Graphical abstract
Keywords: Heavy metal, Baby food, Risk assessment, Arsenic, Cadmium, Mercury, Lead
Highlights
-
•
As, Cd and Pb detected in baby foods containing fruit, grain, and root vegetables.
-
•
Select product HIs exceeded 1 for As and Pb using conservative assumptions.
-
•
Cancer risks exceeded 10−6 and were driven by As from grain products.
-
•
Analysis revealed minimal risk under most scenarios using conservative assumptions.
Abstract
Recently, the U.S. House of Representatives reported on the presence of heavy metals in raw ingredients used in baby foods and in finished baby food products themselves. In light of these concerns, this study aimed to evaluate potential risks associated with the presence of heavy metals in baby food products. We analyzed 36 baby food samples representing four ingredient categories (fruit; leguminous vegetable; root vegetable; or grain) for arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb). We assessed the potential lifetime cancer and non-cancer health risks posed to infants and toddlers following daily consumption of these chemicals in each food type, based on established daily food-specific ingestion rates. Daily doses were compared against selected reference values and oral slope factors to determine non-cancer hazard indices (HIs) and lifetime cancer risks. Hazard indices indicated a potential for non-cancer risk (e.g., HIs > 1.0) under only a few exposure scenarios, including for As and Pb under selected product type and age/concentration assumptions. Increases in lifetime cancer risks for all analytes across the ingredient categories evaluated ranged from 3.75 × 10−5 to 5.54 × 10−5; cancer risks were primarily driven by As from grain products. Though a limited set of exposure scenarios indicated a potential for health risk, the exposure assumptions in this assessment were conservative, and the heavy metal concentrations we found in baby foods are similar to those observed in similar whole foods. Based on these findings and the limited scenarios under which risks were identified, this study indicates that an infant’s typical intake of baby food is unlikely to pose health risks from heavy metals above accepted tolerable risk levels under most exposure scenarios.
1. Introduction
In recent years, concerns have been raised about the presence of heavy metals and metalloids in baby foods sold in the United States [1,2]. More recently, multiple federal lawsuits have been brought against baby food manufacturers, alleging that exposure to “heavy metals” can negatively impact children’s development [3]. These lawsuits were filed shortly after the release of a 2021 U.S. House of Representatives Subcommittee on Economic and Consumer Policy report indicating that baby foods manufactured in the U.S. contain “elevated levels” of “heavy metals,” such as arsenic (As), cadmium (Cd), mercury (Hg), and lead (Pb) [3,4]. These “heavy metals” occur naturally in the environment, and may also enter the environment as industrial pollutants. They also may be introduced into the food supply via plant uptake, livestock consumption of contaminated water or food, and/or agricultural or manufacturing processes [5].
The term “heavy metals” is widely used in the scientific community, but lacks a standardized, authoritative definition. The U.S. Food and Drug Administration (FDA) defines and regulates the metals Hg Pb, and the metalloid As as “heavy metals” in food additive provisions [6]. These elements are also commonly referred to as “heavy metals” in the scientific literature [[7], [8], [9]]. In this publication, we refer to the metals Cd, Hg, Pb, and the metalloid As collectively as heavy metals.
Multiple organizations have detected heavy metals at varying concentrations in baby and toddler foods sold in the U.S. As part of the Total Diet Study (TDS), the FDA samples key foods, including various baby foods, in the U.S. and tests them for various elements, including As, Cd, Hg, and Pb [10]. Additionally, non-governmental organizations, such as Consumer Reports and Healthy Babies Bright Futures (HBBF), tested baby and toddler food samples collected in the U.S. for heavy metals in 2017 and 2019, respectively, focusing specifically on As, Cd, Hg, and Pb [2,11]. A total of 50 packaged foods were sampled in the Consumer Reports study, all of which had detectable concentrations of one or more of these heavy metals. Similarly, the HBBF study detected at least one heavy metal in 95 % of the 168 baby foods tested. Gardener et al. [12] tested 564 baby and toddler formulas and foods and found that 37 % and 57 % of products had detectable levels of Pb and Cd, respectively, with the highest levels of each metal detected in cereals, snacks, and kids’ meals.
Chronic oral exposures to the heavy metals highlighted by these studies (As, Cd, Hg, and Pb)are associated with cancer and adverse non-cancer health effects. Both As and Pb are designated as known human carcinogens when ingested. Oral exposures to As, Cd, Hg, and Pb have also been shown to induce non-cancer systemic and target organ toxicity, including neurological, reproductive, developmental, cardiovascular, hematological, gastrointestinal, renal, musculoskeletal, and dermal adverse health effects, depending on the element [[13], [14], [15], [16]].
The FDA has issued import alerts and draft and final guidance documents to industry detailing Pb and inorganic As levels not to be exceeded in select food products (e.g., infant rice cereal; juice; dried fruits; candy; and spices). More comprehensive guidance or regulations limiting heavy metal levels in food, however, have not been established or promulgated to date [[17], [18], [19], [20], [21], [22]]. Absent any authoritative limits for exposure to As and heavy metals in many foods transparently evaluating any health risk potential associated with these elements detected in food becomes critically important. Many studies have evaluated the presence of heavy metals in food and their associated health risks in general, but only a limited number of peer-reviewed studies have evaluated the exposure and subsequent health risks of select heavy metals in baby foods sold in the U.S. Using probabilistic risk assessment methods to evaluate the health risks of inorganic As in rice cereal consumed by U.S. infants and toddlers, Shibata et al. [23] concluded that median and upper bound consumption of rice cereal exceeded tolerable chronic non-cancer risk levels, but was within an acceptable cancer risk range. Gardener et al. [12] indicated that fewer than 7% of total solid baby food samples collected exceeded FDA and World Health Organization (WHO) limits for both Pb and Cd under high-consumption scenarios, and 0% and 14 % of the infant formulas exceeded the Pb and Cd limits, respectively. Similarly, in an analysis of FDA TDS Cd and Pb concentration data from 2014 to 2016, Spungen [24] demonstrated that dietary Pb exposure typically exceeds toxicity criteria when the upper bound of the data were used, whereas Cd exposures exceed toxicity criteria across upper and lower bounds using conservative toxicity criteria. Additional peer-reviewed publications have evaluated heavy metal exposures and their associated health risks from baby and toddler foods outside the U.S. Martins et al. [25], for example, assessed exposure of infants to total Hg concentrations in infant foods commercially available in Portugal, and found that its provisional tolerable weekly intake (PWTI) in foods other than fish and shellfish was not exceeded under any exposure condition. In their recent study, Gu et al. [26] measured concentrations of inorganic and total As in rice-based baby foods to estimate infant dietary exposure to As. They found that 75 % of samples exceeded maximum levels for As in the EU. Further, under high-consumption scenarios, exposure to As exceeded the benchmark dose lower confidence limit (BMDL), indicating potential for increased health risks associated with excess consumption of As in rice products. In a study of weaning formula in Spain, Camara-Martos et al. [27] performed a probabilistic assessment of Cd exposure, and determined that all exposure scenarios were below the associated PWTI.
Multiple non-peer-reviewed publications have also contributed to the current public understanding of this issue. In 2017, the Environmental Defense Fund (EDF) raised concerns about Pb in its analysis of 11 years of data from the FDA TDS, describing it as a “hidden health threat” in baby food [1]. The EDF did not evaluate human health risk, however, but instead characterized the hazard presence (i.e., the detection of one or more heavy metals). In its 2019 study, HBBF benchmarked the these metal concentrations in baby foods against FDA guidance when possible [11]. In addition, metal concentrations were benchmarked against these non-authoritative limits established by advocacy groups: 1 μg/kg Pb in all categories of food tested (EDF); 1 μg/kg Pb in fruit juices (American Academy of Pediatrics (AAP)); 1 μg/kg Cd and 3 μg/kg As in fruit juices (Consumer Reports); and 25 μg/kg As in infant rice cereal (HBBF). Most recently, in 2021, the U.S. House of Representatives Subcommittee on Economic and Consumer Policy released a report analyzing data provided by commercial baby food manufacturers, and found that the As, Cd, Hg, and Pb concentrations detected in baby foods or their raw ingredients were multiple times higher than the FDA’s bottled water standards [4]. This analysis, though, did not characterize children’s potential exposures to these hazards based on use patterns, but instead compared them to water intake benchmarks, which are inappropriate for characterizing risks associated with food. Additionally, in its 2018 analysis, Consumer Reports identified “troubling” findings, including the detection of “worrisome” levels of at least one of the heavy metals in the products tested. It noted that 15 of the sampled products would pose potential health risks to a child regularly eating one serving or fewer per day [2]. Consumer Reports, however, has not made its dataset publicly available in order to allow for an independent risk assessment.
Based on the available literature to date, few studies have evaluated health risk to children from multiple heavy metals across a variety of food types. The objective of this study was to understand potential health risks associated with heavy metals in specific food product categories, including fruits, grains, leguminous vegetables, and root vegetables, with the understanding that potential risks are likely to differ by food type. In this study, we performed a risk assessment of As, Cd, Hg, and Pb in purchased baby foods within these categories. We purchased food products, analyzed them for heavy metal concentrations, and conducted a complete risk assessment for consuming these foods during early childhood using the resulting concentrations and food type-specific intake rates. This study’s aim is to provide complete and transparent information on the health risks associated with consuming baby foods via established, authoritative risk assessment methods, so as to comprehensively address the recent and emerging concerns emerging about this issue.
2. Methods
2.1. Selection of samples
For this study, we selected infant and toddler foods made by three different manufacturers. Our inclusion criteria included foods that were both targeted toward children between the ages of four months and three years (i.e., stages one through four) and that also contained one or more primary ingredient(s) in the following categories: fruit (e.g., pear, peach, apple, and/or strawberry); leguminous vegetable (e.g., peas and/or green beans); root vegetable (e.g., sweet potatoes and/or carrots); and grain (e.g. rice and/or whole wheat). While dietary exposure to the analytes of interest is not limited to the specific food products or categories described herein, we concentrated on products within these ingredient categories because of their ubiquity in infant and young children’s diets [28]. Because one likely origin of heavy metal contamination in foods is plant uptake from water and soil, we identified the primary ingredient category as the variable of interest.
