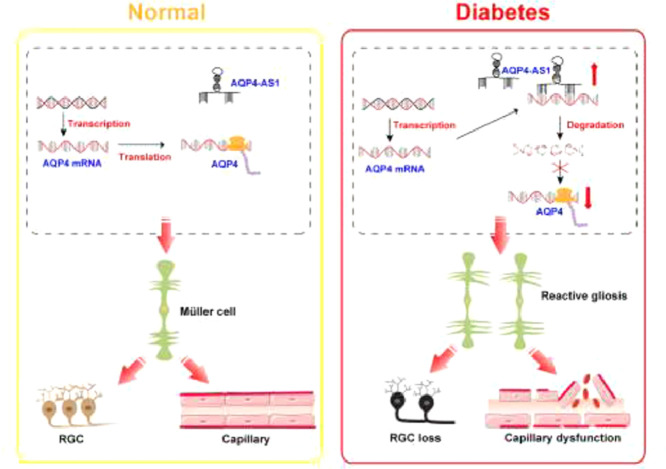
Summary
Background
Diabetic retinopathy (DR) is a leading cause of blindness in the working-age population, which is characterized by retinal neurodegeneration and vascular dysfunction. Long non-coding RNAs (LncRNAs) have emerged as critical regulators in several biological processes and disease progression. Here we investigated the role of lncRNA AQP4-AS1 in retinal neurovascular dysfunction induced by diabetes.
Methods
Quantitative RT-PCR was used to detect the AQP4-AS1 expression pattern upon diabetes mellitus-related stresses. Visual electrophysiology examination, TUNEL staining, Evans blue staining, retinal trypsin digestion and immunofluorescent staining were conducted to detect the role of AQP4-AS1 in retinal neurovascular dysfunction in vivo. MTT assays, TUNEL staining, PI/Calcein-AM staining, EdU incorporation assay transwell assay and tube formation were conducted to detect the role of AQP4-AS1 in retinal cells function in vitro. qRT-PCR, western blot and in vivo studies were conducted to reveal the mechanism of AQP4-AS1-mediated retinal neurovascular dysfunction.
Findings
AQP4-AS1 was significantly increased in the clinical samples of diabetic retinopathy patients, high glucose-treated Müller cells, and diabetic retinas of a murine model. AQP4-AS1 silencing in vivo alleviated retinal neurodegeneration and vascular dysfunction as shown by improved retinal capillary degeneration, decreased reactive gliosis, and reduced RGC loss. AQP4-AS1 directly regulated Müller cell function and indirectly affected endothelial cell and RGC function in vitro. Mechanistically, AQP4-AS1 regulated retinal neurovascular dysfunction through affecting AQP4 levels.
Interpretation
This study reveals AQP4-AS1 is involved in retinal neurovascular dysfunction and expected to become a promising target for the treatment of neurovascular dysfunction in DR.
Funding
This work was generously supported by the grants from the National Natural Science Foundation of China (Grant No. 81800858, 82070983, 81870679 and 81970823), grants from the Medical Science and Technology Development Project Fund of Nanjing (Grant No ZKX17053 and YKK19158), grants from Innovation Team Project Fund of Jiangsu Province (No. CXTDB2017010), and the Science and Technology Development Plan Project Fund of Nanjing (Grant No 201716007, 201805007 and 201803058).
Keywords: Long noncoding RNA, Müller cell, Reactive gliosis, Neurovascular dysfunction
Graphical abstract
Research in Context.
Evidence before this study
Diabetic retinopathy (DR) is a leading cause of blindness in the working-age population, which is characterized by neurovascular dysfunction. Current available therapies for DR have limited efficacy, and new therapeutic strategies need to be developed. Aberrant lncRNA expression is involved in the pathogenesis of several diseases. AQP4-AS1 is a lncRNA that is transcribed from the anti-sense strand of AQP4 gene in chromosome 18. Previous studies have implicated that antisense transcript usually regulates sense transcript at transcriptional or post-transcriptional levels, affecting the expression and biological function of sense transcripts. The role of AQP4 in neurodegeneration and vascular permeability has been demonstrated. However, the role of AQP4-AS1 in neurovascular dysfunction induced by DR remains unknown.
Added value of this study
AQP4-AS1 is significantly up-regulated in diabetic retinas and Müller cells cultured in high glucose medium. AQP4-AS1 knockdown alleviates diabetes-induced retinal impairment of neurovascular unit in vivo. AQP4-AS1 knockdown directly inhibits Müller cell function and indirectly affects endothelial cell and RGC function in vitro. Moreover, AQP4-AS1 knockdown increases AQP4 expression and inhibits reactive gliosis, thereby attenuating neurovascular dysfunction induced by DR.
Implications of all the available evidence
Our study has uncovered that AQP4-AS1 knockdown could alleviates retinal neurodegeneration and vascular dysfunction induced by DR. These findings suggest that AQP4-AS1 is a promising target for treating retinal neurovascular complications.
Alt-text: Unlabelled box
Introduction
Diabetes mellitus is a complex metabolic disease, which can increase the risk of cardiovascular disease, kidney failure, blindness, and lower-limb amputation.1 Diabetic retinopathy (DR) is one of the major complications of diabetes mellitus. It is the leading cause of visual disability and blindness in working-age population.2 DR is generally considered as a microvascular disorder. At the non-proliferative stage, DR is characterized by retinal capillary degeneration, such as microaneurysm, intraretinal hemorrhage, and capillary closure. At the proliferative stage, DR is characterized by vascular leakage, hemorrhaging, angiogenesis, vitreal contraction, and retinal detachment.3 In fact, the retina is actually a nervous system tissue. The pathogenesis of DR is also associated with retinal neuroglial degeneration, such as reactive gliosis, neuronal degeneration, and RGC loss. Previous studies have shown that retinal neurodegeneration participates in the development of retinal vascular dysfunction.4 Retinal neurodegeneration is an earlier event in the pathogenesis of DR, which occurs even before retinal capillary degeneration.5 Thus, identification of the mediators in the crosstalk between neurodegeneration and vascular dysfunction is essential for the development of new therapeutic strategy. Please confirm that the provided emails “dryaojin@126.com” and “jqin710@vip.sina.com” are the correct address for official communication, else provide an alternate e-mail address to replace the existing one, because private e-mail addresses should not be used in articles as the address for communication.
The current treatments of DR mainly include laser photocoagulation, anti-VEGF therapeutics, and corticosteroids therapy.6 However, laser treatment is usually associated with the side effects such as diminished visual field, reduced color vision, and reduced contrast sensitivity.7 Intravitreous injections of anti-VEGF agents may cause deleterious effects for the remaining healthy retina, such as neuronal toxicity8 and geographic atrophy.9 Repetitive injection of corticosteroids may lead to substantial adverse effects such as infection, glaucoma, and cataract formation.10 New insights into the underlying mechanism of DR may provide alternative therapeutic methods.
Long non-coding RNAs (LncRNAs) are defined as the transcripts greater than 200 nucleotides. They have similar structure as mRNAs but do not encode proteins. LncRNAs regulate gene expression through affecting mRNA splicing, transcription, translation, and genomic imprinting.11 Recent studies have shown that lncRNAs are important regulators of cellular differentiation, organogenesis and tissue homeostasis.12 Moreover, abnormal lncRNA expression is implicated in the pathological conditions such as cancers, neurodegenerative diseases, and cardiovascular diseases, providing novel biomarkers and pharmaceutical targets.13 The homeostasis and plasticity of neurovascular crosstalk is required for exquisite gene regulatory mechanism. Given the critical role of lncRNAs in gene regulation and tissue homeostasis, it is not surprising that lncRNAs play important roles in neurovascular crosstalk in DR.
AQP4-AS1 is a long non-coding RNA (lncRNA) that is transcribed from the anti-sense strand of AQP4 gene in chromosome 18. AQP4 is heterogeneously restrictedly expressed in the Müller glial cells and astrocytes in the retina.14 AQP4 not only take part in water transport, but also plays important roles in blood-brain barrier stability,15 cerebral water content,16 glutamate transports17 and nerve inflammation.18 The expression of anti-sense transcript usually affects the expression of the sense transcript. Due to the critical role of AQP4 in neurodegeneration and vascular permeability, we speculate that AQP4-AS1 may be involved in neurodegeneration and vascular dysfunction through regulating AQP4 level in DR. In this study, we investigated the role of AQP4-AS1 in retinal neurovascular dysfunction and estimated whether AQP4-AS1 could serve as a therapeutic target for DR.
