Abstract
Atrial fibrillation (AF) is associated with an increased risk of stroke, which can be prevented by the use of oral anticoagulation. Although non-vitamin K antagonist oral anticoagulants (NOACs) have become the first choice for stroke prevention in the majority of patients with non-valvular AF, adherence and persistence to these medications remain suboptimal, which may translate into poor health outcomes and increased healthcare costs. Factors influencing adherence and persistence have been suggested to be patient-related, physician-related, and healthcare system-related. In this review, we discuss factors influencing patient adherence and persistence to NOACs and possible problem solving strategies, especially involving an integrated care management, aiming for the improvement in patient outcomes and treatment satisfaction.
Keywords: Adherence, Atrial fibrillation, Integrated care, Integrated management, Non-vitamin K antagonist oral anticoagulants, Oral anticoagulation, Persistence
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults and is associated with substantial morbidity and mortality, leading to a significant health and socioeconomic burden.1 Atrial fibrillation is associated with a five-fold increased risk of stroke,2 which can be reduced by the use of oral anticoagulants (OACs).3
Stroke prevention in patients with non-valvular atrial fibrillation (NVAF) requires oral anticoagulation (OAC), whether as vitamin K antagonists (VKAs, e.g. warfarin) or non-VKA oral anticoagulants (NOACs).4 The NOACs are increasing in popularity over the traditional VKAs,5–8 due to better bleeding risk profile, reduced need for laboratory monitoring, more convenient fixed-dose regimen, greater predictability, faster onset of anticoagulation effect, and lower potential for food, alcohol, and drug interactions.8–15
Adherence and persistence to the prescribed OAC regimen is of extreme importance as non-adherence and non-persistence to anticoagulant treatment increase the risk of both ischaemic and haemorrhagic complications, other adverse cardiovascular events, and all-cause mortality, which may translate into poor health outcomes and increased healthcare costs.14,16–19 Due to the shorter elimination half-lives and short duration of anticoagulant effect of NOACs compared with warfarin, strict adherence to NOAC therapy is crucial.20,21 However, adherence and persistence to NOAC therapy remains suboptimal.17
In this review, we discuss factors influencing patient adherence and persistence to NOACs and possible problem solving strategies, especially involving an integrated care management, aiming for the improvement in patient outcomes and treatment satisfaction.
Search strategy
We searched the PubMed database for relevant reviews and original research studies published since January 2015 until the present date. We also consulted some of the reviews and research studies included in the references of these works.
Regarding adherence and persistence to NOAC therapy, we searched for titles and abstracts using the following search strategy: (atrial fibrillation) and (adherence or persistence or compliance or NOAC or DOAC); (NOAC or DOAC) and (adherence or persistence or compliance); (optimizing) and (NOAC or DOAC) and (adherence or persistence or compliance).
Regarding integrated management of AF patients, we searched for titles and abstracts using the following search strategy: (atrial fibrillation) and (integrated care or integrated management).
Specifically regarding NOAC antidotes, we searched for titles and abstracts using the following search strategy: (NOAC or DOAC) and (antidotes).
Definitions of adherence, persistence, compliance, and concordance to medications
Adherence refers to the extent to which the patient’s behaviour matches agreed recommendations from the prescriber, thus emphasizing the need for an agreement.22–25 Persistence refers to the length of time over which the patient kept taking their medication, regardless of whether they consistently followed the exact regimen.23,24 Adherence is usually reported as a percentage (e.g. reflecting the extent to which a patient takes the correct dose, at the correct time, at the prescribed frequency), and persistence is usually reported as a function of time (e.g. proportion of patients who remained on a specific strategy after a predefined time period).23,24
Compliance focuses only in the degree to which the patient follows the prescriber’s recommendations without implying an agreement. Because of its paternalistic authoritarian attitude towards the patient, it has been replaced by the term adherence.22–25 Therefore, we opted to use only the term adherence throughout the document.
Concordance implies an alliance between the clinician and patient with an equal and effective therapeutic relationship. It represents a shift towards a partnership in medicine-taking and highlights the shared decision-making process, being synonymous with patient-centred care.24,25 Therefore, we also opted not to use this term since we already discuss shared decision-making and patient-centred care, which have been frequently associated with medication adherence.
Adherence and persistence to non-vitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation
In a recent systematic review and meta-analysis on real-world adherence and persistence to NOACs in patients with AF, the overall mean adherence was 77%, the overall proportion of patients with good adherence was 66%, and the overall proportion of persistence for all follow-up durations was 69%. The pooled persistence with any NOAC was shown to be higher than for VKAs [odds ratio (OR) 1.44, 95% confidence interval (CI) 1.12–1.86].17
In that same study, NOAC non-adherence was associated with an almost 40% increased risk of stroke (HR 1.39, 95% CI 1.06–1.81) and NOAC non-persistence was associated with an almost five-fold increased risk of stroke/transient ischaemic attack (HR 4.55, 95% CI 2.80–7.39).17
Since NOACs have become the first choice for stroke prevention in the majority of patients with NVAF, it is essential to find effective strategies for improvement of adherence and persistence to anticoagulant medication in this group of patients.