We purchased baby and toddler foods from five supermarket chain locations in and around Pittsburgh, Pennsylvania between December, 2018 and March, 2019. Purchased products included both organic and non-organic foods packaged in jars and pouches. We collected a total of 36 baby and toddler food samples. We procured three different organic or non-organic baby food products for each of four primary ingredient categories: fruits, leguminous vegetables, root vegetables, and grains. Three samples of each product type from three distinct lots were obtained in order to ensure that we captured potential variability in heavy metal concentrations within a product type. Table 1 provides additional detail on the samples, including primary ingredient categorization, brand, organic designation, packaging material, and sample size.
Table 1.
Baby and Toddler Food Samples.
| Sample ID | Primary Ingredient Category | Brand | Organic/Nonorganic | Packaging Material | Size |
|---|---|---|---|---|---|
| 1 | Fruit | Brand 1 | Organic | Plastic pouch | 3.5 oz. |
| 2 | Fruit | Brand 1 | Organic | Plastic pouch | 3.5 oz. |
| 3 | Fruit | Brand 1 | Nonorganic | Plastic container | 2 oz. |
| 4 | Grain | Brand 3 | Organic | Cardboard box | 8 oz. |
| 5 | Grain | Brand 1 | Nonorganic | Plastic container | 8 oz. |
| 6 | Grain | Brand 1 | Nonorganic | Plastic container | 8 oz. |
| 7 | Leguminous Vegetable | Brand 3 | Organic | Glass jar | 2.5 oz. |
| 8 | Leguminous Vegetable | Brand 1 | Nonorganic | Plastic container | 2 oz. |
| 9 | Leguminous Vegetable | Brand 1 | Nonorganic | Plastic container | 2 oz. |
| 10 | Root Vegetable | Brand 2 | Nonorganic | Glass jar | 4 oz. |
| 11 | Root Vegetable | Brand 3 | Organic | Glass jar | 4 oz. |
| 12 | Root Vegetable | Brand 1 | Organic | Plastic pouch | 3.5 oz. |
2.2. Analysis and quantification of As, Cd, Hg, and Pb in baby foods
To determine which elements to evaluate in baby foods, we considered common metals and metalloids characterized by laboratories, and narrowed the list by identifying specific hazards to which children may be sensitive, including reproductive and developmental hazards, carcinogenicity, and mutagenicity. For this screening, we specifically identified metals and metalloids on the California Office of Environmental Health Hazard Assessment (Cal/OEHHA) Proposition 65 list, as well as those that the European Chemicals Agency (ECHA) has designated as Category 1 or 2 carcinogens, mutagens, or reproductive toxicants. Upon identifying the metals and metalloids subset, we further excluded essential minerals (e.g., cobalt; chromium; nickel) and/or elements only identified as carcinogens due to inhalation effects (e.g., antimony; beryllium; selenium; vanadium). The resulting identified elements were As, Cd, Hg, and Pb. Previous studies have identified these elements and the importance of characterizing their risks to infants and young children [2,4,29].
After purchase, the 36 baby food samples were stored under temperature-controlled conditions in an office setting. In March, 2019, all samples were sent in their original, sealed packaging to AGQ Labs USA (Oxnard, CA, USA), an ISO-17025 accredited laboratory. Food samples were analyzed for total As, Cd, Hg, and Pb, using a heat-block assisted acid digestion and inductively-coupled plasma mass spectrometry (ICP-MS) method per the FDA Elemental Analysis Manual (EAM for Food and Related Products, Method 4.7 [30]). Briefly, samples were acid digested and heated using a heat block with half volume acid, and then subsequently diluted to 50 mL total volume. Samples were then analyzed for total As, Cd, Hg, and Pb via ICP-MS. The quantification range for As, Cd, and Pb was 0.010–25.0 mg/kg (or 10–25,000 μg/kg) and 0.010–2.50 mg/kg (or 10–2500 μg/kg) for Hg. The limit of detection (LOD) for As, Cd, Pb, and Hg was 0.003 mg/kg (or 3 μg/kg). Consistent with EPA guidance, non-detects (ND), or samples with concentrations below the LOD, were assumed to have concentrations of the analyte of interest at one-half of the LOD (0.0015 mg/kg, or 1.5 μg/kg) for risk assessment purposes [31]. Samples that were non-quantifiable (NQ), with instrument detections between the LOD and lower limit of quantitation (LLOQ, 0.010 mg/kg, or 10 μg/kg), were assumed to have concentrations of the analyte of interest equal to one-half of the LLOQ (0.005 mg/kg or 5 μg/kg). The uncertainty, which accounts for error in calibration, accuracy, precision, and percent recovery parameters, for As, Cd, Pb, and Hg were 6 %, 15 %, 8 %, and 15 %, respectively. For method validation, analysts at AGQ Labs USA followed the US FDA EAM guidance for method validation. The validation included the demonstration of several figures of merit, including accuracy, precision, sensitivity, selectivity, limit of detection, limit of quantification, linearity, range, and ruggedness of the method. The method used for this study was validated by the US FDA and a multi-laboratory validation.
2.3. Data analysis
In order to calculate exposure point concentrations for As, Cd, Hg, and Pb in each ingredient category, summary statistics (mean, median, and maximum concentrations as well as standard deviation) were generated. Figures for As, Cd, Hg, and Pb concentrations in baby food samples were prepared in GraphPad Prism version 9.1.2 (San Diego, CA). All ingredient categories were characterized by a relatively small sample size (N = 9), and across all analyzed elements and ingredient categories a majority of samples fell below the LOD. For these reasons, we were not able to fit a distribution to the data to identify the best central tendency measure for the risk assessment. Both mean and median concentrations were therefore used to calculate exposure point concentrations as central tendency measures. Similarly, the sample size and number below the LOD precluded us from calculating a valid 95 % upper confidence level. As such, we used maximum analyte concentrations to estimate potential upper-bound exposure concentrations.
2.4. Exposure and risk assessment
2.4.1. Exposure estimate
We estimated exposure to As, Cd, Hg, and Pb via baby food ingestion using deterministic methods for three different age groups: birth to <1 year; 1 year to <2 years; and 2 years to <3 years. Mean, median, and maximum analyte concentrations in each food category were used to calculate an average daily dose (ADD) in order to evaluate non-cancer health effects, using the following Eq. (1):
| (1) |
where ADD is the average daily dose (mg/kg-day), C is the respective mean, median, or maximum concentration of heavy metals in each food category (mg/g; see Table 4), and IR is the average daily intake rate for each ingredient category (g/kg-day; see Table 2).
Table 4.
As, Cd, Hg, and Pb Concentrations (μg/kg) in Baby Food Samples by Ingredient Category.
| Heavy Metal | Ingredient Category | No. of Samples | Detection Frequency n (%) | Concentration (μg/kg)a |
||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Mean | Median | Maximum | Error (±SD)b | ||||
| As | Fruit | 9 | 6 (67) | 1.5 | 3.8 | 5.0 | 5.0 | 1.8 |
| Grain | 9 | 9 (100) | 10.0 | 90.4 | 126.0 | 132.0 | 54.4 | |
| Leguminous Vegetable | 9 | 7 (78) | 1.5 | 4.2 | 5.0 | 5.0 | 1.5 | |
| Root Vegetable | 9 | 9 (100) | 5.0 | 10.8 | 12.0 | 22.0 | 5.4 | |
| Cd | Fruit | 9 | 3 (33) | 1.5 | 4.4 | 1.5 | 16.0 | 5.2 |
| Grain | 9 | 9 (100) | 12.0 | 25.8 | 20.0 | 61.0 | 16.9 | |
| Leguminous Vegetable | 9 | 0 (0) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 | |
| Root Vegetable | 9 | 6 (67) | 1.5 | 3.8 | 5.0 | 5.0 | 1.8 | |
| Hg | Fruit | 9 | 0 (0) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 |
| Grain | 9 | 0 (0) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 | |
| Leguminous Vegetable | 9 | 0 (0) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 | |
| Root Vegetable | 9 | 0 (0) | 1.5 | 1.5 | 1.5 | 1.5 | 0.0 | |
| Pb | Fruit | 9 | 3 (33) | 1.5 | 2.7 | 1.5 | 5.0 | 1.8 |
| Grain | 9 | 9 (100) | 5.0 | 9.7 | 5.0 | 20.0 | 7.0 | |
| Leguminous Vegetable | 9 | 2 (22) | 1.5 | 2.3 | 1.5 | 5.0 | 1.5 | |
| Root Vegetable | 9 | 8 (88) | 1.5 | 15.8 | 5.0 | 48.0 | 15.6 | |
Values below the lower limit of quantitation (LLOQ) were replaced with ½ the LLOQ (10 μg/kg), 5 μg/kg.
Values below the limit of detection (LOD) were replaced with ½ the LOD (3 μg/kg), 1.5 μg/kg.
SD = Standard Deviation.
Table 2.
Mean Baby Food Ingestion Exposure Parameters.
| Parameter | Age Group |
Reference | |||
|---|---|---|---|---|---|
| <1 year | 1-<2 years | 2-<3 years | |||
| BW: Body Weight (kg) | 6.825 | 11.4 | 13.8 | Table 8–1 [32] | |
| ED: Exposure Duration (days) | 365 | – | |||
| AT: Averaging Time (days) | 25,550 | – | |||
| IR: Consumer-Only Mean Intake Rate (g/kg-day) | Fruit | 9.9 | 9.8 | 7.7 | Table 9–1: Mean values for Total Fruits [33] |
| Grain | 3.9 | 6.4 | 6.4 | Table 12-1: Mean values for Total Grains [34] | |
| Leguminous Vegetable | 2.73 | 3.31 | 1.49 | Table 9–6: Mean values for Legumes [33] | |
| Root Vegetable | 3.62 | 2.9 | 2.64 | Table 9–6: Mean values for Root Tuber Vegetables [33] | |
In addition, we calculated a lifetime average daily dose (LADD) to assess lifetime cancer risk, using mean, median, and maximum analyte concentrations according to the following Eq. (2):
| (2) |
where LADD is the lifetime average daily dose (mg/kg-day); C is the respective mean, median, or maximum heavy metal concentration (mg/g; see Table 4); IR is the average daily intake rate for the respective ingredient category (g/kg-day; see Table 2); ED is the exposure duration over which the infant/child in each age group consumed baby foods (days); and AT is the averaging time (i.e., the period over which the exposure is averaged (a 70-year lifetime equates to 25,550 days)).