Methods
Ethics statement
All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Ethics and Experimentation Committee of Nanjing Medical University (NJMUEC-2018-37). Usage of patient samples was approved by the Ethical Committee of the Affiliated Eye Hospital, Nanjing Medical University (NJMUEHEC-2018-10; NJMUEHEC-2021-21). The surgical specimens were handled according to the Declaration of Helsinki. All patients enrolled in this study were given the informed consent before inclusion.
Sample collection
All patients enrolled in this study were given the informed consent before inclusion. The aqueous humor samples were collected from diabetic retinopathy, cataract, glaucoma and trauma patients. The fibrovascular membranes were obtained from the patients undergoing pars plana vitrectomy for proliferative DR treatment or undergoing pars plana vitrectomy for the treatment of idiopathic macular holes. The retinal samples were obtained from patients undergoing traumatic enucleation. The relevant information about the patients was shown in Supplemental Tables 1, 2.
Cell culture and transfection
Human retinal Müller cells were collected from two patients with one eye each (one was 52 years old, and the other was 46 years old). Mouse primary Müller cells were isolated from postnatal day 7-10 mice. Eyes were immersed in Dulbecco's modified Eagle medium (DMEM, Gibco, Cat# C11995500BT) containing 1% Penicillin/Streptomycin at 4°C overnight. Mid-peripheral regions of retina were cut into approximately 1 mm3 pieces and subsequently trypsinized at 37°C for 20 min. After that, the retinas were rinsed with DMEM containing 10% fetal bovine serum (FBS, Gibco, Cat# 10099141) to terminate the digestion reaction and dissociated into small aggregates before attaching to the bottom of dish. Primary Müller cells were identified using antibody against glial acidic fibrillary protein (GFAP) (Abcam Cat# ab68428, RRID: AB_1209224) and glutamine synthetase (GS) (Abcam Cat# ab228590). The third passaged cells were used for the subsequent experiments. Primary RGCs were isolated from postnatal day 0-3 mice and dissociated in a papain solution (15 U/mL) and collagenase (70 U/mL) for 15 min. Subsequently, the suspension of retinal cells was immunopanned on plates incubated with mouse Thy-1.2 antibody (Bio-Rad Cat# MCA02R, RRID: AB_323481) to purify RGCs. Primary RGCs were identified using antibody against βIII-tubulin (Abcam Cat# ab18207, RRID: AB_444319). Human retinal vascular endothelial cells (HRVEC), human retinal pericytes and human retinal pigment epithelial (ARPE-19)(RRID:CVCL_0145) cells were obtained from Cell Systems Corp. (CSC, Kirkland, WA, USA). Cell lines were authenticated using Short Tandem Repeat (STR) analysis. Mycoplasma testing has been performed with Vazyme D101-01 MycoBlue Mycoplasma Detector recently.
Primary Müller cells were cultured in DMEM with 10% FBS, supplemented with 1% penicillin/streptomycin. Purified primary RGCs were plated into 24-well plates precoated with poly-L-lysine and cultured in 1 ml neurobasal growth medium supplemented with penicillin, streptomycin, CNTF, BDNF, 10% FBS, forskolin, and B27. HRVECs were cultured in EGM2-MV medium supplemented with 5% FBS at 5.55 mmol/L D-glucose concentration. RPEs were cultured in DMEM/F-12 (1:1) [Gibco Cat# C11330500BT] with 10% FBS, supplemented with 1% penicillin/streptomycin. Human retinal pericytes were cultured in DMEM at 5.5 mmol/L D-glucose concentration supplemented with 10% FBS and cell growth supplements. These cells were maintained at 37 °C in a 95% humidified atmosphere containing 5% CO2. These cells were transfected with the synthesized small interfering RNAs (20 nM; siRNAs; GenePharm) targeting AQP4-AS1 using the Lipofectamine RNAi Max (Life Technologies, Cat# 13778150) according to the manufacturer's protocol. The siRNA sequences for AQP4-AS1 are provided in Supplemental Table 3.
Cocultures of cells
Co-culture models were established to investigate the effects of Müller cells on the function of HRVECs or RGCs. HRVECs (2.5 × 105) were inoculated on a 24-well plate with 500 μl of EGM2-MV medium supplemented with 5% FBS at 5.55 mmol/L D-glucose concentration. After the cells adhered (6-8h), the culture medium was changed into 1 ml of DMEM containing 10% FBS. Primary RGCs (2.5 × 105) were inoculated on a 24-well plate with 500 μl specific medium. And then Müller cells (1 × 105) were precultured in 0.4 μm membrane inserts (Millipore, USA, Cat# MCHT24H48). Co-cultures were maintained in 37 °C in a 95% humidified atmosphere containing 5% CO2 for additional 24 h. There was a physical separation of the two cell populations, which accessed to media below and above the inserts in each of the wells.
Animals
C57BL/6J (8-week old, male) mice were purchased from the Animal Core Facility of Nanjing Medical University (Nanjing, Jiangsu, China). Aqp4-deficient mice (Aqp4−/− mice) (IMSR Cat# NM-KO-190243, RRID: IMSR_NM-KO-190243) were donated by Jiangsu Key Laboratory of Neurodegeneration (Nanjing Medical University). They were bred and maintained under environmentally controlled conditions (ambient temperature, 24°C; humidity, 40%) on a 12-h light/dark cycle with access to food and water. All experiments were performed on age-and weight-matched littermates (20–45 g).
Induction of diabetic mice
After 12-h fast, two-months old Aqp4−/− mice and wild-type mice received a single daily intraperitoneal injection of streptozotocin (STZ, 50 mg/kg, Biofroxx, Cat# EZ5679D158) over 5 days, dissolved in 0.1 M sodium citrate buffer, pH 4.5. The non-diabetic controls received an equivalent amount of citrate buffer. Seven days after STZ injection, the blood from tail vein was used for the measurement of glucose level by Contour TS meter. Blood glucose levels higher than 16.7 mM were considered diabetic.
Evans blue staining
Retinal vascular leakage was determined using Evans blue dye (Sigma Aldrich, Cat# E2129). Evans blue dye was dissolved in PBS and sonicated for 20 min at a concentration of 100 mg/ml and filtered through a 0.22 μm filter prior to administration. The mice were anesthetized with ketamine (80 mg/kg) and xylazine (4 mg/kg). The dye solution was injected via the femoral vein (45 mg/kg) and filled with heparinized saline. After the dye circulated for 2 h, about 0.2 ml blood was obtained from the anesthetized mice, and then the chest cavity was opened. The animals were perfused via the left ventricle. The cornea, lens, and vitreous humor were removed. The remaining retinas were fixed in 4% PFA in phosphate-buffered saline for 30 min at room temperature, and then dried for 5 h. Evans blue was extracted by incubating each retina in formamide (0.2 ml per retina) for 12 h at 78°C, and then the resulting suspensions were centrifuged at 12,000 g for 45 min. Evans blue dye in the supernatant was determined at 620 nm (blue) and 740 nm (background). Blood samples were treated similarly but not treated with formamide. They were centrifuged for at 3,600 g at 25°C for 15 min. The concentration of Evans blue was calculated from a standard curve and normalized to dry weight of retina and the time-averaged concentration of Evans blue in the plasma, which was calculated as follows:19
Retinal trypsin digestion
The enucleated eyes were fixed in 10% neutral formaldehyde for 24 h. The retina was removed and washed for 30 min at least 4-5 times. The retina was incubated with 3% trypsin (1:250, BioFroxx, Cat# 1004GR025) in 0.1M Tris buffer (pH 7.8) at 37°C until the medium became cloudy. It was gently shook to free vessel network, washed, and mounted on the glass slides for dry. Retinal vessels were stained with periodic acid-Schiff-hematoxylin and observed under a light microscopy. The number of acellular capillary was counted in 30 randomly selected fields (× 600 magnification) in the mid-retina in a masked manner.19
Immunofluorescent staining of retinal slices
Retinal tissues were cryoprotected in 30% sucrose for 24 h and embedded in OCT medium (Thermo Scientific, Cat# 6502). Ten-micrometer tissue sections were cut at -20°C in a cryostat (Thermo Scientific) and collected on the poly-L-lysine coated slides. After blocking with 5% BSA and 0.1% Triton X-100 in PBS, the retinal sections were incubated overnight at 4°C with the following primary antibodies: GFAP (1:200, Abcam Cat# ab68428, RRID: AB_1209224), NeuN (1:300, Abcam Cat# ab177487, RRID: AB_2532109), Calretinin (1:500, Santa Cruz Biotechnology Cat# sc-365956, RRID: AB_10846469), Calbindin (1:200, Santa Cruz Biotechnology Cat# sc-365360, RRID: AB_10841576), Rhodopsin (1:400, Abcam Cat# ab5417, RRID: AB_304874) and PKCα (1:400, Abcam Cat# ab32376, RRID: AB_777294). The retinal sections were washed and incubated for 3 h at room temperature with the fluorophore-conjugated secondary antibodies. The retinal sections were observed using an Olympus IX-73 microscopy and the fluorescent signals were analyzed by Image J.