Although one of the advantages of NOACs over VKAs is their ease of use,20 the lack of required ongoing coagulation monitoring may become a disadvantage as it diminishes clinician oversight of therapeutic regimens, requiring a greater effort in patient education and monitoring for adherence.
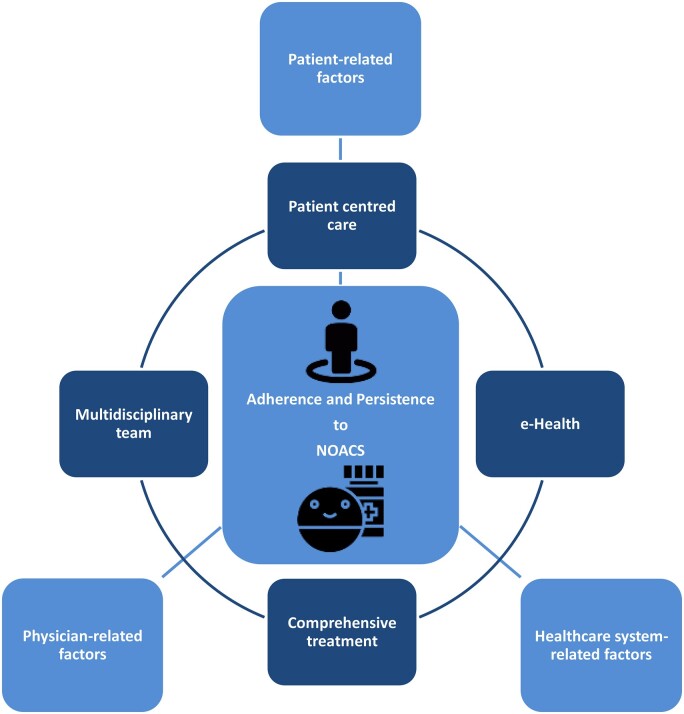
Nonetheless, achieving optimal prevention of stroke in NVAF patients is a multifactorial process. Factors influencing adherence and persistence to NOAC therapy have been suggested to be patient-related, physician-related, and healthcare system-related (Table 1 and Figure 1).14
Table 1.
Factors influencing adherence and persistence to NOAC therapy
| Patient-related | |
| Demographics | Age |
| Ethnicity | |
| Educational level | |
| Socioeconomic status | |
| Presence of caregivers | |
| Medical conditions | Comorbidities |
| Disability | |
| Frailty | |
| Cognitive impairment | |
| Tolerance and side effects of drugs | |
| Polypharmacy | |
| Treatment complexity | |
| Behavioural factors | Social isolation |
| Psychiatric disorders | |
| Addictive behaviours | |
| Daily routine and lifestyle | |
| Patient education and disease management | Awareness of thromboembolic risk |
| Knowledge of the benefits of oral anticoagulation | |
| Awareness of the risk of drug discontinuation | |
| Understanding of the treatment regimen | |
| Understanding of the importance of strict adherence | |
| Knowledge of risk control strategies | |
| Education on drug-specific information | |
| Engagement in treatment decisions | |
| Expectations, values, goals, and preferences | |
| Burden of treatment | |
| Physician-related | |
| Knowledge | Adherence to guidelines |
| Awareness of recommendations | |
| Awareness of risks and preventive measures | |
| Knowledge on management of bleeding | |
| Knowledge on management of side effects | |
| Knowledge on drug characteristics and dose choice | |
| Expertise | |
| Patient follow-up | Risk/benefit reassessment |
| Implementation of adherence strategies | |
| Reassurance | |
| Shared decision-making | |
| Drug regimen adjustments | |
| Outcomes measurement | |
| Quality of life measurement | |
| Healthcare system-related | |
| Work setting | Specialized centres |
| Structures of healthcare system | |
| Continuity in patient–doctor relation | |
| Multidisciplinary team approach | |
| Technology tools support and e-Health | |
| Patient-reported outcomes | |
| Continuous healthcare professional training | |
| Costs of care | Medication availability |
| Access to treatment | |
| Economic concerns | |
| Financial burden to the patient | |
NOAC, non-vitamin K antagonist oral anticoagulant.
Figure 1.
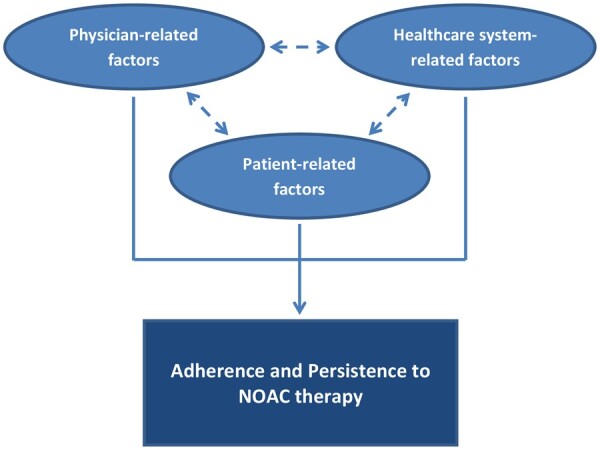
Factors influencing adherence and persistence to non-vitamin K antagonist oral anticoagulant therapy and their interrelation. NOAC, non-vitamin K antagonist oral anticoagulant.