Age- and mean ingredient-specific intake rates, compiled from the U.S. Environmental Protection Agency’s (EPA) Exposure Factors Handbook, are based on U.S. dietary survey data collected between 2005 and 2010 [[32], [33], [34]]. Considerable variability exists in the timing of the transition from breast milk or formula to solid foods among infants and young children between <1 and 3 years old, particularly within the first year [[35], [36], [37]]. As such, consumer-only mean intake rates, representing only those individuals who reported eating the food item during the survey period, were selected as conservative food consumption estimates not biased downward by survey participants who did not consume the foods of interest. These exposure parameters are presented by age group and ingredient category in Table 2 below.
2.4.2. Toxicity criteria identification
We compared the estimated daily exposure for each analyte to available cancer and non-cancer-based oral toxicity criteria or health guidelines. The cancer criteria result from applying low-dose extrapolation procedures, and are presented as the cancer risk per mg/kg/day [38]. The non-cancer criteria are estimates of daily exposure likely to be without an appreciable risk of deleterious health effects, based on the most sensitive endpoint(s) [38].
In accordance with EPA recommendations for selecting toxicity criteria, cancer and non-cancer criteria from EPA’s Integrated Risk Information System (IRIS) were selected when available. When IRIS values were not available, we selected current, transparent, peer-reviewed toxicity criteria from authoritative sources, such as the European Food Safety Authority (EFSA) or Cal/OEHHA [39]. As described herein, the heavy metal analyses estimated the total elemental concentrations, and did not distinguish between organic and inorganic chemical forms The toxicity criteria for both As and Hg, however, are differentiated by chemical form (i.e., organic and inorganic). Both the organic and inorganic forms of As and Hg are found in various foodstuffs. Organic As and Hg are more commonly found in fish and aquatic plants, representing a major route of human exposure to these forms. Comparatively, inorganic As and Hg are the predominant types found in foods other than seafood [[40], [41], [42], [43]]. As such, we selected toxicity criteria associated with the inorganic forms of these elements. In the case of As, applying inorganic As toxicity criteria yields conservative risk estimates, in the event that both types are present in the sample concentrations. Table 3 depicts the selected cancer and non-cancer toxicity criteria for each heavy metal, including the issuing agency and tumor type or most sensitive target organ or system, as applicable.
Table 3.
Oral Toxicity Criteria for As, Cd, Hg, and Pb.
| Heavy Metal | Cancer |
Non-Cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Criterion | Value | Unit | Tumor Type | Reference | Criterion | Value | Unit | Most Sensitive Target Organ/ System | Reference | |
| As, inorganic | EPA IRIS OSF | 1.50E+00 | (mg/kg/day)−1 | Splenic sarcoma | [38] | EPA IRIS RfD | 3.00E-04 | mg/kg/day | Cardiovasculara, Dermalb | [38] |
| Cd | NA | NA | NA | NA | NA | EPA IRIS RfD | 1.00E-03 | mg/kg/day | Urinaryc | [44] |
| Hg, inorganic | NA | NA | NA | NA | NA | EFSA TDI | 5.70E-04 | mg/kg/day | Kidneyd | [45] |
| Pb | Cal/ OEHHA OSF | 8.50E-03 | (mg/kg/day)−1 | Kidney tumors | [46] | Cal/OEHHA MADL | 5.00E-04 | mg/day | Reproductive | [47] |
Key: EPA = U.S. Environmental Protection Agency; IRIS = Integrated Risk Information System; OSF = Oral Slope Factor; RfD = Reference Dose; EFSA = European Food Safety Authority; TDI = Tolerable Daily Intake, calculated by dividing the Tolerable Weekly Intake (TWI) by 7 days/week; Cal/OEHHA = California Office of Environmental Health Hazard Assessment; MADL = Maximum Allowable Dose Level.
Possible vascular complications.
Hyperpigmentation and keratosis.
Significant proteinuria.
Kidney weight change.
2.4.3. Non-cancer risk
To estimate the non-cancer health risk of ingesting heavy metals via baby food consumption, we calculated a hazard quotient (HQ) for As, Cd, and Hg using the standard EPA methodology, as depicted in the following Eq. (3) [48]:
| (3) |
where ADD is the average daily dose (mg/kg-day), the RfD is the reference dose (mg/kg-day), and the TDI is the tolerable daily intake (mg/kg-day). For Pb, we calculated the HQ using the Eq. (4) below, to account for body weight:
| (4) |
where ADD is the average daily dose (mg/kg-day), BW is the age-specific body weight (kg; see Table 2), and MADL is the Maximum Allowable Dose Level (mg/day) established by Cal/OEHHA.
We used hazard indices (HIs) to evaluate the exposure effect from multiple ingredient categories. HIs were calculated by summing HQs for each analyte and age group across ingredient categories, using the Eq. (5) below. Because the RfDs for As, Cd, Hg, and Pb are based on adverse effects on different target organs (Table 3), HQs were not summed across analytes, since the resulting health risks may not be cumulative. HQs and cumulative HIs exceeding 1.0 indicate a potential for human health risk from the associated exposure(s).
| (5) |
2.4.4. Cancer risk
Cancer health risks represent the probability of developing cancer from exposure to a given chemical at a given concentration [48]. The incremental probability of developing cancer (i.e., the theoretical excess cancer risk, or increased lifetime cancer risk) is the additional risk above the cancer risk an individual would face absent the exposures characterized in this study. We calculated the lifetime cancer risks (LCRs) for As and Pb from consuming these foods using the following Eq. (6):
| (6) |
where LADD is the lifetime average daily dose (mg/kg-day) and OSF is the oral slope factor (mg/kg-day)−1. LCRs were summed across all age groups, analytes, and ingredient categories in order to calculate cumulative lifetime cancer risks associated with baby food consumption.
3. Results
3.1. Metal and Metalloid Analyses and Exposure Estimates for Baby Foods
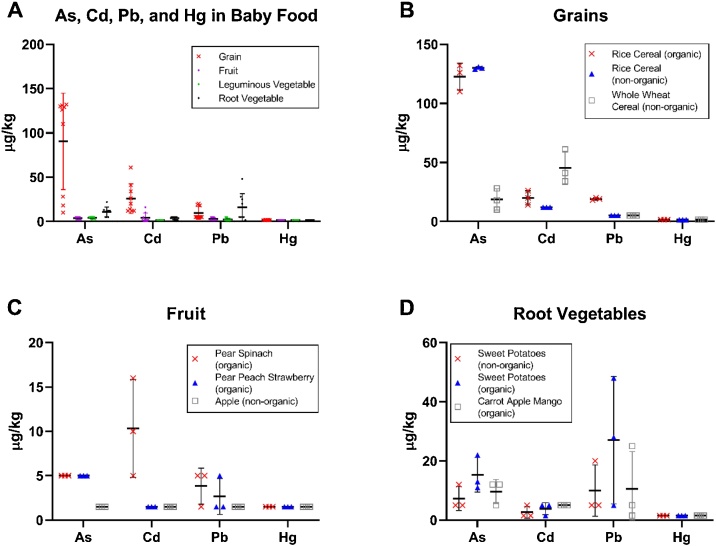
Table 4 presents heavy metal detection frequencies, as well as minimum, mean, median, and maximum concentrations across food categories. As, Cd, and Pb were each detected in samples within at least two ingredient categories, while Hg was not detected in any samples. As was detected in 100 % of grain samples, and had the highest reported mean (90.4 μg/kg), median (126.0 μg/kg), and maximum (132.0 μg/kg) concentrations as compared to the other ingredient categories. As was also detected in 100 %, 67 % and 78 % of the root vegetable, fruit and leguminous vegetable product samples, respectively. Cd was detected in 100 % of grain product samples, resulting in mean and median concentrations of 25.8 μg/kg and 20.0 μg/kg, respectively (range: 12.0–61.0 μg/kg). Cd was detected 33 % of fruit product samples and 67 % of root vegetable samples. The mean and maximum Cd concentrations in the fruit product samples were 4.4 μg/kg and 16.0 μg/kg, respectively, and the mean and maximum Cd concentrations in the root vegetable product samples were 3.8 μg/kg and 5.0 μg/kg, respectively. The minimum and median Cd concentrations in fruit and root vegetable products were non-detected (1.5 μg/kg) or non-quantifiable (5.0 μg/kg). No Cd detections were reported in the leguminous vegetable samples.
Pb was detected in both grain (100 %) and root vegetable products (88 %). The mean and maximum Pb concentrations were highest in the root vegetable ingredient category, with respective values of 15.8 μg/kg and 48.0 μg/kg. The mean and maximum concentrations in the grain product samples were 9.7 μg/kg and 20.0 μg/kg, respectively. Median Pb concentrations in both ingredient categories were non-detected (1.5 μg/kg) or non-quantifiable (5.0 μg/kg). Pb was also detected in the fruit (33 %) and leguminous vegetable (22 %) products. The mean and maximum Pb concentrations in the fruit product samples were 2.7 μg/kg and 5.0 μg/kg, respectively, and the mean and maximum Pb concentrations in the leguminous vegetable product samples were 2.3 μg/kg and 5.0 μg/kg, respectively. The minimum and median Pb concentrations in fruit and leguminous vegetable products were non-detected (1.5 μg/kg) or non-quantifiable (5.0 μg/kg).
Fig. 1A illustrates the distribution of each heavy metal in each ingredient category. Specifically, this figure demonstrates that grain and root vegetable ingredient categories had higher median concentrations, with larger ranges relative to fruit and leguminous vegetable ingredient categories for As, Cd, and Pb. Additional detail is provided for the grain (Fig. 1B), fruit (Fig. 1C), and root vegetable (Fig. 1D) ingredient categories.
Fig. 1.
