Abstract
Introduction
Hidradenitis suppurativa (HS) is a chronic, inflammatory, recurrent disease, usually presenting after puberty with inflammatory lesions that mainly affect the apocrine gland-bearing areas of the body, most commonly the axillary, inguinal and anogenital regions. The treatment of HS is associated with certain challenges due to intrinsic resistance to various treatments and the presence of comorbidities and complications. The antibiotic dapsone is an established treatment for HS, but the current evidence base is limited. The aim of this review is to systematically review the literature on the efficacy of dapsone in the treatment of HS.
Methods
The Cochrane, PubMed and CINAHL databases were searched for relevant articles to be included in the systematic review.
Results
A total of seven studies, with a cumulative patient population of 135 patients, were included. Of these 135 patients, 62.2% demonstrated various degrees of improvement following treatment. However, as only three of the seven studies used dapsone monotherapy it is difficult to assess the effectiveness of dapsone because the benefits observed may be due to concurrently administered treatment.
Conclusion
Overall, the quality of evidence supporting the use of dapsone is weak. However, it is a well established treatment recommended in current, various national guidelines. There is a crucial need for well-designed randomized controlled trials to support its usage.
Keywords: Hidradenitis suppurativa, Acne inversa, Dapsone
Key Summary Points
| Hidradenitis suppurativa (HS) is a chronic inflammatory condition characterized by recurrent inflammatory lesions in the apocrine gland-bearing areas of the skin. |
| Current guidelines recommend the antibiotic dapsone as a third-line treatnent for patients with mild to moderate HS. |
| Dapsone is an oral medication that possesses both anti-inflammatory and antimicrobial effects. |
| Dapsone has mainly been used as an effective treatment in mild to moderate cases of HS, although it has been shown to be helpful in severe cases in some instances. |
| Current evidence for its usage is limited to case reports, case series and uncontrolled retrospective studies. |
| Further studies, especially randomized controlled trials, are needed to further assess the safety and efficacy of dapsone. |
Introduction
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic inflammatory disease of follicular occlusion [1]. It is a part of the ‘follicular occlusion tetrad’ along with acne conglobota, dissecting folliculitis of the scalp and pilonidal cysts [2]. Occlusion of the hair follicle with subsequent perifollicular inflammation is thought to be the key pathogenic event [3, 4]. Although consensus is still lacking on how follicular occlusion is triggered in HS, factors such as immune system dysregulation, genetics, hormonal fluctuations and specific environmental conditions have been implicated. The immune system dysregulation theory is supported by the presence of elevated levels of proinflammatory cytokines, including interleukin (IL)-1b, IL-12, IL-23, IL-17 and tumor necrosis factor alpha, in the inflammatory lesions [5]. The initial stages are characterized by painful subcutaneous nodules with recurrent flares, leading to the development of complications such as the formation of sinus tracts and fibrous scars [6]. Other secondary lesions include pyogenic granulomas in the sinus tract openings, induration and giant multiheaded comedones [7, 8]. HS is more prevalent among women than men, with 12.1 in 100,000 women being affected compared to 5.1 in 100,000 men [9]. It has also been reported that individuals between the ages of 30 and 39 years old are more prone to the disease [10].
The aim of this article is to systematically review the available data on the effectiveness of dapsone in the treatment of HS.
Methods
The reviewers searched the Cochrane, PubMed and CINAHL databases for studies conducted on the use of dapsone therapy for HS using the key words ‘dapsone,’ ‘hidradenitis suppurativa’ and ‘acne inversa.’
Eligibility Criteria
Studies that directly reported the treatment of HS with dapsone were considered for inclusion in this review. Those studies in which dapsone was used in combination with other drugs were also considered to be eligible. Papers and studies that did not fulfil these criteria were excluded.
