Abstract
Honeybee products consist of many substances, which have long been known for their medicinal and health-promoting properties. This study set out to appraise the protective potential of Egyptian propolis (EP) and bee venom (BV) separately or combined against total body irradiation (TBI) induced oxidative injury in rats. Besides, we assessed the bioactive components in EP and BV using HPLC and UPLC/ ESI–MS analysis in the positive ion mode. The animals were subjected to a source of gamma ionizing radiation at a dose of 6 Gy. Propolis and BV were administered independently and in combination before 14 days of γ-irradiation. Liver and kidney functions were estimated besides, DNA damage index (8- OHdG) by ELISA. Antioxidants, including glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) were detected. Gene expression technique investigated for BAX, BCL2, and in plasma also miR125b expression in serum of rats. Besides, the histopathological for the brain, liver, kidney, and heart were investigated. In addition, lipid peroxidation was investigated in plasma and in the previous organs. The present results provide opportunities to advance the use of bee products as promising medicinal sources.
Subject terms: Biological techniques, Cell biology, Structural biology, Zoology, Biomarkers
Introduction
Ionizing radiation has cytotoxic and genotoxic impacts caused fundamentally by the oxidative harm prompted by the free radical liberation1. Recent studies have revealed the close association between the risk of heart disease2, and kidney damage3 in people exposed to ionizing radiation (IR). Successively and on a large scale, there are studies to examine and evaluate protective factors from ionizing radiation to reduce the oxidative stress resulting from these radiations4. The equilibrium between ROS and antioxidants is appropriate, as oxidative and antioxidative stress is harmful to both extremes5 . Oxidative stress arises when the equilibrium between antioxidants and ROS is interrupted6. Therefore, through activation or silencing of genes encoding protective enzymes, transcription factors, and structural proteins, cells attempt to counteract the oxidant effects and restore the redox balance7. Radioprotectors can remove the resulting free radicals; can partially reduce the damage caused by radiation, especially to normal tissues. The honeybees’ products incorporate various ingredients like bee venom, propolis, royal jelly, and bee pollen. They are recognized to have many therapeutic and health-promoting characteristics. Bee products are a great source of natural antioxidants. They comprise flavonoids, phenolic acids, or terpenoids. Consequently, the use of natural substances that can counteract the effects of oxidative stress that causes many diseases, such as diabetes, cancer, neurodegenerative disorders, and atherosclerosis, has become a source of great interest. Propolis is a honeybee product with a very complex chemical structure and antioxidant properties. It contains a high percentage of polyphenols, flavonoids, tannins, terpenoids, and phenolic compounds, and these compounds have free radical Curb activity. Many biological and pharmacological properties of propolis have been noted. They include antibacterial, antifungal, anti-inflammatory, antioxidant, immunomodulatory, antiviral, and anticarcinogenic properties. Interest in propolis as a reliable remedy has increased due to its broad-spectrum biological properties8. Moreover, Russo and others have confirmed that propolis has an anti-proliferative ability on human tumor cell lines KB (human oral human melanoma cells), Caco-2 (colon adenocarcinoma cells), and DU-145 (prostate cancer cells). Insensitive to androgens)9. Also, a study by10 confirmed that propolis is a highly effective UV protection and anti-fungal additive for sunscreens, cosmetics, and food or pharmaceutical products containing plant extracts. Research has shown that bee venom has a unique chemical formula, consisting of organic and inorganic materials, which gives it specific properties. The BV content changes significantly according to the internal bee-related characteristics such as age, strain, and caste and external factors such as seasons and BV collection methods11.
Bee venom contains proteins, peptides, enzymes, phospholipids, amines, sugars, mineral salts, etheric oils, and other volatile substances11. The most important part of the protein is melittin, as it is the most active component. It can increase capillary permeability, increment blood circulation and lower blood pressure, treat cancers and infectious diseases12. Other well-represented fractions are phospholipase A and hyaluronidase. Hyaluronidase is a single polypeptide that contains 349 amino acids. Hyaluronidase shares 30% of the amino acid sequence with human hyaluronidase, and the enzyme forms 1–2% of the toxin content. Hyaluronidase breaks down hyaluronic acid in various tissues (for example, in the synovial bursa of rheumatoid arthritis patients) thus is used as an anti-inflammatory13. The enzyme possesses anti-bacterial properties depending on its concentration, exposure time, and stage of bacterial growth. Apamin is the smallest neuropeptide in BV, made of 18 amino acids. Tertiapin is also a peptide (21 amino acids) that is extremely susceptible to oxidation due to the methionine residue14.
Bee venom has been demonstrated to have a radiation protective effect against oxidative-base DNA damage in rat lymphocytes15. Propolis is often referred to as honey bee gum, which contains essential oils, waxes, amino acids, minerals, and some vitamins (A, B, and E).
Variations in propolis' chemical components, and its biological activity, are linked to its kind, botanical source, bee species, and geographic origin. Moreover, seasonality, illumination, food availability, and activity developed during propolis harvesting can all affect propolis composition16. Chemical composition and biological activity have been studied extensively. Until now, over 300 chemical constituents have been identified in propolis from different regions17. Propolis contains polyphenols (flavonoids, phenolic acids, and esters)18, phenolic aldehydes, and ketones19. Resins and vegetable balsam account for 50%, Bee wax 30%, pollen 5%, essential and aromatic oils 10%, and other substances20. In North America, Europe, non-tropical areas of Asia, and New Zealand, the popular tree (Populus nigra) are found. Propolis from Egypt was found to include poplar tree compounds, caffeic acid esters, and long-chain fatty alcohols (tetradecanol, hexadecanol, and dodecanol)21. Betula verrucosa flavonols and flavones (different from popular propolis) are found in birch propolis from Russia (plant source). Baccharis dracunculifolia leaf resin is the primary source of Brazilian propolis, which contains diterpenes, lignans, prenylated derivatives of p-coumaric acid, acetophenone, and flavonoids. Compared to caffeic acid phenethyl ester, Brazilian propolis includes artepillin C22. Some chemicals, such as sesquiterpenoids such as germacren d, ledol, and spatulenol, are found in tropical areas22 Clusia rosea is the principal source of Cuban propolis, which differs from European and Brazilian propolis, which contains polyisoprenylated benzophenone.
Furthermore, propolis comprises flavonoids responsible for preventing injury caused by free radicals by direct scavenging, resulting in a more stable, less-reactive radical. So, several studies emphasized that flavonoids can be used as potential drugs to prevent oxidative stresses23.
According to Santos et al.24 Brazilian propolis (AF-08) has a radioprotective impact of 30 to 50 µg/ml in irradiated CHO-K1 cells, showing that it could be used in radiomedicine to reduce the harmful effects of ionizing radiation. Cui et al.25 has studied the protective effect of propolis on rats exposed to cobalt 60 irradiation, and the results showed propolis was effective in raising the vitality of SOD in irradiated tissues by 25–36%, increasing in kidney and spleen tissues. Propolis was also potent in inhibiting SOD in irradiated tissues. MDA content as low as 12–18% in kidney and liver tissue.
The researchers have conducted a considerable part of examinations on the protecting impact of some synthetic compounds that have shown that these substances can decrease the death rate when administered before exposure to deadly doses of ionizing radiation, but they hurt cells after a time. In contrast, using natural products as radiation shields has many advantages, as it is safe with proven therapeutic benefits and is harmless. Because of these perceptions, the present study aimed to investigate the biological potency of propolis and/or bee venom in γ-rays induced oxidative stress in rats.
Results
In vitro results
The results indicated that the concentration of total phenolics of propolis were 130.45 ± 0.61 mg Gallic acid/g DW, respectively. Whereas that of flavonoids was 81.92 ± 0.70 mg QE/g DW. As well as the FRAP with an EC50 for two samples (Pr and BV) were 3.172 ± 0.259 µg/ml and 2.254 ± 0.073 µg/ml, respectively. Furthermore, the total antioxidant capacity (propolis) = 4.00 ± 0.049 mg Gallic acid/ gm and 1.76 ± 0.027 mg Gallic acid/ gm (BV), also, DPPH % scavenging activity for propolis and BV with an IC50 value of 0.320 ± 0.02 and 0.511 ± 0.03 respectively. The radical scavenging activities of DPPH and ABTS, its ferric-reducing antioxidant ability (FRAP), and the antioxidant properties of propolis EtOH and BV have been assessed. DPPH scavenging activity with the regular antioxidant BHT was shown by the propolis EtOH extract and BV to have an “IC50 value of 10.45 ± 0.08 μg/ml. The propolis ethanolic extract and BV exhibited an IC50 value of 170 ± 1.66 μg/ml and 163 ± 1.07 μg/ml, respectively, concerning the ABTS∙ + quenching activity, but the regular Trolox had an IC50 concentration of 12.25 ± 1.31 μg/ml.
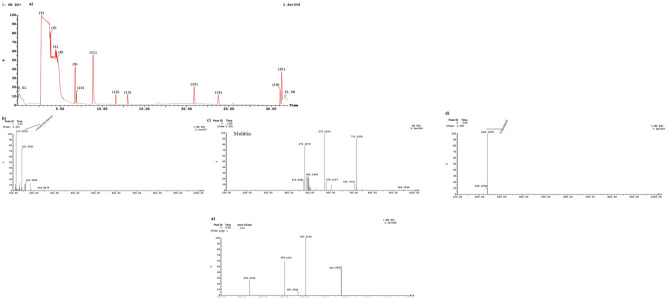
The phytochemical fingerprint of propolis extract was estimated using UHPLC-ESI–MS conditions. The results of the BV showed that melittin and apamin had a strong response and more intense peaks through Curves 3 and 11 (Fig. 1c,e). The bioactive compounds in BV were melittin (m/z 570.2) and apamin (m/z 507.7) (Fig. 1a–c).
Figure 1.
UPLC/ESI–MS analysis and bee venom.
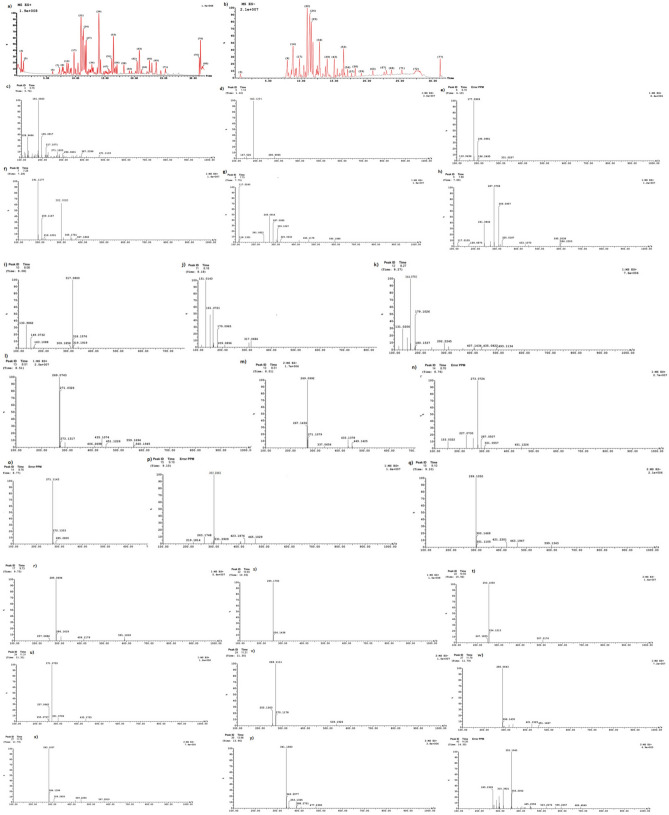
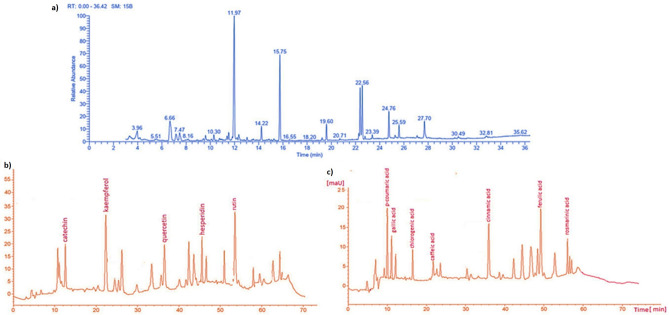
Concerning the analysis of UPLC/ ESI–MS, the results of the biochemical evaluation of propolis and bee venom (Table 1a–c and Figs. 1 and 2), showed the most abundant compounds in propolis were Gallic acid, Catechin, Chlorogenic, and Cinnamic in this extract (Table 1a,b and Fig. 2a–q), these results confirmed by HPLC (Fig. 3a–c).
Table 1.
