Abstract
Introduction
Secukinumab has demonstrated sustained long-term efficacy with a favourable safety profile in various manifestations of psoriatic disease. We investigated effectiveness and safety of secukinumab, other biologics and conventional systemic therapies in patients with chronic plaque psoriasis in a real-world setting.
Methods
REALIA was a non-interventional, multicentre, prospective, parallel group study. Eligible patients were ≥ 18 years old with chronic plaque psoriasis commencing a new treatment with a biologic agent or conventional systemic therapies.
Results
At baseline, 541 patients were divided into three cohorts based on treatment initiated: conventional systemics (173), secukinumab (184) and other biologics (184). A significantly higher proportion of patients achieved almost clear to clear skin based on physician’s judgement in secukinumab versus conventional systemics at month 3 (64.7% versus 22.8%, P < 0.001) and month 6 (61.8% versus 20.8%, P < 0.001). At month 12, clear to almost clear skin was achieved by 52.1% of the patients in secukinumab versus 35.8% in conventional systemics (P = 0.066). The proportion of patients achieving Psoriasis Area Severity Index (PASI) 90 on conventional systemics, secukinumab and other biologics was 18.8%, 59.7% and 40.0% at month 3 and 35.3%, 60.8% and 50.0% at month 12, respectively. Secukinumab patients showed significantly higher change in PASI total score from baseline versus conventional systemics at month 3 {least squares [LS] mean [standard error (SE)]: −14.49 [0.648] versus −8.48 [1.149], P < 0.001} and numerically higher [LS mean (SE): −13.60 (0.475) versus −10.84 (1.733), P = 0.122] at month 12. The proportion of patients with Dermatology Life Quality Index 0/1 score on conventional systemics, secukinumab and other biologics was 22.6%, 65.0% and 41.6% at month 3 and 32.0%, 63.5% and 41.3% at month 12, respectively. Safety profile was comparable across cohorts.
Conclusions
Secukinumab is effective and well tolerated in patients with chronic plaque psoriasis in a real-world setting in an Asia-Pacific and Middle East population, and these results are in agreement with clinical outcomes of secukinumab reported in randomised clinical trials.
Trial registration number
170803-001645.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-021-00675-w.
Keywords: Biologics, Psoriasis, Real-world, Safety, Secukinumab
Key Summary Points
| Why carry out this study? |
| Secukinumab has demonstrated sustained long-term efficacy with a favourable safety profile in various manifestations of psoriatic disease in clinical trials. |
| In the real world, secukinumab is prescribed for patients with chronic plaque psoriasis who may have clinical features that differ from those seen in controlled clinical trials; therefore, the collection of real-world data becomes essential in the characterisation of the patterns of secukinumab use in the everyday clinical practice where other treatment options are available. |
| Real-world data on the effectiveness of secukinumab in the Asia-Pacific and Middle East regions are sparse. |
| REALIA was a non-interventional, multicentre, prospective, parallel group study conducted to investigate effectiveness and safety of secukinumab, other biologics and conventional systemic therapies in patients with chronic plaque psoriasis in a real-world setting. |
| What has been learned from the study? |
| The findings from the REALIA study conducted in a large geographical region of Asia-Pacific and Middle East suggest that secukinumab is prescribed in patients with chronic plaque psoriasis with higher prevalence of psoriatic arthritis, more severe and longer duration of the disease, compared with patients treated with conventional systemics and other biologics. |
| The results confirm that secukinumab is effective and well tolerated in patients with psoriasis in a real-world setting, and these findings are in line with the clinical outcomes of secukinumab reported in clinical trials. |
Introduction
Psoriasis is a chronic inflammatory condition characterised by well-defined erythematous plaques with silver or white scales that can be itchy and vary in severity [1]. Psoriasis affects 2–4% of the adult population in Western countries, with a worldwide prevalence ranging from 0.91% to 8.5% [1–3]. Estimated prevalence of psoriasis is 2.3–6.6% in Australia, 0.24% in Taiwan and 0.45% in South Korea [4]. In Middle East countries such as Egypt, Lebanon, Saudi Arabia and United Arab Emirates, the estimated prevalence of psoriasis in the overall population ranges from 0.40% to 0.58% [5]. An estimated 20–30% of patients with psoriasis have moderate to severe forms of the disease, which represent serious and disabling conditions for patients [6]. Comorbidities frequently associated with psoriasis include obesity, metabolic syndrome, cardiovascular disease and psoriatic arthritis (PsA) [7]. Patients with psoriasis have an increased risk of depression and suicidality as well as impaired quality of life (QoL) and work productivity [8]. Different options available for the management of chronic plaque psoriasis include topical agents (mainly for mild forms), phototherapy, conventional systemic treatments (e.g. methotrexate, ciclosporin, retinoids; in patients suffering from moderate to severe disease) and biologic agents, which act by inhibiting the actions of proinflammatory cytokines [9–13]. Biologic agents approved for the treatment of moderate to severe psoriasis can be grouped into the following classes: tumour necrosis factor alpha (TNFα) inhibitors, interleukin (IL)-12/23 and IL-23 inhibitors, and IL-17A cytokine inhibitors [10, 11, 13].
Secukinumab is a fully human monoclonal antibody that selectively neutralises IL-17A, a cornerstone cytokine involved in the pathogenesis of psoriatic disease. Secukinumab has demonstrated sustained long-term efficacy with a favourable safety profile in various psoriatic disease manifestations [7, 14–19]. Almost clear to clear skin, corresponding to at least 90% reduction in the Psoriasis Area and Severity Index response (PASI 90), was achieved in patients with moderate to severe chronic plaque psoriasis treated with secukinumab in clinical studies of time period ranging from 12 weeks to 5 years [16, 20–26]. The development program showed that almost clear to clear skin correlates with a significant improvement in the patients’ QoL when compared with a PASI 75 response [27]. An about 50% increase in the proportion achieving Dermatology Life Quality Index (DLQI) response of 0/1 was observed in patients who achieved PASI 90 versus patients achieving PASI 75 [27].