Study Selection
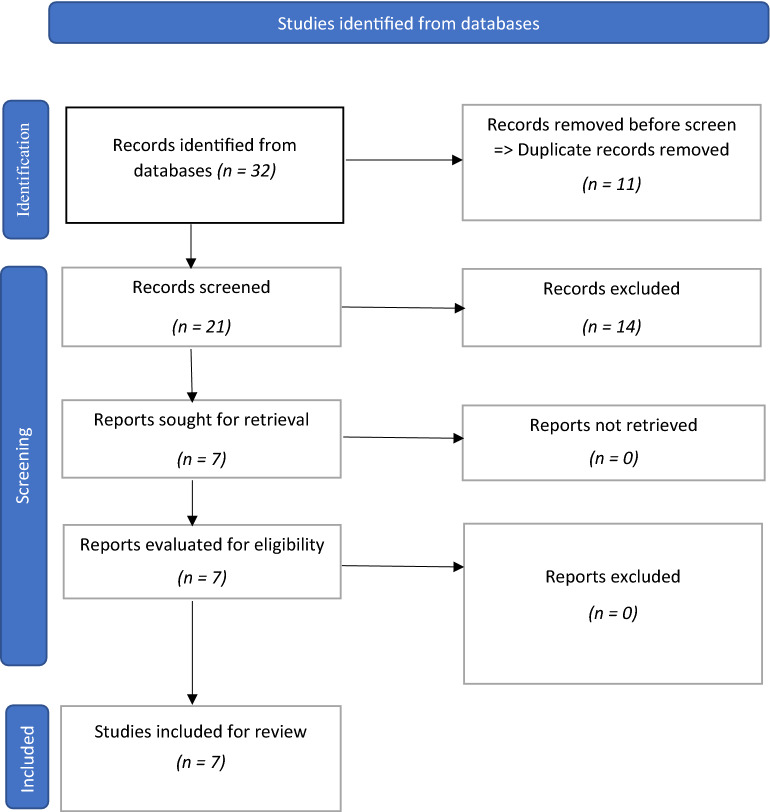
A total of seven studies were included from the 32 (studies) that were identified through the search of the databases. Prior to screening, 11 studies were removed as they were found to be duplicate studies, reducing the number of studies for screening to 21. These 21 studies were then extensively screened for eligibility by the reviewers, leading to the selection of seven studies that were ultimately included in the review (Fig. 1). The reasons for not including 14 of the 21 studies were they were not related to dapsone therapy or HS, as well as some were review papers.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram for study inclusion
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Classification of HS
The classification of HS is intended to reflect the severity of the disease. While there are several methods of classification, the Hurley staging system is the most widely adopted. This system has three stages:
Hurley stage I: This encompasses the formation of single or multiple abscesses without sinus tracts or cicatrization.
Hurley stage II: In this case, there are recurrent abscesses, with single or multiple widely separated lesions along with the presence of sinus tracts and cicatrization.
Hurley stage III: There is diffuse/broad involvement of multiple interconnected sinus tracts and abscesses across the entire area involved [11].
The Modified Hidradenitis Suppurativa Score (MHSS), also known as the Sartorius score, is a more detailed scoring method used to classify HS. It requires recording the measurement of the longest distance between two lesions that are of the same type around each of the seven anatomical regions and subsequently matching a predetermined weightage to various lesion types [12, 13].
Treatment of HS
Although various monotherapy and combination therapy regimens have been used to manage HS, treatment can be extremely challenging [14, 15]. The inherent delay that can be associated with obtaining a diagnosis [16] as well as initial misdiagnosis [14] contribute to the challenges of successful treatment. The average diagnostic delay for HS is reported to be 7 years [17] and, in particular, there is a notable gap between the correct diagnosis and actualization of effective treatment and/or favorable outcomes [7]. Patient outcomes are further complicated by the complications and comorbidities that evolve with the disease [18]. Anti-inflammatory therapies, surgery, antimicrobial therapies and adjuvant therapies have been extensively used in the treatment of HS, and the choice of treatment is, in most cases, dependent upon the severity of the patient’s condition [19]. However, in this context, antimicrobial therapy—though adopted for treating mild cases of the disease—is not associated with significant and/or highly effective outcomes and the issue of drug resistance has also to be considered [19]. Some of the most frequently recommended antimicrobial agents include macrolide antibiotics such as clindamycin [20], tetracyclines [21], rifampicin, metronidazole and moxifloxacin [22]. However, there have been reports of drug resistance associated with some of these antimicrobial agents, which ultimately defeats the purpose of treatment [23]. It is against this backdrop that researchers and physicians have been looking for more effective antimicrobial agents, and dapsone appears to offer some promise in this regard. It should be noted that the main objective of treatment with dapsone is to improve the quality of life together with reduction of pain, lesion formation and flare count [24].
