Highlights
-
•
The P. niruri based nanoemulsion was prepared using plant extract (1%), a fixed amount of oil (5%), sodium alginate solution (1%) (with 0.5% tween 80).
-
•
The nanoemulsion loaded with P. niruri extract showed 192 nm average droplet size.
-
•
The formulated nanoemulsion exhibited higher potency against bacterial and fungal pathogens with respect to P. niruri extract alone.
-
•
The P. niruri nanoemulsion showed the potential to be utilized in pharmaceutical, food, and cosmetic industries in near future.
Keywords: Nanoemulsion, Sodium alginate, Phyllanthus niruri, Antimicrobial activity, Antioxidant
Abstract
In this study, we formulated an oil-in-water nanoemulsion of Citrullus lanatus seed oil in the presence of Phyllanthus niruri methanolic extract using a delivery system based on sodium alginate. The control nanoemulsion was prepared without plant extract and the nanoemulsion loaded with extract was further characterized based on their size, polydispersity index, morphology, and stability. The nanoemulsion showed an average droplet size of about 192 nm, with a polydisperse droplet size with a spherical shape and the zeta potential of -15.0 mV and -18.4 mV. In contrast to the control nanoemulsion, the drug release rate of the nanoemulsion formulation was found to be significant (p <0.05). Antibacterial activity was assessed against a variety of pathogenic bacterial and fungal strains and the formulated nanoemulsion exhibited significantly higher potency against them in comparison to P. niruri extract alone. The results revealed thermodynamically stable nanoemulsion which could be used for various therapeutic applications.
Graphical abstract
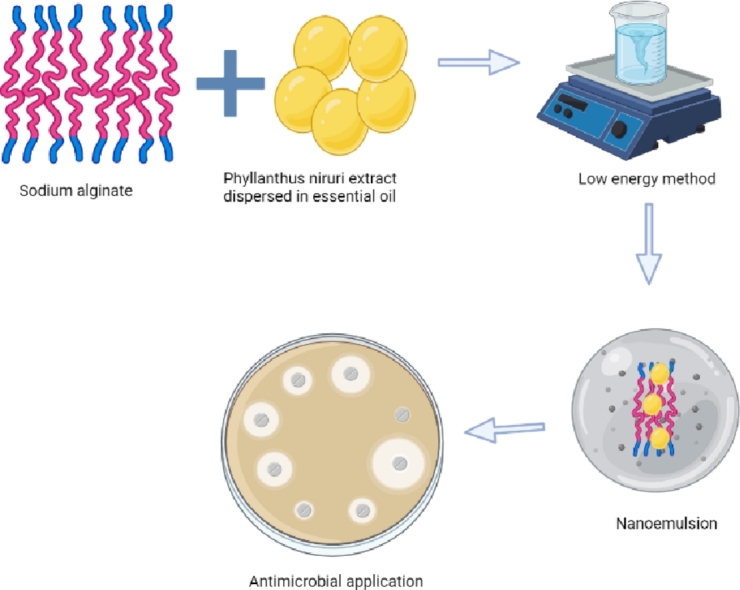
1. Introduction
Over the past years, medicinal plants and oils are attaining more attention than ever due to their numerous advantages to society or indeed to all mankind, particularly in the area of food, nutraceuticals, and medicines [1]. The use of herbal treatment helps to increase the therapeutic values by reducing the toxicity and side effects of drugs and simultaneously it likewise increases bioavailability.[2] Phyllanthus niruri (P. niruri) (Bhumiamla) is an annual herb, that belongs to the family Euphorbiaceae, and is widely distributed throughout subtropical and tropical countries of the world and even in the coastal areas of India [3]. The herb harbor the presence of numerous bioactive components for example alkaloids, flavonoids, tannins, glycosides, lignans, terpenes, phyllanthin, hypophyllanthin, steroids, phenylpropanoids, ricinolic acid, phyltetralin, and niruriside which aids in its various therapeutic and pharmacological properties [4]. The P. niruri plant has been reported to have strong antimicrobial activity against important foodborne pathogens such as Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, Bacillus cereus, and Salmonella typhi [5]. To prevent the explosion of different microorganisms and to replace the synthetic antimicrobial compounds used in foods through natural ways, herbal plants are considered as a good alternative source of antimicrobial additives. The P.niruri is effectively being utilized as anti-inflammatory, anti-fungal, antiviral, antibacterial, antioxidant, hepatoprotective, hypoglycemic, hypotensive, and also shows an inhibitory effect on renal stone formation [6]. Recent studies have shown considerable interest in the search for natural antioxidants derived from plants and oils for their potential antioxidant and anti-free radical properties[7]. Because of the existence of phytochemical components, most herbal extracts and their isolated compounds have shown improved therapeutic efficacy in vitro, although this does not necessarily translate directly to in vivo.This has drawn attention due to the low bioavailability, stability, and distribution of herbal medications when given in traditional dosage forms and as a result, covered the way for research into the formulation of different plant extracts into novel drug delivery systems[8]. Nanoherbal drug delivery systems have an enormous potential future for enhancing the activity and overcoming the problems related to the use of medicinal flora[9].
One of the most promising strategies is the use of nanoemulsions as a carrier for topical drug delivery systems to improve the stability and oral bioavailability of lipophilic functional agents and, therefore, increase the permeation of drugs through the skin [10]. Nanoemulsions are dermatological carriers that increase the penetration of medications, as well as the drug release profile. A nanoemulsion is a transparent or translucent emulsified system with nanometer-size droplets that increases the bioavailability of the herbal product [11]. Plant extracts are considered to have several advantages when incorporated into nanoemulsions which include increased permeability, stability, solubility, bioavailability, therapeutic efficacy, better tissue distribution, and sustained drug delivery.Given the benefits of nanoemulsions, few studies have been conducted on incorporating herbal drugs into these systems [12,13]. Many plant extracts are incorporated into nanoemulsion for the drug delivery system, however, P.nirurihas not been evaluated into nanoemulsion vehicles.In literature, there are a variety of methods for preparing nanoemulsions are reported. Both high-energy and low-energy techniques are often used to create nanoemulsions. High-pressure homogenization, microfluidization, and ultrasonication are examples of high-energy techniques, whereas phase inversion emulsification is an example of low-energy approaches[14]. Low-energy techniques have recently attracted a lot of attention since they are simple and easy to use, non-destructive, and do not damage encapsulated molecules. Furthermore, these technologies are more energy-efficient, making them more appealing for large-scale production[15].In this context, oil in water nanoemulsion was prepared and stabilized by using sodium alginate a naturally occurring polysaccharide. Sodium alginate is a linear polysaccharide having acidic, derived from the cell membrane of seaweed, and is chemically stable at pH values ranging from 5 to 10[16]. Alginate is widely applicable as a suspending, chelating, and thickening agent that acts as a stabilizer, emulsifier, helps in encapsulation, swelling, or is used for forming gels and films in various industries like pharmaceutics, food, beverages, textile, and printing[17]. The P.niruri extract was used in several studies in the literature that shows the potential in vitro antioxidant and antimicrobial potential. However, to the best of our knowledge, no previous attempts have been made for the preparation and characterization of sodium alginate stabilized nanoemulsions from P. niruri plant extract for the release of antioxidant compounds and as a potential antimicrobial agent which seems a favorable strategy for cosmetics, food, and pharmaceutical industry. Hence in the present study, the plant extract-based nanoemulsion containing the extract of the P. niruri plant was prepared by using a low energy method (spontaneous emulsification), which is a novel way to develop an environmentally relevant method for a highly stable emulsion system. We selected Tween 80 as a nonionic surfactant because nonionic surfactants are known to be less influenced by pH [18]. The P. niruri encapsulated nanoemulsion enhances the antimicrobial and antioxidant activities which show the potential of formulated nanoemulsion to be utilized in pharmaceutical, food, and cosmetic industries in near future. This study provides new important information about the formulation of nanoemulsion from the plant-based extract.
