Summary
Background
The efficacy and mechanisms of acupuncture for Crohn's disease (CD) are not well understood. We investigated its effects on symptoms, intestinal microbiota, and circulating inflammatory markers in CD patients.
Methods
This 48-week, randomized, sham controlled, parallel-group clinical trial was performed at a tertiary outpatient clinic in China. From April 2015 to November 2019, 66 patients (mean age 40·4, 62·1% were male, all were Han Chinese) with mild to moderate active CD and unresponsive to drug treatment were enrolled and randomly assigned equally to an acupuncture group or a sham group. The treatment group received 3 sessions of acupuncture plus moxibustion per week for 12 weeks and a follow-up of 36 weeks. Clinicaltrials.gov: NCT02559037.
Findings
At week 12, the clinical remission rate (the primary outcome) and clinical response rate of acupuncture group were significantly higher than that of sham group, with a difference of 42·4% (95% CI: 20·1%-64·0%) and 45·5% (95% CI: 24·0%-66·9%), respectively, both of which maintained at week 48. The acupuncture group had significantly lower CD activity index and C-reactive protein level at week 12, which maintained at 36-week follow-up. The CD endoscopic index of severity, histopathological score, and recurrence rate at week 48 were significantly lower in acupuncture group. The number of operational taxonomic unit of intestinal microbiota and relative abundance of Faecalibacterium prausnitzii and Roseburia faecis were increased. Plasma diamine oxidase, lipopolysaccharide, and Th1/Th17 related cytokines were decreased in 12-week after acupuncture.
Interpretation
Acupuncture was effective in inducing and maintaining remission in patients with active CD, which was associated with increased abundance of intestinal anti-inflammatory bacteria, enhanced intestinal barrier, and regulation of circulating Th1/Th17-related cytokines.
Funding
National Key Basic Research Program of China (2015CB554500 and 2009CB522900), Shanghai Rising-Star Program (19QA1408100).
Keywords: Acupuncture, Inflammatory bowel disease, Gut microbes, Intestinal barrier, Alternative therapy
Abbreviations: CD, Crohn's disease; CDAI, Crohn's disease activity index; CDEIS, Crohn's disease endoscopic index of severity; CRP, C-reactive protein; DAO, diamine oxidase; HCs, healthy control subjects; HS, histopathological score; IBD, inflammatory bowel disease; ITT, intention to treat; LEfSe, linear discriminant analysis effect size; LPS, lipopolysaccharides; OTU, operational taxonomic unit; PP, per-protocol; SCFAs, short chain fatty acids
Research in context.
Evidence before this study
We searched PubMed for studies including clinical trials, randomized controlled trials, systematic reviews, and meta-analyses from the inception of the database to October 31, 2021 using the search terms "acupuncture" or "electroacupuncture" or "moxibustion" in combination with "Crohn's disease". Although four randomized controlled trials (three in English and one in Chinese) as well as two observational trials report acupuncture treatment for Crohn's disease (CD), the quality of this evidence is not optimal and needs to be improved. As described in a meta-analysis, there are very few robust clinical trials of acupuncture for CD. Further, conventional drugs and biologic are effective for only a fraction of patients, but no study has been made on testing the effect of acupuncture and moxibustion on CD patients who are unresponsive, intolerant or dependent on these medications.
Added value of this study
This is the first randomized, sham controlled, parallel-group, 48-week follow-up clinical trial that evaluated the efficacy and safety of acupuncture for mildly and moderately active patients with CD who had poor drug response. The study provides stronger evidence that 12 weeks of acupuncture was safe and effective in inducing and maintaining disease remission, and that this effect maintained for at least 48 weeks. Its therapeutic effects may be related to increased relative abundance of anti-inflammatory bacteria as well as short chain fatty acids-producing bacteria, increased intestinal epithelial barrier function, and inhibition of Th1/Th17-related pro-inflammatory cytokines.
Implications of all the available evidence
CD is poorly controlled by currently available medications and the effects are often short-lasting. The conclusions of this study will encourage the clinical use of acupuncture as a safe and effective alternative treatment for mild to moderately active CD patients, especially those who are poorly responsive or intolerant to medications. Future research may include refining and improving the effect of acupuncture by using differentiated acupuncture protocols based on patient disease subtypes such as disease location or disease behavior, and further exploring inflammatory mechanism.
Alt-text: Unlabelled box
Introduction
Crohn's disease (CD) is a chronic, recurrent inflammatory disease that can affect any part of the digestive tract. The main symptoms include abdominal pain, diarrhea, and weight loss.1 While the highest prevalence of CD is in North America and Europe (range: 319/105–322/105),2 a rapid increase in the incidence of CD occurs in newly industrialized countries such as China, owing to the impact of westernized diets and environmental changes on individual intestinal microbiota.3 The pathogenetic mechanism of CD involves dysfunctional intestinal microbiota, which may impair the integrity of the intestinal barrier through the activation of inflammatory pathways in genetically susceptible individuals.3,4 Recent studies have shown that the intestinal microbiota and immune response in patients with active CD are significantly less stable than in healthy individuals. The active phase of the disease is marked by changes in intestinal microbial diversity, such as depletion of obligate anaerobes [including Faecalibacterium Prausnitzii and Roseburia hominis, short chain fatty acids (SCFAs) producing bacteria], and enrichment of facultative anaerobes such as Escherichia coli.5
Dyshemostasis of CD4+ T cell differentiation in intestinal mucosa and peripheral circulation is another feature of CD, manifested by Th1 and Th17 cell-mediated immune inflammatory responses.6, 7, 8, 9 Studies have demonstrated increased expressions of Th1 and Th17 cells, as well as those of IFN-γ, IL-17A, and other cytokines secreted by these cells in the intestinal mucosa and peripheral blood of patients with active CD.8 The impaired integrity of intestinal epithelial barrier and the consequent enhanced intestinal permeability is believed to promote the infiltration of intestinal microorganisms and antigens from the intestinal lumen to the lamina propria; this further induces aberrant immune response in CD.9
The efficacy of currently available drugs for CD (such as aminosalicylic acid, glucocorticoids, immunomodulators, and biological agents) is rather unsatisfactory and some of these drugs are also costly and have several side effects.10 About 1/3–2/3 of patients fail to respond to TNF-α antagonists or have decreased response over time.11 Therefore, developing effective therapies for CD is imperative.12 Acupuncture is an alternative therapy that has been used for the treatment of inflammatory bowel diseases around the world.13,14 Acupuncture and moxibustion are often used together to achieve synergistic effect.15 Joos et al.16 and our research team17 found that acupuncture can reduce the CD activity index (CDAI) and improve the quality of life of patients with mild to moderate active CD. It can also improve the general well-being of patients and reduce the concentrations of serum α1-acid glycoprotein16 and C-reactive protein (CRP), and increase hemoglobin level.17 However, a meta-analysis18 revealed a paucity of robust clinical trials of acupuncture in patients with CD. The main shortcomings of previous studies include uncertain evidence of improvement in endoscopic performance, inadequate duration of follow-up, and lack of standardization of acupuncture technique and evaluation of the underlying mechanisms. In addition, intestinal microbiota and inflammation are believed to play a key role in the pathogenesis of CD,3,4 while acupuncture is shown to regulate them.19, 20, 21 The effects of acupuncture may be achieved through regulating the function of the central nervous system, the cholinergic and the splenic sympathetic anti-inflammatory pathway and the hypothalamic-pituitary-adrenal axis.13,22,23
We hypothesized that acupuncture may improve clinical symptoms of CD by regulating intestinal microbial composition and Th1/Th17 cell-mediated inflammatory response. Based on improved acupuncture methods, we conducted a randomized, sham-controlled, parallel grouping, 48-week follow-up trial in patients with mild to moderate active CD who were not responding, intolerant, dependent, or refusing to use medications.