In real-life settings, secukinumab is prescribed to patients with chronic plaque psoriasis who may exhibit clinical characteristics different from clinical trial population. Clinicians’ judgement on the benefit–risk of secukinumab for individual patients, and the characteristics of their local health systems, may lead to treatment regimens that differ from the label or the current treatment recommendations. The collection of real-world data becomes therefore essential in the characterisation of the patterns of secukinumab use in the everyday clinical practice. The REALIA study investigated the effectiveness, safety and treatment patterns of secukinumab, other biologics and conventional systemic therapies in patients with chronic plaque psoriasis eligible for systemic treatment in a real-world setting.
Methods
Study Design
REALIA was a 12-month non-interventional, multicentre, prospective, parallel group study conducted at 59 sites in 11 countries in the Asia-Pacific and Middle East region including Australia, Egypt, Lebanon, Malaysia, Philippines, Saudi Arabia, Singapore, South Korea, Taiwan, the United Arab Emirates and Vietnam. Data collection spanned from 26 December 2016 to 31 July 2019.
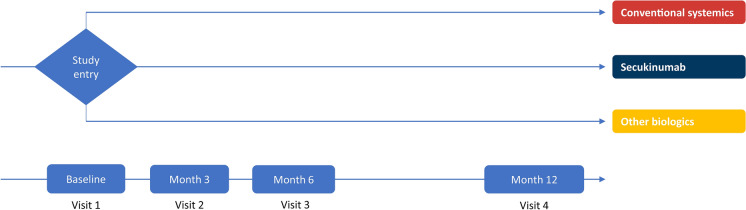
At baseline, patients were enrolled in three treatment cohorts, as shown in Fig. 1.
Cohort 1: conventional systemic drugs (non-biologic), such as methotrexate, ciclosporin or retinoids.
Cohort 2: secukinumab.
Cohort 3: other biologic agents (proprietary or biosimilars), such as TNFα inhibitors (etanercept, adalimumab and infliximab), IL-12/23 inhibitors (ustekinumab) or IL-17A inhibitors (ixekizumab only).
Fig. 1.
Study design
Treatment at baseline was used to assign patients to the baseline treatment cohort [e.g., a patient who entered the study in cohort 3 (other biologics) remained in this baseline treatment cohort even if during the study the patient switched treatment to conventional systemics or secukinumab]. The baseline treatment cohort may differ from the actual treatment cohort, which refers to the treatment cohort the patient was on at any time during the study; each patient could be in more than one actual treatment cohort over the course of the study. Recording changes as add-on therapy, switch in therapy, treatment withdrawal and treatment regimen adjustment accounted for treatment change. All types of changes, including treatment adjustment, were recorded for each patient.
All patients provided written informed consent before enrolling in the study. The study protocol was approved by the institutional review board/ethics committee of each participating centre. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) and in compliance with all federal, local or regional requirements.
Patients
Patients enrolled in the study were aged ≥ 18 years with chronic plaque psoriasis and active skin lesions diagnosed by a dermatologist, commencing a new treatment with a biologic agent or conventional systemic therapy for psoriasis in compliance with the local prescribing information (either treatment-naïve or switching to a different agent) due primarily to their active skin lesions in the case of concurrent joint involvement. Inclusion and exclusion criteria are detailed in the supplementary appendix.
Objectives
The primary objective was to determine the real-world effectiveness of secukinumab, other biologics and conventional systemic therapies by assessing the achievement of almost clear to clear skin based on the physician’s judgement at month 3. The secondary objectives were to describe: patients’ clinical characteristics; real-world effectiveness of therapies by the achievement of almost clear to clear skin at months 6 and 12; the association/correlation of almost clear to clear skin with the psoriasis assessment tool(s) used in clinical practice over the observation period [i.e. PASI, Physician’s Global Assessment (PGA)/Investigator’s Global Assessment (IGA), body surface area (BSA)]; treatment patterns of therapies; the impact on health-related quality of life (HR-QoL) measured with the Dermatology Life Quality Index (DLQI); and safety and tolerability.
Statistical Analysis
The following analysis sets were used for data analysis:
The enrolled set (ENS) included all patients who provided informed consent.
The exposed set (EXS) included all patients in the ENS who fulfilled the inclusion and exclusion criteria and received at least one dose of any of the medications of interest during the treatment period after enrolment into the study. Patients who switched to a compound associated with a different cohort at some point in the study who would then be allocated to all relevant treatment cohorts (i.e. a patient could be counted under multiple treatment cohorts) were also part of the EXS.
The full analysis set (FAS) was subdivided into FAS3, FAS6 and FAS12 analysis sets to account for the timing of treatment changes. FAS3, FAS6 and FAS12 included all patients in the ENS who fulfilled the inclusion and exclusion criteria and received at least one dose of baseline treatment of the medication of interest without a treatment change prior to their month 3, 6 or 12 visit, respectively. Further details about statistical analysis are included in the supplementary appendix.
Results
Patient Disposition
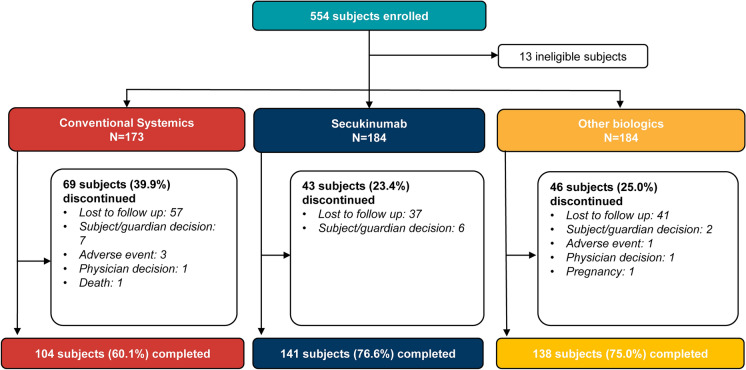
Overall, 554 patients were enrolled in the study, of whom 541 were divided into conventional systemics (n = 173), secukinumab (n = 184) and other biologics (n = 184) cohorts. A total of 383 patients (70.8%) of EXS completed the 12-month study; 60.1%, 76.6% and 75.0% in the conventional systemic, secukinumab and other biologics groups, respectively. The most common (≥ 1%) reasons for discontinuation were lost to follow-up (24.4%) and subject/guardian decision (2.7%) (Fig. 2). Of the 13 ineligible patients (2.3%), 9 patients did not meet inclusion criteria and 4 patients met exclusion criteria (Supplementary Table S1).