2. Materials and methods
2.1. Materials
Phyllanthus niruri plant was collected from district Kangra. (Elevation: 2749 ft, Latitude: 31˚ 21′ to 32˚ 59′ N and Longitude: 75˚ 47′ 55″ to 77˚ 45′ E) Himachal Pradesh, India in July 2019. The seeds of Citrullus lanatus were purchased from the local market of Solan, Himachal Pradesh (India). Sodium alginate, tween 80, bovine serum albumin, gallic acid were purchased from Sigma Aldrich Co. St. Louis, MO, USA. l-ascorbic acid, Methanol, yeast extract, Mueller Hinton Agar, Mueller Hinton Broth, sabouraud dextrose agar (SDA), sabouraud dextrose broth (SDB), DMSO (dimethyl sulfoxide), Chloramphenicol, Ketoconazole, Hydrogen peroxide, Phosphate buffer, sodium chloride were provided by Hi-Media Laboratories Pvt. Ltd., Mumbai, India. Plastic dishes and plates were obtained from Corning (Corning, NY, USA). The analytical reagent grade and triple distilled cellular grade water, and acid-washed glassware were used throughout the experiments.
3. Methods
3.1. Preparation of the methanolic extract from the Phyllanthus niruri plant
Fresh P. niruri plants were collected and the taxonomic identification was confirmed from the Botanical Survey of India, Dehradun (Uttrakhand), India, havingvoucher specimen no. 277.The whole plant was surface sterilized with 0.1% HgCl2 and then washed with triple distilled water for 8–10 min. The plant was dried for 1 to 2 weeks at room temperature. The part of the dry plant was ground to make a fine powder. Briefly, 10 g of powder was placed in 100 ml of organic solvent (methanol) in a conical flask and subjected to shaking by using an orbital shaker (Orbitek Lt, Scigenics Biotech PVT. Ltd., Chennai, India) for 48 h. The extract was then filtered through Whatman filter paper No. 1. The crude extract was concentrated and dried using a modified solvent evaporation technique. The extract was then stored in air-tight glass vials at −20°C for further analysis[19].
3.2. Extraction of the Citrullus lanatus oil
C. lanatus seeds (200 g) were powdered in a grinder (Philips, HR1720, Amsterdam) and the volatile oil was gathered from the water-distillation process by using a Clevenger type apparatus for 5 h. Anhydrous sodium sulfate was used to dry the oil and after filtration kept in an amber bottle flask and stored at 4°C until tested and analyzed[20].
3.3. Preparation of sodium alginate – P. niruri extract-based nanoemulsions
Oil-in-water nanoemulsion was prepared by a low-energy method by Yildirim et al.[21]. with some modifications. To obtain P. niruri based nanoemulsion a fixed amount of (1 g) weight of P. niruriextract was dissolved in the volume of (5 ml) C. lanatus seed oil. Then, the oil phase was mixed with a 1% weight of sodium alginate solution (with 500 µl Tween 80). For the preparation of nanoemulsion, tween 80 was found to be a suitable surfactant for the nanoemulsion formulation since it is less affected by pH, and is considered to be nontoxic and biocompatible.Therefore, the nonionic surfactant stabilizes the emulsions by generating a steric barrier across the bulky molecular groups which are directed towards the continuous medium. Water was added to the organic phase containing oil and surfactant under constant stirring at 1000 rpm for 30 min using a magnetic stirrer (DIGIMAG). Along with this, a blank nanoemulsion (control) with the same surfactant, oil, and aqueous phase without extract was prepared following the same procedure, and it was considered as a control. After the preparation of the nanoemulsion, the prepared samples were stored in glass vials at room temperature for further analysis, and the stability was evaluated after 15 and 30 days.
4. Characterization of nanoemulsion
4.1. Droplet size, zeta potential, and polydispersity index
The droplet size, zeta potential, and polydispersity index were determined using Zetasizer Nano ZS Particle Sizer (Malvern Instruments Limited, Malvern, WR14 1XZ, UK) at a temperature of 25 °C. The 1% nanoemulsion was suitably diluted in triple-distilled water prior to droplet size measurement. Three measurements were acquired for each emulsion sample. The average droplet size was measured during the 1st, 15th, and 30th days of storage.
4.2. Transmission electron microscopy (TEM)
To determine the shape and morphology of the formulated nanoemulsion, TEM was carried out. To perform the TEM observations, nanoemulsions were placed on a carbon-coated copper grid (200 mesh/inches) and then stained with 1% phosphotungstic acid. The excess phosphotungstic acid on the sample was gently wiped off using filter paper and examined after drying for about half an hour at room temperature. The micrographs were acquired using a transmission electron microscope (Tecnai-10, Phillips) with a tungsten source and operating at 80 kV.
4.3. Field emission scanning electron microscopy (FESEM)
The prepared nanoemulsion was also analyzed by a FESEM. A small amount of nanoemulsion sample was first fixed on a carbon-coated copper grid. The fixed sample on the grid was allowed to dry for about 10–15 min before FESEM imaging. (FEI QUANTA 250 Field Emission Scanning Electron Microscope (FEICO, USA) at high tension range 200–30,000 volts.
4.4. Refractive index and viscosity of the nanoemulsion
The refractive index and viscosity of the selected nanoemulsion were evaluated by following the proposed method of Ahmad et al.[22]. The samples were repeated three times whereas the mean value and standard deviation (SD) for the data were calculated.
5. In vitro antioxidant activity
5.1. Free radical scavenging activity (DPPH)
The radical scavenging activity of C. lanatus oil, P. niruri extract, and P. niruri based nanoemulsion was determined using the method DPPH described by Sundararajan et al.[23]. with a slight modification.Test solution i.e.C. lanatus oil, P. niruri extract, and 1 ml nanoemulsion were added to 4 ml of DPPH (0.10 mM) solution at varying concentrations of 10, 20, 40, 60, 80, and 100 μg/ml. A UV–Vis spectrophotometer was used to assess the absorbance of the samples after incubation at 517 nm. Positive control was used, which was ascorbic acid. Linear regression analysis was used to determine the 50% DPPH inhibitory concentration (IC50). The experiment was carried out three times, and the percentage of DPPH free radical inhibition was estimated using the following equation:
| (1) |
Where Abs control = DPPH radical with methanol absorbance; Abs sample = absorbance of the sample solution[23].
5.2. Hydrogen peroxide scavenging method (H2O2)
The ability of C. lanatus oil, P. niruri extract, and P. niruri based nanoemulsion to scavenge hydrogen peroxide were determined using the method reported by Vinodhini et al.[24]. with slight modifications. In phosphate buffer, a 20 mM hydrogen peroxide solution was prepared (pH 7.4). In 0.6 ml of hydrogen peroxide solution, the varied amounts (10, 20, 40, 60, 80, and 100 g/ml) were added. Using a UV-visible spectrophotometer, the absorbance of samples was measured at 230 nm after 10 min against a blank solution comprising phosphate buffer without hydrogen peroxide. Whereas, in the case of positive control ascorbic acid was used. Linear regression analysis was used to determine the 50% DPPH inhibitory concentration (IC50). The experiment was carried out three times, and the percentage of DPPH free radical inhibition was estimated using the following equation:
| (2) |
Where Abs control = absorbance of control; Abs sample = absorbance of the sample solution[24].
5.3. In vitro drug release study
In vitro drug release for the nanoemulsion formulation was performed in phosphate buffer using a dialysis membrane (pore size: 12 KDa, Sigma Chemical Co., USA)[25]. The membrane was activated overnight in phosphate buffer. It was exposed to running water for a few hours to get rid of glycerin-based contents. Prepared (10% v/v) nanoemulsion formulation was loaded into the dialysis membrane and placed in 150 ml of 10 mM phosphate buffer solution (pH 7.4) in a shaking incubator (maintained at 100 rpm and 37 °C). At specific time intervals, 1 ml of the sample was withdrawn and replaced with the same volume of the fresh buffer solution. The samples were then filtered and assayed for the drug content using a UV/Visible spectrophotometer at 210 nm against the control sample. The experiments were carried out in triplicate.
5.4. Release kinetics study
In order to study the release kinetics of nanoemulsion formulation, the data which was obtained from the in vitro release study was evaluated using several kinetic models to describe the process of drug release from nanoemulsions. On the release behavior, two kinetic models, (1) zero-order, (2) first-order, were applied as follows:
-
1Zero-order equation:
(3)
Where Qt = percentage of drug released at time t and, ko= release rate constant
-
1First order equation:
(4)
Where Q0= is the initial concentration of the drug, k= is the first-order rate constant, t = release time[23], [24].
