDM and impaired glucose tolerance (n = 200) | Randomized to intensive or routine dietary advice | Median follow-up 51 months | No significant difference in prevalence of diabetes and impaired glucose tolerance was found. |
Amongst pharmacological agents, there is evidence to support metformin as an intervention for reducing the risk of diabetes in women with previous GDM. In the Diabetes Prevention Program (DPP), overweight adults with pre-diabetes (impaired glucose tolerance or impaired fasting glucose) were randomized to placebo, intensive health behaviour change, metformin, or the older thiazolidinedione troglitazone.66 While intensive lifestyle intervention yielded the greatest reduction in risk of developing diabetes in the overall study population,66 DPP participants who had a previous history of GDM comprised a subgroup in which metformin matched lifestyle modification, with both interventions yielding ∼50% risk reduction compared to placebo.67 Moreover, in the long-term follow-up of DPP participants, the effect of metformin on reduction of incident diabetes in this subgroup persisted over 10 years68 and 15 years.69 However, it should be recognized that, on average, this subgroup of women with previous GDM were 12 years postpartum at the start of the DPP, such that this effect on diabetes prevention may not be generalizable to women who are in the early years after pregnancy. Indeed, given that they had not progressed to overt diabetes during the early postpartum years (when the highest risk women may progress to T2DM),27,70 it is likely that these DPP participants comprise a comparatively lower-risk subset within the overall population of women with previous GDM.
Other pharmacological agents have been studied as intervention to prevent diabetes after a GDM.71 However, as with the interpretation of metformin in the DPP, these studies have caveats and limitations that preclude definitive conclusions on their role in women with recent GDM (Table 2). In the Troglitazone in Prevention of Diabetes (TRIPOD) and Pioglitazone in Prevention of Diabetes (PIPOD) studies, the insulin-sensitizing thiazolidinediones troglitazone and pioglitazone were shown to significantly reduce the risk of progression to T2DM in Hispanic-American women with previous GDM.72,73 However, safety concerns have limited the applicability of these findings to current practice (troglitazone was withdrawn from the market due to hepato-toxicity and concerns of off-target effects have markedly reduced clinical initiation of pioglitazone). In a study of 40 women with previous GDM, the combination of metformin and the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin yielded improvement in beta-cell function and insulin sensitivity after 16-weeks (compared to baseline),74 while a placebo-controlled trial in 113 women found that the DPP-4 inhibitor vildagliptin did not reduce the risk of diabetes in this patient population.75 In addition, a study of 49 women reported that the combination of metformin and the sodium glucose co-transporter-2 (SGLT-2) inhibitor dapagliflozin reduced weight and improved cardiometabolic risk factors after 24-weeks.76 Recognizing the limitations of this literature, we are currently conducting a double-blind, placebo-controlled RCT to evaluate the impact of the SGLT-2 inhibitor empagliflozin on beta-cell function and glucose tolerance over 1 year in women with recent GDM (ClinicalTrials.Gov NCT03215069).
Table 2.
Studies of postpartum pharmacologic interventions for reducing the risk of progression to type 2 diabetes in women with a history of GDM.