LC–ESI/MS data.
| Compound | RT (min) | m/z (+ /− ESI) | Exact mass | Peak | |
|---|---|---|---|---|---|
| (a) For ethanolic propolis extracts | |||||
| C6H6O4 | Octane, 3,5-dimethyl- | 0.2 | 142.00 | 142.20 | 1 ES + |
| C4H6N2O2 | 1-Methylhydantoin | 0.49 | 114.00 | 114.10 | 2 ES + |
| C3H4O4 | Malonate | 0.75 | 104.10 | 104.60 | 3ES + |
| C9H11NO3 | Alpha-amino-p-hydroxyhydrocinnamic acid | 0.75 | 181.07 | 181.10 | 3 ES− |
| C9H11NO3 | Alpha-amino-p-hydroxyhydrocinnamic acid | 0.93 | 179.17 | 179.07 | 4 ES + |
| C9H8O4 | Caffeic acid | 1.14 | 180.13 | 180.16 | 5 ES + |
| C10H10O4 | Ferulic acid | 6.10 | 195.10 | 194.186 | 6 ES + |
| C15H10O7 | Quercetin | 7.28 | 302.102 | 302.2 | 7 ES + |
| C3H7N3O2 | Glycocyamine | 7.75 | 117.024 | 117.05 | 8 ES + |
| C17H21NO3 | Galantamine | 7.89 | 287.10 | 287.15 | 9 ES + |
| C15H12O5 | Kaempferol | 7.89 | 285.13 | 285.10 | 9 ES− |
| C15H10O8 | Myricetin | 8.08 | 317.08 | 316.70 | 10 ES + |
| C15H10O8 | Myricetin | 8.08 | 315.10 | 316.70 | 10 ES− |
| C5H9NO3 | 5-Aminolevulinic acid | 8.18 | 131.014 | 131.06 | 11ES + |
| C9H8O3 | P-Coumaric acid | 8.27 | 164.07 | 164.047 | 12ES + |
| C15H10O5 | Apigenin | 8.51 | 269.074 | 269.1 | 13 ES + |
| C15H10O5 | Apigenin | 8.51 | 269.1 | 269.1 | 13 ES− |
| C15H15NO2S | Modafinil | 8.76 | 273.072 | 273.08 | 14 ES + |
| C15H10O5 | Galangin | 8.76 | 271.11 | 271.1 | 14 ES− |
| C14H6O8 | Ellagic acid | 9.10 | 302.186 | 302.199 | 15ES + |
| C11H12O5 | 3,5-Diphenyl-4 hydroxy cinnamic acid | 9.10 | 299.1 | 299.105 | 15ES− |
| C16H20F3NO3 | L-Valine, N-(2-trifluoromethylbenzoyl)-, propyl ester | 9.26 | 331.33 | 331.11 | 16 ES + |
| C13H16N3O3 | 2,3 Di-O-acetyl-5-deoxy-S-fluorocytidine | 9.20 | 329.13 | 329.09 | 16 ES− |
| C15H12O5 | Kaempferol | 9.73 | 285.10 | 285.10 | 17 ES + |
| C17H17NO3 | Coumaroyl tyramine | 9.73 | 283.1 | 283.3 | 17 ES− |
| C20H17NO3 | 3,5-Diphenyl-4 hydroxy cinnamic acid | 9.95 | 299.1 | 299.1 | 18 ES− |
| C7H11NO3 | N-Tigloylglycine | 157.07 | 157.07 | 19 ES + | |
| (b) For ethanolic propolis extract | |||||
| C18H19NO5 | Lycorine-monoacetate | 10.41 | 329.114 | 329.13 | 20 ES− |
| C22H43NO | Erucamide | 10.68 | 337.28 | 337.33 | 21 ES + |
| C18H23O4 | 5,8-Dihydroxy 9,12-octadecadienoic acid | 10.68 | 313.00 | 313.23 | 21ES− |
| C15H12O4 | Pinocembrin | 10.93 | 255.18 | 256.1 | 22ES + |
| C15H10O4 | Chrysin | 10.93 | 253.105 | 254.057 | 22ES− |
| C12H16CINO3 | Meclofenoxate | 11.22 | 257.08 | 257.08 | 23 ES + |
| C15H12O5 | Naringenin | 11.31 | 271.1 | 272.1 | 24 ES + |
| C15H10O5 | Naringenin | 11.30 | 270.05 | 270.11 | 24 ES− |
| C17H14O6 | Kampferol-dimethyl-ether | 11.53 | 313.11 | 313.00 | 25 ES− |
| C15H14O5 | Flavon-3-Ols + 3O | 11.60 | 274.26 | 274.27 | 26 ES + |
| C15H12O5 | Kampferol | 11.79 | 285.10 | 285.10 | 27 ES + |
| C17H14O4 | Chrysin-6-methyl-ether | 11.79 | 283.1 | 283.00 | 27 ES− |
| C12H18N4O4PS | Thiamine monophosphate | 11.99 | 345.1 | 345.08 | 28 ES + |
| C22H33NO5 | Fumaric acid, monoamide, N-(3,4-dimethoxyphenethyl)-, 2-ethylhexyl ester | 12.20 | 391.20 | 391.50 | 29 ES− |
| C21H30FN3O2 | Pipamperone | 12.25 | 375.15 | 375.23 | 30 ES + |
| C14H17NO8 | P-Acetamidophenylglcuronide | 12.32 | 327.1 | 327.10 | 31ES− |
| C3H7N3O2 | Glycocyamine | 12.45 | 117.03 | 117.05 | 32ES + |
| C19H21NO2 | Caffeic acid cinnamylester | 12.45 | 295.16 | 295.00 | 32ES− |
| C22H33NO5 | Fumaric acid, monoamide, N-(3,4-dimethoxyphenethyl)-, 2-ethylhexyl ester | 12.56 | 391.16 | 391.50 | 33ES + |
| C11H19N10S2 | 2-Hydroxy-3butenyl-glucosinolate | 12.56 | 389.20 | 389.40 | 33ES− |
| C18H19NO5 | Lycorine-monoacetate | 12.78 | 329.14 | 329.13 | 34ES + |
| C14H17NO8 | P-Acetamidophenyl-glcuronide | 12.78 | 327.12 | 327.10 | 34ES− |
| C3H7NO3 | L-Serine | 12.89 | 105.04 | 105.04 | 35ES + |
| C13H17NO2 | 2-Dimethylaminoethyl-cinnamate | 13.01 | 219.20 | 219.30 | 36ES + |
| C17H14O4 | Chrysin-6-methyl-ether | 13.30 | 283.10 | 283.00 | 37ES + |
| C21H30FN3O2 | Pipamperone | 13.30 | 375.21 | 375.23 | 37ES− |
| C3H7N3O2 | Glycocyamine | 13.69 | 117.03 | 117.05 | 38 ES + |
| C15H10O5 | Apigenin | 13.96 | 269.2 | 269.10 | 39ES + |
| C20H23NO4 | Coumarin | 13.96 | 341.16 | 341.16 | 39 ES− |
| C15H10O6 | Luteolin | 14.26 | 285.06 | 286.00 | 40ES + |
| C16H18O9 | Chlorogenic acid | 14.26 | 355.20 | 354.095 | 40 ES− |
| (c) For BV | |||||
| C4H6N2O2 | 1- Methylhydantion | 0.25 | 113.92 | 113.92 | 1 ES− |
| C17H32O2 | 7-Hexadecenoic acid, methyl ester | 0.78 | 268.40 | 268.83 | 2 ES + |
| C131H229N39O31 | Melittin | 2.80 | 570.28 | 570.28 | 3 ES + |
| C15H24O2 | Curcumol | 3.90 | 236.35 | 236.20 | 4 ES + |
| C13H21N3O | Procainamide | 4.47 | 235.325 | 235.327 | 5 ES + |
| C13H21N3O | Procainamide | 4.54 | 235.325 | 235.33 | 6 ES + |
| C14H21NO2 | Isonicotinic acid, octyl ester | 4.61 | 235.322 | 235.317 | 7 ES + |
| C12H10FNOS | Acetamide, N-(4-fluorophenyl)-2-(2-thienyl)- | 4.73 | 235.277 | 235.276 | 8 ES + |
| C8H9NO2 | Phenol, 4-methyl-2-nitro- | 6.78 | 153.14 | 153.05 | 9 ES + |
| C15H14O5 | Phenol, 2,6-dimethyl-4-nitroso- | 6.79 | 151.16 | 151.04 | 9 ES− |
| C15H10O4 | Genistein | 6.93 | 269.074 | 269.15 | 10 ES + |
| C79H131N31O24S4 | Apamin | 8.91 | 507.62 | 507.62 | 11 ES + |
| C12H22N2O5 | Sarcosylsarcosine, n-propoxycarbonyl-, ethyl ester | 11.60 | 274. 31 | 274.29 | 12 ES + |
| C11H9NO4 | Acetoxyacetic acid, 4-cyanophenyl ester | 13.01 | 219.194 | 219.197 | 13 ES + |
| C14H19NO4 | l-Valine, N-(m-anisoyl)-, methyl ester | 14.16 | 265.31 | 265.212 | 14 ES− |
| C13H9N3S | Isothiocyanic acid, (p-phenylazo) phenyl ester | 20.89 | 239.30 | 239.23 | 15 ES + |
| C15H22F5NO3 | l-Proline, n-pentafluoropropionyl-, heptyl ester | 23.75 | 359.33 | 359.36 | 16 ES + |
| C15H10O5 | Apigenin | 27.65 | 117.00 | 116.92 | 17 ES− |
| C5H11NO2 | Valine | 27.98 | 117.15 | 116.94 | 18 ES− |
| C5H10O2 | Butanoic acid, 2-methyl- | 31.12 | 102.13 | 102.08 | 19 ES + |
| C6H14O2 | 3-Methylpentane-2,3-diol | 31.12 | 117.916 | 118.17 | 20 ES− |
| C6H6FN | 2-Fluoro-6 methylpyridine | 31.25 | 111.12 | 111.104 | 21 ES + |
Figure 2.
UPLC/ESI–MS analysis in the positive ion mode for propolis.
Figure 3.
HPLC for propolis showed the flavonoid and phenolic compounds.
In vivo results
Acute toxicity
No animals died during the experimental period. We performed an acute toxicity test to determine LD50 values of the bee venom and aqueous extract of propolis for screening their medical potential for promoting the antioxidant indices and the oxidative damage that occurred in the rats because of the exposure to γ-rays. Under the condition of this in vivo test, the propolis extract produced no signs of toxicity, up to 3000 mg/kg of body weight. The LD50 of bee venom IP was 1.56 mg/kg of body weight.
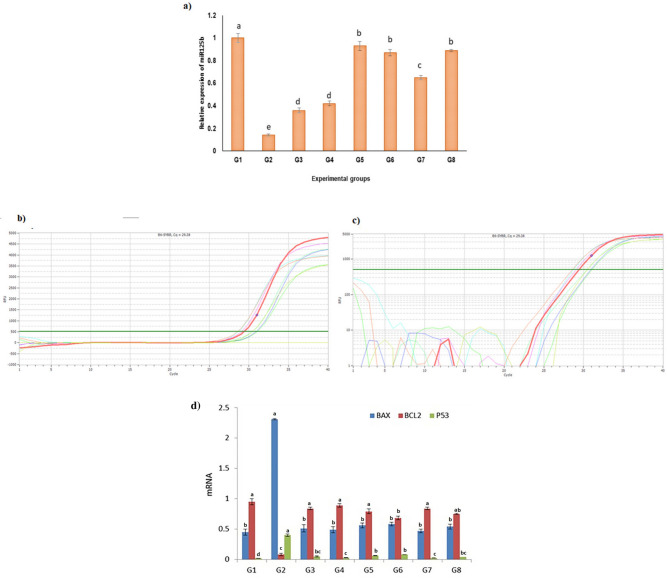
Effect of propolis and/or bee venom on miR125b expression in serum of irradiated rats
The obtained qPCR events showed significant (P ≤ 0.05) downregulation of miRNA125b expression level in the serum of radiated rats as corresponded to the control group (G1) and treated groups (G3-G8) (Table 2 Fig. 4a–c). This expression was significantly upregulated following administration of 300 mg propolis (G3), 0.05 mg bee venom (G4), and 300 mg propolis + 0.05 mg bee venom (G5), also groups 6–8 (Normal rats administered propolis (Pr), BV, and combined Pr + BV). However, the expression levels in G2 remained significantly lower than that in all groups.
Table 2.
