Abstract
Introduction
Actinic keratoses (AK) are epithelial lesions caused by chronic skin exposure to ultraviolet light that can progress into squamous cell carcinoma. Although several treatments are effective, they are associated with severe skin reactions, which might be related to the extent of the disease. This study aimed to examine the relationship between the severity of local skin reactions during treatment with 5-fluorouracil 4% cream and the number of AK lesions at baseline.
Methods
This post hoc analysis pooled data from two multicentre randomised phase III studies (HD-FUP3B-048, HD-FUP3B-049) in patients with AK treated with topical 5-fluorouracil 4% once daily (OD) or 5% twice daily (BID) for 4 weeks. First, we compared the severity, assessed using a numerical rating scale, of the local skin reactions between 5-fluorouracil 4% and 5%. Then, we investigated the relationship between the number of lesions at baseline and severe skin reactions with 5-fluorouracil 4% OD.
Results
Safety data were included from 397 patients who had received 5-fluorouracil 4% (348 in study HD-FUP3B-048, 49 in study HD-FUP3B-049) OD and 342 (HD-FUP3B-048) who had received 5-fluorouracil 5% BID. For most skin reactions, severe ones were more common in patients treated with 5-fluorouracil 5% cream BID than in those treated with 5-fluorouracil 4% cream OD (P < 0.05). With 5-fluorouracil 4% OD, the incidence of severe erythema was significantly higher in patients with at least 10 lesions (46%) than in patients with 5–10 lesions (28%; P < 0.001). Similar results were observed for the other local skin reactions.
Conclusion
Treatment with 5-fluorouracil 4% cream OD was associated with less severe local skin reactions than 5-fluorouracil 5% BID. The number of AK lesions at baseline seems to have predictive value regarding the severity of local skin reactions that appear during treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00668-9.
Keywords: Actinic keratosis, 5-Fluorouracil, Local skin reactions, Topical
Plain Language Summary
In order to prevent recurrence of actinic keratosis lesions and their progression to invasive squamous cell carcinoma, topical treatment of actinic keratosis often involves treating the field of cancerisation to clear visible and subclinical lesions. The occurrence of local adverse skin reactions during treatment can affect patient adherence to therapy and therefore compromise efficacy. A thorough understanding of the relationships between changes in lesions, actinic keratosis remission and tolerability of topical treatments over time is necessary to manage patient expectations, ensure treatment adherence and optimise clinical outcomes. This exploratory study used pooled data from two pivotal phase III studies to compare local skin reaction severity between 5-fluorouracil 4% cream applied once daily and 5-fluorouracil 5% cream applied twice daily, then to examine the relationship between the severity of local skin reactions during treatment and the number of actinic keratosis lesions at baseline. Local skin reactions appeared to be more severe in patients with more than 10 actinic keratosis lesions at baseline than in those with 5 to 10 lesions. Using a cut-off value of 10 lesions pre-treatment, healthcare practitioners can forewarn patients accordingly of potentially more severe local adverse skin reactions in order to motivate them to complete their treatment, thus ensuring treatment efficacy. If patients are likely to experience intolerable severe skin reactions, practitioners can plan to introduce an appropriate rescue treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00668-9.
Key Summary Points
| Why carry out this study? |
| The occurrence of severe local adverse skin reactions during topical treatment can affect patient adherence to therapy and therefore compromise efficacy. |
| By their mode of action, topical treatments for actinic keratosis (AK) may cause local skin reactions. The severity of local skin reactions may be due to the extent of the field of cancerisation. |
| What was learned from the study? |
| Treatment of AK with topical 5-fluorouracil 4% once daily showed less severe local skin reactions compared with a 5% formulation twice daily. |
| A cut-off value of 10 pre-treatment lesions seems to be meaningful for anticipating possibly severe skin reactions during topical 5-fluorouracil treatment. |
Introduction
Actinic keratoses (AK) are epithelial lesions composed of proliferating neoplastic keratinocytes that manifest as macules, papules, or superficial scaly plaques [1–3]. They occur on sun-exposed areas including the face, scalp, ears, arms and hands [1, 3, 4], and affect mostly fair-skinned individuals [5].
AK are in situ dysplastic lesions that can progress to invasive squamous cell carcinoma (SCC) [4, 5]. Histopathological studies have revealed that clinically visible AK lesions are often accompanied by subclinical or invisible lesions which, if untreated, might result in field cancerisation [6]. Thus, early diagnosis and therapy initiation, even at the stage of grade I lesions, are of great importance for patient prognosis [3, 7, 8].