Graphical Abstract.
Integrated management of AF patients taking into account factors influencing adherence and persistence to NOAC therapy. Abbreviations: AF Atrial fibrillation; NOAC Non-vitamin K antagonist oral anticoagulant.
Patient-related factors influencing non-vitamin K antagonist oral anticoagulant adherence
Non-adherence to oral anticoagulation appears more prevalent in younger patients, those of lower socioeconomic status, and those less well informed about their disease and medications.14,26,27 Other commonly reported reasons for non-adherence to oral anticoagulation are depressive symptoms or pessimistic attitude, psychiatric illness, cognitive impairment, frailty and risk of falling, comorbidity burden and impaired quality of life, lack of social support, alcohol, and drug abuse.14,26,28,29 Male gender and living alone have also been associated with a higher risk of therapy discontinuation.30
The cultural and geographical background of the patient is also possibly related to adherence and persistent rates.31,32 An international survey found that perceived AF seriousness, concern about stroke, and self-reported adherence, as well as the willingness for shared decision-making or self-empowerment differ between patients from diverse geographical regions.32
Low adherence is also related to polypharmacy, especially when including antiplatelet drugs or more complex drug regimens, the fear of bleeding, and worries about worsening health outcomes. Active employment, especially with busy work schedules, frequent social activities and greater education are also known to affect negatively adherence to treatment.14,26,28,33–35
Patient-perceived treatment burden, defined as the workload imposed by healthcare on patients and its effect on patient functioning and well-being apart from specific treatment side-effects, may also affect adherence to treatment.36–39 The impact on daily routine and lifestyle restrictions must be taken into consideration.26,40
Adherence to therapy may be also limited by the complexity of the regimen.26,27,41 An European survey revealed that the fixed dosing without the need for routine laboratory monitoring of the anticoagulation effect is the strongest driver for NVAF patients choosing a NOAC over a VKA, and the main reasons for NOAC refusal were the fear of bleeding and under-appreciation of stroke risk.14,42
A recent study based on 1-year follow-up phase III data from GLORIA-AF registry43 found that drug persistence was higher with NOACs than with VKAs, without a significant difference in persistence when comparing NOAC dosing regimens (once daily vs. twice daily).44 Nonetheless, in other studies, once daily NOACs were shown to increase adherence in comparison to twice daily NOACs.14,27,45–48
However, it is still uncertain whether any regimen is superior in terms of thromboembolic prevention and safety profile, especially in the case of omitted doses.21,45,49 Therefore, it is essential to ensure that drugs are taken according to the prescribed regimen.20
Stroke avoidance can be highly valued by patients once they became aware of it, but the bleeding risk, availability of an antidote and interactions with food or drugs are commonly reported concerns.26,41,48 An international survey revealed that stroke prevention is the most important factor for 47% of AF patients when choosing OAC, followed by the risk of major bleeding (15%), other side effects (10%), dosing frequency (8%), antidote availability (7%), dietary restrictions (6%), and the need for intake with/without food (5%).32
Although the already mentioned advantages of NOACs may be the answer for many of these concerns,9,11,26,48 there is yet limited availability of specific antidotes.9,50 The non-requirement for bridging therapy when planning a procedure or surgery is another highly valued characteristic of NOACs.41
Patients with AF and recent stroke, especially with higher grades of functional disability, are also more likely to perceive AF as a serious condition and to be more concerned about stroke. However, these patients report significantly lower adherence to OAC, poorer knowledge of their condition, and less willingness for self-empowerment and shared decision-making compared with AF patients without a recent stroke.32
Physician-related factors influencing non-vitamin K antagonist oral anticoagulant adherence
Physicians often overestimate bleeding risk, and this apprehension about feeling responsible for a major bleed seems to outweigh their concern about risk of stroke.14,51–53 A study using warfarin identified a previous major bleed as a limiting factor for OAC prescription, although prescription was not influenced by the occurrence of an ischaemic stroke in an untreated AF patient.54
Clinicians must be aware that the clinical benefit of stroke prevention will nearly always prevail over bleeding risk, and that reversible bleeding risk factors which are incorporated in bleeding scores must be identified and corrected instead of limiting OAC therapy.55–57
In a report from the EuroObservational Research Programme Pilot survey on Atrial Fibrillation (EORP-AF Pilot) General Registry, only 60% of the patients were correctly treated with an OAC according to current guidelines.58
Various factors have been related with non-guideline-adherence by the prescribing physicians, including geographical region, observation setting, patient’s advanced age, male gender, comorbidities (some of them represented in the CHA2DS2-VASc score), clinical presentation, and previous pharmacological cardioversion.58–60 On the other hand, patient’s previous electrical cardioversion and catheter ablation were associated with a higher likelihood of being prescribed guideline-adherent treatment.58 Also, while asymptomatic AF patients may be under-treated, symptomatic patients are more likely to be over-treated, perhaps reflecting patients’ values and preferences potentially driven by symptom-triggered anxieties.52,58 Furthermore, cardiologists and electrophysiologists are more inclined to prescribe NOACs in preference to warfarin compared to primary care physicians.60–63
