Abstract
Treatment of locally advanced rectal cancer includes chemoradiation and surgery, but patient response to treatment is variable. Patients who have a complete response have improved outcomes; therefore, there is a critical need to identify mechanisms of resistance to circumvent them. DNA-PK is involved in the repair of DNA double-strand breaks caused by radiation, which we found to be increased in rectal cancer after treatment. We hypothesized that inhibiting this complex with a DNA-PK inhibitor, Peposertib (M3814), would improve treatment response.
We assessed pDNA-PK in a rectal cancer cell line and mouse model utilizing western blotting, viability assays, γH2AX staining, and treatment response. The three treatment groups were: standard of care (SOC) (5-fluorouracil (5FU) with radiation), M3814 with radiation, and M3814 with SOC.
SOC treatment of rectal cancer cells increased pDNA-PK protein and increased γH2AX foci, but this was abrogated by the addition of M3814. Mice with CT26 tumors treated with M3814 with SOC did not differ in average tumor size but individual tumor response varied. The clinical complete response rate improved significantly with the addition of M3814 but pathological complete response did not. We investigated alterations in DNA repair and found that Kap1 and pATM are increased after M3814 addition suggesting this may mediate resistance.
When the DNA-PK inhibitor, M3814, is combined with SOC treatment, response improved in some rectal cancer models but an increase in other repair mechanisms likely diminishes the effect. A clinical trial is ongoing to further explore the role of DNA-PK inhibition in rectal cancer treatment.
Keywords: DNA-PK, DNA damage, Chemoradiaiton resistance, DNA-PK inhibitor, Rectal cancer
Introduction
Colorectal cancer is the third leading cause of cancer related death in the United States. Rectal cancer makes up one-third of colorectal cancer cases and approximately 45,230 Americans are diagnosed with rectal cancer annually.1 Traditionally, locally advanced rectal cancer treatment includes 5-fluorouracil chemotherapy, radiation, and surgery.2 Neoadjuvant treatment can be difficult for patients and surgery can result in morbidity such as debilitating bowel or urinary dysfunction or a permanent ostomy. This series of treatments has been shown to diminish quality of life for these patients.3 Response to neoadjuvant treatment is variable among patients. Some patients achieve a complete pathological response to their neoadjuvant therapy and these patients have significantly better 5-year disease-free survival rates compared to partial responders.4 Unfortunately, only 15–25% of patients are complete responders.5,6 Recent studies have even shown that it may be safe to omit surgery in these complete responders and non-operative clinical trials are ongoing.7,8 Thus, there is a substantial interest in improving patient response to neoadjuvant chemoradiation therapy to increase the number of complete responders.
Radiation therapy causes double-strand breaks (DSBs) in DNA leading to cancer cell death if not repaired. DNA-dependent protein kinase (DNA-PK) is a complex involved in the repair of DNA DSBs via non-homologous end-joining repair. Studies have shown that when this complex is inhibited, radiation response in glioblastoma cells and non-small cell lung cancer cells is enhanced.9,10 While there have been trials combining targeted agents with standard of care such as EGFR inhibitors, these agents have not improved response rates in rectal cancer.11., 12., 13. Although other mechanisms of DNA repair exist such as via ATM and ATR, we and others have found that DNA-PK is increased after chemoradiation in rectal cancer.14
Inhibitors of DNA repair have not been fully assessed in rectal cancer. We hypothesized that inhibition of DNA-PK mediated DNA repair would improve response to standard 5FU and radiation in rectal cancer. Peposertib, formerly M3814, is a DNA-PK inhibitor and was chosen due to its clinical relevance and selectivity. In this manuscript, we show that phosphorylated DNA-PK (pDNA-PK) is increased after chemoradiation treatment and that this increase is abrogated by addition of a DNA-PK inhibitor both in vitro and in vivo. We found that addition of M3814 increased the number of clinical complete responders in vivo but that there was no significant difference in pathological complete response. In order to try and understand why inhibition of DNA-PK did not improve the pathologic response, we assessed other mechanisms of DNA repair.
Methods
Cell culture
SW837 cell line was obtained from American Type Culture Collection (ATCC). The Leopold lab shared the CT26 cell line. The SW837 cell line was grown in Advanced DMEM with 5% FBS and antibiotic/antimitotic supplements and the CT26 cell line was grown in DMEM with 10% FBS and antibiotic/glutamate supplements (Gibco). All cell lines were verified by short tandem repeat testing by sending samples of cultured cells from the lab to ATCC at regular intervals. Cell lines were used at low passage numbers only (SW837 <15, CT26 <25). For treatment in vitro, there were three treatment groups that were compared to vehicle: standard of care (SOC) (5-fluorouracil (5FU) with 5 Gy radiation), the DNA-PK inhibitor M3814 with 5 Gy radiation, and M3814 with SOC. The protocol used for chemoradiation treatment of all cell lines was as follows: cells were treated with 3 µM 5-fluorouracil (5FU) (Selleckchem) or 10 µM M3814 (CTEP) or combination of both 30 min prior to radiation (5 Gy) (Kimtron Medical IC-320) and media was changed the following day.