Statistical analysis
Data analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, CA). All results are expressed as means ± SD. For the normally distributed data with equal variance, the significant difference was determined by Student's t-test (when two groups were compared) or one-way or two-way ANOVA to test the effect of group (when > 2 groups were compared). For the non-normally distributed data or data with unequal variances, the significant difference was determined by non-parametric Mann-Whitney's U-test (when two groups were compared) or Kruskal-Wallis's test followed by post-hoc Bonferroni's test (when > 2 groups were compared). P < 0.05 was considered statistically significant.
Role of funding source
No entity other than the authors listed played any role in the design of this study; the collection, analyses, or interpretation of the data; writing of manuscript or decision to submit study for publication.
Results
AQP4-AS1 level is significantly up-regulated under diabetic condition
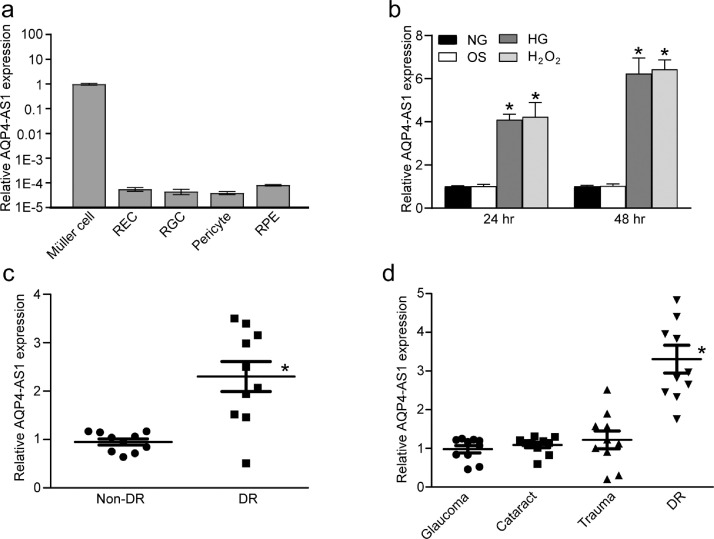
AQP4-AS1 is a long non-coding RNA that is transcribed from the anti-sense strand of AQP4 gene. qRT-PCR assays were conducted to detect the expression pattern of AQP4-AS1 in a wide array of retinal cells, including Müller cells, retinal endothelial cells (REC), retinal ganglion cell (RGCs), pericytes, and RPE cells. The results showed that AQP4-AS1 was specially expressed in Müller cells, but not in other retinal cells (Fig. 1a). Müller cells were exposed to high glucose or H2O2 to mimic diabetes-related stresses for 24 h or 48 h in vitro. Increased level of AQP4-AS1 were observed in response to high glucose or oxidative stress (Fig. 1b). The levels of AQP4-AS1 in the fibrovascular membranes of diabetic patients were significantly higher than those in the idiopathic epiretinal membranes of non-diabetic controls (Fig. 1c). Aqueous humor is the body fluid in ocular tissue, and its condition is tightly associated with the pathogenesis of several ocular diseases. AQP4-AS1 levels were significantly up-regulated in the aqueous humor of diabetic patients, but not in other patients with glaucoma, cataract, or trauma (Fig. 1d). Together, these results suggest that AQP4-AS1 is a potential regulator of diabetic retinopathy in vivo and in vitro.
Fig. 1.
AQP4-AS1 level is significantly up-regulated under diabetic condition. (a) Quantitative reverse transcriptase-PCRs (qRT-PCRs) were performed to detect the levels of AQP4-AS1 in a wide array of retinal cells, including Müller cells, retinal endothelial cells (RECs), retinal ganglion cells (RGCs), pericytes, and RPE cells (n = 4). (b) qRT-PCRs were performed to detect the level of AQP4-AS1 gene in Müller cells after the following treatments for 24 h or 48 h, including normal glucose (5.55 mM, NG), 5.55 mM glucose + 24.45 mM mannitol (Osmotic control, OS), high glucose (30 mM, HG) or H2O2 (200 μM). *P < 0.05, n = 4, one-way ANOVA followed by the Bonferroni post hoc test. (c) qRT-PCR assays were performed to detect AQP4-AS1 levels in the fibrovascular membranes of diabetic patients and idiopathic epiretinal membranes of non-diabetic patients (*P < 0.05, 2-tailed Student's t test). (d) qRT-PCR assays were performed to detect AQP4-AS1 levels in aqueous humor of patients with diabetes mellitus, glaucoma, cataract, or trauma (*P < 0.05, one-way ANOVA, Bonferroni test).
AQP4-AS1 directly regulates Müller cell function and indirectly regulates endothelial cell and RGC function in vitro
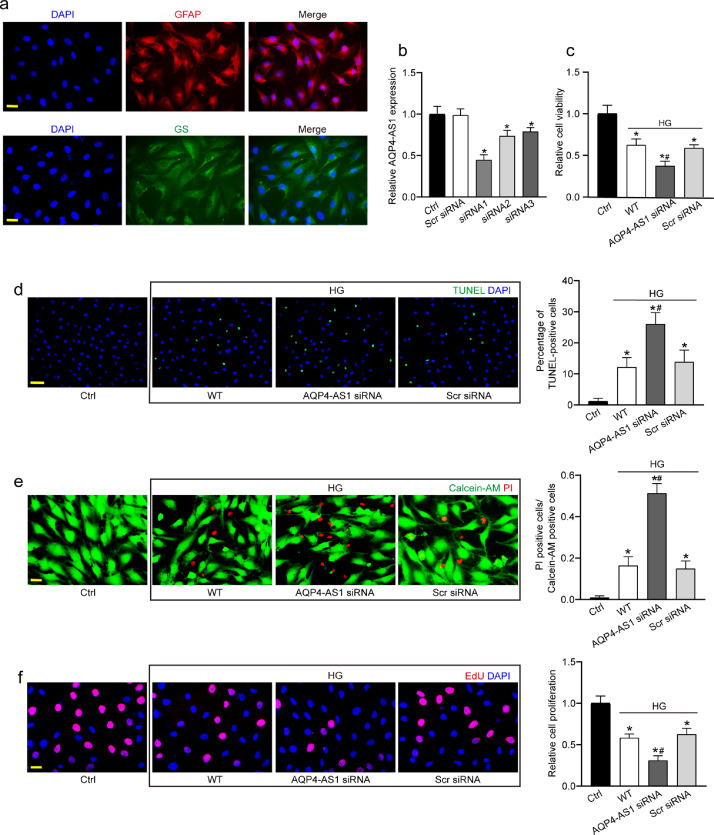
Since AQP4-AS1 is mainly expressed in Müller cells. Of note, Müller cells are the principal glial cells of the retina, assuming many of the functions carried out by astrocytes, oligodendrocytes and ependymal cells in other central nervous system (CNS) regions.20 Human primary Müller cells were cultured and identified with GFAP and GS protein immunofluorescence. The result showed that all of the primary Müller cells were strongly GFAP and GS positive (>90%; Fig. 2a). Then we determined the functional significance of AQP4-AS1 alteration in Müller cells. We performed AQP4-AS1 small interfering RNA (siRNA) transfection to decrease the levels of AQP4-AS1 and indeed led to a marked reduction of AQP4-AS1 levels (Fig. 2b). We selected AQP4-AS1 siRNA1 for subsequent experiment due to its highest silencing efficiency.
Fig. 2.
AQP4-AS1 regulates Müller cell function in vitro. (a) Primary Müller cells were identified with GFAP and GS protein immunofluorescence. GFAP, red; GS, green; DAPI, blue. Scale bar, 20 μm. (b) Müller cells were transfected with AQP4-AS1 siRNA1-3, scrambled (Scr) siRNA, or left untreated (Ctrl) for 48 h. qRT-PCRs were conducted to detect AQP4-AS1 expression (n = 4, *P < 0.05 versus Ctrl, one-way ANOVA followed by Bonferroni's post-hoc test). (c–f) Müller cells were transfected with AQP4-AS1 siRNA1, Scr siRNA, or left untreated, and then these cells were exposed with 30 mM high glucose for 48 h. The group without high glucose treatment was taken as the Ctrl group. The viability of Müller cells was determined by MTT assay (c, n = 4). Apoptotic cells were analyzed using TUNEL staining and quantitated.DAPI, blue; TUNEL, green. Scale bar 50 μm (d, n = 4). Apoptotic cells were also analyzed using Calcein-AM/PI staining, and quantitative analysis were conducted to detect the apoptotic percentage of Müller cells. Calcein-AM, green; PI, red. Scale bar, 20 μm (e, n = 4). EdU incorporation assay was performed to detect cell proliferation and the EdU positive cells were quantitated. DAPI, blue; EdU, red. Scale bar, 20 μm (f, n = 4). *P < 0.05 versus Ctrl; #P < 0.05 AQP4-AS1 siRNA versus Scr siRNA. The significant difference was determined by one-way ANOVA followed by Bonferroni test.