Dapsone (chemical name: 4,4-diaminodiphenylsulfone), is a white, odorless crystalline powder that causes inhibition of dihydrofolic acid synthesis by binding to the active site of dihydropteroate synthetase [25]. Dapsone has been found to have a dual function as it has both antimicrobial and anti-inflammatory properties, thus mimicking the mechanism of action of non-steroidal anti-inflammatory drugs [25]. Broadly speaking, the anti-inflammatory action of dapsone is triggered by the inhibition of neutrophil migration as the drug adheres to extravascular sites (such as the pilosebaceous unit) while the antimicrobial action results in the local microbiome being modified [26]. Hyper-reactive neutrophil-mediated pathogenesis of HS has been demonstrated, and theoretically the effectiveness of dapsone could be attributed to reduction of this inflammation [27]. Dapsone has not only been used to treat HS, but also other diseases, including acne conglobata [28], dermatitis herpetiformis [29], pemphigus vulgaris and IgA pemphigus [30]. Reported adverse effects include hematological complications. Dapsone is usually given orally at dosages varying from 50 to 200 mg [25].
Results
All except two of the reports were retrospective studies. The seven studies that were reviewed had a cumulative sample size of 135 patients. Of all the studies identified, the gender trend was only reported in six studies, and 58.6% (68) of the patients in these studies were female; male patients affected by HS were the majority in just one study. This observation confirms the gender-based prevalence rate of the disease. The patients were administered dapsone in varying doses; the lowest being 25 mg/day and the highest (dosage) was 200 mg/day (Table 1). Of these 135 patients, 62.2% demonstrated various degrees of improvement. However, only three out of the seven studies used dapsone as monotherapy; the remaining studies used it in combination with other agents. Consequently, it is difficult to assess the effectiveness of dapsone monotherapy as the benefits seen may be due to concurrently administered treatments.
Table 1.
Description of the individual studies
| Paper | Patients’ characteristic | Study type | Level of evidence | HS classificationa | Drug(s) used in treatment | Dapsone dosage/duration of treatment | Treatment outcome/comments | Adverse events |
|---|---|---|---|---|---|---|---|---|
| Hofer and Itin (2001) [28] |
5 patients (all females) Mean age: 33 years |
Case report | 4 | Not reported | Dapsone | 50–100 mg/day | All patients had good (clinically significant) outcome. Adverse events were reported in 40% (2) of patients | Icteric sclera, increased bilirubin levels |
| Kaur and Lewis (2006) [31] |
5 patients (3 females; 2 males) Mean age: 49 years |
Retospective case study | 4 | Severe HS | Dapsone | 25–150 mg/day for between 6 and 12 weeks | Clinically significant improvement was observed in all patients although the use of dapsone had to be continued to prevent recurrence or relapse of HS. Adverse events in 40% (2) of patients | Tiredness, hemolysis, methemoglobinemia, agranulocytosis |
| Yazdanyar et al. (2011) [32] |
24 patients (22 females; 2 males) Mean age: 44.1 years |
Uncontrolled open longitudinal study | 4 |
Hurley I: 33% Hurley II: 50% Hurley III: 17% |
Dapsone [+ ciprofloxacin and/or rifampicin given to three patients] | 50–200 mg/day for 28 days | Six (25%) patients had clinically significant improvement while 3 (12.5%) others had slight improvement. The condition of 13 (54.1%) patients remained unchanged and the remaining two patients had deteriorating health status. Patients who were given additional treatment did not show any improvement and treatment with dapsone had to be discontinued in two cases where side effects were reported. Adverse events were reported in 10 patients | Dizziness, tiredness, nausea, anemia, headache, low hemoglobin, general malaise, gloominess |
| Kozub and Simaljakova (2012) [33] |
1 female patient Age: 53 years |