Explanations for these may include the fact that such comorbidities may sometimes preclude the initiation of guideline-adherent therapy or that some interventions may distract physicians from assessing stroke risk, the problem of therapeutic inertia and negative herding behaviour, the absence of anticoagulation reversal antidotes, and the fact that non-specialized clinicians may lack expertise for dealing with NOACs and eventually choose pharmacological agents with which they are more familiar, comfortable and knowledgeable.52,58,60,62,64–68
NOAC prescription rates may also be affected by the perception that even minor deviations from strict adherence can significantly decrease the efficacy of NOACs, due to their shorter half-lives. On the other hand, the fact that NOACs have fewer food and drug interactions, resulting in much easier longitudinal management, favour NOAC prescription and adherence over VKAs.52
Healthcare system-related factors influencing non-vitamin K antagonist oral anticoagulant adherence
Patients are drug cost sensitive, and cost is a considered prescribing factor in eligible patients.26,48,52 The out-of-pocket costs to patients depends on the level of public coverage and/or private insurance.26,52 Despite being more expensive than VKAs, NOACs have nevertheless been shown to be more cost-effective in the longer term.52,69,70
On the one hand, the presence of health insurance is associated with better persistence rates, but on the other hand, lower socio-economic status and capacity to afford medications impacts the ability to follow prescriptions, with lower adherence and persistence rates.26,71–73 Furthermore, the insurance type granting greater prescription coverage may substantially increase the use of NOACs.72 Lower income and lower social support are already known to affect medication taking as a whole.
However, clinicians can deliver patient-tailored evidence-based care more equitably when they appropriately apply the available clinical tools to assess the risk of stroke (CHA2DS2-VASc), the risk of bleeding (HAS-BLED), and the likelihood of having poor international normalized ratio (INR) control (SAMe-TT2R2).52,74
Apart from the financial burden, the emotional support from healthcare professionals is also important for NOAC patients, and the reduced opportunity for patients to be in regular contact with their healthcare provider (HCP) may concern some of them and even affect adherence.26
Strategies to improve adherence and persistence to non-vitamin K antagonist oral anticoagulants in atrial fibrillation
Identification of factors accounting for non-adherence and non-persistence to NOACs is essential for targeting patient management and improving overall adherence and persistence to medication.14 Adherence should be measured and patterns of and reasons for non-adherence are valuable in developing individualized strategies to improve adherence and outcomes.75 Patient’s well-being and personal circumstances must be covered.
Despite the intuitive potential benefit of educational interventions and clinical decision tools, currently there is little robust evidence,51,53,76 sometimes even with contradictory findings.53,77,78 However, regular scheduled contact with healthcare professionals, patient anticoagulation card recordings, and an active multidisciplinary approach have been proposed to improve patient’s adherence.14,79
Patient-centred strategies
Patient engagement and shared decision-making
The decision of whether to initiate NOAC therapy, and which anticoagulant to use, should be made by the treating clinician after consultation with the patient.40 Patient’s values, goals, and preferences shall guide shared decision-making.4,80 Patients want to be involved in the decision-making process and wish to feel reassured about the diagnosis and treatment. A good physician–patient relationship and communication is required for good patient adherence and persistence with NOACs.40
Engaging patients in treatment decisions about their own health, taking into account their feelings and beliefs on the subject, gives healthcare professionals the opportunity to address any concerns about medication, correct misconceptions, increase patient knowledge about their condition and the therapeutic options, and will help patients make informed decisions about their healthcare, being ware of the importance of OAC therapy.40,48,81
The importance attached by the patient to OAC therapy and the perceived risk of death, stroke, and major bleeding, as well as the burden of treatment, must be thoroughly assessed and respected. The discussion of treatment burden should be an integral part of shared decision-making, and it can be assessed using a validated questionnaire.4,80
A shared decision-making is also intimately related to patient knowledge about AF and its management, but education alone is not sufficient to produce and maintain medication adherence and lifestyle modifications.4,41 Different other patient-related, physician-related, and healthcare system-related factors may affect adherence to treatment (Table 1).
Patient education and behavioural interventions
Ensuring patients are appropriately informed about their condition, treatment options, how to adhere to treatment, risks and benefits of treatment, potential consequences of non-adherence, in addition to managing patient’s expectations, are crucial to NOAC treatment success.14,26,40,81 Regular patient review is crucial to identify non-adherence and implement strategies to improve adherence.79
The need for NOAC therapy and the importance of strict and sustained adherence must be thoroughly explained to the patient, so that the patient will be able to understand the potential consequences of non-adherence.20 Patient involvement in treatment decisions and patient adherence to an accorded therapeutic regimen are dependent on patient knowledge about AF, stroke, and drug-specific information.81–83
Information should be provided using appropriate language, in a variety of formats, and confirmation of patients’ understanding should be checked.14 The mode of delivery and complexity of information should be adapted to the individual patient.20,48,75 Many simultaneous approaches can help the clinician providing straightforward information, including leaflets, a patient anticoagulation card, group sessions, and online patient support websites.
