Summary
Background
There is currently insufficient evidence on the safety and efficacy of antenatal corticosteroids in preventing mortality and severe morbidity amongst late preterm newborns in low-resource countries.
Methods
We conducted a double-blind, randomized trial in four hospitals in India between 26 December 2017 to 21 May 2020. Pregnant women at risk of imminent preterm birth between 34 weeks 0 days and 36 weeks 0 days of gestation were recruited. Women were randomly assigned (1:1) to a course of 6 mg intramuscular dexamethasone or an identical placebo. All trial participants, research staff and outcome assessors were masked to allocation. Primary outcomes were neonatal death, any baby death (stillbirth or neonatal death), severe neonatal respiratory distress and possible maternal bacterial infection. The study was registered with ANZCTR (ACTRN12617001494325) and CTRI (CTRI/2017/05/008721).
Findings
We randomized 782 women, 391 to each arm. Neonatal death occurred in 11 of 412 liveborn babies (2.7%) in the dexamethasone group and 12 of 425 liveborn babies (2.8%) in the placebo group (RR 0.95; 95% CI 0.42–2.12). Any baby death occurred in 16 of 417 infants (3.8%) in the dexamethasone group and 19 of 432 infants (4.4%) in the placebo group (RR 0.87; 95% CI 0.45–1.67). Severe neonatal respiratory distress was infrequent in both groups (0.8% vs 0.5%; RR 1.56; 95% CI 0.26–9.29). Possible maternal bacterial infection did not differ between groups (2.3% vs. 3.8%, RR 0.60; 95% CI 0.27–1.35). Fewer neonates in the dexamethasone group required resuscitation at birth (RR 0.38, CI 0.15–0.97). Other secondary outcomes were similar in the two arms. The trial was stopped due to lower than expected prevalence of primary outcomes and slow recruitment.
Interpretation
Antenatal dexamethasone did not result in a reduction in neonatal death, stillbirth or neonatal death, or severe neonatal respiratory distress in this trial. The overall trend of effects suggests that potential benefit of dexamethasone in late preterm cannot be excluded, and further trials are required.
Funding
This trial was primarily funded by the Bill and Melinda Gates Foundation (Grant OPP1136821). Additional support was provided by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research; and Department of Maternal, Newborn, Child, Adolescent Health, and Ageing, of the World Health Organization, Geneva, Switzerland.
Keywords: Dexamethasone, India, Maternal, Newborn, Preterm birth
Research in context.
Evidence before this study
An update of the Cochrane review on antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth was published in December 2020. On 3 September 2020, the authors searched the Cochrane Pregnancy and Childbirth's Trials Register, which is based on searches of CENTRAL, MEDLINE, Embase and CINAHL, as well as handsearched journals and conference proceedings. The review included any randomized trial on the use of antenatal corticosteroids compared to placebo or no treatment in pregnant women expected to give birth before 37 weeks of pregnancy. The review included 27 trials (11,272 randomised women and 11,925 neonates), though few trials and data were available for the subgroup ≥34 weeks gestation. There were too few neonatal deaths in this subgroup to conclude whether there is mortality benefit (6/1831 vs 4/1817, RR 1.51, 95% CI 0.49 – 4.61), though respiratory distress syndrome might be reduced with steroid treatment (115/2077 vs 152/2065; RR 0.75, 95% CI 0.60 – 0.95).
Added value of this study
This is the largest efficacy trial of antenatal corticosteroids conducted in the late preterm period (≥34 weeks gestation) in a low-resource country setting. We pooled the results of the current trial (ACTION-II) with the Cochrane review to update meta-analyses and found no substantive changes in the strength or direction of risk estimates for neonatal death, perinatal death, or respiratory distress syndrome, both overall and for the subgroup of women receiving antenatal corticosteroids at 34 weeks gestation or greater.
Implications of all the available evidence
This trial did not detect reductions in neonatal death or severe respiratory morbidity. However, there was no evidence of maternal or newborn harms and there were reductions in a secondary newborn respiratory morbidity outcome with dexamethasone. Updated meta-analyses also suggest benefit for severe neonatal respiratory distress, though mortality benefit cannot yet be excluded. Further efficacy trials are required.
Alt-text: Unlabelled box
Introduction
An estimated 15 million neonates are born preterm annually, accounting for 11% of live births worldwide, nearly 85% of which occur from 32 to <37 weeks gestation.1 Complications of preterm birth are the leading cause of mortality in neonates and children less than 5 years of age.2 Compared to babies born at term, preterm babies have higher rates respiratory, infectious and neurological morbidities; elevated risks of adverse health outcomes persisting into childhood and later life.2, 3, 4 While the risk of morbidities for preterm babies are most frequent at earlier gestations, babies born in the late preterm period (34 to <37 weeks) experience a significantly higher rate of morbidity and mortality than those born at term.5, 6, 7, 8
In 2015, the World Health Organization (WHO) recommended that antenatal corticosteroids (ACS) should only be used for pregnant women at risk of preterm birth from 24 to 34 weeks’ gestation.9,10 This gestational age range was selected based on a meta-analysis conducted for the WHO guideline that indicated a lack of evidence of benefit of ACS beyond 34 weeks.10 The WHO recommendations also specify that ACS should only be used in settings where certain conditions – accurate gestational age (GA) assessment, imminent preterm birth, absence of maternal infection, and adequate childbirth and preterm newborn care – can be met.10 These criteria were informed by the findings of the Antenatal Corticosteroids Trial (ACT), a cluster-randomized trial conducted in six low-resource countries that aimed to scale up ACS use for women at risk of preterm birth up to 36 weeks.11 Unexpectedly, ACT reported no mortality benefit amongst babies born with a birth weight less-than-fifth-centile (a proxy for preterm birth), while neonatal mortality, stillbirth and possible maternal infections in the overall population were significantly higher in the intervention clusters. The WHO guideline panel acknowledged that while these consensus-based treatment criteria were intended to maximize benefit and minimize possible harms from ACS, further efficacy trials in low-resource countries on ACS use in both early and late preterm populations were a high research priority.9
The Antenatal Late Preterm Steroid (ALPS) trial was published in 2016, reporting that intramuscular (IM) betamethasone administered to women at risk of late preterm birth significantly reduced a composite newborn outcome of respiratory morbidity treatment, stillbirth or neonatal death in the first 72 h after birth.13 ALPS was conducted in tertiary hospitals in the USA where there was a high level of care available for preterm infants and their mothers. While ALPS has led to updated recommendations in favour of late preterm ACS use in some high-resource countries,13, 14, 15 no efficacy trials have evaluated the use of ACS to support policy change for late preterm births in low-resource countries. Despite this, observational evidence suggests that ACS is used variably in late preterm period in low-resource countries.16
To address these knowledge gaps, the WHO ACTION (Antenatal CorticosTeroids for Improving Outcomes in preterm Newborn) Trials collaboration was established.17,18 The results of the WHO ACTION-I trial on the efficacy of dexamethasone in the early preterm period have been reported previously.17 Here we describe the findings of the WHO ACTION-II trial, which aimed to assess safety and efficacy of dexamethasone when given to women at risk of late preterm birth, in hospitals in low-resource countries.