Western blotting
SW837 and CT26 cells were lysed in RIPA (radioimmunoprecipitation assay) buffer and Halt protease and phosphatase inhibitor cocktail (Fisher Scientific) 4 h, 1 day or 5 days after treatment. Mouse model tumors were harvested and dissociated 2 h after completion of treatment to assess changes in protein. Samples underwent electrophoresis and protein transfer to Polyvinylidene fluoride (PVDF) membranes and were blocked in 5% nonfat dry milk in 1X Tris buffered saline and 0.1% Tween-20 (TBST). Blots were probed with 1:1,000 dilution of antibodies against pDNA-PK (Abcam), pATM (Novus Biologicals), and Kap1 (Novus Biologicals). Protein loading was verified using 1:10,000 dilution of β-Actin (Abcam). Membranes were incubated with appropriate secondaries and imaged using enhanced chemiluminescence.
Immunofluorescent staining and quantification of γH2AX foci
SW837 cells were plated in 24 well plates on sterile glass coverslips with 125,000 cells/well. Cells were treated according to our chemoradiation protocol. Six hours after treatment, cells were fixed and permeabilized with ice-cold methanol for 10 min. Slides were incubated with γH2AX antibody (EMD Millipore Corp) at a 1:1,000 dilution for one hour at room temperature. Following incubation with primary antibody, the slides were incubated with Alexa488 secondary antibody (Invitrogen) at 1:500 dilution for one hour at room temperature in the dark. Coverslips were mounted on slides using DAPI mounting media (Invitrogen). Five high powered fields per slide manually counted by a blinded assessor for both number of cells (nuclear DAPI positive) and number of γH2AX positive cells. Greater than 10 foci of γH2AX staining in a cellular nucleus was defined as a γH2AX positive cell.
Clonogenic survival assay
Clonogenic assays were performed using standard techniques previously described.15 Cells were plated at varying cell densities, from 1,000 to 100,000 cells. Cells were treated with various doses of M3814 (0.5-15 µM) and various radiation doses (0–10 Gy). Cells were grown for 12 days then the surviving fraction was determined by counting cell colonies. The radiation enhancement ratio was calculated as previously described.16
Cell Titer-Glo Viability Assays
SW837 cells were plated in 96 well plates with 3,000 cells/well. Cells were treated according to our treatment protocol. Media was changed 24 h after treatment and every other day thereafter. Cell Titer-Glo Luminescent Cell Viability Assay reagent (Promega) was added on day 5 in volume equal to the media. Cells were left at room temperature for one hour then luminescence was measured using a plate reader (Infinite M200 Pro, Tecan).
Mouse model studies
All animal studies were approved by the Institute Animal Care and Use Committee (protocol # 21742) of the University of Alabama at Birmingham. This study was carried out in strict accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. CT26 cells were grown in culture in DMEM with 10% FBS media and 1 × 106 cells were subcutaneously implanted into the flank of BALB/c mice (Jackson Laboratories) in accordance with our approved IACUC protocol. Mice were randomized into groups once tumor volume reached 100 mm3 as measured by calipers. Mice were randomly split into the following treatment groups: vehicle, SOC (100 mg/kg capecitabine (LC Laboratory) with 2 Gy radiation), 50 mg/kg M3814 (obtained through the National Cancer Institute, Cancer Therapy Evaluation Program) with 2 Gy radiation, and SOC plus M3814. Capecitabine and/or M3814 were given via oral gavage on radiation treatment days. Mice were placed in a custom gig for shielding and radiation was delivered to tumors via X-RAD 320 (Precision X-Ray Inc.) daily. Mice were treated 5 days per week for 2 weeks. Subcutaneous tumor volume and body weights were measured 3 times per week. A clinical complete response was defined as no measurable or palpable tumor. Animals were euthanized one week after the end of treatment. The area where the tumor had been implanted was harvested including the skin and flank muscle if not grossly visible tumor was found. Pathologic complete response was defined as lack of identification of tumor cells on H and E slides from the harvested area.
Statistical analysis
Western blots were quantified using Image J and analyzed using a Student's T-test when comparing just 2 groups. For assessment of more than 2 groups, an ANOVA was used, followed by pairwise tests for statistical comparison. γH2AX positivity and tumor size were compared using logistical regression. For the Cell Titer-Glo viability assays, measurements of relative light units at 5 Gy for each cell type were converted to fold change values by dividing each 5 Gy measurement by the corresponding 0 Gy mean value of the same cell type within each experiment. Subsequently, the log fold change was fit using a linear mixed model with cell type treated as a fixed effect and experiment as a random effect. The model assessed for a change in viability with radiation. For assessment of tumor response in the in vivo model, a pairwise t-test was used at 14 days with adjustment for multiple testing. Experiments in cell lines were repeated using different passages in at least 3 separate experiments.
