Introduction
Albright hereditary osteodystrophy (AHO) is an autosomal dominant disorder characterized by short stature, brachydactyly, and subcutaneous ossifications, caused by reduced expression or function of the G protein Gαs.1 Gαs is a product of one of several GNAS transcripts and couples to many 7-transmembrane-spanning hormone receptors, including the parathyroid hormone (PTH) receptor, follicle-stimulating hormone receptor, and luteinizing hormone receptor. Gαs modulates adenylyl cyclase to increase intracellular cyclic adenosine monophosphate to further amplify cellular signaling.2 Homozygous variants that abolish Gαs are embryonically lethal in mice.3 In most tissues, Gαs is biallelically expressed, and heterozygous variants produce sufficient Gαs to compensate for a defective allele. In some tissues, however, Gαs is paternally imprinted and expressed only by the maternal allele. Therefore, heterozygous variants may affect only certain tissue types, depending on the parent of origin of the affected allele. For example, resistance to certain hormones, such as PTH, can be observed in maternally, but not paternally, inherited GNAS-inactivating variants.3 Imprinting explains why patients with variants inactivating the maternal allele exhibit pseudohypoparathyroidism (PHP), which is characterized by the AHO phenotype in addition to tissue-selective resistance to Gαs-dependent hormones. STX16 is a gene located near GNAS that has a role in imprinting via methylation of GNAS locus alternative transcripts. Maternally inherited deletions in STX16 have been associated with PHP type 1b and occasionally mild brachydactyly without other AHO features.4 Apart from imprinting-related differences, individuals with similar GNAS variants can have differences in the severity of endocrine resistance and/or the extent of the AHO phenotype. The poorly understood genotype-phenotype correlation makes reporting novel GNAS variants and their associated clinical presentations valuable for understanding this complex locus. Here we report a novel variant with associated clinical findings.
Case report
A 32-year–old woman presented to the dermatology clinic with firm lesions behind her right knee and on her lower back. The lesions had first appeared 10 years previously and had slowly progressed to affect ambulation, cause pain, and become cosmetically disfiguring. Computed tomography of the right lower extremity demonstrated soft tissue calcification along the fascial planes of the posterior right thigh, initially raising concern for dermatomyositis. Creatine phosphokinase, calcium, phosphorus, vitamin D, PTH, glomerular filtration rate, and complete blood count were within normal limits. An antinuclear antibody test was reactive at 1:160 with diffuse patterning; antibodies to extractable nuclear antigens were negative, interpreted as nonspecific and without immediate clinical relevance. She was well aside from the progressively debilitating lesions on her back and right thigh, as well as hypothyroidism diagnosed at age 16. Review of systems was negative for muscle weakness, joint pain, dysphagia, shortness of breath, chest pain, oral or genital ulcers, and symptoms of Raynaud phenomenon.
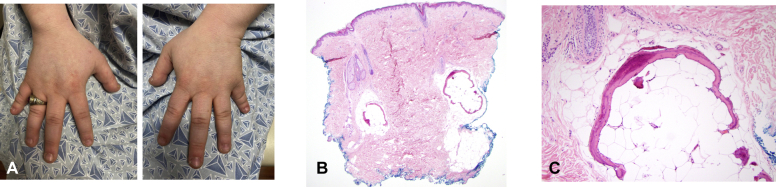
On examination, her height was relatively short at 152 cm. Her weight was 76.2 kg, yielding a body mass index of 33. She had truncal obesity. Her face was rounded, with mild facial hirsutism. Hand examination revealed brachydactyly, most prominently of the bilateral fifth fingers (Fig 1, A). She also had brachytelephalanges of both thumbs. The shortness of the fifth fingers was mostly accounted for by the significantly short middle phalanges but also by the proximal and distal phalanges, as radiographically confirmed. Other phalanges appeared short to variable degrees, more notably on the fourth fingers. Examination of the feet revealed short toes and toenails. She had extensive subcutaneous, firm, immobile, lesions on her posterior right thigh extending to the popliteal region and throughout her lower back. No epidermal changes were seen. The results of a strength examination were normal. Written informed consent for a skin biopsy was obtained. A punch biopsy of the lower back revealed osteoma cutis (Fig 1, B and C). The patient had hypothyroidism but normal serum levels of PTH, calcium, phosphate, growth hormone, insulin-like growth factor 1, luteinizing hormone, and follicle-stimulating hormone.
Fig 1.
Key clinical and pathologic findings of Albright hereditary osteodystrophy. A, Brachydactyly. Punch biopsy of the lower back demonstrating osteoma cutis at (B) low power and (C) high power.