Treatment of AK ranges from the removal of individual lesions (e.g. cryosurgery, excision) to the treatment of the wider field (e.g. topical therapies, photodynamic therapy) [1, 9–11] and depends on patient preference and their clinical circumstances. The number of visible AKs is a poor indicator of the real number of lesions present in the field of cancerisation, underlying the importance of treating the whole field of cancerisation using field-directed treatments for multiple AKs [12].
The aim of AK therapy is to achieve a good response rate (i.e. reduction of the extent of field cancerisation) and/or clearance (complete and partial) of clinical and subclinical lesions, to prevent progression to invasive SCC, to improve skin appearance, to minimise pain and other symptoms, and to reduce lesion recurrence [1, 3, 6, 13].
Since its approval 50 years ago, 5-fluorouracil (5-FU) has been the mainstay treatment for AK. Owing to its efficacy, ease of use and cost-effectiveness, 5-FU is one of the most commonly used topical medications for both lesion-directed and field-directed treatment of AK [1, 9, 14–18]. However, 5-FU topical treatment is also associated with adverse local skin reactions, which are probably due to a local inflammatory response related to its mechanism of action.
5-FU 4% cream (Tolak®) is indicated for the topical treatment of non-hyperkeratotic, non-hypertrophic actinic keratosis (Olsen grade I and II) of the face, ears, and/or scalp in adults [19, 20], and was approved on the basis of data from two well-controlled phase III primary efficacy studies (HD-FUP3B-048 and HD-FUP3B-049). The results of the study HD-FUP3B-048 have been published previously alongside those of a phase II study (HD-FUDR-045) [21]. In these two studies, 5-FU 4% cream once daily demonstrated similar rates of complete (54.4% vs 57.9%) and partial clearance of AK lesions (80.5% vs 80.2%) to the original formulation of 5-FU 5% cream applied twice daily [21].
The occurrence of severe local adverse skin reactions during treatment can affect patient adherence to therapy and therefore compromise efficacy. This pooled analysis of data from the two phase III trials (HD-FUP3B-048 and HD-FUP3B-049) had two aims. The first was to compare severe local skin reactions between 5-FU 4% cream applied once daily and 5-FU 5% cream applied twice daily. The second was to explore the relationship between the severity of local skin reactions during treatment with once-daily 5-FU 4% cream and the number of AK lesions at baseline.
Methods
Study Design and Patients
This was a post hoc, exploratory analysis of pooled data of safety populations from two multicentre, randomised phase III studies: HD-FUP3B-048 [21] and HD-FUP3B-049 (unpublished). The design of each study was comparable, with similar active treatment regimens and study endpoints. Study HD-FUP3B-048 had two active treatments (5-FU 4% and 5% cream) and vehicle control groups, whereas study HD-FUP3B-049 had one active treatment (5-FU 4%) and a vehicle control group.
Participants from both studies were eligible for inclusion if they had at least five clinically recognisable AK on either their face, ears and/or scalp, were more than 18 years old, and were not pregnant nor intended to be during the duration of the treatment and agreed to use contraceptives. Participants were excluded if they had skin lesions suspected to be SCC, had been treated with any systemic cancer treatment or any other AK treatments including steroids or retinoids in the past 2 months prior to the study, or had or were suspected to develop sensitivity to any of the ingredients in the study medication.
Patients applied 5-FU cream to the entire affected field (i.e. face, ears and/or scalp), either once daily (4% cream) or twice daily (5% cream) for 4 weeks. The creams (4% and 5%) were applied to the entire involved field at the same time each day.
The number and severity of skin lesions were evaluated at baseline, week 2 and week 4. For each patient, the same investigator, who was blinded to treatment allocation, evaluated local skin reactions by visual assessment of erythema, scaling/dryness, oedema, crusting and erosions and by patient questioning about the presence, severity and impact of symptoms (stinging/burning, pruritus) in the preceding 24 h. The reactions were graded at each visit (without reference to baseline or previous assessments) as an average across all treated areas using a four-point numerical scale, where 0 was used to denote none (no evidence of reaction), 1 mild (faintly detectable reaction), 2 moderate (clearly distinguishable reaction) and 3 severe (marked/extensive/bothersome reaction).