Changes in relative expression of miRNA125b gene in serum of radiated rats following treatment by propolis and/or bee venom.
| Groups | Fold change mean ± SEM |
|---|---|
| G1: Control | 1.00 ± 0.04a |
| G2: Gamma rays (γ rays) | 0.14 ± 0.01e |
| G3: Propolis 300 mg/kg + γ rays | 0.36 ± 0.02d |
| G4: BV 0.05 mg/kg + γ rays | 0.42 ± 0.02d |
| G5: Pr 300 mg/kg + BV 0.05 mg/kg + γ rays | 0.93 ± 0.04b |
| G6: Normal rats + pr 300 mg/kg | 0.87 ± 0.03b |
| G7: Normal rats + BV 0.05 mg/kg | 0.65 ± 0.02c |
| G8: Normal rats + Pr 300 + BV 0.05 mg/kg | 0.89 ± 0.01b |
Means within carrying different superscript letters are significantly different (P ≤ 0.05).
Pr propolis, BV bee venom.
Figure 4.
(a) miR125b expression by real time PCR in serum of irradiated rats and after propolis and bee venom; (b,c) amplification curves for miR125b expression by real time PCR. (d) Effect of propolis and/ or bee venom on BAX, BCL2 and P53 in the irradiated rats. Data are expressed as mean ± SE. Means having different superscript letters within the same column are significantly different at p < 0.05.
Effect of propolis and/or bee venom on BAX, BCL2 and P53 expression in serum of irradiated rats
The data illustrated in Fig. 4d delineated that the single-dose exposure of 6 Gy gamma rays induced apoptotic signals. Significant upregulation of BAX and P53 (5.75 and 40-fold, respectively p < 0.05) and the downregulation of Bcl2 (30-fold ≈ 96.70%, p < 0.05) as compared to the controls were noted post-exposure.
Effect of propolis and/or bee venom on liver and kidney functions in serum of irradiated rats
Gamma rays induced severe biochemical alterations and an imbalance of oxidants and antioxidants in rats. We assessed serum levels of creatinine and urea as markers of renal and liver functions. As the result of the present work, a significant increase in serum creatinine, urea levels, ALT, AST, ALP, total protein and albumin in the irradiated rats compared to the control group. Gamma rays showed a significant increase of lipid peroxidation in plasma (Table 3) and tissues of rats (Fig. 5a–d). Concomitant treatment of rats with propolis and/ or bee venom significantly (P < 0.05) diminished the raised values of urea and creatinine in the serum of the irradiated group near control (Table 3). Also, results revealed that total protein, albumin, ALT, AST, and ALP levels were notable (P < 0.05) declined in propolis and/or bee venom treated rats as compared to control irradiated ones (Table 3).
Table 3.
Effect of propolis and/or bee venom on liver and kidney functions and plasma lipid peroxidation.
| Parameters | Control | Irradiated rats | Normal rats | |||||
|---|---|---|---|---|---|---|---|---|
| γ rays | γ rays + Pr | γ rays + BV | Pr + BV + γ rays | + Pr | + BV | Pr + BV | ||
| 6 Gy | 300 mg/kg | 0.05 mg/kg | 300 + 0.05 mg/kg | 300 mg/kg | 0.05 mg/kg | 300 + 0.05 mg/kg | ||
| Urea(mg/dl) | 30.02 ± 0.53f | 103.45 ± 11.22a | 42.31 ± 5.63cd | 46.08 ± 2.66bc | 38.31 ± 1.23de | 34.31 ± 2.34ef | 32.24 ± 1.47f | 51.31 ± 4.03b |
| Creatinine (mg/dl) | 0.35 ± 0.011e | 1.28 ± 0.02a | 0.39 ± 0.04cd | 0.4 ± 0.02c | 0.37 ± 0.03de | 0.41 ± 0.03c | 0.36 ± 0.03e | 0.43 ± 0.03b |
| Total protein (g/dl) | 4.36 ± 0.25d | 3.76 ± 0.21f | 4.82 ± 0.23b | 4.56 ± 0.32c | 5.24 ± 0.22a | 4.61 ± 0.23c | 5.21 ± 0.33a | 4.01 ± 0.20e |
| Albumin (g/dl) | 3.10 ± 0.14b | 1.03 ± 0.02g | 2.65 ± 0.21d | 2.91 ± 0.25c | 3.22 ± 0.16a | 2.54 ± 0.08e | 3.02 ± 0.20bc | 1.88 ± 0.03f |
| Globulin(g/dl) | 1.26 ± 0.11g | 2.73 ± 0.15a | 2.17 ± 0.02b | 1.65 ± 0.07f. | 2.02 ± 0.06e | 2.07 ± 0.15d | 2.19 ± 0.13b | 2.13 ± 0.17c |
| ALT (U/l) | 28.76 ± 3.68g | 165.23 ± 12.20a | 32.10 ± 2.87e | 37.52 ± 4.03c | 26.18 ± 1.49h | 35.41 ± 2.12d | 30.04 ± 0.53f | 43.17 ± 5.17b |
| AST (U/l) | 40.35 ± 2.55h | 204.43 ± 14.02a | 44.29 ± 4.01e | 48.39 ± 3.68d | 42.31 ± 3.20g | 50.35 ± 4.15c | 43.15 ± 2.30f | 53.28 ± 4.08b |
| ALP (U/l) | 65.24 ± 7.04h | 187.67 ± 12.15a | 70.08 ± 8.14e | 72.62 ± 6.98d | 67.14 ± 4.87g | 80.13 ± 6.11c | 68.61 ± 5.37f | 85.11 ± 5.81b |
| MDA (ng/dl) | 55.42 ± 4.23f | 134.13 ± 10.22a | 58.32 ± 4.43e | 61.15 ± 4.30c | 55.97 ± 4.52f | 58.70 ± 4.35d | 52.70 ± 3.72h | 71.27 ± 6.24b |
Values with same superscript in the same raw are not statistically different. Data expressed as mean ± SE, n = 6. The groups are statistically significant (P < 0.05) as compared with control, using one-way ANOVA followed by Duncan’s test as a post-hoc test.
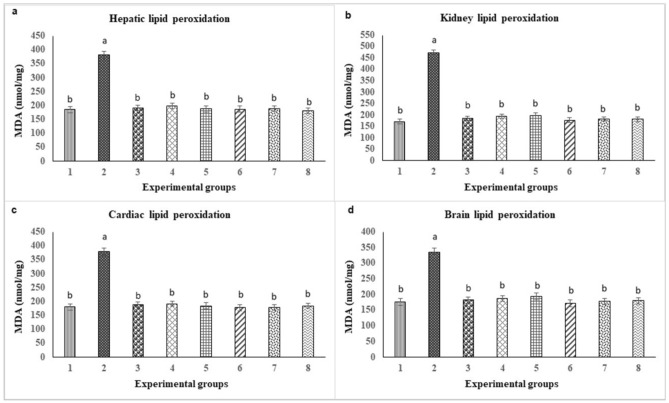
Figure 5.
Effect of propolis and BV on the lipid peroxidation in (a) liver, (b) kidney, (c) heart and (d) brain. Data are expressed as mean ± SE. Means having different superscript letters within the same column are significantly different at p < 0.05.
Effect of propolis and/or bee venom on cytokines (IL-6, IL-1β, TNF-α & IL-10)
In the irradiated rats, there are significant changes in the cytokines (P < 0.05) treatment with propolis and bee venom stressed these consequences if compared to the non-treated group (P < 0.05) (Table 4).
Table 4.
Effect of propolis and/ or bee venom on cytokines.
| Parameters | Control | Irradiated rats | Normal rats | |||||
|---|---|---|---|---|---|---|---|---|
| γ rays | Pr + γ rays | BV + γ rays | Pr + BV + γ rays | Pr | BV | Pr + BV | ||
| 6 Gy | 300 mg/ kg | 0.05 mg/kg | 300 + 0.05 mg/kg | 300 mg/ kg | 0.05 mg/kg | 300 + 0.05 mg/kg | ||
| IL-6 (pg/ml) | 30.02 ± 0.53h | 103.45 ± 11.22a | 42.31 ± 5.63d | 51.31 ± 4.03b | 38.31 ± 1.23e | 32.24 ± 1.47g | 46.08 ± 2.66c | 34.31 ± 2.34f |
| IL1-β (pg/ml) | 27.31 ± 2.25h | 86.74 ± 4.11a | 32.01 ± 2.67d | 46.23 ± 2.81b | 27.97 ± 1.09g | 28.44 ± 1.46f | 30.18 ± 1.13e | 40.53 ± 2.14c |
| TNF (pg/ml) | 30.57 ± 1.60h | 102.34 ± 8.40a | 40.12 ± 2.03d | 51.26 ± 2.66b | 34.34 ± 1.91g | 36.44 ± 2.32f | 38.12 ± 1.27e | 42.05 ± 3.12c |
| IL10 (pg/ml) | 40.28 ± 5.23a | 21.03 ± 1.10h | 35.08 ± 1.67d | 28.49 ± 1.07g | 38.92 ± 2.44b | 38.87 ± 2.33c | 31.46 ± 1.93f | 33.27 ± 2.56e |
Values with same superscript in the same raw are not statistically different. Data expressed as mean ± SE, n = 6. The groups are statistically significant (P < 0.05) as compared with control, using one-way ANOVA followed by Duncan’s test as a post-hoc test.
Gamma rays provoke severe fluctuations in the inflammatory cytokines, as shown in Table 4. The irradiated group showed a significant increase (P < 0.05).in both IL-6, IL-1 B, and TNF-α, while it revealed a significant decrease in IL-10 compared to the control group. The treatment of irradiated rats with propolis and bee venom resulted in a considerable decline in IL- 6, IL-1B, and TNF, also, a significant increase in IL10 (P < 0.05).
Effect of propolis and/or bee venom on antioxidant activities and OHdG in serum of irradiated rats
Table 5 displays the abnormally declined activities of antioxidant enzymes (SOD, CAT, and GPx) and the level of a non-antioxidant enzyme (GSH), significantly decreased as a sign of damage provoked by gamma rays when compared with control. These previous parameters were restored to near control values by propolis and bee venom. The level of 8-OHdG was a significant increase in the irradiated rats compared to controls. The administration of propolis and bee venom had a dramatic reduction in 8-OHdG levels compared to untreated.
Table 5.
Effect of propolis and/ or bee venom on the antioxidant enzymes and 8-OHdG.
| Parameters | Control | Irradiated rats | Normal rats + | |||||
|---|---|---|---|---|---|---|---|---|
| γ rays | Pr + γ rays | BV + γ rays | Pr + BV | Pr | BV | Pr + BV | ||
| 6 Gy | 300 mg/kg | 0.05 mg/kg | 300 + 0.05 mg/kg | 300 mg/kg | 0.05 mg/kg | 300 + 0.05 mg/kg | ||
| SOD(U/ml) | 30.71 ± 2.25b | 10.03 ± 1.32g | 28.90 ± 1.97c | 24.64 ± 1.41d | 29.14 ± 1.86c | 36.41 ± 2.83a | 17.25 ± 1.30f | 21.38 ± 1.55e |
| CAT(U/ml) | 22.86 ± 2.01b | 8.12 ± 0.51h | 18.56 ± 1.42d | 17.86 ± 1.83e | 20.18 ± 1.23c | 24.02 ± 2.16a | 12.08 ± 1.33g | 15.34 ± 1.40f |
| GPx(U/ml) | 4.02 ± 0.08a | 1.57 ± 0.03f | 3.72 ± 0.22c | 3.36 ± 0.24d | 4.01 ± 0.05a | 3.85 ± 0.40b | 2.81 ± 0.03e | 3.65 ± 0.21c |
| GSH(U/ml) | 17.25 ± 1.57a | 7.03 ± 0.78h | 11.22 ± 1.67f | 12.48 ± 1.93d | 15.10 ± 2.44b | 14.32 ± 2.33c | 9.23 ± 1.07g | 11.38 ± 2.56c |
| 8-OHdG (ng/ml) | 343.12 ± 15.70g | 789.65 ± 20.43a | 366.07 ± 11.80d | 366.07 ± 14.51d | 350.06 ± 12.54f | 354.12 ± 10.76e | 470.11 ± 18.85b | 401.13 ± 21.23c |
Values with same superscript in the same raw are not statistically different. Data expressed as mean ± SE, n = 6. The groups are statistically significant (P < 0.05) as compared with control, using one-way ANOVA followed by Duncan’s test as a post-hoc test.
Histopathological findings
The histopathological inspection of the liver, kidney, heart and brain tissues of different animal groups is presented in Figs. 6, 7, 8 and 9, respectively. Table 6 illustrated the histopathological changes in the liver, kidney, heart and brain of rats exposed to radiation and the treatment effect of propolis and/or bee venom, based on scoring severity of injury.