It is important to utilize each patient visit and every prescription renewal for re-education, discussing intake modalities, the importance of strict adherence to the prescribed dosing regimen to reduce the likelihood of serious adverse events and to convince patients that NOACs therapy should not be discontinued.
The fear of bleeding still has a major impact in patient adherence to NOAC therapy. However, proper education and shared decision-making can overcome this barrier, since AF patients are willing to accept certain bleeding risks for a decrease in the probability of experiencing a stroke.41,81,84
The patient must also be instructed about what to do in case of an overdose and, especially in the advent of antidotes to NOACs, it is even more important that patients know what drug they are taking.
Education may be more effective if directed to specific knowledge gaps of the patient, which can be measured by validated questionnaires.85,86 Educational group sessions, tailored to each NOAC and considering social, ethnic, cultural, and geographical differences may be developed to improve adherence to NOACs.
Some patients may prefer VKA over NOAC therapy, because they feel safer with regular laboratory monitoring and the existence of an antidote.26,41 Patient education must include the discussion of these preferences in the context of available clinical trial data, especially regarding the bleeding risk.20,82
In NOAC patients in whom low adherence is suspected despite proper education and additional tools, conversion to VKAs may be considered, given the ability to monitor INR and the potential for higher time in therapeutic range for patients managed at anticoagulation clinics.17,20,87 However, poor adherence to VKA therapy is equally associated with INR fluctuations and worst outcomes.20
The IMPACT-AF study demonstrated that education of patients and their families as well as of HCPs, with regular monitoring and feedback, increased OAC use by three-fold with a consequent reduction in stroke risk by 52% after 1 year compared with the non-intervention group.77
The AEGEAN study was not able to show an incremental benefit of an education program focused in patients that initiated therapy with apixaban at baseline, probably because this study had more than 90% adherence and 85% persistence rates in both the intervention and usual care groups after 1 year. On the one hand, this highlights the challenge of enrolling a control group representative of general practice, but on the other hand, this may reflect the fact that both groups received appropriate NOAC-specific information at initiation, used an electronic monitoring device, and had a continued and structured follow-up.78
Technological aids and mobile health applications
Technological aids for drug intake monitoring have been explored, and include the day-marked blister pack format, medication boxes (conventional or with electronic verification of intake), smart packages (with electronic detection of package entry), smart pills (with direct electronic detection of pills in the stomach), and smartphone applications (with reminders to alert the patient about the next intake, and some even requiring confirmation that the dose has been taken).20,45,88 However, the long-term effects of such tools are still unknown and one tool may not suit all patients or healthcare settings.20,88
A previous review have already shown that electronic monitoring feedback interventions are potentially effective approaches to enhance patient adherence to medications.89 Especially in cases where suboptimal adherence is suspected, electronic monitoring may expose patterns of missed doses, serving as a basis for patient education.20,88,90 A telemonitoring-based service, with or without feedback to the patient, was shown to result in high NOAC adherence, and could eventually become a cost-effective approach in high-risk patients deemed poorly adherent. However, its impact in clinical practice still needs confirmation in larger trials.88,90
The use of Mobile Health technology (mHealth) in the management of AF has been shown to improve patient quality of life, knowledge, anticoagulation satisfaction, drug adherence and persistence, and clinical outcomes.51,91–94 These mobile AF applications incorporate inbuilt personal health records, clinical decision support tools, and education programmes in keeping with integrated care principles.51,92–94 Further studies on the applicability of these technological advancements in healthcare systems and integrated care are needed.51,91
Patient-reported outcomes
Patient-reported outcomes (PROs) are subjective measures of patient experience and well-being about any particular condition or treatment they are receiving and quantify assessment of patient’s expectations, satisfaction, adherence or health-related quality of life (HRQoL). Patient-reported outcomes can be obtained from patient interviews, questionnaires or specifically developed tools to capture and enable analysis of valuable patient reported data. There is an increasing body of evidence for the importance of PROs in patients treated with NOACs.95 Therefore, PROs can be used to assist in prescription choices, according to patient’s experience.