Pooled Analysis
Patients from the active treatment arms of both studies were eligible for inclusion in this pooled analysis. Study HD-FUP3B-048 enrolled a total of 841 patients from 26 sites in the USA [21]; data from 690 enrolled patients were included in the pooled analysis, of whom 348 were in the 5-FU 4% once daily treatment group and 342 were in the 5-FU 5% twice daily treatment group. Of the 100 patients included in study HD-FUP3B-049 from six sites in the USA, data from 49 enrolled patients receiving 5-FU 4% cream once daily were included in the pooled analysis. The remaining patients enrolled in the two studies either received placebo or had no local skin reaction data available after study inclusion.
Outcome Measures
We compared the severity of local skin reactions (i.e. erythema, scaling/dryness, oedema, crusting, erosions, stinging/burning or pruritus) with 5-FU 4% cream once daily and 5% cream twice daily at week 4 of treatment using the data from study HD-FUP3B-048. Then, any associations between the number of AK lesions at baseline (categorised in groups of five) and the severity of local skin reactions at week 4 of treatment were assessed for 5-FU 4% cream once daily using pooled data from study HD-FUP3B-049 and study HD-FUP3B-048.
Statistical Analyses
Since these were exploratory post hoc analyses, there was no formal statistical protocol. Baseline demographics and patient characteristics are presented descriptively as percentages, means, medians and standard deviations, and were compared using the Student t test, Fisher’s exact test, chi-squared test, Cochran–Mantel–Haenszel (CMH) test and Wilcoxon test, as appropriate. The severity of local skin reactions with the two 5-FU regimens was compared using a chi-squared test and the odds ratio (OR) and 95% confidence intervals (CI) calculated using logistic regression (unadjusted and after adjustment for age). A CMH analysis was also undertaken, stratifying for the number of treated areas (1, 2 or 3). Any associations between the number of baseline lesions and the severity of local skin reactions were examined at week 4 using Fisher’s exact test and two-sided P values were calculated. Because differences were noted in the baseline characteristics of patients using 5-FU 4% once daily versus 5-FU 5% twice daily, we conducted a post hoc logistic regression analysis on the difference in skin reactions between the groups, adjusting for baseline age, Fitzpatrick skin type, number of lesions and disease severity.
Ethical Considerations
This pooled analysis was based on previously conducted studies and does not contain any direct investigation with human participants or animals performed by any of the authors. Studies HD-FUP3B-048 and HD-FUP3B-049 received ethical approval from the institutional review boards of all relevant study sites and were conducted in compliance with US Food and Drug Administration regulations, the ethical principles of the Declaration of Helsinki, and the current International Conference on Harmonisation Good Clinical Practice guidelines. Patients provided written informed consent to participate in the studies.
Results
Patient Characteristics
Baseline demographics and clinical characteristics of the study participants were generally similar between the various treatment arms (5-FU 4% and 5%) in study HD-FUP3B-048 (Table 1). Compared with patients receiving 5-FU 4% once daily in study HD-FUP3B-048, those receiving 5-FU 4% once daily in study HD-FUP3B-049 had significantly more lesions (P = 0.0031) and more severe AK (P = 0.0045) and included significantly more patients with darker Fitzpatrick skin types (P = 0.0083) (Table 1). In the overall patient population, the majority of patients were aged at least 60 years old (range 36.7–94.1 years) and were male (81%). Most patients had skin type I–III (94.6%) and the number of AK ranged from 5 to 83 lesions. Disease severity at baseline was mild to moderate in the majority of patients (88.4%; Table 1).
Table 1.