Methods
Study design
The study design was a multicountry, multicentre, individually-randomized, parallel-group, double-blind, placebo-controlled trial. We planned to conduct the trial across six study sites in Bangladesh, India, Kenya, Nigeria (two sites) and Pakistan. The trial protocol was approved by ethics committees and regulatory agencies for each country and participating hospital, as well as the WHO Ethics Review Committee (Supplementary File S1). The trial protocol was registered prior to participant recruitment (Australia and New Zealand Clinical Trials Registry number ACTRN12617001494325; Clinical Trials Registry-India number, CTRI/2017/05/008721). The statistical analysis plan (Supplementary File S2) was finalised prior to trial stoppage. The trial steering group comprised a trial co-ordinating unit, principal investigators (including obstetricians and neonatologists at each site) and independent technical advisors. The findings are reported as per the Consolidated Standards of Reporting Trials (CONSORT) guidelines.19 Study hospitals were selected to participate through a standardized assessment of maternal and newborn healthcare services to ensure that the ACS treatment criteria described in the WHO guidelines could be reasonably met.9 This trial was planned to run concurrently with ACTION-I (early preterm) trial across all sites. However, as a result of several logistical challenges encountered with concurrent set up of two trials, only the four hospitals at the India site (in two different states) recruited into the ACTION-II trial (Table S1).
Participants
Eligible participants were pregnant women with a singleton or multiple pregnancy (with confirmed live fetus/es) who were at risk of preterm birth between 34 weeks 0 days and 36 weeks 0 days. Pregnant women were eligible if birth was planned or expected in the next 48 h, following preterm prelabour rupture of membranes, spontaneous labour, or a decision for provider-initiated preterm birth. The gestational age estimate was based on the earliest obstetric ultrasound available. If a dating ultrasound of reasonable quality had not been performed prior to hospital presentation, one was performed prior to screening for eligibility. Women were ineligible if they had clinical signs of severe infection; suspicion or evidence of clinical chorioamnionitis; major congenital fetal anomalies; concurrent or recent (within the past two weeks) use of systemic steroids; participation in another maternal or newborn health trial; or contraindication to steroids. Written informed consent and/or assent was obtained from every participant prior to randomisation.
Randomization and masking
Eligible women were randomized in a 1:1 ratio to dexamethasone or placebo arms. As the trial was planned to be conducted across 6 sites, site-stratified individual randomization with balanced permuted blocks of size 10 were used. The randomization sequence was computer-generated by WHO.
All study sites received purpose-designed dispensers containing 10 sequentially numbered, identical treatment packs. Each pack contained eight 4 mg/mL ampoules of dexamethasone or placebo (thus providing enough ampoules for four 6 mg injections). Once a woman was screened for eligibility and her informed consent was obtained, randomization was performed immediately by withdrawing and opening the next treatment pack from the dispenser. The trial steering group, clinical and research staff and participants were blind to group assignments.
Procedures
The intervention arm comprised a single course of IM dexamethasone sodium phosphate. Participants received a 6 mg injection every 12 h, to a maximum of four doses, or until hospital discharge or birth (whichever came first). Women in the control arm received an identical placebo regimen. Dexamethasone and matching placebos were procured from Fresenius Kabi/Labesfal, Portugal and packaged and shipped to study sites by Ivers-Lee CSM, Switzerland. At participating hospitals, dispensers and treatment packs were kept in a secure, temperature-controlled (15 to 25 °C) area. Participating hospitals were provided with obstetric ultrasound systems (Philips HD5, Netherlands), continuous positive airway pressure (CPAP) machines (Diamedica, Sweden), pulse oximeters (Masimo, Switzerland) and glucometers. Standardized trainings were conducted for research and clinical staff on trial-related activities, as well as training workshops for clinical staff performing dating ultrasounds. Eligible women were screened at the time of presentation to hospital, and randomized women and their babies were followed up at day 7 and at day 28 after birth, either at the hospital or at home.
Outcomes
The trial primary outcomes were neonatal death (death of a liveborn neonate by 28 completed days of life); any baby death (any death of a fetus, or death of a liveborn neonate within 28 completed days of life); severe respiratory distress in a liveborn neonate; and possible maternal bacterial infection (occurrence of maternal fever ≥ 38 °C or clinically suspected or confirmed infection for which therapeutic antibiotics were used, during hospital admission). Severe neonatal respiratory distress was based on clinical assessment during the initial admission after birth, up to a maximum of 7 completed days, death or discharge (whichever came first). It was defined as the presence of fast breathing (respiratory rate ≥ 70 breaths per min) and at least one of: (a) marked nasal flaring during inspiration, or (b) expiratory grunting audible with naked ear, or (c) severe chest in drawing; and SpO2< 90% or supplemental oxygen is used. Clinical assessments for outcomes occurring during hospital admission were performed by maternal and neonatal care clinical staff, collected and entered in paper study forms by study data collectors (research nurses or midwives) and monitored by hospital investigators (including obstetricians and neonatologists). Home visits for ascertaining vital status of trial participants during follow up were performed by trained data collectors using paper forms. For neonatal hypoglycaemia, all liveborn newborns had a screening glucose level recorded at 6 and 36 h. These screening tests were timed to precede feeding or IV fluid administration. Hypoglycaemia could also be identified at any other time during first 7 days of postnatal admission on the basis of clinical testing when indicated. Secondary outcomes include maternal and newborn mortality and morbidities, and process of care outcomes (see Supplementary File S3). Any adverse event (AEs) and serious adverse events (SAEs) occurring from randomization to day 28 after birth were documented using standardized forms.
