Abstract
Jabuticaba is a Brazilian berry known for its therapeutic potential against cancer, obesity, insulin resistance (IR), and others. It is a natural source of bioactive compounds, leading to better glucose metabolism, and attenuating obesity and IR through the reduction of pro-inflammatory status. The present study aimed to observe the prebiotic effect of freeze-dried jabuticaba peel (J) consumption on gut bacteria profile and describe its effects on IR derived from the lipopolysaccharides/Toll-like receptor-4 inflammatory pathway. Jabuticaba peel was chemically characterized, and its bioactive compounds were quantified. Twenty-four C57BL/6 mice were feed with a control diet (n = 6), control diet + J (n = 6), high-fat diet (HF) (n = 6), and HF + J (n = 6) for thirteen weeks. Gut bacteriota (16s RNA sequencing), glucose metabolism (fasting glucose and insulin, OGTT, ITT, HOMA-IR, and β, QUICKI), and inflammatory status (serum lipopolysaccharide, and protein expression) were assessed. The main bioactive compounds found in J were dietary fiber, and anthocyanins, and its consumption along with a healthy diet reduced the abundance of Firmicutes and Actinobacteriota phyla (p < 0.01), increased the Muribaculaceae and Lachnospiraceae families, and Faecalicatena genus (p < 0.05). The correlation test indicates a negative correlation between the Muribaculaceae and glucose metabolism. Jabuticaba peel is a nutritive source of bioactive compounds with prebiotic effects.
Keywords: Berries, Dysbiosis, Prebiotics, Obesity, Insulin resistance, Bioactive compounds
Graphical abstract
Highlights
-
•
Jabuticaba peel is a natural source of bioactive compounds with high anti-oxidant power.
-
•
Jabuticaba peel in a healthy diet intake can modulate the gut bacteriota and increase short-chain fatty acids production.
-
•
The consumption of freeze-dried jabuticaba peel does not alter the glucose metabolism via LPS-TLR4 pathway.
1. Introduction
Excessive intake of saturated and trans fatty acids and simple sugars promotes dysbiosis in addition to obesity (Cani, 2019; Wilkins et al., 2019). Dysbiosis (related to obesity) is characterized by the predominance of intestinal bacteria from the phylum Firmicutes and reduction of Bacteroidetes, generating metabolites such as lipopolysaccharide (LPS) and trimethylamine-N-oxide (Wang et al., 2019; Wilkins et al., 2019). Thanks to the literary and technological expansion of -omic techniques, microbial signatures and profile (microbiome) and their metabolites (metabolomics) have been linked to the health and/or disease status of each host (Davies, 2001; Peterson et al., 2009; Proctor et al., 2019).
The LPS is a molecule found in the cell wall of gram-negative bacteria, such a molecule is responsible for activating the pro-inflammatory response via Toll-like receptor 4 (TLR4), producing inflammatory cytokines and cellular oxidative stress, causing phosphorylation of insulin receptors resulting in insulin resistance (IR) impairing glucose metabolism (Bagarolli et al., 2017; Miura et al., 2017; Wang et al., 2019). Thus, the modulation of the profile of gut bacteriota as well as its metabolites through the ingestion of berries became a therapeutic target aiming at the resolution of comorbidities related to insulin resistance and LPS-inflammation (Gasparrini et al., 2021; Lail et al., 2021).
Jabuticaba (Plinia cauliflora) is a typical Brazilian berry (Schreckinger et al., 2010) whose pulp is widely consumed in natura and has high technological applicability in the food industry, but its dark violet skin is usually discarded (Leite-Legatti et al., 2012). Due to its chemical composition, the jabuticaba peel has therapeutic potential with high levels of dietary fibers, and polyphenols, mainly anthocyanins, helping to prevent and treat diseases such as insulin resistance, obesity, cancer, Alzheimer and others (Batista et al., 2017, 2018; Dragano et al., 2013; Lamas et al., 2020).
Therefore, we seek to identify, for the first time, through the next sequencing generation the profile of gut bacteriota of animals fed an obesity/dysbiosis-inducing diet, supplemented with jabuticaba peel freeze-dried, as its chemical composition may be able to modulate the gut microbiota, avoiding insulin resistance via inflammatory signaling induced by LPS/TLR4.
2. Material and methods
2.1. Sample characterization
The jabuticaba fruits (Plinia cauliflora (Mart.) Kausel) were donated by a producer from the city of Casa Blanca, São Paulo, Brazil. The fruits were washed, sanitized, manually pulped, the peels were frozen (−18 °C), and then freeze-dried (Liobras®). After the drying, the sample was pulverized, packed, and stocked protected from light.
The proximate composition of freeze-dried jabuticaba peel (J) was determined by the content of humidity (constant drying at 100 °C), total protein (Kjeldahl 6.25 conversion factor), ashes (muffle incineration at 550 °C) (AOAC, 1995), total lipids (Bligh and Dyer, 1959), and carbohydrate was calculated by the following formula (%carbohydrate = 100 - %humidity - %total protein - %ash - %total lipids). The energy content was estimated by the sum of carbohydrates (excluding fiber content) and protein multiplied by 4 calories/g, and lipids by 9 calories/g.
The extract was obtained using 1g of J in 60 mL of an acidified 70% hydro-methanolic solution (0.1 HCl/14.9/85; v/v/v) under magnetic agitation for 1 h, at room temperature (20 °C), and then filtered. Total polyphenol content, monomeric anthocyanin, ferric reduction antioxidant power (FRAP), and hydrophilic oxygen radical absorbance capacity (ORAC) were determined by UV–Vis and fluorescence emission spectroscopy assay, and phenolics compounds were determined by high-performance liquid chromatography (HPLC) equipped with a photodiode array detector (DAD) and fluorescence detectors (FLD) (according to protocols validated by da Silva et al. (da Silva et al., 2016)).
2.2. Preclinical study
This experiment was based on the ethics requirements of the National Council for Animal Experimentation Control (CONCEA) and was approved by the Ethics Committee on Animal Use (CEUA/UNICAMP protocol 4895-1/2018). The access to Brazilian genetic heritage was registered at the Ministry of the Environment via SISGEN (protocol A2C81F8).
Twenty-four male black mice (C57BL/6), 5 weeks old, provided by the Multidisciplinary Center of Biological Investigation (CEMIB/UNICAMP), were allocated collectively in polyethylene boxes, under environmental controlled conditions of temperature (22±2 °C), humidity (60–70%), a light-dark cycle of 12 h, with free access to water and food during the whole experimental period.
The AIN-93G diet (Reeves et al., 1993) was offered during the acclimatization, followed by the AIN-93M diet (normolipidic and hyperlipidic, 35% of fat) offered during thirteen weeks of experimentation, besides both diets supplemented with 4% of J (this dosage had a better impact over glucose metabolism according to Dragano et al., 2013), resulting in four experimental groups of 6 animals. The diet's formulations are described in Table 1. After being manufactured the diets were kept in a conventional refrigerator (10 °C), hidden from light, to avoid phenolic degradation.
Table 1.
Nutritional composition of experimental diets.
| Nutrient (g/kg) | C | CJ | HF | HFJ |
|---|---|---|---|---|
| Casein | 137.24 | 137.24 | 137.24 | 137.24 |
| Cornstarch | 467.47 | 467.47 | 267.15 | 267.15 |
| Dextrinized cornstarch | 155.59 | 155.59 | 88.92 | 88.92 |
| Sucrose | 100.38 | 100.38 | 57.36 | 57.36 |
| Soybean Oil | 40 | 40 | 40 | 40 |
| Lard | – | – | 310 | 310 |
| Cellulose | 50 | 50 | 50 | 50 |
| Mineral mix | 35 | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 | 10 |
| L-cystine | 2 | 2 | 2 | 2 |
| Choline Bitartrate | 3 | 3 | 3 | 3 |
| tBHQ | 0.008 | 0.008 | 0.008 | 0.008 |
| J | – | 40 | – | 40 |
| Total weight | 1000.00 | 1040.00 | 1000.00 | 1040.00 |
| Total polyphenols | – | 3.28 | – | 3.28 |
| Total anthocyanin | – | 0.30 | – | 0.30 |
| Total dietary fiber | 50 | 63.5 | 50 | 63.5 |
| Total energycal | 3802 | 3888 | 5032 | 5118 |
C: Control group (AIN93-M diet). J: Freeze-dried jabuticaba peel. HF: High-Fat group (AIN93-M with adjusted 35% from lard). tBHQ: Tert-butylhydroquinone. cal: Calories.