| Study | Population analyzed | Intervention | Key findings | Caveats and limitation |
|---|---|---|---|---|
| Diabetes Prevention Program (DPP)67 | Subset of DPP participants with pre-diabetes and previous GDM (n = 350) | Randomized to intensive lifestyle, metformin or placebo | Both lifestyle and metformin reduced incident diabetes by ∼50% compared to placebo | Women were mean 12 years postpartum and thus may be low-risk subset of GDM patient population |
| TRIPOD72 | Women with previous GDM (n = 236) | Randomized to troglitazone or placebo | Troglitazone reduced incident diabetes by >50% compared to placebo | Troglitazone has been withdrawn from the market due to liver toxicity |
| PIPOD73 | TRIPOD participants who did not have diabetes (n = 86) | Open-label observational follow-up of women treated with pioglitazone | Pioglitazone stabilized beta-cell function over 3 years | Concerns of off-target effects have markedly reduced clinical initiation of pioglitazone in current practice |
| Daniele et al74 | Women with pre-diabetes and previous GDM (n = 40) | Randomized to metformin, sitagliptin or combination (metformin + sitagliptin) | Metformin and sitagliptin combination improved beta-cell function and insulin sensitivity | Short duration (16-week study) with drop-out of 24.5% of randomized women |
| Hummel et al75 | Women with previous insulin-treated GDM (n = 113) | Randomized to vildagliptin or placebo | Vildagliptin did not reduce incident diabetes (low incidence led to stoppage of trial) | 46% of randomized women withdrew before completing treatment |
| Elkind-Hirsch et al.76 | Women with overweight/obesity and previous GDM (n = 49) | Randomized to metformin, dapagliflozin or combination (metformin + dapagliflozin) | Metformin and dapagliflozin combination reduced weight and improved cardiometabolic risk factors | Short duration (24-week study) with drop-out of 25.8% of randomized women |
While definitive early postpartum intervention remains to be established, it should be noted that, if achieved, the reduction of incident T2DM following GDM will likely also ultimately reduce the risk of CVD, but not fully mitigate it.7 Accordingly, attention to cardiometabolic risk factors (such as lipids and blood pressure) is ideally warranted in future studies to determine appropriate intervention for modifying the long-term risks of both T2DM and CVD in this patient population. Moreover, the recent emergence of anti-diabetic medications such as SGLT-2 inhibitors that may offer cardiovascular risk reduction above and beyond their glucose-lowering activity77 raises the tantalizing possibility of single interventions that potentially may enable the primary prevention of both T2DM and CVD.
Future perspectives
Debate around the optimal approach to screening and diagnosis of GDM has been ongoing since the initial description of this condition in 1964. Resolution of this debate remains an important focus for the future since standardization of practices across jurisdictions should reduce the uncertainty and frustration this lack of consensus may engender in both patients and providers. However, since the relationships between maternal glycemia and the respective future risks of T2DM and CVD extend to milder degrees of dysglycemia below the GDM diagnostic range,27, 28, 29,33,34 the current lack of universal standardization of diagnostic criteria and screening protocols does not necessarily preclude the opportunity for pursuing primary prevention of these outcomes. Rather, this unique opportunity should be pursued in tandem with efforts to standardize the identification of GDM.
In this context, a critical step to be undertaken now is a fundamental shift in the perception of GDM from that of a medical complication of pregnancy to one of a chronic cardiometabolic condition (first identified in pregnancy) that carries lifelong implications (Figure 2). This shift in perception on the part of both providers and patients could address some of the challenges and barriers identified in this review. Specifically, broader appreciation of this life course perspective of GDM should help to improve adherence with the recommended postpartum glucose tolerance testing. Moreover, this awareness would enhance recognition of the need for further studies to determine the optimal clinical strategies for cardiometabolic surveillance and risk modification in women with a history of GDM. More broadly, in the design of GDM studies, there should be a shift from focusing exclusively on immediate pregnancy outcomes to also considering long-term maternal and offspring outcomes. Ultimately, the coupling of enhanced recognition of the life course perspective of GDM with further research to delineate risk-modifying strategies in practice may enable this diagnosis to fulfill its potential as a unique opportunity for the primary prevention of T2DM and CVD in women.
Search strategy and selection criteria
References for this review were identified through searches on PubMed for articles published from Jan 1, 1980 to Oct 1, 2021, with the terms: “gestational diabetes mellitus”, “screening”, “diagnostic criteria”, “long-term complications”, “cardiovascular disease”, and “prevention”. Relevant articles from these searches and relevant references that were cited in these articles were reviewed. Articles published in English were included in this review.
Contributions
JF and RR each wrote sections of the first draft. Both authors contributed to critical revision of the manuscript for important intellectual content and both authors approved the manuscript.
Funding
None.
Declaration of interests
Dr. Fu has nothing to disclose. Dr. Retnakaran reports grants and other from Boehringer Ingelheim, grants and personal fees from Novo Nordisk, personal fees from Sanofi, personal fees from Eli Lilly, other from Sun Life Financial, outside the submitted work. Dr. Retnakaran also holds the Boehringer Ingelheim Chair in Beta-cell Preservation, Function and Regeneration at Mount Sinai Hospital.
Acknowledgements
Dr. Retnakaran's research program is supported by the Sun Life Financial Program to Prevent Diabetes in Women.