6. Antimicrobial activity
6.1. Collection of bacterial strains
The bacterial strains E. coli (MTCC-739), Klebsiella pneumonia (MTCC-39), S. aureus (MTCC-737), Pseudomonas aruginosa(MTCC-741), S. typhimurium (MTCC 3232), Streptococcus mutans (MTCC-2567), B. subtilis (MTCC-441) were collected from IMTECH, Chandigarh. The microbial strains were subcultured in Mueller Hinton Broth and incubated at 37°C for 24 h before the experiment.
6.2. Collection of fungal strains and inoculum preparation
The fungal pathogens Trichophyton rubrum (NCCPF no. 900,001) and Trichophyton mentagrophytes (NCCPF no. 800,009) were obtained from the National Culture Collection of Pathogenic Fungi (NCCPF), Chandigarh (India), and was maintained on SDA. The slant medium was supplemented with 0.1% yeast extract and stored at 4°C for six months as active cultures and revived after that. For inoculum preparation, stock suspensions of these fungal pathogens were prepared from a sporulating 15 days old culture grown on the SDA plate at 28°C. The colonies were enclosed with 5 ml of sterile distilled water, having 0.05% Tween 20 and surface scraped with a sterilized loop. The conidia and hyphal fragments mixture was filtered with a sterile filter of 8 mm pore size and collected in a sterile test tube. This method removed most of the hyphae and gives rise to the inocula composed mainly of spores. The turbidity of the final inocula was made to 1.0 × 106 spores ml−1, at a wavelength of 520 nm, and transmission adjusted to 70% in a spectrophotometer[26].
6.3. In-vitro antibacterial activity of P. niruri based nanoemulsion
Antibacterial activity of C. lanatus oil, P. niruri extract, and P. niruri based nanoemulsion was evaluated by the Agar well diffusion method[27]. Thesubcultured microorganism's turbidity was adjusted with sterile distilled water using 0.5 McFarland as standard (∼1.5 × 108 cells/ml). Mueller Hinton Agar powder was prepared in triple distilled water. Agar glass plates were prepared and inoculated with the tested microorganisms by the spread plate method. The plates were kept in the laminar airflow for 30 min. A cork borer was used to punch wells of 6 mm diameter in the seeded agar plates. Then, the C. lanatus oil, P. niruri extract, and P. niruri loaded nanoemulsion were added to the wells at a constant concentration of 100 μl in each well. The standard antibiotic chloramphenicol was used as a positive control, whereas triple distilled water was used as a negative control in each agar plate. Then, the plates were incubated at 37 °C for 24 h. After incubation, the antibacterial activity was evaluated around the well by measuring the diameter of the inhibition zone. The experiment was repeated thrice.
6.4. In vitro antifungal activity of P. niruri based nanoemulsion
The antifungal potential was assessed by the agar well diffusion method. Briefly, 25 ml of sterile sabouraud dextrose agar (SDA) media was poured into the petri dishes. 100 µl of fungal suspension was evenly distributed on solidified SDA plates using a sterilized spreader. In the center of these SDA plates, wells with a diameter of 6 mm were made using a sterilized cork borer. Then, C. lanatus oil, P. niruri extract, and P. niruri nanoemulsion loaded at a constant concentration of 100 µl were added to the wells in each well and allowed to diffuse at room temperature for one hour. Triple distilled water was used as a negative control and the antibiotic ketoconazole (10 mg) was used for the positive control. Then, for 3 days, the plates were incubated at 28–30 °C. The antifungal activity was evaluated around the well by measuring the diameter of the zone of inhibition[28]. The experiment was repeated three times.
6.5. Statistical analysis
All results were examined and showed as mean ± SEM (standard error of the mean) in Microsoft Excel, 2016 (Microsoft Corp., Redmond, WA). Data analyses and IC50 value was calculated with the help of GraphPad Prism 5.0 software.
7. Results and discussion
7.1. Preparation and physicochemical characteristics of the nanoemulsion
In this work, a P. nirurinanoemulsion was successfully prepared by the low energy method. The P. niruribased nanoemulsion was prepared using plant extract (1%), a fixed amount of oil (5%), sodium alginate solution (1%) (with 0.5% tween 80). Whereas, the blank nanoemulsion was prepared without plant extract. The nanoemulsion containing P. niruri extract had a milky appearance and a light brown color, whereas the nanoemulsion containing no plant extract (control) showed white color (Fig. 1). The stability of a nanoemulsion system is an important factor for significant usage in food and pharmacological applications. Therefore, the nanoemulsion sample was evaluated for thermodynamic stability tests and was found to behomogeneous, with no precipitate formation or no phase separation.