Fig. 2.
Patient disposition (baseline treatment cohort). Percentages are based on the number of patients in the enrolled set in the respective baseline treatment cohort
In total, 405 patients were included in the FAS3 (conventional systemics, 133 patients; secukinumab, 118 patients; other biologics, 154 patients), 338 patients were included in the FAS6 (conventional systemics, 98 patients; secukinumab, 108 patients; other biologics, 132 patients) and 266 patients were included in the FAS12 (conventional systemics, 72 patients; secukinumab, 89 patients; other biologics, 105 patients; Table 1). All 541 eligible patients were included in the EXS.
Table 1.
Analysis sets
| Analysis set | Conventional systemics, n | Secukinumab, n | Other biologics, n | Total, n |
|---|---|---|---|---|
| Enrolled and eligible patients | 173 | 184 | 184 | 541 |
| Full analysis set (FAS) | ||||
| FAS3 | 133 | 118 | 154 | 405 |
| FAS6 | 98 | 108 | 132 | 338 |
| FAS12 | 72 | 89 | 105 | 266 |
| Exposed set (EXS) | 182 | 211 | 213 | 541 |
Enrolled and eligible patients, FAS3, FAS6 and FAS12 are summarised based on the baseline treatment cohort. EXS is based on the actual treatment cohort. The total of the EXS is the number of enrolled and eligible patients who fulfil the inclusion and exclusion criteria and receive at least one dose of any of the medications of interest. It is not the sum of the EXS of the three treatment cohorts as patients can be exposed to more than one treatment cohort. EXS, exposed set; FAS3/6/12, full analysis set at month 3, 6 or 12; n, number of patients
Baseline Demographics and Disease Characteristics
Patients were predominantly male (68.6%), with a mean age of 42.5 years and body mass index (BMI) of 28.11 kg/m2 (Table 2). Overall, 27.0% (n = 146) of the patients had concomitant PsA at a higher proportion in the secukinumab versus the conventional systemics treatment cohort [30.4% (n = 56) versus 20.2% (n = 35), respectively]. The mean duration since diagnosis of psoriasis was longer in the secukinumab treatment cohort [14.23 versus 9.78 (conventional systemics) and 12.64 years (other biologics)]. Patients in the secukinumab treatment cohort had more severe disease [PASI > 20 or BSA > 20%: 44.2% (n = 76)] versus conventional systemics [39.0% (n = 64)] and other biologics [34.5% (n = 60)].
Table 2.
Baseline demographics and disease characteristics (EXS, baseline treatment cohort)
| Variable | Conventional systemics (N = 173), n (%) | Secukinumab (N = 184), n (%) | Other biologics (N = 184), n (%) | Total (N = 541), n (%) |
|---|---|---|---|---|
| Age (years), mean (SD) | 43.1 (14.98) | 43.3 (13.33) | 41.2 (13.34) | 42.5 (13.89) |
| Age < 65 years, n (%) | 158 (91.3) | 173 (94.0) | 175 (95.1) | 506 (93.5) |
| Male, n (%) | 119 (68.8) | 133 (72.3) | 119 (64.7) | 371 (68.6) |
| Race, n (%) | ||||
| Caucasian | 77 (44.5) | 89 (48.4) | 91 (49.5) | 257 (47.5) |
| Asian | 96 (55.5) | 89 (48.4) | 88 (47.8) | 273 (50.5) |
| Pacific Islander | 0 | 1 (0.5) | 3 (1.6) | 4 (0.7) |
| Other | 0 | 5 (2.7) | 2 (1.1) | 7 (1.3) |
| BMI (kg/m2), mean (SD) | 28.40 (5.76) (n = 143) | 28.18 (5.83) (n = 151) | 27.78 (5.92) (n = 153) | 28.11 (5.83) (n = 447) |
| Time since PsO diagnosis (years), mean (SD) | 9.78 (10.19) (n = 172) | 14.23 (10.70) (n = 183) | 12.64 (9.61) (n = 182) | 12.27 (10.32) (n = 537) |
| PsA, yes | 35 (20.2) | 56 (30.4) | 55 (29.9) | 146 (27.0) |
| PASI total score, mean (SD) | 15.45 (9.17) (n = 124) | 17.35 (11.12) (n = 131) | 15.63 (8.53) (n = 125) | 16.17 (9.71) (n = 380) |
| PASI total score category, n (%) | ||||
| < 10 | 33 (26.6) | 30 (22.9) | 27 (21.6) | 90 (23.7) |
| ≥ 10 to ≤ 20 | 67 (54.0) | 60 (45.8) | 71 (56.8) | 198 (52.1) |
| > 20 | 24 (19.4) | 41 (31.3) | 27 (21.6) | 92 (24.2) |
| BSA, mean (SD) | 29.0 (24.26) (n = 106) | 30.0 (21.28) (n = 99) | 24.5 (19.24) (n = 125) | 27.6 (21.64) (n = 330) |
| BSA category, n (%) | ||||
| Patients with data available (m) | 106 | 99 | 125 | 330 |
| Mild (BSA < 10%) | 22 (20.8) | 12 (12.1) | 17 (13.6) | 51 (15.5) |
| Moderate (BSA ≤ 10% to ≤ 20%) | 31 (19.2) | 31 (31.3) | 57 (45.6) | 119 (36.1) |
| Severe (BSA > 20%) | 53 (50.0) | 56 (56.6) | 51 (40.8) | 160 (48.5) |
| Severity of psoriasis | ||||
| Patients with data available (m) | 164 | 172 | 174 | 510 |
| Mild (BSA < 10% or PASI total score < 10) | 38 (23.2) | 31 (18.0) | 24 (13.8) | 93 (18.2) |
| Moderate (10% ≤ BSA ≤ 20% or 10 ≤ PASI total score ≤ 20) | 62 (37.8) | 65 (37.8) | 90 (51.7) | 217 (42.5) |
| Severe (BSA > 20% or PASI total score > 20) | 64 (39.0) | 76 (44.2) | 60 (34.5) | 200 (39.2) |
Percentages are based on the number of patients (n) or the number of patients available (m) in the EXS in the respective baseline treatment cohort
BMI body mass index, BSA body surface area, EXS exposed set, m number of patients available for assessment, N total number of patients, n number of patients, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, PsO psoriasis, SD standard deviation
Prior Psoriasis Medications
Overall, the most commonly used treatment class prior to enrolment was topical therapies (95.2%, n = 515), followed by conventional systemics (60.3%, n = 326) and phototherapy including psoralen and ultraviolet light A (PUVA), (51.9%, n = 281) (Supplementary Table S2). Biologic agents were used by 149 patients (27.5%); 61 patients (11.3%) had used two or more biologic agents. A total of 74.5% and 44.6% of patients received conventional systemics and biologic treatment prior to the secukinumab baseline treatment cohort, respectively (Supplementary Table S2). A higher percentage (23.4%) of patients in secukinumab group received two or more prior biologic agents than the other biologics (8.7%) and conventional systemics (1.2%) groups (Supplementary Table S2).