Based on the findings suggestive of AHO, the patient was referred to genetics. She underwent next-generation sequencing and insertion and deletion analysis of the GNAS and STX16 loci. DNA was extracted from peripheral blood leukocytes and randomly fragmented according to Illumina sequencing-by-synthesis technology. The human reference genome GRCh37/hg19 was used for mapping of the reads. One GNAS allele was found to carry a c.43G>T variant in the first exome of the Gαs transcript.
Discussion
The GNAS c.43G>T variant has not been previously described and is predicted to introduce a Glu15X premature stop codon to the Gαs transcript. Since the wild-type Gαs protein is 394 amino acids in length, this novel variant is presumed to be pathogenic as a result of producing severely truncated peptides or producing no protein due to messenger RNA decay. The patient had no measured hormone abnormalities aside from being treated for hypothyroidism, which is relatively prevalent in the female population. Given the high penetrance in childhood of multihormonal resistance in PHP,5 this patient's hypothyroidism was judged to be unrelated to her underlying genetic variant and she was given a diagnosis of AHO-associated pseudopseudohypoparathyroidism. The observed variant is expected to have been inherited or arisen de novo on the paternally inherited allele. The patient’s mother and father, who did not have similar symptoms, underwent GNAS allele sequencing. Both parents were wild-type, confirming a de novo origin.
Although short fourth and fifth metacarpals are typically seen in cases of AHO,6 the metacarpals of this patient did not appear overly disproportionate. The finding of hypoplastic fourth and fifth phalanges was unexpected, since the AHO phenotype, to our knowledge, is classically associated with brachydactyly type E, which is associated with shortened metacarpals but not with shortened phalanges.7 This finding may expand the skeletal phenotype of AHO to include prominent hypoplastic phalanges other than the first distal phalanx. If the patient bears children carrying the same GNAS variant, their phenotype will be expected to be that of PHP with endocrinopathies and AHO, as opposed to the more limited AHO phenotype of the patient. This shift in offspring phenotype is due to the maternal origin of the variant and the imprinting properties of the GNAS locus.
In summary, we present a novel GNAS variant leading to AHO-associated pseudopseudohypoparathyroidism and describe how this condition can initially present to a dermatologist. This case further adds to our understanding of the GNAS locus.
Conflicts of interest
Dr Merola is a consultant and/or investigator for Merck, Abbvie, Dermavant, Eli Lilly, Novartis, Janssen, UCB, Celgene, Sanofi, Regeneron, Arena, Sun Pharma, Biogen, Pfizer, EMD Sorono, Avotres, and Leo Pharma. Drs Smith, Aldeeri, Elman, and Krier have no conflicts of interest to declare.
Footnotes
Authors Smith and Aldeeri contributed equally to this article.
Funding sources: None.
IRB approval status: Not applicable.
Contributor Information
Joel B. Krier, Email: jkrier@partners.org.
Joseph F. Merola, Email: jfmerola@bwh.harvard.edu.
References
- 1.Patten J.L., Johns D.R., Valle D., et al. Mutation in the gene encoding the stimulatory G protein of adenylate cyclase in Albright’s hereditary osteodystrophy. New Eng J Med. 1990;322:1412–1419. doi: 10.1056/NEJM199005173222002. [DOI] [PubMed] [Google Scholar]
- 2.Smith J.S., Lefkowitz R.J., Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018;17:243–260. doi: 10.1038/nrd.2017.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu S., Yu D., Lee E., et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein alpha-subunit (Gsalpha) knockout mice is due to tissue-specific imprinting of the gsalpha gene. Proc Natl Acadu S A. 1998;95:8715–8720. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linglart A., Gensure R.C., Olney R.C., Jüppner H., Bastepe M. A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Genet. 2005;76:804–814. doi: 10.1086/429932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelfand I.M., Eugster E.A., DiMeglio L.A. Presentation and clinical progression of pseudohypoparathyroidism with multi-hormone resistance and Albright hereditary osteodystrophy: a case series. J Pediatr. 2006;149:877–880. doi: 10.1016/j.jpeds.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 6.Usardi A., Mamoune A., Nattes E., Carel J.C., Rothenbuhler A., Linglart A. Progressive development of PTH resistance in patients with inactivating mutations on the maternal allele of GNAS. J Clin Endocrinol Metab. 2017;102:1844–1850. doi: 10.1210/jc.2016-3544. [DOI] [PubMed] [Google Scholar]
- 7.Pereda A., Garin I., Garcia-Barcina M., et al. Brachydactyly E: isolated or as a feature of a syndrome. Orphanet J Rare Dis. 2013;8:141. doi: 10.1186/1750-1172-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]