Fig. 1.
Physical appearance of the prepared nanoemulsion obtained by low energy method–(PN) nanoemulsion prepared with Phyllanthus niruri extract (P. niruri nanoemulsion); and (CO) nanoemulsion without extract (control nanoemulsion).
7.2. Droplet size, zeta potential,and polydispersity index
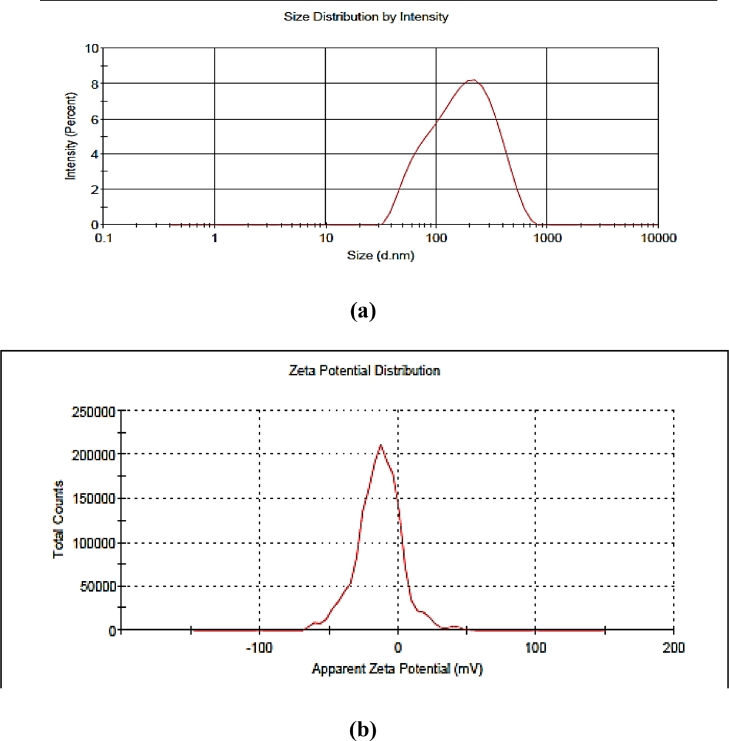
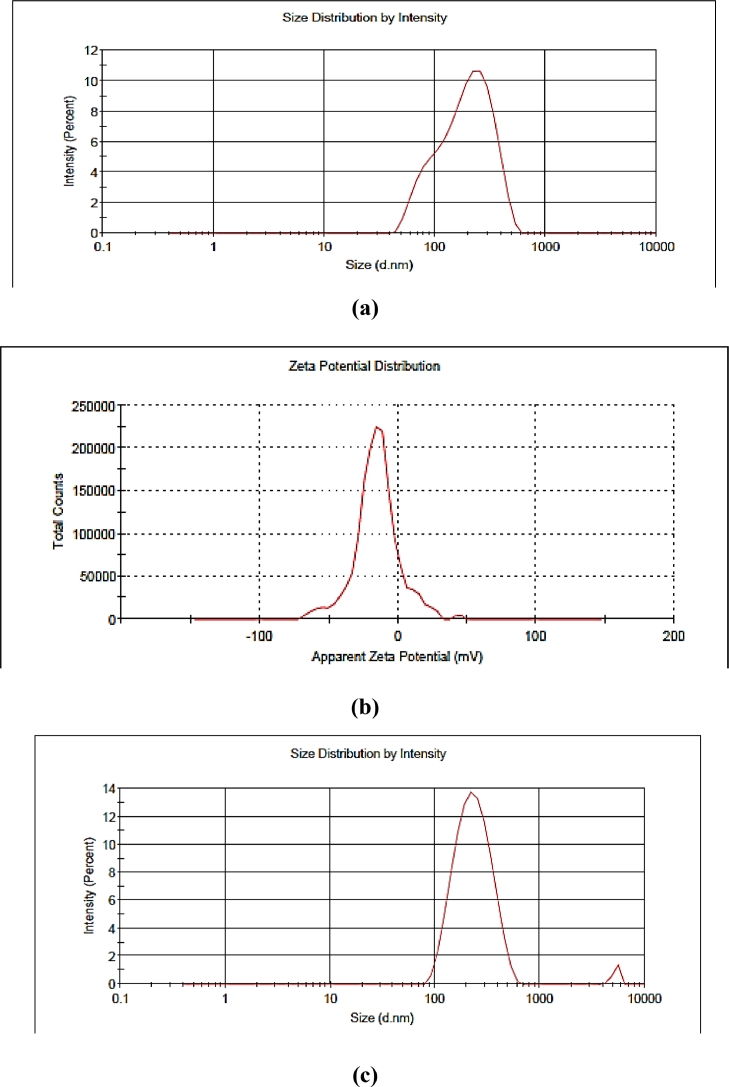
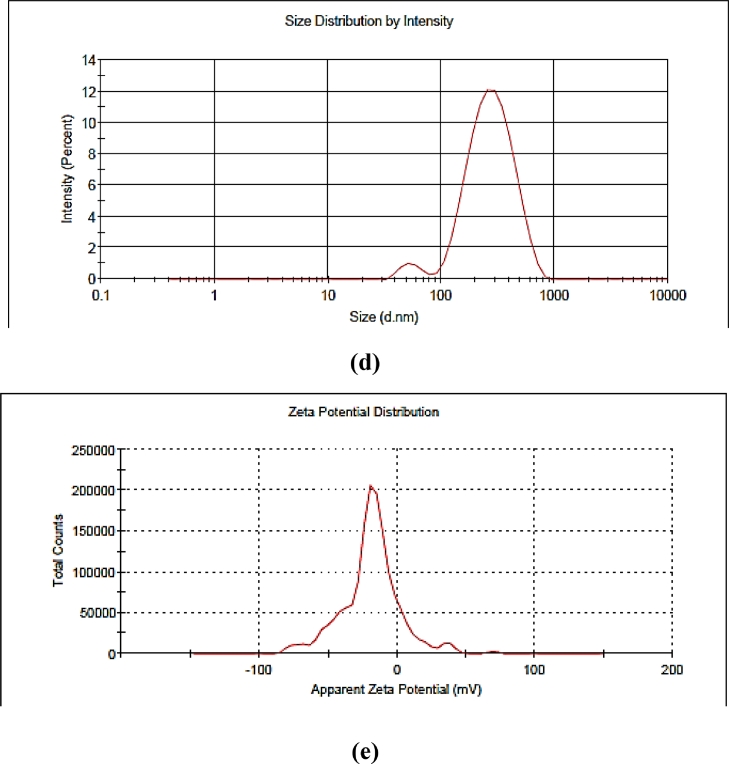
Droplet size, zeta potential, and polydispersity index are considered essential parameters for the nanoemulsion formulation for its better stability and efficacy. In terms of particle size distribution, PDI measures the broadness of size distribution and indicates the deviation from mean particle size. On the other hand, zeta potential is an indicator of the degree of repulsion between the charged droplets in dispersion, and it indirectly denotes the stability of colloidal dispersion. The positive or negative sign indicates the charge present at the particle surface. The formulation having a zeta potential value higher than +25 mV and lower than −25 mV indicates that the formulation system is stable. The control nanoemulsion without plant extract presented the lowest droplet size (138 nm) having a polydispersity index (0.26) value and zeta potential of −13.6 ± 0.15 mV (Table 1). On the other hand, when the extract was incorporated into the nanoemulsion, the average size was remarkably higher. i.e. nanoemulsion loaded with P. niruri extract shows mean particle size 192 nm i.e.< 200 nm, after 15 and 30 days increase in particle size, was observed ranged between 211 and 234 nm (Fig. 2) whereas, PDI values remained between 0.2 – 0.4 and zeta potential value was found to be in the range of −15.0 ± 0.21 mV to −18.4 ± 0.24 mV, showed that the formulations were with accepted stability (Fig. 3). Similarly, Rodrigues et al.[29]. and Restrepo et al.[30]. reported the mean particle size of astaxanthin nanoemulsion and rosemary nanoemulsion in a range from 197 to 676 nm. Our results were in accordance with Mazzarino et al.[31]. they prepared jaboticaba extract-based nanoemulsions with dropletsizesrangingbetween 122 and 211 nm. In the end, nanoemulsion prepared without extract and nanoemulsion containing P. niruri extract displayed pH values of 4.3- 6.8 respectively. The low energy method was used to obtain the small droplet size of nanoemulsion within a range of 200 nm after 30 days of storage. Based on the physicochemical properties and the results of the stability study, the nanoemulsion formulation was further investigated for FESEM and in vitro evaluations.
Table 1.
Average droplet distribution, poly dispersity index and Zeta potential of P. niruri based nanoemulsion and control nanoemulsion.
| Sample (P.niruri based nanoemulsion) | Average droplet distribution | poly dispersity index (PDI) | Zeta potential (mV) |
|---|---|---|---|
| 1st day | 192.6 | 0.4 | −15.0±0.21 |
| 15th day | 211.2 | 0.3 | −18.4±0.24 |
| 30th day | 234.3 | 0.2 | −18.4±0.24 |
| Control nanoemulsion | 138.2 | 0.2 | −13.6±0.15 |
Fig. 2.
(a) Average droplet distribution of control nanoemulsion without plant extract (b) Zeta potential of control nanoemulsion.
Fig. 3.
(a) Average droplet distribution of P. niruri based nanoemulsion–1st day (b) Zeta potential of P. niruri based nanoemulsion–1st day (c) Average droplet distribution of P. niruri based nanoemulsion–15 days (d) Average droplet distribution of P. niruri based nanoemulsion–30 days (e) Zeta potential of P. niruri based nanoemulsion–30 days.
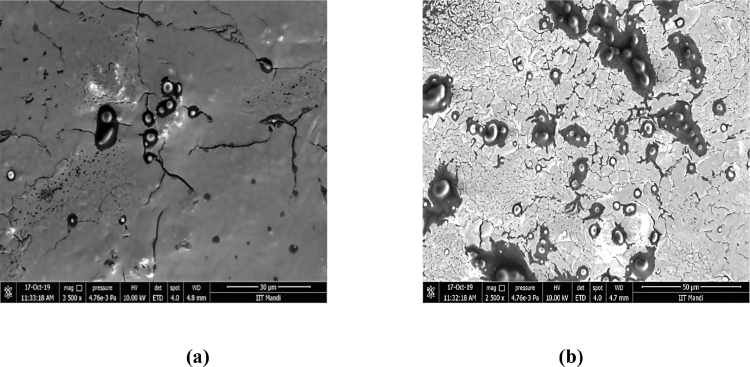
7.3. Transmission electron microscopy (TEM) and field emission scanning electron microscope (FESEM)
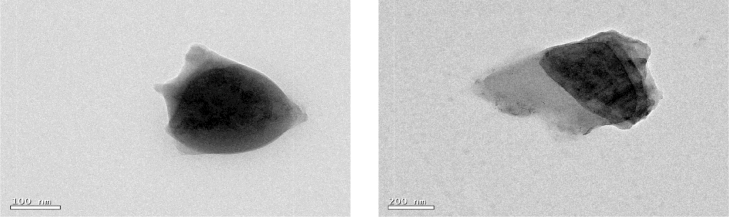
The control nanoemulsion and plant extract loaded oil in water nanoemulsion was carried out for morphological characteristics. The TEM results (Fig. 4) revealed nanoemulsions with spherical shape and an average size of approximately 200 nm, similar to that determined by the dynamic light scattering technique. On the other hand, FESEM of the prepared nanoemulsion (Fig. 5) showed homogeneous and spherical shapes the micrograph displays droplets were spherical. Our results were in accordance with the previous reports from other researchers indicating the spherical morphology of nanoemulsion Hussain et al.[32]. and Moghimi et al.[33].
Fig. 4.
Morphology characterization of nanoemulsion analyzed by Transmission Electron Microscopy (a) Control nanoemulsion without plant extract 100 nm scale (b) P. niruri extract loaded nanoemulsion 200 nm scale.
Fig. 5.
Morphology characterization of nanoemulsion analyzed by Field Emission Scanning Electron Microscope (a) Control nanoemulsion without plant extract (30 µm) at 3500x (b) P. niruri extract loaded nanoemulsion (50 µm) at 2500x.