Methods
Study design
The protocol was approved by the Institutional Review Board of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (2015–007), and was registered on ClinicalTrials.gov: NCT 02,559,037. All participants provided written consent. The study adheres to the CONSORT guidelines for reporting randomized trials.
Computerized random numbers were generated by an independent investigator and kept in a sealed, opaque envelope. When eligible subjects were included, the envelopes were opened by an unblinded acupuncturist (C.H.B.) in order of numbering on the envelopes to view the randomly assigned group. The acupuncturist will give the subject the corresponding intervention. The eligible patients were randomly assigned to the acupuncture group or sham acupuncture group in a 1:1 ratio. The subjects received 3 treatment sessions per week for 12 consecutive weeks, which was followed by a follow-up period of 36 weeks. During the treatment, the patients were blinded from the treatment process by an opaque curtain and asked to close their eyes, and were not allowed to communicate with each other to avoid unblinding. At the end of treatment, the subjects were asked to give their blinded guess about specific treatment they received. The treating physicians who prescribed the medication were blinded to the grouping and separated from two independent specialists who performed the acupuncture. The outcome evaluators (J.Z.Z. and D.W.) and statisticians (L.M.C. and G.N.L.) were also blinded to the grouping.
The CDAI score24 and serum CRP level were used to assess disease severity at baseline and at weeks 12, 24, 36, and 48. Ileocolonoscopy and biopsy were performed at baseline and week 48. The findings were central reading and graded according to the Crohn's disease endoscopic index of severity (CDEIS) score25 and histopathological score (HS),26 respectively. Plasma immunological indicators, fecal calprotectin concentation,27 and intestinal microbiota structure and diversity were assessed at baseline and 12 weeks. Blood samples (serum, plasma) and fecal samples were stored at −80 °C within 2 h after collection. A schematic illustration of the study protocol is shown in Supplementary Fig. S1.
Subjects
From April 1, 2015 to November 30, 2019, CD patients were screened and referred from the gastroenterology departments of five general hospitals in China. All patients received systematic and gastrointestinal examinations, including ileocolonoscopy and histopathology, MRI or CT examination of the small intestine and the diagnosis28 was confirmed by a gastroenterologist. The eligible patients were enrolled at the outpatient clinic of the inflammatory bowel disease (IBD) specialty at the Shanghai Research Institute of Acupuncture and Meridian. Healthy control subjects (HCs) were recruited from the Shanghai University of Traditional Chinese Medicine. The investigators responsible for subject enrollment were D.W. and J.Z.Z., who were unaware of the grouping. Inclusion/exclusion criteria for patients and HCs are described in Table 1.
Table 1.
The selection criteria for patients and healthy control subjects (HCs).
| Patients with CD |
| Inclusion criteria: |
| 1. age 16–70 years; 2. patients in mild to moderate active phase (150≤CDAI<450) and with evidence of active inflammation (serum CRP concentration ≥5 mg/L, fecal calprotectin concentration ≥250 μg/g, or endoscopy with obvious evidence of ulcerations during a 4-week screening period); 3. patients who were not responsive, intolerant, dependent, or refused to use any one of the following medications: mesalazine, glucocorticoid, immunomodulator (azathioprine, methotrexate), and anti-TNF-α agents; 4. not taking medicine or taking one or more of the following: prednisone ≤15 mg/d (at least for 1 month); azathioprine (≤1 mg/kg/d), methotrexate (≤15 mg/wk), or mesalazine (≤4 g/d) (at least for 3 months); 5. no history of treatment with anti-TNF-α agents within the 3 months immediately preceding the enrolment; 6. no history of acupuncture therapy; 7. provision of written informed consent. |
| Exclusion criteria: |
| 1. Pregnant or lactating women, and those desirous of conceiving in the near future; 2. patients with severe organic diseases; 3. patients with mental illness; 4. patients receiving antibiotics, probiotics, prebiotics, traditional Chinese medicine, or other drugs; 5. patients with multiple comorbid conditions that require long-term therapy with drugs, which may affect the outcome measures; 6. patients with severe extraintestinal manifestations, such as severe skin diseases, eye diseases, or thromboembolic diseases; 7. severe intestinal fistula in the abdomen, abdominal abscess, intestinal stenosis and intestinal obstruction, perianal abscess, gastrointestinal bleeding, bowel perforation, or other complications; 8. patients with short bowel syndrome; 9. patients with a history of abdominal or gastrointestinal surgery in the past six months; 10. patients with skin diseases or defects at the sites of acupuncture that prevents the application of acupuncture. |
| HCs Inclusion criteria:
|
| Exclusion criteria: |
|
Intervention and control
Acupuncture group: We selected acupoints including Zhongwan (CV12) and bilateral Shangjuxu (ST37), Sanyinjiao (SP6), Gongsun (SP4), Taichong (LR3), Taixi (KI3), Hegu (LI4), and Quchi (LI11)17 according to the World Health Organization standard29 (Supplementary Fig. S2a). Single-use 0·30 × 40 mm or 0·30 × 25 mm acupuncture needles (Hwato, Suzhou, China)30,31 were vertically inserted into each acupoint to 20–30 mm depth to obtain a deqi sensation (a soreness, distention, numbness or heaviness sensation). Bilateral Zusanli (ST36) and Tianshu (ST25) were selected for moxibustion. Pure moxa sticks (diameter: 2·8 cm; Hanyi, Nanyang, China) were ignited and fixed on a moxibustion stand at a distance of 3–5 cm to the surface of acupoints. The temperature of skin surface at the acupoints was maintained at 43 ± 1 °C and monitored with a miniature infrared thermometer (Fluke 62, Fluke Corporation, Everett, WA, USA). Acupuncture and moxibustion were concomitantly performed for 30 min (Supplementary Fig. S2b).
Sham acupuncture group: Sham acupuncture needles (0·35 × 40 mm) with flat tips (Hwato, Suzhou, China) were inserted towards the same acupoints to induce slight pain but without penetrating the skin. Sham moxibustion was made by igniting the same type of moxa sticks but fixing them at a distance of 8–10 cm from the skin of acupoints to maintain the temperature at 37 ± 1 °C. Sham acupuncture and moxibustion were concomitantly performed for 30 min (Supplementary Fig. S2c).
After enrollment, no additional drugs or interventions were allowed during the treatment period. For patients who received combination treatment with mesalazine, corticosteroids, azathioprine, or methotrexate, the original dose was kept unchanged. During follow-up, if the serum CRP level of the subjects returned to normal (< 5 mg/L), the dosage of glucocorticoid (prednisone) was gradually reduced at a dose of 2·5 mg every two weeks. In the event of aggravation of patient's condition or occurrence of serious adverse reactions that required an increased dose of corticosteroids, the investigator should be informed for record. Subjects were withdrawn from the study if medications or other therapies other than those prescribed in this study were required.