References
- 1.Hod M., Kapur A., Sacks D.A., et al. The International Federation of Gynecology and Obstetrics (FIGO) initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet. 2015;131(Suppl 3):S173–S211. doi: 10.1016/S0020-7292(15)30033-3. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation . 9th ed. International Diabetes Federation; 2019. IDF Diabetes Atlas.https://www.diabetesatlas.org/en Accessed 10 October 2021. [Google Scholar]
- 3.McIntyre H.D., Catalano P., Zhang C., Desoye G., Mathiesen E.R., Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. doi: 10.1038/s41572-019-0098-8. [DOI] [PubMed] [Google Scholar]
- 4.Saravanan P., Diabetes in Pregnancy Working Group. Maternal Medicine Clinical Study Group. Royal College of Obstetricians and Gynaecologists, UK Gestational diabetes: opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020;8(9):793–800. doi: 10.1016/S2213-8587(20)30161-3. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes after gestational diabetes a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 6.Vounzoulaki E., Khunti K., Abner S.C., Tan B.K., Davies M.J., Gillies C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020;369:m1361. doi: 10.1136/bmj.m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer C.K., Campbell S., Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62:905–914. doi: 10.1007/s00125-019-4840-2. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan T.A. Pancreatic beta-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:989–993. doi: 10.1210/jcem.86.3.7339. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan T.A., Xiang A.H. Gestational diabetes mellitus. J Clin Investig. 2005;115(3):485–491. doi: 10.1172/JCI24531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.HAPO Study Cooperative Research Group. Metzger B.E., Lowe L.P., et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 11.Crowther C.A., Hiller J.E., Moss J.R., McPhee A.J., Jeffries W.S., Robinson J.S. Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial group. effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 12.Landon M.B., Spong C.Y., Thom E., et al. Eunice kennedy shriver national institute of child health and human development maternal-fetal medicine units network. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boney C.M., Verma A., Tucker R., Vohr B.R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 14.Fraser A., Lawlor D.A. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diabetes Rep. 2014;14(5):489. doi: 10.1007/s11892-014-0489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowe W.L., Scholtens D.M., Lowe L.P., et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;32(10):1005–1016. doi: 10.1001/jama.2018.11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sauder K.A., Ritchie N.D. Reducing intergenerational obesity and diabetes risk. Diabetologia. 2021;64(3):481–490. doi: 10.1007/s00125-020-05341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIntyre H.D., Kapur A., Divakar H., Hod M. Gestational diabetes mellitus-innovative approach to prediction, diagnosis, management, and prevention of future NCD - mother and offspring. Front Endocrinol (Lausanne) 2020;11 doi: 10.3389/fendo.2020.614533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Retnakaran R. Diabetes in pregnancy 100 years after the discovery of insulin: hot topics and open questions to be addressed in the coming years. Metabolism. 2021;119 doi: 10.1016/j.metabol.2021.154772. [DOI] [PubMed] [Google Scholar]
- 19.Howe C.G., Cox B., Fore R., et al. Maternal gestational diabetes mellitus and newborn DNA methylation: findings from the pregnancy and childhood epigenetics consortium. Diabetes Care. 2020;43(1):98–105. doi: 10.2337/dc19-0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popova P.V., Vasileva L.B., Tkachuk A.S., et al. Association of tribbles homologue 1 gene expression in human umbilical vein endothelial cells with duration of intrauterine exposure to hyperglycaemia. Genet Res (Camb) 2018;100:e3. doi: 10.1017/S0016672318000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillman M.W., Oakey H., Baghurst P.A., Volkmer R.E., Robinson J.S., Crowther C.A. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33(5):964–968. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landon M.B., Rice M.M., Varner M.W., et al. Eunice kennedy shriver national institute of child health and human development Maternal-Fetal Medicine Units (MFMU) network. Mild gestational diabetes mellitus and long-term child health. Diabetes Care. 2015;38(3):445–452. doi: 10.2337/dc14-2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanky E., Ødegård R. Metformin in pregnancy-safe or sorry? Nat Rev Endocrinol. 2018;14(10):570–572. doi: 10.1038/s41574-018-0081-6. [DOI] [PubMed] [Google Scholar]