Baseline demographics and clinical characteristics of the enrolled study populations
| Study HD-FUP3B-048 | Study HD-FUP3B-049 | ||
|---|---|---|---|
| 5-FU 4% cream (N = 353) |
5-FU 5% cream (N = 349) |
5-FU 4% cream (N = 50) |
|
| Age, years | |||
| Mean (SD) | 67.7 (9.8) | 67.4 (10.0) | 67.9 (11.7) |
| Range | 36.7–88.9 | 37.6–94.1 | 44.7–85.1 |
| Between-group P value | 0.6586a1 | 0.9439b1 | |
| Sex, n (%) | |||
| Male | 287 (81) | 282 (81) | 39 (78) |
| Female | 66 (19) | 67 (19) | 11 (22) |
| Between-group P value | 0.8656a2 | 0.5782b2 | |
| Race, n (%) | |||
| White | 348 (99) | 347 (99) | 50 (100) |
| American Indian/Alaska Native | 1 (< 1) | 0 | 0 |
| Other | 4 (1) | 2 (1) | 0 |
| Between-group P value | 0.6862a3 | 1.000b3 | |
| Skin type, n (%) | |||
| I | 84 (24) | 91 (26) | 7 (14) |
| II | 178 (50) | 155 (44) | 17 (34) |
| III | 75 (21) | 80 (23) | 25 (50) |
| IV | 13 (4) | 21 (6) | 1 (2) |
| V | 3 (1) | 2 (1) | 0 |
| VI | 0 | 0 | 0 |
| Between-group P value | 0.6136a4 | 0.0084b4 | |
| Number of lesions | |||
| Median | 11 | 12 | 14 |
| Range | 5–76 | 5–76 | 5–83 |
| Between-group P value | 0.1846a5 | 0.0031b5 | |
| Disease severityc, n (%) | |||
| Mild | 171 (48) | 154 (44) | 13 (26) |
| Moderate | 138 (39) | 162 (46) | 27 (54) |
| Severe | 44 (12) | 33 (9) | 10 (20) |
| Between-group P value | 0.7960a4 | 0.0045b4 | |
5-FU 5-fluorouracil, SD standard deviation
aComparison of the 5-FU 4% vs 5-FU 5% group in study HD-FUP3B-048
bComparison of the 5-FU 4% group in study HD-FUP3B-049 with the 5-FU 4% group in study HD-FUP3B-048
cMild disease severity was defined as 5–10 lesions, moderate as 11–25 lesions and severe as > 25 lesions
1Student t test
2Chi-squared test
3Fisher test
4Cochran–Mantel–Haenszel test
5Wilcoxon test
Local Skin Reaction Severity: 5-FU 4% vs 5-FU 5% Cream
Severe skin reactions were more common with 5-FU 5% cream applied twice daily than with 5-FU 4% cream applied once daily at week 4 when skin reactions were maximal (Table 2). Significantly more patients treated with 5-FU 5% versus 4% cream reported severe erythema (47% vs 37%), severe scaling/dryness or crusting (both 25% vs 18%), severe stinging/burning (27% vs 18%) and severe pruritus (22% vs 13%; all P < 0.05). The rates of severe oedema (8% vs 5% of patients; P = 0.2081) and severe skin erosions (12% vs 8%; P = 0.1495) were similar between treatment groups (Table 2). Similar significant differences were seen in the logistic regression analyses, including the unadjusted analysis, the age-adjusted analysis, and after adjustment for age, skin type, lesion number and disease severity. In addition, the results of the CMH analysis stratified by the number of treated areas were also consistent, with significant between-group differences in erythema (P = 0.0080), scaling/dryness (P = 0.0131), crusting (P = 0.0179), stinging/burning (P = 0.0085) and pruritus (P = 0.0032), but no significant difference in oedema (P = 0.1773) or erosions (P = 0.1356).
Table 2.
Summary of local skin reactions at week 4 of treatment with either 5-fluorouracil 4% or 5% cream (safety population study HD-FUP3B-048)
| Reaction by severity, n (%a) | 5-FU 4% cream OD n = 348 |
5-FU 5% cream BID n = 342 |
Unadjusted OR (95% CI) | Age-adjusted OR (95% CI) | Multivariate-adjusted OR (95% CI)b |
|---|---|---|---|---|---|
| Erythema | |||||
| Severe |
120 (37) |
140 (47) |