Outcomes
Efficacy outcome
The primary outcome measure was the proportion of patients with clinical remission at the completion of treatment (defined as CDAI score < 150 and decrease in CDAI score by ≥ 70 from baseline).32
The secondary outcome measures were: proportion of patients with clinical remission at weeks 24, 36, and 48; clinical response ratio (defined as a decrease in CDAI score by ≥ 70 points from baseline),32 mean changes in CDAI score and serum CRP level at weeks 12, 24, 36, and 48; proportion of patients with corticosteroid-free remission at weeks 48; the mean change in CDEIS score and HS at week 48; cumulative proportions of patients with recurrence at weeks 24, 36, and 48. Recurrence was defined as CDAI > 150 and an increase of ≥ 70 points, or need to adjust medications to control the disease.33 The safety outcomes included vital signs (blood pressure, body temperature, heart rate, pulse), adverse effects linked to acupuncture and moxibustion.
Fecal microbiological and calprotectin outcome
We quantified changes in the operational taxonomic unit (OTU) number and composition of intestinal microbiota including relative abundance of targeting SCFAs producing bacteria (Coprococcus, Lachnospira, Oscillospira, Roseburia, and Roseburia faecis) and anti-inflammatory bacteria (Faecalibacterium, and Faecalibacterium prausnitzii), α-diversity indices (sobs, chao1, ace, shannon, simpson, and coverage), β-diversity tests (unweighted, and weighted UniFrac metrics) and the change in fecal calprotectin concentration at different time points.
Circulating inflammatory markers
We used enzyme-linked immunosorbent assay to measure plasma concentrations of diamine oxidase (DAO), lipopolysaccharides (LPS), d-lactic acid, and Th1/Th17-related cytokines IFN-γ, IL-17A, IL-23, TNF-α, and IL-1β.
Statistical analysis
Based on a previous study,17 the difference between the proportion of patients with clinical remission in the acupuncture and sham groups was set to 45% (with the acupuncture and sham groups being 65% and 20%, respectively). Incorporating a test level α = 0·05, and power 1-β = 0·9, each group required at least 33 patients (including a 15% dropout rate).
The categorical variables were analyzed using Chi squared test or Fisher's exact test and expressed as frequency (percentage); the continuous variables were expressed as mean ± standard deviation. The 95% confidence intervals (CIs) of the rate difference and median difference were estimated by Wald method (without continuity correction)34 and Hodges-Lehmann, respectively. The mean change in CDAI score and serum CRP level at multiple time-points were measured by the mixed effect model and two independent samples t-test; the fecal calprotectin concentration, CDEIS score, HS and the circulating inflammatory markers were compared using the t-test or one-way analysis of variance (ANOVA).
The α-diversity and relative abundance of the genera and species of intestinal microbiota were analyzed using Kruskal-Wallis H test and Nemenyi test or Wilcoxon signed-rank/rank-sum test and expressed as median (interquartile range). Linear discriminant analysis effect size (LEfSe) was used to analyze the differences of the microbial population between the acupuncture and sham group at the completion of treatment and the HCs. Linear discriminant analysis > 2·0 was considered as microbes with significant impact. The microbial bioinformation was analyzed by R version 3·1·1 (Vienna, Austria). Linear regression was used to analyze the correlation between clinical variables, bacterial flora, and inflammatory markers. p ≤ 0·05 was considered statistically significant. See Appendix S4 in the Supplementary materials for further details. Statistical analysis was performed using SPSS Version 24·0 (IBM Corp., Armonk, NY, USA).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Patient characteristics
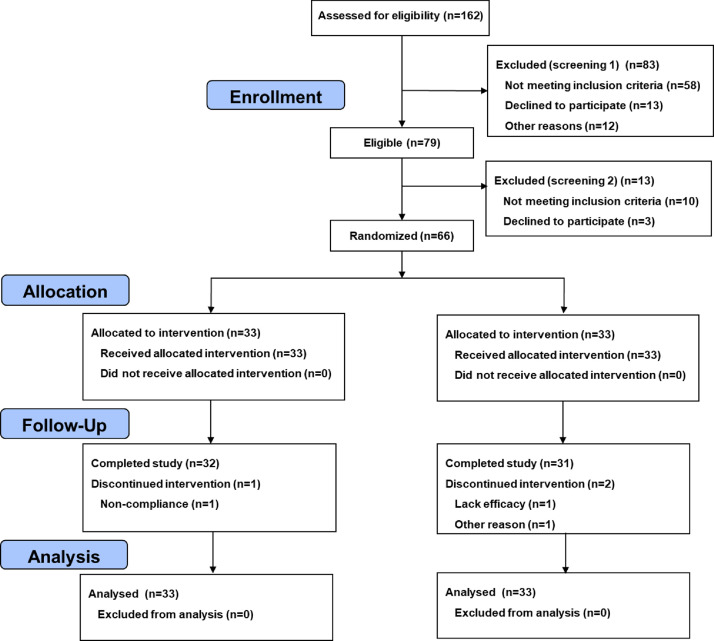
A total of 162 patients were screened, and 96 patients were excluded after 2 screenings. Finally, 66 patients with a mean age of 40·4 (SD 14·2) years, 62·1% male, all Han Chinese and a duration of CD of 41·9 (SD 34·6) months were included. The Montreal classification: A1 (n = 4, 6·1%), A2 (n = 38, 57·6%), A3 (n = 24, 36·4%); L1 (n = 23, 34·8%), L2 (n = 12, 18·2%), L3 (n = 31, 47·0%); B1 (n = 19, 28·8%), B2 (n = 8, 12·1%), B3 (n = 39, 59·1%), B1p (n = 6, 9·1%), B2p (n = 2, 3·0%), B3p (n = 17, 25·8%). Concomitant medication: glucocorticoid (n = 14, 21·2%), 5-aminosalicylicacid (n = 29, 42·9%), Azathioprine (n = 21, 31·8%), Methotrexate (n = 4, 6·1%). Twenty-seven (40·9%) subjects had moderate activity. The mean CDAI for all subjects was 208·7 (SD 48·5). There were no significant differences between the two groups of patients with respect to baseline demographic and clinical characteristics (Table 2). All 66 patients (33 in each group) were included in the intention to treat (ITT) analysis and safety analysis. Among these, 32 patients in the acupuncture group (1 dropout due to long distance traveling) and 31 cases in the sham group (2 patients withdrew voluntarily due to either lack of therapeutic effect or time conflict) completed treatment and 48-week follow-up (Figure 1). The baseline characteristics of the sub-study population are presented in Supplementary Table S1.
Table 2.
Demographic and baseline characteristics of the patient population.