- 24.Retnakaran R., Qi Y., Sermer M., Connelly P.W., Hanley A.J., Zinman B. Beta-cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. 2010;33(8):1798–1804. doi: 10.2337/dc10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang A.H., Kjos S.L., Takayanagi M., Trigo E., Buchanan T.A. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59(10):2625–2630. doi: 10.2337/db10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Retnakaran R., Qi Y., Ye C., et al. Hepatic insulin resistance is an early determinant of declining beta-cell function in the first year postpartum following glucose intolerance in pregnancy. Diabetes Care. 2011;34:2431–2434. doi: 10.2337/dc11-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer C.K., Swaminathan B., Hanley A.J., et al. Each degree of glucose intolerance in pregnancy predicts distinct trajectories of beta-cell function, insulin sensitivity and glycemia in the first 3 years postpartum. Diabetes Care. 2014;37:3262–3269. doi: 10.2337/dc14-1529. [DOI] [PubMed] [Google Scholar]
- 28.Retnakaran R., Qi Y., Sermer M., Connelly P.W., Hanley A.J., Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008;31:2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Retnakaran R., Shah B.R. Abnormal screening glucose challenge test in pregnancy and future risk of diabetes in young women. Diabet Med. 2009;26(5):474–477. doi: 10.1111/j.1464-5491.2009.02712.x. [DOI] [PubMed] [Google Scholar]
- 30.Retnakaran R., Shah B.R. Role of type 2 diabetes in determining retinal, renal and cardiovascular outcomes in women with previous gestational diabetes. Diabetes Care. 2017;40(1):101–108. doi: 10.2337/dc16-1400. [DOI] [PubMed] [Google Scholar]
- 31.Rawal S., Olsen S.F., Grunnet L.G., et al. Gestational diabetes mellitus and renal function: a prospective study with 9- to 16-year follow-up after pregnancy. Diabetes Care. 2018;41(7):1378–1384. doi: 10.2337/dc17-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Retnakaran R., Luo J., Shah B.R. Gestational diabetes in young women predicts future risk of serious liver disease. Diabetologia. 2019;62(2):306–310. doi: 10.1007/s00125-018-4775-z. [DOI] [PubMed] [Google Scholar]
- 33.Retnakaran R. Hyperglycemia in pregnancy and its implications for a woman's future risk of cardiovascular disease. Diabetes Res Clin Pract. 2018;145:193–199. doi: 10.1016/j.diabres.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Retnakaran R., Shah B.R. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective population-based study. Lancet Diabetes Endocrinol. 2019;7:378–384. doi: 10.1016/S2213-8587(19)30077-4. [DOI] [PubMed] [Google Scholar]
- 35.Retnakaran R., Qi Y., Sermer M., Connelly P.W., Zinman B., Hanley A.J. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–677. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Retnakaran R., Qi Y., Connelly P.W., Sermer M., Hanley A.J., Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of LDL cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. 2010;95:4345–4353. doi: 10.1210/jc.2010-0361. [DOI] [PubMed] [Google Scholar]
- 37.Retnakaran R. The insulin-like growth factor axis: a new player in gestational diabetes? Diabetes. 2016;65(11):3246–3248. doi: 10.2337/dbi16-0048. [DOI] [PubMed] [Google Scholar]
- 38.Tisi D.K., Burns D.H., Luskey G.W., Koski K.G. Fetal exposure to altered amniotic fluid glucose, insulin and insulin-like growth factor binding protein 1 occurs prior to screening for gestational diabetes mellitus. Diabetes Care. 2011;34:139–144. doi: 10.2337/dc10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sovio U., Murphy H.R., Smith G.C. Accelerated fetal growth prior to diagnosis of gestational diabetes mellitus: a prospective cohort study of nulliparous women. Diabetes Care. 2016;39(6):982–987. doi: 10.2337/dc16-0160. [DOI] [PubMed] [Google Scholar]
- 40.Gunderson E.P., Quesenberry C.P., Jacobs D.R., Feng J., Lewis C.E., Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am J Epidemiol. 2010;172:1131–1143. doi: 10.1093/aje/kwq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedderson M.M., Darbinian J.A., Quesenberry C.P., Ferrara A. Pregravid cardiometabolic risk profile and risk for gestational diabetes mellitus. Am J Obstet Gynecol. 2011;205:55. doi: 10.1016/j.ajog.2011.03.037. e1-7. [DOI] [PubMed] [Google Scholar]