7.4. Refractive index and viscosity of nanoemulsion
Both refractive index and viscosity are important parameters for the physicochemical characterization of nanoemulsion. Refractive index tells transparency of nanoemulsion, whereas, Viscosity measurement tells physical stability of nanoemulsion.The measurement of the refractive index of the nanoemulsion is related to the clarity of the prepared nanoemulsion influenced by the particle size. A less dense and clear formulation was observed in P. niruriextract-based nanoemulsion with an RI value of 1.46 ± 0.039. However, In addition, the viscosity was observed as 38 ± 7 cp.
7.5. DPPH radical scavenging activity
The DPPH method was used to assess the antioxidant activity of C. lanatus oil, P. niruri extract, and a nanoemulsion comprising P. niruri extract. DPPH is a stable free radical that is commonly employed to assess plant extracts' radical scavenging abilities.This approach is based on the capacity of the free radical DPPH i.e. violet color in methanol solution to react with a hydrogen donor molecule to produce the reduced form i.e. yellow color, which is measured by spectrophotometer by a reduction in absorbance. The substances that can carry out this reaction can be considered antioxidants and radical scavengers. Table 2 shows the findings of the DPPH assay used to determine the antioxidant activity of C. lanatus seed oil, P. niruri extract, and P. niruri loaded nanoemulsion which was prepared by using the low energy technique. As for C. lanatus oil, both the extract and the fabricated nanoemulsion demonstrated a similar free radical scavenging ability of DPPH in a concentration-dependent manner. The IC50 values for C. lanatus oil, P. niruri extract, and P. niruri loaded nanoemulsion were 64.3%, 61.9%, and 56.4%, respectively. Gallic acid, on the other hand, has an IC50 value of 49.7% . As assessed by DPPH free radical scavenging, the C. lanatus oil and plant exhibited good antioxidant activity. It might be possible that bioactive chemicals found in plants and oil might help in the healing process. Our findings were consistent with those of Adaramola et al.[34]. suggesting the free radical scavenging activity was 56% respectively in C. lanatus oil.
Table 2.
DPPH radical scavenging activity of P.niruri extract, C. lanatus oil, and nanoemulsion.
| Concentration (µg/ml) | % Inhibition | |||
|---|---|---|---|---|
| P. niruri extract | C. lanatus oil | Nanoemulsion | Gallic acid | |
| 10 | 12.0 ± 0.08 | 12.83 ± 0.62 | 11.46 ± 0.65 | 18.10 ± 0.16 |
| 20 | 22.15 ± 0.67 | 22.56 ± 0.54 | 24.9 ± 0.14 | 28.86 ± 0.85 |
| 40 | 36.53 ± 1.08 | 37.53 ± 0.41 | 38.9 ± 0.09 | 42.93 ± 1.14 |
| 60 | 51.83 ± 0.235 | 49.73 ± 0.75 | 58.4 ± 0.63 | 56.10 ± 1.44 |
| 80 | 63.06 ± 0.329 | 60.66 ± 0.47 | 64.93 ± 0.73 | 73.30 ± 0.96 |
| 100 | 72.80 ± 0.82 | 69.2 ± 0.69 | 80.43 ± 0.68 | 89.86 ± 0.20 |
| IC 50 | 61.9 | 64.3 | 56.4 | 49.7 |
These results are consistent with the IC50 values obtained by the DPPH method. There is a slight difference between the P. niruri nanoemulsion and the free extract which can be explained by the encapsulation of the antioxidant compounds within the droplets of the nanoemulsion, which avoids an optimal interaction with the reagent. Our study has reported a positive correlation with other studies reported by Khaled et al.[35]., Zorzi et al.[36]. and Shahbazi[37].
7.6. Hydrogen peroxide scavenging method
Hydrogen peroxide is extremely important because of its capacity to permeate cellular membranes, although peroxide is not particularly reactive, it can be harmful to cells because it produces hydroxyl radicals within them[38]. The ability of an extract to scavenge H2O2 might also be due to their phenolics, which could transfer electrons to H2O2 and so neutralize it to water[39]. The findings revealed that the extract exhibited strong H2O2 scavenging activity, which may be attributed to the antioxidant components. Because the antioxidant components in the extracts are having strong electron donors that convert H2O2 to H2O. The IC50 value for hydrogen peroxide radical scavenging activity of C. lanatus seed oil was 62.4%, 59.3% for P. niruri extract, and 57.1% for P. niruri based nanoemulsions, respectively, while the IC50 value for Gallic acid was 53.% (Table 3). However, when associated with the nanoemulsions, the antioxidant effect of P. niruri extract-loaded nanoemulsions increased significantly.
Table 3.
Hydrogen peroxide scavenging activity of P. niruri extract, C.lanatus oil, and nanoemulsion.
| Concentration (µg/ml) | % inhibition | |||
|---|---|---|---|---|
| P. niruri extract | C. lanatus oil | Nanoemulsion | Gallic acid | |
| 10 | 8.33 ± 0.62 | 11.01 ± 0.81 | 10.16 ± 0.62 | 9.66 ± 0.47 |
| 20 | 15.63 ± 0.32 | 23.80 ± 0.86 | 24.26 ± 0.61 | 22.66 ± 0.40 |
| 40 | 34.3 ± 0.49 | 39.86 ± 1.64 | 39.00 ± 0.89 | 38.93 ± 1.36 |
| 60 | 43.06 ± 0.82 | 50.33 ± 0.92 | 57.06 ± 1.24 | 55.60 ± 0.43 |
| 80 | 65.43 ± 0.42 | 63.33 ± 1.24 | 65.66 ± 0.33 | 71.50 ± 0.45 |
| 100 | 84.07 ± 0.72 | 70.00 ± 0.81 | 79.5 ± 0.37 | 90.46 ± 0.41 |
| IC 50 | 59.3 | 62.4 | 57.1 | 53.8 |
7.7. In-vitro drug release study
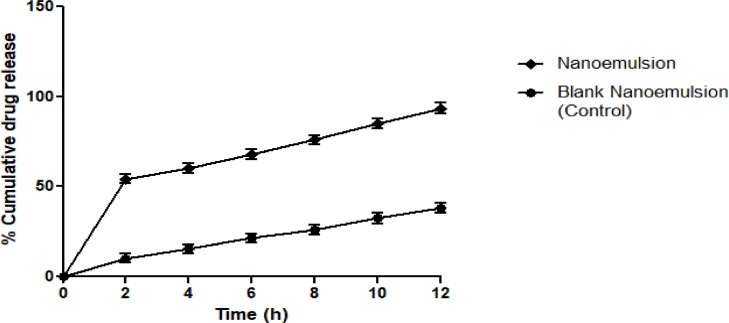
In vitro drug release of the blank nanoemulsion (control) without extract and P. niruri based nanoemulsion was carried out in phosphate buffer. The results showed that the blank nanoemulsion, due to its lipophilic nature and low water solubility, had the maximum in vitro drug release of 38.22±1.74% in 12 h. Whereas, the dissolution rate of the P. niruri-based nanoemulsion was increased, with a maximum drug release of 93.37± 1.82% in 12 h. This may be due to the tiny droplet size, which offered a wide surface area for the drug to be released. Consequently, the nanoemulsion offered a much higher release profile (P <0.05) than the nanoemulsion control drug release (Fig. 6). Our results are in agreement with a previous study by Laxmi et al.[40].
Fig. 6.
In vitro drug release study of control and P. niruri based nanoemulsion.