Statistical analysis and trial monitoring
We hypothesised that the use of dexamethasone would result in a reduction in the neonatal mortality outcomes and severe neonatal respiratory distress, without increasing the risk of maternal bacterial infection. Therefore, we applied a superiority hypothesis to the neonatal outcomes and a non-inferiority hypothesis to the possible maternal infection outcome. Data were double-entered into a web-based data management platform, and managed centrally by Centro Rosarino Estudios Perinatales (Rosario, Argentina). Independent monitors conducted regular in-person visits to participating hospitals. These visits included assessment that all study activities were being conducted according to the trial protocol and Manual of Operations, and in accordance with Good Clinical Practice standards. Visits included verification of collected data against source documents (such as medical records). Additional trial monitoring visits were conducted by WHO staff and site principal Investigators. In addition, data in the web-based platform on recruitment rate, AE/SAEs, and other key progress indicators were monitored on an ongoing basis by the trial co-ordinating unit.
Based on a superiority hypothesis, a total of 22,589 women were needed to detect a reduction of 15% or more in neonatal deaths (from 8.0% deaths to 6.8%) amongst neonates of women who received ACS at 34 to 36 weeks, in a two-sided 5% significance test with 90% power, including 10% loss to follow-up. This sample size would provide 93% power to detect a relative reduction of 20% (from 5.0% to 4.0%) for severe neonatal respiratory distress in a two-sided 5% significance test with 10% loss to follow up. It would also provide > 99% power at the 2.5% significance level to detect if dexamethasone is non-inferior to placebo for the maternal infection outcome, within a non-inferiority margin of 2.5% on the absolute scale, and assuming a 10% baseline rate of maternal infection. Clustering by site or multiple births was not considered in the sample size calculation.
Primary analyses were based on intention-to-treat (ITT), analysing all participants with outcome data available, and corrected for multiplicity of primary outcomes. The dexamethasone arm was compared against the placebo arm for the primary outcomes using relative risk with 95% confidence intervals, based on a logistic model with a binomial distribution and the log link to obtain relative risks. The stratifying variable, study hospital, was included in the model, as well as a clustering feature for multiple births for neonatal outcomes. For continuous variables, means and standard deviations or medians, quartiles and interquartile range by group were reported. Treatment groups were compared using mean or median differences and 95% confidence intervals based on a general linear model that included study site as stratifying variable. Separate models were fitted for each of the primary and secondary outcomes. Results for secondary outcomes are presented as point estimates and 95% confidence interval without correction for multiple comparisons. We prespecified several subgroup analyses of the primary outcomes, however as the trial recruited substantially less than the planned sample size, these analyses were not performed. All models were fitted using SAS Software version 9.4 (SAS Institute Inc., Cary, NC, USA). The Cochrane review on efficacy of antenatal corticosteroids was updated in December 2020.20 In that review, fixed-effects meta-analyses have been used for the neonatal death outcome as the intervention, populations and methods from available trials were judged by review authors as sufficiently similar. Neonatal death data from the current trial were added to estimate the pooled effect of ACS on neonatal death, both overall and for the subgroup gestational age at trial entry. A per-protocol analysis (pre-specified in the statistical analysis plan) was also performed.
Blind, aggregate data were monitored in confidence by the Data Safety Monitoring Board (DSMB) during the trial. Three pre-planned interim analyses specified that the DSMB would consider the Haybittle-Peto stopping rule21 for the primary mortality outcomes to guide decision-making. In May 2020, however, the DSMB recommended stopping the trial given the lower than expected primary outcome rates and the low recruitment rate. In consultation with study sponsor and steering committee, recruitment was stopped, and all ethics committees and regulatory authorities were informed. The funder had no role in the decision to stop the trial.
