Summary
Bullous pemphigoid (BP) is an autoimmune subepidermal blistering dermatological condition that can be triggered by several external factors. Here, we present a case of an immunocompetent patient with no prior dermatological history, who developed BP as a result of autologous skin graft surgery. It is an uncommon surgical complication and was most likely triggered by the trauma of the surgery itself.
Our patient's bullae first developed a month after his surgery at both surgical sites and subsequently became widespread. The diagnosis was confirmed histologically using punch biopsies of a bulla and the perilesional skin for direct immunofluorescence together with indirect immunofluorescence of the serum for anti-skin antibodies. Initial topical treatment and regular wound care were not improving the patient's condition at a satisfactory rate. Therefore, the patient was started on systemic steroids, which unfortunately resulted in a presumed split skin graft infection requiring admission. After histological diagnosis confirmation was achieved, the Dermatology team formulated a treatment plan, which combined both topical and systemic medication. The patient is currently making a good recovery and the graft loss resulting from the condition is only partial, requiring no further surgery.
We present this case as a reminder to all clinicians that, although rare, BP can be triggered by skin grafting, even in patients with no prior history of it or any predisposing conditions. This autoimmune condition needs to be recognised and treated promptly to ensure optimal clinical outcomes and minimise graft loss.
Keywords: skin grafting, plastic surgery, autoimmune disease, dermatopathology
An 83-year-old gentleman underwent evacuation of a right lower limb haematoma with split skin grafting (SSG) ∼3% total body surface area. His rivaroxaban for atrial fibrillation was held pre-operatively, and he was commenced on broad-spectrum antibiotics. Medical history was insignificant with no personal or family history of dermatological or rheumatological conditions.
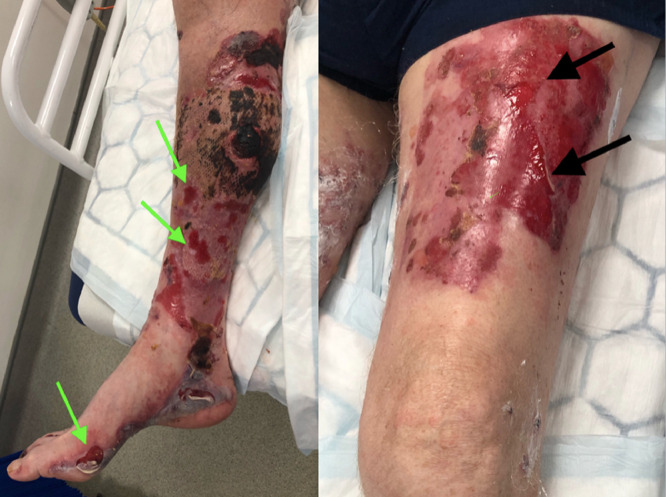
One month after surgery, new tense bullae developed across both the graft and left thigh donor site (Figure 1) as well as the remaining bilateral lower limbs, upper limbs, and trunk. The patient was free from peri-oral blistering and systemically well, with no obvious cause identified nor the previous occurrence. These bullae were suspicious of BP, and a Dermatology opinion was sought. Dermatology agreed with the clinical impression and arranged an anti-skin antibody test (indirect immunofluorescence), diagnostic 8 mm bulla punch biopsy, and a 4 mm punch biopsy from perilesional skin (direct immunofluorescence). Daily topical Dermol emollient and Dermovate ointment were recommended with regular wound care while awaiting the results. Six weeks post-surgery, oral Prednisolone was initiated, (30 mg daily for 4 weeks followed by a reducing regime of 20 mg daily for 4 weeks) as there was no clinical improvement with topical management. However, the steroids were stopped after four days due to presumed infection at the SSG site requiring intravenous antibiotics. The patient reported steroids did provide some resolution. Wound swabs showed mixed growth only, and bloods showed only mildly raised white cell count (13.1) and neutrophils (12.2).
Figure 1.
Right lower limb (SSG recipient site) and left thigh (SSG donor site). Various size bullous lesions present on both SSG donor and recipient site one-month post-surgery with tense bullae already deroofed (demonstrated by arrows). Note the patchy graft loss with a small dry necrotic area right medial calf.
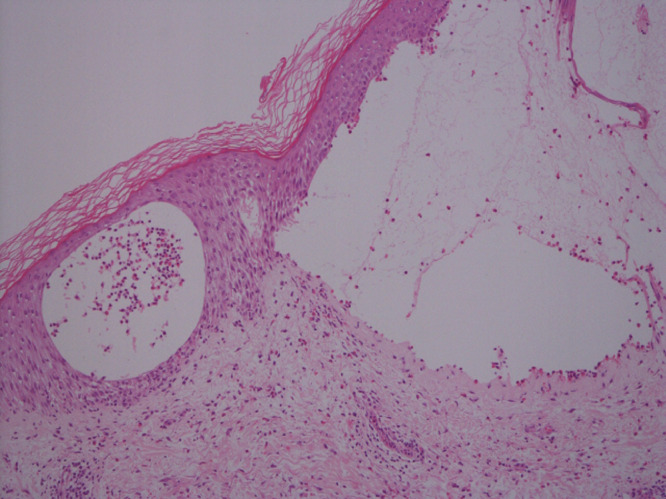
Histology demonstrated a subepidermal blister with a moderate eosinophil infiltrate in the dermis and fluid cavity (Figure 2). The direct immunofluorescence showed IgG and C3 along the dermo-epidermal junction, confirming BP. The patient has since been treated with Dermol cream, Dermol soap substitute, Dermovate ointment (to active areas), hydrocortisone cream (to face), a reducing prednisolone regime, and long-term doxycycline 100 mg daily with six-month review. At the time of submission, the Dermatology department is continuing to review the patient who is showing signs of improvement.