In a recent systematic review of PROs associated with the use of NOACs, patients prescribed with a NOAC showed enhanced treatment satisfaction and patients prescribed with warfarin had more expectations of adverse events, patients with a greater knowledge of their anticoagulant treatment were more likely to adhere, and a lower HRQoL was observed in patients prescribed with warfarin with poor INR control.95
The International Consortium for Health Outcomes Measurement (ICHOM) assembled a working group to develop a standardized set of outcomes for benchmarking care delivery in AF. Specifically regarding PROs, this working group found a standard set of outcomes that matter most to patients with AF: HRQoL, emotional functioning, physical functioning, exercise tolerance, symptom severity, ability to work, and cognitive functioning. Although patients also reported health literacy as a very important outcome, it was not included in the core set as it covered aspects outside of the scope of the project, and despite haemorrhagic stroke, life-threatening/major bleeding, serious adverse events post-intervention, and medication side effects being measured in a different domain of outcomes, they were also highly considered by patients.96
Physician-centred and Healthcare system-centred strategies
Anticoagulant monitoring services and patient follow-up
The long-term management of patients receiving NOACs may be efficiently handled by centralized anticoagulation clinics. This specialized management is associated with better anticoagulation control (Table 2). Both nurse-led and pharmacist-led AF centres may be helpful in coordinating patient follow-up and checking on adherence with improved outcomes.97–103 The involvement of the patient’s general practitioner and family members or caregivers is also crucial to reinforce adherence.20,79 Thus, success on patient adherence and persistence to a NOAC is frequently highly dependent on a multidisciplinary team.79,104
Table 2.
Anticoagulation clinic core activities
| Confirm appropriate indication |
| Selecting the optimal anticoagulant |
| Anticoagulant initiation and dosing |
| Patient education and behavioural intervention |
| Long-term anticoagulation follow-up |
| Anticoagulant dose management when needed |
| Monitoring of drug interactions |
| Invasive procedures planning |
| Monitoring of adherence |
| Safe and effective transitions between anticoagulants when needed |
There should be a pre-specified follow-up schedule for the NOAC patient, which must be known and shared by all people involved in patient care (general practitioners, cardiologists, pharmacists, nurses, family members, etc.). Everyone’s actions regarding NOAC patient should be communicated to the others, e.g. by filling out a line on the NOAC anticoagulation card. All HCPs should be responsible for reinforcing general educational messages.
A guidelines-based and software-supported nurse-led integrated chronic care program supervised by a cardiologist was shown to result in better medication adherence and a significant reduction in cardiovascular hospitalizations and cardiovascular mortality, probably because it allows a more systematic care and coordinated follow-up.98,102
In the mAFA II trial, an integrated care of AF patients using the ABC (Atrial Fibrillation Better Care) pathway approach and supported by mobile health technology (mobile AF Application—mAFA) reduced the risk of the composite outcome of stroke/thromboembolism, all-cause death and rehospitalization by 61% compared to usual care.93 In the mAFA II trial long-term extension cohort, consisting in the subgroup of patients with follow-up over 1 year, 71% of the patients in the ABC/mAFA intervention group had good management adherence and the persistence of use was 92%, with a sustained reduction in the risk of the primary outcome by 82% compared to usual care.94
An increased follow-up and adherence monitoring by pharmacists may also improve NOAC adherence. Thus, especially in countries with a highly networked pharmacy database, which can help track the number of NOAC prescriptions that individual patients claim, pharmacists should be involved in adherence monitoring, and this information could also be used to cross-check appropriate prescription and dosing.20,99,101
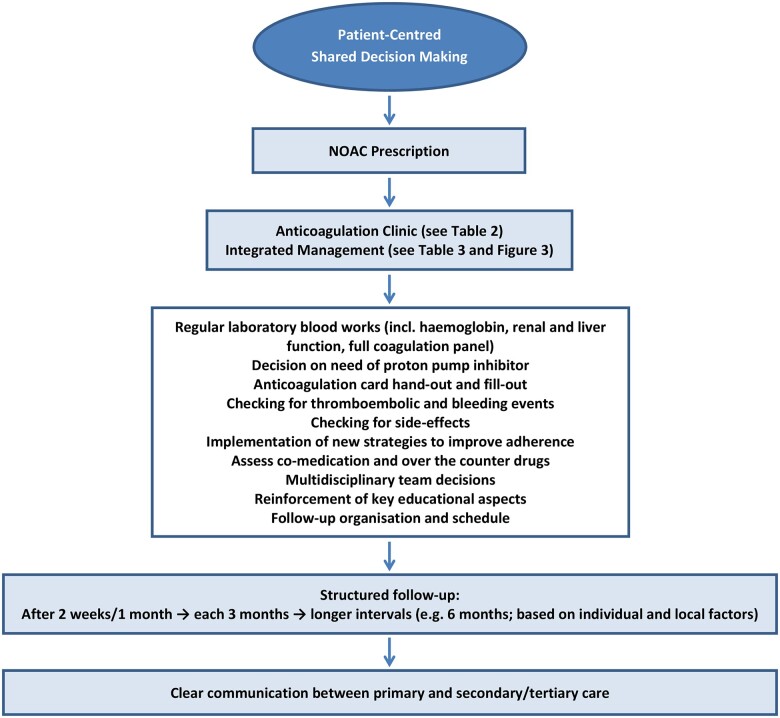
After a full discussion of the problem with the patient, the first step on optimal prevention of stroke in patients with NVAF consists on the prescription of the most appropriate NOAC drug according to patient’s characteristics, values, goals, and preferences.4,14,20,75 The initial prescriber should be responsible for including the patient on a follow-up programme.20 During follow-up, a regular patient review is important to identify non-adherence and implement strategies to improve adherence where appropriate (Figure 2).4,20,79,100
Figure 2.