Role of the funding source
Neither the funder nor manufacturers of trial medicines or equipment had any role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Recruitment and characteristics of study population
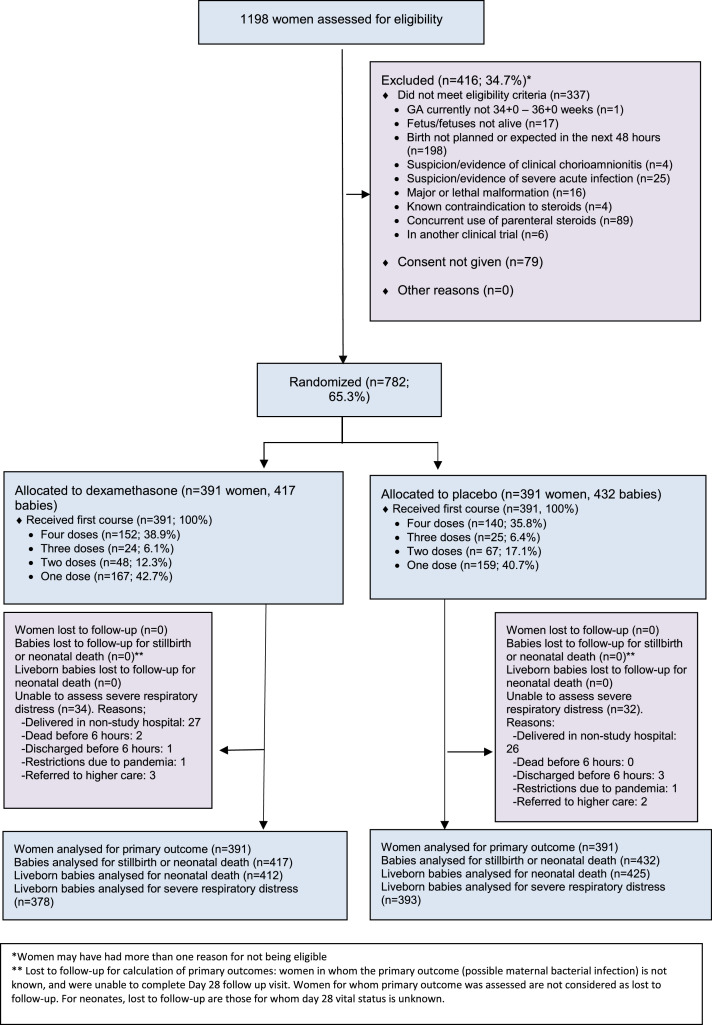
Women were recruited into the study from 26 December 2017 to 21 May 2020. Of the 1198 women who were screened for eligibility, 782 women were randomized - 391 women and their 417 babies to the dexamethasone group, and 391 women and their 432 babies to the placebo group (Figure 1). Birth occurred before 37 weeks for 90.4% of infants in the dexamethasone group and 90.1% of infants in the placebo group. All randomized women and newborns completed follow-up for the mortality outcomes. However, a total of 34 liveborn babies in the dexamethasone group and 32 liveborn babies in the placebo were unable to be fully assessed for the severe neonatal respiratory distress outcome, largely due to babies born at or immediately referred to non-study hospitals, where standardized clinical assessments for trial morbidity outcomes could not be performed.
Figure 1.
Study flowchart.
*Women may have had more than one reason for not being eligible
** Lost to follow-up for calculation of primary outcomes: women in whom the primary outcome (possible maternal bacterial infection) is not known, and were unable to complete Day 28 follow up visit. Women for whom primary outcome was assessed are not considered as lost to follow-up. For neonates, lost to follow-up are those for whom day 28 vital status is unknown.
Characteristics of women in the dexamethasone and placebo groups were similar at baseline (Tables 1, S2). All women received at least one dose of their allocated treatment, while a total of 152 of 391 women (38.9%) in the dexamethasone group and 140 of 391 women (35.8%) in the placebo group received the maximum four doses (Figure 1). The most common reason for non-administration of a scheduled dose was that birth had occurred between doses.
Table 1.
Characteristics of women at trial entry.
| Characteristic | Dexamethasone N = 391 | Placebo N = 391 | ||
|---|---|---|---|---|
| Clinical assessment of imminent preterm birth at trial entry – no. (%) | ||||
| Spontaneously-initiated preterm birth | 205 | (52.4) | 200 | (51.2) |
| Preterm prelabour rupture of membranes | 73 | (18.7) | 76 | (19.4) |
| Spontaneous preterm labour | 132 | (33.8) | 124 | (31.7) |
| Provider-initiated preterm birth | 186 | (47.6) | 191 | (48.8) |
| Gestational age at trial entry – no. (%) | ||||
| 34 weeks 0 days to 34 weeks 6 days | 145 | (37.1) | 150 | (38.4) |
| 35 weeks 0 days to 35 weeks 6 days | 223 | (57.0) | 213 | (54.5) |
| 36 weeks 0 days | 23 | (5.9) | 28 | (7.2) |
| Gestational age at trial entry (wks) – mean (SD) | 34.7 | (0.6) | 34.7 | (0.6) |
| Maternal age (yrs) – mean (SD) | 25.4 | (4.5) | 25.2 | (4.3) |
| No. of fetuses in the current pregnancy – no. (%) | ||||
| Single | 363 | (92.8) | 350 | (89.5) |
| Twin | 28 | (7.2) | 40 | (10.2) |
| Higher order multiples | 0 | (0.0) | 1 | (0.3) |
| Nulliparity |
192 |
(49.1) |
193 |
(49.4) |
| History of preterm birth* – no. (%) | 30 | (15.1) | 26 | (13.0) |
| Obstetric conditions currently present – no. (%)⁎⁎ | ||||
| Gestational diabetes | 13 | (3.3) | 17 | (4.3) |
| Pre-eclampsia or eclampsia | 69 | (17.6) | 86 | (22.0) |
| Gestational hypertension (excl. preeclampsia or eclampsia) | 24 | (6.1) | 35 | (9.0) |
| Oligohydramnios (known or suspected) | 102 | (26.1) | 109 | (27.9) |
| Polyhydramnios (known or suspected) | 14 | (3.6) | 7 | (1.8) |
| Intrauterine growth restriction (known or suspected) | 52 | (13.3) | 73 | (18.7) |
| Macrosomia | 10 | (2.6) | 6 | (1.5) |
| Abruptio placentae | 6 | (1.5) | 7 | (1.8) |
| Placenta praevia | 17 | (4.3) | 17 | (4.3) |
| Other obstetric haemorrhage | 3 | (0.8) | 1 | (0.3) |
| Trimester of pregnancy when ultrasound for gestational age estimate was performed – no. (%) | ||||
| 1st trimester (up to 13 weeks 6 days) | 150 | (38.4) | 152 | (38.9) |
| 2nd trimester (14 weeks 0 days to 27 weeks 6 days) | 192 | (49.1) | 174 | (44.5) |
| 3rd trimester (28 weeks 0 days and beyond) | 49 | (12.5) | 65 | (16.6) |
| Medication administered prior to randomization – no. (%) | ||||
| Tocolytic | 78 | (19.9) | 89 | (22.8) |
| Magnesium sulfate for neuroprotection | 2 | (0.5) | 6 | (1.5) |
Only amongst women with a previous pregnancy.
Women may have had more than one condition; There was no significant difference between treatment groups at an experimentwise error rate of 5%.