| Characteristics | Acupuncture group (n = 33) | Sham group (n = 33) | Total (n = 66) |
|---|---|---|---|
| Gender (male), n (%) | 20 (60·6) | 21 (63·6) | 41(62·1) |
| Age (years), mean (SD) | 39·5 (15·0) | 41·3 (13·5) | 40·4(14·2) |
| Height (cm), mean (SD) | 168·1 (7·4) | 168·2 (8·3) | 168·2(7·8) |
| Weight (kg), mean (SD) | 53·9 (8·1) | 54·8 (10·5) | 54·3(9·3) |
| BMI (kg/m2), mean (SD) | 19·1 (2·5) | 19·3 (2·8) | 19·2(2·7) |
| Ethnicity (Han Chinese), n (%) | 33 (100·0) | 33 (100·0) | 66(100·0) |
| Duration of CD (months), mean (SD) | 41·4 (35·9) | 42·4 (33·8) | 41·9(34·6) |
| The Montreal classification, n (%) | |||
| A1 | 1 (3·0) | 3 (9·1) | 4(6·1) |
| A2 | 19 (57·6) | 19 (57·6) | 38(57·6) |
| A3 | 13 (39·4) | 11 (33·3) | 24(36·4) |
| L1 | 11 (33·3) | 12 (36·4) | 23(34·8) |
| L2 | 6 (18·2) | 6 (18·2) | 12(18·2) |
| L3 | 16 (48·5) | 15 (45·5) | 31(47·0) |
| B1 | 22 (66·7) | 17 (51·5) | 39(59·1) |
| B2 | 6 (18·2) | 13 (39·4) | 19(28·8) |
| B3 | 5 (15·2) | 3 (9·1) | 8(12·1) |
| B1p | 7 (21·2) | 10 (30·3) | 17(25·8) |
| B2p | 2 (6·1) | 4 (12·1) | 6(9·1) |
| B3p | 1 (3·0) | 1 (3·0) | 2(3·0) |
| Concomitant medication, n (%) | 26 (78·8) | 27 (81·8) | 53(80·3) |
| Glucocorticoid (prednisone) | 9 (27·3) | 5 (15·2) | 14(21·2) |
| 5-ASA | 12 (36·4) | 17 (51·5) | 29(43·9) |
| AZA | 12 (36·4) | 9 (27·3) | 21(31·8) |
| MTX | 1 (3·0) | 3 (9·1) | 4(6·1) |
| Surgical history, n (%) | 10 (30·3) | 14 (42·4) | 24(36·4) |
| CDAI, mean (SD) | 211·3 (52·2) | 206·0 (45·1) | 208·7(48·4) |
| CDAI > 220, n (%) | 13 (39·4) | 14 (42·4) | 27(40·9) |
| CRP (mg/L), mean (SD) | 14·8 (16·5) | 12·7 (15·7) | 13·8(16·0) |
| CDEIS, mean (SD) | 9·2 (4·9) | 9·5 (5·2) | 9·3 (5·0) |
| Ulcers of small intestine, n (%)a | 15 (56·6) (n = 27) | 14 (51·9) (n = 27) | 29(53·7) (n = 54) |
| Edema of small intestine, n (%)a | 20 (74·1) (n = 27) | 21 (77·8) (n = 27) | 41(75·9) (n = 54) |
| FC (µg/g), mean (SD) | 560·1(159·4) (n = 15) | 534·4(173·7) (n = 15) | 547·2(164·3) (n = 30) |
NOTE: 5-ASA, 5-aminosalicylicacid; A1, age > 17; A2, age 17–40; A3, age < 40; AZA, Azathioprine; B1, non-structuring and non-penetrating; B2, structuring; B3, penetrating; BMI, body mass index; CD, Crohn's Disease; CDAI, Crohn's Disease Activity Index; CDEIS, Crohn's disease endoscopic index of severity; CRP, C-reactive protein; FC, fecal calprotectin; L1, ileal; L2, colonic; L3, ileocolic; MTX, Methotrexate; P, Perianal disease; SD, standard deviation.
Sum of L1 and L3.
Figure 1.
The CONSORT flowchart of the patient flow throughout the study.
Primary efficacy outcome
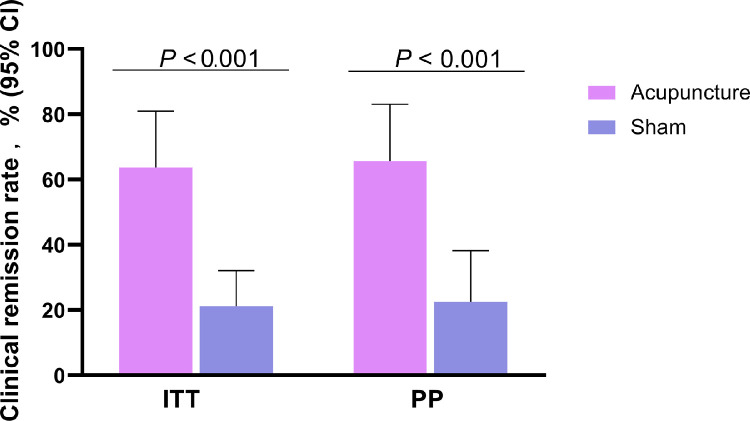
ITT and per-protocol (PP) analysis showed that the proportion of patients with clinical remission at week 12 in the acupuncture group (63·6%, 65·6%, respectively) was significantly higher than that in the sham group (21·2%, 22·6%, respectively) with difference of 42·4% (95% CI: 20·1%−64·0%, p < 0·001 for ITT) and 43·0% (95% CI: 20·3%−65·1%, p < 0·001 for PP) (Figure 2).
Figure 2.
Acupuncture treatment significantly increased the rate of CD remission in 12 weeks after treatment, ITT analysis (n = 33 in each group) and PP analysis (n = 32 in acupuncture group, n = 31 in sham group). Proportion of patients with clinical remission (CDAI score <150 and decrease ≥70 from baseline) at the completion of 12-week treatment.
Secondary efficacy outcomes
Clinical remission rate
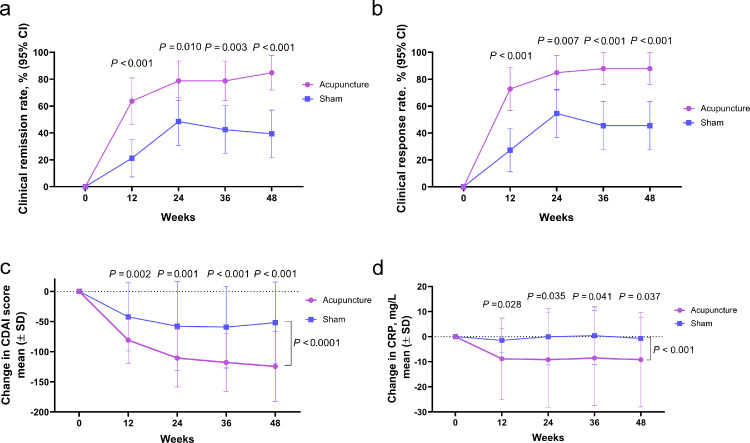
The proportions of patients with clinical remission at weeks 24, 36, and 48 of the acupuncture group (78·8%, 78·8%, 84·9%, respectively) were significantly greater than that of the sham group (48·5%, 42·4%, 39·4%, respectively) (wk 24: difference: 30·3%, 95%CI: 8·3%−52·3%, p = 0·011; wk 36: difference: 36·4%, 95%CI: 14·5%−58·3%, p = 0·003; wk 48: difference: 45·5%, 95%CI: 24·8%−66·1%, p = 0·001, respectively) (Figure 3a). The PP analysis results were consistent with the ITT analysis (Supplementary Fig. S3a). 78·8% of patients in the acupuncture group and 27·3% in the sham group were corticosteroid-free remission at week 48, with a difference of 51·5% (95%CI: 30·9%−72·1% (p < 0·001).
Figure 3.
Acupuncture treatment had a better effect on clinical remission and clinical response to CD, as well as on decreasing the CDAI score and CRP level at week 12 and 36-week follow-up (n = 33 in each group). Proportion of patients with (a) clinical remission (CDAI score < 150 and decreased ≥ 70 from baseline) and (b) Clinical response (decrease in CDAI score by ≥ 70 from baseline) at weeks 12, 24, 36, and 48 for ITT analysis. The mean change in (c) CDAI score and (d) CRP level from baseline at weeks 12, 24, 36, and 48 for ITT analysis.