Case report | 4 | Not reported | Infliximab and dapsone | 100–200 mg/day for 21 weeks | The patient showed a clinically significant improvement with the normalization of C-reactive protein level achieved | No side effects |
| Bashyam et al. (2020) [34] | 19 patients | Retrospective case series | 4 | Moderate—severe HS | Dapsone [+ adalimumab in 12 cases] | 25–100 mg/day for 1–3 months | Clinically significant improvement was observed in 3 (15.8%) patients while the condition of 10 (52.6%) patients slightly improved. The remaining 6 (31.6%) patients had no change in their health status. Adverse events were reported in 4 cases | Nausea |
| Murray et al. (2020) [24] |
25 patients (18 females; 7 males) Median age: 42 years |
Uncontrolled retrospective study | 4 |
Hurley I: 28% Hurley II: 60% Hurley III: 12% |
Dapsone | 50–200 mg/day for 1–3 months | Sixteen (64%) patients had clinically subjective improvement while no improvement was seen in 8 (32%) patients. The use of dapsone was discontinued in 2 patients due to adverse events | Anemia |
| Lopez-Llunell et al. (2021) [35] |
56 patients (19 females; 37 males) Median age: 33 years |
Uncontrolled retrospective single-center study | 4 |
Hurley I: 53.6% Hurley II: 23.2% Hurley III: 23.2% |
Dapsone [+ isotretinoin or acitretin in 4 cases] | 50–150 mg/day for 3–14 months | Thirty-five (62.5%) patients had clinically significant improvement while others did not show such response. Dapsone therapy was discontinued as a result of adverse events and due to the intention for pregnancy in the future | Anemia, nausea, headache, diarrhoea |
HS Hidradenitis suppurativa
aSee section Classification of HS for explanation of HS categories
Discussion
Dapsone for the treatment of HS resulted in 62.2% (84) of the patient population showing varying degrees of improvement, although no change was seen in 35.5% (48) of the patients. However, only three of the seven studies used dapsone monotherapy, with the other studies reporting the effects of using dapsone in combination with other medications. More than half of the patients in each of the studies—except the study by Kaur and Lewis [31]—had either slightly or clinically significant improved health status following treatment. Another aspect that has to be examined is the correlation between drug effectiveness and severity of HS. Current guidelines [36] recommend dapsone for mild to moderate HS (i.e. Hurley stage I and II); however, the findings from this systematic review show that dapsone can also be effective in the treatment of severe HS (i.e. Hurley stage III), as seen in the studies conducted by Kaur and Lewis [31] and Yazdanyar et al. [32]. This efficacy may be related to the dosage and duration of dapsone therapy and, as observed in this review, the dosage of dapsone was varied based on how well a patient was able to tolerate the drug. Some patients responded to a lower dose while others required a relatively higher dose. The studies by Murray et al. [24] and Lopez-Llunell et al. [35] did not show any significant treatment response with dapsone. One study reported the need for maintenance dosage therapy to sustain positive patient outcomes [31]. Although most of the studies did not report the anti-inflammatory marker levels during HS treatment, Kozub and Simaljakova reported that dapsone contributed to the normalization of C-reactive protein levels [33].
Drug Safety
Based on the results of this systematic review, dapsone was relatively well tolerated when used in the management of HS. The incidence rate of adverse events was 25.1%, with nausea and anemia being the most commonly reported adverse events [24, 32, 34]. Furthermore, the effects of the adverse events on treatment outcomes were not significant in most cases. However, adverse events such as headache and nausea did result in the discontinuation of dapsone therapy [24, 32, 35], as well as the deterioration of the condition only in three patients. Concerns over the safety and side effects of dapsone can sometimes lead to dose reductions and shorter duration of drug administration, both of which can affect its effectiveness.