Effectiveness
Patients Achieving Almost Clear to Clear Skin Based on Physician’s Assessment
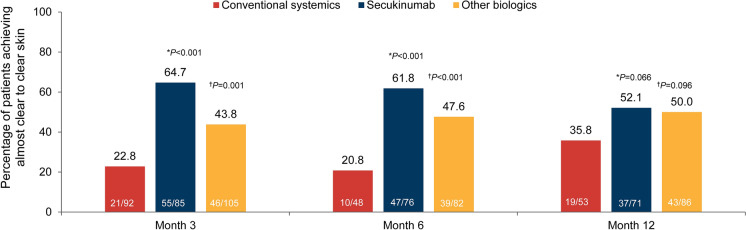
The proportion of patients achieving almost clear to clear skin was significantly higher in secukinumab versus conventional systemics treatment cohort at month 3 [64.7% versus 22.8%, risk difference (RD): 41.9 (95% CI 28.6, 55.2), P < 0.001] and month 6 [61.8% versus 20.8%, RD: 41.0 (95% CI 25.2, 56.9), P < 0.001], and numerically higher at month 12 [52.1% versus 35.8%, RD: 16.3 (95% CI −1.1, 33.6), P = 0.066] (Fig. 3). Similarly, in the other biologics cohort, the proportion of patients achieving almost clear to clear skin based on the physician’s assessment was significantly higher versus the conventional systemics cohort at month 3 [43.8%, RD: 21.0 (95% CI 8.2, 33.8), P = 0.001] and month 6 [47.6%, RD: 26.7 (95% CI 11.0, 42.5), P < 0.001], and numerically higher at month 12 [50.0%, RD: 14.2 (95% CI −2.5, 30.8), P = 0.096].
Fig. 3.
Proportion of patients achieving almost clear to clear skin (baseline treatment cohort). Percentages are based on the number of patients in the FAS3, FAS6 and FAS12 with data available (m) at each visit of interest in the respective baseline treatment cohort. *Secukinumab versus conventional systemics, †other biologics versus conventional systemics. FAS3/6/12, full analysis set at month 3, 6 or 12
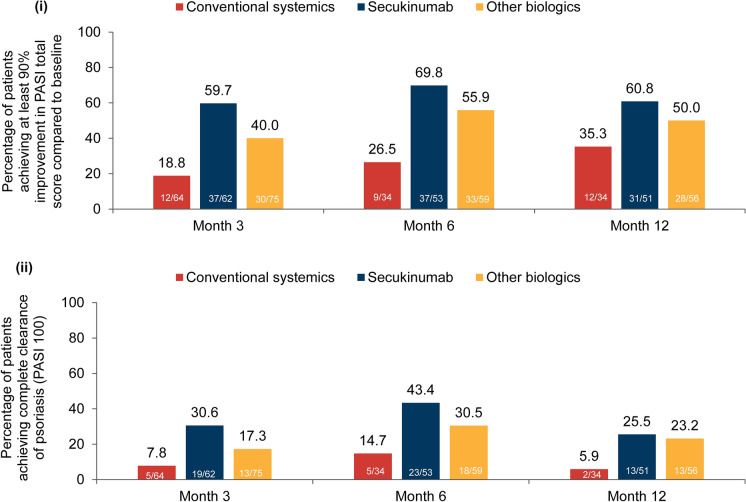
PASI 90 and PASI 100
The proportion of patients achieving PASI 90 in the conventional systemics, secukinumab and other biologics cohorts was 18.8%, 59.7% and 40.0% at month 3, 26.5%, 69.8% and 55.9% at month 6 and 35.3%, 60.8% and 50.0% at month 12, respectively (Fig. 4i). The proportion of patients achieving PASI 100 (%) in the conventional systemics, secukinumab and other biologics cohorts was 7.8%, 30.6% and 17.3% at month 3, 14.7%, 43.4% and 30.5% at month 6 and 5.9%, 25.5% and 23.2% at month 12, respectively (Fig. 4ii).
Fig. 4.
Proportion of patients achieving (i) PASI 90 and (ii) PASI 100 response (baseline treatment cohort). Percentages are based on the number of patients in the FAS3, FAS6 and FAS12 with data available (m) at each visit of interest in the respective baseline treatment cohort. FAS3/6/12, full analysis set at month 3/6/12; PASI Psoriasis Area and Severity Index
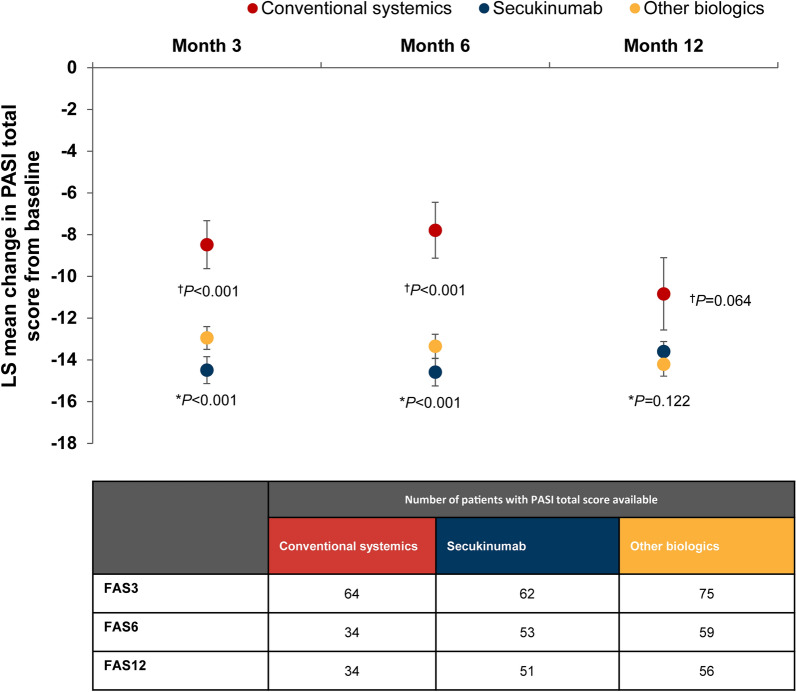
Change in PASI and BSA Total Score
Secukinumab patients showed significantly higher difference in least squares (LS) means in PASI total score from baseline versus conventional systemics at month 3 (−6.02; 95% CI for difference: −8.55, −3.49; P < 0.001) and numerically higher at month 12 (−2.76; 95% CI for difference: −6.27, 0.75; P = 0.122) (Fig. 5). Patients treated with other biologics also had significantly higher difference in LS means in PASI total score from baseline vs. conventional systemics at Month 3 (−4.47; 95% CI for difference: −6.90, −2.04; P < 0.001) and numerically higher change at Month 12 (−3.37; 95% CI for difference: −6.93, 0.19; P = 0.064) (Fig. 5). Change in BSA total score from baseline are presented in supplementary section.