Results
Chemoradiation induces DNA damage and increase in pDNA-PK in colorectal cancer cell lines
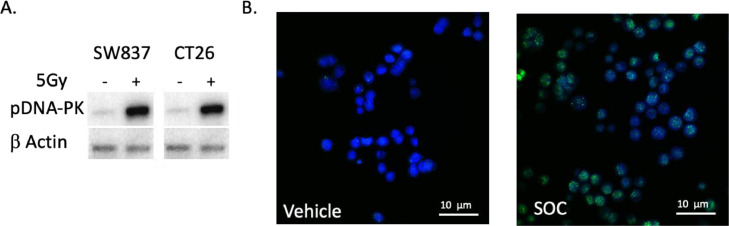
To evaluate the role of pDNA-PK in chemoradioresistance in rectal cancer cells, SW837 and CT26 cells were treated with 5FU and 5 Gy radiation. pDNA-PK was compared between treated and untreated cells 4 h after treatment and treated cells had increased pDNA-PK protein (SW837: 21.0-fold increase compared to vehicle, p = 0.002, CT26: 3-fold-increase compared to vehicle, p < 0.005, Fig. 1A). γH2AX is a marker of DNA damage from radiation that can be assessed via immunofluorescent staining. We found that γH2AX foci positive cells increased from 3.5% to 65.8% at 6 h after chemoradiation in SW837 cells (N = 5 slides per treatment group, N = 3 experiments, p < .001, Fig. 1B). This led to the hypothesis that because rectal cancer cells increase DNA-PK mediated DNA repair after chemoradiation, inhibition of this complex by M3814 would improve the response of these tumor cells to treatment.
Fig. 1.
Chemoradiation induces increase in pDNA-PK and DNA damage in rectal cancer cell lines. A: Western identifies increase in pDNA-pk after chemoradiation in rectal cancer cell lines SW837 and CT26 (N = 4, p < 0.001). B: Increased γH2Ax staining in SW837 rectal cancer cells treated with chemoradiation compared to vehicle indicating increased DNA damage (N = 5 slides per treatment group, N = 3, p < .001).
Inhibition of pDNA-PK improves response to chemoradiation and decreases pDNA-PK in rectal cancer cell line
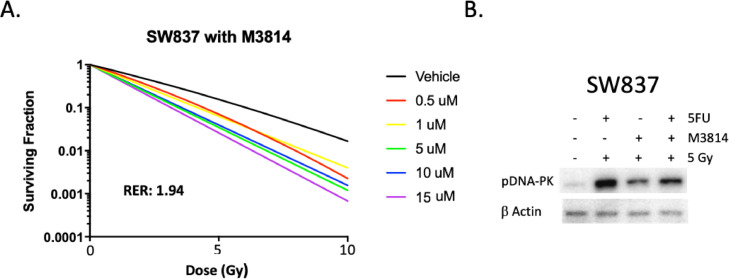
In a standard clonogenic assays in SW837 cells, M3814 was found to be a potent inhibitor of DNA-PK with a radiation enhancement ratio of 1.94 (Fig. 2A). The dose 10 µM was chosen for subsequent studies as this dose elicited the highest radiation enhancement ratio (RER). To assess for pDNA-PK protein, SW837 cells were treated with SOC, M3814 plus radiation, or SOC plus M3814 and were harvested 4 h after treatment. Cells treated with SOC had a 21-fold increase in pDNA-PK compared to vehicle, as expected (Fig. 2B). When SW837 cells were treated with 10 µM M3814 in combination with either radiation alone or SOC, pDNA-PK decreased 8-fold (p = 0.03, N = 3) and 14-fold (p = 0.01, N = 3), respectively.
Fig. 2.
M3814 is a potent radiation sensitizer in SW837 rectal cancer cell line. A: Clonogenic assay of SW837 cells plus addition of M3814. Radiation enhancement ratio for this cell line is 1.94 indicating that M3814 is a radiation sensitizer. B. Western showing that the increase in pDNA-PK after chemoradiation is abrogated with the addition of M3814 in SW837 cell line with radiation alone (p = 0.03, N = 3) and SOC (p = 0.01, N = 3).
M3814 decreases cell viability and increases DNA damage when added to SOC in rectal cancer cells
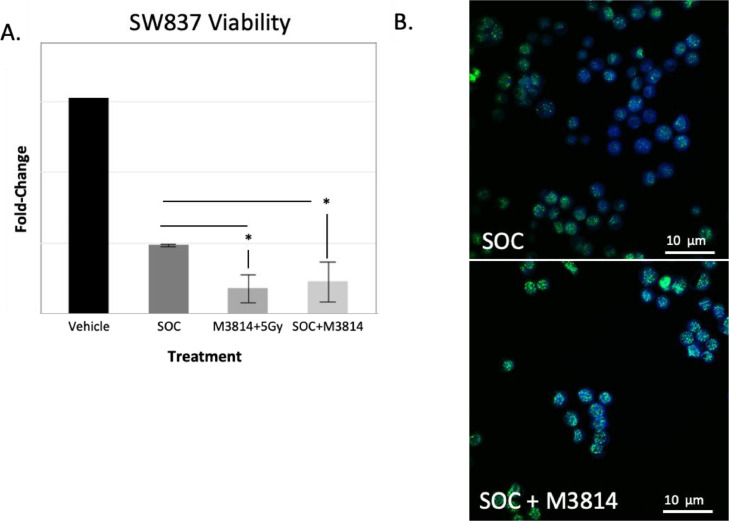
To assess for differences in cell viability after treatment, SW837 cells were treated with SOC, M3814 plus 5 Gy radiation, or SOC plus M3814 and collected 5 days later for assessment. In SW837 cells, addition of M3814 to treatment led to a significant reduction in viability compared to SOC (n = 4, Fig. 3A, * represents p < 0.05). Additionally, the number of cells staining positive for γH2AX foci increased upon addition of M3814 compared to SOC alone (65.8% to 97.3%, p < .001, Fig. 3B) indicating increased DNA damage.