Müller cells were exposed to high glucose to mimic diabetic condition in vitro. MTT assay showed that AQP4-AS1 silencing significantly decreased the viability of Müller cells (Fig. 2c). We then determined whether AQP4-AS1 affected the apoptosis of Müller cells using TUNEL and Calcein-AM/PI staining. AQP4-AS1 silencing aggravated high glucose-induced apoptosis of Müller cells as shown by increased TUNEL positive cells (Fig. 2d) and increased number of PI-positive cells (Fig. 2e). EdU incorporation assay revealed that AQP4-AS1 silencing could significantly reduce the proliferation ability of Müller cells (Fig. 2f). A similar event was observed in Müller cells in response to oxidative stress (Fig. S1).
Müller cells are intermediaries between neurons and blood vessels in neurovascular interaction, owing to their ability to release vasoactive factors in response to neuronal activity.21 We then used the co-culture model to investigate the effects of AQP4-AS1 silencing in Müller cells on the function of endothelial cells. Co-culture with Müller cells could increase the migration, tube formation, and proliferation ability of HRVECs. By contrast, AQP4-AS1 silencing in Müller cells significantly reduced the migration, proliferation, and tube formation of endothelial cells (Fig. S2).
Müller cells play a dual role in maintaining retinal function. It can protect retinal tissue from further damage by the release of antioxidants and neurotrophic factors. However, abnormal Müller cell gliosis can also contribute to neurodegeneration and impede regenerative processes in the retinal tissue. To determine whether AQP4-AS1 silencing in Müller cells affected the function of RGCs, we purified and cultured primary RGCs. Immunocytochemical staining of βIII-tubulin showed that the percentage of RGCs was about 90%. Primary RGCs were co-cultured with mouse primary Müller cells, MTT assay and PI staining showed that co-culture with Müller cells could protect RGCs against high glucose-induced stress at the 12 h, AQP4-AS1 silencing in Müller cells promoted the protective effects of Müller cell on RGC function. By contrast, co-culture with Müller cells could aggravate RGC injury induced by high glucose at the 24 h, AQP4-AS1 silencing in Müller cells interrupted the detrimental effects of Müller cell on RGC function (Fig. S3). These results suggest that AQP4-AS1 silencing can directly regulate the function of Müller cells and indirectly regulate the function of endothelial cells or RGCs.
AQP4-AS1 protects against diabetes mellitus-induced retinal vascular dysfunction
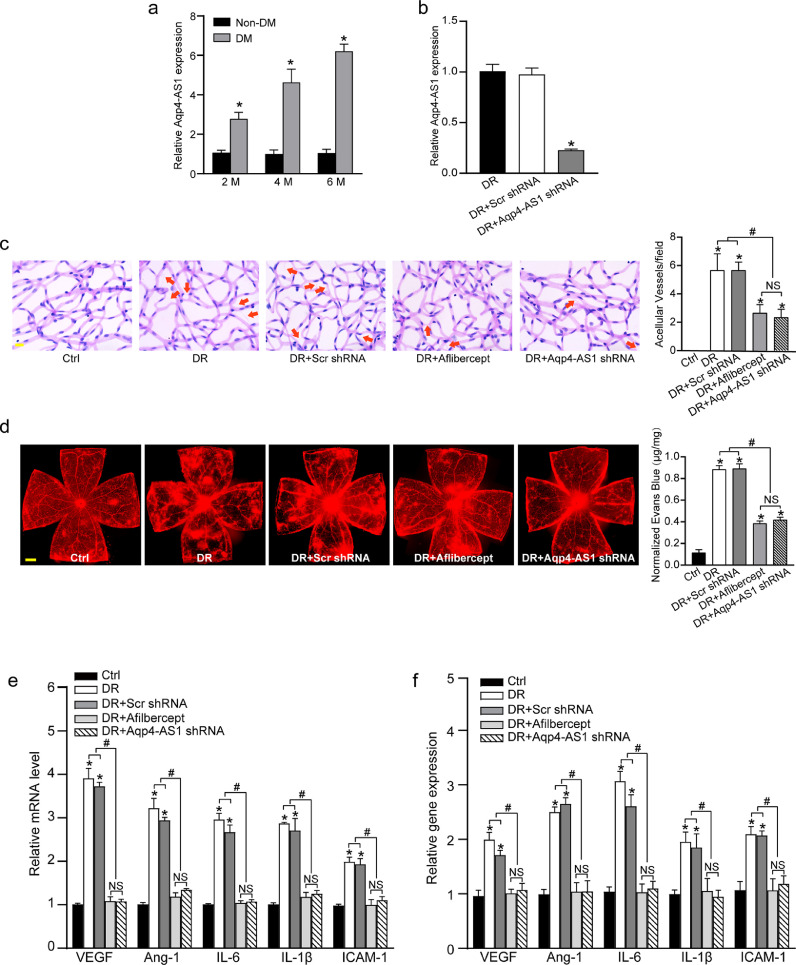
To investigate the role of AQP4-AS1 in vivo, we first searched for the homologous gene of Aqp4-AS1 in mouse genome. The homologous sequence located at chr18:15477383-15478277 in mouse genome was aligned to the sequence of AQP4-AS1 in human genome, showing over 90% sequence identity. We showed that the levels of Aqp4-AS1 were significantly up-regulated in the retinas of diabetic mouse, compared with those of non-diabetic mouse (Fig. 3a). DR is characterized by retinal vascular dysfunction, such as microaneurysm, vascular leak, and vascular inflammation.22 To reveal the role of Aqp4-AS1 in diabetes mellitus-induced vascular dysfunction in vivo, the diabetic mice received an intravitreal injection of Aqp4-AS1 shRNA. Aqp4-AS1 shRNA did not alter blood glucose level and body weight of diabetic mice (Supplemental Table 5). qRT-PCRs showed that Aqp4-AS1 shRNA injection significantly reduced the levels of Aqp4-AS1 in the retinas (Fig. 3b). Trypsin digestion of retinal vessels showed that Aqp4-AS1 silencing could ameliorate capillary degeneration in diabetic mice as shown by decreased number of acellular capillary, which was similar to the effect of Aflibercept (Fig. 3c). Evans blue assay showed that compared with diabetic retina, Aqp4-AS1 silencing decreased diabetes-induced retinal vascular leakage, showing the similar effect of Aflibercept on vascular leakage (Fig. 3d). Pro-inflammatory factors, including vascular endothelial growth factor (VEGF), Angiogenin-1 (Ang-1), interleukin-6 (IL-6), IL-1β, and intercellular adhesion molecule-1 (ICAM-1), were significantly up-regulated in diabetic retinas. qRT-PCR and ELISA assays showed that Aqp4-AS1 silencing reduced diabetes-induced retinal inflammation as shown by decreased amount of VEGF, Ang-1, IL-6, IL-1β, and ICAM-1 (Fig. 3e and f). Collectively, these results suggest that Aqp4-AS1 silencing exerts a protective effect on diabetes-induced retinal vascular dysfunction in vivo.
Fig. 3.
Aqp4-AS1 silencing protects against diabetes mellitus-induced retinal vascular dysfunction. (a) qRT-PCR assays were conducted to compare Aqp4-AS1 levels in non-diabetic retinas and diabetic retinas after 2-month, 4-month, and 6-month induction of diabetes (n = 6). (b) Diabetic C57BL/6J mice (2-month-old, male) received an intravitreous injection of scrambled (Scr) shRNA, Aqp4-AS1 shRNA, or left untreated (Ctrl). Six months after the induction of diabetes, qRT-PCRs were conducted to detect Aqp4-AS1 levels (n = 6). (c) Retinal trypsin digestion was performed to detect acellular capillaries. Acellular capillaries were quantified in 30 random fields per retina and averaged. Red arrows indicated acellular capillaries (n = 6). Scale bar, 10 μm. (d) Mice were infused with Evans blue dye for 2 h. The fluorescence signal of flat-mounted retina was detected using a fluorescence microscope. A representative image was shown. Meanwhile, the quantification of Evans blue leakage was conducted (n = 6). Scale bar, 200 μm. (e) qRT-PCR assays were conducted to determine the relative levels of VEGF, Ang-1, IL-1β, IL-6, and ICAM-1 mRNA (n = 6). (f) ELISA assays were conducted to detect the amount of VEGF, Ang-1, IL-1β, IL-6, and ICAM-1 in retinal lysates (n = 6). *P < 0.05 versus Ctrl group; #P < 0.05 DR+Aqp4-AS1 shRNA versus DR+Scr shRNA or DR. The significant difference was evaluated by the Kruskal-Wallis test followed by the post hoc Bonferroni test.