The dietary consumption and weight gain of animals were monitored every two days. Feed efficiency and energy intake were calculated (Novelli et al., 2007) based on the total energy of each experimental diet. Blood was collected by cardiac puncture under anesthesia (ketamine and xylazine); the liver and the sum of adipose tissue (mesenteric, retroperitoneal, and epididymal) were weighted, and liver fragments were immediately frozen in liquid nitrogen.
After 6 h of fasting, the glucose was measured in the peripheral tail vein, using a glucose meter (Abbott®) to register the time zero. After the gavage of 2g of glucose/kg of body weight, the glycemia was registered at 15, 30, 60, 90, and 120 min to determine the oral glucose tolerance test (OGTT). To perform the Insulin Tolerance Test (ITT), fasting glycemia was measured to register the time zero, also after 6 h fasting, then a peritoneal injection of human insulin (0.75UI/kg) was administrated in the animals, measuring glycemia at 5, 10, 15, 20, and 25 min. Results were used to plot the area under the curve (AUC) and the graph of glucose decay through the times.
Biochemical analyses (glycemia, insulin, and LPS levels) were measured in the serum using commercial kits according to the manufactures (LaborLab®, Millipore©, and Thermo scientific™). The Homeostatic Model Assessment (HOMA) of IR, HOMA-β (Matthews et al., 1985), and Quantitative Insulin Sensitivity Check Index (QUICK) (Katz et al., 2000) were calculated.
For western blotting assay, protein from frozen liver fragments was extracted with RIPA buffer using the Polytron (Brinkmann®). Then, the insoluble content was removed by centrifugation at 4 °C for 20 min, and the protein concentration in homogenates was determined using Bradford reagent. Thus, samples containing 75 μg of protein were boiled in Laemmli buffer for 5 min (1:1). The electrophoresis separation was carried into SDS-PAGE of 8%, and 15% (for high and low protein molecular weight respectively), following that, proteins were transferred to a nitrocellulose membrane. The membranes were blocked within none to 3% bovine serum albumin, incubated with specific antibodies overnight 4 °C mouse anti-TLR4 (sc-53462, Santa Cruz Biotechnology); rabbit anti-MyD88 (bs-1047R, Bioss); mouse anti-nuclear factor-kappa B (NFκB) (ab13594, Abcam); mouse anti-tumor necrosis factor-alpha (TNF-α) (ab48394, Abcam); goat anti-phospho-insulin receptor substrate (IRS) tyrosine (sc-17196, Santa Cruz Biotechnology); and mouse anti-β-actin (ab8227, Abcam), then incubated with specific secondary antibodies for 2 h, at room temperature. To detect reactive band was used chemiluminescent substrate, and the densitometry of antigen bands was quantified by UN-Scan Software. β-actin was used as an endogenous control.
2.3. Metataxonomic analysis
After euthanasia, the colon of animals was removed using sanitized scissors, and all fecal content was placed in sterile RNA/DNA-free tubes, this content was extracted from the gut bacterial genetic material (using QIAmp DNA stool kit).
The DNA library followed the manufacturer's instructions (Illumina). The V4 region of the 16S rRNA gene was amplified, including Illumina sequencing adapters (forward: 5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-specific-locus sequencing; reverse: 5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-specific-locus sequencing). Libraries were quantified and pooled in equimolar amounts. For sequencing, the Illumina MiSeq platform was used, with reads of 2 × 250bp.
The DADA2 program (Callahan et al., 2016) was used for amplicon modeling and error correction without the construction of operational taxonomic units (OTU), with a complete pipeline, implemented to transform sequencer fastq files into inferred, dismembered, and chimera-free sample sequences. The assigned taxonomy function takes as input a set of amplicom sequencing variants (ASV) to be classified, and a reference sequence training set with known taxonomy and assigns taxonomies. The GTDB: Genome Taxonomy Database was used as a reference (Parks et al., 2017). The taxonomic classifications generated by DADA2, and their quantifications, were imported into the phyloseq program.
Alpha (Shannon diversity) and beta (Detrended Correspondence Analysis) diversity analyses were performed on the phyloseq package as described (Callahan et al., 2016). ASVs that were not classified at least to the family level were filtered, and ASVs marked as the same species were clustered. After applying these filters, the tables of gross abundance and relative abundance counts were obtained. The taxonomic counts contained in the phyloseq were imported into the edgeR package for normalization of the library sizes of each sample; subsequently, the counts were transformed to the base 2 logarithms of the counts per million (logCPM) of each sample (voom transformation). After adjusting the linear limma model, differential taxonomic abundance was tested for each group with moderate t-tests (Smyth, 2004). For more details of the bioinformatic analysis, check supplementary material.
Gas chromatography with a flame ionization detector (GC-FID) was used to determine short-chain fatty acids in the feces collected from the animal's boxes before the euthanasia begin (G. Zhao et al., 2006). Chromatography conditions were: injector and detector temperatures set at 200 °C, injected volume was 1 μL (autosampler, Shimadzu®), split mode 1:5 for 1 min; carrier helium gas at 1.0 mL min−1. The column oven was programmed at 100 °C, then heated at 8 °C/min until reaching 190 °C, held for 3.25 min; validation parameters are presented in the supplementary material (Table S1). The total cholesterol was determined by a commercial kit (LaborLab®) in lipids extracted from the feces collected (FOLCH et al., 1957).
2.4. Statistics
The results of the chemical analysis are expressed as mean ± standard deviation and those obtained from in vivo experimental analysis as mean ± standard error of the mean. The data were submitted to analysis of variance (ANOVA) with post-hoc on Tukey's test with a p-value <0.05 within the experimental groups. For metataxonomic analysis, a moderated t-test (p-value <0.05) was used to compare the profile of gut bacteria of every single diet distinctly. The Pearson correlation test was carried out comparing the better and the worst phenotypes for glucose metabolism (CJ and HF respectively), considering p-value <0.05 as a significant difference.
3. Results
3.1. Freeze-dried jabuticaba peel characterization
J major nutrient is carbohydrate, although dietary fiber represents 43% of its total (Batista et al., 2017). As following comes protein and lipid, resulting in 2.1 calories per gram of J. The sample showed high content of polyphenols (Rufino et al., 2010), anthocyanins (total content of 769 mg/100g), and antioxidant capacity (Table 2). Furthermore, five different types of anthocyanins and gallic acid were tentatively identified and quantified in J with external standards (Table 3).
Table 2.
Chemical composition and bioactive compounds in freeze-dried jabuticaba peel.
| Nutrient (g/100g) | Mean ± SD |
|---|---|
| Humidity | 12.01 ± 0.3 |
| Ash | 2.06 ± 0.07 |
| Total protein | 5.94 ± 0.4 |
| Lipids | 1.42 ± 0.01 |
| Carbohydrate | 44.88 |
| Total fiber# | 33.77 ± 1.20 |
| Insoluble fiber | 25.34 ± 0.33 |
| Soluble fiber | 8.49 ± 0.48 |
| Energycal | 216.06 |
| Antioxidant capacity (mg/100g) |
Mean ± SD |
| Total phenolic content (GAE) | 8219.94 ± 99.56 |
| Monomeric anthocyanin* | 663.27 ± 11.91 |
| ORAC (TE) | 44.96 ± 1.75 |
| FRAP (TE) | 92.04 ± 2.08 |
SD: Standard deviation. #: values obtained from Batista et al. (2017). cal: Calories. GAE: Gallic acid equivalent. *: Kuromanin. ORAC: Oxygen radical antioxidant capacity. FRAP: Ferric reducing antioxidant power. TE: Trolox equivalent.