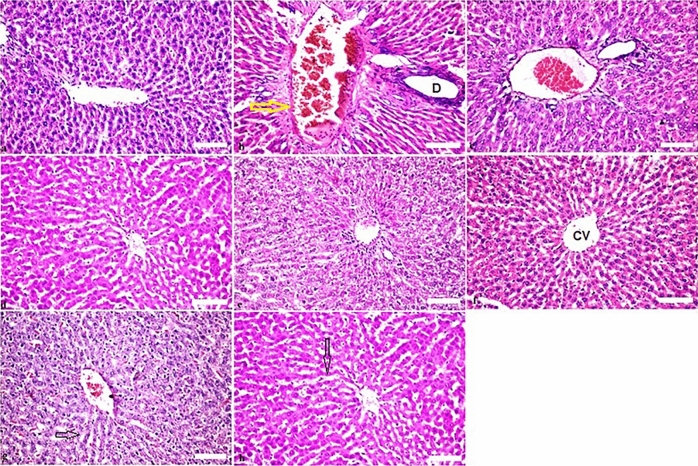
Figure 6.
Histopathological findings in liver; (a) non-treated rats manifesting the typical histological architecture of the liver, (a), (b) gamma rays at dose 6 Gy showing congestion in the portal vein (score 3) (yellow arrow) and dilatation in the bile ducts (D) (score 2), associated with fibrosis (score 2) in the liver. (c) Rats were received of BV 0.05 mg/kg showed there was no histopathological alteration (score 0) as recorded in liver. (d) Rats were administered of propolis 300 mg/kg showed no alteration (score 0) of liver. (e) Effect of combination treatment of propolis + BV 300 & 0.05 mg/kg showed normal histological structure (score 0) in liver. (f–h) Effect of propolis 300 mg/kg and BV 0.05 separately and combined (respectively) on the normal rats for two weeks without any alterations in liver. Normal hepatocytes radiating from the central vein (CV) and separated by blood sinusoids (arrows) (f–h). H&E stain used.
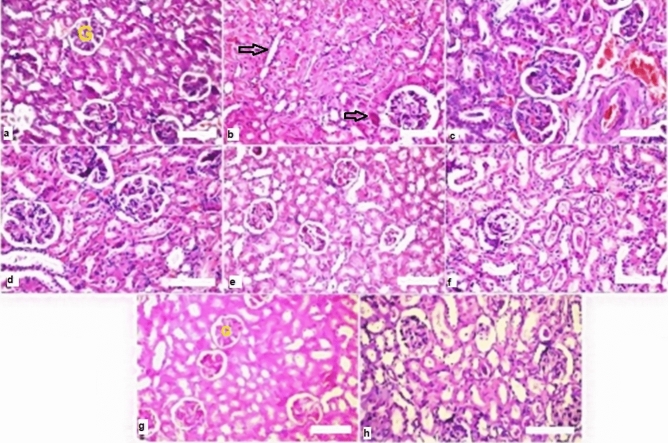
Figure 7.
Histopathological findings in kidney (a) normal glomeruli (G) and tubules at the cortex in kidney. (b) Focal inflammatory cells infiltration with fibrosis in between the glomeruli and tubules at the cortex in kidney (arrows) of exposed rats. (c,d) Irradiated rats were administered of BV 0.05 or propolis 300 mg/kg respectively showed no alteration (score 0) of kidney. (e) Effect of combination treatment of propolis + BV 300 & 0.05 mg/kg respectively showed normal histological structure (score 0) in kidney. (f–h) Effect of propolis 300 mg/kg and BV 0.05 separately and combined (respectively) on the normal rats for two weeks without any alterations in kidney. H&E stain used.
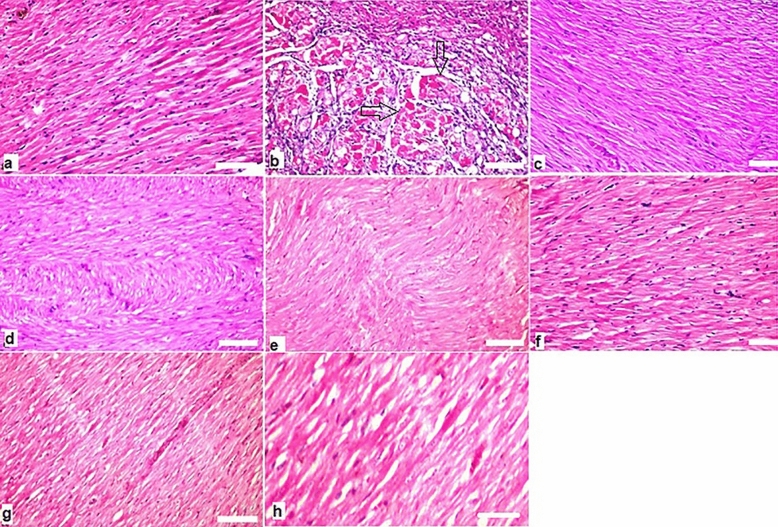
Figure 8.
Histopathological findings in heart (a) showing normal histopathological structure of the myocardial bundles. (b) Focal degenerative changes with Zenkers necrosis (infarction) (arrows) (score 3) after exposure to gamma rays. (c) Irradiated rats were received of BV 0.05 mg/kg showed there was no histopathological alteration (score 0) as recorded in heart. (d) Irradiated rats were received of propolis 300 mg/kg showed there was no histopathological alteration (score 0) as recorded in heart. (e) Effect of combination treatment of propolis + BV 300 & 0.05 mg/kg respectively showed normal histological structure (score 0) in heart. (f,g) Effect of propolis 300 mg/kg and BV 0.05 separately respectively and (h) combined on the normal rats for two weeks without any alterations in heart. H&E stain used.
Figure 9.
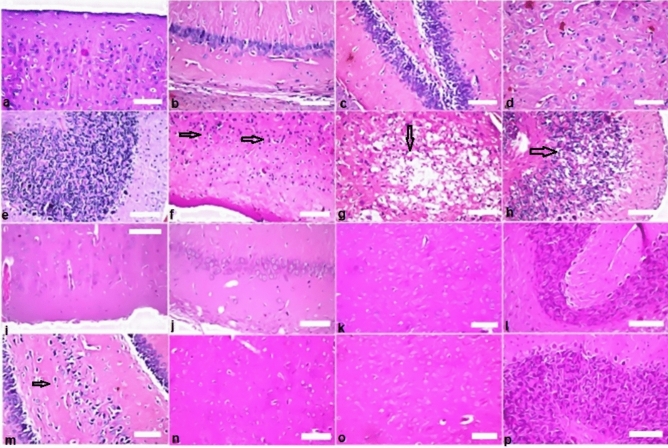
Histopathological findings in brain (a–e) showing normal histopathological of the cerebral cortex of rat. (a) The control group showing normal appearance of the nerve cells. (f–h) Rats which exposed to gamma rays at dose 6 Gy revealed nuclear pyknosis (arrows) (score 3), degeneration (score 2), and cellular vacuolization (score 2) were detected in the neurons of central cortex, striatum and cerebellum respectively. (i,j) Rats were administered BV 0.05 mg/kg showed no alteration (score 0) of the brain. (k,l) The group of animals were administered propolis (300 mg/kg): there was no histopathological alteration as recorded in brain. (m) Nuclear pyknosis and degeneration (arrow) (score 1) were in some neurons of fascia dentata in the group of rats administered propolis 300 + BV 0.05 mg/kg. (n–p) Normal histological structures (score 0) of brain occurred in the normal rats administered propolis, BV, and combined treatment (Pr + BV) respectively.
Table 6.
Histopathological changes in the heart of male rats exposed to γ- rays and the treatment effect of propolis and/or bee venom, based on scoring the severity of the injury.
| Organs Histopathological alteration |
Control | Gamma rays | Normal rats | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| γ- rays (6 Gy) | Pr + γ rays | BV + γ rays | Pr + BV + γ rays | Pr (300 mg/kg) | BV (0.05 mg/kg) | Pr + BV (300 + 0.05 mg/kg) | ||||
| Liver | Congestion | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Dilatation in bile duct | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Inflammatory cells infiltration in portal area | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Fibrosis | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Kidney | Tubular degeneration | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Focal fibrosis | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Focal infiltration cells | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Heart | Infarction areas in myocardium | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Focal hemorrhage in myocardium | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Focal infiltration cells in pericardium | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Brain | Cerebral cortex | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hippocampus | Nuclear pyknosis and degeneration of the neurons | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Striatum | Nuclear pyknosis of the neurons | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vacuolization of the neurons | 0 | 2 | 0 | 0 | 0 | 0 | 0 | |||
| Cerebellum | Nuclear pyknosis of the neurons | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
Normal (0), moderate (2), mild (1) severe (3).
Liver
There was no histopathological alteration and the normal histological structure (score 0) of the liver was recorded in control group (Fig. 6a). While rats which exposed to gamma rays at dose 6 Gy revealed congestion in the portal vein (score 3) and dilatation in the bile ducts (score 2), associated with fibrosis in liver (score 2) (Fig. 6b). On the contrast, rats were administered BV 0.05 mg/kg showed no alteration (score 0) of the liver (Fig. 6c). Similarly, the group of animals were administered propolis (300 mg/kg): there was no histopathological alteration as recorded in liver (Fig. 6d). Moreover, group of rats exposed by combination (propolis + BV 300 + 0.05 mg/kg respectively). Normal histological structures (score 0) of liver were shown (Fig. 6e). No alteration changes occurred of histopathological structures in the normal rats administered propolis, BV, and combined treatment (Pr + BV) (Fig. 6f–h respectively).
Kidney
Normal glomeruli, normal Bowman's spaces, normal proximal tubules with preserved brush boundaries, normal distal tubules, and normal interstitium were seen in the control group (Fig. 7a).
The irradiated group showed focal inflammatory cells infiltration (score 2) (with fibrosis (score 1) in between the glomeruli and tubules at the cortex in kidney (Fig. 7b). On the contrast, rats were administered BV 0.05 mg/kg showed no alteration (score 0) of the kidney (Fig. 5c). Similarly, the group of animals were administered propolis (300 mg/kg): there was no histopathological alteration as recorded in kidney (Fig. 7d).
Moreover, group of rats exposed by combination (propolis + BV 300 + 0.05 mg/kg respectively). Normal histological structures (score 0) of kidney was shown (Fig. 7e). No alteration changes occurred of histopathological structures in the normal rats administered propolis, BV, and combined treatment (Pr + BV) (Fig. 7f–h respectively).
Heart
There was no histopathological alteration and the normal histological structure (score 0) of the heart was recorded in control group (Fig. 8a). While rats which exposed to gamma rays at dose 6 Gy revealed focal areas in the myocardium showed Zenkers necrosis (score 3), focal hemorrhage (score 2) and myofibroblasts proliferation (infarction) (score 3) (Fig. 8b). On the contrast, rats were administered BV 0.05 mg/kg or propolis (300 mg/kg) showed no alteration (score 0) of the, heart (Fig. 8c,d respectively). Similarly, the group of rats exposed by combination (propolis + BV 300 + 0.05 mg/kg respectively) revealed normal histological structures (score 0) of heart (Fig. 8e). Moreover, no alteration changes occurred of histopathological structures in the normal rats administered propolis, BV separately, and combined treatment (Pr + BV) (Fig. 8f–h respectively).
Brain
There was no histopathological alteration and the normal histological structure (score 0) of the brain was recorded in control group (Fig. 9a–e). While rats which exposed to gamma rays at dose 6 Gy revealed nuclear pyknosis (score 3), degeneration (score 2), and cellular vacuolization (score 2) were detected in the neurons of central cortex, striatum and cerebellum (Fig. 9f–h respectively). On the contrast, rats were administered BV 0.05 mg/kg showed no alteration (score 0) of the brain (Fig. 9 i,j). Similarly, the group of animals were administered propolis (300 mg/kg): there was no histopathological alteration as recorded in brain (Fig. 9k,l). We recorded nuclear pyknosis and degeneration (score 1) were in some neurons of fascia dentata in the group of rats administered propolis 300 mg/kg (Fig. 9m).
Moreover, group of rats exposed by combination (propolis + BV 300 + 0.05 mg/kg respectively). Normal histological structures (score 0) of brain were shown (Fig. 9n–p). Moreover, no alteration changes occurred of histopathological structures in the normal rats administered propolis and BV (Fig. 9n,o respectively), and combined treatment (Pr + BV) (Fig. 9p).
Discussion
Radiation exposure produces free radicals that cause oxidative stress decreases antioxidant activity and increases lipid peroxidation26. Ionized radiation contributes to a considerable rise in physiological and metabolic processes, also biochemical disorders of the blood8, and oxidation chain reaction27. The results included in this study revealed that exposure to whole-body gamma rays led to changes in the vital signs of the liver, kidneys, and heart, besides severe damage to these organs, along with nerve cells.