To further test for the function of the orthologous Aqp4-AS1 through the gain-of-function of Aqp4-AS1, the diabetic mice received an intravitreal injection of the orthologous mouse Aqp4-AS1 or human AQP4-AS1. qRT-PCRs showed that Aqp4-AS1 OE-AAV and AQP4-AS1 OE-AAV injection significantly increased the levels of Aqp4-AS1 and AQP4-AS1 in the retinas. Retinal trypsin digestion assays revealed that, in comparison with the diabetic group (diabetic retinopathy), gain-of-function of orthologous mouse Aqp4-AS1 or human AQP4-AS1 significantly increased acellular capillary number. Evans blue assays showed that gain-of-function of orthologous mouse Aqp4-AS1 or human AQP4-AS1 significantly increased diabetes mellitus-induced retinal vascular leakage (Fig. S4).
AQP4-AS1 silencing protects against diabetes -induced retinal neurodegeneration
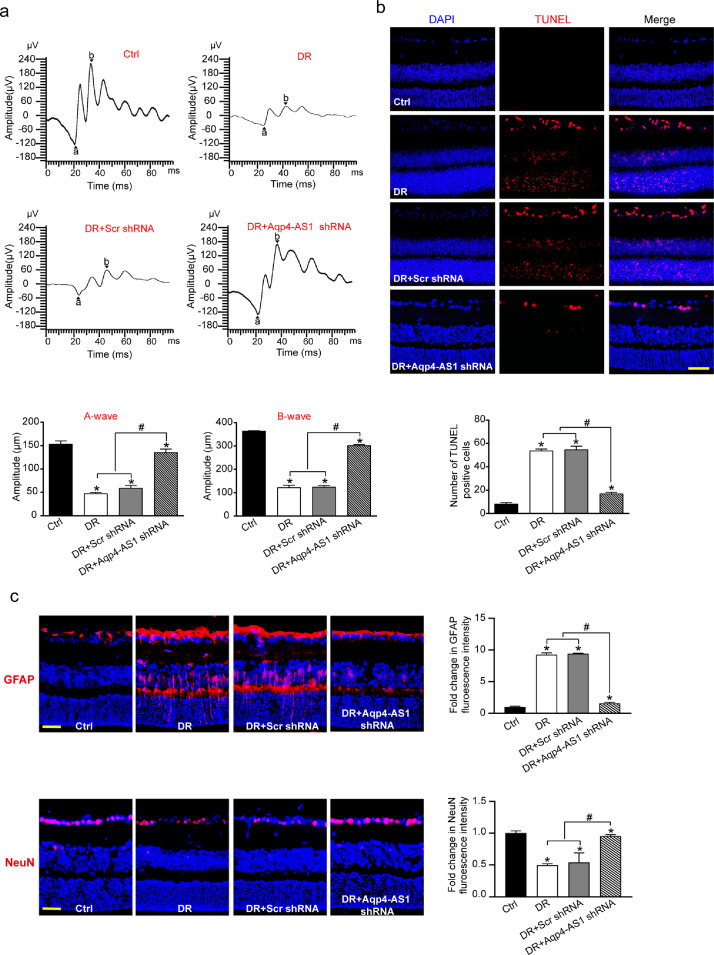
Retinal neurodegeneration is another critical event in the pathogenesis of DR.4 To reveal the role of Aqp4-AS1 in retinal neurodegeneration, we first used retinal electrophysiology to investigate the overall function of retinas. Electrophysiology assays showed that the amplitude of A-wave or B-wave was significantly reduced while the B-wave latency was significantly increased in diabetic mice, whereas Aqp4-AS1 silencing could partially reverse the decreased amplitude of A-wave and B-wave, the increased B-wave latency and ameliorate retinal dysfunction (Fig. 4a). TUNEL assays were used to detect the apoptotic percentage of retinal cells. Aqp4-AS1 silencing could reduce the number of TUNEL-positive cells in diabetic retinas (Fig. 4b).
Fig. 4.
Aqp4-AS1 silencing protects against diabetes-induced retinal neurodegeneration. (a) Electrophysiology was conducted to detect the retinal function in non-diabetic mice (Ctrl), diabetic mice, Scr shRNA-injected, and Aqp4-AS1 shRNA-injected mice at 6-month after diabetes induction. Amplitudes of A and B waves were statistically analyzed (n = 6). (b) TUNEL assays and quantitative analysis was conducted at 6 months after diabetes induction (n = 6). Nuclei, blue; TUNEL-positive cells, red. Scale bar, 50 μm. (c) Immunofluorescence and quantitative analysis of GFAP and NeuN staining were conducted to determine retinal reactive gliosis and RGC survival. The representative images were shown (n = 6). Scale bar, 50 μm. *P < 0.05 versus Ctrl group; #P < 0.05 DR+Aqp4-AS1 shRNA versus DR+Scr shRNA or DR. The significant difference was evaluated by the Kruskal-Wallis test followed by the post hoc Bonferroni test.
We performed protein immunofluorescence to determine the role of Aqp4-AS1 in retinal neurodegeneration in vivo. Compared with diabetic group, Aqp4-AS1 silencing significantly attenuated reactive gliosis as shown by decreased number of GFAP positive Müller cells and improved RGC survival as shown by reduced loss of NeuN-positive RGCs (Fig. 4c). We also showed that Aqp4-AS1 silencing did not affect the staining signaling of amacrine cells, horizontal cells, rod photoreceptor, and bipolar cells (Fig. S5). We further investigated the gain-of-function of Aqp4-AS1 in retinal neurodegeneration. Compared with the diabetic group, the gain-of-function of orthologous mouse Aqp4-AS1 or human AQP4-AS1 significantly aggravated reactive gliosis as shown by increased number of GFAP-positive Müller cells and increased RGC injury as shown by decreased number of NeuN-positive RGCs (Fig. S6). These results indicate that Aqp4-AS1 silencing protects against diabetes-induced retinal neurodegeneration through regulating reactive gliosis and RGC survival.
AQP4-AS1 negatively regulates AQP4 expression
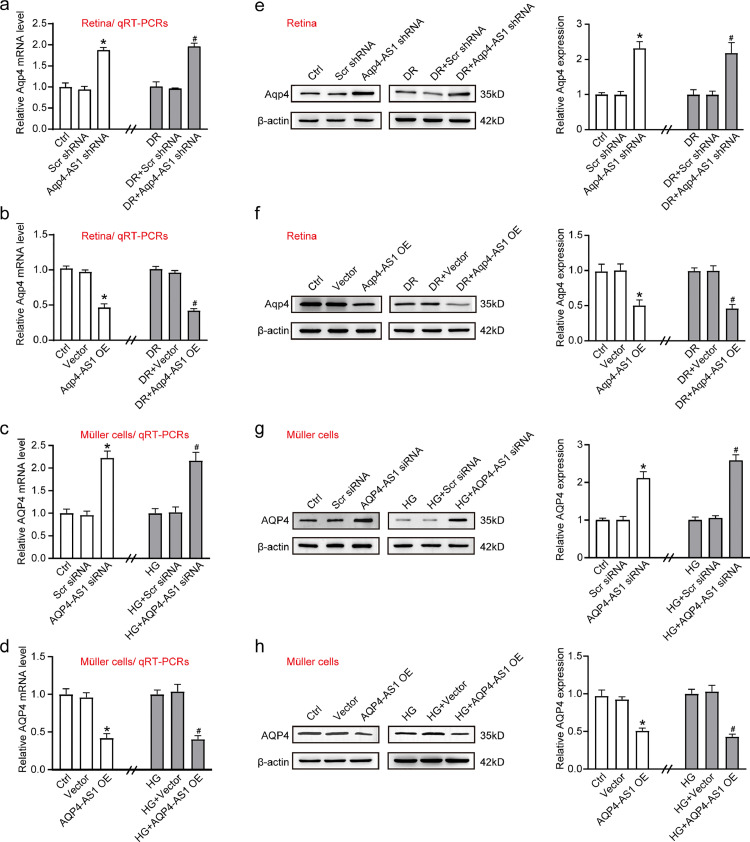
We then investigated the potential mechanism of AQP4-AS1 in regulating neurovascular dysfunction in diabetic condition. AQP4-AS1 is transcribed from the anti-sense strand of AQP4. We conducted qRT-PCRs to detect whether AQP4-AS1 intervention could affect the levels of AQP4. qRT-PCR assays showed that Aqp4-AS1 silencing by Aqp4-AS1 shRNA could lead to increased levels of Aqp4 mRNA in diabetic retinas and non-diabetic retinas (Fig. 5a), whereas Aqp4-AS1 overexpression led to decreased levels of Aqp4 mRNA (Fig. 5b). Meanwhile, AQP4-AS1 silencing by AQP4-AS1 siRNA could lead to increased levels of AQP4 mRNA in Müller cells both at normal glucose and high glucose condition (Fig. 5c), whereas AQP4-AS1 overexpression led to decreased levels of AQP4 mRNA (Fig. 5d). We then conducted Western blot analysis to detect whether AQP4-AS1 intervention could affect the expression of AQP4 protein. The results showed that Aqp4-AS1 silencing by Aqp4-AS1 shRNA could lead to increased Aqp4 expression in diabetic retinas and non-diabetic retinas, whereas Aqp4-AS1 overexpression led to decreased Aqp4 protein expression (Fig. 5e and f). Moreover, similar results were observed in Müller cells both at normal glucose and high glucose condition (Fig. 5g and h). These results suggest that AQP4-AS1 negatively regulates AQP4 expression in vivo and in vitro.