Table 3.
Phenolic compounds extracted from freeze-dried jabuticaba peel.
| nma | Retention time | Compound | mg/L | mg/100 g J Mean ± SD |
|---|---|---|---|---|
| 280 | 9.42 | Gallic acid | 0.99 | 11.39 ± 1.58 |
| 520 | 27.59 | Cyanidin-3-O-glucoside | 58.76 | 675.74 ± 51.57 |
| 520 | 24.62 | Delphinidin- 3-O-glucoside | 5.54 | 63.67 ± 2.67 |
| 520 | 37.44 | Malvidin-3-O-glucoside | 1.50 | 17.27 ± 1.57 |
| 520 | 30.99 | Pelargonidin-3-O-glucoside | 0.90 | 10.3 ± 0.17 |
| 520 | 34.68 | Peonidin | 0.24 | 2.79 ± 0.84 |
Wavelength (DAD) emission/excitation (FLD). Retention time in minutes.
3.2. Preclinical study
The supplementation of J softens the weight gain for normocaloric and high-fat diet groups. The lean groups showed a higher dietary intake compared to obese groups, also reflecting on higher energy intake. However, despite these observations, the obese groups showed a greater feed efficiency when compared to lean groups, resulting in the highest values of weight gain and adipose tissue (Table 4).
Table 4.
Body composition and dietary consumption of animals.
| Parameter | C | CJ | HF | HFJ |
|---|---|---|---|---|
| Weight gaing | 11.38 ± 0.93a | 10.13 ± 0.46b | 16.08 ± 1.63a | 12.75 ± 0.99ab |
| Adipose tissueg | 12.86 ± 1.63b | 12.36 ± 1.51b | 19.75 ± 2.06a | 20.91 ± 1.77a |
| Feed intakeg | 3.65 ± 0.05a | 3.74 ± 0.04a | 2.77 ± 0.03b | 2.67 ± 0.03b |
| Energy intakecal | 13.87 ± 0.18b | 14.51 ± 0.16a | 13.92 ± 0.12ab | 13.62 ± 0.14b |
| Feed efficiency% | 83.65 ± 1.16c | 70.05 ± 0.82d | 117.24 ± 1.04a | 95.04 ± 1.06b |
C: Control. CJ: Control + J. HF: High-fat diet. HFJ: High-fat diet + J. g: Grams. cal: Calories. %: Percentage. n = 6 animals/group. The values are expressed as mean ± standard error of the mean. Different letters in the same row present statistical differences (of at least p < 0.05).
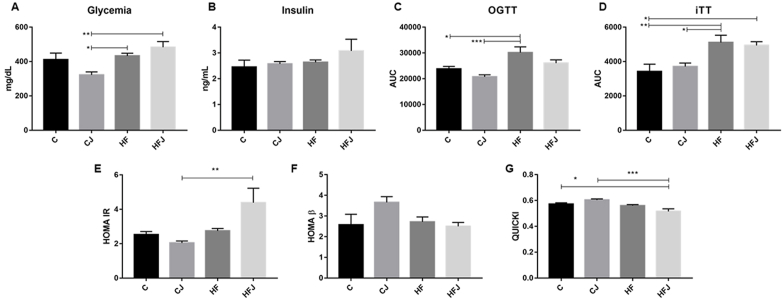
The high-fat diets successfully induced animals to IR, animals fed with the C and CJ diet presented improved glucose metabolism (fasting glycemia, OGTT, ITT, HOMA-IR, and QUICKI), even though J consumption was not enough to prevent IR in obese animals (HFJ - Fig. 1).
Fig. 1.
Serum and clinical parameters of glucose metabolism from experimental animals fed with different diets (n = 6/group). (A) Fasting glycemia, and (B) Fasting insulin serum levels, (C) Area under the curve of oral glucose tolerance test, (D) Area under the curve of insulin tolerance test, Homeostatic model assessment of (E) insulin resistance, and (F) beta cells, and (G) Quantitative insulin sensitivity check index. Statistical differences (*p < 0.05; **p < 0.01; ***p < 0.001).
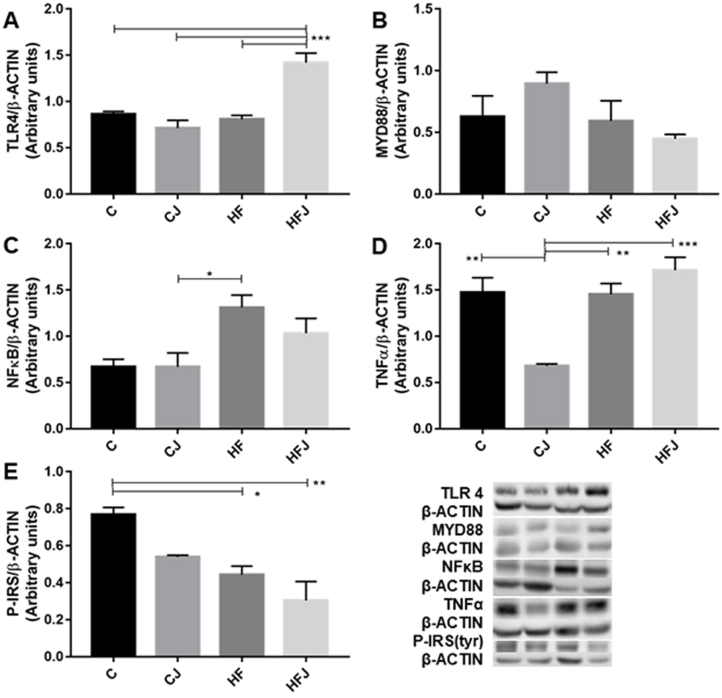
The western blotting analysis showed lower inflammatory markers expression in the CJ group compared to the HF groups and confirmed the improvement in glucose metabolism by presenting better levels of the active form of insulin receptor (phosphorylated-tyrosine). The LPS inflammatory pathway does not influence these markers since it did not differ statistically among the groups (Supplementary material - Table S2).
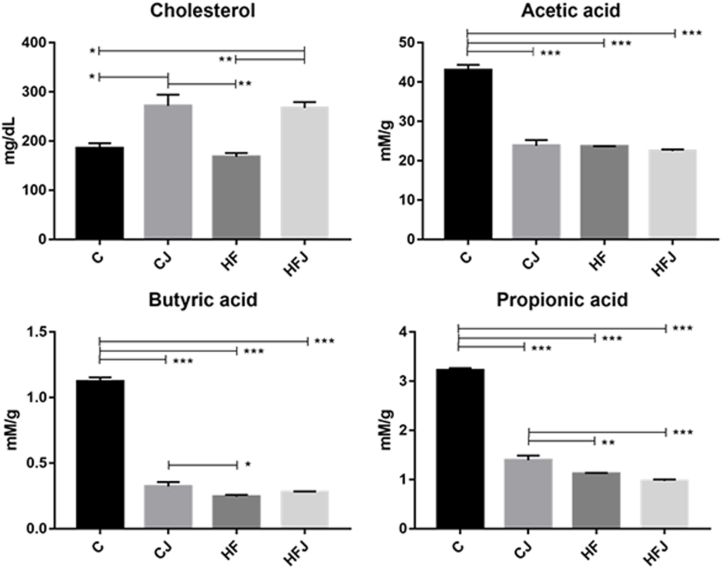
The consumption of J increased the cholesterol excretion in feces; also, the lean groups presented significantly higher levels of short-chain fatty acids (SCFA) in fecal content when compared to both obese groups (Fig. 3).
Fig. 3.