Recently, there is a global concern to profoundly identify and utilize the natural antioxidant products that are pharmaceutically effective and of moderate or no side consequences for facing different diseases28. Advantages of products of honeybees such as honey, propolis, and bee venom, against various kinds of diseases, have been recognized. Honey bees’ products are well known for their nutritional and medicinal implications to have been employed for ages for a range of therapeutic purposes29.
Polyphenols derived from bee products are characterized by their swift, efficient access to distinct metabolic processes without any harmful effects as they do with their synthetic counterparts. Previous studies also indicated a relationship between dietary polyphenols, heart and liver protective, anti-inflammatory, antibacterial, anticancer, and immunomodulatory30. Egyptian propolis includes several compounds that have an anti-bacterial and anti-fungal effect, including, for example, aliphatic and phenolic acids, phenolic esters, and flavonoids. The substantial antioxidant activity of propolis recorded in the current study was inconsistent with the analysis of two specimens of propolis requested from the reclaimed area in Egypt31. The data of this study demonstrated that the EtOH propolis contains significant amounts of antioxidant.
Phenolics, which can quench free radicals. Natural antioxidants are considered biocompatible intriguing alternatives and can prevent diseases and oxidized complex food systems29 . Dietary polyphenols act as antioxidants, so, antioxidants neutralize ROS and minimize oxidative stress. Therefore, the quercetin, caffeic acid, kaempferol, and apigenin found in propolis and melittin, apamine, adolapin, and other components in BV might play a role in reducing oxidative stress induced by gamma rays. Quercetin reduces oxidative stress by scavenging free radicals, chelating metal ions, and inhibiting xanthine oxidase and lipid peroxidation32. Caffeic acid inhibits oxidative stress in irradiated rats by reducing lipid peroxidation and scavenging free radicals32. Kaempferol is a flavonoid that can reduce oxidative stress caused by glutamate in the mouse hippocampal cell line HT-22 by blocking ROS generation33. Kaempferol also blocks oxidative stress in granule cells during low potassium-induced apoptosis33. Apigenin is a common flavonoid that can reduce oxidative stress, and it protects endothelium-dependent vasorelaxation of the aorta in male Sprague–Dawley rats. Mohdaly et al.34 confirmed that propolis with higher phenolic content showed significantly greater activity over pollen extracts. The crucial phenolic compounds in propolis extract, caffeic acid, ferulic acid, rutin, and p-coumaric acid, were found. Others supported our findings, demonstrating that much higher quantities of Hydroxycinnamic (caffeic, p-coumaric, and ferulic) acids have considerable in vitro antioxidant activity34. The natural flavonoid compound pinocembrin is found in bee products that have shown anti‐inflammatory, neuroprotective, and antioxidative effects. In contrast to the presented results, literature data indicate that the concentration of ethanol used for extraction influences the DPPH free radical scavenging activity and Fe3+ reducing power assay of propolis extract34.
In the current study, it was observed that oxidative stress was exerted by 6 Gy gamma radiation, represented by increased MDA level, associated with reduced GSH level, SOD, GSH-PX, and CAT activities.
Radiation-derived free radicals replied with hydroperoxide-generating unsaturated lipids. The lipid bilayer structure, membrane permeability, fluidity, and induced lipid peroxidation were subsequently changed35. Thus, the use of chemicals or radiation for cancer prevention seems closely linked to the use of LPO, where antioxidants like SOD, GPx, and CAT have declined with the rising of LPO36. ROS can also interrupt the balance of endogenous protective systems, including the enzymic antioxidants (CAT and GSH-Px) defense system. Our study showed that propolis and BV separately or combined prevented the degradation of antioxidants by removing free radicals.
Bee venom is a translucent liquid that drains distinctly to room temperature, smell, bee sniffing, sour taste, or a water mixture of proteins with a basic pH (4.5–5). Our results revealed that the peak shapes, responses of melittin, and apamin were better in the ESI positive mode by adding formic acid. These observations were followed the previous data for37, where they obtained similar findings on the bee venom lyophilized powder from Apis mellifera. Bee venom (BV) is a natural toxin produced by the honeybee, and it contains different peptides including melittin, apamin, and mast cell degranulating peptides. Therefore, in recent years the popularity of the BV as an alternative therapy has been increased37. In this study, an analysis of bee venom was found to contain, along with melittin and apamin, BV anti-inflammatory properties are associated with melittin and phospholipase A238. Melittin is associated with the underexpression of proinflammatory cytokines, including nuclear factor-kappa B, extracellular signal-regulated kinases (ERK1/2), and protein kinase Akt38.
The flavonoids return oxygen radicals (-R) such as O2, peroxyl, alkoxyl, and hydroxyl radical and the resulting flavonoxy root (Flav-O-) can interact with another one to produce a stable quinone structure. In the propolis extricate range, the most power is that of m / z 299, which may be the 3,5-diphenyl-4-hydroxycinnamic acid (DPHC) phenolic compound or the flavonoid kaempferide, both present in propolis39.
Several mechanisms may be the cornerstone of both the natural product propolis and bee venom against oxidative stress. Enhancing the endogenous antioxidant such as GSH, SOD, GPx, and CAT are among these mechanisms. The findings obtained from our analysis showed that propolis with or without bee venom therapy mitigates the harmful impacts of 6 Gy radiation by increasing antioxidant enzyme activity and decreasing levels of MDA. Therefore, such natural products reduce the free radicals that hurt living cells and stimulate the antioxidant defense system to prevent the rise in MDA induced by radiation34.
The other mechanism appears through the strong sweeping effect of some antioxidants such as naringenin, pinostrombin, and galangin, which present in propolis of ROS electrons. The positive effect can accumulate in the cells of the coiled tubules close to the kidneys, as was reported in a previous study on the effect of propolis on these tubes40. The current investigation is intended to address the impact of utilizing BV as a characteristic cell reinforcement in the improvement of harm impacts coming about because of gamma-radiation presentation. Gamma-irradiation of rats, in the present work, resulted in increased serum TNF-α and IL-6 indicating the role of these cytokines in irradiation-induced toxicity. Similarly, the study of Shah41 found that the production of TNF-α and IL-6 was elevated significantly after radiation exposure. Notably, treatment with BV + propolis before gamma irradiation showed a significant decrease in both TNF-α and IL-6 levels, compared to the untreated group. The anti-inflammatory activity of phenolic acids and flavonoids is a result of their antioxidative properties. Propolis exhibits anti-inflammatory efficacy in acute and chronic inflammatory processes due to its high contents of polyphenol compounds31. Propolis affects the metabolic pathway of arachidonic acid significantly. The appearance and distribution of inflammatory reactions are significantly influenced by interleukin IL1β. Therefore, the inhibiting effect of propolis on its synthesis was demonstrated by inhibiting the expression of mRNA IL1β and synthase NO. Chrysin, kaempferol, quercetin, and galangin present in propolis affect the expression of mRNA. Propolis extract experiments have shown that they have a suppressive effect on the inflammation of cells. This effect can be used without degrading the conditions for cell repair processes to regulate the inflammatory response31. The high content of caffeic acid is a consequence of this phenomenon. The extract of propolis synergistically enhanced the efficacy of BV, proving synergistic action between each other. However, low doses of this BV compound can induce anti-inflammatory effects. It acts by inhibiting inflammatory cytokines like interleukin-6 (IL-6), IL-8, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ). Many reports investigated the anti-inflammatory mechanisms of melittin in different diseases38. By blocking their primary signaling pathways, melittin inhibited inflammatory cytokines, leading to reduced inflammation in the skin, liver, joint, and neuronal tissue41.
Apoptosis, along with OHDG, BCL-2, and BAX, is systemic at the cellular level by different genes. The effector that induces cell degradation in the final stages of apoptosis is caspase. In regulating intrinsic apoptotic signaling, BCL-2 plays a critical role and is considered a survival enhancer protein, while BAX is known as the opposite of BCL2, which is a pro-apoptotic protein42. As two major proteins of the BCL family that restrain and promote apoptosis, BCL-2 and BAX play pivotal roles in regulating the effect of mitochondrial membrane permeability, mitochondrial function, and Cyt-c release42. The apoptotic factors in the mitochondrial intermembrane space are released into the cytoplasm and transfer to the nucleus where they bind to DNA, leading to nuclear condensation, DNA fragmentation, and induction of the mitochondrial caspase-independent apoptosis pathway43. BCL-2 can stabilize the barrier function of the mitochondrial membrane and inhibit the transfer of apoptosis-inducing factors to the nucleus42.
MicroRNAs are short non-coding RNA molecules that change cell biology by affecting both the stability and translation of mRNAs44. Scarce novel investigations have shown the noteworthy function of miR in controlling radiation-prompted harm45. MiRNAs include a thoroughly conserved 18–22 bp RNA family that binds to mRNA and either tackle transcription or promote mRNA degradation and thus serve a unique mechanism for regulating gene expression. In targeting signaling pathways, radiation-induced miRs may also play critical roles45. A little number of studies have shown an effect of radiation on miRNA expression patterns both in vitro, and in vivo46. Moreover, the overexpression of miR-125b inhibited cell proliferation, migration and provoked cell cycle arrest in the G1 stage in some cancers like bladder cancer. Therefore, a recent studies demonstrated that miR-125b acts as a tumor suppressor in breast47 and liver cancer48. Data from the current study revealed improvements in miRNA expression levels relative to the control group in the serum of irradiated rats. This present study recorded that the radiation produced downregulation of miRNA when compared to the control group. On the contrary, the expression was adjusted for miRNA when using propolis with or without bee venom, and the improvement was apparent in the propolis plus bee venom at the concentrations used for each. We marked the radiation-actuated concealment of miRs, an effect reversible by pretreatment of propolis or/and bee venom. The expression of miR-125b has been shown to associate with erythropoiesis49. Exposure to ionizing radiation has been shown to cause a substantial decrease (P < 0.05) in miR-125b expression, which can adversely affect healthy blood production. Moreover, pretreatment of propolis plus BV prevents the radiation-induced suppression of miR-125b. Ghosh et al.50 indicated that miR-125b might be engaged with shielding spleen tissues from radiation-actuated demolition. The role of miR-125b has also been stated in the control of essential inflammatory genes in various cell types, indicating its role in inflammation and in stimulating the immune system51. Moreover, P53 expression is altered after irradiation and some studies have confirmed that it regulates hematopoiesis52. Also, after DNA damage, p53 alters the expression of a significant number of miRs, particularly in the counterargument to DNA disability, p53 is reasonably the most grounded miR-34a inducer53. It is noticeable that there is an inverse relationship between expression P53 and miR-125b, so the increase in expression of the first is followed by a decrease in the second and vice versa. Another study discovered that radiation presentation triggered extended p53 guidelines in the mucous layer of patients undergoing radiotherapy54.
In the present work, total exposure to gamma rays has led to tissue toxicity, including liver and kidneys, represented by significant increases in ALT, AST, ALP, urea, and creatinine. Also, a notable increase in liver enzymes was associated with a decrease in total protein and albumin post-irradiation. The changes in the biochemical parameters in the liver and kidney induced by radiation reflect on the pathological alteration in biliary flow and the drastic dysfunction of the liver cells. Therefore, gamma rays led to damage to all tissues of the heart, brain, and spleen. These findings are in line with the results of previous studies55. Propolis extracts also contribute to the improvement of the activity of hepatic enzymes, kidney functions and bilirubin content in the case of inflammation and toxic liver damage. The effect was manifested by the lowered level of IL-1B, IL-6 and TNF-α. The histopathological findings confirmed the potential to mitigate propolis + BV against gamma-ray damage.
Materials and methods
Collection of propolis and bee venom
A sample of Egyptian propolis (EP) and bee venom was collected from Aga—Dakahlia in the Center of Egyptian Delta (Abdelbaky Honey apiary) in 2019 and stored for five months at less than 20 °C. Lyophilized Apis mellifera purified Bee venom (1 mg/vial) was used. EP was extracted using 70% ethanol following the previously described protocol56 in our lab (Cell Biology Unite, Radioisotopes Department, Atomic Energy Authority.
Chemicals
ABTS+ [2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] (PubChem CID: 5815211), BHT (butylated hydroxytoluene) (PubChem CID: 31404), DPPH˙ (2,2-diphenyl-1-picrylhydrazyl) (Code/ PubChem CID: 2735032), Folin-Ciocalteau’s reagent, gallic acid (PubChem CID: 370), potassium persulfate (PubChem CID: 24412), quercetin (Code/PubChem CID: 5280343), potassium ferricyanide (Code/ PubChem CID: 26250), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) (PubChem Substance ID 24854347) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) standards of phenolics and flavonoids, and other chemicals and solvents were further of reasonable evaluation.