Determination of As, Cd, Pb, and Hg in Baby Foods. Heavy metal concentrations depicted by (A) All Ingredient Categories, (B) Grain Products, (C) Fruit Products, and (D) Root Vegetable Products. Individual concentrations are plotted for each ingredient category (A) or subcategory (B-D), with mean concentrations depicted by a black horizontal bar. The Leguminous Vegetable Ingredient Category was not depicted, as all detections (n = 9) were between the LOD and LLOQ.
Table 5, Table 6 present the heavy metal ADDs and LADDs for each age group and ingredient category, calculated using mean, median, and maximum metal concentrations.
Table 5.
As, Cd, Hg, and Pb Average Daily Doses (μg/kg-day) for Children Ages <1 to <3 Years Consuming Baby Foods.
| Heavy Metal | Ingredient Category | Age Categories |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1 year |
1-<2 years |
2-<3 years |
||||||||
| Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | ||
| As | Fruit | 2.59E-01 | 3.38E-01 | 3.38E-01 | 4.28E-01 | 5.59E-01 | 5.59E-01 | 4.07E-01 | 5.31E-01 | 5.31E-01 |
| Grain | 2.41E+00 | 3.35E+00 | 3.51E+00 | 6.60E+00 | 9.19E+00 | 9.63E+00 | 7.99E+00 | 1.11E+01 | 1.17E+01 | |
| Leguminous Vegetable | 7.87E-02 | 9.32E-02 | 9.32E-02 | 1.59E-01 | 1.89E-01 | 1.89E-01 | 8.68E-02 | 1.03E-01 | 1.03E-01 | |
| Root Vegetable | 2.66E-01 | 2.96E-01 | 5.44E-01 | 3.56E-01 | 3.97E-01 | 7.27E-01 | 3.93E-01 | 4.37E-01 | 8.02E-01 | |
| Cd | Fruit | 3.00E-01 | 1.01E-01 | 1.08E+00 | 4.97E-01 | 1.68E-01 | 1.79E+00 | 4.72E-01 | 1.59E-01 | 1.70E+00 |
| Grain | 6.86E-01 | 5.32E-01 | 1.62E+00 | 1.88E+00 | 1.46E+00 | 4.45E+00 | 2.28E+00 | 1.77E+00 | 5.39E+00 | |
| Leguminous Vegetable | 2.79E-02 | 2.79E-02 | 2.79E-02 | 5.66E-02 | 5.66E-02 | 5.66E-02 | 3.08E-02 | 3.08E-02 | 3.08E-02 | |
| Root Vegetable | 9.47E-02 | 1.24E-01 | 1.24E-01 | 1.27E-01 | 1.65E-01 | 1.65E-01 | 1.40E-01 | 1.82E-01 | 1.82E-01 | |
| Hg | Fruit | 1.01E-01 | 1.01E-01 | 1.01E-01 | 1.68E-01 | 1.68E-01 | 1.68E-01 | 1.59E-01 | 1.59E-01 | 1.59E-01 |
| Grain | 3.99E-02 | 3.99E-02 | 3.99E-02 | 1.09E-01 | 1.09E-01 | 1.09E-01 | 1.32E-01 | 1.32E-01 | 1.32E-01 | |
| Leguminous Vegetable | 2.79E-02 | 2.79E-02 | 2.79E-02 | 5.66E-02 | 5.66E-02 | 5.66E-02 | 3.08E-02 | 3.08E-02 | 3.08E-02 | |
| Root Vegetable | 3.71E-02 | 3.71E-02 | 3.71E-02 | 4.96E-02 | 4.96E-02 | 4.96E-02 | 5.46E-02 | 5.46E-02 | 5.46E-02 | |
| Pb | Fruit | 1.80E-01 | 1.01E-01 | 3.38E-01 | 2.98E-01 | 1.68E-01 | 5.59E-01 | 2.83E-01 | 1.59E-01 | 5.31E-01 |
| Grain | 2.57E-01 | 1.33E-01 | 5.32E-01 | 7.05E-01 | 3.65E-01 | 1.46E+00 | 8.54E-01 | 4.42E-01 | 1.77E+00 | |
| Leguminous Vegetable | 4.24E-02 | 2.79E-02 | 9.32E-02 | 8.59E-02 | 5.66E-02 | 1.89E-01 | 4.68E-02 | 3.08E-02 | 1.03E-01 | |
| Root Vegetable | 3.91E-01 | 1.24E-01 | 1.19E+00 | 5.23E-01 | 1.65E-01 | 1.59E+00 | 5.77E-01 | 1.82E-01 | 1.75E+00 | |
Table 6.
As, Cd, Hg, and Pb Lifetime Average Daily Doses (μg/kg-day) for Children Ages <1 to <3 Years Consuming Baby Foods.
| Age Categories |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Heavy Metal | Ingredient Category | <1 year |
1-<2 years |
2-<3 years |
||||||
| Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | Mean (μg/kg-day) | Median (μg/kg-day) | Max (μg/kg-day) | ||
| As | Fruit | 3.70E-03 | 4.83E-03 | 4.83E-03 | 6.12E-03 | 7.98E-03 | 7.98E-03 | 5.82E-03 | 7.59E-03 | 7.59E-03 |
| Grain | 3.44E-02 | 4.79E-02 | 5.02E-02 | 9.43E-02 | 1.31E-01 | 1.38E-01 | 1.14E-01 | 1.59E-01 | 1.67E-01 | |
| Leguminous Vegetable | 1.12E-03 | 1.33E-03 | 1.33E-03 | 2.28E-03 | 2.70E-03 | 2.70E-03 | 1.24E-03 | 1.47E-03 | 1.47E-03 | |
| Root Vegetable | 3.80E-03 | 4.24E-03 | 7.76E-03 | 5.09E-03 | 5.67E-03 | 1.04E-02 | 5.61E-03 | 6.25E-03 | 1.15E-02 | |
| Cd | Fruit | 4.29E-03 | 1.45E-03 | 1.54E-02 | 7.09E-03 | 2.39E-03 | 2.55E-02 | 6.75E-03 | 2.28E-03 | 2.43E-02 |
| Grain | 9.80E-03 | 7.61E-03 | 2.32E-02 | 2.69E-02 | 2.08E-02 | 6.36E-02 | 3.25E-02 | 2.52E-02 | 7.70E-02 | |
| Leguminous Vegetable | 3.99E-04 | 3.99E-04 | 3.99E-04 | 8.09E-04 | 8.09E-04 | 8.09E-04 | 4.41E-04 | 4.41E-04 | 4.41E-04 | |
| Root Vegetable | 1.35E-03 | 1.76E-03 | 1.76E-03 | 1.81E-03 | 2.36E-03 | 2.36E-03 | 2.00E-03 | 2.60E-03 | 2.60E-03 | |
| Hg | Fruit | 1.45E-03 | 1.45E-03 | 1.45E-03 | 2.39E-03 | 2.39E-03 | 2.39E-03 | 2.28E-03 | 2.28E-03 | 2.28E-03 |
| Grain | 5.70E-04 | 5.70E-04 | 5.70E-04 | 1.56E-03 | 1.56E-03 | 1.56E-03 | 1.89E-03 | 1.89E-03 | 1.89E-03 | |
| Leguminous Vegetable | 3.99E-04 | 3.99E-04 | 3.99E-04 | 8.09E-04 | 8.09E-04 | 8.09E-04 | 4.41E-04 | 4.41E-04 | 4.41E-04 | |
| Root Vegetable | 5.29E-04 | 5.29E-04 | 5.29E-04 | 7.08E-04 | 7.08E-04 | 7.08E-04 | 7.81E-04 | 7.81E-04 | 7.81E-04 | |
| Pb | Fruit | 2.57E-03 | 1.45E-03 | 4.83E-03 | 4.26E-03 | 2.39E-03 | 7.98E-03 | 4.05E-03 | 2.28E-03 | 7.59E-03 |
| Grain | 3.68E-03 | 1.90E-03 | 7.61E-03 | 1.01E-02 | 5.21E-03 | 2.08E-02 | 1.22E-02 | 6.31E-03 | 2.52E-02 | |
| Leguminous Vegetable | 6.06E-04 | 3.99E-04 | 1.33E-03 | 1.23E-03 | 8.09E-04 | 2.70E-03 | 6.69E-04 | 4.41E-04 | 1.47E-03 | |
| Root Vegetable | 5.59E-03 | 1.76E-03 | 1.69E-02 | 7.48E-03 | 2.36E-03 | 2.27E-02 | 8.24E-03 | 2.60E-03 | 2.50E-02 | |
3.2. Non-cancer risk
Table 7 presents the As, Cd, Hg, and Pb non-cancer HQs and HIs for each ingredient category and age range. We calculated HQs (unitless) using the mean, median, and maximum analyte concentrations reported in Table 4, in accordance with Eqs. (3) and (4) above. HQs for As exceeded 1.0 for children in all age groups consuming grain products represented by mean, median, and maximum concentrations. For each concentration, As HQs were lower for children <1 year old, compared to the two older age groups, which had identical HQs. Across age groups, consuming grain products represented by mean As concentrations resulted in lower HQs than median concentrations. In children <1 year old, the HQs ranged from 1.18 (based on the mean As concentration) to 1.72 (based on the maximum As concentration). HQs ranged from 1.93 to 2.82 for the two older age groups. Regarding As non-cancer health risks from consuming all ingredient category products, HIs ranged from 1.47 in children <1 year (based on the mean As concentration) to 3.25 in children ages 1 to <2 years (based on the maximum As concentration). Cumulative HIs for As were driven by HQs for grain products.
Table 7.
Non-Cancer Hazard Quotients for Children Ages <1 to <3 Years Consuming Baby Foods Containing As, Cd, Hg, and Pb.
 |
Note: HQs and HIs >1 are shaded in grey; HQs and HIs derived from mean, median, and max concentrations are provided in the table.