Total cholesterol and short-chain fatty acids from animal feces. n = 6 animals/groups. Statistical differences (*p < 0.05; **p < 0.01; ***p < 0.001).
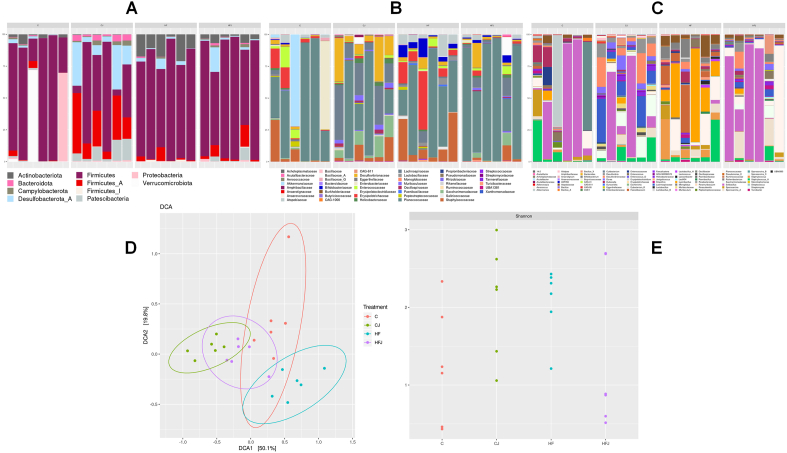
As observed in DCA2 (Fig. 4C), different types of diets (C or HF) can cluster the bacteria profile community towards opposed directions (19.8% of variance). In contrast, J intake is capable of clustering bacteria communities towards a distinct direction from both C and HF diets, across DCA1 varying 50%. There was no difference indicated by alpha diversity between the groups (Fig. 4B). When inserted in the control diet J supplementation reduced the relative abundance of Actinobacteriota, Firmicutes, and Firmicutes_I, while in the high-fat diet it reduced the Patescibacteria phylum abundance (Fig. 4A and Table 5).
Fig. 4.
Relative abundance of the gut bacterial (A) phylum, (B) family, and (C) genera. Each bar represents each animal's (n = 6) relative abundance in the four different experimental groups (C, CJ, HF, and HFJ). (D) Beta and (E) Alpha diversity (Detrended correspondence analysis and Shannon index, respectively) of gut bacteriota of experimental groups. No statistical difference for ecological tests.
Table 5.
Comparison of differential relative abundance of OTUs among the controls and supplemented groups.
| Phylum | logFC | p-value* | C | CJ | ||
|---|---|---|---|---|---|---|
| Actinobacteriota | 6415 | 4,6E-06 | + | – | ||
| Firmicutes | 4476 | 3,1E-03 | + | – | ||
| Firmicutes_I | 7329 | 3,4E-03 | + | – | ||
|
Phylum |
logFC |
p-value* |
HF |
HFJ |
||
| Patescibacteria | −6557 | 3,3E-05 | – | + | ||
|
Genera |
logFC |
p-value* |
C |
CJ |
||
| GCA-900066575 | −5,44 | 4,44E-05 | – | + | ||
| Leaf454 | −5,78 | 4,44E-05 | – | + | ||
| Anaerotignum | −3,61 | 8,91E-05 | – | + | ||
| Dubosiella | 5,42 | 8,91E-05 | + | – | ||
| Lachnospiraceae# | −5,42 | 8,91E-05 | – | + | ||
| Paramuribaculum | −5,01 | 8,91E-05 | – | + | ||
| CAG-95 | −5,00 | 9,13E-05 | – | + | ||
| Lactobacillus | 4,96 | 1,47E-04 | + | – | ||
| Atopobiaceae# | 7,38 | 1,71E-04 | + | – | ||
| Bifidobacterium | 5,26 | 2,19E-04 | + | – | ||
| Faecalicatena | −5,63 | 3,54E-04 | – | + | ||
| Acutalibacter | −4,09 | 6,97E-04 | – | + | ||
| Aerococcus | −4,70 | 1,88E-03 | – | + | ||
| Muribaculaceae# | −4,86 | 2,29E-03 | – | + | ||
| Dorea | −3,15 | 2,65E-03 | – | + | ||
| Staphylococcus | 6,20 | 2,78E-03 | + | – | ||
| Streptococcus | −3,18 | 3,82E-03 | – | + | ||
| Ruthenibacterium | −3,29 | 4,67E-03 | – | + | ||
| CAG-611# | 3,69 | 6,97E-03 | + | – | ||
| Pseudomonas_E | 3,15 | 1,08E-02 | + | – | ||
| Acutalibacteraceae# | −2,98 | 1,57E-02 | – | + | ||
| Oscillospiraceae# | −3,46 | 1,73E-02 | – | + | ||
| Erysipelatoclostridium | 2,83 | 2,25E-02 | + | – | ||
| Sporosarcina | 6,60 | 2,29E-02 | + | – | ||
| Adlercreutzia | 2,90 | 2,41E-02 | + | – | ||
| Paenibacillus | 4,14 | 2,76E-02 | + | – | ||
| CAG-41 | −2,64 | 4,19E-02 | – | + | ||
| Enterobacteriaceae# | 5,09 | 4,36E-02 | + | – | ||
|
Genera |
logFC |
p-value* |
HF |
HFJ |
||
| Anaerotignum | −3,70 | 1,53E-04 | – | + | ||
| Eggerthellaceae# | −4,59 | 1,05E-03 | – | + | ||
| Lysinibacillus | 10,91 | 1,05E-03 | + | – | ||
| Lactobacillus_B | 5,01 | 1,45E-03 | + | – | ||
| Pseudomonas_E | 3,94 | 3,91E-03 | + | – | ||
| Lactobacillus | 4,63 | 5,82E-03 | + | – | ||
| Leaf454 | −3,17 | 5,86E-03 | – | + | ||
| Oscillibacter | −3,15 | 6,51E-03 | – | + | ||
| Streptococcus | 3,23 | 6,88E-03 | + | – | ||
| Saccharimonadaceae# | −5,20 | 8,09E-03 | – | + | ||
| Duncaniella | −2,95 | 8,09E-03 | – | + | ||
| Staphylococcus | 6,12 | 8,09E-03 | + | – | ||
| CAG-611# | 3,31 | 8,43E-03 | + | – | ||
| Erysipelatoclostridium | 3,59 | 8,82E-03 | + | – | ||
| Faecalibaculum | 4,37 | 9,32E-03 | + | – | ||
| Peptostreptococcaceae# | 4,83 | 1,26E-02 | + | – | ||
| GCA-900066575 | −2,39 | 1,37E-02 | – | + | ||
| Faecalicatena | −3,23 | 3,39E-02 | – | + | ||
| Kineothrix | −3,68 | 3,39E-02 | – | + | ||
| Lactobacillus_H | 3,40 | 3,95E-02 | + | – | ||
| Staphylococcus_A | 3,74 | 4,15E-02 | + | – | ||
C: Control. CJ: Control + J. HF: High-fat diet. HFJ: High-fat diet + J. LogFC: Obtained by log2 (C/CJ or HF/HFJ). n = 6 animals/group. A positive sign indicates major relative abundance, whereas a negative sign indicates minor relative abundance. *: Adjusted p-value (<0.05). #: Family identification.
The most abundant family observed in all groups was Planococcaceae. The Muribaculaceae is observed only in groups supplemented with J, and Lachnospiraceae had greater relative abundance in supplemented groups than in controls. CJ shows a greater abundance of Desulfovibrionaceae and Saccharimonadaceae, while groups HF and HFJ Atopobiaceae and Bifidobacteriaceae (Fig. 4B).
The most observed genera are Solibacillus, and Staphylococcus_A among all groups; Faecalibaculum has greater abundance in the HF and HFJ groups, while the J supplemented groups have a greater abundance of Faecalicatena (Fig. 4C). The statistically different genera between the groups are shown in Table 5.