Fig. 5.
AQP4-AS1 negatively regulates AQP4 level. (a, b, e, and f) qRT-PCR and Western blot assays were performed to detect the levels of Aqp4 mRNA and Aqp4 protein in the non-diabetic and diabetic retinas after the injection of scrambled (Scr) shRNA, Aqp4-AS1 shRNA, vector, Aqp4-AS1 OE, or left untreated for 2-month (n = 6; *P < 0.05 versus Ctrl and #P < 0.05 versus DR, Kruskal-Wallis test followed by Bonferroni's post-hoc test). (c, d, g, and h) qRT-PCR and Western blot assays were performed to detect the levels of AQP4 mRNA and AQP4 protein in Müller cells after transfection of scrambled (Scr) siRNA, AQP4-AS1 siRNA, vector, AQP4-AS1 OE, or left untreated for 48 h both at normal glucose and high glucose condition (n = 4, *P < 0.05 versus Ctrl and #P < 0.05 versus HG, One-way ANOVA followed by Bonferroni's post-hoc test).
Aqp4 knockout aggravates neurovascular dysfunction in diabetic mice
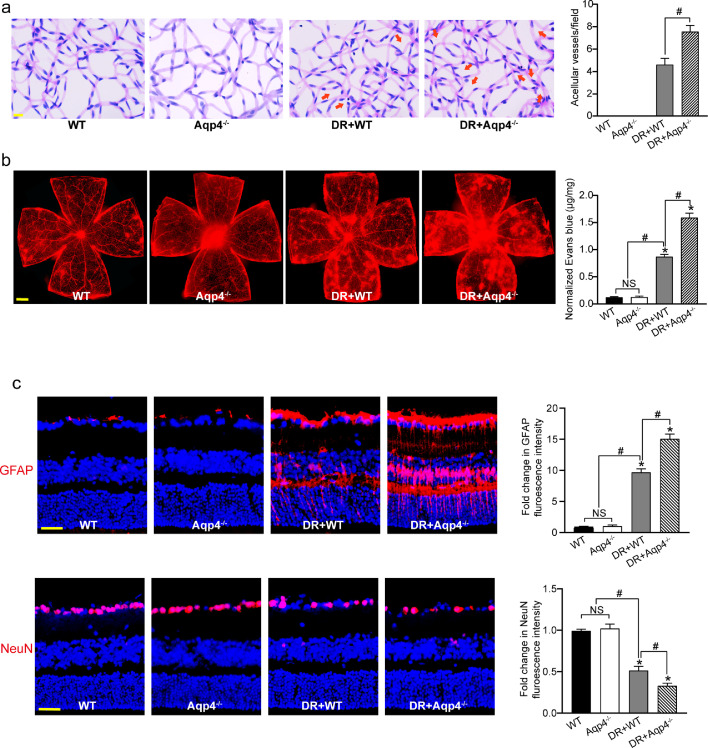
We then used Aqp4 knockout mice to determine whether Aqp4 was involved in neurovascular dysfunction in diabetic mice. Retina trypsin assays showed that Aqp4 knockout could aggravate diabetes -induced retinal capillary degeneration (Fig. 6a). Evans blue assays showed that Aqp4 knockout could aggravate hyperglycemia-induced retinal vascular leakage in diabetic mouse (Fig. 6b). Immunofluorescence staining showed that compared with the diabetic group, Aqp4 knockout could accelerate retinal reactive gliosis as shown by increased GFAP staining and aggravate RGC injury as shown by decreased number of NeuN-positive RGCs (Fig. 6c). These results indicate that Aqp4 plays a crucial role in diabetes-induced retinal neurovascular dysfunction.
Fig. 6.
Aqp4 knockout aggravates neurovascular dysfunction in diabetic mice. (a) Six months after diabetes induction, retinal trypsin digestion was conducted to detect acellular capillaries in non-diabetic wild-type (WT) mice, non-diabetic Aqp4−/− mice, diabetic wild-type (DR+WT) mice, and diabetic Aqp4−/− mice. Red arrows indicated acellular capillaries. Acellular capillaries were quantified in 30 random fields per retina and averaged (n = 6). Scale bar, 10 μm. (b) The mice were perfused with Evans blue dye for 2 h. The fluorescence signal of flat-mounted retina was detected using the fluorescence microscope. The representative images were shown. Meanwhile, the quantification of Evans blue leakage was conducted (n = 6). Scale bar, 200 μm. (c) Immunofluoresence analysis of GFAP and NeuN were conducted to detect retinal reactive gliosis and RGC survival. The representative images were shown (n = 6). Scale bar, 50 μm. *P < 0.05 versus WT group; #P < 0.05 significant difference between the marked groups; NS, no significant difference. The significant difference was evaluated by the Kruskal-Wallis test followed by the post hoc Bonferroni test.
Aqp4/Aqp4-AS1 crosstalk is involved in the regulation of diabetes-induced neurovascular dysfunction
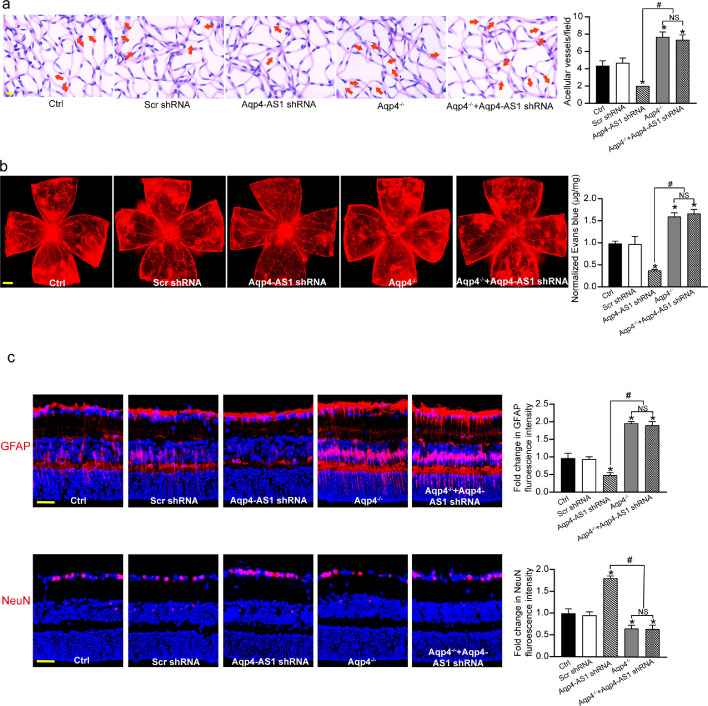
We next determined whether the protective effect of Aqp4-AS1 silencing on retinal neurovascular dysfunction was mediated by altered Aqp4 expression. Retina trypsin assays showed that compared with Ctrl group, Aqp4-AS1 silencing led to decreased number of acellular capillaries. Aqp4 knockout could interrupt the protective effect of Aqp4-AS1 silencing on diabetes-induced retinal capillary degeneration (Fig. 7a). Evans blue assays showed that Aqp4 knockout interrupted the protective effect of Aqp4-AS1 on retinal vascular leakage in diabetic mouse (Fig. 7b). Compared with Ctrl group, Aqp4-AS1 silencing reduced the degree of diabetes-induced retinal reactive gliosis. By contrast, Aqp4 knockout could lead to increased retinal reactive gliosis. Compared with Ctrl group, Aqp4-AS1 silencing significantly decreased the number of diabetes-induced RGC injury. Aqp4 knockout could interrupt the protective effects of Aqp4-AS1 silencing on RGC survival (Fig. 7c). These results indicate that Aqp4/Aqp4-AS1 crosstalk is involved in the regulation of diabetes-induced neurovascular dysfunction.