Fig. 3.
pDNA-PK inhibition improves response to chemoradiotherapy in rectal cancer cell line and increase DNA damage A. Cell titer glo viability assay in SW837 cells. SW837 cells treated with M3814 plus SOC as well as M3814 plus radiation have decreased viability compared to standard of care alone (N = 3), * indicates p < 0.05. B. Significantly increased γH2Ax staining in SW837 cells treated with SOC+M3814 compared to SOC (N = 5 slides per treatment group, N = 3, p < .001). Representative photographs are shown.
M3814 treatment of in vivo Colorectal (CRC) model
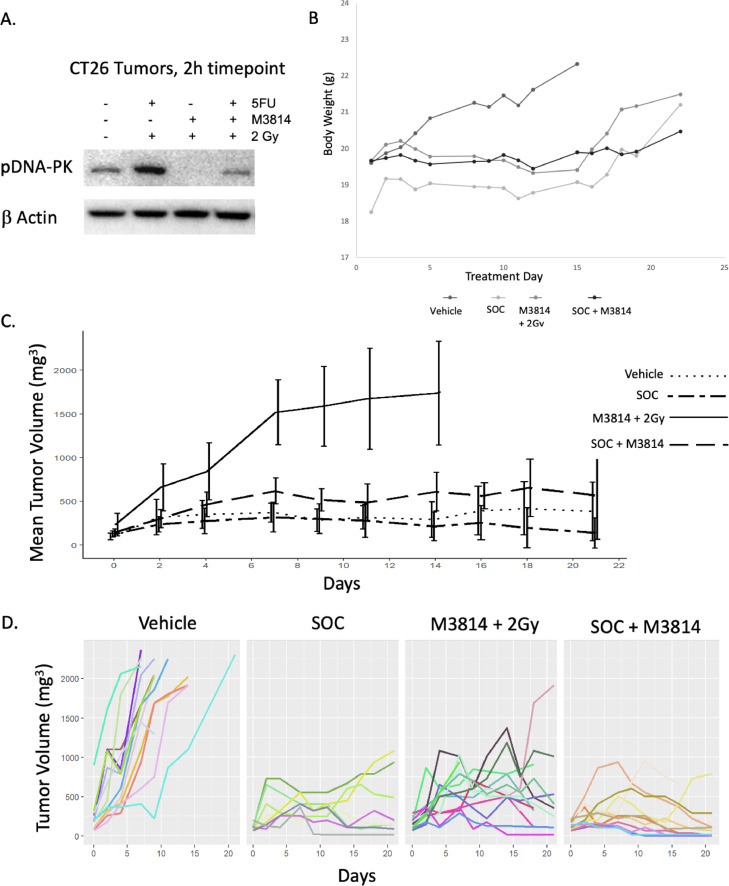
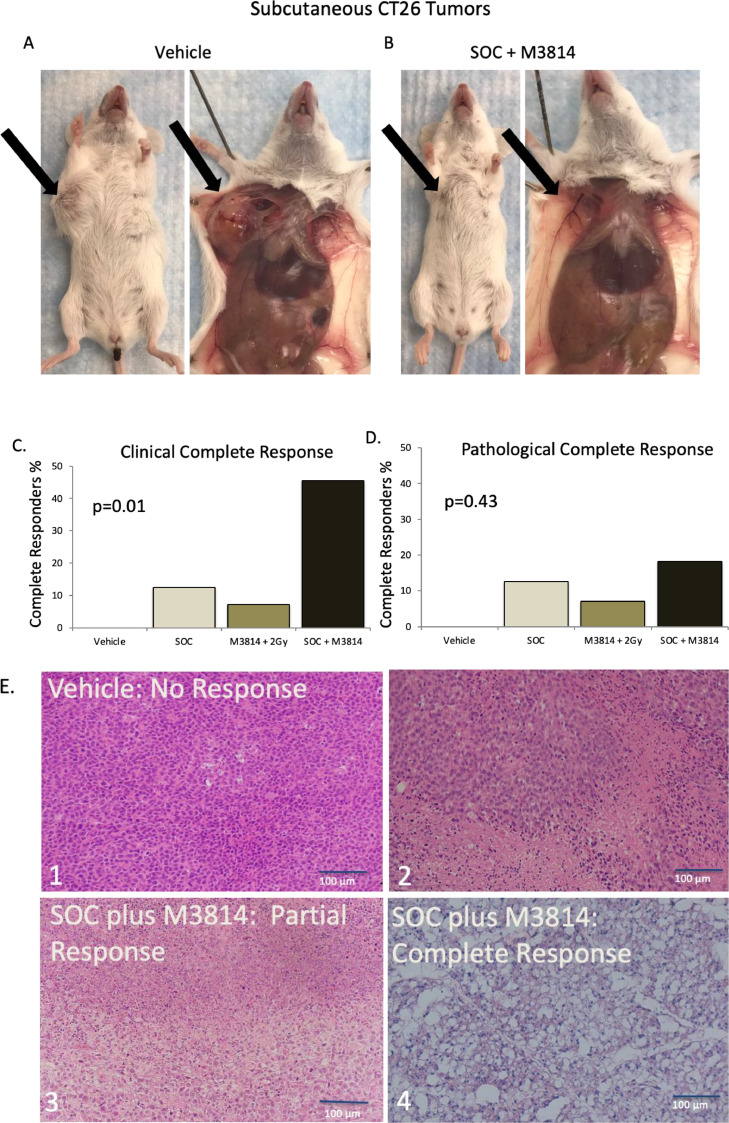
To assess the addition of inhibition of DNA-PK to standard treatment in vivo, BALB/c mice were implanted subcutaneously in the interscapular area with the CT26 cell line which is a well described immune competent CRC model.17 Tumors were allowed to grow until they reached 100 mm3 and mice were randomly assigned to the following groups: Vehicle, 100 mg/kg capecitabine with 2 Gy radiation daily (SOC), 50 mg/kg of M3814 with 2 Gy radiation daily, and SOC plus 50 mg/kg of M3814. Mice (n = 8-10 per group) were treated 5 days per week for 2 weeks. Mice were sacrificed 1 week after completion of treatment with the exception of 2 mice per group that were sacrificed 2 h after treatment to assess for inhibition of the target protein. pDNA-PK was assessed in the tumors collected 2 h after treatment via western blotting, whereupon we found that tumor pDNA-PK is increased with SOC (Fig. 4A 1.14-fold increase from vehicle, p = 0.03, n = 3) but this increase is effectively abrogated by the addition of the DNA-PK inhibitor, M3814. Body weight was largely unchanged throughout this study indicated mice tolerated the treatment well (Fig. 4B). Tumor size significantly decreased over the study period in each treatment group compared to vehicle (p < .001). There was no statistical difference between the SOC plus M3814 or SOC but both were smaller than M3814 plus radiation (p = .03, Fig. 4C). To assess individual tumors, each individual tumor size was plotted for each treatment group (Fig. 4D). Some treated tumors initially shrank during treatment and subsequently progressed leading us to hypothesize that some tumors may have developed therapeutic resistance during treatment.
Fig. 4.