Measurement of total flavonoid (TF) content
The number of flavonoids was measured depending on the aluminum chloride (AlCl3) compound57. 1 ml of each propolis extract or bee venom, 4 ml of distilled water, and 0.3 ml of 5 percent of NaNO2 were mixed thoroughly. 0.30 ml of 10% of AlCl3 was added after a 5 min incubation at room temperature. In the sixth minute of incubation, 2 ml of 1 M NaOH is added, and the mixture was formulated in distilled water with a total volume of up to 10 ml and mixed well. Using a “Unicum UV-300” Spectrophotometer, at 510 nm, the absorbance was determined. Total flavonoids have been documented as dry weight (QE/g) mg quercetin/g, and findings represented as mean values (Mn) ± standard deviation (SD).
HPLC examination of composites for phenolics and flavonoids
Using the slightly changed method adopted by a study of58, the phenolic compounds and flavonoids present in propolis extract were analyzed. 1 gm of propolis was dissolved in 10 ml of ethanolic alcohol at 99% concentration for an hour at room temperature. Each mixture was subsequently ultrasonicated for half an hour and then centrifuged at "10,000 × g" for 5 min. Propolis was filtered, let it evaporate and then dry, and then re-dissolve it in 1 ml of deionized water. Immediately before injecting it into the HPLC, the collected extraction supernatant was filtered through a 0.45 μm Whatman® GF/F membrane. A GBC Scientific Equipment, LC-1110 Pump, Australia and a Kromasil column (Eka Chemical Inc., Bohus, Sweden) were used to conduct chromatographic separations. Liquid chromatographic testing was conducted using water–acetic acid as a mobile phase at concentration (99:1, volume/volume) and water-acetonitrile-acetic acid as a mobile phase B at concentration (67:32:1, volume/volume/volume) in slope mode (0–10 min: 90% A and 10% B, 10–16 min: 80% A and 20% B, 16–20 min: “60% A and 10% B, 10–16 min: 80% A and 20% B, 16–20 min: 60% A. Notably, the flow rate was 0.8 and 1 ml/min, respectively for flavonoids and phenols. The injected sample volume was 20 μl and the separation was done at 25° C. Then, at 280 nm, the phenols were detected while the flavonoids were tested with the GBC-UV/Vis at 356 nm. By examining their retention times with those of absolute norms, the description of phenolics, and flavonoids were examined. Chromatogram peaks were investigated using the chromatographic programme WinCrome V1.3. Flavonoid and phenolic acid quantities were represented as microgram per gram “μg/g DW”.
Screening of bioactive compounds in propolis and BV by UPLC/ESI–MS
The quantitative of bioactive compounds were identified by using Ultra-performance liquid chromatography (UPLC) with electrospray ionization quadrupole- linear ion trap tandem mass spectrometry analysis. Where the UPLC/ESI–MS positive and negative ion acquisition modes were performed on a XEVO TQD triple quadrupole instrument. The samples were dissolved in HPLC grade methanol, filtered through a 0.2 μm membrane disc filter, and resulting solution concentrations were in the range of 0.2 to 0.5 mg/mL, depending on each crude extract. The UPLC system was a mass spectrometer, Waters Corporation, Milford, USA. LC Conditions: Column: ACQUITY UPLC—BEH C18 1.7 µm—2.1 × 50 mm Column. Flow rate: 0.2 mL/min. Solvent system: consisted of water containing 0.1% formic acid and Acetonitrile containing 0.1% formic acid. Gradient: Start with 5% B, at 30 min 50% B, Flow rate: 0.2 mL/min, Column temperature: 40 °C and Injection volume: 10 μl. Electrospray ionization (ESI) was performed in both negative and positive ion modes to obtain more data. The analysis was set using negative ion mode as follows: source temperature 150 °C, cone voltage 30 eV, capillary voltage 3 kV, desolvation temperature 440 °C, cone gas flow 50 L/h, and desolvation gas flow 900 L/h. Mass spectra were detected in the ESI between m/z 100 and 1000 atomic mass units. Chemical constituents were identified by their ESI–QqQLIT–MS/MS spectra and fragmentation patterns. The peaks and spectra were processed using the Mass Lynx 4.1 software and tentatively identified by comparing their retention time (Rt) and mass spectrum with reported data and library search (such as Mass Bank (https://massbank.eu/MassBank).
Antioxidant assays in vitro
DPPH radical scavenging activity
The strategy portrayed by Chu et al.59 was implemented to survey the DPPH˙ (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activities of the fluid concentrate of propolis and honey bee venom. Summarily, 1 ml of each concentrate was added to 0.5 ml of 100 µM DPPH˙ arrangement (broke up in methanol). The blend was stirred well to be homogeneous and permitted to settle at room temperature within half an hour. The absorbance was estimated at 515 nm utilizing Unicum UV-300 UV/Vis spectrophotometer. Butylated hydroxytoluene (BHT), commercially convenient cell reinforcement, was utilized as positive control while the negative control just contained DPPH˙ and ethanol or distilled water.
As for the data evaluation and calculation stage, it was expressed as a DPPH˙% radical scavenging activity, and by the end of the evaluation phase it was calculated as follows:
Where AC is the absorbance of the control reaction, and AS is the absorbance of the bee venom and propolis extract. All assessments in three replicates were performed.
The lower the absorption of the reaction mixture, the higher the anti-radical efficiency.
Radical curbing activity of ABTS+ [2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)]
The test was conducted by Arnao et al.60. In brief, ABTS radical cations (ABTS.+) were created by responding to the solid oxidizing specialist potassium persulfate (2.6 mM) with ABTS salt (7.4 mM). The reaction was made by combining equivalent volumes (five ml) of the two reagents and kept in dimness for 12–16 h at room temperature. After that, the solution was diluted by combining 60 ml methanol to 1 ml of ABTS.+ radical cations with acquiring an absorbance estimation of 1.1 ± 0.02 at 734 nm utilizing Unicum UV-300 UV/Vis spectrophotometer. Approximately 150 μl of each examined substance was permitted to respond with 2850 μl of the newly arranged ABTS.+ radicals for two hours in obscurity and at room temperature. The absorbance was estimated at 734 nm. Trolox was utilized as a positive control. Data were communicated as ABTS.+ % and defined as the following:
Where AC is the absorbance of the control, and AS is the absorbance of the propolis extract.
Ferric-reducing antioxidant power (FRAP)
The reducing capacity of bee venom and ethanolic extract of propolis were calculated using the Kuda et al. process61. 1 ml of each diluted specimen (propolis extract or BV) was blended completely with 2.5 ml of 50 mM phosphate support (pH 6.6), and 2.5 ml of 1% potassium ferricyanide. After the hatching of the blend at 50 ºC for 20 min. Then a solution of about 2.5 ml of 10% trichloroacetic acid was added, and this mixture was centrifuged at 3000 × g for 10 min. The top layer was collected at 1.25 ml and mixed in 1.25 ml of deionized H2O2 as well as 0.25 ml of 0.1% FeCl3, and the absorbance was assessed at 700 nm using a Unicum UV-300 UV/Vis spectrophotometer. The test was completed, its precision was established by performing it in triplicate, and the results were inferred from the Mn (μg/ml) ± SD.
Animal experimentations (in vivo)
Animals' protocol of care and ethics
In the present examination, forty-eight Wistar male rats were used. The rats were obtained from the Nuclear Research Center's animal house in Cairo, Egypt. In plastic rat cages supplied with food and water ad libitum, rats weighing 180 ± 10 g (at aged of 3–4 months), were maintained under ordinary experimental conditions (25 ± 3 °C temperature, RH ≈ 65%, and 12/12 h light/dark cycle).
The protocol was revised and approved by the Ethics Research Committee of National Center for Radiation and Technology- Egyptian Atomic Energy Authority Cairo, Egypt (REC-NCRRT-34A/21). This research was done in compliance with the ARRIVE guidelines and regulations (https://arriveguidelines.org).
Gamma-irradiation
The Gamma cell-40 irradiation unit was supported with a gamma-cesium-137 (137Cs) radiation resource at the National Center for Radiation Science and Technology, Cairo, Egypt. At a rate equivalent to 0.47 Gy/min, the experimental animals were exposed to a single radiation dose of 6 Gy.
Acute toxicity (LD50) test for the bee venom (BV), and propolis extract
The lethal toxicity of 50% of experimental animals (LD50) was ascertained following a strategy62. Briefly, thirty rats were primarily used to investigate the acute toxicity of propolis and BV (orally and intraperitoneally, respectively) and were split into two major groups for each material.
The rats were divided into three subgroups in the first category, with five individuals each. These rats were treated orally for propolis and intraperitoneally (IP) in bee venom at: stage one [low doses: 10.0, 100.0, and 1000.0 mg/kg b.wt. (Propolis)], and 0.02, 0.05, 0.08 (BV) concentrations, and were observed for mortality for two weeks. The second group of rats was also divided into three subgroups and treated at higher doses with propolis extract, i.e., stage 2: 1500, 2000, and 5000 mg/kg b.wt, and BV. Animals were observed for two weeks, and the ultimate LD50 value was calculated as follows:
Where M0 is the highest concentration (dose) with survival effects of the propolis extract and venom, and M1 is the minimum death-causing extract concentration.
Design of experiments
The rats in this experiment were divided into eight groups, each group comprised six rats. Group one representing the non-treated control group (G1). The second group (G2) rats that were irradiated with a single dose of TBI at a dose of 6 Gy to stimulate oxidative stress. The third group (G3) represented rats treated with ethanolic extract of propolis at a dose (300 mg/kg b.wt.) orally63 for two consecutive weeks, and on the 16th day they exposed to a single dose of TBI at 6 Gy. The fourth and fifth subgroups (G4) represented the rats administrated intraperitoneally (IP) the BV at a concentration of 0.05 mg/kg b.wt.11 for two consecutive weeks. Also, on the 16th day rats exposed to γ-irradiation similar to the second and third group. The fifth group (G5) represented the rats administrated the propolis extract (300 mg/ kg) plus bee venom at a concentration of 0.05 two weeks before exposure to γ-irradiation as the same manner of G3 &4). The sixth group (G6) normal rats treated with ethanolic extract of propolis at a dose (300 mg/kg b.wt.) orally for two weeks. The seventh group (G7) normal rats administrated intraperitoneally (IP) the BV at a concentration of 0.05 mg/kg b.wt. for two consecutive weeks. The eighth group (G8) the animals treated with combined propolis 300 mg/kg orally and BV 0.05 mg/kg IP for 2 weeks.
The rats (irradiated groups) were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg) and chlorpromazine (10 mg/kg)64, and then the rats in groups 2 to 5 were exposed to a whole-body gamma radiation dose of 6 Gy.
To confirm the oxidative stress due to the exposure to γ-rays, and also to appraise the implied efficacy of the propolis and bee venom used in this study, the blood samples were taken from the orbital venous plexus on the 7th day post-irradiation. Rats were benumbed with Na-pentobarbital (60 mg/kg I.P.). The blood samples were collected from all animals and apportioned into two portions, one portion was taken in EDTA containing tubes and used for antioxidant enzymes, lipid peroxidation, P53 analyses and apoptotic indexes such as BAX and BCL2. The other portion of blood was left in clean tubes at room temperature to clot, after an hour, then serum was segregated by centrifugation at 3000 × g for 15 min, divided into aliquots and then stored at −20 °C for further biochemical assays.
Peroxidation of lipid and nitric oxide (NO)
Throughout the generation of malondialdehyde (MDA), lipid peroxidation was achieved in plasma and tissues, and was accomplished through a reactive Thiobarbituric acid technique by an approach recently acquainted by Ohkawa et al.65 . Besides, the indirect calorimetric technique (BioAssay pack) was used to evaluate plasma NO.
Serum transaminases, and ALP
Kits from Biovision (Avenue, USA) were used for the examination of serum, alanine and aspartate aminotransferase (ALT & AST respectively), and alkaline phosphatase (ALP).
Total protein, albumin, urea, and creatinine
According to the method of Gornall et al.66 the total protein evaluation was carried out. Albumin was also determined in the serum of rats according to Doumas67. Besides, a serum urea and creatinine analyses were performed according to the method of Barham & Trinder68.
Plasma antioxidant enzymes
In the current study, the antioxidant activities of GSH, GPX, CAT, and SOD were examined in the according to the methodology of Tietze69, Rotruck et al.70, and Kakkar et al.71 respectively.