Conclusion
Overall, the quality of evidence supporting the use of dapsone is weak. However, it is a well-established treatment recommended in current guidelines [36]. There is a crucial need for well-designed randomized controlled trials to support its usage.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. Article processing charges were funded independently by the authors themselves.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
AR contributed to the concept, screening the articles and drafting the manuscript. BJ contributed to screening the articles and drafting the manuscript.
Disclosures
Aswatha Rabindranathnambi and Balasubramanian Jeevankumar have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539–561. doi: 10.1016/j.jaad.2008.11.911. [DOI] [PubMed] [Google Scholar]
- 2.Pink A, Anzengruber F, Navarini AA. Acne and hidradenitis suppurativa. Br J Dermatol. 2018;178:619–631. doi: 10.1111/bjd.16231. [DOI] [PubMed] [Google Scholar]
- 3.Von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164:367–371. doi: 10.1111/j.1365-2133.2010.10034.x. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Desai N, Emtestam L, et al. European S1 for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29:619–644. doi: 10.1111/jdv.12966. [DOI] [PubMed] [Google Scholar]
- 5.Collier EK, Parvataneni RK, Lowes MA, et al. Diagnosis and management of hidradenitis suppurativa in women. Am J Obstet Gynecol. 2020;224(1):54–61. doi: 10.1016/j.ajog.2020.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revuz JE. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2009;23(9):985–998. doi: 10.1111/j.1468-3083.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 7.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med. 2012;366:158–164. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 8.Lipsker D, Severac F, Freysz M, et al. The ABC of hidradenitis suppurativa: a validated glossary on how to name lesions. Dermatology. 2016;232:137–142. doi: 10.1159/000443878. [DOI] [PubMed] [Google Scholar]
- 9.Garg A, Lavian J, Lin G, Strunk A, Alloo A. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118–122. doi: 10.1016/j.jaad.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Garg A, Kirby JS, Lavian J, Lin G, Strunk A. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760–764. doi: 10.1001/jamadermatol.2017.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa, and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, editors. Dermatologic surgery. New York: Marcel Dekker; 1989. pp. 729–739. [Google Scholar]
- 12.Sartorius K, Lapins J, Emtestam L, Jemec GB. Suggestions for uniform outcome variables when reporting treatment effects in hidradenitis suppurativa. Br J Dermatol. 2003;149:211–213. doi: 10.1046/j.1365-2133.2003.05390.x. [DOI] [PubMed] [Google Scholar]
- 13.Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Del MV, Mrowietz U, et al. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology. 2015;231(2):184–190. doi: 10.1159/000431175. [DOI] [PubMed] [Google Scholar]
- 15.Gulliver W, Zouboulis CC, Prens E, Jemec GB, Tzellos T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev Endocr Metab Disord. 2016;17(3):343–351. doi: 10.1007/s11154-016-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg A, Neuren E, Cha D, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey of Impact and Healthcare Needs (VOICE) project. J Am Acad Dermatol. 2020;82:366–376. doi: 10.1016/j.jaad.2019.06.1301. [DOI] [PubMed] [Google Scholar]
- 17.Saunte DM, Boer J, Stratigos A, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol. 2015;173:1546–1549. doi: 10.1111/bjd.14038. [DOI] [PubMed] [Google Scholar]