M3814 treatment of in vivo CRC model: A: Western of CT-26 flank tumors after treatment in mice showing pDNA-PK increases after SOC treatment but decreases with addition of M3814 (N = 3, P = 0.03) B: Weight of mice over time showed no significant weight loss indicating that treatment was tolerated well. C. Tumor size was lower in all treatment groups compared to vehicle (N ≥ 8 mice per group, p < 0.01). There was no statistical difference between the SOC plus M3814 versus SOC, but both were smaller than M3814 plus radiation (p = 0.03). D. Spaghetti plots of each treatment group reveal that some tumors initially shrank and then grew again indicting possible outgrowth of resistant tumor.
Clinical but not pathologic complete response significantly improved with SOC plus M3814 in vivo
Since complete response is an important clinical outcome in humans, we wanted to evaluate complete response in the CT26 mouse model. An example of a mouse with a complete response from the SOC plus M3814 group (no measurable tumor) is shown in Fig. 5B compared to a large tumor in an animal from the vehicle group (Fig. 5A). We identified a 45.5% clinical complete response rate 1 week after completion of treatment with SOC plus M3814 which is significantly higher than SOC alone or M3814 plus radiation (Fig. 5C, N≥8 mice per group; p = 0.01. Because clinical complete response does not necessarily equate to lack of any viable tumor, we assessed specimens for pathologic complete response (pCR). Coded, blinded, hematoxylin and eosin slides of the tumors were sent to our pathologist (EKC) to assess for pCR, which did not identify a difference between groups (18.2% pCR for SOC plus M3814 vs. 12.5% pCR for SOC, Fig. 5D, N≥8 mice per group; p = 0.43). Representative H&E slides of each treatment outcome are shown in Fig. 5.
Fig. 5.
Clinical but not pathologic complete response significantly improved with SOC plus M3814 in vivo. A. Vehicle mouse with large tumor B. Mouse treated with SOC plus M3814 with a clinical and pathologic complete response (no measurable tumor) C. Percent of clinical complete responders after treatment shows a 45.5% complete response rate after treatment compared to 12.5% in the SOC group (N≥8 mice per group, p = 0.01). D. Percent of pathological compete responders after treatment shows 18.8% in SOC plus M3814 group compared to 12.5% in SOC (p = 0.43) E. Representative H&E images of tumors after treatment. 1: vehicle showing no response 2: SOC showing partial response 3: SOC plus M3814 showing partial response 4: SOC plus M3814 showing complete response.