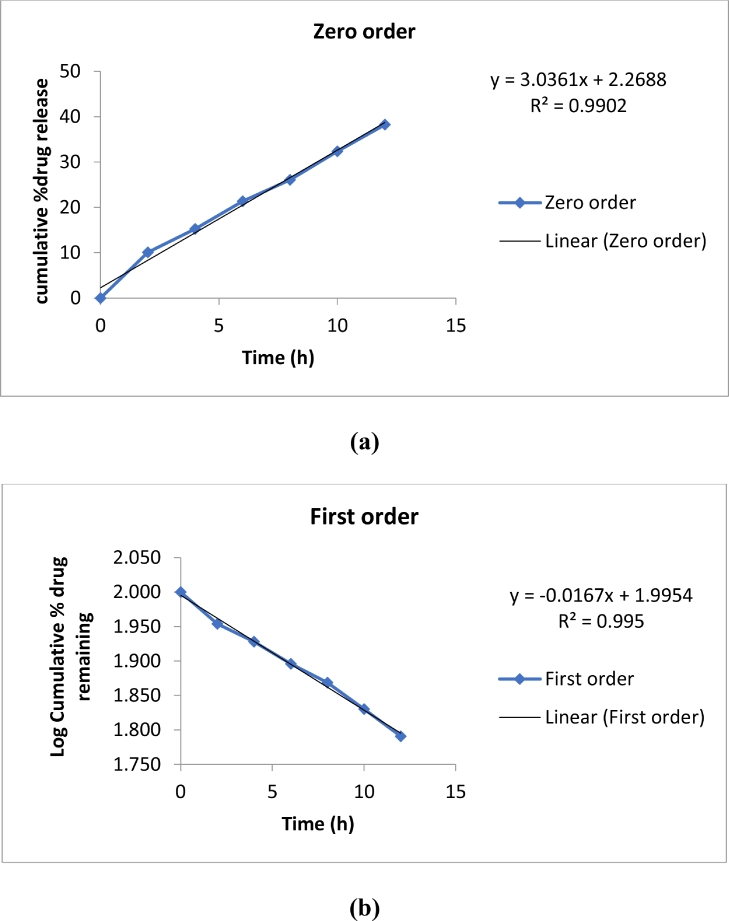
7.8. Drug release kinetics
Release kinetics of formulated nanoemulsion was studied. Sustained drug release was observed during the entire period of the study showed a burst release. The release kinetics was computed by fitting the release rate data to zero-order and first-order models. The best fit with the highes r2 value was found to be shown by first-order permeation which was near 0.995, thus; the release of drug from nanoemulsion formulations followed first-order release kinetic with R2 = 0.995 (Fig. 7).
Fig. 7.
In vitro drug release kinetics of P. niruri extract loaded nanoemulsion (a) zero-order (b) First-order.
7.9. Antibacterial and antifungal activity
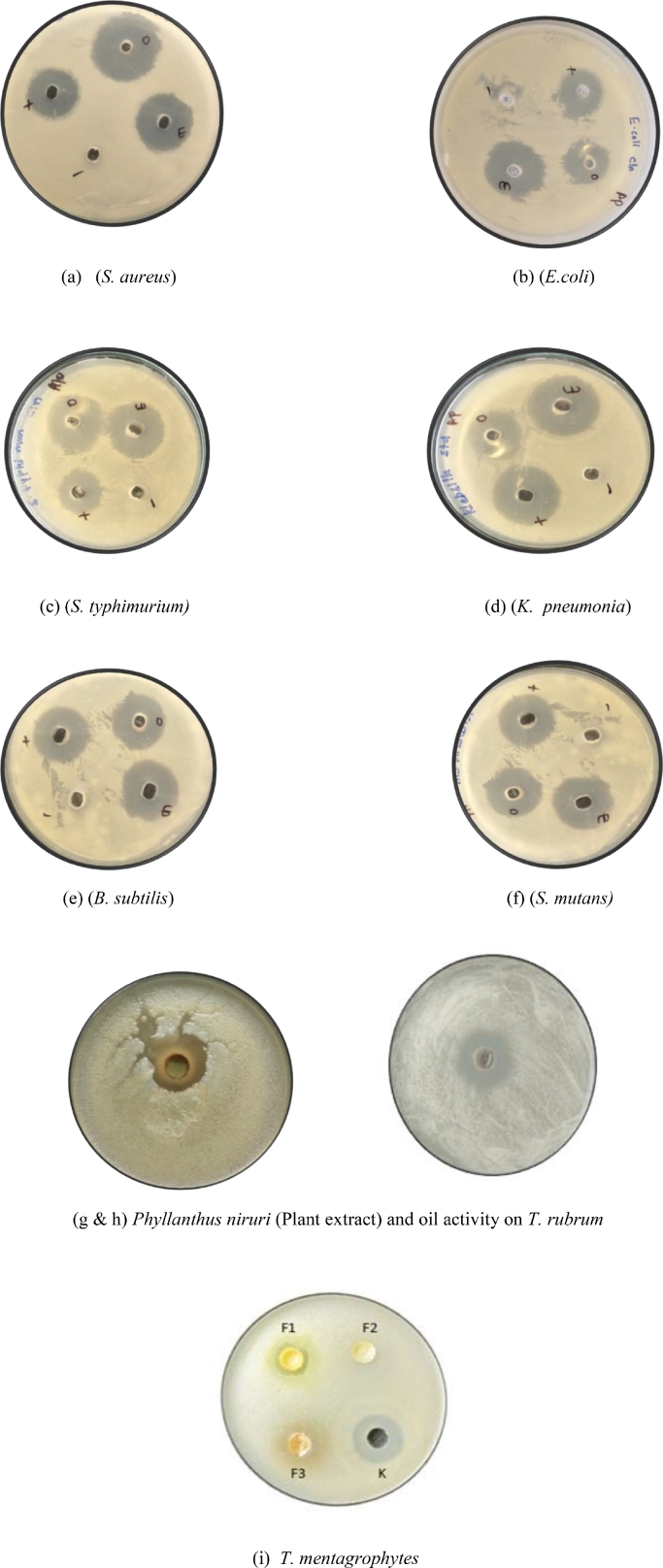
The antibacterial activity of C. lanatus seed oil, P. niruri extract, and P. niruri loaded nanoemulsion was tested against Gram-positive and Gram-negative bacteriausing an agar well diffusion method (Fig. 8 & Table 4). According to the findings of the current study, the effect of P. niruri extract was found to be more susceptible to S. aureus and Bacillus as compared to other bacterial pathogens. The previous studies had proven that the P. niruri plant extract was found to be more effective against the bacterial strains as compared to C. lanatus seed oil[41,42]. The antimicrobial activity of the P. niruri extract is because of the presence of components i.e. flavonoids, triterpenoids, phyllanthin, glycosides, and tannins[43]. Whereas, the P. niruri based nanoemulsions exhibited potent activity having a zone of inhibition in the range of 18–19 mm against E. coli, S. aureus, and B. subtilis. Whereas, the commercially available drug chloramphenicol had a zone of inhibition of 24–25 mm. The antibacterial assay indicated that the P. niruri based nanoemulsion had shown better potency as compared to P. niruri extract. Hence, the results suggested that the formulated plant-based nanoemulsion was active against all the tested bacterial strains. This was mostly due to the nanoemulsion tiny droplet size, which allowed it to penetrate the bacteria more easily than C. lanatus oil or P. niruri extract. The bacteria have a thick lipid coating and high lipid content, making them less susceptible to antimicrobial treatments. When compared to the bulk material, the nanoemulsion prepared with the surfactant was capable of crossing lipid barriers and therefore increasing the susceptibility of bacterial cells[44]. The antifungal activity of C. lanatus seed oil, P. niruri extract, and P. niruri loaded nanoemulsion was tested against the two dermatophytes strains i.e. T. rubrum and T. mentagrophytes (Table 4). The P. niruri based nanoemulsion exhibited potent antifungal activity having a zone of inhibition in the range of 18 mm, whereas, positive control Ketoconazole was found to have a 20 mm zone of inhibition. The finding of the current study agrees with the previous studies of different nanoemulsions by Jerobinetal[45]., Hassanzad et al.[27]. and Najmeh and Motakef[46] observed similar outcomes.
Fig. 8.
Representation of Antimicrobial activity of P. niruri plant extract, C. lanatus seed oil, formulated nanoemulsion on different bacterial and fungal strains.
Table 4.