Molecular detection of miRNA125b in serum
The mirVana™ PARIS™ Kit is for the purification of both native protein and RNA (including small RNA) (Ambion, Life Technologies, #AM1556). To sequester miRNA from serum, briefly, 100% ethanol was added for samples to attain a concentration of 25% ethanol. The large RNAs are immobilized at the point where the serum/ethanol mixture passes through a fiberglass channel, and the small RNA species are gathered in the filter. Then the ethanol affinity for the filter is extended to 55%, and as the small RNA is frozen or immobilized, it disappears through a fiberglass tube. This RNA is washed a few times, and discarded in the low ionic content order. Utilizing this novel methodology comprising of two consecutive filtrations with various ethanol concentrations, miRNA (< 200 nt) can be obtained. Briefly, a serum sample volume of 400 μl was mixed at room temperature of 2× Denaturing Solution. Then a volume of 800 μl acid-Phenol: Chloroform (equal to the total volume of the sample plus the 2× Denaturing Solution) was added and the mixture was vortexed for 60 s and then centrifuged at 1000 × g for 5 min at room temperature to split up he blend into watery (upper) and organic (lower) stages. Without stirring the lower phase or the interphase, the upper the phase was purposefully skipped and moved to a new tube. 1/3 volume of 100% ethanol was added to the recouped fluid stage and blended completely before being moved into a Filter Cartridge. The filter Cartridge (FC) like a channel comprising tubes was centrifuged for thirty seconds or until the blend has gone through the channel. A hundred percent ethanol was applied to the filtrate at room temperature of 2/3 volume. For example, if 400 μl of filtrate has been retrieved, it is appropriate to add 266 μl of 100 percent ethanol. The filtrate/ethanol blend was gone during a time FC, yet this time the course through was disposed of. A volume of 700 μl of miRNA of washing Solution 1 was used on the filter cartridge, and the blend was centrifuged for approximately fifteen seconds. The flow was removed from the collector tube, and a similar collection tube was replaced with the Filter Cartridge. As in the previous method, a volume of 500 μl Wash Solution 2/3 was added and was drawn through the FC. After abandoning the flow-through from the last wash, the Filter Cartridge was substituted in a similar Collection Tube and the gathering was centrifuged for 1 min to eliminate leftover liquid from the channel. Finally, the Filter Cartridge was moved into a new Collection Tube and 100 μL of preheated (95 °C) Elution Solution or nuclease-free water was applied to the focal point of the filter and centrifuged for ~ 30 s to recuperate the RNA.
cDNA synthesis
Quanti-Mir RT kit (SBI, System Biosciences, and Cat. # RA420A-1) which depends on the poly (A) method. In this strategy, a poly (A) tail is added to the 3' finish of each develop miRNA done by poly (A) polymerase. Tailed miRNAs are then exposed to RT utilizing an all-inclusive RT preliminary containing 2 to 3 savage nucleotides at 3' end followed by an oligo (dT) and widespread opposite groundwork grouping. The incorporated cDNA is intensified with explicit forward and widespread opposite preliminaries. In nuclease-free tube 5 μl miRNA (1–5 µg) was combined with 2 μl 5× PolyA Buffer, 0.5 μl PolyA Polymerase, 1 μl 25 mM MnCl2, and 1.5 μl 5 mM ATP. The blend was incubated at the optimal temperature of 37 °C for half an hour. A volume of 0.5 μl Oligo dT Adaptor was annexed and the mixture was heated at 60 °C for 5 min and then left to chill for 2 min at room temperature. The following components were added to the mixture; 4 μl of 5× RT Buffer, 2 μl dNTP mix, 1.5 μl 0.1 M DTT, 1.5 μl RNase free water, and 1 μl reverse transcriptase to give a total volume of 20.5 μl mixture. The latter was incubated at 42 °C for 60 min and was heated at 95 °C for 10 min.
Real time PCR quantitative (qPCR)
Constant PCR with SYBR Green was used to test the serum of miRNA125b, with the internal reference being U6. Using 2× Maxima SYBR Green / ROX qPCR Master Mix, the separated cDNA was escalated after the maker demonstrate (Thermo consistent, USA, # K0221) and miRNA expression forward readiness (Table 7) and an inescapable primer included with the Quanti-Mir RT device. To guarantee preliminary succession is interesting for the layout arrangement; we verified compatibility with other known groupings by BLAST (www.ncbi.nlm.nih.gov/blast/Blast.cgi).
Table 7.
The oligonucleotide primers sequence of studied genes for BCL2, BAX, P53 & miRNA-125b.
| BCL2 gene | Forward primer: 5’ CGGGAGAACAGGGTATGA 3’ Reverse primer: 5’ CAGGCTGGAAGGAGAAGAT 3’ |
|---|---|
| BAX gene |
Forward primer: 5’ GTTGCCCTCTTCTACTTTG 3’ Reverse primer: 5’ AGCCACCCTGGTCTTG 3’ |
| P53 gene |
Forward: 5′-GTTCCGAGAGCTGAATGAGG-3′ Reverse: 5′-TTTTATGGCGGGACGTAGAC-3′ |
| GAPDH |
Forward 5’ TGCTGGTGCTGAGTATGTCG 3’ Reverse 5’ TTGAGAGCAATGCCAGCC 3’ |
|
miRNA-125b U6 |
/5 TCCCTGAGACCCTAACTTGTGA /3 /5 CTCGCTTCGGCAGCACA/3 |
Primer’s preparation
As per the assembling guidelines, preliminaries were set up as the accompanying: Lyophilized preliminary at − 20 °C was equilibrated at room temperature. At that point, an equilibrated preliminary was turned down for 3 s using the swirl of the turn vortex. Lyophilized preliminary with RNase free water was weakened (both forward and turn around) (the volume was added to get 100 μM stock) and the cylinder was subsequently altered at room temperature for 2 min. With RNase free water support (pH 8.0), the preliminary stock was weakened to obtain 5 μM and held at− 20 °C until used. There was a 30-μl polymerase chain response blend. In a Phase One Plus constant warm cycler (Applied Biosystems, Life innovation, USA), the last response blend was placed, and the PCR application was performed in Table 7 with the PCR stipulations as nitty–gritty. Toward the finish of the latest sequence, the temperature was expanded from 60 to 95 °C to deliver a liquefy bend. The housekeeping quality (miRNA16) spoke to as standardize that utilized to compute the relativistic quality articulation or overlay change in target quality The specific quantity limit (Ct) of target quality was then standardized with quantities (Ct) of household quality (U6) using the 2-Ct technique72 . In brief, the benchmark community was used as a calibrator, while in both objective and reference quality, numerous gatherings spoke as test bunches. In both the experimental groups and the benchmark group, the edge cycle numbers (Ct) of target quality were standardized to that of reference quality:
- The ∆Ct of the test genes were normalized to the ∆Ct of the calibrator:
Fold change of relative gene expression was calculated as follow: Fold change = (2−∆∆Ct).
Gene expression for BAX, BCL2 and P53
RNA extraction
The total RNA was confined to plasma using the RNA simple purging reagent (Qiagen, Germany), which was strictly and consistently followed by the product instructions. RNA assembly, using spectrophotometry, was obtained by gel electrophoresis (Gene Quant 1300, Uppsala, Sweden).
The synthetization of cDNA
Using the basic Oligo (dT)12–18 and Superscript II RNase Reverse Transcriptase, the main strand cDNA was mixed from 4 μg of hard and fast RNA, this mix was generated at 42 °C for 1 h, the pack was given by (Life Technologies, Breda, the Netherlands).
Quantitative polymerase chain reaction in real time (RT-PCR)
RT-PCR intensification was allocated with 10 μl rejuvenation blends consisting of SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA USA), equivalent to 8 ng of converse translated ribonucleic acid and preliminary 300 nM.
ABI PRISM 7900 HT revelation structure (Applied Biosystems) PCR responses, consisting of 95 °C for 10 min (1 cycle), 94 °C for 15 s, and 60 °C for 1 min (40 cycles) reactions were performed, data was settled with the programming of the ABI Prism plan affirmation method and evaluated using the PE Biosystems (Foster City, CA) v1·7 Sequence Detection Software. Using the relative limit period technique, we determined the complete articulation of qualities. Besides, glyceraldehyde-3-phosphate dehydrogenase (GADPH) has been standardized for all characteristics72 . The series of primers are reported in Table 7.
Histological studies
Samples of the liver, kidney, heart, and brain were taken from the control and experimental groups, and formalin was stabilized for about 24 h at 10 percent. All samples were then washed for half an hour in tap water and then dried with progressive alcohol levels (70, 80, & 90 percent, absolute ethanol). Samples were wiped with xylene, impregnated at a temperature of 55 °C with paraffin wax, and these slices were divided into 4 μM, then were stained73.
The histopathological alterations scoring was performed in the liver, kidney, heart, and brain of different experimental groups. Scored was between 0 and 3 (where (0) denotes no change and 1, 2, and 3 indicate mild, moderate, and severe changes, respectively74.
Statistical examination
The results were described as mean ± standard error (S.E.) for “six” animals utilizing SPSS version 24 (SPSS, Cary, NC, USA). Variations between bunches were surveyed by one-way analysis of variance (ANOVA), when diversity was notable followed by Duncan’s test for multiple comparisons between groups.
Statement of ethics
The guidelines for the ethical use and maintenance of laboratory animals issued by the National Institutes of Health and authorized by the Scientific Committee of the Nuclear Research Center, Atomic Energy Authority, Cairo, Egypt, were complied with in all procedures used in caring for rats and taking blood and tissue samples for this experiment.
Conclusions
We concluded that pretreatment with propolis and BV has a significant antioxidant property against ionizing radiation. This protective potential restrains the injury that occurs to vital tissues because of radiation exposure. Besides, the histopathology confirmed the biochemical and molecular data, which are supporting the presumption that the efficacy of bee products has a substantial potential to frustrate and obstruct free radicals generated by ionizing radiation, leading to several undesired consequences. Future studies concerning bee products could be a promising adjuvant in treating oxidative stress-related disorders or diseases in humans.
Acknowledgements
The authors would like to express their sincere gratitude to Prof. Dr. Adel Bakeer, Professor of Pathology at the University of Cairo, Faculty of Veterinary Medicine, for his professional assistance in conducting the histopathological test. The authors also appreciate Angie Kamel for supplying them with propolis and bee venom.
Abbreviations
- BV
Bee venom
- TBI
Total body irradiation
- UPLC/ ESI–MS
Ultra-performance liquid chromatography–electrospray ionization–tandem mass spectrometry
- 8-OHdG
8-Hydroxy-2'-deoxyguanosine
- ELISA
The enzyme-linked immunosorbent assay
- GSH
Glutathione
- CAT
Catalase
- SOD
Superoxide dismutase
- GPx
Glutathione peroxidase
- P53
Tumor suppressor protein
- miR125b
MicroRNAs
- HPLC
High-performance liquid chromatography
- IR
Ionizing radiation
- ROS
Reactive oxygen species
- KB
Human oral human melanoma cells
- Caco-2
Colon adenocarcinoma cells
- DU-145
Prostate cancer cells
- UV
Ultraviolet
- IgE
Immunoglobulin E
- AlCl3
Aluminum chloride
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- BHT
Butylated hydroxytoluene
- ABTS+
2,2'-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid
- FRAP
Ferric-reducing antioxidant power
- EDTA
Ethylene diamine tetra-acetic acid
- NO
Nitric oxide
- MDA
Malondialdehyde
- ALP
Alkaline phosphatase
- AST
Aspartate aminotransferase
- ALT
Alanine aminotransferase
- EtOH
Ethanol
Author contributions
All authors conceived and designed the experiment performed all the in vivo experiments. E.K.E.A. and A.I.H. performed the in vivo experiments and data management and statistics. M.M.D. performed all the in vitro assays and all authors contributed in writing and reviewing the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-024-59116-1
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/17/2024
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41598-024-59116-1
References
- 1.Jaeger A, Weiss DG, Jonas L, Kriehuber R. Oxidative stress-induced cytotoxic and genotoxic effects of nano-sized titanium dioxide particles in human HaCaT keratinocytes. Toxicology. 2012;296:27–36. doi: 10.1016/j.tox.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Ping, Z. et al. Oxidative stress in radiation-induced cardiotoxicity. Oxid. Med. Cell. Longev.2020 (2020). [DOI] [PMC free article] [PubMed]
- 3.Abou-Zeid SM, El-Bialy BE, El-Borai NB, AbuBakr HO, Elhadary AMA. Radioprotective effect of date syrup on radiation-induced damage in rats. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-25586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Üstün K, et al. Radio-protective effects of Nigella sativa oil on oxidative stress in tongue tissue of rats. Oral Dis. 2014;20:109–113. doi: 10.1111/odi.12082. [DOI] [PubMed] [Google Scholar]
- 5.Poljsak, B., Šuput, D. & Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev.2013 (2013). [DOI] [PMC free article] [PubMed]
- 6.Materska M, Konopacka M, Rogoliński J, Ślosarek K. Antioxidant activity and protective effects against oxidative damage of human cells induced by X-radiation of phenolic glycosides isolated from pepper fruits Capsicum annuum L. Food Chem. 2015;168:546–553. doi: 10.1016/j.foodchem.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Surai PF, Kochish II, Fisinin VI, Kidd MT. Antioxidant defence systems and oxidative stress in poultry biology: An update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azzam EI, Jay-Gerin J-P, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo A, et al. Chilean propolis: Antioxidant activity and antiproliferative action in human tumor cell lines. Life Sci. 2004;76:545–558. doi: 10.1016/j.lfs.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Angelo G, Lorena C, Marta G, Antonella C. Biochemical composition and antioxidant properties of Lavandula angustifolia Miller essential oil are shielded by propolis against UV radiations. Photochem. Photobiol. 2014;90:702–708. doi: 10.1111/php.12229. [DOI] [PubMed] [Google Scholar]
- 11.Hanafi MY, et al. The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp. Ther. Med. 2018;15:5091–5099. doi: 10.3892/etm.2018.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpena M, Nuñez-Estevez B, Soria-Lopez A, Simal-Gandara J. Bee venom: An updating review of its bioactive molecules and its health applications. Nutrients. 2020;12:3360. doi: 10.3390/nu12113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abd El-Wahed, A. A. et al. Bee venom composition: From chemistry to biological activity. in Studies in Natural Products Chemistry. Vol. 60. 459–484. (Elsevier, 2019).