ATM/ Kap1 as potential mechanism of resistance to M3814 treatment
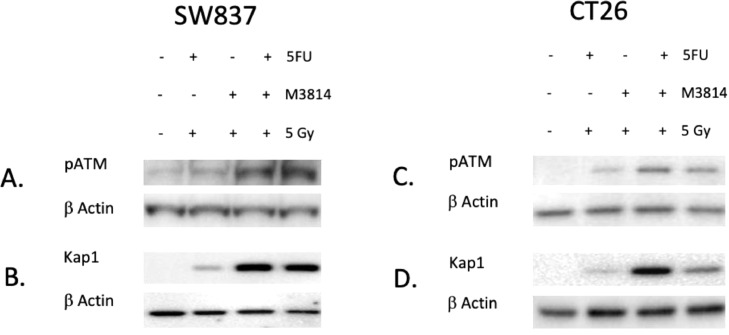
Due to the variable response of the CT26 tumors (see spaghetti plots, Fig. 4D), we hypothesized that an alternate means of DNA repair was being upregulated in our rectal cancer models to overcome inhibition of DNA-PK. In order to test this hypothesis, we treated SW837 and CT26 cell lines according to our treatment protocol and assessed for changes in a different mechanism of DNA repair. We assessed pATM and downstream Kap1 in both cell lines and found that expression of both increase when groups receiving M3814 were compared to SOC. In SW837 cells, pATM had a 4.6-fold increase in the M3814 plus radiation group compared to SOC (1.02-fold increase) and vehicle (Fig. 6A, p = 0.03, N = 3). Kap1 had a 304-fold increase in the M3814 plus radiation group compared to SOC (44.7-fold increase) (Fig. 6B, p < .001, N = 3). Similarly, we assessed ATM and Kap1 in CT26 cells grown and treated in vitro and identified an increase in pATM which had a 25.3-fold increase in the M3814 plus radiation group compared to SOC (8.9-fold increase) (Fig. 6C, p = 0.02, N = 3). Kap1 was increased in the M3814 plus radiation group (45.8-fold increase) compared to SOC (2.9-fold increase) and vehicle (Fig. 6D, p = 0.03, N = 3).
Fig. 6.
ATM/Kap1 as a potential mechanism of resistance to M3814. A/C. Western (N = 3) identified an increase in pATM in the M3814 groups compared to SOC in the SW837 cell line (p = 0.03) and in CT-26 cell line (p = 0.02) B/D. Western showing an increase in Kap1 (downstream of ATM) in the M3814 groups compared to SOC in the SW837 cell line (p < .001) and in the CT26 cell line (p = 0.03).
Discussion
Rectal cancer incidence has been increasing18 but therapeutics have remained unchanged. Studies have shown that patients who are complete responders to neoadjuvant chemoradiation have better 5-year survival.4 In order to reduce morbidity and increase survival, there is a need to improve response to treatment through improved therapeutics. One possible therapeutic approach is to inhibit radiation-induced DNA repair. DNA-PK is a prominent mechanism of DNA repair. In this study, we examined how M3814, a DNA-PK inhibitor, affects treatment response in the SW837 cell line and the CT26 mouse model. We found that DNA-PK is increased in SW837 cells when treated with radiation and this increase is abrogated by M3814. In addition, cell viability in vitro in SW837 cells is decreased with the addition of M3814 to SOC compared to vehicle. Tumors resulting from subcutaneously implanted CT26 colorectal cells in mice show variable response with some tumors re-growing after initially shrinking. There was no difference in average tumor size or pathologic complete response between treatment groups despite addition of M3814 to treatment and inhibition of the target (DNA-PK) in the tumors. We hypothesized that another mechanism of DNA-repair could be increased to compensate for DNA-PK inhibition. We found that pATM and Kap1, which is downstream of pATM, are increased after treatment with M3814. This may diminish the effectiveness of M3814 in treatment of rectal cancer.
Studies have assessed DNA-PK inhibitors in other cancer models but very little has been done in colorectal cancer. Studies of DNA-PK inhibition in glioblastoma and ovarian cancer have identified improved response when combining with other therapies.9,19 Fok et al. examined AZD7648, a DNA-PK inhibitor in non-small-cell lung cancer cells.20 They found this compound to be a potent radiosensitizer, similar to M3814, and showed that it causes persistence of DNA damage following radiation. In mouse xenografts, addition of AZD7648 achieved up to 85% tumor regression compared to 60% with radiation alone. Their improved response may be attributable to a number of factors, including compound differences with dissimilar pharmacokinetic exposure profiles or differences in crosstalk with other DNA repair pathways. While they found that pATM was increased in a delayed fashion subsequent to DNA-PK inhibition, we found that it was not delayed but rather increased early after the DNA-PK inhibitor was added. ATM is recruited to chromatin in response to DSB in DNA similar to DNA-PK. This kinase phosphorylates multiple sites that alter checkpoint activation, apoptosis, and transcription including p53. This kinase allows for a pause of the cell-cycle progression (checkpoint activation) and DNA repair which can contribute to chemoradiation resistance.21 Sun et al., found that DNA-PK inhibition increased pATM and Kap1 expression in adenocarcinoma human alveolar basal epithelial cells and fibrosarcoma cancer cell lines treated with M3814.22 They reported an increase in ATM expression in response to radiation alone that was substantially augmented upon treatment with M3814.