Non-vitamin K antagonist oral anticoagulant prescription and involvement of the non-vitamin K antagonist oral anticoagulant patient in a follow-up programme. NOAC, non-vitamin K antagonist oral anticoagulant.
Improving the prescription of oral anticoagulants by healthcare providers
Healthcare providers must be aware of potential NOAC non-adherence and non-persistence in order to ensure that anticoagulation therapy for stroke prevention is optimized.17 On the other hand, there is still limited evidence of clinicians’ perspectives of NOACs, necessitating further research.105
All HCPs should receive constant updates on available evidence on NOACs with role-appropriate levels of complexity, and flow charts, software and e-support should be made available for guiding treatment. Healthcare providers should also be responsible for reinforcement of key educational messages about anticoagulant therapy, assessment of patient understanding, periodic contact to follow-up, and active interactions with the other members of the multidisciplinary team.14,79
Digital clinical decision support systems have emerged in order to easily provide evidence-based guidelines, clinical pathways, and algorithms for personalized, timely, and evidence-based treatment.4,51,79,104 These clinical support systems work best when decisions are shared and the same evidence is available to both parties, resulting in improvements in patient involvement and clinician satisfaction and reductions in decision conflict.40,51,106 Although several shared decision-making tools are available, their efficacy in an integrated management of AF patients still need rigorous testing.40,106
Electronic health records facilitate clinical coordination, registration of data for patient risk stratification, remote patient monitoring, and reviews of prescribing. These clinical information systems may allow targeted interventions, which impact in integrated care still need further testing.45,51,53,107,108
Interventions based on HCPs education, implementing guidelines or protocols, and medical care programmes, were shown to be effective despite some inconsistent results. Some success was also reported when local opinion leaders promoted evidence-based practice. Therefore, interventions enabling clinicians to seek consensus with, and receive expert opinion from, their peer-group may be effective for prompting behavioural change. However, large-scale studies are still required to determine the most effective intervention.53
At a broader level, strategies for improving the affordability of NOACs must be implemented, so that these drugs can become readily available to those who need them.109
Physician-reported outcomes
The ICHOM AF working group included in the standardized set of outcomes for benchmarking care delivery in AF two other outcome domains besides PROs: long-term consequences of disease and complications of treatment.96
Long-term consequences of disease include mortality (all-cause and cardiovascular), ischaemic stroke, systemic embolism, unclassified stroke, heart failure, cardiovascular hospitalization, freedom from rapid and/or symptomatic atrial arrhythmia post-treatment, anticoagulation management, and clinician-reported patient cognitive functioning. Complications of treatment include haemorrhagic stroke, life-threatening/major bleeding, serious adverse events post-intervention, and medication side effects.96
In combination with PROs, this set of outcomes may enable institutions to monitor, compare, and improve the quality of their care for patients with AF.96
Integrated management of atrial fibrillation patients and the ‘atrial fibrillation better care’ pathway
Currently, NOAC patients lack a structured follow-up. There is no agreement on follow-up responsibilities between general practitioners and medical specialists, and there is a lack in clarity about the role of each HCP within the anticoagulation chain. The absence of a common electronic system and of a standard communication method makes a multidisciplinary collaboration even more difficult.110
The concept of an integrated management for all patients on any form of antithrombotic medication, and not only on VKAs, is receiving high consideration, and different strategies have been suggested to improve anticoagulation care of NOAC patients (Table 3).45,51,79,88,97,98,100–111 Adherence to OAC for stroke prevention is only one part of the integrated management of AF patients, which involves a holistic approach.88 Similar holistic approaches have also been proposed for aortovascular disease and for stroke.112,113
Table 3.
How to improve anticoagulation care with NOACs in an integrated management for AF patients
| Clarification of roles and responsibilities for the medical specialists involved |
| Structured monitoring of therapy compliance and dosage |
| Structured follow-up (ideally in a NOAC clinic or structured care pathway) |
| Involvement of the local pharmacist |
| Optimization of the information transfer between professionals and to families or caregivers (ideally through a common system/actual medication overview) |
| Improvement of healthcare professionals’ knowledge about NOACs (particularly primary care professionals) |
| Training of healthcare professionals in integrated care for AF |
| Use of electronic decision support systems |
| Adequate patient education about the disease and NOAC treatment |
| Involvement and education of family members or caregivers |
| Implementation of technical aids (medication boxes, phone apps, reminder systems) |
| Electronic monitoring if poor adherence suspected |
AF, atrial fibrillation; NOAC(s), non-vitamin K antagonist oral anticoagulant(s).