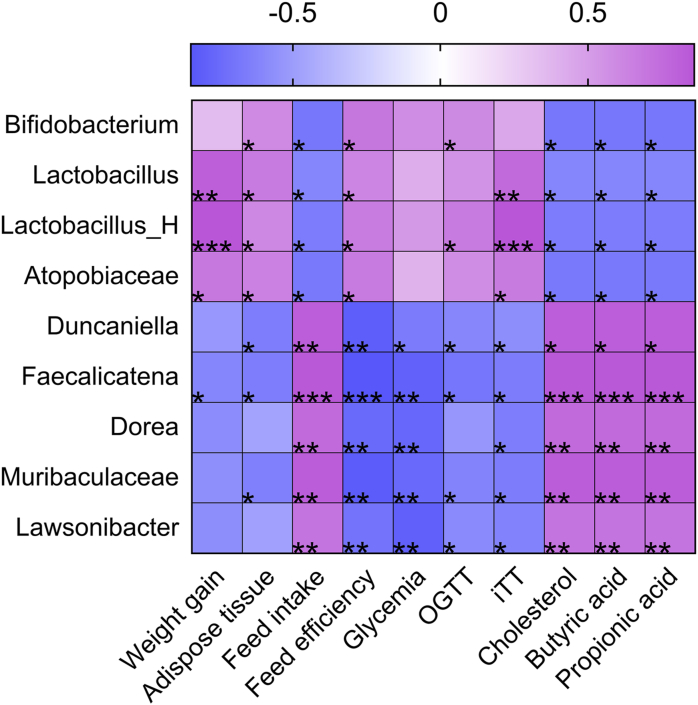
Comparing the better and the worst parameters for glucose metabolism related to the gut microbiota the HF animals presented a higher abundance of Actinobacteriota, Fimicutes_I, and Firmicutes, whereas CJ had a higher abundance of Bacteroidota and Patescibacteria phylum. Thirty-one genera of bacteria statistically differ between CJ and HF groups (Table 6). The Pearson test indicated twenty-two bacteria genera correlated with, at least, one parameter evaluated (Supplementary Material – Fig. S1). Nine bacteria genera are represented in the heatmap (Fig. 5); these bacteria represent at least seven statistical, positive or negative, correlations with the observed parameters, besides being cited for other authors who also worked with gut bacteriota, preclinical experiments, or glucose metabolism.
Table 6.
Comparison of differential relative abundance of OTUs among the better and the worst phenotypes for glucose metabolism.
| Phylum | logFC | p-value* | CJ | HF |
|---|---|---|---|---|
| Actinobacteriota | −8,35 | 1,10E-07 | – | + |
| Patescibacteria | 7,27 | 3,67E-06 | + | |
| Bacteroidota | 2,57 | 8,17E-04 | + | |
| Firmicutes_I | −5,34 | 6,73E-03 | – | + |
| Firmicutes | −3,54 | 1,11E-02 | – | + |
|
Genera |
logFC |
p-value* |
CJ |
HF |
| Bifidobacterium | −11,51 | 3,09E-08 | – | + |
| Lactobacillus | −9,03 | 3,20E-07 | – | + |
| Lactobacillus_H | −8,66 | 1,44E-06 | – | + |
| Leaf454 | 6,15 | 8,18E-06 | + | – |
| Atopobiaceae# | −8,93 | 2,20E-05 | – | + |
| GCA-900066575 | 5,12 | 2,36E-05 | + | – |
| Paramuribaculum | 5,37 | 2,49E-05 | + | – |
| Anaerotignum | 3,80 | 2,49E-05 | + | – |
| CAG-95 | 5,36 | 2,52E-05 | + | – |
| Staphylococcus | −9,71 | 2,62E-05 | – | + |
| Saccharimonadaceae# | 8,45 | 3,63E-05 | + | – |
| Duncaniella | 5,17 | 4,17E-05 | + | – |
| Acutalibacter | 4,69 | 1,07E-04 | + | – |
| Ruthenibacterium | 4,23 | 3,55E-04 | + | – |
| Erysipelatoclostridium | −4,73 | 4,75E-04 | – | + |
| Faecalibaculum | −5,73 | 5,74E-04 | + | – |
| Faecalicatena | 5,15 | 1,13E-03 | + | – |
| Dubosiella | −3,97 | 1,21E-03 | – | + |
| Dorea | 3,11 | 2,52E-03 | + | – |
| Muribaculaceae# | 4,65 | 2,56E-03 | + | – |
| Pseudomonas_E | −3,61 | 4,02E-03 | – | + |
| Aerococcus | 4,18 | 5,15E-03 | + | – |
| Oscillospiraceae# | 4,05 | 5,21E-03 | + | – |
| CAG-41 | 3,55 | 5,48E-03 | + | – |
| Lysinibacillus | −8,16 | 5,66E-03 | – | + |
| Paenibacillus | −4,74 | 1,10E-02 | – | + |
| Lawsonibacter | 3,33 | 1,36E-02 | + | – |
| Acutalibacteraceae# | 2,91 | 1,70E-02 | + | – |
| Lachnospiraceae# | 2,73 | 2,42E-02 | + | – |
| Helicobacteraceae# | 2,02 | 4,60E-02 | + | – |
| Sporosarcina_C | −4,06 | 4,82E-02 | – | + |
CJ: Control + J. HF: High-fat diet. LogFC: Obtained by log2 (CJ/HF). n = 6 animals/group. A positive sign indicates major relative abundance, whereas a negative sign indicates minor relative abundance. *: Adjusted p-value (<0.05). #: Family identification.
Fig. 5.
Correlations between gut bacteria genera and weight gain, adipose tissue, feed intake, feed efficiency, glycemia, OGTT, ITT, cholesterol, butyric, and propionic acids. The correlations are indicated by color (positive: purple; negative: blue). Statistical differences (*p < 0.05; **p < 0.01; ***p < 0.001). Atopobiaceae and Muribaculaceae are represented as bacteria family because both genera are not identified yet. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Several studies have contributed to a better understanding of the link among bacteria, intestinal health, and their effects on host health status. This unprecedented study evaluated the prebiotic effect of freeze-dried jabuticaba peel in dysbiosis/obesity-induced preclinical model, and through classical LPS endotoxin-activated inflammation observed the anti-diabetic and inflammatory effects of J related to the profile of gut bacteria.
4.1. Freeze-dried jabuticaba peel characterization
To date, to most regulatory authorities, the criteria to be met for classifying a prebiotic food and/or compound still are: (i) being resistant to biochemical transformations and not being absorbed throughout the gastrointestinal tract, (ii) undergoing biotransformation (fermentation) by gut bacteria, and (iii) may induce growth and/or activity of certain gut bacteria, resulting in improved health of the host (Gibson et al., 2010).
The anti-obesogenic, -diabetogenic, and -inflammatory effects attributed to J come from the phenolics, more specifically anthocyanins (the major phenolic present in its composition), which are absorbed (around 20%) by gastric cells; due to pH, digestive enzymes, bile acids, and even food matrix (control or high-fat diets), these compounds pass through a chemical transformation that can be absorbed in the small intestine; however, a significant portion of them reaches the colon, where they serve as a substrate to gut microbiota (Fernandes et al., 2014; Quatrin et al., 2020; Tarko and Duda-Chodak, 2020).
The biotransformation of J's anthocyanins by the bacteriota results in many phenolic metabolites that can be absorbed or utilized by gut microbiota, increasing short-chain fatty acids production, and inhibiting the growth of pathological bacteria (Quatrin et al., 2020); thus improving the bacteriota profile and health parameters of CJ group that ingested about 150 mg of anthocyanins/day along with a healthy diet (Table 3, Table 4). It is important to emphasize that some phenolic compounds (extracted from the jabuticaba peel) have antibacterial activity, inhibiting the growth of gram-negative bacteria (Albuquerque et al., 2019).