Primary and secondary outcomes
There were 11 (2.7%) neonatal deaths in the dexamethasone group, as compared with 12 (2.8%) neonatal deaths in the placebo group (RR 0.95; 95% CI, 0.42–2.12; P = 0.89) (Table 2). Any baby death (stillbirth or neonatal death) occurred in 16 of 417 infants (3.8%) in the dexamethasone group and in 19 of 432 infants (4.4%) in the placebo group (RR 0.87; 95% CI 0.45–1.67; P = 0.89). Severe neonatal respiratory distress was low in both groups (3/378, 0.8% vs 2/393, 0.5%; RR 1.56; 95% CI 0.26–9.29; P = 0.89). Possible maternal bacterial infection occurred in 9 of 391 women (2.3%) in the dexamethasone group and 15 of 391 women (3.8%) in the placebo group (RR 0.60; 95% CI 0.27 to 1.35; risk difference −1.53; 95% CI −3.95 to 0.88; P = 0.002 for non-inferiority), a result consistent with noninferiority at the prespecified margin of 2.5% on the absolute scale. Primary causes of neonatal mortality events are provided in Table S3.
Table 2.
Primary outcomes.
| Primary outcome | Dexamethasone n/N (%) | Placebo n/N (%) | Relative risk (95% CI)* | P-value§ |
|---|---|---|---|---|
| Neonatal death | 11/412 (2.7) | 12/425 (2.8) | 0.95 (0.42 – 2.12) | 0.8916§ |
| Any baby death (stillbirth or neonatal death) | 16/417 (3.8) | 19/432 (4.4) | 0.87 (0.45 - 1.67) | 0.8916§ |
| Severe respiratory distress of the newborn | 3/378 (0.8) | 2/393 (0.5) | 1.56 (0.26 - 9.29) | 0.8916§ |
| Possible maternal bacterial infectionǂ | 9/391 (2.3) | 15/391 (3.8) | 0.60 (0.27 – 1.35) | 0.0020¶ |
Relative risk and 95% CI, calculated from modelling, adjusting for study sites and taking into account the clustering due to multiple birth.
P-value adjusted for multiplicity for the four primary outcomes using the False Discovery Rate approach.
P-value for non-inferiority for possible maternal bacterial infection.
Defined as occurrence of maternal fever of ≥ 38 °C or clinically suspected or confirmed infection, for which therapeutic antibiotics were used. Suspected or confirmed infection included obstetric infection (chorioamnionitis, postpartum endometritis, or wound infection) or non-obstetric infection (respiratory tract infection [pneumonia, pharyngitis, sinusitis or similar], urinary tract infection (excluding pyelonephritis), pyelonephritis, acute cholecystitis or other system infection), captured during hospital admission/s only.
Amongst secondary outcomes, neonatal resuscitation at birth (positive pressure ventilation for more than one minute) was significantly lower in the dexamethasone group compared with the placebo group (1.5% vs 3.8%; RR 0.38, 95% CI 0.15–0.97; p = 0.043) (Table 3). There were no differences in other secondary outcomes relating to maternal and neonatal morbidity and mortality, including neonatal hypoglycaemia and neonatal sepsis (Tables 3 and 4, S4 and S5). There were no differences in process of care outcomes. Although not statistically significant, use of respiratory support was somewhat lower in the dexamethasone group (use of oxygen therapy: RR 0.86, CI 0.61–1.21, use of CPAP: RR 0.55, CI 0.29–1.08) and use of mechanical ventilation: RR 0.65, CI 0.21–1.97). A total of 68/396 infants (17.2%) in the placebo arm and 56/381 infants (14.7%)in the dexamethasone arm received supplemental oxygen, CPAP or mechanical ventilation. The rates of maternal secondary outcomes were also similar between groups; there were no events for chorioamnionitis or endometritis (Tables 3, S4 and S5). Postpartum maternal readmission was rare in both groups (1 woman in dexamethasone group and 2 women in placebo group). Two maternal deaths occurred, both in the placebo group – one due to pulmonary embolism and the other due to multiorgan failure following postpartum haemorrhage. There were 4 maternal adverse events (AEs) in the dexamethasone group and 3 in the placebo group and serious adverse events (SAEs) did not differ between groups (6 SAEs in dexamethasone group and 6 SAEs in placebo group). There was one neonatal AE in the dexamethasone group and one in the placebo group and one SAE in the placebo group (Table S6). All differences in AEs and SAEs were non-significant by Fisher's exact tests (p-value=1 for all). The per-protocol analysis of the primary outcomes had similar findings to the ITT analysis as only five newborns were excluded (Table S7).
Table 3.
Secondary maternal and neonatal outcomes.
| Outcome | Dexamethasone n/N (%) | Placebo n/N (%) | Relative risk (95% CI) |
|---|---|---|---|
| Neonatal outcome | |||
| Stillbirth | 5/417 (1.2) | 7/432 (1.6) | 0.74 (0.24 – 2.31) |
| Early neonatal death (≤7 days) | 9/412 (2.2) | 9/425 (2.1) | 1.03 (0.41 - 2.58) |
| Neonatal sepsis | 3/378 (0.8) | 4/393 (1.0) | 0.78 (0.18 - 3.46) |
| Hypoglycemia* | 31/377 (8.2) | 30/393 (7.6) | 1.09 (0.65 - 1.81) |
| Major resuscitation at birth (positive pressure ventilation for more than 1 min) | 6/412 (1.5) | 16/425 (3.8) | 0.38 (0.15 – 0.97) |
| Use of oxygen therapy* | 54/381 (14.2) | 66/396 (16.7) | 0.86 (0.61 - 1.21) |
| Use of CPAP* | 14/381 (3.7) | 25/396 (6.3) | 0.55 (0.29 - 1.08) |
| Use of mechanical ventilation* | 5/381 (1.31) | 8/396 (2.0) | 0.65 (0.21 - 1.97) |
| Use of parenteral therapeutic antibiotics for 5 days or more ǂ | 44/381 (11.6) | 39/396 (9.9) | 1.21 (0.79 - 1.85) |
| Admission to a special care unit | 193/381 (50.7) | 223/396 (56.3) | 0.90 (0.79 - 1.03) |
| Maternal outcome | |||
| Maternal death | 0/391 (0.0) | 2/391 (0.5) | – |
| Maternal fever | 4/390 (1.0) | 8/390 (2.1) | 0.50 (0.15 - 1.65) |
| Chorioamnionitis | 0/391 (0.0) | 0/391 (0.0) | – |
| Endometritis | 0/391 (0.0) | 0/391 (0.0) | – |
| Wound infection | 2/391 (0.5) | 4/391 (1.0) | 0.50 (0.09 - 2.71) |
| Non-obstetric infection | 6/390 (1.5) | 9/391 (2.3) | 0.67 (0.24 - 1.86) |
| Therapeutic antibiotics | 9/391 (2.3) | 15/391 (3.8) | 0.60 (0.27 – 1.35) |
| Any antibiotic use | 378/390 (96.9) | 380/389 (97.7) | 0.99 (0.97 – 1.02) |
Measured during initial postnatal hospitalization only, until death, discharge or completed day 7 (whichever came first); h, hours; CPAP, continuous positive airway pressure.