Clinical response rate
The proportions of clinical response of patients at weeks 12, 24, 36, and 48 in the acupuncture group (72·7%, 84·9%, 87·9%, 87·9% respectively) were significantly greater than that in the sham group (27·3%, 54·6%, 45·5%, 45·5% respectively) (wk 12: difference: 45·5%, 95%CI: 24·0%−66·9%, p < 0·001; wk 24: difference: 30·3%, 95%CI: 9·4%−51·2%, p = 0·007; wk 36: difference: 42·4%, 95%CI: 22·1%−62·7%, p < 0·001; wk 48: difference: 42·4%, 95%CI: 22·1%−62·7%, p < 0·001, respectively) (Figure 3b). The PP analysis results were consistent with the ITT analysis (Supplementary Fig. S3b).
Disease activity
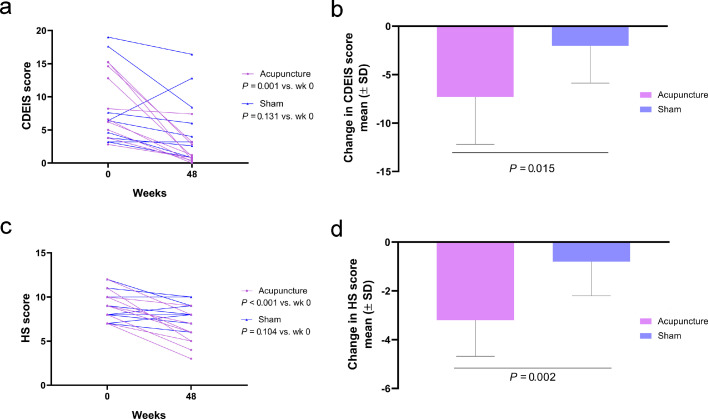
The mean decreases in CDAI score from baseline at weeks 12, 24, 36, and 48 in the acupuncture group were significantly greater than that in the sham group (wk 12: difference: 38·5, 95%CI: 14·8–62·2, p = 0·002; wk 24: difference: 52·6, 95%CI: 21·9–83·3, p = 0·001; wk 36: difference: 58·7, 95%CI: 29·8–87·6, p = 0·001; wk 48: difference: 72·5, 95%CI: 41·5–103·5, p < 0·001, respectively) (Figure 3c). The mean decreases in CRP levels from baseline at weeks 12, 24, 36, and 48 in the acupuncture group were significantly greater than those in the sham group (wk 12: difference: 7·4, 95%CI: 0·8–13·9, p = 0·028, wk 24: difference: 9·1, 95%CI: 0·7–17·6, p = 0·034, wk 36: difference: 8·9, 95%CI: 0·4–17·4, p = 0·041, wk 48: difference: 8·5, 95%CI: 0·5–16·4, p = 0·037, respectively) (Figure 3d). At week 48, the acupuncture group had significantly lower CDEIS score and HS than the baseline (difference: −7·3, 95%CI: −3·8-(−10·8), p = 0·001; difference: −3·2, 95%CI: −2·1-(−4·3), p < 0·001, respectively), while the sham group did not (difference: −2·0, 95%CI: −4·8–0·7, p = 0·131; difference: −0·8, 95%CI: −1·8–0·2, p = 0·104, respectively) (Figure 4a and c). The mean change in the CDEIS score and HS from baseline in the acupuncture group were significantly better than that in the sham group (CDEIS: difference: 4·1, 95%CI: 0·8–7·4, p = 0·015; HS: difference: 2·4, 95%CI: 1·0–3·8, p = 0·002, respectively) (Figure 4b and d).
Figure 4.
Acupuncture treatment significantly decreased CDEIS score and HS at the end of 48-week follow-up (n = 10 in each group). (a) CDEIS score at baseline and week 48; (b) Mean change in CDEIS score from baseline at week 48; (c) HS at baseline and week 48; (d) Mean change in HS from baseline at week 48.
Recurrence rate
The cumulative proportions of patients with recurrence at weeks 24, 36, and 48 in the acupuncture group (all 6·1%) were significantly lower than those in the sham group (27·3%, 33·3%, and 45·5%, respectively) (wk 24: difference: 21·2%, 95%CI: 4·0%−38·4%, p = 0·021; wk 36: difference: 27·3%, 95%CI: 9·2%−45·3%, p = 0·005; wk 48: difference: 39·4%, 95%CI: 20·6%−58·2%, p < 0·001, respectively).
Blinding assessment
In the acupuncture and sham groups, 87·9% (29/33) and 78·8% (26/33), respectively, considered that they had received actual acupuncture treatment (difference: 9·1%, 95%CI: −8·8%−26·9%, p = 0·322).
Safety outcome
A total of 2 adverse events (2/66) occurred in this study; both were in the acupuncture group. One patient developed mild blood stasis in the left SP6, while the other patient developed mild scalding due to falling of moxibustion ash at the left ST25. Both patients healed completely after symptomatic treatment. No serious adverse events occurred.
Fecal microbiological and calprotectin outcome
OTU analysis
At baseline, the numbers of OTUs unique to HCs, the acupuncture and sham groups were 256, 81 and 39, respectively (Supplementary Fig. S4a). After treatment, the number of unique OTUs in acupuncture group increased by 13 (65 to 78), and the number of OTUs coinciding with the HCs increased by 14 (Supplementary Fig. S4b, c). The number of unique OTUs in the sham group increased by one (75 to 76), while the number of OTUs coinciding with the HCs decreased by five (Supplementary Fig. S4b, d). This suggests that CD caused a decrease in species diversity in the intestinal microbiota while acupuncture improved it. Acupuncture was better than sham acupuncture in this respect.
α-Diversity index analysis
The intestinal flora coverage index of all the subjects was close to 1, indicating that the sample sequencing was relatively complete. The baseline sobs, chao1, and ace indices of the two patient groups were significantly lower than HCs, which suggests a low intestinal bacterial community richness in patients. The median change in ace index from baseline in the acupuncture group was significantly better than that in the sham group (difference: 38·0, 95%CI: 3·9–66·7, p = 0·032) and the chao1 index was nearly significant (difference: 30·3, 95%CI: −2·7–61·4, p = 0·062), suggesting a better effect of acupuncture on the enrichment of intestinal microorganisms in patients with CD (Supplementary Tables S2, S3).
Microbial structure analysis
At the genus level, the baseline relative abundance of Faecalibacterium, Lachnospira, Coprocococcus, Roseburia and Oscillospira in the two patient groups were all significantly lower than that in HCs (Supplementary Table S4). At the completion of treatment, the median change of Faecalibacterium and Lachnospira from baseline in the acupuncture group was significantly better than that in the sham group (difference: 2·1, 95%CI: 0·0–5·3, p = 0·009; difference: 0·6, 95%CI: 0·0–1·8, p = 0·045, respectively; Supplementary Table S5).
At the species level, the relative abundance of baseline Faecalibacterium prausnitzii and Roseburia faecis in the two patient groups was significantly lower than that in HCs (Supplementary Table S4). At the completion of treatment, the median change of these two species of bacteria from baseline in the acupuncture group was significantly better than that in the sham group (difference: 2·1, 95%CI: 0·0–5·3, p = 0·009; difference: 0·6, 95%CI: 0·0–2·0, p = 0·043, respectively; Supplementary Table S5).