Pb HQs exceeded 1.0 for a subset of age ranges for fruit, grain, and root vegetable products under at least one scenario (e.g., mean or maximum concentration). HQ exceedances ranged from 1.06 to 1.12 for children ages 1 to <3 years consuming only fruit products containing the maximum Pb concentrations. The lower HQ was reported for the older age group. The fruit product HQs were calculated using only Pb concentrations below the LLOQ. HQs calculated for children consuming grain and root vegetable products demonstrated a contrasting trend of higher HQs reported for the older age group. Grain and root vegetable product HQs derived using mean and maximum Pb concentrations were greater than 1.0 for children ages 1 to <3 years old, and were higher in the older population. Comparatively, consuming grain and root vegetable products represented by median Pb concentrations resulted in HQs less than 1.0 for these ages. For children <1 year, HQs only exceeded 1.0 for consuming grain and root vegetable products containing the maximum Pb concentrations. Pb HI exceedances, accounting for consuming all ingredient category products, ranged from 1.51 for children 1 to <2 years (based on the median Pb concentration) to 8.30 for children 2 to <3 years (based on the maximum Pb concentration).
Consuming Cd- and Hg-containing baby foods across all ingredient categories did not result in increased non-cancer risks for any age group at even the maximum concentrations.
3.3. Cancer risk
Table 8 summarizes lifetime cancer risks for children ages <1 to <3 years from consuming As- and Pb-containing baby foods. We calculated lifetime cancer risks using the mean, median, and maximum metal concentrations reported in Table 4, in accordance with Eq. (6). Increased cancer risk for general population exposures is typically characterized as cumulative cancer risk above 1 in 1,000,000, or 10−6 (unitless). Consuming mean, median, and maximum As concentrations in fruit, grain, and root vegetable products resulted in cancer risks greater than 10−6. Total cancer risks from As across all ingredient categories ranged from 3.74 × 10-5 (based on the mean As concentration) to 5.53 × 10-5 (based on the maximum As concentration). Comparatively, cancer risks from consuming Pb across all food categories were well below the threshold of 10−6, ranging from 2.21 × 10-8 to 1.15 × 10-7. Combined lifetime cancer risks from consuming As and Pb across all ingredient categories were driven by As concentrations, and ranged from 3.75 × 10-5 (based on the mean concentrations) to 5.54 × 10-5 (based on the maximum concentrations).
Table 8.
Lifetime Cancer Risks (LCRs) for Children Ages <1 to <3 Years Consuming Baby Foods Containing As, Cd, Hg, and Pb.
 |
Note: CRs >10−6 are shaded in grey. LCRs using the mean, median, and max sample concentrations are provided in the table.
4. Discussion
Recently, public interest concerning heavy metals in baby foods has grown, thanks in part to widely publicized studies from Consumer Reports and the U.S. House of Representatives [2,4]. These studies, however, have been limited in their interpretation by: 1) reporting only concentrations in ingredients or finished products, and not calculating health risk; 2) comparing measured concentrations to inappropriate screening values in an effort to estimate risk (e.g., comparing food concentrations to drinking water limits); or 3) not providing details on risk assessment assumptions, preventing transparency. This risk assessment provides a transparent and conservative estimate of potential health risk to enhance understanding of this issue.
The analyses in this study focused on measuring As, Cd, Hg, and Pb levels in different baby food types (e.g., fruits, grains, root vegetables, and leguminous vegetables). Previous studies identified these heavy metals as chemicals of concern for children’s exposures, owing either to their concentrations in foods or to childhood-specific hazards [2,4,11,29]. We used results of these analyses to estimate non-cancer and cancer health risks for children <1 year, 1 to <2 years, and 2 to <3 years, along with cumulative cancer risks across all ages. Based on these analyses, human health risks, including non-cancer and cancer (where applicable), are not expected from Cd or Hg exposure, based on the concentrations measured in these baby food types. This finding is consistent with the existing available literature regarding these metals [12,25,27,49,50]. The As and Pb concentrations reported in certain product types, however, may represent potential health risks under the exposure assumptions used in this assessment.
4.1. Arsenic
Health risks associated with As were not identified from exposure to fruit or vegetable (root or leguminous) products. Overall, non-cancer and cancer risks from As are driven by its presence in grain products. This finding is consistent with the published literature, which indicates that both non-cancer and cancer risks are associated with arsenic in rice-based products [[51], [52], [53], [54]]. Such conclusions, though, may differ by rice type and source, and/or the risk assessment’s underlying assumptions [53,55]. Elevated concentrations of As in grain products, particularly rice-based products, is common, owing to its natural occurrence in soil [26,53,54,[56], [57], [58], [59]]. In the most recent FDA TDS, rice and rice-based products were among the products listed with the highest measured As concentrations [10]. As was detected in all white rice samples and in 97 % of crisped rice cereal samples, with mean concentrations of 66 μg/kg and 159 μg/kg, respectively. Of the three grain-based baby foods evaluated in this study, two were rice-based. The rice-based products in this study contained much higher As concentrations than did the wheat-based product (Fig. 1B), and therefore accounted for the increased risk from As associated with the grain-based products. As concentrations in these rice products exceed the guidance value established by the FDA for rice-based cereal and baby food of 100 μg/kg [19]. Gu et al. [26] found that 75 % of all rice-based baby foods had As concentrations above 100 μg/kg in Australia, indicating this phenomenon is common. Although both non-cancer and cancer risks associated with As could be anticipated based on other studies of As in rice-based products, the risks herein are likely overestimated because of the selected exposure assumptions and guidance values. The assumptions regarding daily intake of grains used in this assessment are based on total grain consumption [34]. In its risk assessment of rice and rice products for <1 year olds, the FDA assumed ingestion rates of 0.664 and 0.925 g/kg/d for rice-based cereal and all rice products, respectively [60]. Comparatively, we assumed that a child’s consumption of grain-based baby foods was equivalent to his or her total daily grain consumption across grain products (e.g., 3.9 g/kg-d for < 1 year olds). Because the selected grain products were primarily rice-based cereals that represented the highest As concentrations in grain-based baby foods, using consumption rates for all grains will overestimate daily As exposure associated with any specific grain product type (including rice). In addition to overestimating the mass of consumption for any specific grain product, this risk assessment also conservatively assumed all As measured in the samples was inorganic, as no differentiation of organic and inorganic As was conducted. Previous researchers have demonstrated that inorganic As represents only a fraction of total As in grain products, including rice-based products, though the fraction of inorganic As ranges widely, depending on the study [51,[56], [57], [58],61,62]. FDA estimated that inorganic As could range from 12 % to 100 % of total As in rice and rice products [62]. Because organic As offers different hazard and dose response profiles than inorganic forms, and because only inorganic As screening values were used in this risk assessment, this risk assessment offers a worst-case prediction of human health risk from inorganic As. Risks would be reduced if some As in the products were organic.
In addition to the uncertainties regarding the estimated exposure to As, uncertainties also exist regarding selecting screening values to use in As-based risk assessments. For cancer-based risk assessments, we relied on an oral slope factor from EPA IRIS, which is more conservative than those used by the EPA to establish drinking water standards, or by the. FDA to evaluate risk associated with rice and rice products [60]. In establishing the slope factor under IRIS, EPA acknowledged that uncertainty exists regarding the potency of As carcinogenicity [38]. One primary uncertainty associated with the slope factor is related to assumptions regarding consuming As in food. The underlying study providing the basis for the slope factor involves exposure to contaminated drinking water. In modeling the data, the. EPA assumed that 2 μg/d of As from food contributed to the overall As exposure. These data, however, were not based on empirical information provided in the study, and therefore have a high level of uncertainty. EFSA relies on an alternate screening value to determine inorganic As risk from foods of 0.3 to 8 μg/kg/d. Average daily As exposure from baby food in the current study was comparable to the EFSA screening range, estimated to range from 0.1 to 11.7 μg/kg/d [63]. This result is consistent with several other studies that indicate exposures to As in rice products are at or below the EFSA screening range [51,52,64].
Collectively, these inherent conservatisms in the risk assessment are likely to overpredict non-cancer and cancer risks associated with As exposure in baby foods. Overall, exceedances of non-cancer screening values for As were modest. HIs for all scenarios, including risks based on maximum As concentration in any sample, were 3.25 or lower. For cancer risk, cumulative risks from As ranged from 3.74 × 10−5 to 5.53 × 10−5, which would be considered an increased risk for general population exposures by most agencies. Although both non-cancer and cancer risk estimates for As in grain-based baby foods are likely to be overestimated, and daily exposure estimates are within acceptable ranges as defined by EFSA, this study provides additional evidence that further investigating As in rice-based baby foods may be warranted. In response to EU imposed As limits in rice-based baby foods (100 μg/kg, similar to FDA’s guideline), the presence of mixed cereal types (containing rice and another grain) has increased in the UK market [65]. At the time of its 2016 risk assessment, FDA reported that 53%–62% of infant rice cereal products exceeded the 100 μg/kg limit [60]. For concerned parents, limiting children’s rice-based product consumption remains a possible mitigation strategy. The. FDA has recommended feeding infants a variety of grain-based cereals in order to limit heavy metal exposure from food [66].
4.2. Lead
For Pb, potential non-cancer health risks were observed in fruit, grain, and root vegetable ingredient categories. Across all product categories, Pb was only detected in selected products (mostly grains and root vegetables), consistent with Gardener et al. [12], who reported 37 % detection frequency for Pb across all baby food products, with the highest concentrations found in rice products. Pb was not detected in any evaluated fruit-based baby foods, and risks were therefore determined based only on the LOD and LLOQ. As such, the HQs may be substantially overestimated for fruits (the exceedance was already small, with HQs ranging from 1.06 to 1.12). For grain-based baby foods, Pb was measured above the LLOQ in one product (an organic rice-based cereal), and screening values were exceeded only when assuming mean or maximum Pb concentrations for all grains (Fig. 1B). As with As, this risk assessment assumed that ingesting only grain-based baby foods accounted for a child’s total daily grain consumption. Pb was not detected in most rice cereal samples in the TDS (non-detect in 94 %). Maximum Pb concentrations in TDS products was 13 μg/kg. The maximum Pb concentration detected in grain products in this study was 20 μg/kg, while the mean concentration for all grain products was 9.7 μg/kg (approximating the LLOQ in this study and the range of TDS LODs).