Parenteral therapeutic antibiotics for 5 days or more, even if interrupted, excluding neonates who died before 5 completed days; referral for treatment not presented because of very few events.
Table 4.
Secondary neonatal outcomes.
| Neonatal outcome | Dexamethasone |
Placebo |
Median difference (95% CI) | ||
|---|---|---|---|---|---|
| N | Median(IQR) | N | Median(IQR) | ||
| Duration of oxygen therapy (hours) | 54 | 24 (12–60) | 66 | 24 (12–60) | 0.0 (−14.3 to 14.3) |
| Duration of CPAP ventilation (hours) | 14 | 48 (24–60) | 25 | 24 (12–48) | 24.0 (−1.4 to 49.4) |
| Duration of use of mechanical ventilation (hours) | 5 | 60 (36–60) | 8 | 48 (21–84) | 12.0 (−42.9 to 66.9) |
| Duration of parenteral therapeutic antibiotic use (hours) | 44 | 7.0 (6.0–7.0) | 39 | 6.8 (6.0–7.0) | 0.2 (−0.2 to 0.7) |
| Length of hospital stay after birth (days) | 390 | 7.0 (5.0–9.0) | 400 | 7.0 (5.0–9.0) | 0.0 (−0.7 to 0.7) |
We pooled the ACTION-II trial findings with the Cochrane review on antenatal corticosteroids for accelerating fetal lung maturation that was published in 2020 to update its meta-analyses. We found no substantial changes in the strength or direction of risk estimates of neonatal death, either overall, or for the subgroup of babies at 34 weeks gestation or greater (Figs. S1, S2).
Discussion
This hospital-based randomized trial of antenatal dexamethasone for women at risk of late preterm birth did not identify any differences in fetal or neonatal mortality, severe neonatal respiratory distress or possible maternal bacterial infection. The trial was halted prior to reaching its target sample size, however we did not find any evidence of maternal or neonatal harms due to dexamethasone, including neonatal hypoglycaemia. Though there were no differences between the groups for secondary maternal and newborn health outcomes, the use of major resuscitation at birth for newborns was significantly lower with dexamethasone.
This trial of 782 women in four hospitals in India is, at time of writing, the largest efficacy trial of ACS for late preterm birth conducted in a low-resource country context. Women were carefully selected for inclusion based on standardized screening criteria, including clinical assessments by obstetric physicians and verification of gestational age using ultrasound prior to randomisation. Over 80% of trial participants had a first or second-trimester ultrasound, conferring a relatively high accuracy of gestational age dating. In addition, study hospitals had the necessary minimum standards of maternal and preterm newborn care available, including access to oxygen and CPAP. Neonatal survival was evaluated with minimal loss to follow up through in-person visits at 28 days after birth. It is reassuring that neonatal hypoglycaemia was not significantly different between trial arms, though with few events it is not possible to draw any conclusions in this trial population. Comparatively, the multicentre trial in the USA by Gyamfi-Bannerman et al. reported that betamethasone administration increased the incidence of neonatal hypoglycemia by 60%.12 Rates of neonatal sepsis were low but similar between trial arms; the prevalence is similar to the 1.5% prevalence of neonatal systemic infections in the first 48 h of life described by Attawattanakul et al. in their trial of ACS in 194 women at risk of preterm birth in Thailand.22 It is noteworthy that prophylactic parenteral antibiotics for babies perceived as being at risk of infection is common practice in the participating hospitals.
Our sample size calculation assumed a neonatal mortality prevalence of 8% in the placebo arm based on data from the WHO Multi-Country Survey on Maternal and Newborn Health,23 however overall neonatal mortality for participants at the study site was only 2.7%. While this is considerably higher than neonatal mortality rates in other late preterm trials (Porto et al.24 and Gyamfi-Bannerman et al.12 reported neonatal mortality prevalence of 0.07% and 0.7%, respectively), the current trial lacked statistical power to detect any difference in the primary mortality outcome, if such a difference exists. The lower-than-expected outcome rate contributed to the independent DSMB decision to halt the trial. Slow recruitment was also a factor, in part driven by unexpectedly high pre-hospital use of ACS in the late preterm period (26% of women screened were women ineligible for the trial for this reason) – we are unable to determine whether certain groups of women were more or less likely to receive pre-hospital ACS. The somewhat narrow window of gestational age eligibility (34 weeks 0 days to 36 weeks 0 days) is another contributing factor. While Gyamfi-Bannerman et al. used an upper limit of 36 weeks 5 days,12 we opted for the more conservative limit of 36 weeks 0 days, considering the inclusion of women planned or expected to deliver within 48 h, the time required for administering the full regimen (36 h), the need to minimise the risk of women delivering at term, and the error margin for gestational age estimation in the third trimester.25 The original sample size calculation for the trial did not consider the possible effect of clustering due to site or multiple births, as we did not have intra-cluster correlation estimates for the primary outcomes in these settings. Any such clustering effects might have resulted in a slightly increased sample size, however the trial was halted at a considerably lower sample size.