Fig. 5.
Adjusted mean change in PASI total score from baseline (baseline treatment cohort). Error bars represent SE. Analysed using MMRM model including baseline treatment cohort, psoriatic arthritis, visit as fixed effect factors, baseline PASI total score value as covariate and the baseline treatment cohort by visit interaction, psoriatic arthritis by visit interaction, and baseline PASI total score by visit interaction. †Other biologics versus conventional systemics; *secukinumab versus conventional systemics. FAS3/6/12 full analysis set at month 3, 6 or 12; LS least squares; PASI, Psoriasis Area and Severity Index; MMRM mixed model repeated measures; SE standard error
PGA/IGA 0 or 1 Score
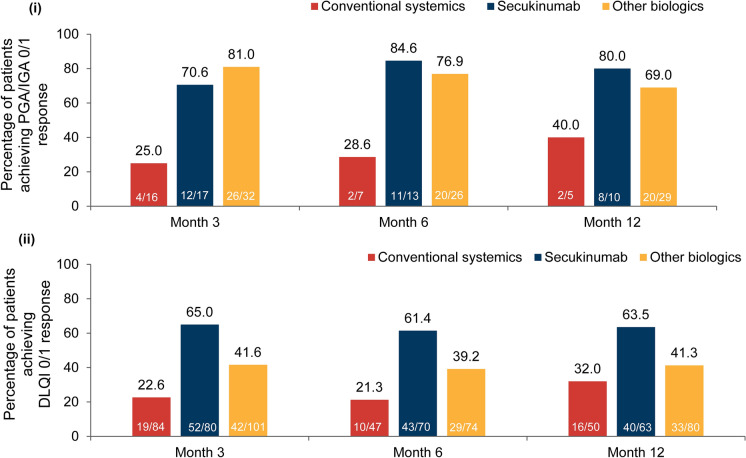
At month 3, the proportion of patients with PGA/IGA 0 or 1 score was highest in the other biologics group (81.0%), followed by the secukinumab group (70.6%) and the conventional systemics group. Secukinumab group had the highest proportion of patients (84.6% and 80.0%) with PGA/IGA 0 or 1 score at months 6 and 12 followed by other biologics (76.9% and 69.0%) and conventional systemics (28.6% and 40.0%) (Fig. 6i).
Fig. 6.
Proportion of patients achieving (i) PGA/IGA 0/1 response; (ii) DLQI 0/1 score (baseline treatment cohort). Percentages are based on the number of patients in the FAS3, FAS6 and FAS12 with data available (m) at each visit of interest in the respective baseline treatment cohort. FAS3/6/12 full analysis set at month 3/6/12; DLQI Dermatology Life Quality Index; IGA Investigator’s Global Assessment; PGA Physician’s Global Assessment
Health-Related Quality of Life
At months 3, 6 and 12, the proportion of patients with DLQI score of 0 or 1 was higher in the secukinumab group (65.0%, 61.4% and 63.5%) than in the other biologics (41.6%, 39.2% and 41.3%) and the conventional systemics groups (22.6%, 21.3% and 32.0%) (Fig. 6ii). Change in DLQI total score from baseline is presented in the supplementary section.
Treatment Patterns
Exposure
The mean (SD) time of exposure in conventional systemics, secukinumab and other biologics actual treatment cohorts was 236.8 (160.55) days, 300.4 (132.25) days and 291.8 (136.45) days, respectively.
Baseline Treatment
Treatments for psoriasis, including previous biologics used at baseline, are presented per baseline treatment cohort for the EXS in Supplementary Table S3. In the conventional systemics baseline treatment cohort, the most commonly used baseline medication (≥ 5.0%) was methotrexate (67.6%, n = 117). In the secukinumab baseline treatment cohort, 8.7% (n = 16) patients also received conventional systemics at baseline; each conventional systemic medication was received by < 5.0% of patients. In other biologics baseline treatment cohort, the most commonly used baseline medication was ustekinumab (50.0%, n = 92).
Actual Treatment
Actual treatments for psoriasis used during the study are presented per actual treatment cohort for the EXS in Supplementary Table S4. In the conventional systemics actual treatment cohort (N = 182), the most commonly used medication (≥ 5.0%) was methotrexate (74.7%, n = 136). In the secukinumab actual treatment cohort (N = 211), during treatment with secukinumab, 31 patients (14.7%, n = 31) also received conventional systemics, and 5 patients (2.4%) also received other biologics. In the other biologics actual treatment cohort (N = 213), the most commonly used medications was ustekinumab (47.4%, n = 101) followed by conventional systemics (22.1%; n = 47).