1.517 (1.102; 2.087) |
1.506 (1.094; 2.074) |
1.550 (1.114; 2.154) |
| Non-severe (none/mild/moderate) |
208 (63) |
160 (53) |
|||
| Not reported | 20 | 42 | |||
| P value | 0.0104c | 0.0106d | 0.0121d | 0.0092d | |
| Scaling/dryness | |||||
| Severe |
57 (17) |
75 (25) |
1.592 (1.081; 2.345) |
1.581 (1.073; 2.330) |
1.691 (1.131; 2.527) |
| Non-severe (none/mild/moderate) |
271 (83) |
224 (75) |
|||
| Not reported | 20 | 43 | |||
| P value | 0.0181c | 0.0186d | 0.0205d | 0.0104d | |
| Oedema | |||||
| Severe |
18 (5) |
24 (8) |
1.498 (0.796; 2.818) |
1.487 (0.790; 2.801) |
1.503 (0.783; 2.887) |
| Non-severe (none/mild/moderate) | 310 (95) | 276 (92) | |||
| Not reported | 20 | 42 | |||
| P value | 0.2081c | 0.2106d | 0.2188d | 0.2210d | |
| Crusting | |||||
| Severe |
56 (17) |
74 (25) |
1.590 (1.077; 2.347) |
1.615 (1.092; 2.387) |
1.703 (1.140; 2.544) |
| Non-severe (none/mild/moderate) | 272 (83) | 226 (75) | |||
| Not reported | 20 | 42 | |||
| P value | 0.0190c | 0.0195d | 0.0163d | 0.0094d | |
| Erosions | |||||
| Severe |
27 (8) |
35 (12) |
1.472 (0.868; 2.498) |
1.500 (0.883; 2.549) |
1.571 (0.914; 2.701) |
| Non-severe (none/mild/moderate) |
301 (92) |
265 (88) |
|||
| Not reported | 20 | 42 | |||
| P value | 0.1495c | 0.1513d | 0.1340d | 0.1022d | |
| Stinging/burning | |||||
| Severe |
60 (18) |
81 (27) |
1.652 (1.131; 2.412) |
1.659 (1.135; 2.423) |
1.652 (1.121; 2.433) |
| Non-severe (none/mild/moderate) |
268 (82) |
219 (73) |
|||
| Not reported | 20 | 42 | |||
| P value | 0.0090c | 0.0094d | 0.0089d | 0.0111d | |
| Pruritus | |||||
| Severe |
43 (13) |
66 (22) |
1.869 (1.227; 2.849) |
1.876 (1.231; 2.860) |
1.875 (1.215; 2.894) |
| Non-severe (none/mild/moderate) |
285 (87) |
234 (78) |
|||
| Not reported | 20 | 42 | |||
| P value | 0.0033c | 0.0036d | 0.0034d | 0.0045d | |
Data from 348 patients who received 5-FU 4% cream and 342 patients who received 5-FU 5% cream in study HD-FUP3B-048 were considered for the safety analysis
5-FU 5-fluorouracil
aPercentage values calculated using the total number of patients with available data as the denominator (excludes patients with missing values)
bAdjusted for age, Fitzpatrick skin type (types IV and V were pooled together because of the low number of patients with type V), number of lesions, and disease severity
cChi-squared test
dLogistic regression analysis
Severity of Local Skin Reactions by Number of Baseline Lesions
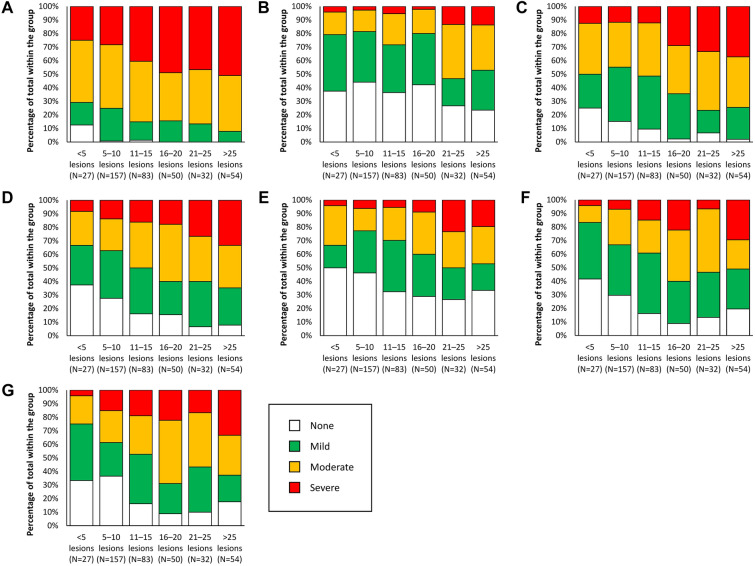
Figure 1, Table 3 and Supplementary Table 1 show a potential relationship between the number of AK lesions at baseline and the severity of local skin reactions after 4 weeks of treatment with 5-FU 4% cream once daily. In general, local skin reactions appeared to be more severe in patients with more than 10 lesions at baseline than in patients with fewer pre-treatment lesions (Fig. 1). For example, the number of patients with erythema was higher in patients with more than 10 lesions at baseline, with 46% of patients experiencing severe erythema in comparison with 28% of patients who had 5–10 lesions at baseline (Table 3). In addition, a greater proportion of patients with more than 10 lesions at baseline showed severe oedema, nevertheless, the proportion of patients with severe skin oedema was relatively low (less than 15%) irrespective of the number of lesions at baseline (Fig. 1b and Supplementary Table 1).
Fig. 1.