For root vegetable products, Pb was detectable in selected sweet potato and carrot-based product samples, and screening values were exceeded only when assuming Pb concentrations at the mean or maximum (HQs ranged from 1.05 to 3.5). Pb was not detected in all lots of these products (Fig. 1D), suggesting variability in Pb concentration, even within one product. Risk estimates based on the maximum measured concentration are therefore likely to overestimate risk, even in instances in which parents feed their children a single product brand. Risk estimates based on mean concentration are more likely to approximate actual risk to children. Although exposures at the mean concentration may exceed the screening value in this risk assessment, the exceedance is quite small (HQs ranged from 1.05 to 1.15). Given the conservative assumptions used in this risk assessment, then, this risk is unlikely to be appreciable.
The Cal/OEHHA MADL of 0.5 μg/d was used to evaluate non-cancer health risk for Pb. In the 1990s, the FDA established a provisional tolerable total daily intake (PTTDI) level of 6 μg/d for Pb in young children [67]. In 2018, the FDA reduced its daily intake level for Pb in food from 6 μg/d to an interim limit of 3 μg/d for children, corresponding to the Pb level that would result in a blood Pb level of 5 μg/dL, the level at which clinical monitoring is recommended [67]. All daily dose estimates in this study were well below the FDA limit of 3 μg/d. The highest daily dose predicted by this study from Pb in baby food is 1.77 μg/d. Because it uses the most sensitive screening value available, this risk assessment may overestimate Pb risk from baby foods. The FDA does not have recommended or enforceable Pb limits in vegetables, although it has provided recommendations for other food products, including candy, dried fruits (for import), and fruit juices. These guidance values are all 50 μg/kg or higher, and the Pb concentrations in all baby products tested herein are below 50 μg/kg. This study is also consistent with Gardener et al. [12], who identified that Pb exposures from concentrations in baby food products typically do not exceed the FDA limits that were in place at the time of publication (either 6 μg/d or 50 μg/kg). Daily doses, however, can occasionally exceed the Cal/OEHHA MADL. Further, our findings are consistent with Spungen [24], who determined that Pb exposures from baby food products typically do not exceed the interim FDA limit (3 μg/d), except under upper bound exposure conditions.
The collective, inherent conservatisms in this risk assessment are likely to overpredict risks associated with current Pb exposure from baby foods. Within the context of this study, exceedances of non-cancer screening values for Pb were modest. HQs for all scenarios, including risks based on maximum Pb concentration in any sample, were 3.53 or lower. Data variability further indicates that even within a brand, a range of potential exposures to Pb exist, indicating that daily exposure at the maximum concentration is highly unlikely. Cancer risk associated with Pb was not found to increase in this study. Estimated daily Pb exposure from these products remains below daily dose limits established by the FDA for Pb. Although this study likely overestimates non-cancer Pb risk estimates (and thus consuming these foods may not represent health risks to children), Pb should, however, remain a focus of ongoing testing for food companies, so as to ensure children’s safety, owing to children’s sensitivity to Pb’s effects.
4.3. Uncertainties and limitations
Although this study provides a transparent and conservative risk assessment of the selected heavy metals in baby foods in order to aid understanding of whether these products pose risks to children, it is not without some limitations and/or uncertainties. In addition to the uncertainties and limitations in specific risk assessments associated with Pb and As exposure, this study only evaluated a small subset of available products on the market. Though these products were selected from national suppliers in order to include a range of foods that babies may eat, their representativeness of the entire market as a whole is unknown. Identifying specific risks (and, contrastingly, the elements identified as unlikely to be a risk) are consistent with the published literature, indicating that these results might actually be representative of other products [12,[24], [25], [26], [27],50,68]. Furthermore, the As and Pb concentrations found in the sampled baby foods in this study and identified as potential health risks are consistent with concentrations found in many whole foods and non-baby food products reported in the TDS, as depicted in Table 9. In spite of the similarities to existing data on these elements in both baby food products and whole foods, more comprehensive analyses could bolster these conclusions. Follow-up studies with more robust sample sizes could elucidate the relationship between heavy metal concentrations in baby foods and brand-specific manufacture processes and formulations, including variables such as specific ingredients, source location, agricultural practices, and/or production methods.
Table 9.
TDS As and Pb Concentrations in Whole Foods Compared to Current Study Baby Food Samples.
| Food Type | As Concentration (μg/kg) |
Pb Concentration (μg/kg) |
||||
|---|---|---|---|---|---|---|
| Minimum | Mean | Maximum | Minimum | Mean | Maximum | |
| Current Study: Grain | 10.0 | 90.4 | 132 | 5.0 | 9.7 | 20.0 |
| TDS: Rice, white, enriched, cooked | 36 | 66 | 111 | 0 | 0 | 0 |
| TDS: Shredded wheat cereal | 0 | 1 | 18 | 0 | 0.3 | 11 |
| Current Study: Fruit | 1.5 | 3.8 | 5.0 | 1.5 | 2.7 | 5.0 |
| TDS: Orange (navel/Valencia), raw | 0 | 0 | 0 | 0 | 1 | 21 |
| TDS: Pineapple, canned in juice | 0 | 0 | 0 | 0 | 7 | 46 |
| Current Study: Leguminous Vegetable | 1.5 | 4.2 | 5.0 | 1.5 | 2.3 | 5.0 |
| TDS: Peas, green, fresh/frozen, boiled | 0 | 0 | 0 | 0 | 0 | 0 |
| TDS: Pinto beans, dry, boiled | 0 | 0 | 0 | 0 | 0.3 | 11 |
| Current Study: Root Vegetable | 5.0 | 10.8 | 22.0 | 1.5 | 15.8 | 48.0 |
| TDS: Sweet potatoes, canned | 0 | 0.3 | 11 | 0 | 12 | 23 |
| TDS: Carrot, fresh, peeled, boiled | 0 | 0 | 0 | 0 | 2 | 19 |
| TDS: Carrot, baby, raw | 0 | 0.4 | 13 | 0 | 1 | 9 |
Note: Current study ND = 1.5 μg/kg, NQ = 5 μg/kg; TDS ND = 0 μg/kg.
Further, heavy metals were not present above limits of detection and quantitation in most product samples. Pb and As were detected in <75 % of all products tested. Uncertainty therefore remains regarding actual concentrations of the elements in the products tested. For the purposes of this risk assessment, we assumed that non-detected element concentrations were present in the food at one-half of the LOD, which may over- or under-estimate the actual metal concentration in the product.
Lastly, this risk assessment relies on inherent assumptions regarding food intake and body weight that are intended to represent typical use conditions, but may not represent all children’s food consumption patterns and body weights. In an effort to understand potential risk variability related to food intake rate variation, Table 10 presents the upper bound (95th percentile) ingredient-specific consumer-only intake rates and body weights, compiled from the EPA Exposure Factors Handbook. The upper-bound food intake rates are approximately 1.8- to 9.4-fold higher than the intake rates used in this risk assessment. Correspondingly, for children with very high food consumption rates, calculated risk estimates would be approximately 1.8- to 6.8-fold higher than this risk assessment reports (with high-end estimates based on total vegetable consumption, rather than type-specific consumption). Contrastingly, upper bound body weights are approximately 25 % higher than those used in this risk assessment, which would reduce the estimated daily dose and corresponding risk estimates in a similar fashion. Furthermore, children with higher consumption rates could reasonably be expected to have higher body weights. Collectively, then, although this risk assessment did not consider all combinations of intake rate, body weight, and concentration, the results presented herein represent the most typical exposure scenarios for children exposed to heavy metals via food products.
Table 10.
95th Percentile Baby Food Ingestion Exposure Parameters.
| Parameter | Age Group |
Reference | |||
|---|---|---|---|---|---|
| <1 year | 1-<2 years | 2-<3 years | |||
| BW: Body Weight (kg) | 8.475 | 14 | 17.1 | Table 8–3 [32] | |
| IR: Consumer-Only 95th Percentile Intake Rate (g/kg-day) | Fruit | 27.2 | 24.0 | 20.5 | Table 9–1: 95th percentile values for Total Fruits [33] |
| Grain | 8.7 | 12.7 | 11.7 | Table 12-1: 95th percentile values for Total Grains [34] | |
| Leguminous Vegetablea | 18.7 | 16.3 | 14.0 | Table 9–1: 95th percentile values for Total Vegetables [33] | |
| Root Vegetablea | 18.7 | 16.3 | 14.0 | Table 9–1: 95th percentile values for Total Vegetables [33] | |
Age-specific intake rates were not available for leguminous or root vegetables. Age-specific consumer-only upper bound intake rates for total vegetables were therefore assigned to each vegetable category.
5. Conclusions
Overall, this risk assessment indicates that, except for select exposure scenarios and products, when consumed, baby foods are unlikely to pose risks from heavy metals. The primary exception, however, is As in rice-based foods, a recognized issue worldwide because of the natural occurrence of As in soil and its high uptake into rice. Though Pb risks were potentially identified for some product categories, Pb exposures routinely were below FDA guidelines, and daily doses were very close to the Cal/OEHHA MADL of 0.5 μg/d, if not below. This study can provide additional information and support for decision-makers regarding concerns about dietary heavy metal exposures to children, particularly in terms of understanding potential risks by baby food product type.
Conflict of interest
The authors declare no conflict of interest.
Funding
This research was not funded by any grants from any agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The author report no declaration of interest. Four of the authors (GHP, CEG, JVM, MLK) are or were employed by Cardno ChemRisk now Stantec, a consulting firm that provides scientific advice to governmental bodies, corporations, law firms, and various scientific/professional organizations. The remaining author (DEB) is a graduate student at Carnegie Mellon University and former Cardno ChemRisk employee. No outside funding was received for planning or executing the study, analyzing the samples, interpreting the data, or writing this manuscript. The study design, execution, results, and interpretation are the sole responsibility of the authors, and the text was prepared and written exclusively by the authors.
CRediT authorship contribution statement
Gwendolyn H. Parker: Conceptualization, Methodology, Formal analysis, Writing - original draft, Writing - review & editing, Visualization, Project administration. Caroline E. Gillie: Conceptualization, Methodology, Resources, Formal analysis, Writing - original draft, Writing - review & editing, Visualization. Julie V. Miller: Conceptualization, Methodology, Resources, Writing - review & editing, Visualization. Deanna E. Badger: Conceptualization, Methodology, Formal analysis, Resources, Writing - original draft, Project administration. Marisa L. Kreider: Conceptualization, Methodology, Writing - original draft, Writing - review & editing, Project administration, Supervision.