Lefse analysis
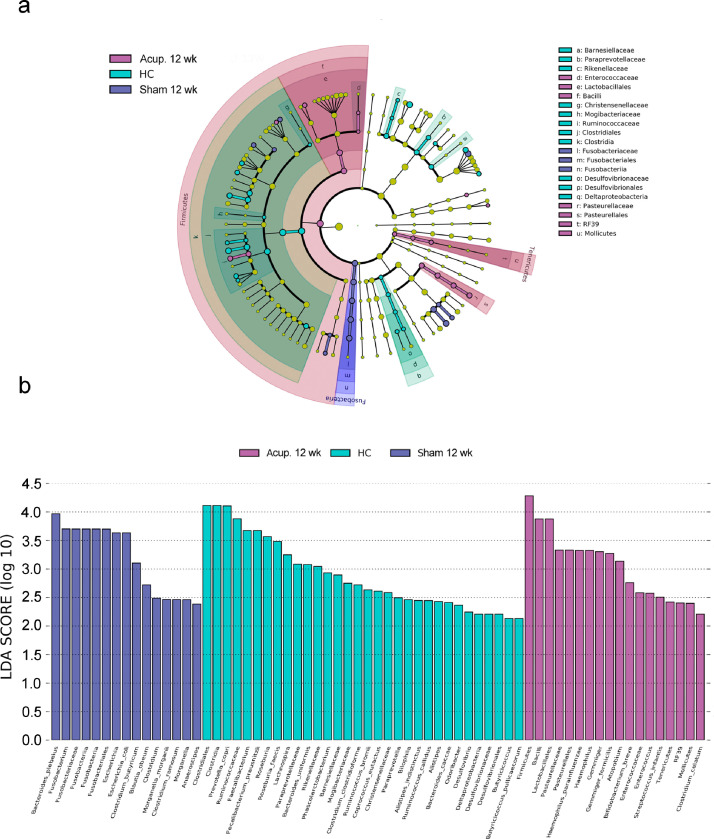
The intestinal microbiota of HCs was significantly enriched with Clostridia, Clostridiales, Ruminococcaceae, Mogibacteriaceae and Christensenellaceae of phylum Firmicutes; δ-Proteobacteria, Desulfovibrionales and Desulfovibrionaceae of phylum Proteobacteria; and Barnesiellaceae, Paraprevotellaceae and Rikenellaceae of phylum Bacteroidetes. The intestinal microbiota in the acupuncture group was significantly enriched with Bacilli, Lactobacillales and Enterococcaceae of phylum Firmicutes; Mollicutes and RF39 of phylum Tenericutes, and γ-Proteobacteria, Pasteurellaceae and Pasteurellales of phylum Proteobacteria; while the intestinal microbiota in the sham group was significantly enriched with Fusobacteriia, Fusobacteriales and Fusobacteriaceae of phylum Fusobacteria at the completion of treatment (Figure 5).
Figure 5.
(a) Cladogram of the linear discriminant analysis (LDA) effect size (Lefse) analysis of significant difference of microbial population. (b) Histogram of the LDA score of microbes that showed a significant impact in the acupuncture group (n = 15), sham group (n = 15) at the end of treatment and HCs (n = 30).
Fecal calprotectin concentration
At the completion of treatment, the fecal calprotectin concentration in the acupuncture group was significantly lower (difference: −139·8, 95%CI: −94·1-(−185·5), p < 0·001), while there was no significant change in the sham group (difference: −13·0, 95%CI: −65·4–39·4, p = 0·603) compared to baseline. The mean change in fecal calprotectin concentration from baseline in the acupuncture group was significantly better than that in the sham group (difference: 126·8, 95%CI: 60·4–193·2, p < 0·001) (Supplementary Fig. S5).
Circulating inflammatory marker outcome
The baseline plasma levels of LPS, DAO, and d-lactic acid (which reflects the function of the intestinal epithelial barrier)35,36 in these two patient groups were significantly higher than that in the HCs (Supplementary Table S6). The mean changes in plasma DAO and LPS levels from baseline in the acupuncture group were significantly greater than those in the sham group (LPS: difference: 51·19, 95%CI: 12·6–89·9, p = 0·01, DAO: difference: 1·37, 95%CI: 0·1–2·7, p = 0·037, respectively; Supplementary Table S7).
The baseline IFN-γ, TNF-α, and IL-1β levels in the two patient groups were significantly higher than those in the HCs (Supplementary Table S8). The mean change from baseline in the acupuncture group was significantly greater than that in the sham group (IFN-γ: difference: 8·1, 95%CI: 2·3–13·9, p = 0·008; TNF-α: difference: 16·2, 95%CI: 0·4–32·1, p = 0·041; IL-1β: difference: 5·5, 95%CI: 0·2–10·8, p = 0·036, respectively; Supplementary Table S9). The baseline plasma IL-17A and IL-23 levels in the two patient groups were significantly higher than those in HCs (Supplementary Table S8). The mean change from baseline in the acupuncture group was significantly higher than that in the sham group (IL-17A: difference: 7·2, 95%CI: 3·0–11·4, p = 0·002; IL-23: difference: 16·3, 95%CI: 1·8–30·7, p = 0·029, respectively; Supplementary Table S9).
Correlation analysis
At the completion of treatment, the change in serum CRP from baseline in the acupuncture group exhibited a significant inverse correlation with the changes in the relative abundance of Roseburia (B=−0·755, p = 0·007) and Lachnospira (B=−2·720, p = 0·002). In contrast, the change in serum CRP of the sham group showed a significant inverse correlation with the changes in the relative abundance of Oscillospira (B=−7·058, p = 0·035).
Discussion
To the best of our knowledge, this study is the first randomized, sham controlled, parallel group, 48-week follow up clinical trial of acupuncture for the treatment of patients with mild to moderate active CD and poor response to drugs. The results may support our hypothesis that acupuncture improves CD by regulating intestinal microbial composition and Th1/Th17 cell-mediated inflammation.
We found that 12-week acupuncture improved disease activity of patients with mild to moderate active CD who responded poorly to conventional drug therapy and that acupuncture induced disease remission maintained for nearly one year. The low dropout rate (3%) indicates that this type of acupuncture treatment is generally acceptable to the patients. We excluded patients who were using biologics because our team's clinical experience over the past 30 years has found that the use of biologics reduces the effects of acupuncture, although the mechanism for this is unclear. The proportion of patients with clinical remission in the acupuncture group at week 12 was significantly higher than that in the sham group. It is worth mentioning that we set a more stringent criteria for clinical remission to provide more reliable results. In addition to a CDAI score of less than 150, a reduction of more than 70 points from baseline was required to ensure that patients with mild activity achieved clinically significant remission.
During the follow-up, the therapeutic effect of the acupuncture remained stable, and the proportion of patients in clinical remission and clinical response increased by 12%−21%, compared with those at the completion of treatment. This may be related to the significant post-treatment effects of acupuncture, which can promote self-healing and continue to restore the body to normal condition even after stopping acupuncture. Similar post-effects have been observed in two recent large-scale acupuncture studies.37,38 In contrast, the clinical remission and clinical response rates in the sham group increased to some extent, but showed fluctuation; the difference in both rates between the two groups was maintained between 30%−45%. The clinical improvement in the sham group may be due to a psychological effect caused by contact of acupuncture with the skin and mild warm stimulation with 37 °C moxibustion, or the interaction between patients and acupuncturists during the intervention period. This placebo effect may also explain the fluctuation during the follow-up.