Some dietary fibers are classified as prebiotics by the criteria mentioned before, which along with (poly)phenols and anthocyanins, favored the reduction of Actinobacteriota, and Firmicutes in the control diet; also, when supplemented in a high-fat diet it could favor the growth of Patescibacteria phylum (Table 5 and Fig. 4). Supporting our results, the consumption of fresh berries, their extracts, or isolated anthocyanins has the capacity of modulating gut bacteria, reflecting beneficially in several health parameters related to glucose metabolism (De Filippo et al., 2010; Esposito et al., 2015; Foito et al., 2018; Lee et al., 2018).
The results observed in the present study are in agreement with the new definition of prebiotics, ‘a substrate that is selectively utilized by host microorganisms conferring a health benefit’, including (poly)phenols, some amino- and bile acids, vitamins, and other molecules in the list of food and components with gut microbiota modulation power (Gibson et al., 2017).
4.2. Preclinical study
Studies on several animal species, mainly mammals, confirm that feed efficiency can be influenced by the greater abundance of the phylum Firmicutes and by the genus Lactobacillus (Jami et al., 2014; Turnbaugh et al., 2006; Yan et al., 2017). The bacteria genera Lactobacillus, Lactobacillus_B, and Lactobacillus_H found greater in the HF group are positively correlated to feed efficiency, suggesting that despite lower feed intake than C, and CJ, the HF group obtained a higher conversion of feed intake into body mass, which reflects on the higher fat accumulation also evidenced by the reduction of phylum Firmicutes_I and Firmicutes in lean groups.
The IR occurs by the recognition of TLR4 to LPS, stimulating a serial cell signalization through MYD-88, IRAK, and TRAF-6 (Roquetto et al., 2015). As a consequence, NFκB and IKB activate nucleus transcription factors that contribute to the reduction of glucose uptake by expressing pro-inflammatory cytokines, like TNF-α and interleukin-6, causing impairment in tyrosine phosphorylation of insulin receptor (IRS) (Bagarolli et al., 2017; Rogero and Calder, 2018).
Due to the greater abundance of phylum Firmicutes, serum LPS levels increase, signaling TLR4 activating the inflammatory cascade (Rogero and Calder, 2018; Roquetto et al., 2015). This scenario observed in obesity-related dysbiosis was not found in our experiment. Although phylum Firmicutes is less abundant in C and CJ groups (Table 5), the serum LPS did not differ among different types of diet or the supplementation of J (Table S2), and molecular analyzes did not show a change in protein expression activating the LPS/TLR-4/MYD-88 pathway (Fig. 2).
Fig. 2.
Western blotting analysis of (A) TLR4, (B) MYD88, (C) NFκB, (D) TNF-α, and (E) P-IRS (tyrosine) levels from the liver of 3 animals per experimental group. Statistical differences (*p < 0.05; **p < 0.01; ***p < 0.001).
The correlation test of our data indicates that genera found in HF (Bifidobacterium, Lactobacillus, Lactobacillus_H, and Atopobiaceae) have a statistically positive relation with impaired peripheral glucose metabolism (Fig. 5). On the other hand, CJ had a greater abundance of bacteria from the phylum Bacteroidota and showed a negative statistic correlation between glucose metabolism and the Muribaculaceae family.
It is known that the Bacteroidota phylum plays a role in obesity/metabolic health, although its greater abundance or Bacteroidota/Firmicutes ratio is not sufficient to assess the glucose metabolism (Johnson et al., 2017; Magne et al., 2020) the supplementation of different berries to animals indeed improve its abundance and glycemic parameters (Esposito et al., 2015; Lee et al., 2018).
As well as CJ, the intake of Saskatoon berry and Cyanidin-3-glucoside was able to improve the glucose metabolism showing a negative correlation to the greater abundance of the Muribaculaceae family (Huang et al., 2020; R. Zhao et al., 2021). Classified as a degrading polysaccharide microorganism (Lagkouvardos et al., 2019) the Muribaculaceae growth was stimulated by the ingestion of isolated fiber from cranberry but not to its isolated phenolics ingestion (Rodríguez-Daza et al., 2020).
Besides the abundance caused by the substrate offer Muribaculaceae, per se, was not able to improve the glucose metabolism of animals from this study (Rodríguez-Daza et al., 2020), fostering a classical hypothesis about the effect of complex food matrix versus isolated compounds (nutrient or non-nutrient) and its potential bioactivity (Jacobs and Tapsell, 2007). It is suggested that anthocyanins are more bioaccessible and tend to exert their bioactivity if embedded into food matrices thanks to a synergistic interaction with different phytochemicals groups and nutrients (Koss-Mikołajczyk et al., 2019; Tarko and Duda-Chodak, 2020).
Our research group already elucidated the anti-diabetic and inflammatory effects of J, and so these results are attributed to the (poly)phenolic compounds (mainly the anthocyanins) reducing the oxidative stress, improving parameters of antioxidant capacity in vitro, and endogenous antioxidant capacity in vivo and clinical trials, optimizing the glucose homeostasis by avoiding the insulin resistance via Akt/forkhead box protein pathway (Batista et al., 2017; Dragano et al., 2013; Plaza et al., 2016).
According to the reduced expression of pro-inflammatory proteins, fasting glucose levels, OGTT and ITT AUCs, considerable improvement in clinical indexes of pancreatic function (HOMA-IR and β, and QUICKI) (Fig. 1), and the maintenance of tyrosine phosphorylation of IRS (Fig. 2), we can imply that J, along with a healthy diet, may have contributed to these parameters through its composition, by reducing the oxidative cell stress (Dragano et al., 2013).
However, the supplementation of J in the HF diets did not prevent obesity per se, it was capable to attenuate the inflammatory liver biomarkers and reducing IR in mice treated for six up to ten weeks (Batista et al., 2017; Dragano et al., 2013; Lenquiste et al., 2012). In contrast, in the present experiment, the animals were fed during 13 weeks with HFJ, and we could only observe the numerical reduction of liver NFᴋB; we suggest that future papers can better explore the J's health effects influenced by time, and diets composition.
The bacteria communities found are divergent from each other, and yet all groups did not present statistical differences in species diversity (Fig. 4B and C), suggesting that the composition of diets modulated gut bacteria more than the supplementation of J. Although beta diversity overlaps CJ and HFJ bacteria's communities toward different directions from C and HF, the prebiotic effect of J is dependent on what diet it is inserted in.
The excretion of cholesterol in feces increased in groups supplemented with J, and the minimum intake of 100 mg of J/day was sufficient to increase this parameter and reduce weight gain, even though there was a difference in feed intake between CJ and HFJ. These effects result from soluble dietary fibers by adsorbing lipids from the gastrointestinal tract and increasing their fecal excretion, reaffirming the ability to reduce circulating lipids in serum and liver by supplementing J (Batista et al., 2018; Cazarin et al., 2014).
The SCFAs, obtained from the fermentation of polysaccharides by the gut bacteriota, are suggested as a theory of obesity's etiology since they increase the energy absorbed by the enterocytes; these molecules are commonly observed in serum, cecum, and feces of obese animals and humans (Greiner and Bäckhed, 2011; Khan et al., 2016). However, in this experiment, the bacteria found in non-obese groups indicate a positive correlation between higher dietary fiber intake and higher SCFAs production and excretion (Fig. 3, Fig. 5).
The decreased levels of Firmicutes along with Actinobacteria and Bifidobacterium are associated with SCFA production (Chung et al., 2020; Schnorr et al., 2014) evidenced in CJ (Table 5 and Fig. 3). These findings support an emerging theory where the influence on the energetic homeostasis of the host is due to the intestinal bacteria profile and not to the harvesting energy from fermentation (Sharma and Tripathi, 2018).