Fig. 7.
Aqp4/Aqp4-AS1 crosstalk is involved in the regulation of diabetes-induced neurovascular dysfunction. (a) Six months after diabetes induction, retinal trypsin digestion was conducted to detect acellular capillaries in diabetic retinas (Ctrl), diabetic retinas injected with Scr shRNA, Aqp4-AS1 shRNA, diabetic Aqp4 knockout mice (Aqp4−/−), and diabetic Aqp4 knockout mice (Aqp4−/−) injected with Aqp4-AS1 shRNA. Red arrows indicated acellular capillaries. Acellular capillaries were quantified in 30 random fields per retina and averaged (n = 6). Scale bar, 10 μm. (b) The mice were perfused with Evans blue dye for 2 h. The fluorescence signal of flat-mounted retina was detected using a fluorescence microscope. The representative images were shown. Meanwhile, the quantification of Evans blue leakage was conducted (n = 6). Scale bar, 200 μm. (c) Immunofluorescence analysis of GFAP and NeuN was conducted to detect retinal reactive gliosis and RGC survival. A representative image was shown (n = 6). Scale bar, 50 μm. *P < 0.05 versus Ctrl group; #P < 0.05 significant difference between the marked groups; NS, no significant difference. The significant difference was evaluated by the Kruskal-Wallis test followed by the post hoc Bonferroni test.
Discussion
Diabetic retinopathy (DR) begins with the non-proliferative stage in which retinal vessels and neurons are damaged due to the hyperglycemia injury, which is followed by the over-compensatory pathological neovascularization at the proliferative stage. Retinal vessels are intimately associated with and governed by neurons and glia. Dysregulation of neurovascular crosstalk is responsible for the pathogenesis of DR both at the non-proliferative stage and advanced proliferative stage.23 Here, we show that a lncRNA, AQP4-AS1, is significantly increased in the clinical samples of diabetic patients, high glucose-treated Müller cells, and diabetic retinas. Aqp4-AS1 silencing in vivo alleviates retinal neurodegeneration and vascular dysfunction. AQP4-AS1 directly regulates Müller cell function and indirectly affects endothelial cell and RGC function in vitro. This study reveals a novel mechanism underlying neurovascular crosstalk and dysregulation in DR. Modulating AQP4-AS1 level may lead to improved means to treat retinopathy and better maintain vision.
LncRNAs have gained increasing attentions due to their versatile roles in biological processes and human disorders. Notably, the critical roles of lncRNAs in vascular and neurodegenerative diseases have been recognized. LncRNA-NEXN-AS1 regulates the expression of the actin-binding protein NEXN and exerts a protective role against atherosclerosis.24 LncRNA-MIAT regulates retinal microvascular dysfunction by functioning as a competing endogenous RNA.25 LncRNA-MEG3 can improve the cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer's disease.26 LncRNA SNHG14 silencing reduces dopaminergic neuron damage in dopaminergic cells and Parkinson's disease (PD) mouse model.27 These evidences suggest that lncRNAs are potential regulators of vascular function and neurological function. Here, we identified a therapeutic target for the treatment of neurodegeneration and impaired neurovascular coupling. LncRNA-AQP4-AS1 is specially expressed in Müller cells and the level of AQP4-AS1 is significantly increased under diabetic conditions. AQP4-AS1 silencing in vivo alleviates retinal neurodegeneration and vascular dysfunction as shown by reduced vessel impairment, decreased reactive gliosis, and reduced RGCs loss.
Retinal capillaries are composed of endothelial cells and pericytes but also have intimate associations with glial cells and neural cells.28 The conceptualization of DR as a disease of neurovascular unit broadens our appreciation of cell types that contribute to the progression of DR. Müller cells can secrete the guidance cues in response to injury or metabolic stress that may regulate vascular outgrowth.29 We used the co-culture model to investigate the effects of AQP4-AS1 silencing in Müller cells on the function of endothelial cells or RGCs. AQP4-AS1 silencing in Müller cells significantly reduces the migration, proliferation, and tube formation of endothelial cells. AQP4-AS1 silencing in Müller cells decreases the deleterious effects of Müller cells on RGC function. These results imply that AQP4-AS1 silencing in Müller cells has a potential role in anti-angiogenesis and plays a protective role in RGC function. A great understanding of the interactions of these cellular elements and their pathogenic contributions can provide great possibilities for new therapeutic strategies.
The astrocytes and Müller cells are the components of the neurovascular unit. The homeostatic function of these cells is vulnerable to be damaged by diabetes, which leads to altered retinal blood flow, water balance, and blood-retina barrier function.30 Müller cells undergo reactive gliosis characterized by the up-regulation of GFAP and increased expression of innate immune-related pathways reflected by pro-inflammatory cytokine secretion.31 Previous study has revealed the pivotal role of Müller cells in retinal vascular dysfunction. Conditional KO mice with disrupted VEGF in Müller cells exhibits a decrease in biomarkers of retinal inflammation, including TNF-α and ICAM-1, as well as a reduction in retinal vascular abnormalities including leakage.21 This finding suggests that dysfunctional Müller cells could act in paracrine fashion to promote vascular dysfunction in DR. We show that silencing of Müller cell-specific lncRNA, AQP4-AS1, can induce decreased GFAP expression and reduced inflammation reaction, suggesting that AQP4-AS1 silencing exerts a protective effect on diabetes-induced retinal retinopathy.
LncRNAs are emerging as potential key regulators of gene expression. They are involved in several biological processes, such as chromatin remodeling, RNA stabilization, and transcription regulation.32 Here, we identify a natural anti-sense transcript for AQP4-AS1. AQP4 is the main water channel protein expressed in the CNS. AQP4 is highly expressed in retina, brain, prostate, and lung and is densely expressed in Müller cells or astrocytes.33 Changes in AQP4 activity and expression have been implicated in several CNS disorders, including epilepsy, edema, stroke, and glioblastoma.34, 35, 36 Furthermore, it has been reported that downregulation of AQP4 exacerbates diabetic retinopathy through aggravating inflammatory response.37 Thus, it is important to control the activity of AQP4 for treating these disorders. Four regulatory methods have been reported, including regulation of AQP4 expression via microRNAs, regulation of AQP4 channel gating/trafficking via phosphorylation, regulation of water permeability using heavy metal ions, and regulation of water permeability using small molecule inhibitors. However, there are still great challenges for controlling AQP4 function due to great variability and low efficiency of these methods. AQP4-AS1 is expressed temporally and spatially in vivo with its native target, AQP4. AQP4-AS1 selectively binds AQP4 mRNA in vivo and regulates AQP4 transcript levels. AQP4-AS1 may broadly serve to fine-tune the expression of AQP4 genes with remarkable tissue specificity, highlighting our rapidly evolving understanding of the non-coding genome. Hybridization of the anti-sense LncRNA to its target destabilizes AQP4, which may lead to the loss of AQP4 protein. The presence of anti-sense noncoding transcript at the same time and space as its native endogenous transcript provides a novel regulatory paradigm.
In conclusion, this study reveals the role of AQP4-AS1 in diabetes retinopathy. AQP4-AS1 directly regulates the biological functions of Müller cells, and indirectly regulates the functions of endothelial cells and RGCs. Clinical and animal experiments indicate that AQP4-AS1 is implicated in retinal neurovascular dysfunction. Mechanistically, AQP4-AS1 silencing leads to increased AQP4 expression, alleviating diabetes-induced neurovascular dysfunction. Taken together, our findings suggest that AQP4-AS1 is a promising target for treating retinal neurovascular complications.
Declaration of interests
No potential conflicts of interest relevant to this article were reported.