Acknowledgement
The authors thank Carrie Kahn for her thoughtful suggestions and editorial support.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.EDF . Environmental Defense Fund; 2017. Lead in Food: A Hidden Health Threat.https://www.edf.org/health/lead-food-hidden-health-threat#:∼:text=Food%20is%20a%20meaningful%20%E2%80%93%20and,in%20blood%20has%20been%20identified.&text=EDF%20analyzed%2011%20years%20of,and%20surprising%20-%20source%20of%20lead June 14, 2017. Retrieved April 6, 2021 from: [Google Scholar]
- 2.Hirsch J. Consumer Reports; 2018. Heavy Metals in Baby Food: What You Need to Know Heavy Metals in Baby Food: What You Need to Know.https://www.consumerreports.org/food-safety/heavy-metals-in-baby-food/ Page Last Reviewed August 16, 2018. Retrieved February 26, 2019 from. [Google Scholar]
- 3.Slisco A. Newsweek; 2021. Lawsuits Allege Baby Food Manufacturers Failed to Disclose Lead, Arsenic in Product.https://www.newsweek.com/lawsuits-allege-baby-food-manufacturers-failed-disclose-lead-arsenic-product-1572214 Feb. 25, 2021. Retrieved March 21, 2021 from. [Google Scholar]
- 4.ECP . Subcommittee on Economic and Consumer Policy (ECP)-Committee on Oversight and Reform-U.S. House of Representatives; Washington, D.C: 2021. Baby Foods are Tainted With Dangerous Levels of Arsenic, Lead, Cadmimum and Mercury: Staff Report.https://oversight.house.gov/sites/democrats.oversight.house.gov/files/2021-02-04%20ECP%20Baby%20Food%20Staff%20Report.pdf Feb. 4, 2021. Retrieved March 30, 2021 from. [Google Scholar]
- 5.FDA . Food and Drug Administration (FDA); White Oak, MD: 2021. Metals and Your Food.https://www.fda.gov/food/chemicals-metals-pesticides-food/metals-and-your-food Current as of March 5, 2021. Retrieved March 15, 2021 from. [Google Scholar]
- 6.FDA . Food and Drug Administration (FDA); Silver Spring, MD: 2020. Code of Federal Regulations, Title 21, Vol. 3, Ch. I, Subchapter B, Part 172, Subpart A, Sec. 172.5: General Provisions for Direct Food Additives.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172&showFR=1 Revised April 1, 2020. Last Updated Nov. 10, 2020. Retrieved June 16, 2021 from. [Google Scholar]
- 7.Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011;2011 [Google Scholar]
- 8.Tchounwou P.B., Yedjou C.G., Patlolla A.K., Sutton D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012;101:133–164. doi: 10.1007/978-3-7643-8340-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaishankar M., Tseten T., Anbalagan N., Mathew B.B., Beeregowda K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Tox. 2014;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA . U.S. Food and Drug Administration (FDA)-Center for Food Safety and Applied Nutrition-Office of Analytics and Outreach-Exposure Assessment Branch; College Park, MD: 2017. Total Diet Study: Elements Results Summary Statistics. Market Baskets 2006 Through 2013. April 15, 2014. Revised April, 2017. [Google Scholar]
- 11.Houlihan J., Brody C. Healthy Babies/Bright Futures (HBBF); 2020. What’s in My Baby’s Food?https://www.healthybabyfood.org/sites/healthybabyfoods.org/files/2020-04/BabyFoodReport_ENGLISH_R6.pdf Oct., 2019. Version R6, March, 2020. Retrieved March 14, 2021 from. [Google Scholar]
- 12.Gardener H., Bowen J., Callan S.P. Lead and cadmium contamination in a large sample of United States infant formulas and baby foods. Sci. Total Environ. 2019;651(Pt 1):822–827. doi: 10.1016/j.scitotenv.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 13.ATSDR . U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Washington, D.C: 1999. Toxicological Profile for Mercury. March, 1999. [Google Scholar]
- 14.ATSDR . U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Washington, D.C: 2007. Toxicological Profile for Arsenic. August, 2007. [PubMed] [Google Scholar]
- 15.ATSDR . U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Washington, D.C: 2012. Toxicological Profile for Cadmium. September, 2012. [PubMed] [Google Scholar]
- 16.ATSDR . U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry; Washington, D.C: 2020. Toxicological Profile for Lead. August, 2020. [PubMed] [Google Scholar]
- 17.FDA . Food and Drug Administration (FDA); Rockville, MD: 2006. Guidance Document: Guidance for Industry: Lead in Candy Likely to Be Consumed Frequently by Small Children. Recommended Maximum Level and Enforcement Policy.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-lead-candy-likely-be-consumed-frequently-small-children Nov., 2006. Retrieved March 8, 2021 from. [Google Scholar]
- 18.FDA . Center for Food Safety and Applied Nutrition-Food and Drug Administration (FDA)-U.S. Dept. of Health and Human Services; College Park, MD: 2013. Guidance for Industry: Arsenic in Apple Juice: Action Level. Draft Guidance. July, 2013. [Google Scholar]
- 19.FDA . Center for Food Safety and Applied Nutrition-Food and Drug Administration (FDA)-U.S. Dept. of Health and Human Services; College Park, MD: 2020. Inorganic Arsenic in Rice Cereals for Infants: Action Level. Guidance for Industry. Aug., 2020. [Google Scholar]
- 20.FDA . U.S. Food and Drug Administration (FDA)-Division of Import Operations; Rockville, MD: 2020. Import Alert 20-05: Detention Without Physical Examination and Surveillance of Fruit Juices and Fruit Juice Concentrates Due to Heavy Metal Contamination.https://www.accessdata.fda.gov/cms_ia/importalert_56.html Dec. 9, 2020. Retrieved March 14, 2021 from. [Google Scholar]
- 21.FDA . U.S. Food and Drug Administration (FDA)-Division of Import Operations; Rockville, MD: 2021. Import Alert 20-03: Detention Without Physical Examination of Dried Fruits Due to Lead.https://www.accessdata.fda.gov/cms_ia/importalert_55.html Jan. 27, 2021. Retrieved March 14, 2021 from. [Google Scholar]
- 22.FDA . U.S. Food and Drug Administration (FDA)-Division of Import Operations; Rockville, MD: 2021. Import Alert 28-13: Detention Without Physical Examination of Spices and Spice Products Due to Lead Contamination.https://www.accessdata.fda.gov/cms_ia/importalert_1143.html Feb. 5, 2021. Retrieved March 14, 2021 from. [Google Scholar]
- 23.Shibata T., Meng C., Umoren J., West H. Risk assessment of arsenic in rice cereal and other dietary sources for infants and toddlers in the U.S. Int. J. Environ. Res. Pub. Health. 2016;13(4):361. doi: 10.3390/ijerph13040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spungen J.H. Children’s exposures to lead and cadmium: FDA total diet study 2014-16. Food Addit. Contam. A. 2019;36(6):893–903. doi: 10.1080/19440049.2019.1595170. [DOI] [PubMed] [Google Scholar]
- 25.Martins C., Vasco E., Paixao E., Alvito P. Total mercury in infant food, occurrence and exposure assessment in Portugal. Food Addit. Contam. B. 2013;6(3):151–157. doi: 10.1080/19393210.2013.775603. [DOI] [PubMed] [Google Scholar]
- 26.Gu Z., de Silva S., Reichman S.M. Arsenic concentrations and dietary exposure in rice-based infant food in Australia. Int. J. Environ. Res. Pub. Health. 2020;17(2) doi: 10.3390/ijerph17020415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camara-Martos F., Ramirez-Ojeda A.M., Jimenez-Mangas M., Sevillano-Morales J., Moreno-Rojas R. Selenium and cadmium in bioaccessible fraction of organic weaning food: Risk assessment and influence of dietary components. J. Trace Elem. Med. Biol. 2019;56:116–123. doi: 10.1016/j.jtemb.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Nastasescu V., Mititelu M., Goumenou M., Docea A.O., Renieri E., Udeanu D.I., Oprea E., Arsene A.L., Dinu-Pirvu C.E., Ghica M. Heavy metal and pesticide levels in dairy products: evaluation of human health risk. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111844. [DOI] [PubMed] [Google Scholar]
- 29.Callen C., Bhatia J., Czerkies L., Klish W.J., Gray G.M. Challenges and considerations when balancing the risks of contaminants with the benefits of fruits and vegetables for infants and toddlers. Nutrients. 2018;10(11) doi: 10.3390/nu10111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.FDA . U.S. Food and Drug Administration (FDA); 2015. Elemental Analysis Manual (EAM), Method 4.7, Version 1.1, Inductively Coupled Plasma-Mass Spectrometric Determination of Arsenic, Cadmium, Chromium, Lead, Mercury, and Other Elements in Food Using Microwave Assisted Digestion. [Google Scholar]
- 31.EPA . U.S. Environmental Protection Agency (EPA); Washington, D.C: 2017. Regional Guidance on Handling Chemical Concentration Data Near the Detectioln Limit in Risk Assessments: Regional Technical Guidance Manual, Risk Assessment.https://www.epa.gov/risk/regional-guidance-handling-chemical-concentration-data-near-detection-limit-risk-assessments Last Updated Aug. 29, 2017. Retrieved June 16, 2021 from. [Google Scholar]
- 32.EPA . U.S. Environmental Protection Agency (EPA), Office of Research and Development, National Center for Environmental Assessment; Washington, D.C: 2011. Exposure Factors Handbook: 2011 Edition. EPA/600/R-090/052F. Sept., 2011. [Google Scholar]
- 33.EPA . U.S. Environmental Protection Agency (EPA), Office of Research and Development, National Center for Environmental Assessment; Washington, D.C: 2018. Update for Chapter 9 of the Exposure Factors Handbook: Intake of Fruits and Vegetables. EPA/600/R-18/098F. Aug., 2018. [Google Scholar]