- 14.Bellik Y. Bee venom: Its potential use in alternative medicine. Anti-Infect. Agents. 2015;13:3–16. doi: 10.2174/2211352513666150318234624. [DOI] [Google Scholar]
- 15.Andrade ER, et al. Evaluation of the potential protective effects of ad libitum black grape juice against liver oxidative damage in whole-body acute X-irradiated rats. Food Chem. Toxicol. 2011;49:1026–1032. doi: 10.1016/j.fct.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Ristivojević P, Trifković J, Andrić F, Milojković-Opsenica D. Poplar-type propolis: Chemical composition, botanical origin and biological activity. Nat. Prod. Commun. 2015;10:1934578X1501001117. [PubMed] [Google Scholar]
- 17.Ahangari Z, Naseri M, Vatandoost F. Propolis: Chemical composition and its applications in endodontics. Iran. Endod. J. 2018;13:285. doi: 10.22037/iej.v13i3.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurek-Górecka A, et al. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2014;19:78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang H, et al. Characterization of Chinese crude propolis by pyrolysis–gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis. 2015;113:158–164. doi: 10.1016/j.jaap.2014.12.006. [DOI] [Google Scholar]
- 20.Chandna, P., Adlakha, V. K., Das, S. & Singh, S. Complementary and alternative medicine (CAM): A review of propolis in dentistry. Technology4 (2014).
- 21.Anjum SI, et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019;26:1695–1703. doi: 10.1016/j.sjbs.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moise AR, Bobiş O. Baccharis dracunculifolia and Dalbergia ecastophyllum, main plant sources for bioactive properties in green and red Brazilian propolis. Plants. 2020;9:1619. doi: 10.3390/plants9111619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koudoufio M, et al. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants. 2020;9:982. doi: 10.3390/antiox9100982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos GS, Tsutsumi S, Vieira DP, Bartolini P, Okazaki K. Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with 60Co gamma-radiation. Mutat. Res. Toxicol. Environ. Mutagen. 2014;762:17–23. doi: 10.1016/j.mrgentox.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Cui F, Nie J, Li J, Tong J. Experimental study of propolis on anti-radiation protective effect in mice. Ind. Heal. Occup. Dis. 2006;32:283. [Google Scholar]
- 26.Asadi N, Bahmani M, Kheradmand A, Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagnostic Res. (JCDR) 2017;11:IE01. doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Guo C, Kong J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012;7:376. doi: 10.3969/j.issn.1673-5374.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdel-Daim, M. M. et al.Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. (2018). [DOI] [PMC free article] [PubMed]
- 29.Al Naggar, Y. et al. Fighting against the second wave of COVID-19: Can honeybee products help protect against the pandemic? Saudi J. Biol. Sci. (2020). [DOI] [PMC free article] [PubMed]
- 30.Cianciosi D, et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules. 2018;23:2322. doi: 10.3390/molecules23092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegazi, A. G. & Abd El Hady, F. K. Egyptian propolis: 3. Antioxidant, antimicrobial activities and chemical composition of propolis from reclaimed lands. Z. Naturforsch. C57, 395–402 (2002). [DOI] [PubMed]
- 32.Belinha I, et al. Quercetin increases oxidative stress resistance and longevity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2007;55:2446–2451. doi: 10.1021/jf063302e. [DOI] [PubMed] [Google Scholar]
- 33.Bai H, et al. Enhanced antioxidant effect of caffeic acid phenethyl ester and Trolox in combination against radiation induced-oxidative stress. Chem. Biol. Interact. 2014;207:7–15. doi: 10.1016/j.cbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 34.Mohdaly AAA, Mahmoud AA, Roby MHH, Smetanska I, Ramadan MF. Phenolic extract from propolis and bee pollen: Composition, antioxidant and antibacterial activities. J. Food Biochem. 2015;39:538–547. doi: 10.1111/jfbc.12160. [DOI] [Google Scholar]
- 35.Srinivasan M, Sudheer AR, Rajasekaran KN, Menon VP. Effect of curcumin analog on γ-radiation-induced cellular changes in primary culture of isolated rat hepatocytes in vitro. Chem. Biol. Interact. 2008;176:1–8. doi: 10.1016/j.cbi.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Jagetia GC, Rajanikant GK, Rao SK, Baliga MS. Alteration in the glutathione, glutathione peroxidase, superoxide dismutase and lipid peroxidation by ascorbic acid in the skin of mice exposed to fractionated γ radiation. Clin. Chim. Acta. 2003;332:111–121. doi: 10.1016/S0009-8981(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 37.Goh VSL, Mok C-K, Chu JJH. Antiviral natural products for arbovirus infections. Molecules. 2020;25:2796. doi: 10.3390/molecules25122796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasozi KI, et al. Bee venom—A potential complementary medicine candidate for SARS-CoV-2 infections. Front. Public Heal. 2020;8:755. doi: 10.3389/fpubh.2020.594458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, et al. Quantification of melittin and apamin in bee venom lyophilized powder from Apis mellifera by liquid chromatography–diode array detector–tandem mass spectrometry. Anal. Biochem. 2010;404:171–178. doi: 10.1016/j.ab.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Guha M, et al. Melatonin inhibits free radical-mediated mitochondrial-dependent hepatocyte apoptosis and liver damage induced during malarial infection. J. Pineal Res. 2007;43:372–381. doi: 10.1111/j.1600-079X.2007.00488.x. [DOI] [PubMed] [Google Scholar]
- 41.Shah KG, et al. Human ghrelin ameliorates organ injury and improves survival after radiation injury combined with severe sepsis. Mol. Med. 2009;15:407–414. doi: 10.2119/molmed.2009.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, Q. et al. The relationship between the Bcl-2/Bax proteins and the mitochondria-mediated apoptosis pathway in the differentiation of adipose-derived stromal cells into neurons. PLoS One11, e0163327 (2016). [DOI] [PMC free article] [PubMed]
- 43.Popgeorgiev N, Jabbour L, Gillet G. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front. cell Dev. Biol. 2018;6:13. doi: 10.3389/fcell.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ransohoff JD, Wei Y, Khavari PA. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. cell Biol. 2018;19:143–157. doi: 10.1038/nrm.2017.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng Y, et al. MiR-181b promotes chemoresistance in breast cancer by regulating Bim expression. Oncol. Rep. 2016;35:683–690. doi: 10.3892/or.2015.4417. [DOI] [PubMed] [Google Scholar]
- 46.Chakravarty G, et al. Nelfinavir targets multiple drug resistance mechanisms to increase the efficacy of doxorubicin in MCF-7/Dox breast cancer cells. Biochimie. 2016;124:53–64. doi: 10.1016/j.biochi.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Hu G, et al. miR-125b regulates the drug-resistance of breast cancer cells to doxorubicin by targeting HAX-1. Oncol. Lett. 2018;15:1621–1629. doi: 10.3892/ol.2017.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hua S, et al. miR-125b-5p inhibits cell proliferation, migration, and invasion in hepatocellular carcinoma via targeting TXNRD1. Cancer Cell Int. 2019;19:203. doi: 10.1186/s12935-019-0919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sengupta S, et al. MicroRNA 92b controls the G1/S checkpoint gene p57 in human embryonic stem cells. Stem Cells. 2009;27:1524–1528. doi: 10.1002/stem.84. [DOI] [PubMed] [Google Scholar]
- 50.Ghosh SP, et al. Gamma-tocotrienol modulates radiation-induced microRNA expression in mouse spleen. Radiat. Res. 2016;185:485–495. doi: 10.1667/RR14248.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mimura N, Hideshima T, Anderson KC. Novel therapeutic strategies for multiple myeloma. Exp. Hematol. 2015;43:732–741. doi: 10.1016/j.exphem.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacombe J, Zenhausern F. Emergence of miR-34a in radiation therapy. Crit. Rev. Oncol. Hematol. 2017;109:69–78. doi: 10.1016/j.critrevonc.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jeong S-H, Wu H-G, Park W-Y. LIN28B confers radio-resistance through the posttranscriptional control of KRAS. Exp. Mol. Med. 2009;41:912–918. doi: 10.3858/emm.2009.41.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones MF, Lal A. MicroRNAs, wild-type and mutant p53: More questions than answers. RNA Biol. 2012;9:781–791. doi: 10.4161/rna.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kregel KC, Zhang HJ. An integrated view of oxidative stress in aging: basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol. Integr. Comp. Physiol. 2007;292:R18–R36. doi: 10.1152/ajpregu.00327.2006. [DOI] [PubMed] [Google Scholar]
- 56.Nna, V. U., Bakar, A. B. A., Lazin, M. R. M. L. M. & Mohamed, M. Antioxidant, anti-inflammatory and synergistic anti-hyperglycemic effects of Malaysian propolis and metformin in streptozotocin-induced diabetic rats. Food Chem. Toxicol.120, 305–320 (2018). [DOI] [PubMed]
- 57.Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 58.Rodríguez-Bernaldo de Quirós A, Lage-Yusty MA, López-Hernández J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010;121:634–638. doi: 10.1016/j.foodchem.2009.12.078. [DOI] [Google Scholar]
- 59.Chu Y, Chang C, Hsu H. Flavonoid content of several vegetables and their antioxidant activity. J. Sci. Food Agric. 2000;80:561–566. doi: 10.1002/(SICI)1097-0010(200004)80:5<561::AID-JSFA574>3.0.CO;2-#. [DOI] [Google Scholar]
- 60.Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- 61.Kuda T, Tsunekawa M, Goto H, Araki Y. Antioxidant properties of four edible algae harvested in the Noto Peninsula, Japan. J. Food Compos. Anal. 2005;18:625–633. doi: 10.1016/j.jfca.2004.06.015. [DOI] [Google Scholar]
- 62.Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54:275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 63.Muhammad MMA, Mouchira M, Naglaa RA. Physiological effects of bee venom and propolis on irradiated Albino rats. Danish J. Agric. Anim. Sci. 2015;15:11–21. [Google Scholar]
- 64.Mihandoost, E., Shirazi, A., Mahdavi, S. R. & Aliasgharzadeh, A. Consequences of lethal-whole-body gamma radiation and possible ameliorative role of melatonin. Sci. World J.2014 (2014). [DOI] [PMC free article] [PubMed]
- 65.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 66.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177:751–766. doi: 10.1016/S0021-9258(18)57021-6. [DOI] [PubMed] [Google Scholar]
- 67.Doumas BT. Standards for total serum protein assays—A collaborative study. Clin. Chem. 1975;21:1159–1166. doi: 10.1093/clinchem/21.8.1159. [DOI] [PubMed] [Google Scholar]
- 68.Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972;97:142–145. doi: 10.1039/an9729700142. [DOI] [PubMed] [Google Scholar]
- 69.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal. Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 70.Rotruck JT, et al. Selenium: Biochemical role as a component of glutathione peroxidase. Science (80-) 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 71.Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol. Cell. Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- 72.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 73.Chaplin AJ. Manual of Histological Techniques. JD Bancroft and HC Cook. Churchill Livingstone; 1985. [Google Scholar]
- 74.El-Hamoly T, El-Denshary ES, Saad SM, El-Ghazaly MA. 3-aminobenzamide, a poly (ADP ribose) polymerase inhibitor, enhances wound healing in whole body gamma irradiated model. Wound Repair Regen. 2015;23:672–684. doi: 10.1111/wrr.12330. [DOI] [PubMed] [Google Scholar]