- 42.Retnakaran R., Shah B.R. Divergent trajectories of cardiovascular risk factors in the years before pregnancy in women with and without gestational diabetes mellitus: a population-based study. Diabetes Care. 2020;43(10):2500–2508. doi: 10.2337/dc20-1037. [DOI] [PubMed] [Google Scholar]
- 43.Retnakaran R., Shah B.R. Impact of pregnancy on the trajectories of cardiovascular risk factors in women with and without gestational diabetes. Diabetes Obes Metab. 2021;23(10):2364–2373. doi: 10.1111/dom.14479. [DOI] [PubMed] [Google Scholar]
- 44.Bruns D.E., Metzger B.E., Sacks D.B. Diagnosis of gestational diabetes mellitus will be flawed until we can measure glucose. Clin Chem. 2020;66(2):265–267. doi: 10.1093/clinchem/hvz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Sullivan J.B., Mahan C.M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 46.Coustan D.R., Dyer A.R., Metzger B.E. One-step or two-step testing for gestational diabetes mellitus: which is best? Am J Obstet Gynecol. 2021;225(6):634–644. doi: 10.1016/j.ajog.2021.05.009. [DOI] [PubMed] [Google Scholar]
- 47.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 48.Carpenter M.W., Coustan D.R. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 49.O'Sullivan J.B., Mahan C.M., Charles D., Dandrow R.V. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895–900. doi: 10.1016/s0002-9378(16)33833-9. [DOI] [PubMed] [Google Scholar]
- 50.National Institutes of Health Consensus Development Conference Statement: Diagnosing gestational diabetes mellitus, March 4–6, 2013. Obstet Gynecol. 2013;122:358–369. doi: 10.1097/AOG.0b013e31829c3e64. [DOI] [PubMed] [Google Scholar]
- 51.American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Obstetrics. American College of Obstetricians and Gynecologists ACOG practice bulletin no. 190 summary: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):406–408. doi: 10.1097/AOG.0000000000002498. [DOI] [PubMed] [Google Scholar]
- 52.Metzger B.E., Gabbe S.G., Persson B., et al. International associations of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization . World Health Organization; 2013. Diagnostic Criteria and Classification of Hyperglycemia First Detected in Pregnancy.https://apps.who.int/iris/handle/10665/85975 Accessed 15 October 2021. [PubMed] [Google Scholar]
- 54.American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 55.Bilous R.W., Jacklin P.B., Maresh M.J., Sacks D.A. Resolving the gestational diabetes diagnosis conundrum: the need for a randomized controlled trial of treatment. Diabetes Care. 2021;44:858–864. doi: 10.2337/dc20-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillay J., Donovan L., Guitard S., et al. Screening for gestational diabetes: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;326(6):539–562. doi: 10.1001/jama.2021.10404. [DOI] [PubMed] [Google Scholar]
- 57.Hillier T.A., Pedula K.L., Ogasawara K.K., et al. A pragmatic randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;384:895–904. doi: 10.1056/NEJMoa2026028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pastore I., Chiefari E., Vero R., Brunetti A. Postpartum glucose intolerance: an updated overview. Endocrine. 2018;59:481–494. doi: 10.1007/s12020-017-1388-0. [DOI] [PubMed] [Google Scholar]
- 59.Nielson K.K., Kapul A., Damm P., et al. From screening to postpartum follow-up-the determinants and barriers for gestational diabetes mellitus services, a systematic review. BMC Pregnancy Childbirth. 2014;14:41. doi: 10.1186/1471-2393-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Battarbee A.N., Yee L.M. Barriers to postpartum follow-up and glucose tolerance testing in women with gestational diabetes mellitus. Am J Perinatol. 2018;35(4):354–360. doi: 10.1055/s-0037-1607284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stuebe A.M., Rich-Edwards J.W., Willet W.C., et al. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294(20):2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 62.Shah B.R., Lipscombe L.L., Feig D.S., Low J.M. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG. 2011;118(12):1484–1490. doi: 10.1111/j.1471-0528.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 63.Bennett W.L., Ennen C.S., Carrese J.A., et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Womens Health (Larchmt) 2011;20(2):239–245. doi: 10.1089/jwh.2010.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goveia P., Canon-Montanez W., Santos D.P., et al. Lifestyle intervention for the prevention of diabetes in women with previous gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne) 2018;9:583. doi: 10.3389/fendo.2018.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li N., Yang Y., Cui D., et al. Effects of lifestyle intervention on long-term risk of diabetes in women with prior gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2021;22(1):e13122. doi: 10.1111/obr.13122. [DOI] [PubMed] [Google Scholar]