Changes in Treatment
An overall summary of treatment changes, including regimen adjustments in the EXS, is provided in Supplementary Fig. S3, and treatment changes since the previous visit in Supplementary Fig. S4. The overall proportion of patients with no change in treatment regimen during the study in the secukinumab, conventional systemics and other biologics baseline treatment cohort was 67.9% (n = 125), 62.5% (n = 115) and 45.1% (n = 78), respectively (Supplementary Fig. S3i). The most common type of change was treatment withdrawal and treatment regimen adjustment (each 15.2%) for the secukinumab baseline treatment cohort, treatment regimen adjustment (26.6%) for the conventional systemics baseline treatment cohort and treatment withdrawal (16.8%) for other biologics baseline treatment cohort (Supplementary Fig. S3ii). The secukinumab baseline treatment cohort had the lowest proportion of patients with treatment changes (15.8%), followed by other biologics baseline treatment cohort (29.5%); the conventional systemics baseline treatment cohort had the highest proportion of patients with a change of treatment (42.1%) (Supplementary Fig. S4). The most common primary reasons for treatment change are presented in Supplementary Table S5.
Safety
The overall safety profile was comparable across actual treatment cohorts (Table 3). Proportion of patients experiencing treatment emergent adverse event was comparable (conventional systemics, 34.6%, secukinumab, 33.6%; other biologics, 30.5%). Among the patients who were not taking medication at certain times during the study, 20 of 125 patients (16.0%) experienced a treatment emergent adverse event (TEAE). The majority of TEAEs that occurred during the study were mild, across all actual treatment cohorts. Severe TEAEs occurred in four patients (2.2%) in the conventional systemics actual treatment cohort, in seven patients (3.3%) in the secukinumab actual treatment cohort, in eight patients (3.8%) in other biologics actual treatment cohort, and in four patients (3.2%) in the ‘None’ actual treatment cohort. There was one death during the study in the conventional systemics actual treatment cohort. The cause of death was diffuse large B-cell lymphoma, which was not considered to be related to baseline treatment. Adverse events of special interest are presented in Table 4. A higher proportion of patients reported infections in secukinumab group (16.6%) than other biologics group (11.7%) and conventional systemics (5.5%). The higher proportion of patients reporting infections is mainly driven by fungal and other skin structure infections. No cases of inflammatory bowel disease were reported.
Table 3.
Overall safety profile (EXS, actual treatment cohort)
| Conventional systemics (N = 182), n (%) | Secukinumab (N = 211), n (%) | Other biologics (N = 213), n (%) | Nonea (N = 125), n (%) | |
|---|---|---|---|---|
| AEs | 63 (34.6) | 71 (33.6) | 65 (30.5) | 20 (16.0) |
| Related to baseline treatment | 47 (25.8) | 19 (9.0) | 21 (9.9) | 11 (8.8) |
| Related to other treatment | 9 (4.9) | 3 (1.4) | 2 (0.9) | 1 (0.8) |
| SAEs | 4 (2.2) | 9 (4.3) | 6 (2.8) | 5 (4.0) |
| Related to baseline treatment | 0 | 4 (1.9) | 2 (0.9) | 3 (2.4) |
| Related to other treatment | 1 (0.5) | 0 | 0 | 0 |
| Fatal SAEs | 1 (0.5) | 0 | 0 | 0 |
| AEs leading to discontinuation | 3 (1.6) | 0 | 1 (0.5) | 0 |
| AEs leading to dose adjustment | 13 (7.1) | 3 (1.4) | 4 (1.9) | 0 |
aPatients who were not taking medication at certain times during the study. These AEs could not be allocated to a cohort owing to technical set-up of the electronic case report form and were manually analysed; most of these AEs occurred between the loading and maintenance dose. Percentages are based on the number of patients from the EXS (N) for each respective actual treatment cohort. Actual treatment cohort may differ from baseline treatment
AE adverse event, EXS exposed set, N total number of patients, n number of patients, SAE serious adverse event
Table 4.
Adverse events of special interest (EXS, actual treatment cohort)
| Grouping | Conventional systemic N = 182 n (%) |
Secukinumab N = 211 n (%) |
Other biologics N = 213 n (%) |
Nonea N = 125 n (%) |
|---|---|---|---|---|
| Number of subjects with at least one event | 22 (12.1) | 47 (22.3) | 40 (18.8) | 9 (7.2) |
| Hypersensitivity | 2 (1.1) | 9 (4.3) | 11 (5.2) | 2 (1.6) |
| Immune/administration reactions | 0 | 1 (0.5) | 4 (1.9) | 0 |
| Infections | 10 (5.5) | 35 (16.6) | 25 (11.7) | 6 (4.8) |
| Infections (infectious pneumonia) | 0 | 1 (0.5) | 1 (0.5) | 1 (0.8) |
| Infections (fungal) | 0 | 7 (3.3) | 4 (1.9) | 2 (1.6) |
| Infections (viral herpes) | 1 (0.5) | 1 (0.5) | 1 (0.5) | 0 |
| Infections (mycobacterial) | 1 (0.5) | 0 | 0 | 0 |
| Infections (skin structure) | 5 (2.7) | 15 (7.1) | 12 (5.6) | 4 (3.2) |
| Infections (staphylococcal) | 0 | 1 (0.5) | 4 (1.9) | 0 |
| Major adverse cardiovascular events (MACE) | 7 (3.8) | 5 (2.4) | 8 (3.8) | 0 |
| Malignant or unspecified tumours | 3 (1.6) | 1 (0.5) | 0 | 1 (0.8) |
| Malignant or unspecified tumours (except NMSC) | 3 (1.6) | 1 (0.5) | 0 | 1 (0.8) |
| Neutropenia | 0 | 1 (0.5) | 0 | 0 |
aPatients who were not taking medication at certain times during the study. Percentages are based on the number of patients from the EXS for each respective actual treatment cohort
AE adverse event, EXS exposed set, MACE major adverse cardiac events, N total number of patients, n number of patients, NMSC non-melanoma skin cancer
Discussion
REALIA study generated real-world evidence in patients from a large geographical region, where little has been published on the real-life treatment patterns and effectiveness of the different treatment modalities used in patients with chronic plaque psoriasis eligible for systemic treatment. Patients in secukinumab baseline treatment cohort had more severe psoriasis than other biologics and conventional systemics cohorts and presented with PsA more often than in the conventional systemics baseline treatment cohort. A higher percentage of patients treated with secukinumab received more than two prior biologic agents than the other treatment groups, which indicates that these patients seemed to suffer from more severe psoriasis; nevertheless, secukinumab treatment demonstrated similar or better effectiveness compared with conventional systemics and other biologic groups.