5. Conclusions
The jabuticaba peel is a natural source of bioactive compounds, such as dietary fiber and (poly)phenols (mainly anthocyanins), with considerable antioxidant capacity. Its consumption leads to the attenuation of parameters of non-communicable diseases. This investigation confirms the prebiotic role of the jabuticaba peel. The gut microbiota composition and its metabolites are positively modulated by freeze-dried jabuticaba peel intake when inserted into a healthy diet, improving glucose metabolism by reducing inflammation. We highlight the role of the Muribaculaceae family in modulating the glucose metabolism and suggest future studies regarding these microorganisms and complex food matrices and isolated compounds of berries to better understand theirs exact mechanisms of action. The high-fat diets also were capable of modulating the gut bacteriota into a dysbiotic obesity status, impairing glucose metabolism, and SCFA production, plus improving obesity per se through the feed efficiency.
Funding
The authors thank the National Council for Scientific and Technological Development (CNPq - 403328/2016-0; 301496/2019-6) for financial support. VHAC and MRMJ thank São Paulo Research Foundation (FAPESP - 2015/50333-1; 2018/11069-5; 2015/13320-9). MRMJ acknowledges Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543). This study was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
CRediT authorship contribution statement
Paulo Sérgio Loubet Filho: carried the animal design, experimentation, and all posterior analysis. Andressa Mara Baseggio: collaborated with the blotting analysis. Milena Morandi Vuolo: carried the animal design, experimentation, and all posterior analysis. Lívia Mateus Reguengo: the gas-chromatography analysis. Aline Camarão Telles Biasoto: the chromatography analysis. Luiz Claudio Correa: the chromatography analysis. Stanislau Bogusz Junior: the gas-chromatography analysis. Valéria Helena Alves Cagnon: collaborated with the blotting analysis. Cinthia Baú Betim Cazarin: made extensive revisions to the manuscript. Mário Roberto Maróstica Júnior: made extensive revisions to the manuscript, All authors contributed equally to the manuscript's elaboration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
PSLF and MMV carried the animal design, experimentation, and all posterior analysis. AMB and VHAC collaborated with the blotting analysis. ACTB and LCC with the chromatography analysis. LMR and SBJ with the gas-chromatography analysis. CBBC and MRMJ made extensive revisions to the manuscript. All authors contributed equally to the manuscript's elaboration.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2022.02.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Albuquerque B.R., Pereira C., Calhelha R.C., José Alves M., Abreu R.M.V., Barros L., Oliveira M.B.P.P., Ferreira I.C.F.R. Jabuticaba residues (Myrciaria jaboticaba (Vell.) Berg) are rich sources of valuable compounds with bioactive properties. Food Chem. 2019 doi: 10.1016/j.foodchem.2019.125735. [DOI] [PubMed] [Google Scholar]
- AOAC . 1995. Official Methods of Analysis of the Association of Official Analytical Chemists. [Google Scholar]
- Bagarolli R.A., Tobar N., Oliveira A.G., Araújo T.G., Carvalho B.M., Rocha G.Z., Vecina J.F., Calisto K., Guadagnini D., Prada P.O., Santos A., Saad S.T.O., Saad M.J.A. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. JNB (J. Nutr. Biochem.) 2017;50:16–25. doi: 10.1016/j.jnutbio.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Batista Ângela G., Soares E.S., Mendonça M.C.P., da Silva J.K., Dionísio A.P., Sartori C.R., da Cruz-Höfling M.A., Maróstica Júnior M.R. Jaboticaba berry peel intake prevents insulin-resistance-induced tau phosphorylation in mice. Mol. Nutr. Food Res. 2017;61(10) doi: 10.1002/mnfr.201600952. [DOI] [PubMed] [Google Scholar]
- Batista Ângela Giovana, da Silva-Maia J.K., Mendonça M.C.P., Soares E.S., Lima G.C., Bogusz Junior S., da Cruz-Höfling M.A., Maróstica Júnior M.R. Jaboticaba berry peel intake increases short chain fatty acids production and prevent hepatic steatosis in mice fed high-fat diet. J. Funct.Foods. 2018;48:266–274. doi: 10.1016/j.jff.2018.07.020. July. [DOI] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37(1):911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P.D. Handbook of Obesity. Taylor & Francis; 2019. Gut microbiome and obesity; pp. 183–192. [Google Scholar]
- Cazarin C.B.B., Da Silva J.K., Colomeu T.C., Zollner R.L., Maróstica Junior M.R. Capacidade antioxidante e composição química da casca de maracujá (Passiflora edulis) Ciência Rural. 2014:1699–1704. doi: 10.1590/0103-8478cr20131437. [DOI] [Google Scholar]
- Chung Y.W., Gwak H.J., Moon S., Rho M., Ryu J.H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J.K., Cazarin C.B.B., Correa L.C., Batista Â.G., Furlan C.P.B., Biasoto A.C.T., Pereira G.E., de Camargo A.C., Maróstica Junior M.R. Bioactive compounds of juices from two Brazilian grape cultivars. J. Sci. Food Agric. 2016;96(6):1990–1996. doi: 10.1002/jsfa.7309. [DOI] [PubMed] [Google Scholar]
- Davies J. In a map for human life, count the microbes, too. Science. 2001;291(5512):2316b–2316. doi: 10.1126/science.291.5512.2316b. [DOI] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J.B., Massart S., Collini S., Pieraccini G., Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 2010;107(33):14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragano N.R.V., Marques A.Y.C., Cintra D.E.C., Solon C., Morari J., Leite-Legatti A.V., Velloso L.A., Maróstica-Júnior M.R. Freeze-dried jaboticaba peel powder improves insulin sensitivity in high-fat-fed mice. Br. J. Nutr. 2013;110(3):447–455. doi: 10.1017/S0007114512005090. [DOI] [PubMed] [Google Scholar]
- Esposito D., Damsud T., Wilson M., Grace M.H., Strauch R., Li X., Lila M.A., Komarnytsky S. Black currant anthocyanins attenuate weight gain and improve glucose metabolism in diet-induced obese mice with intact, but not disrupted, gut microbiome. J. Agric. Food Chem. 2015;63(27):6172–6180. doi: 10.1021/acs.jafc.5b00963. [DOI] [PubMed] [Google Scholar]
- Fernandes I., Faria A., Calhau C., de Freitas V., Mateus N. Bioavailability of anthocyanins and derivatives. J. Funct.Foods. 2014;7(1):54–66. doi: 10.1016/j.jff.2013.05.010. [DOI] [Google Scholar]
- Foito A., McDougall G.J., Stewart D. Annual Plant Reviews. John Wiley & Sons, Ltd; 2018. Evidence for health benefits of berries; pp. 1–43. [DOI] [Google Scholar]
- Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226(1):497–509. doi: 10.1016/s0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- Gasparrini M., Forbes-Hernandez T.Y., Cianciosi D., Quiles J.L., Mezzetti B., Xiao J., Giampieri F., Battino M. Trends in Food Science and Technology. Elsevier Ltd; 2021. The efficacy of berries against lipopolysaccharide-induced inflammation: a review. [DOI] [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., Gareau M., Murphy E.F., Saulnier D., Loh G., Macfarlane S., Delzenne N., Ringel Y., Kozianowski G., Dickmann R., Lenoir-Wijnkoop I., Walker C., Buddington R. Dietary prebiotics: current status and new definition. 2010. 7(1), 1–19. [DOI]
- Greiner T., Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol. Metabol. 2011;22(4):117–123. doi: 10.1016/j.tem.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Huang F., Zhao R., Xia M., Shen G.X. Impact of cyanidin-3-glucoside on gut microbiota and relationship with metabolism and inflammation in high fat-high sucrose diet-induced insulin resistant mice. Microorganisms. 2020;8(8):1238. doi: 10.3390/MICROORGANISMS8081238. Page 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs D.R., Tapsell L.C. Food, not nutrients, is the fundamental unit in nutrition. Nutr. Rev. 2007;65(10):439–450. doi: 10.1111/J.1753-4887.2007.TB00269.X. [DOI] [PubMed] [Google Scholar]