Relationship between the number of lesions at baseline and severity of a erythema, b oedema, c scaling, d crusting, e erosion, f pruritus and g stinging during treatment of actinic keratosis with 5-fluorouracil 4% cream once daily at week 4. Local skin reactions were graded as a visual average across all treated areas using a 4-point numerical scale: 0 (none; no evidence of reaction), 1 (mild; faintly detectable reaction), 2 (moderate; clearly distinguishable reaction) or 3 (severe; marked/extensive/bothersome reaction)
Table 3.
Comparison of the severity of local skin reactions at week 4 of actinic keratosis with 5-fluorouracil 4% cream in patients with 0–10 vs > 10 lesions at baseline (pooled 5-fluorouracil 4% populations from study HD-FUP3B-048 and study HD-FUP3B-049)
| Reaction by severity, n (%) | Number of actinic keratosis lesions at baseline |
P value (2-sided) |
|
|---|---|---|---|
| 0–10 (N = 169) |
> 10 (N = 200) |
||
| Severity of erythema | 0.0004 | ||
| None/mild/moderate | 122 (72) | 108 (54) | |
| Severe | 47 (28) | 92 (46) | |
| Severity of oedema | 0.0430 | ||
| None/mild/moderate | 164 (97) | 184 (92) | |
| Severe | 5 (3) | 16 (8) | |
| Severity of scaling | 0.0009 | ||
| None/mild/moderate | 149 (88) | 149 (74) | |
| Severe | 20 (12) | 51 (25) | |
| Severity of crusting | 0.0212 | ||
| None/mild/moderate | 147 (86) | 155 (77) | |
| Severe | 22 (13) | 45 (22) | |
| Severity of erosions | 0.0334 | ||
| None/mild/moderate | 159 (94) | 175 (87) | |
| Severe | 10 (6) | 25 (12) | |
| Severity of pruritus | 0.0004 | ||
| None/mild/moderate | 158 (93) | 162 (81) | |
| Severe | 11 (7) | 38 (19) | |
| Severity of stinging | 0.0230 | ||
| None/mild/moderate | 146 (86) | 154 (77) | |
| Severe | 23 (14) | 46 (23) | |
The severity of local reactions was measured at week 2 and week 4; week 4 data are shown in this table. Local skin reactions were graded as a visual average across all treated areas using a 4-point numerical scale: 0 (none; no evidence of reaction), 1 (mild; faintly detectable reaction), 2 (moderate; clearly distinguishable reaction) or 3 (severe; marked/extensive/bothersome reaction)
Data were missing for 34 patients
For some skin reactions, the distinction was notable at the 15-lesion cut-off; for example, patients with more than 15 lesions at baseline had more than twice the rate of severe scaling in comparison with patients who had 11–15 skin lesions at baseline (Fig. 1c and d, Supplementary Table 1). Pruritus and stinging were typically mild or moderate in severity regardless of the number of lesions, but when the number of lesions was greater than 15 at baseline, there was a higher incidence of moderate or severe pruritus or stinging than in patients with fewer lesions (Fig. 1f and g, Supplementary Table 1).
To confirm these graphical trends, we compared the proportion of patients presenting with a severe reaction using the 10-lesion cut-off. All local skin reactions were significantly more severe in the subgroup of patients with more than 10 baseline lesions than in patients with 5–10 lesions (P < 0.05; Table 3).
Discussion
Results from this exploratory analysis confirm that severe local skin reactions were less frequent with 5-FU 4% cream applied once daily compared with the 5% formulation applied twice daily. More interestingly, the occurrence of severe local skin reactions was related to the number of lesions at baseline. Local skin reactions (i.e. erythema, scaling/dryness, oedema, crusting, erosions, stinging/burning and pruritus) were maximal after 4 weeks of treatment with 5-FU 4% cream and appeared to be more severe in patients with more than 10 lesions at baseline compared with those with 5–10 lesions. This suggests that a threshold of 10 lesions pre-treatment could be considered a good predictor of more severe local skin reactions.
The established treatment for AK is generally 5-FU 5% cream applied once or twice daily for 4 weeks [10, 16, 18]. However, while 5-FU has proven efficacy, this treatment is often associated with adverse events that some patients may find difficult to tolerate, discontinuing their treatment early and thus reducing the duration of treatment and compromising its efficacy [9]. As shown in this analysis, the frequency of severe local skin reactions is lower when using the once-daily 5-FU 4% cream than the 5-FU 5% cream twice daily. In addition, given the effect of tolerability on treatment adherence, predicting which patients may have more severe skin reactions during treatment with 5-FU cream could be important in ensuring that these patients are adequately informed and managed. This post hoc analysis helped to define a cut-off value of 10 lesions pre-treatment that could help healthcare practitioners to inform patients and to prevent the potentially more severe local adverse skin reactions. This could help improve patient treatment adherence.