The outcome severe respiratory distress of the newborn was largely based on clinical assessment. Very few events were identified - only 5 of 771 babies in our cohort met this definition - even though 16% of liveborn babies received supplemental oxygen, CPAP or mechanical ventilation, a possible reflection of the inherent subjectivity in the assessment of clinical signs. In addition, prospective assessments of newborn clinical status by research staff commenced at 6 h after birth – it is possible that clinicians may have commenced some neonates on respiratory support interventions prior to or between these assessments. Notably, the need for major neonatal resuscitation at birth was significantly lower in the dexamethasone group. Outcomes related to oxygen, CPAP and mechanical ventilation use were lower, though not statistically significant, in the dexamethasone arm. Taken together, this may suggest a respiratory morbidity benefit in the late preterm population. Future trials would be better served by endpoints that are based on objective use of respiratory support interventions for measuring severe neonatal respiratory morbidity, rather than definitions based solely on clinical features. While overall loss to follow up for mortality outcomes was minimal, approximately 9% of babies were unable to be assessed for the severe respiratory distress outcome, almost entirely due to babies born at non-study hospitals where standardized clinical assessments for morbidity outcomes amongst trial participants could not be performed. We consider it unlikely that the outcome prevalence is different for these babies.
While this trial is underpowered to detect differences, additional considerations that may impact efficacy include the number of doses administered and duration of fetal exposure prior to birth. The study protocol specified that randomized women were eligible for a maximum of four doses, though more than 40% of randomized women delivered after receiving a single dose. Comparatively, the ALPS trial used a regimen of two IM injections of 12 mg betamethasone or placebo 24 h apart, and reported a reduction in the primary outcome with 59.6% of women receiving a full course (i.e. 2 doses) and 40.2% of women receiving a single dose.12 The minimum time required for corticosteroids to have a clinically significant effects in late preterm infants is not yet clearly delineated. Animal studies suggest that fetal lung changes are detectable within 12 h of corticosteroid exposure,26 and a 2020 pharmacokinetic/pharmacodynamic study of 48 non-pregnant, healthy women reported that 6 mg of IM or oral dexamethasone reached maximum concentration in a median of 3 h with a half-life of 5.2 h.27 The WHO ACTION-I trial, which demonstrated the clinical benefits of dexamethasone administered in the early preterm period in low-resource countries, also found that increasing time from first dose to birth increased benefit.16 Additionally, approximately 10% of babies in the current trial were born after 37 weeks – these more mature babies are at considerably lower risk of mortality and respiratory morbidity. These factors may have contributed to the lack of clinical benefit identified. Future efficacy trials of late preterm corticosteroids need to carefully balance the need for enroling women at risk of imminent preterm birth against the time and number of doses required to have clinical effects.
The role of ACS in the late preterm period remains an important clinical and public health question as more than 12 million preterm babies are born after 32 weeks’ gestation worldwide, with significantly higher rates of mortality and morbidity than term babies.1,8 There is also variation amongst national guidelines on whether to use ACS in the late preterm period, though it is not standard practice in India where the trial was conducted. For example, the National Institute of Clinical Excellence (NICE) in the United Kingdom and the American College of Obstetrics and Gynaecology advise that ACS be considered for women in the late preterm period, whereas the Society of Obstetrics and Gynaecology of Canada guidelines indicate that they should not be used routinely, as, the balance of risks and benefits from 35 weeks 0 days to 36 weeks 6 days favours their use only in selected clinical situations.13, 14, 15
While this trial did not detect reductions in neonatal death or severe respiratory distress, we identified a reduction in neonatal resuscitation at birth for newborns, and there was no evidence of difference between groups for neonatal hypoglycemia. The possibility of clinical benefits cannot yet be excluded and further efficacy trials in low-resource countries are required. Ideally, any such trials should explore possible benefits of ACS on composite outcome of newborn mortality and use of respiratory support interventions.
Authors contributions
This trial was initially conceived during a meeting convened by WHO, held in Geneva on 12–13 November 2015. JPV, OTO, AMG and RB co-ordinated the writing of the study protocol, with input from the country principal investigators and the technical advisory group. GP prepared the statistical analysis plan and led all statistical analysis with support from JC and statistical programming team. The trial steering group reviewed and interpreted the final data at a workshop convened by WHO. The first drafts for various sections of the manuscript were prepared by five writing subgroups drawn from the trial steering group. OTO and JPV consolidated the first draft, which was then reviewed and revised critically for intellectual content by all authors. All authors approved the final version and approved the manuscript for publication. The manuscript represents the views of the named authors only.
Data sharing
Request for access to these data can be made to the World Health Organization through srhmph@who.int. Data sharing with any individual or organization will be subject to WHO data sharing policy.
Declaration of interests
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgements
We thank the women and infants who participated in this trial; the physicians, midwives, pharmacists, data managers and research assistants who helped conduct the trial. We are grateful to the following experts for their contributions to various aspects of trial implementation: Betty Kirkwood (chair), Jon Deeks (independent statistician), Siddarth Ramji, Elizabeth Bukusi, Robert Pattinson (2015–2019), and G. Justus Hofmeyr (from 2019) (Data Safety Monitoring Board); Cynthia Pileggi-Castro (trial protocol development); Liana Campodonico, Gabriela Camacho Garcia (data management); Vania A. Nilsson, Luciana Abreu (statistical programming and analysis); Lynn Coppola, Sandhya Maranna, and Devasenathipathy Kandasamy (obstetric and neonatal ultrasound training and quality assurance). We thank Janna Patterson, Jerker Liljestrand and Hilary Gammill for their technical input and support as program managers for WHO ACTION Trials Collaboration at the Bill and Melinda Gates Foundation.
Funding
This trial was primarily funded by the Bill and Melinda Gates Foundation (Grant OPP1136821). Additional support was provided by UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexual and Reproductive Health and Research; and Department of Maternal, Newborn, Child, Adolescent Health, and Ageing, of the World Health Organization, Geneva, Switzerland.