The decreases in CRP level and the CDAI score in the acupuncture group were significantly better than sham group at the completion of treatment and at the 36-week follow-up. This is consistent with previous acupuncture studies that involved a follow-up period of 12 weeks, although a longer-term follow-up was not made.16,17 Recently, mucosal healing has become a new target of the therapeutic effect of CD. It is related to the reduction in recurrence rate and surgical rate of patients.39 The improvement in the mean decrease in CDEIS score in the acupuncture group was greater than that in the sham group at week 48, suggesting a better mucosal healing ability of true acupuncture. This finding is different from the results of our previous study in which the CDEIS score was negative at week 12.17 The discrepancy may be related to the re-design of acupuncture treatment and the extended follow-up. In this study, manipulation of moxibustion was more convenient and the thermal effect of the moxa stick (diameter: 2·8 cm) was greater than that of the former study (diameter: 1.5 cm); the 48-week follow-up for the endoscopic outcome was to assess a long-term effect of acupuncture on mucosal healing; many previous studies have assessed long-term endpoints at 46–52 weeks.40,41 The cumulative recurrence rate in the acupuncture group was significantly lower than that in the sham group (difference of 39.4%); moreover, no serious adverse events were observed. The results support that acupuncture can be recommended as a treatment option to maintain remission in mild to moderate CD patients.
Consistent with previous studies19, 20, 21 that demonstrated positive effects of acupuncture on intestinal microbiota, 12-week acupuncture in our study increased the number of OTUs, ace index, and the relative abundance of SCFAs producing bacteria (Lachnospira, Coprococcus, Roseburia and Roseburia faecis) and anti-inflammatory bacteria (Faecalibacterium and F. prausnitzii). The distribution of microbiota in the acupuncture group trended towards that of HCs, both of which were mainly enriched with phylum Firmicutes (including the anti-inflammatory bacteria and SCFAs producing bacteria). In contrast, the sham acupuncture was mainly enriched with phylum Fusobacteria. This suggests that acupuncture may help restore the balance of intestinal microbiota. Specifically, F. prausnitzii has been shown to inhibit intestinal inflammation of CD and production of SCFAs, e.g., butyric acid.42 The abundances of Genus Roseburia, Lachnospira and Coprococcus were reduced in CD patients, and may lead to insufficient SCFAs production. In contrast, SCFAs may regulate the integrity of the intestinal barrier by inducing the intestinal epithelial cells to secrete IL-18, antimicrobial peptides, mucins and by up-regulating the expression of tight junctions; these directly affect the transformation of naive T cells into Th1 or Th17 cells according to the cytokine milieu.43 After acupuncture treatment, the increases in the relative abundance of genus Roseburia and Lachnospira showed an inverse correlation with the decrease in serum CRP level. This suggests that acupuncture may increase the abundance of SCFAs producing bacteria, which strengthens the intestinal barrier function and inhibits the intestinal proinflammatory cytokines. The absence of significant differences in β diversity between acupuncture and sham may be due to the inability of 12 weeks of acupuncture to distinguish differences in the degree of bacterial community differentiation between the two, and sampling at 48 weeks, when the effect of acupuncture was more robust, may be beneficial in reinforcing the differences.
Acupuncture decreased the plasma levels of DAO and LPS and Th1/Th17-related cytokine levels, indicating that acupuncture augmented the integrity of the intestinal barrier and reduced the intestinal secretion and migration of proinflammatory cytokines. Previous studies44, 45, 46 have also demonstrated that acupuncture may increase the intestinal epithelial tight junction protein, reduce the apoptosis of intestinal epithelial cells, restore the ratio of intestinal mucosa Th17/Treg cells. Considerable evidence demonstrated that gut inflammation per se can lead to changes in microbiota, for example, in animal models of colitis and infectious gastroenteritis.47 The improvement in CD activity by acupuncture is therefore likely to be associated with increasing the abundance of intestinal anti-inflammatory bacteria and SCFAs producing bacteria, repairing intestinal epithelial barrier, and inhibiting the production and release of intestinal Th1/Th17-related proinflammatory cytokines.
Some limitations of this study should be acknowledged. First, there was no waiting-list group. Inclusion of a waiting-list may help eliminate the effect of non-specific factors such as natural remission of the disease itself. Second, the fecal calprotectin level was not assessed at week 48. However, studies27,48 have shown that serum CRP, CDAI score and endoscopic findings are positively correlated with fecal calprotectin concentration, which could indirectly reflect the trend of fecal calprotectin and the severity of intestinal inflammation. Third, the intestinal flora and peripheral inflammatory markers were only observed for 12 weeks; the long-term effect of acupuncture on these indices could not be determined. Fourth, direct evidence that support a potential mechanism of acupuncture in regulating intestinal microbiota is still not available, which may be further validated using sterile animal fecal bacteria transplantation experiment. Future studies should improve these deficiencies to provide more definitive results. The relatively low prevalence of CD in China, as well as patients' doubts about the efficacy of acupuncture (lack of knowledge about acupuncture for CD), led to a long recruitment period for this trial. Since CD is a lifelong disease, we may explore the effect of acupuncture treatment (e.g., once every 1–2 weeks) for long-term maintenance of disease remission in patients (with a broader population) after induction of remission.
In conclusion, this study is the first to demonstrate the long-term effect of acupuncture in patients with mild to moderate active CD who responded poorly to conventional drugs. We show acupuncture is a safe and effective treatment for induction and long-term maintenance of remission. The therapeutic effects of acupuncture are associated with increasing the relative abundance of intestinal anti-inflammatory bacteria and SCFAs producing bacteria, enhancing intestinal barrier function, and inhibiting Th1/Th17-related proinflammatory cytokines. This study provides evidence that acupuncture is a safe and effective treatment for patients with mild to moderate CD, especially for those who show poor response to conventional drug therapy.
Contributors
CHB, HGW and HRL conceived and designed the study; LYW, DW, YS, JZZ and XQZ acquired the data; CHB, XMJ and JHC analyzed and interpreted the data; CHB, HGW, DW and JJZ accessed and were responsible for the raw data associated with the study; LMC and GNL performed the statistical analysis; CHB and LYW drafted the manuscript; HRL, XMJ and JHC critically revised the important intellectual content of the manuscript; HGW took the decision to submit the manuscript for publication.
Data sharing statement
The data collected in this study, including de-identified participant data and the data dictionary are available to researchers through corresponding author Prof. Huangan Wu upon reasonable request. These data will be available for a period of 6 months to 3 years after publication. Data requests require a methodologically sound proposal as well as a data access agreement and approval by the local ethics committee.
Funding
This work was supported by the National Key Basic Research Program of China (2015CB554500 and 2009CB522900), the Shanghai Rising-Star Program (19QA1408100).
Declaration of interests
All the authors disclose no conflicts of interest.
Acknowledgements
All authors would like to thank the subjects who participated in the study. They also thank Prof. Jie Zhong from Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Prof. Lixin Yin from Longhua Hospital, Shanghai University of Traditional Chinese Medicine and Prof. Yiqi Du from Changhai Hospital of Shanghai for their recruitment and screening of patients; Dr. Quanlin Li from Zhongshan Hospital, Fudan University for the endoscopic examination and evaluation, as well as Prof. Ye Yao from Department of Biostatistics, Fudan University and Prof. Weijun Zheng from Department of Medical Statistics, Zhejiang University of Traditional Chinese Medicine for their guidance on the statistical analysis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101300.
Contributor Information
Chunhui Bao, Email: baochunhui@shutcm.edu.cn.
Huirong Liu, Email: liuhuirong@shutcm.edu.cn.
Huangan Wu, Email: wuhuangan@shutcm.edu.cn.