The effectiveness and safety of conventional systemics, secukinumab and other biologics in a real-world setting in the treatment of chronic plaque psoriasis are in agreement with clinical outcomes reported in randomised clinical trials [14]. The proportion of patients achieving almost clear to clear skin was significantly higher in secukinumab versus conventional systemics baseline treatment cohorts at month 3 and at month 6, and numerically higher at month 12. Similar results were observed for other biologics versus conventional systemics baseline treatment cohorts at months 3, 6 and 12.
There were significant differences between secukinumab and other biologics versus the conventional systemics baseline treatment cohorts in PASI total score at month 3 and month 6, but not at month 12, similar to the physician’s assessment of disease severity. A potential explanation for this observation might be that patients who need to switch due to efficacy or safety concerns are more likely to do so before 12 months of treatment. The secukinumab baseline treatment cohort was numerically better than conventional systemics and other biologics baseline treatment cohorts at attaining PASI 90 and PASI 100 responses at every timepoint in the study. The secukinumab and other biologics baseline treatment cohorts were significantly better than conventional systemics baseline treatment cohort at improving the BSA total score at every timepoint.
Secukinumab was numerically better than both conventional systemics and other biologics at attaining a PGA/IGA score of 0 at month 6 and month 12, but not at month 3. However, as only a small number of patients had available PGA/IGA assessments throughout the study, these results should be interpreted with caution. Secukinumab and other biologics have led to significantly higher improvements of the DLQI total score versus conventional systemics at month 3 and month 6, but not at month 12. However, the DLQI was an optional assessment, and at month 12, fewer patients answered the DLQI questionnaire than at month 3 and month 6.
The findings of secukinumab treatment in this study were comparable to the results of a meta-analysis of 43 studies, which examined real-world evidence of secukinumab in psoriasis treatment [28]. The meta-analysis reported drug survival of 80% at 12 months [28]. The PASI 90 scores in the meta-analysis were 50%, 53% and 60%, and PASI 100 scores were 36%, 46% and 51%, at 3, 6 and 12 months, respectively [28]. Overall, in the meta-analysis, 57%, 55% and 65% of patients achieved DLQI score of 0 or 1 at 3, 6 and 12 months, respectively [28].
Patients in the secukinumab baseline treatment cohort were the least likely to change treatment within the 12-month observation period. The main reason for secukinumab treatment changes was disease control, and the main changes made were treatment withdrawal or regimen adjustment. Due to the relatively small numbers of patients and the magnitude of the differences, these observations should be interpreted with caution.
The safety profile of conventional systemics, secukinumab and other biologics showed no new or unexpected signals. There was one death reported in the conventional systemics baseline treatment cohort (diffuse B-cell lymphoma), which was considered as related to concomitant cancer treatment. The frequency of AEs was similar among the actual treatment cohorts, with only headache and nausea occurring more often with conventional systemics treatment. Patients treated with secukinumab reported a slightly higher rate of infections compared with other biologics which was, nevertheless, similar to that observed in controlled clinical studies [29]. Notably, cases of inflammatory bowel disease were not reported in this study.
Limitations of this study were inherent to its non-interventional design, and hence missing or incomplete data and selection bias are to be expected as with other multicentre prospective observational trials. In addition, the clinical judgement of almost clear to clear skin (yes/no) depended strongly on the subjective assessment of the physician. The lack of standardisation across the participating countries has led to limited interpretation of the association of the clinical judgement with the different disease severity assessments tools. Furthermore, patient-reported outcomes (DLQI) from non-blinded patients should also be carefully interpreted. Finally, secukinumab was analysed separately, whereas other biologics were grouped and analysed together; therefore, the results obtained for the other biologics were the average for the group of biologic treatment, and gave no measure of effectiveness of the individual biologic treatments. In addition, while evaluating findings of the current research, the heterogeneity of the biologic drug group should be considered.
Conclusion
In conclusion, real-world evidence in patients with chronic plaque psoriasis from a large geographical region of Asia-Pacific and Middle East suggests that, despite the higher incidence of PsA, more severe disease and a longer disease duration, secukinumab is at least as effective as conventional systemics and other biologics. These results are in agreement with the clinical outcomes of secukinumab reported in its clinical development program.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Sponsorship for this study and Rapid Service Fee were funded by Novartis Pharma AG, Basel, Switzerland.
Medical Writing, Editorial, and Other Assistance
The authors thank Avinash Thakur and Sumeet Sood (Novartis Healthcare Pvt. Ltd, Hyderabad) for editorial and medical writing support, which was funded by Novartis Pharma AG, Basel, Switzerland in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to manuscript concept and design. Data collection was done by Peter Foley, Tsen-Fang Tsai, Karl Rodins, Issam Ribhi Hamadah, Alfred Ammoury, Hussein Abdel Dayem, Mahmoud Abdallah and Yu-Huei Huang. Susanne Crowe conducted the statistical analyses on the data. All authors interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the manuscript.
Prior Presentation
This study was presented in part at 29th European Academy of Dermatology and Venereology Virtual Congress, 29–31st October, 2020.
Disclosures
Peter Foley has served on the advisory board and/or as a consultant/investigator and/or received research grant/speaker’s honoraria/travel grants from AbbVie, Akaal, Amgen, Aslan, Astra Zeneca, BMS, Boehringer Ingelheim, Botanix, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Eli Lilly, Galderma, Genentech, GenesisCare, GlaxoSmithKline/Stiefel, Hexima, Janssen, Leo Pharma, Mayne Pharma, MedImmune, Melaseq/Geneseq, Merck, Novartis, Pfizer, Regeneron Pharmaceuticals Inc., Reistone, Roche, Sanofi Genzyme, Sun Pharma, Teva, UCB Pharma, Valeant. Tsen-Fang Tsai has served as investigator and/or speaker and/or advisor for Allergan, AbbVie, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, Galderma, GSK, Janssen, Leo Pharmaceuticals, MSD, Novartis, Pfizer, Sanofi Aventis and Tanabe. Karl Rodins: Nothing to disclose. Issam Ribhi Hamadah served as speaker and advisory board for AbbVie, Novartis, Janssen, Celgene, Sanofi, Newbridge and Pfizer. Alfred F. Ammoury served as a speaker and participated in advisory boards for Novartis, AbbVie, Pfizer, Eli Lilly, Janssen, Celgene and Leo Pharmaceuticals. Hussein Abdel Dayem: Speaker and member of the advisory board for Novartis, Eli Lilly, Abbvei, Janssen, Leo, Sanofi. Participation in clinical studies for Novartis, Eli Lilly, Sanofi, and Abbvei. Mahmoud Abdallah served as speaker for Janssen, Novartis, AbbVie and Pfizer, investigator for AbbVie and Novartis and participated in advisory board for Novartis, Eli Lilly, Janssen, AbbVie. Susanne Crowe, Silvia Haas, Effie Pournara and Piotr Jagiello are employed by Novartis. E. Pournara and S. Crowe are shareholders of Novartis. Yu-Huei Huang: conducted clinical trials or received honoraria as a consultant and speaker for Abbvie, Celgene, Janssen-Cilag Pharmaceuticals, Galderma, Novartis, Pfizer Pharmaceuticals and Sanofi Aventis.