Antimicrobial effect of C. lanatus oil, P. niruri extract, and nanoemulsion against bacterial and fungal strains.
| Bacterial strains | Zone of inhibition (mm ± SD) | |||
|---|---|---|---|---|
| C. lanatus oil | Phyllanthus niruri extract | Nanoemulsion | Positive control (Chloramphenicol) | |
| Escherichia coli | 12± 0.8 | 13.6± 0.4 | 18.0± 0.0 | 24.0± 0.0 |
| Klebsiella pneumonia | 9.3± 0.4 | 11.3± 0.9 | 14.0± 0.8 | 22.3± 0.4 |
| Staphylococcus aureus | 8.6± 0.4 | 18.0± 0.8 | 17.0± 0.8 | 22.6± 0.4 |
| Pseudomonas aruginosa | 8.3± 0.4 | 10.0± 0.0 | 9.0± 0.8 | 23.3± 0.4 |
| Salmonella typhimurium | 9.0± 0.8 | 15.6± 1.2 | 11.6± 1.2 | 23.0± 0.0 |
| Streptococcus mutans | 9.6± 0.4 | 14.6± 1.2 | 11.3± 0.4 | 24.0± 0.0 |
| Bacillus subtilis | 12.0± 0.8 | 17.3± 0.4 | 19± 0.8 | 25.0± 0.0 |
| Fungal strains | C. lanatus oil | Phyllanthus niruri extract | Nanoemulsion | Positive control (Ketoconazole) |
|---|---|---|---|---|
| Trichophyton rubrum | 20.3 ± 0.4 | 13.3± 0.5 | 18.5± 0.4 | 20.0± 0.0 |
| Trichophyton mentagrophytes | 17.6 ± 0.4 | 12.1± 0.7 | 18.0± 0.7 | 19.3± 0.1 |
8. Summary and conclusion
Nanoemulsions are proven to be drug delivery carriers that improve the drug penetration level and drug release profile. Plant extracts, when incorporated into nanoemulsions, are thought to possess good sort of benefits which include enhanced permeability, solubility, bioavailability, therapeutic activity, stability, improved distribution within tissues, and sustained delivery. Nowadays, there are several methods for the preparation of nanoemulsionsaredescribed in the literature. The nanoemulsions are often prepared by both high-energy and low-energy methods. The interest in low-energy methods has grown considerably because these methods are more energy-saving and thus more attractive for large-scale production. Our study firstly fabricated P. niruri extract loaded nanoemulsion which was successfully prepared by the low energy method.
The control nanoemulsion without plant extract presented the lowest droplet size i.e. 138 nm whereas, the nanoemulsion loaded with P. niruri extract showeda mean dropletsize of 192 nm i.e.< 200 nm, after fifteen and thirty days increase in particle size, was observed ranged between 211 nm--234 nm. When the extract was incorporated into the nanoemulsion, the average size was remarkably higher. This could be due to the interaction between the polymer and plant extract structure, the particle size increases with a rise in the addition of plant extract. Results of TEM were found to be in agreement with the results obtained from particle size analysis which assured that the droplet sizes were in the nano range. From FESEM, it was revealed that nanoemulsion showed homogeneous and spherical shapes displaying that the droplets were spherical.
The present results demonstrated the antioxidant activity of P.niruri nanoemulsion due to its excellent ability to eliminate free radicals using the DPPH and H2O2 method. Also, in vitro release profile of P. niruri based nanoemulsion demonstrated a sustained release after 12 h. The in vitro antioxidant, antibacterial and antifungal activity of P. niruriextract could be successfully enhanced by loading it into the nanoemulsion fabricated with sodium alginate and a nonionic surfactant. Therefore, the prepared nanoemulsion can be cost-effective and promising delivery systems having good antioxidant, antibacterial, and antifungal potential and could be utilized in a variety of research fields like cosmetics, food, and pharmaceutical industries in the future. However, in vivo skin irritation and performance testing are necessary for further investigation.
CRediT author statement
R.P. conceived and carried experiments, interpreted the data and wrote the original draft of the manuscript. A.N. conceived, designed experiment and supervised the research and revised the manuscript. P.C. was responsible for editing, analyzed the data and visualization. R.K. and M.A.K. was responsible for validation, formal analysis and investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Authors are gratefully acknowledged Shoolini University, Solan, Himachal Pradesh, India.
Contributor Information
Agnieszka Najda, Email: agnieszka.najda@up.lublin.pl.
Prince Chawla, Email: princefoodtech@gmail.com.
Mohammed Azhar Khan, Email: mk.azhar1@gmail.com.
References
- 1.Silva-Espinoza B.A., Ortega-Ramírez L.A., González-Aguilar G.A., Olivas I., Ayala-Zavala J.F. Antifungal protection and antioxidant enrichment of strawberry with essential oil of cinnamon leaf. Mex. Herb. Mag. 2013;36(3):217–224. [Google Scholar]
- 2.Kesarwani K., Gupta R. Bioavailability enhancers of herbal origin–An overview. Asian Pac. J. Trop. Biomed. 2013;3(4):253–266. doi: 10.1016/S2221-1691(13)60060-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee N.Y., Khoo W.K., Adnan M.A., Mahalingam T.P., Fernandez A.R., Jeevaratnam K. The pharmacological potential of Phyllanthus niruri. J. Pharm. Pharmacol. 2016;(8):953–969. doi: 10.1111/jphp.12565. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S., Singh A., Bajpai V., Singh B., Kumar B. Development of a UHPLC–MS/MS method for the quantitation of bioactive compounds in Phyllanthus species and its herbal formulations. J. Sep. Sci. 2017;40(17):3422–3429. doi: 10.1002/jssc.201601361. [DOI] [PubMed] [Google Scholar]
- 5.Sunitha J., Krishna S., Ananthalakshmi R., Jeeva J.S., Girija A.S., Jeddy N. Antimicrobial effect of leaves of Phyllanthus niruri and Solanum nigrum on caries causing bacteria–An in vitro study. J. Clin. Diagn. Res. 2017;11(6):KC01. doi: 10.7860/JCDR/2017/23602.10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nisar M.F., He J., Ahmed A., Yang Y., Li M., Wan C. Chemical components and biological activities of the genus phyllanthus–A review of the recent literature. Molecules. 2018;23(10):2567. doi: 10.3390/molecules23102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods–Impact on human health. Pharmacogn. Rev. 2010;4(8):118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaturvedi M., Kumar M., Sinhal A., Saifi A. Recent development in novel drug delivery systems of herbal drugs. Int. J. Green Pharm. 2011;5(2):87–94. doi: 10.30574/gscarr.2021.8.2.0158. [DOI] [Google Scholar]
- 9.Ee S.L., Duan X., Liew J., Nguyen Q.D. Droplet size and stability of nano-emulsions produced by the temperature phase inversion method. Chem. Eng. J. 2008;140(1-3):626–631. [Google Scholar]
- 10.Pathania R., Kaushik R., Khan M.A. Essential oil nanoemulsions and their antimicrobial and food applications. Curr. Res. Nutr. Food Sci. J. 2018;6(3):626–643. [Google Scholar]
- 11.Sutradhar K.B., Amin M.L. Nanoemulsions–Increasing possibilities in drug delivery. Eur. J. Nanomed. 2013;5(2):97–110. [Google Scholar]
- 12.Quintão F.J., Tavares R.S., Vieira-Filho S.A., Souza G.H., Santos O.D. Hydroalcoholic extracts of Velloziasquamata–Study of its nanoemulsions for pharmaceutical or cosmetic applications. Rev. Bras. Farmacogn. 2013;23(1):101–107. [Google Scholar]
- 13.Bidone J., Zorzi G.K., Carvalho E.L., Simões C.M., Koester L.S., Bassani V.L., Teixeira H.F. Incorporation of Achyroclinesatureioides (Lam.) DC extracts into topical nanoemulsions obtained by means of spontaneous emulsification procedure. Ind. Crops Prod. 2014;62:421–429. [Google Scholar]
- 14.Porras M., Solans C., Gonzalez C., Gutierrez J.M. Properties of water-in-oil (W/O) nano-emulsions prepared by a low-energy emulsification method. Colloids Surf. A Physicochem. Eng. Asp. 2008;324(1–3):181–188. [Google Scholar]