Figure 2.
Haematoxylin and eosin stain from the patient's diagnostic punch biopsy showing subepidermal blister with a moderate eosinophil infiltrate in the dermis (x10).
Discussion
BP is a subepidermal autoimmune condition, arising with equal gender frequency, presenting classically in the elderly and rarer in infants and children. The aetiology can be linked to trauma, radiotherapy, ultraviolet exposure, photodynamic therapy, vaccinations, skin infections, and surgery.1 BP association with autologous skin grafting and/or skin graft donor sites is rare with few instances reported over the last three decades.1, 2, 3
Punch biopsy showing subepidermal bullae and eosinophilic infiltrates confirms the diagnosis. Direct immunofluorescence highlights deposits of complement and IgG at the dermo-epidermal junction.1 BP arises from polyclonal circulating IgG autoantibodies binding to epidermal antigens with the major antigens (BP 180 and BP 230) in the basement membrane, which is also found in the normal epidermis. One hypothesis is that undergoing SSG can “unmask” the antigens, resulting in antibody binding, complement pathway activation, local inflammatory response, and bullae formation which then spreads throughout the body.2 Lysosomal enzymes are released and disrupt the dermo-epidermal junction leading to the subepidermal blisters.1 Another theory is delayed appearance of BP weeks after grafting could be due to local changes secondary to poor wound healing, as basement membrane integrity is lost, precipitating antigenicity, and faster spread of the bullae.1 Other hypotheses have been described.2
Oral immunosuppressants are the best-established treatment for BP with a quick response – here, prednisolone was initiated; however, one must be mindful of the adverse effects associated with steroid use. In this instance, steroids seemed to precipitate infection at the SSG site. Topical steroid treatment may be used alone or in conjunction with systemic steroids (e.g., in widespread disease) – both being the mainstay and highly evidenced. An oral prednisolone dosage regime of 0.5 mg/kg/day is effective for mild to moderate disease, as confirmed by the Bullous Pemphigoid Steroids and Tetracyclines (BLISTER) Study.4
Tetracyclines have also been used for BP treatment due to anti-inflammatory action, but less evidenced. The BLISTER study demonstrates the effectiveness of Doxycycline with a better long-term safety profile than that of systemic steroids.4 In this case, whole-body application of topical steroids was deemed impractical; therefore, oral Doxycycline was commenced alongside topical and systemic steroid therapy.
Other treatment options in the literature include immunosuppressing and immunomodulating drugs5 (Methotrexate, Azathioprine, Mycophenolate mofetil, Dapsone and Sulphonamides, intravenous immunoglobulins, Chlorambucil, Cyclophosphamide, and Ciclosporin). Topical tacrolimus, biologic agents, plasmapheresis, and immunoapheresis have also been described.5
The annual incidence of BP is 2.4–23/million in the general population; however, this rises to 312 cases/million in the over 80s cohort.6 Incidence has increased steadily over the past two decades, but with that in mind, it is very rare and its unknown incidence in patients receiving skin grafts makes it unlikely to have any specific medico-legal implications or require adding as a risk to the consent form.
To conclude, we present a patient who developed BP precipitated by SSG. This was initially localised to the donor and recipient sites but then became widespread. It is interesting to note that this patient did not have any predisposing factors. Prompt recognition and early specialist involvement for appropriate management were vital in the post-operative care of this patient. This consequence to skin grafting surgery is not widely reported in the literature. In addition, as surgeons, we should be mindful and proceed with caution when considering skin grafting in patients with a history of BP, as this may trigger a recurrence.
Declaration of Competing Interest
None.
Acknowledgments
Funding
None.
Ethical Approval
Not required.
Footnotes
Presented At: Nil
References
- 1.Hafejee A, Coulson IH. Localized bullous pemphigoid 20 years after split skin grafting. Clin Exp Dermatol. 2005;30:187–188. doi: 10.1111/j.1365-2230.2004.01689.x. [DOI] [PubMed] [Google Scholar]
- 2.Ghura HS, Johnstone GA, Milligan A. Development of bullous pemphigoid after split skin grafting. Br J Plastic Surg. 2001;54:447–449. doi: 10.1054/bjps.2001.3601. [DOI] [PubMed] [Google Scholar]
- 3.McGrath J, Black M. Split skin grafting and bullous pemphigoid. Clin Exp Dermatol. 1991;16:72–73. doi: 10.1111/j.1365-2230.1991.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams HC, Wojnarowska F, Kirtschig G, et al. on behalf of the UK Dermatology Clinical Trials Network BLISTER Study Group. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. 2017;389:1630–1638. doi: 10.1016/S0140-6736(17)30560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venning VA, Taghipour K, Mohd Mustapa MF, Highet AS, Kirtschig G. British Association of Dermatologists‘ guidelines for the management of bullous pemphigoid 2012. Br J Dermatol. 2012 Dec;167(6):1200–1214. doi: 10.1111/bjd.12072. [DOI] [PubMed] [Google Scholar]
- 6.Kridin K, Ludwig RJ. The Growing Incidence of Bullous Pemphigoid: Overview and Potential Explanations. Front Med (Lausanne) 2018 Aug 20;5:220. doi: 10.3389/fmed.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]