Acknowledgments
Contributors
Q.J. conceived and supervised this study. Q.J. and J.Y. were responsible for all aspects of study design. X.M.L., J.Y.Z., Y.L.Z., C.L., M.D.Y., Y.N.S., W.Y., X.S.N. and F.Z., conducted the experiments. X.M.L., J.Y.Z., Y.L.Z., C.L., M.D.Y. and Y.N.S. interpreted all results. Q.J., X.M.L., and J.Y.Z. wrote the manuscript. Y.L.Z., C.L. and M.D.Y. did the statistical analysis. J.Y. and M.D.Y. provided advice. X.M.L., J.Y.Z., Y.L.Z., C.L., M.D.Y., Y.N.S., W.Y., X.S.N. and F.Z. verified the underlying data. All authors critically reviewed the manuscript and approved the final version of the manuscript.
Acknowledgements
We thank the Affiliated Eye Hospital, Nanjing Medical University for their skilled technical assistance. This work was generously supported by the grants from the National Natural Science Foundation of China (Grant No. 81800858, 82070983, 81870679 and 81970823), grants from the Medical Science and Technology Development Project Fund of Nanjing (Grant No ZKX17053 and YKK19158), grants from Innovation Team Project Fund of Jiangsu Province (No. CXTDB2017010), and the Science and Technology Development Plan Project Fund of Nanjing (Grant No 201716007, 201805007 and 201803058).
Data sharing statement
The data sets generated and analyzed during this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103857.
Contributor Information
Jin Yao, Email: dryaojin@126.com.
Qin Jiang, Email: jqin710@vip.sina.com.
Appendix. Supplementary materials
References
- 1.Schwartz S.S., Epstein S., Corkey B.E., et al. A unified pathophysiological construct of diabetes and its complications. Trends Endocrinol Metab. 2017;28(9):645–655. doi: 10.1016/j.tem.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Wong T.Y., Cheung C.M., Larsen M., Sharma S., Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. doi: 10.1038/nrdp.2016.12. [DOI] [PubMed] [Google Scholar]
- 3.Moutray T., Evans J.R., Lois N., Armstrong D.J., Peto T., Azuara-Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database Syst Rev. 2018;3(3) doi: 10.1002/14651858.CD012314.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simó R., Stitt A.W., Gardner T.W. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61(9):1902–1912. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng Y., Cao D., Yu H., et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019;103(12):1747–1752. doi: 10.1136/bjophthalmol-2018-313582. [DOI] [PubMed] [Google Scholar]
- 6.Mugisho O.O., Green C.R., Zhang J., Acosta M.L., Rupenthal I.D. Connexin43 hemichannels: A potential drug target for the treatment of diabetic retinopathy. Drug Discov Today. 2019;24(8):1627–1636. doi: 10.1016/j.drudis.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Simó R., Hernández C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retinal Eye Resh. 2015;48:160–180. doi: 10.1016/j.preteyeres.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Robinson G.S., Ju M., Shih S.C., et al. Nonvascular role for VEGF: VEGFR-1, 2 activity is critical for neural retinal development. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15(7):1215–1217. doi: 10.1096/fj.00-0598fje. [DOI] [PubMed] [Google Scholar]
- 9.Grunwald J.E., Daniel E., Huang J., et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva P.S., Cavallerano J.D., Sun J.K., Aiello L.M., Aiello L.P. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat Rev Endocrinol. 2010;6(9):494–508. doi: 10.1038/nrendo.2010.122. [DOI] [PubMed] [Google Scholar]
- 11.Li M., Duan L., Li Y., Liu B. Long noncoding RNA/circular noncoding RNA-miRNA-mRNA axes in cardiovascular diseases. Life Sci. 2019;233 doi: 10.1016/j.lfs.2019.04.066. [DOI] [PubMed] [Google Scholar]
- 12.Batista P.J., Chang H.Y. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz S.U., Grote P., Herrmann B.G. Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci CMLS. 2016;73(13):2491–2509. doi: 10.1007/s00018-016-2174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verkman A.S., Anderson M.O., Papadopoulos M.C. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13(4):259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan Y., Wu D., Huber M., et al. New endovascular approach for hypothermia with intrajugular cooling and neuroprotective effect in ischemic stroke. Stroke. 2020;51(2):628–636. doi: 10.1161/STROKEAHA.119.026523. [DOI] [PubMed] [Google Scholar]
- 16.Li X., Gao J., Ding J., Hu G., Xiao M. Aquaporin-4 expression contributes to decreases in brain water content during mouse postnatal development. Brain Res Bull. 2013;94:49–55. doi: 10.1016/j.brainresbull.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Hoshi A., Tsunoda A., Yamamoto T., Tada M., Kakita A., Ugawa Y. Altered expression of glutamate transporter-1 and water channel protein aquaporin-4 in human temporal cortex with Alzheimer's disease. Neuropathol Appl Neurobiol. 2018;44(6):628–638. doi: 10.1111/nan.12475. [DOI] [PubMed] [Google Scholar]
- 18.Sun H., Liang R., Yang B., et al. Aquaporin-4 mediates communication between astrocyte and microglia: implications of neuroinflammation in experimental Parkinson's disease. Neuroscience. 2016;317:65–75. doi: 10.1016/j.neuroscience.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Shan K., Liu C., Liu B.H., et al. Circular noncoding RNA HIPK3 mediates retinal vascular dysfunction in diabetes mellitus. Circulation. 2017;136(17):1629–1642. doi: 10.1161/CIRCULATIONAHA.117.029004. [DOI] [PubMed] [Google Scholar]
- 20.Vecino E., Rodriguez F.D., Ruzafa N., Pereiro X., Sharma S.C. Glia-neuron interactions in the mammalian retina. Prog Retinal Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Xu X., Elliott M.H., Zhu M., Le Y.Z. Müller cell-derived VEGF is essential for diabetes-induced retinal inflammation and vascular leakage. Diabetes. 2010;59(9):2297–2305. doi: 10.2337/db09-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stitt A.W., Curtis T.M., Chen M., et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retinal Eye Res. 2016;51:156–186. doi: 10.1016/j.preteyeres.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Moran E.P., Wang Z., Chen J., Sapieha P., Smith L.E., Ma J.X. Neurovascular cross talk in diabetic retinopathy: pathophysiological roles and therapeutic implications. Am J Physiol Heart Circ Physiol. 2016;311(3):H738–H749. doi: 10.1152/ajpheart.00005.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y.W., Guo F.X., Xu Y.J., et al. Long noncoding RNA NEXN-AS1 mitigates atherosclerosis by regulating the actin-binding protein NEXN. J Clin Invest. 2019;129(3):1115–1128. doi: 10.1172/JCI98230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan B., Yao J., Liu J.Y., et al. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ Res. 2015;116(7):1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 26.Yi J., Chen B., Yao X., Lei Y., Ou F., Huang F. Upregulation of the lncRNA MEG3 improves cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer's disease through inactivating the PI3K/Akt signaling pathway. J Cell Biochem. 2019;120(10):18053–18065. doi: 10.1002/jcb.29108. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L.M., Wang M.H., Yang H.C., et al. Dopaminergic neuron injury in Parkinson's disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/α-synuclein pathway. Aging. 2019;11(21):9264–9279. doi: 10.18632/aging.102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruggiero D., Lecomte M., Michoud E., Lagarde M., Wiernsperger N. Involvement of cell-cell interactions in the pathogenesis of diabetic retinopathy. Diabetes Metab. 1997;23(1):30–42. [PubMed] [Google Scholar]
- 29.Aung M.H., Park H.N., Han M.K., et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci Off J Soc Neurosci. 2014;34(3):726–736. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rungger-Brändle E., Dosso A.A., Leuenberger P.M. Glial reactivity, an early feature of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2000;41(7):1971–1980. [PubMed] [Google Scholar]
- 31.Luna G., Keeley P.W., Reese B.E., Linberg K.A., Lewis G.P., Fisher S.K. Astrocyte structural reactivity and plasticity in models of retinal detachment. Exp Eye Res. 2016;150:4–21. doi: 10.1016/j.exer.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L.L. Linking long noncoding RNA localization and function. Trends Biochem Sci. 2016;41(9):761–772. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Kitchen P., Salman M.M., Halsey A.M., et al. Targeting Aquaporin-4 subcellular localization to treat central nervous system edema. Cell. 2020;181(4):784–799. doi: 10.1016/j.cell.2020.03.037. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeppenfeld D.M., Simon M., Haswell J.D., et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA neurology. 2017;74(1):91–99. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- 35.Manley G.T., Fujimura M., Ma T., et al. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6(2):159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 36.Wolburg H., Noell S., Fallier-Becker P., Mack A.F. Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Mol Asp Med. 2012;33(5-6):579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Cui B., Sun J.H., Xiang F.F., Liu L., Li W.J. Aquaporin 4 knockdown exacerbates streptozotocin-induced diabetic retinopathy through aggravating inflammatory response. Exp Eye Res. 2012;98:37–43. doi: 10.1016/j.exer.2012.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.