Supplementary materials
References
- 1.Torres J., Mehandru S., Colombel J.F. Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389:1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2017 Inflammatory Bowel Disease Collaborators The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: 10.1016/S2468-1253(19)30333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan G.G., Ng S.C. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321. doi: 10.1053/j.gastro.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.Z., Li Y.Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Price J., Arze C., Ananthakrishnan A.N., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strober W., Fuss I.J. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hisamatsu T., Erben U., Kühl A.A. The role of T-Cell subsets in chronic inflammation in celiac disease and inflammatory bowel disease patients: more common mechanisms or more differences? Inflamm Intest Dis. 2016;1:52–62. doi: 10.1159/000445133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang W., Su J., Zhang X., et al. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm Res. 2014;63:943–950. doi: 10.1007/s00011-014-0768-7. [DOI] [PubMed] [Google Scholar]
- 9.Geremia A., Biancheri P., Allan P., Corazza G.R., Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. doi: 10.1016/j.autrev.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Ooi C.J., Hilmi I., Banerjee R., et al. Best practices on immunomodulators and biologic agents for ulcerative colitis and Crohn's disease in Asia. J Gastroenterol Hepatol. 2019;34:1296–1315. doi: 10.1111/jgh.14648. [DOI] [PubMed] [Google Scholar]
- 11.Roda G., Jharap B., Neeraj N., Colombel J.F. Loss of response to Anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7:e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheifetz A.S., Gianotti R., Luber R., Gibson P.R. Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. 2017;152:415–429. doi: 10.1053/j.gastro.2016.10.004. e15. [DOI] [PubMed] [Google Scholar]
- 13.Song G., Fiocchi C., Achkar J.P. Acupuncture in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:1129–1139. doi: 10.1093/ibd/izy371. [DOI] [PubMed] [Google Scholar]
- 14.Stein D.J. Massage acupuncture, moxibustion, and other forms of complementary and alternative medicine in inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46:875–880. doi: 10.1016/j.gtc.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Deng H., Shen X. The mechanism of moxibustion: ancient theory and modern research. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/379291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joos S., Brinkhaus B., Maluche C., et al. Acupuncture and moxibustion in the treatment of active Crohn’s disease: a randomized controlled study. Digestion. 2004;69:131–139. doi: 10.1159/000078151. [DOI] [PubMed] [Google Scholar]
- 17.Bao C.H., Zhao J.M., Liu H.R., et al. Randomized controlled trial: moxibustion and acupuncture for the treatment of Crohn's disease. World J Gastroenterol. 2014;20:11000–11011. doi: 10.3748/wjg.v20.i31.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langhorst J., Wulfert H., Lauche R., et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis. 2015;9:86–106. doi: 10.1093/ecco-jcc/jju007. [DOI] [PubMed] [Google Scholar]
- 19.Wei D., Xie L., Zhuang Z., et al. Gut microbiota: a new strategy to study the mechanism of electroacupuncture and moxibustion in treating ulcerative colitis. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/9730176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi Q., Liu Y.N., Jin X.M., et al. Moxibustion treatment modulates the gut microbiota and immune function in a dextran sulphate sodium-induced colitis rat model. World J Gastroenterol. 2018;24:3130–3144. doi: 10.3748/wjg.v24.i28.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao C.H., Wang C.Y., Li G.N., et al. Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome. World J Gastroenterol. 2019;25:4696–4714. doi: 10.3748/wjg.v25.i32.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao C., Wang D., Liu P., et al. Effect of electro-acupuncture and moxibustion on brain connectivity in patients with Crohn’s Disease: a resting-state fMRI study. Front Hum Neurosci. 2017;11:559. doi: 10.3389/fnhum.2017.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N., Guo Y., Gong Y., et al. The anti-inflammatory actions and mechanisms of acupuncture from acupoint to target organs via neuro-immune regulation. J Inflamm Res. 2021;14:7191–7224. doi: 10.2147/JIR.S341581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Best W.R., Becktel J.M., Singleton J.W., Kern F. Development of a Crohn's disease activity index. National cooperative Crohn's disease study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 25.Mary J.Y., Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID) Gut. 1989;30:983–989. doi: 10.1136/gut.30.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Haens G.R., Geboes K., Peeters M., Baert F., Penninckx F., Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–267. doi: 10.1016/s0016-5085(98)70476-7. [DOI] [PubMed] [Google Scholar]
- 27.Sipponen T., Savilahti E., Kolho K.L., Nuutinen H., Turunen U., Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. doi: 10.1002/ibd.20312. [DOI] [PubMed] [Google Scholar]
- 28.Van Assche G., Dignass A., Panes J., et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.WHO Regional Office for the Western Pacific . World Health Organization; Manila: 2008. WHO Standard Acupuncture Point Locations in the Western Pacific Region. [Google Scholar]
- 30.Park J.B., White A., Lee H.J., Ernst E. Development of a new sham needle. Acupuncture Med. 1999;17:110–112. [Google Scholar]
- 31.Liang Z.H., Xie C.C., Li Z.P., Zhu X.P., Lu A.P., Fu W.B. Deqi sensation in placebo acupuncture: a crossover study on Chinese medicine students. Evid Based Complement Alternat Med. 2013;2013 doi: 10.1155/2013/620671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomollón F., Dignass A., Annese V., et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. doi: 10.1093/ecco-jcc/jjw168. [DOI] [PubMed] [Google Scholar]
- 33.Colombel J.F., Sandborn W.J., Reinisch W., et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 34.Newcombe R.G. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 35.Song W.B., Lv Y.H., Zhang Z.S., et al. Soluble intercellular adhesion molecule-1, d-lactate and diamine oxidase in patients with inflammatory bowel disease. World J Gastroenterol. 2009;15:3916–3919. doi: 10.3748/wjg.15.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulkens J., Vergauwen G., Van Deun J., et al. Increased levels of systemic LPS-positive bacterial extracellular vesicles in patients with intestinal barrier dysfunction. Gut. 2020;69:191–193. doi: 10.1136/gutjnl-2018-317726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S., Yu L., Luo X., et al. Manual acupuncture versus sham acupuncture and usual care for prophylaxis of episodic migraine without aura: multicentre, randomised clinical trial. BMJ. 2020;368:m697. doi: 10.1136/bmj.m697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Z.S., Liu Y., Xu H.F., et al. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence a randomized clinical trial. JAMA. 2017;317:2493–2501. doi: 10.1001/jama.2017.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baert F., Moortgat L., Van Assche G., et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 40.Alfaro I., Masamunt M.C., Planell N., et al. Endoscopic response to tumor necrosis factor inhibitors predicts long term benefits in Crohn's disease. World J Gastroenterol. 2019;25:1764–1774. doi: 10.3748/wjg.v25.i14.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall B., Holleran G., Chin J.L., et al. A prospective 52 week mucosal healing assessment of small bowel Crohn's disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601–1609. doi: 10.1016/j.crohns.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Sokol H., Pigneur B., Watterlot L., et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun M., Wu W., Liu Z., Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1–8. doi: 10.1007/s00535-016-1242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shang H.X., Wang A.Q., Bao C.H., et al. Moxibustion combined with acupuncture increases tight junction protein expression in Crohn's disease patients. World J Gastroenterol. 2015;21:4986–4996. doi: 10.3748/wjg.v21.i16.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao C., Bao C., Li J., et al. Moxibustion and acupuncture ameliorate Crohn's disease by regulating the balance between Th17 and Treg cells in the intestinal mucosa. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/938054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao C.H., Wu L.Y., Shi Y., et al. Moxibustion down-regulates colonic epithelial cell apoptosis and repairs tight junctions in rats with Crohn's disease. World J Gastroenterol. 2011;17:4960–4970. doi: 10.3748/wjg.v17.i45.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ni J., Wu G.D., Albenberg L., Tomov V.T. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin W.C., Wong J.M., Tung C.C., et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566–13573. doi: 10.3748/wjg.v21.i48.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.