Footnotes
Corresponding authors: Olufemi T. Oladapo oladapoo@who.int (UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Sexualand Reproductive Health and Research, World Health Organization, 20 Avenue Appia, Geneva, Switzerland).
Rajiv Bahl bahlr@who.int (Department of Maternal, Newborn, Child, Adolescent Health, and Ageing, World Health Organization, 20 Avenue Appia, Geneva, Switzerland).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101285.
Supplementary materials
References
- 1.Chawanpaiboon S., Vogel J.P., Moller A.B., et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Inter-Agency Group for Child Mortality Estimation. Levels and trends in child mortality. https://data.unicef.org/resources/levels-and-trends-in-child-mortality/:UNICEF; 2019. [DOI] [PMC free article] [PubMed]
- 3.Platt M.J. Outcomes in preterm infants. Public Health. 2014;128(5):399–403. doi: 10.1016/j.puhe.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 5.Bird T.M., Bronstein J.M., Hall R.W., Lowery C.L., Nugent R., Mays G.P. Late preterm infants: birth outcomes and health care utilization in the first year. Pediatrics. 2010;126(2):e311–e319. doi: 10.1542/peds.2009-2869. [DOI] [PubMed] [Google Scholar]
- 6.Engle W.A., Tomashek K.M., Wallman C., Committee on Fetus and Newborn AeAoP Late-preterm" infants: a population at risk. Pediatrics. 2007;120(6):1390–1401. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 7.Woythaler M.A., McCormick M.C., Smith V.C. Late preterm infants have worse 24-month neurodevelopmental outcomes than term infants. Pediatrics. 2011;127(3):e622–e629. doi: 10.1542/peds.2009-3598. [DOI] [PubMed] [Google Scholar]
- 8.Teune M.J., Bakhuizen S., Gyamfi Bannerman C., et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374. doi: 10.1016/j.ajog.2011.07.015. .e1-9. [DOI] [PubMed] [Google Scholar]
- 9.Vogel J.P., Oladapo O.T., Manu A., Gülmezoglu A.M., Bahl R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob Health. 2015;3(10):e589–e590. doi: 10.1016/S2214-109X(15)00183-7. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 2015. WHO recommendations on interventions to improve preterm birth outcomes. [PubMed] [Google Scholar]
- 11.Althabe F., Belizan J.M., McClure E.M., et al. A population-based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low-income and middle-income countries: the ACT cluster randomized trial. Obstet Gynecol Surv. 2015;70(6):379–381. doi: 10.1016/S0140-6736(14)61651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyamfi-Bannerman C., Thom E.A., Blackwell S.C., et al. Antenatal betamethasone for women at risk for late preterm delivery. N Engl J Med. 2016;374(14):1311–1320. doi: 10.1056/NEJMoa1516783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Care Excellence. Preterm labour and birth. https://www.nice.org.uk/guidance/ng25/resources/preterm-labour-and-birth-pdf-1837333576645, 2015. [PubMed]
- 14.Committee opinion No. 713 summary: antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2017;130(2):493–494. doi: 10.1097/AOG.0000000000002231. [DOI] [PubMed] [Google Scholar]
- 15.Skoll A., Boutin A., Bujold E., et al. 364-Antenatal corticosteroid therapy for improving neonatal outcomes. J Obstet Gynaecol Can. 2018;40(9):1219–1239. doi: 10.1016/j.jogc.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 16.Vogel J.P., Souza J.P., Gülmezoglu A.M., et al. Use of antenatal corticosteroids and tocolytic drugs in preterm births in 29 countries: an analysis of the WHO multicountry survey on maternal and newborn health. Lancet. 2014;384(9957):1869–1877. doi: 10.1016/S0140-6736(14)60580-8. [DOI] [PubMed] [Google Scholar]
- 17.Vogel J.P., Oladapo O.T., Pileggi-Castro C., et al. Antenatal corticosteroids for women at risk of imminent preterm birth in low-resource countries: the case for equipoise and the need for efficacy trials. BMJ Glob Health. 2017;2(3) doi: 10.1136/bmjgh-2017-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO ACTION Trials Collaborators Antenatal dexamethasone for early preterm birth in low-resource countries. N Engl J Med. 2020;383(26):2514–2525. doi: 10.1056/NEJMoa2022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz K.F., Altman D.G., Moher D., Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3) doi: 10.1371/journal.pmed.1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGoldrick E., Stewart F., Parker R., Dalziel S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2020;12 doi: 10.1002/14651858.CD004454.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peto R., Pike M.C., Armitage P., et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attawattanakul N., Tansupswatdikul P. Effects of antenatal dexamethasone on respiratory distress in late preterm infant: a randomized controlled trial. Thai J Obstet Gynaecol. 2015;23:25–33. [Google Scholar]
- 23.Souza J.P., Gülmezoglu A.M., Vogel J., et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross-sectional study. Lancet. 2013;381(9879):1747–1755. doi: 10.1016/S0140-6736(13)60686-8. [DOI] [PubMed] [Google Scholar]
- 24.Porto A.M., Coutinho I.C., Correia J.B., Amorim M.M. Effectiveness of antenatal corticosteroids in reducing respiratory disorders in late preterm infants: randomised clinical trial. BMJ. 2011;342:d1696. doi: 10.1136/bmj.d1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch C.D., Zhang J. The research implications of the selection of a gestational age estimation method. Paediatr Perinat Epidemiol. 2007;21 Suppl 2:86–96. doi: 10.1111/j.1365-3016.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 26.Ikegami M., Polk D., Jobe A. Minimum interval from fetal betamethasone treatment to postnatal lung responses in preterm lambs. Am J Obstet Gynecol. 1996;174(5):1408–1413. doi: 10.1016/s0002-9378(96)70581-1. [DOI] [PubMed] [Google Scholar]
- 27.Jobe A.H., Milad M.A., Peppard T., Jusko W.J. Pharmacokinetics and pharmacodynamics of intramuscular and oral betamethasone and dexamethasone in reproductive age women in India. Clin Transl Sci. 2020;13(2):391–399. doi: 10.1111/cts.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.