- 66.Knowler W.C., Barrett-Connor E., Fowler S.E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ratner R.E., Christophi C.A., Metzger B.E., et al. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle intervention. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aroda V.R., Christophi C.A., Edelstein S.L., et al. The effect of lifestyle intervention and metformin on preventing or delaying diabetes among women with and without gestational diabetes: the diabetes prevention program outcomes study 10-year follow-up. J Clin Endocrinol Metab. 2015;100(4):1646–1653. doi: 10.1210/jc.2014-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diabetes Prevention Program Research Group Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2019;42:601–608. doi: 10.2337/dc18-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim C., Newton K.M., Knopp R.H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 71.Pancer J., Wu N., Mahmoud I., Dasgupta K. Pharmacological intervention for diabetes after pregnancy prevention in women with prior gestational diabetes: a scoping review. Diabetes Res Clin Pract. 2020;160 doi: 10.1016/j.diabres.2020.107998. [DOI] [PubMed] [Google Scholar]
- 72.Buchanan T.A., Xiang A.H., Peters R.K., et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 73.Xiang A.H., Peters R.K., Kjos S.L., et al. Effect of pioglitazone on pancreatic beta-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55(2):517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Daniele G., Tura A., Dardano A., et al. Effects of treatment with metformin and/or sitagliptin on beta-cell function and insulin resistance in prediabetic women with previous gestational diabetes. Diabetes Obes Metab. 2020;22(4):648–657. doi: 10.1111/dom.13940. [DOI] [PubMed] [Google Scholar]
- 75.Hummel S., Beyerlein A., Pfirrmann M., et al. Efficacy of vildagliptin for prevention of postpartum diabetes in women with a recent history of insulin-requiring gestational diabees: a phase II, randomized, double-blind, placebo-controlled study. Mol Metab. 2018;9:168–175. doi: 10.1016/j.molmet.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Elkind-Hirsch K.E., Seidemann E., Harris R., et al. A randomized controlled trial of dapagliflozin and metformin, alone and combined, in overweight women after gestational diabetes mellitus. Am J Obstet Gynecol MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100139. [DOI] [PubMed] [Google Scholar]
- 77.Inzucchi S.E., Zinman B., Fitchett D., et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME Trial. Diabetes Care. 2018;41(2):356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 78.Perez-Ferre N., Del Valle L., Torrejon M.J., et al. Diabetes mellitus and abnormal glucose tolerance development after gestational diabetes: a three-year, prospective, randomized, clinical-based, mediterranean lifestyle interventional study with parallel groups. Clin Nutr. 2015;34(4):579–585. doi: 10.1016/j.clnu.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Shek N.W.M., Ngai C.S.W., Lee C.P., et al. Lifestyle modifications in the development of diabetes mellitus and metabolic syndrome in Chinese women who had gestational diabetes mellitus: a randomized interventional trial. Arch Gynecol Obstet. 2014;289(2):319–327. doi: 10.1007/s00404-013-2971-0. [DOI] [PubMed] [Google Scholar]
- 80.Hu G., Tian H., Zhang F., et al. Tianjin gestational diabetes mellitus prevention program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract. 2012;98(3):508–517. doi: 10.1016/j.diabres.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Wein P., Beischer N., Harris C., et al. A trial of simple versus intensified dietary modification for prevention of progression to diabetes mellitus in women with impaired glucose tolerance. Aust NZ J Obstet Gynaecol. 1999;39(2):162–166. doi: 10.1111/j.1479-828x.1999.tb03363.x. [DOI] [PubMed] [Google Scholar]