- 34.EPA . U.S. Environmental Protection Agency (EPA), Office of Research and Development, National Center for Environmental Assessment; Washington, D.C: 2018. Update for Chapter 12 of the Exposure Factors Handbook: Intake of Grain Products. EPA/600/R-18/095F. July, 2018. [Google Scholar]
- 35.Fein S.B., Labiner-Wolfe J., Shealy K.R., Li R., Chen J., Grummer-Strawn L.M. Infant feeding practices study II: study methods. Pediatrics. 2008;122(Suppl. 2):S28–S35. doi: 10.1542/peds.2008-1315c. [DOI] [PubMed] [Google Scholar]
- 36.Karmaus W., Soto-Ramirez N., Zhang H. Infant feeding pattern in the first six months of age in USA: a follow-up study. Int. Breastfeed. J. 2017;12:48. doi: 10.1186/s13006-017-0139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mayo Clinic . Mayo Foundation for Medical Education and Research (MFMER); Rochester, MN: 2018. Healthy Lifestyle--Infant and Toddler Health: Solid Foods: How to Get Your Baby Started.https://www.mayoclinic.org/healthy-lifestyle/infant-and-toddler-health/in-depth/healthy-baby/art-20046200 Retrieved Oct. 26, 2018 from. [Google Scholar]
- 38.EPA . U.S. Environmental Protection Agency (EPA)-National Center for Environmental Assessment; Washington, D.C: 1991. Integrated Risk Information System (IRIS) Chemical Assessment Summary: Arsenic; Inorganic; CASRN 7440-38-2. Last revision September 1, 1991. [Google Scholar]
- 39.EPA . U.S. Environmental Protection Agency (EPA); Washington, D.C: 2003. Human Health Toxicity Values in Superfund Risk Assessments, OSWER Directive 9285.7-53. [Google Scholar]
- 40.Park J.D., Zheng W. Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health. 2012;45(6):344–352. doi: 10.3961/jpmph.2012.45.6.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC . Centers for Disease Control and Prevention (CDC); Atlanta: 2017. Arsenic Factsheet.https://www.cdc.gov/biomonitoring/Arsenic_FactSheet.html#:∼:text=People Page Last Reviewed April 7, 2017. Retrieved March 14, 2021 from. [Google Scholar]
- 42.Li R., Wu H., Ding J., Fu W., Gan L., Li Y. Mercury pollution in vegetables, grains and soils from areas surrounding coal-fired power plants. Sci. Rep. 2017;7:46545. doi: 10.1038/srep46545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renieri E.A., Safenkova I.V., Alegakis A., Slutskaya E.S., Kokaraki V., Kentouri M., Dzantiev B.B., Tsatsakis A.M. Cadmium, lead and mercury in muscle tissue of gilthead seabream and seabass: risk evaluation for consumers. Food Chem. Toxicol. 2019;124:439–449. doi: 10.1016/j.fct.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 44.EPA . U.S. Environmental Protection Agency (EPA)-National Center for Environmental Assessment; Washington, D.C: 1989. Integrated Risk Information System (IRIS) Chemical Assessment Summary: Cadmium; CASRN 7440-43-9. Last revised October 1, 1989. [Google Scholar]
- 45.EFSA Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012;10(12):2985. [Google Scholar]
- 46.OEHHA . California Office of Environmental Health Hazard Assessment (OEHHA); 2011. Air Toxics Hot Spots Program Technical Support Document for Cancer Potencies. Appendix B. Chemical-Specific Summaries of the Information Used to Derive Unit Risk and Cancer Potency Values. Updated 2011; pp. B368–B375. [Google Scholar]
- 47.OEHHA . California Office of Environmental Health Hazard Assessment (OEHHA); 2021. Proposition 65 No Significant Risk Levels (NSRLs) for Carcinogens and Maximum Allowable Dose Levels (MADLs) for Chemicals Causing Reproductive Toxicity. Updated March 25, 2021. [Google Scholar]
- 48.EPA . U.S. Environmental Protection Agency (EPA)-Office of Emergency and Remedial Response; Washington, D.C: 1989. Risk Assessment Guidance for Superfund, Volume I, Human Health Evaluation Manual (Part A). Interim Final Guidance. December, 1989. [Google Scholar]
- 49.Winiarska-Mieczan A., Kiczorowska B. Determining the content of lead and cadmium in infant food from the Polish market. Int. J. Food Sci. Nutr. 2012;63(6):708–712. doi: 10.3109/09637486.2011.644765. [DOI] [PubMed] [Google Scholar]
- 50.Sadeghi N., Oveisi M.R., Jannat B., Hajimahmoodi M., Behfar A., Behzad M., Norouzi N., Oveisi M., Jannat B. Simultaneous measurement of zinc, copper, lead and cadmium in baby weaning food and powder milk by DPASV. Iran. J. Pharm. Res. 2014;13(1):345–349. [PMC free article] [PubMed] [Google Scholar]
- 51.Guillod-Magnin R., Bruschweiler B.J., Aubert R., Haldimann M. Arsenic species in rice and rice-based products consumed by toddlers in Switzerland. Food Addit. Contam. 2018;35(6):1164–1178. doi: 10.1080/19440049.2018.1440641. [DOI] [PubMed] [Google Scholar]
- 52.Liao N., Seto E., Eskenazi B., Wang M., Li Y., Hua J. A comprehensive review of arsenic exposure and risk from rice and a risk assessment among a cohort of adolescents in Kunming, China. Int. J. Environ. Res. Public Health. 2018;15(10):2191. doi: 10.3390/ijerph15102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharafi K., Nodehi R.N., Yunesian M., Mahvi A.H., Pirsaheb M., Nazmara S. Human health risk assessment for some toxic metals in widely consumed rice brands (domestic and imported) in Tehran, Iran: uncertainty and sensitivity analysis. Food Chem. 2018;277:145–155. doi: 10.1016/j.foodchem.2018.10.090. [DOI] [PubMed] [Google Scholar]
- 54.Pirsaheb M., Hadei M., Sharafi K. Human health risk assessment by Monte Carlo simulation method for heavy metals of commonly consumed cereals in Iran – uncertainty and sensitivity analysis. J. Food Anal. 2021;96 [Google Scholar]
- 55.Garcia-Rico L., Valenzuela-Rodriguez M.P., Meza-Montenegro M.M., Lopez-Duarte A.L. Arsenic in rice and rice products in Northwestern Mexico and health risk assessment. Food Addit. Contam. 2019;13(1):25–33. doi: 10.1080/19393210.2019.1678672. [DOI] [PubMed] [Google Scholar]
- 56.Rintala E.M., Ekholm P., Koivisto P., Peltonen K., Venalainen E.R. The intake of inorganic arsenic from long grain rice and rice-based baby food in Finland – low safety margin warrants follow up. Food Chem. 2014;150:199–205. doi: 10.1016/j.foodchem.2013.10.155. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz-de-Cenzano M., Rochina-Marco A., Cervera M.L., de la Guardia M. Evaluation of the content of antimony, arsenic, bismuth, selenium, tellurium and their inorganic forms in commercially baby foods. Biol. Trace Elem. Res. 2017;180(2):355–365. doi: 10.1007/s12011-017-1018-y. [DOI] [PubMed] [Google Scholar]
- 58.Tenni D., Martin M., Barberis E., Beone G.M., Miniotti E., Sodano M., Zanzo E., Fontanella M.C., Romani M. Total As and As speciation in Italian rice as related to producing areas and paddy soils properties. J. Agric. Food Chem. 2017;65(17):3443–3452. doi: 10.1021/acs.jafc.7b00694. [DOI] [PubMed] [Google Scholar]
- 59.Upadhyay M.K., Shukla A., Yadav P., Srivastava S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019;276:608–618. doi: 10.1016/j.foodchem.2018.10.069. [DOI] [PubMed] [Google Scholar]
- 60.FDA . Center for Food Safety and Applied Nutrition-Food and Drug Administration (FDA)-U.S. Dept. of Health and Human Services; College Park, MD: 2016. Arsenic in Rice and Rice Products: Risk Assessment Report. Version Released for Public Comment. March, 2016. [Google Scholar]
- 61.Tao S.S., Bolger P.M. Dietary arsenic intakes in the United States: FDA Total Diet Study, September 1991-December 1996. Food Addit. Contam. 1999;16(11):465–472. doi: 10.1080/026520399283759. [DOI] [PubMed] [Google Scholar]
- 62.FDA . Food and Drug Administration (FDA); Silver Spring, MD: 2013. Analytical Results From Inorganic Arsenic in Rice and Rice Products Sampling. Sept., 2013. [Google Scholar]
- 63.EFSA Scientific opinion on arsenic in food. EFSA J. 2009;7(10):1351. [Google Scholar]
- 64.Gonzalez N., Calderon J., Rubies A., Bosch J., Timoner I., Castell V., Marques M., Nadal M., Domingo J.L. Dietary exposure to total and inorganic arsenic via rice and rice-based products consumption. Food Chem. 2020;141 doi: 10.1016/j.fct.2020.111420. [DOI] [PubMed] [Google Scholar]
- 65.Carey M., Donaldson E., Signes-Pastor A.J., Meharg A.A. Dilution of rice with other gluten free grains to lower inorganic arsenic in foods for young children in response to European Union regulations provides impetus to setting stricter standards. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0194700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.FDA . U.S. Food and Drug Administration (FDA); 2021. FDA Response to Questions About Levels of Toxic Elements in Baby Food, Following Congressional Report.https://www.fda.gov/food/cfsan-constituent-updates/fda-response-questions-about-levels-toxic-elements-baby-food-following-congressional-report Page Last Reviewed February 16, 2021. Retrieved April 2, 2021 from. [Google Scholar]
- 67.Flannery B.M., Dolan L.C., Hoffman-Pennesi D., Gavelek A., Jones O.E., Kanwal R., Wolpert B., Gensheimer K., Dennis S., Fitzpatrick S. U.S. Food and Drug Administration’s interim reference levels for dietary lead exposure in children and women of childbearing age. Regul. Toxicol. Pharmacol. 2020;110 doi: 10.1016/j.yrtph.2019.104516. [DOI] [PubMed] [Google Scholar]
- 68.Eklund G., Oskarsson A. Exposure of cadmium from infant formulas and weaning foods. Food Addit. Contam. 1999;16(12):509–519. doi: 10.1080/026520399283650. [DOI] [PubMed] [Google Scholar]