There are a limited number of literature reports on the anticancer effects of M3814. A study by Haines et al. showed that M3814 in combination with topoisomerase 2 inhibitors boosts the ATM/p53 response in acute myeloid leukemia cells leading to elevation in p53 protein which leads to enhanced p-53 dependent antitumor activity in tumor cells. They also show that M3814 in combination with CPX-351, was efficacious against leukemia cells in vitro and in vivo without incurring increasing hematopoietic toxicity.23 Zenke, et al., showed that M3814 is a potent inhibitor of DNA-PK and DSB repair across multiple cancer cell lines and that this drug sensitized cancer cells to radiation using colony formation assay and that M3814 synergistically enhances the activity of DSB-inducing agents.24 They measured the inhibitory effect of 72 different drugs and found that bleomycin and the topoisomerase inhibitors were synergistic with M3814. They also looked at xenograft models of hypopharyngeal carcinoma and non-small cell lung cancer and found that treatment with combination M3814 and radiation enhanced tumor growth inhibition in both tumor models and response was dose dependent. The first reported human study evaluating M3814 as a single agent treatment and included thirty-one patients with advanced solid organ tumors (colorectal being the most common) who received M3814 once daily or twice daily in 21-day cycles.25 This study found that the drug was well tolerated and demonstrated modest efficacy in unselected tumors. The best overall response was stable disease (12 patients), lasting for > 12 weeks in seven patients.
In our mouse study, there are more clinical complete responders than pathological complete responders when M3814 is added to SOC, meaning that more tumors had substantial shrinkage such that they were no longer palpable but the pathology data shows that it did not full eradicate the tumor. While this finding is not novel, it does highlight the importance of post chemoradiation tissue examination and surgical removal in similar studies. While there are studies that support close surveillance after chemoradiation if there is a clinical complete response in human rectal cancer similar to the way that anal squamous cell cancer is managed, there is concern that a subset of patients that may not have a pathological complete response and would benefit from surgical resection will have a delay in their surgery or even present when they are not resectable.5,6
Our study has multiple limitations. We only used SW837 and CT26 cell lines, so our response assessment is limited. In addition, we used flank rather than orthotopic in vivo tumors due to ease of measurement of flank tumors. Lastly, we found that caliper measurement of tumor size over-estimated complete response, which is not dis-similar to issues surrounding clinical response in patients. Clinical trials to assess responses across a variety of genetically heterogeneous rectal adenocarcinomas will be important and are ongoing.26
Conclusions
Improving response to neoadjuvant treatment of rectal cancer is needed. We found that when the DNA-PK inhibitor, M3814, is combined with SOC treatment, response is improved in the SW837 rectal cancer cell line but not the CT-26 mouse model and that ATM/Kap1 are activated when DNA-PK is inhibited potentially abrogating the effectiveness of M3814.
CRediT authorship contribution statement
Mary Smithson: Data curation, Investigation, Methodology, Validation, Writing – original draft. Regina K. Irwin: Data curation, Investigation, Methodology, Validation, Writing – review & editing. Gregory Williams: Data curation, Investigation, Validation, Methodology. M. Chandler McLeod: Methodology, Software, Formal analysis, Validation. E. Karen Choi: Investigation, Validation, Methodology, Writing – review & editing. Anutosh Ganguly: Resources, Writing – review & editing. Ashley Pepple: Resources, Writing – review & editing. Clifford S. Cho: Resources, Writing – review & editing. Christopher D. Willey: Resources, Writing – review & editing. Judith Leopold: Resources, Project administration, Supervision, Conceptualization, Writing – review & editing. Karin M. Hardiman: Resources, Project administration, Supervision, Conceptualization, Writing – review & editing, Data curation, Investigation, Methodology.
Acknowledgments
Disclosure
The M3814 used in these studies was provided through the Cancer Therapy Evaluation Program (CTEP) which allows the drug provider (Merck KGaA Darmstadt Germany) to review the manuscript prior to submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the manuscript reflect solely those of the authors.
The study abides by the Declaration of Helsinki principles.
Footnotes
Funding: KH funded by National Institutes of HealthK08CA190645; Research reported in this publication was supported by the National Human Genome Research Institute of the National Institutes of Health under Award Number 1T32HG008961-01.