- Jami E., White B.A., Mizrahi I. Potential role of the Bovine Rumen microbiome in modulating milk composition and feed efficiency. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0085423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.L., Heaver S.L., Walters W.A., Ley R.E. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017;95(1):1–8. doi: 10.1007/s00109-016-1492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A., Nambi S.S., Mather K., Baron A.D., Follmann D.A., Sullivan G., Quon M.J. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metabol. 2000;85(7):2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Khan M.J., Gerasimidis K., Edwards C.A., Shaikh M.G. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J. Obes. 2016 doi: 10.1155/2016/7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss-Mikołajczyk I., Kusznierewicz B., Bartoszek A. The relationship between phytochemical composition and biological activities of differently pigmented varieties of berry fruits; comparison between embedded in food matrix and isolated anthocyanins. Foods. 2019;8(12):646. doi: 10.3390/FOODS8120646. Page 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I., Lesker T.R., Hitch T.C.A., Gálvez E.J.C., Smit N., Neuhaus K., Wang J., Baines J.F., Abt B., Stecher B., Overmann J., Strowig T., Clavel T. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome. 2019;7(1):1–15. doi: 10.1186/S40168-019-0637-2/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lail H.L., Feresin R.G., Hicks D., Stone B., Price E., Wanders D. vol. 13. MDPI AG; 2021. Berries as a treatment for obesity-induced inflammation: evidence from preclinical models; pp. 1–18. (Nutrients). Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas C.A., Kido L.A., Montico F., Collares-Buzato C.B., Maróstica M.R., Cagnon V.H.A. A jaboticaba extract prevents prostatic damage associated with aging and high-fat diet intake. Food Funct. 2020 doi: 10.1039/C9FO02621E. [DOI] [PubMed] [Google Scholar]
- Lee S., Keirsey K.I., Kirkland R., Grunewald Z.I., Fischer J.G., de La Serre C.B. Blueberry supplementation influences the gut microbiota, inflammation, and insulin resistance in high-fat-diet–fed rats. J. Nutr. 2018;148(2):209–219. doi: 10.1093/jn/nxx027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite-Legatti A.V., Batista A.G., Dragano N.R.V., Marques A.C., Malta L.G., Riccio M.F., Eberlin M.N., Machado A.R.T., de Carvalho-Silva L.B., Ruiz A.L.T.G., de Carvalho J.E., Pastore G.M., Maróstica M.R. Jaboticaba peel: antioxidant compounds, antiproliferative and antimutagenic activities. Food Res. Int. 2012;49(1):596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- Lenquiste S.A., Batista A.G., Marineli R. da S., Dragano N.R.V., Maróstica M.R. Freeze-dried jaboticaba peel added to high-fat diet increases HDL-cholesterol and improves insulin resistance in obese rats. Food Res. Int. 2012;49(1):153–160. doi: 10.1016/j.foodres.2012.07.052. [DOI] [Google Scholar]
- Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. 2020;12(5):1474. doi: 10.3390/NU12051474. Page 1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Miura K., Ishioka M., Iijima K. 2017. The Roles of the Gut Microbiota and Toll-like Receptors in Obesity and Nonalcoholic Fatty Liver Disease; pp. 86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli E.L.B., Diniz Y.S., Galhardi C.M., Ebaid G.M.X., Rodrigues H.G., Mani F., Fernandes A.A.H., Cicogna A.C., Novelli Filho J.L.V.B. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007;41(1):111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Rinke C., Chuvochina M., Chaumeil P.A., Woodcroft B.J., Evans P.N., Hugenholtz P., Tyson G.W. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2017;2(11):1533–1542. doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- Peterson J., Garges S., Giovanni M., McInnes P., Wang L., Schloss J.A., Bonazzi V., McEwen J.E., Wetterstrand K.A., Deal C., Baker C.C., Di Francesco V., Howcroft T.K., Karp R.W., Lunsford R.D., Wellington C.R., Belachew T., Wright M., Giblin C., et al. The NIH human microbiome project. Genome Res. 2009;19(12):2317–2323. doi: 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza M., Batista Â.G., Cazarin C.B.B., Sandahl M., Turner C., Östman E., Maróstica Júnior M.R. Characterization of antioxidant polyphenols from Myrciaria jaboticaba peel and their effects on glucose metabolism and antioxidant status: a pilot clinical study. Food Chem. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- Proctor L.M., Creasy H.H., Fettweis J.M., Lloyd-Price J., Mahurkar A., Zhou W., Buck G.A., Snyder M.P., Strauss J.F., Weinstock G.M., White O., Huttenhower C. The integrative human microbiome project. Nature. 2019;569(7758):641–648. doi: 10.1038/s41586-019-1238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatrin A., Rampelotto C., Pauletto R., Maurer L.H., Nichelle S.M., Klein B., Rodrigues R.F., Maróstica Junior M.R., Fonseca B. de S., de Menezes C.R., Mello R. de O., Rodrigues E., Bochi V.C., Emanuelli T. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct.Foods. 2020;65 doi: 10.1016/j.jff.2019.103714. [DOI] [Google Scholar]
- Reeves P.G., Nielsen F.H., Fahey G.C. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition Ad Hoc Writing Committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123(11):1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Daza M.C., Roquim M., Dudonné S., Pilon G., Levy E., Marette A., Roy D., Desjardins Y. Berry polyphenols and fibers modulate distinct microbial metabolic functions and gut microbiota enterotype-like clustering in obese mice. Front. Microbiol. 2020;11:2032. doi: 10.3389/FMICB.2020.02032/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero M., Calder P. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10(4):432. doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquetto A.R., Monteiro N.E.S., Moura C.S., Toreti V.C., de Pace F., Santos A. dos, Park Y.K., Amaya-Farfan J. Green propolis modulates gut microbiota, reduces endotoxemia and expression of TLR4 pathway in mice fed a high-fat diet. Food Res. Int. 2015;76(Pt 3):796–803. doi: 10.1016/j.foodres.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Rufino M. do S.M., Alves R.E., de Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121(4):996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- Schnorr S.L., Candela M., Rampelli S., Centanni M., Consolandi C., Basaglia G., Turroni S., Biagi E., Peano C., Severgnini M., Fiori J., Gotti R., De Bellis G., Luiselli D., Brigidi P., Mabulla A., Marlowe F., Henry A.G., Crittenden A.N. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014;5 doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckinger M.E., Lotton J., Lila M.A., de Mejia E.G. Berries from South America: a comprehensive review on chemistry, health potential, and commercialization. J. Med. Food. 2010;13(2):233–246. doi: 10.1089/jmf.2009.0233. [DOI] [PubMed] [Google Scholar]
- Sharma S., Tripathi P. Gut microbiome and type 2 diabetes: where we are and where to go? J. Nutr. Biochem. 2018;63:101–108. doi: 10.1016/J.JNUTBIO.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Smyth G.K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 2004;3(1) doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Tarko T., Duda-Chodak A. Influence of food matrix on the bioaccessibility of fruit polyphenolic compounds. J. Agric. Food Chem. 2020;68(5):1315–1325. doi: 10.1021/ACS.JAFC.9B07680. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wang J., Gu X., Yang J., Wei Y., Zhao Y. Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front. Cell. Infect. Microbiol. 2019;9:409. doi: 10.3389/fcimb.2019.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins L.J., Monga M., Miller A.W. Defining dysbiosis for a cluster of chronic diseases. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-49452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W., Sun C., Yuan J., Yang N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017;7 doi: 10.1038/srep45308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G., Nyman M., Åke Jönsson J. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006;20(8):674–682. doi: 10.1002/bmc.580. [DOI] [PubMed] [Google Scholar]
- Zhao R., Huang F., Shen G.X. Dose-responses relationship in glucose lowering and gut dysbiosis to Saskatoon berry powder supplementation in high fat-high sucrose diet-induced insulin resistant mice. Microorganisms. 2021;9(8):1553. doi: 10.3390/MICROORGANISMS9081553. Page 1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.