Compliance with Ethics Guidelines
The study protocol was approved by the institutional review board/ethics committee of each participating centre. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) and in compliance with all federal, local, or regional requirements.
Data Availability
The datasets generated and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
References
- 1.Johnson-Huang LM, Lowes MA, Krueger JG. Putting together the psoriasis puzzle: an update on developing targeted therapies. Dis Model Mech. 2012;5(4):423–433. doi: 10.1242/dmm.009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 3.Springate DA, Parisi R, Kontopantelis E, et al. Incidence, prevalence and mortality of patients with psoriasis: a UK population-based cohort study. Br J Dermatol. 2017;176(3):650–658. doi: 10.1111/bjd.15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tada Y, Jo SJ, Huang Y-H, et al. Uncovering the unmet needs among psoriasis patients in the Asia-Pacific region. J Dermatol. 2021;48(11):1665–1674. doi: 10.1111/1346-8138.16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parisi R, Iskandar IYK, Kontopantelis E, et al. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo G, Altomare G, Peris K, et al. Moderate and severe plaque psoriasis: cost-of-illness study in Italy. Ther Clin Risk Manag. 2008;4(2):559–568. doi: 10.2147/TCRM.S2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials. J Dermatolog Treat. 2020:1–9. [DOI] [PubMed]
- 8.Korman NJ, Zhao Y, Pike J, et al. Relationship between psoriasis severity, clinical symptoms, quality of life and work productivity among patients in the USA. Clin Exp Dermatol. 2016;41(5):514–521. doi: 10.1111/ced.12841. [DOI] [PubMed] [Google Scholar]
- 9.Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486. doi: 10.1016/j.jaad.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 10.Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 11.Nast A, Spuls PI, van der Kraaij G, et al. European S3-guideline on the systemic treatment of psoriasis vulgaris—update Apremilast and Secukinumab—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2017;31(12):1951–1963. doi: 10.1111/jdv.14454. [DOI] [PubMed] [Google Scholar]
- 12.Nast A, Jacobs A, Rosumeck S, et al. Methods report: European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):e1–22. doi: 10.1111/jdv.13353. [DOI] [PubMed] [Google Scholar]
- 13.Nast A, Gisondi P, Ormerod AD, et al. European S3-guidelines on the systemic treatment of psoriasis vulgaris—update 2015—short version—EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol. 2015;29(12):2277–2294. doi: 10.1111/jdv.13354. [DOI] [PubMed] [Google Scholar]
- 14.Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 16.Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371(4):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 17.McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 18.Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73(3):400–409. doi: 10.1016/j.jaad.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Zeichner JA, Armstrong A. The role of IL-17 in the pathogenesis and treatment of psoriasis. J Clin Aesthet Dermatol. 2016;9(6 Suppl 1):S3–s6. [PMC free article] [PubMed] [Google Scholar]
- 20.Blauvelt A, Prinz JC, Gottlieb AB, et al. Secukinumab administration by pre-filled syringe: efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE) Br J Dermatol. 2015;172(2):484–493. doi: 10.1111/bjd.13348. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb AB, Blauvelt A, Prinz JC, et al. Secukinumab self-administration by prefilled syringe maintains reduction of plaque psoriasis severity over 52 weeks: results of the FEATURE trial. J Drugs Dermatol. 2016;15(10):1226–1234. [PubMed] [Google Scholar]
- 22.Paul C, Lacour JP, Tedremets L, et al. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE) J Eur Acad Dermatol Venereol. 2015;29(6):1082–1090. doi: 10.1111/jdv.12751. [DOI] [PubMed] [Google Scholar]
- 23.Lacour JP, Paul C, Jazayeri S, et al. Secukinumab administration by autoinjector maintains reduction of plaque psoriasis severity over 52 weeks: results of the randomized controlled JUNCTURE trial. J Eur Acad Dermatol Venereol. 2017;31(5):847–856. doi: 10.1111/jdv.14073. [DOI] [PubMed] [Google Scholar]
- 24.Bissonnette R, Luger T, Thaçi D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177(4):1033–1042. doi: 10.1111/bjd.15706. [DOI] [PubMed] [Google Scholar]
- 25.Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE) J Am Acad Dermatol. 2015;73(1):27–36.e1. doi: 10.1016/j.jaad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study) J Eur Acad Dermatol Venereol. 2018;32(9):1507–1514. doi: 10.1111/jdv.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elewski BE, Puig L, Mordin M, et al. Psoriasis patients with psoriasis Area and Severity Index (PASI) 90 response achieve greater health-related quality-of-life improvements than those with PASI 75–89 response: results from two phase 3 studies of secukinumab. J Dermatol Treat. 2017;28(6):492–499. doi: 10.1080/09546634.2017.1294727. [DOI] [PubMed] [Google Scholar]
- 28.Augustin M, Jullien D, Martin A, et al. Real-world evidence of secukinumab in psoriasis treatment—a meta-analysis of 43 studies. J Eur Acad Dermatol Venereol. 2020;34(6):1174–1185. doi: 10.1111/jdv.16180. [DOI] [PubMed] [Google Scholar]
- 29.Deodhar A, Mease PJ, McInnes IB, et al. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. 2019;21(1):111. doi: 10.1186/s13075-019-1882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymised to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.