Conflict of interest: No conflicts of interest.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021 Mar 2;19(3):329-359. doi: 10.6004/jnccn.2021.0012. PMID: 33724754.NCCN. Accessed September 10, 2021. [DOI]
- 3.Battersby N.J., Juul T., Christensen P., et al. Predicting the risk of bowel-related quality-of-life impairment after restorative resection for rectal cancer: a multicenter cross-sectional study. Dis Colon Rectum. 2016;59(4):270–280. doi: 10.1097/DCR.0000000000000552. [DOI] [PubMed] [Google Scholar]
- 4.Maas M., Nelemans P.J., Valentini V., et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 5.Habr-Gama A. Assessment and management of the complete clincial response of rectal cancer to chemoradiotherapy. Colorectal Dis. 2006;8:21–24. doi: 10.1111/j.1463-1318.2006.01066.x. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A., Perez R.O., Nadalin W., et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–717. doi: 10.1097/01.sla.0000141194.27992.32. discussion 717-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habr-Gama A., Perez R.O., Wynn G., Marks J., Kessler H., Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum. 2010;53(12):1692–1698. doi: 10.1007/DCR.0b013e3181f42b89. [DOI] [PubMed] [Google Scholar]
- 8.Smith J.J., Chow O.S., Gollub M.J., et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. doi: 10.1186/s12885-015-1632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Timme C.R., Rath B.H., O'Neill J.W., Camphausen K., Tofilon P.J. The DNA-PK inhibitor VX-984 enhances the radiosensitivity of glioblastoma cells grown in vitro and as orthotopic xenografts. Mol Cancer Ther. 2018;17(6):1207–1216. doi: 10.1158/1535-7163.MCT-17-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L., Shang Z., Hsu F., et al. NSCLC cells demonstrate differential mode of cell death in response to the combined treatment of radiation and a DNA-PKcs inhibitor. Oncotarget. 2015;6(6):3848–3860. doi: 10.18632/oncotarget.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glynne-Jones R., Mawdsley S., Harrison M. Cetuximab and chemoradiation for rectal cancer–is the water getting muddy? Acta Oncol. 2010;49(3):278–286. doi: 10.3109/02841860903536010. [DOI] [PubMed] [Google Scholar]
- 12.Leichman C.G., McDonough S.L., Smalley S.R., et al. Cetuximab combined with induction oxaliplatin and capecitabine, followed by neoadjuvant chemoradiation for locally advanced rectal cancer: SWOG 0713. Clin Colorectal Cancer. 2018;17(1):e121–e125. doi: 10.1016/j.clcc.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauer R., Becker H., Hohenberger W., et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 14.Burger K., Ketley R.F., Gullerova M. Beyond the trinity of ATM, ATR, and DNA-PK: multiple kinases shape the DNA damage response in concert with RNA metabolism. Front Mol Biosci. 2019;6:61. doi: 10.3389/fmolb.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelke C.G., Parsels L.A., Qian Y., et al. Sensitization of pancreatic cancer to chemoradiation by the Chk1 inhibitor MK8776. Clin Cancer Res. 2013;19(16):4412–4421. doi: 10.1158/1078-0432.CCR-12-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1(5):2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 17.Castle J., Boegel S., Graaf J., Bender C., Tadmor A.D., Boisguerin V., Bukur T., Sorn P., PAret C., Diken M., Kreiter S., Tureci O., Sahin U. Immunonomic, genomic, and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. 2014;15(190):1471–2164. doi: 10.1186/1471-2164-15-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 19.Wise H.C., Iyer G.V., Moore K., et al. Activity of M3814, an oral DNA-PK inhibitor, in combination with topoisomerase ii inhibitors in ovarian cancer models. Sci Rep. 2019;9(1):18882. doi: 10.1038/s41598-019-54796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fok J.H.L., Ramos-Montoya A., Vazquez-Chantada M., et al. AZD7648 is a potent and selective DNA-PK inhibitor that enhances radiation, chemotherapy and olaparib activity. Nat Commun. 2019;10(1):5065. doi: 10.1038/s41467-019-12836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackford A.N., Jackson S.P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol Cell. 2017;66(6):801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Sun Q., Guo Y., Liu X., et al. Therapeutic implications of p53 status on cancer cell fate following exposure to ionizing radiation and the DNA-PK inhibitor M3814. Mol Cancer Res. 2019;17(12):2457–2468. doi: 10.1158/1541-7786.MCR-19-0362. [DOI] [PubMed] [Google Scholar]
- 23.Haines E., Nishida Y., Carr M.I., et al. DNA-PK inhibitor peposertib enhances p53-dependent cytotoxicity of DNA double-strand break inducing therapy in acute leukemia. Sci Rep. 2021;11(1):12148. doi: 10.1038/s41598-021-90500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zenke F.T., Zimmermann A., Sirrenberg C., et al. Pharmacologic Inhibitor of DNA-PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther. 2020;19(5):1091–1101. doi: 10.1158/1535-7163.MCT-19-0734. [DOI] [PubMed] [Google Scholar]
- 25.van Bussel M.T.J., Awada A., de Jonge M.J.A., et al. A first-in-man phase 1 study of the DNA-dependent protein kinase inhibitor peposertib (formerly M3814) in patients with advanced solid tumours. Br J Cancer. 2021;124(4):728–735. doi: 10.1038/s41416-020-01151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Library of Medicine (U.S.). (2019, March 20 - 2022, Februrary 17). Study of peposertib in combination with capecitabine and radiotherpay in rectal cancer. Identifier NCT03770689. Accessed 3/28/2021, 2021.