- 15.Forgiarini A., Esquena J., González C., Solans C. In: Trends in Colloid and Interface Science XV. Koutsoukos P.G., editor. Vol. 18. Springer; Berlin, Heidelberg: 2001. Formation and stability of nano-emulsions in mixed nonionic surfactant systems; pp. 184–189. [Google Scholar]
- 16.Meng X., Tian F., Yang J., He C.N., Xing N., Li F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. 2010;21(5):1751–1759. doi: 10.1007/s10856-010-3996-6. [DOI] [PubMed] [Google Scholar]
- 17.Hamedi H., Moradi S., Tonelli A.E., Hudson S.M. Preparation and characterization of chitosan–Alginate polyelectrolyte complexes loaded with antibacterial thyme oil nanoemulsions. Appl. Sci. 2019;9(18):3933. [Google Scholar]
- 18.Mason T.G., Wilking J.N., Meleson K., Chang C.B., Graves S.M. Nanoemulsions–Formation, structure, and physical properties. J. Condens. Matter Phys. 2006;18(41):R635. [Google Scholar]
- 19.Chawla P., Kumar N., Kaushik R., Dhull S.B. Synthesis, characterization and cellular mineral absorption of nanoemulsions of Rhododendron arboreum flower extracts stabilized with gum arabic. J. Food Sci. Technol. 2019;56(12):5194–5203. doi: 10.1007/s13197-019-03988-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayoola G.A., Lawore F.M., Adelowotan T., Aibinu I.E., Adenipekun E., Coker H.A., Odugbemi T.O. Chemical analysis and antimicrobial activity of the essential oil of Syzigiumaromaticum (clove) Afr. J. Microbiol. Res. 2008;2(7):162–166. [Google Scholar]
- 21.Yildirim S.T., Oztop M.H., Soyer Y. Cinnamon oil nanoemulsions by spontaneous emulsification–Formulation, characterization and antimicrobial activity. LWT - Food Sci. Technol. 2017;84:122–128. [Google Scholar]
- 22.Ahmad N., Alam M.A., Ahmad F.J., Sarafroz M., Ansari K., Sharma S., Amir M. Ultrasonication techniques used for the preparation of novel Eugenol-Nanoemulsion in the treatment of wounds healings and anti-inflammatory. J. Drug Deliv. Sci. Technol. 2018;46:461–473. [Google Scholar]
- 23.Sundararajan B., Moola A.K., Vivek K., Kumari B.R. Formulation of nanoemulsion from leaves essential oil of Ocimumbasilicum L. and its antibacterial, antioxidant and larvicidal activities (Culex quinquefasciatus) Microb. Pathog. 2018;125:475–485. doi: 10.1016/j.micpath.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 24.Vinodhini V., Lokeswari T.S. Antioxidant activity of the isolated compounds, methanolic and hexane extracts of Toona ciliata leaves. Int. J. Eng. Technol. 2014;4(3):135–138. [Google Scholar]
- 25.Yu W.P., Wong J.P., Chang T.M. Sustained drug release characteristics of biodegradable composite poly (d, l) Lactic Acidpoly (I) lactic acid microcapsules containing ciprofloxacin. Artif. Cells Nanomed. Biotechnol. 2000;28(1):39–55. doi: 10.3109/10731190009119784. [DOI] [PubMed] [Google Scholar]
- 26.Balakumar S., Rajan S., Thirunalasundari T., Jeeva S. Antifungal activity of Aegle marmelos(L.) Correa (Rutaceae) leaf extract on dermatophytes. Asian Pac. J. Trop. Biomed. 2011;1(4):309–312. doi: 10.1016/S2221-1691(11)60049-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassanzad Azar H., Ghafari A., Yousefizadeh S., Fathollahi M., Aminzare M. Antimicrobial effects of the nanoemulsion of rosemary essential oil against important foodborne pathogens. J. Hum. Environ. Health Promot. 2019;5(2):79–85. doi: 10.29252/jhehp.5.2.6. [DOI] [Google Scholar]
- 28.CLSI/Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium-Forming Filamentous Fungi.Wayne. (Approved Standard M38-A). 2002.
- 29.Rodrigues F.V., Diniz L.S., Sousa R.M., Honorato T.D., Simão D.O., Araújo C.R., Gonçalves T.M., Rolim L.A., Goto P.L., Tedesco A.C., Siqueira-Moura M.P. Preparation and characterization of nanoemulsion containing a natural naphthoquinone. Quim. Nova. 2018;41(7):756–761. [Google Scholar]
- 30.Restrepo A.E., Rojas J.D., García O.R., Sánchez L.T., Pinzón M.I., Villa C.C. Mechanical, barrier, and color properties of banana starch edible films incorporated with nanoemulsions of lemongrass (Cymbopogon citratus) and rosemary (Rosmarinus officinalis) essential oils. Food Sci. Technol. Int. 2018;24(8):705–712. doi: 10.1177/1082013218792133. [DOI] [PubMed] [Google Scholar]
- 31.Mazzarino L., da Silva Pitz H., Lorenzen Voytena A.P., Dias Trevisan A.C., Ribeiro-Do-Valle R.M., Maraschin M. Jaboticaba (Plinia peruviana) extract nanoemulsions–Development, stability, and in vitro antioxidant activity. Drug Dev. Ind. Pharm. 2018;44(4):643–651. doi: 10.1080/03639045.2017.1405976. [DOI] [PubMed] [Google Scholar]
- 32.Hussain A.I., Anwar F., Sherazi S.T., Przybylski R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimumbasilicum) essential oils depends on seasonal variations. Food chem. 2008;108(3):986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Moghimi R., Aliahmadi A., McClements D.J., Rafati H. Investigations of the effectiveness of nanoemulsions from sage oil as antibacterial agents on some food borne pathogens. LWT - Food Sci. Technol. 2016;71:69–76. [Google Scholar]
- 34.Adaramola B., Onigbinde A. Physicochemical properties and mineral compositions of pawpaw and watermelon seed oils. Pak. J. Nutr. 2016;15(1):23. [Google Scholar]
- 35.Khaled F.M., Ramadan A.K., Ashoush I.A. Nanoencapsulation and nanoemulsion of bioactive compounds to enhance their antioxidant activity in food. Int. J. Food Sci. Technol. 2014;4(3):1–22. [Google Scholar]
- 36.Zorzi G.K., Caregnato F., Moreira J.C., Teixeira H.F., Carvalho E.L. Antioxidant effect of nanoemulsions containing extract of Achyroclinesatureioides (Lam) DC—Asteraceae. AAPS PharmSciTech. 2016;17(4):844–850. doi: 10.1208/s12249-015-0408-8. [DOI] [PubMed] [Google Scholar]
- 37.Shahbazi Y. Antioxidant, antibacterial, and antifungal properties of nanoemulsion of clove essential oil. Nanomed. Res. J. 2019;4(4):204–208. [Google Scholar]
- 38.Srinivasan R., Chandrasekar M.J., Nanjan M.J., Suresh B. Free radical scavenging activity of Ipomoea obscura (L.) Ker-Gawl. J. Nat. Remedies. 2007;7(2):184–188. [Google Scholar]
- 39.Gülçin İ., Huyut Z., Elmastaş M., Aboul-Enein H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010;3(1):43–53. [Google Scholar]
- 40.Laxmi M., Bhardwaj A., Mehta S., Mehta A. Development and characterization of nanoemulsion as carrier for the enhancement of bioavailability of artemether. Artif. Cells Nanomed. Biotechnol. 2015;43(5):334–344. doi: 10.3109/21691401.2014.887018. [DOI] [PubMed] [Google Scholar]
- 41.Bello H.S., Ismail H.Y., Goje M.H., Mangga H.K. Antimicrobial activity of Citrullus Lanatus (Watermelon) seeds on some selected bacteria. Res. J. Biotechnol. 2016;2(6):39–43. [Google Scholar]
- 42.Singh R.P., Pal A., Pal K. Antioxidant activity of ethanolic and aqueous extract of Phyllanthus niruri–In vitro. World J. Pharm. Pharm. Sci. 2016;5:1994–2000. [Google Scholar]
- 43.Hossain M.A., Rahman S.M. Structure characterization and quantification of a new isoflavone from the arial parts of Phyllanthus niruri. Arab. J. Chem. 2019;12(8):2257–2261. [Google Scholar]
- 44.Gupta, S.; Bansal, R.; Ali, J.; Gabrani, R.; Dang, S. Development and characterization of polyphenon 60 and caffeine microemulsion for enhanced antibacterial activity. Biomed. Res. Int. 2014. [DOI] [PMC free article] [PubMed]
- 45.Jerobin J., Makwana P., Kumar R.S., Sundaramoorthy R., Mukherjee A., Chandrasekaran N. Antibacterial activity of neem nanoemulsion and its toxicity assessment on human lymphocytes in vitro. Int. J. Nanomed. 2015;10(Suppl 1):77. doi: 10.2147/IJN.S79983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Najmeh Feizi Langaroudi N., Motakef Kazemi N. Preparation and characterization of O/W nanoemulsion with mint essential oil and parsley aqueous extract and the presence effect of chitosan. Nanomed. Res. J. 2019;4(1):48–55. [Google Scholar]