In the current study, statistically significant associations between the number of baseline lesions and the severity of local skin reactions were shown after 4 weeks of treatment. Hence, future studies could include a formal evaluation of the relationship between the intensity and frequency of local reactions at week 2 with severe local reactions at week 4 (i.e. the pattern of development of local skin reactions over time). Additionally, it would also be useful to know whether the intensity of local reactions (moderate to severe) is predictive of better clearance of lesions, by analysing the severity of reactions at weeks 2–4 versus the complete clearance rate 4 weeks after completing treatment.
A limitation of the current study is its post hoc exploratory nature and thus confirmation of these findings in a prospective study would be preferable. Additional limitations included the lack of assessment of treatment adherence, and whether any patients who experienced moderate or severe local skin reactions discontinued treatment or skipped treatment applications; examination of these issues would be useful in future studies. In addition, the grading scale for the assessment of local skin reactions was not validated and inter-reliability was not evaluated prior to its use in the study.
Conclusions
The results from this analysis provide further evidence to support the treatment of patients with AK with 5-FU 4% cream. Treatment with 5-FU 4% cream once daily was associated with less severe local skin reactions compared with twice daily application of 5-FU 5%. The number of lesions at baseline seems to have predictive value regarding the severity of local skin reactions that appear during treatment. Using a cut-off value of 10 lesions pre-treatment, healthcare practitioners can forewarn patients accordingly of potentially more severe local adverse skin reactions. Given that a higher number of lesions at baseline is likely to be predictive of a greater severity of local skin reactions during treatment, healthcare practitioners should plan appropriately for the management of these patients, with the aim of helping patients persist with their treatment despite such reactions. All patients using 5-FU topical cream should be counselled to use moisturisers and topical steroids to manage severe reactions. However, in patients with more than 10 lesions, proactive patient management may also include continuous monitoring, patient education to manage their expectations of treatment, and other preventive treatments for, or prompt management of, local skin reactions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for these analyses, the Rapid Service Fee and medical writing services were funded by Pierre Fabre Dermatologie.
Medical Writing Assistance
We would like to thank Andrea Bothwell who wrote the outline of this manuscript on behalf of Springer Healthcare Communications and Alma Orts-Sebastian, of Springer Healthcare Communications, who wrote the first draft. This medical writing assistance was funded by Pierre Fabre Dermatologie.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors played equal roles in the concept and planning of the work described; acquisition, analysis and interpretation of the data; critical revision of the manuscript; and approved the final submitted version of the manuscript.
Disclosures
Eggert Stockfleth has received fees from Pierre Fabre for acting as a medical and scientific advisor, speaker and investigator. Nathalie Bégeault and Alain Delarue are employees of Pierre Fabre.
Compliance with Ethics Guidelines
This pooled analysis was based on previously conducted studies and does not contain any direct investigation with human participants or animals performed by any of the authors. Studies HD-FUP3B-048 and HD-FUP3B-049 received ethical approval from the institutional review boards of all relevant study sites and were conducted in compliance with US Food and Drug Administration regulations, the ethical principles of the Declaration of Helsinki, and the current International Conference on Harmonisation Good Clinical Practice guidelines. Patients provided written informed consent to participate in the studies.
Data Availability
The datasets analysed during the current study are available from the corresponding authors on reasonable request.