- 18.Tzellos T, Zouboulis CC, Gulliver W, et al. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2015;173:1142–1155. doi: 10.1111/bjd.14024. [DOI] [PubMed] [Google Scholar]
- 19.Andersen RK, Jemec GBE. Treatments for hidradenitis suppurativa. Clin Dermatol. 2017;35:218–224. doi: 10.1016/j.clindermatol.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Clemmensen OJ. Topical treatment of hidradenitis suppurativa with clindamycin. Int J Dermatol. 1983;22:325–328. doi: 10.1111/j.1365-4362.1983.tb02150.x. [DOI] [PubMed] [Google Scholar]
- 21.Jemec GB, Wendelbee P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol. 1998;39:971–974. doi: 10.1016/S0190-9622(98)70272-5. [DOI] [PubMed] [Google Scholar]
- 22.Join-Lambert O, Coignard H, Jais JP, et al. Efficacy of rifampin-moxifloxacin-metronidazole combination therapy in hidradenitis suppurativa. Dermatology. 2011;222:49–58. doi: 10.1159/000321716. [DOI] [PubMed] [Google Scholar]
- 23.Fischer AH, Haskin A, Okoye GA. Patterns of antimicrobial resistance in lesions of hidradenitis suppurativa. J Am Acad Dermatol. 2017;76:309–313. doi: 10.1016/j.jaad.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Murray G, Hollywood A, Kirby B, Hughes R. Dapsone therapy for hidradenitis suppurativa. Br J Dermatol. 2020;183(4):767–768. doi: 10.1111/bjd.19136. [DOI] [PubMed] [Google Scholar]
- 25.Wozel G, Blasum C. Dapsone in dermatology and beyond. Arch Dermatol Res. 2014;306(2):103–24 [DOI] [PMC free article] [PubMed]
- 26.Booth SA, Moody CE, Dahl MV, Herron MJ, Nelson RD. Dapsone suppresses integrin-mediated neutrophil adherence function. J Invest Dermatol. 1992;98:135–140. doi: 10.1111/1523-1747.ep12555654. [DOI] [PubMed] [Google Scholar]
- 27.Lapins J, Asman B, Gustafsson A, Bergstrom K, Emtestam L. Neutrophil-related host response in hidradenitis suppurativa—a pilot study of patients with inactive disease. Acta Derm Venereol. 2001;81:96–99. doi: 10.1080/000155501750208146. [DOI] [PubMed] [Google Scholar]
- 28.Hofer T, Itin PH. Acne inverse. Eine dapson-sensitive dermatose [Acne inverse: a dapsone-sensitive dermatitis] Hautarzt. 2001;52:989–992. doi: 10.1007/s001050170015. [DOI] [PubMed] [Google Scholar]
- 29.Zhu YI, Stiller MJ. Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol. 2001;45:420–434. doi: 10.1067/mjd.2001.114733. [DOI] [PubMed] [Google Scholar]
- 30.Kasperkiewicz M, Schmidt E. Current treatment of autoimmune blistering diseases. Curr Drug Discov Technol. 2009;6:270–280. doi: 10.2174/157016309789869065. [DOI] [PubMed] [Google Scholar]
- 31.Kaur MR, Lewis HM. Hidradenitis suppurativa treated with dapsone: a case series of five patients. J Dermatolog Treat. 2006;17(4):211–213. doi: 10.1080/09546630600830588. [DOI] [PubMed] [Google Scholar]
- 32.Yazdanyar S, Boer J, Ingvarsson G, Szepietowski JC, Jemec GBE. Dapsone therapy for hidradenitis suppurativa: a series of 24 patients. Dermatology. 2011;222:342–346. doi: 10.1159/000329023. [DOI] [PubMed] [Google Scholar]
- 33.Kozub P, Simaljakova M. Hidradenitis suppurativa treated with combination of infliximab and dapsone. Bratisl Lek Listy. 2012;113(5):319–323. doi: 10.4149/bll_2012_074. [DOI] [PubMed] [Google Scholar]
- 34.Bashyam AM, Baroudi B, Feldman S, Pichardo RO. Dapsone to treat moderate to severe hidradenitis suppurativa: a retrospective case series. J Am Acad Dermatol. 2020;83(6). [DOI] [PubMed]
- 35.Lopez-Llunell C, Riera-Marti N, Gamissans M, Romani J. Dapsone in hidradenitis suppurativa: a case series of 56 patients. Dermatol Ther. 2021:15161. 10.1111/dth.15161. [DOI] [PubMed]
- 36.Ingram JR, Collier F, Brown D, et al. British Association of Dermatologists guidelines for the management of hidradenitis suppurativa (acne inversa) 2018. Br J Dermatol. 2019;180(5):1009–1017. doi: 10.1111/bjd.17537. [DOI] [PubMed] [Google Scholar]