References
- 1.de Berker D, McGregor JM, Mohd Mustapa MF, Exton LS, Hughes BR. British Association of Dermatologists' guidelines for the care of patients with actinic keratosis 2017. Br J Dermatol. 2017;176(1):20–43. doi: 10.1111/bjd.15107. [DOI] [PubMed] [Google Scholar]
- 2.Stockfleth E, Kerl H, Zwingers T, Willers C. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165(5):1101–1108. doi: 10.1111/j.1365-2133.2011.10387.x. [DOI] [PubMed] [Google Scholar]
- 3.Szeimies R-M, Atanasov P, Bissonnette R. Use of lesion response rate in actinic keratosis trials. Dermatol Ther (Heidelb) 2016;6(4):461–464. doi: 10.1007/s13555-016-0145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 5.Traianou A, Ulrich M, Apalla Z, et al. Risk factors for actinic keratosis in eight European centres: a case-control study. Br J Dermatol. 2012;167(Suppl 2):36–42. doi: 10.1111/j.1365-2133.2012.11085.x. [DOI] [PubMed] [Google Scholar]
- 6.Dirschka T, Gupta G, Micali G, et al. Real-world approach to actinic keratosis management: practical treatment algorithm for office-based dermatology. J Dermatolog Treat. 2017;28(5):431–442. doi: 10.1080/09546634.2016.1254328. [DOI] [PubMed] [Google Scholar]
- 7.Huyke C, Reuter J, Rödig M, et al. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J Dtsch Dermatol Ges. 2009;7(2):128–133. doi: 10.1111/j.1610-0387.2008.06865.x. [DOI] [PubMed] [Google Scholar]
- 8.Stockfleth E, von Kiedrowski R, Dominicus R, et al. Efficacy and safety of 5-fluorouracil 0.5%/salicylic acid 10% in the field-directed treatment of actinic keratosis: a phase III, randomized, double-blind, vehicle-controlled trial. Dermatol Ther (Heidelb) 2017;7(1):81–96. doi: 10.1007/s13555-016-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulin Y, Lynde CW, Barber K, et al. Non-melanoma skin cancer in Canada Chapter 3: management of actinic keratoses. J Cutan Med Surg. 2015;19(3):227–238. doi: 10.1177/1203475415583414. [DOI] [PubMed] [Google Scholar]
- 10.Werner RN, Jacobs A, Rosumeck S, Erdmann R, Sporbeck B, Nast A. Methods and Results Report—evidence and consensus-based (S3) guidelines for the treatment of actinic keratosis—International League of Dermatological Societies in cooperation with the European Dermatology Forum. J Eur Acad Dermatol Venereol. 2015;29(11):e1–e66. doi: 10.1111/jdv.13179. [DOI] [PubMed] [Google Scholar]
- 11.Fleming P, Zhou S, Bobotsis R, Lynde C. Comparison of the treatment guidelines for actinic keratosis: a critical appraisal and review. J Cutan Med Surg. 2017;21(5):408–417. doi: 10.1177/1203475417708166. [DOI] [PubMed] [Google Scholar]
- 12.Stockfleth E. The importance of treating in the field of actinic keratosis lesions. J Eur Acad Dermatol Venereol. 2017;31(Suppl 2):8–11. doi: 10.1111/jdv.14092. [DOI] [PubMed] [Google Scholar]
- 13.Ezzedine K, Painchault C, Brignone M. Use of complete clearance for assessing treatment efficacy for 5-fluorouracil interventions in actinic keratoses: how baseline lesion count can impact this outcome. J Mark Access Health Policy. 2020;8(1):1829884. doi: 10.1080/20016689.2020.1829884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piquero-Casals J, Morgado-Carrasco D, Gilaberte Y, et al. Management pearls on the treatment of actinic keratoses and field cancerization. Dermatol Ther (Heidelb) 2020;10(5):903–915. doi: 10.1007/s13555-020-00425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen MHE, Kessels J, Merks I, et al. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol. 2020;183(4):738–744. doi: 10.1111/bjd.18884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen MHE, Kessels J, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380(10):935–946. doi: 10.1056/NEJMoa1811850. [DOI] [PubMed] [Google Scholar]
- 17.Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev. 2012;12(12):CD004415. doi: 10.1002/14651858.CD004415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Y, Tang N, Cai L, Li Q. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32(3):e12822. doi: 10.1111/dth.12822. [DOI] [PubMed] [Google Scholar]
- 19.Pierre Fabre Dermatologie. Tolak 40 mg/g cream: summary of product characteristics. 2020. https://docetp.mpa.se/LMF/Tolak%20cream%20ENG%20SmPC_09001bee809dbaa1.pdf. Accessed 8 Dec 2020.
- 20.Hill Dermaceuticals Inc. TOLAK (fluorouracil) cream, 4%, for topical use. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/022259Orig1s000TOC.cfm. Accessed 8 Dec 2020.
- 21.Dohil MA. Efficacy, safety, and tolerability of 4% 5-fluorouracil cream in a novel patented aqueous cream containing peanut oil once daily compared with 5% 5-fluorouracil cream twice daily: meeting the challenge in the treatment of actinic keratosis. J Drugs Dermatol. 2016;15(10):1218–1224. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analysed during the current study are available from the corresponding authors on reasonable request.