Coordinated systems of care are already recognized as a mechanism to provide high-quality programs towards improved patient outcomes in other areas of cardiovascular care.79,104 Integrated care refers to organization and delivery of healthcare services in a coordinated, efficient, and effective way with the aim of optimizing patient care.51 It leads to improved treatment adherence, reduced perceived treatment burden and better outcomes.51,114,115
Integrated care places the patient at its centre and implies an informed and active shared decision-making process and disease management between the patient, their family or caregivers, and HCPs. The practice of integrated care therefore involves a multidisciplinary team and a close collaboration between different healthcare services across primary, secondary, and tertiary care settings.51,114,115
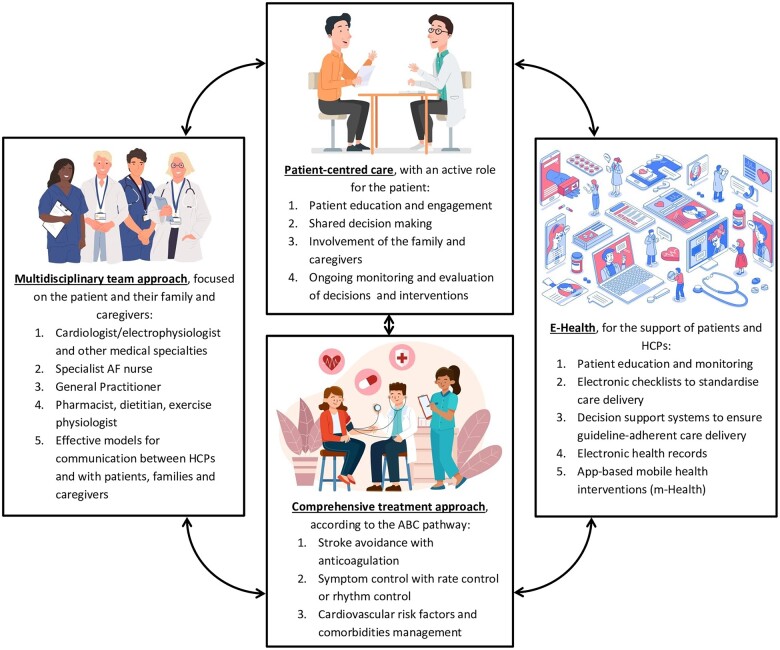
The integrated management of AF patients includes the delivery of patient-centred care, with an active role for the patient; a multidisciplinary team approach; the use of e-health to support patients and HCPs; and a comprehensive treatment approach that includes a full suite of options for managing AF and associated conditions (Figure 3).4,51,79,104,116
Figure 3.
Integrated management of atrial fibrillation patients including the ABC pathway. This figure was designed using resources from Freepik.com. ABC, Atrial Fibrillation Better Care; m-Health, Mobile Health technology.
A systematic review and meta-analysis has shown that an integrated care approach in AF reduced all-cause mortality and cardiovascular hospitalizations without a significant impact on cerebrovascular events.104 This focused on studies before the widespread use of NOACs and the concept of Atrial Fibrillation Better Care (ABC) pathway,117 which has since been incorporated into guidelines.4,118,119
However, a recent systematic review and meta-analysis showed that patients who received an integrated care with the ABC pathway approach had a lower risk of stroke (OR 0.55, 95% CI 0.37–0.82) and major bleeding (OR 0.69, 95% CI 0.51–0.94), besides a reduction of all-cause mortality (OR 0.42, 95% CI 0.31–0.56) and cardiovascular death (OR 0.37, 95% CI 0.23–0.58). The length of follow-up was also directly associated with an increase in the effectiveness of this management strategy.111 Another recent review also highlighted the possible reduction in the risk of myocardial infarction and hospitalizations.115
The ABC pathway stands on three main pillars: ‘A’—Avoiding stroke with the adequate use of anticoagulants, ‘B’—Better symptom management with the adequate use of rate and rhythm control therapies, and ‘C’—optimal management of Cardiovascular risk factors and Comorbidities.4,117 Although this comprehensive treatment approach for AF patients has been associated with a better prognosis, clinical management adherent to the ABC pathway remains suboptimal, around 21%, meaning that more efforts are needed to follow this strategy.111,115
Nonetheless, optimized AF treatment also requires a well-structured healthcare system and significant financial resources, implying the judicious use of available resources.4,109 Various studies have suggested that integrated care is the way forward for managing the growing heath care burden associated with AF, leading to a more cost-effective care.120–122
Therefore, this holistic strategy for AF treatment, including the ABC pathway approach, may help overcome patient-related, physician-related, and healthcare system-related factors affecting adherence and persistence to NOAC therapy, supporting both patients and clinicians in their shared decisions and disease management.
Conclusion
Atrial fibrillation is the most common sustained cardiac arrhythmia in adults and is associated with an increased risk of stroke, which can be prevented by the use of OACs. Although NOACs have become the first choice for stroke prevention in the majority of patients with NVAF, adherence and persistence to these medications remain suboptimal, which may translate into poor health outcomes and increased healthcare costs.
Factors influencing adherence and persistence to NOAC therapy are patient-related, physician-related, and healthcare system-related. Identification of factors accounting for non-adherence and non-persistence to NOACs is essential for targeting patient management and improving overall adherence and persistence to medication.
Patient education, behavioural interventions and engagement in a shared decision-making process, implementation of anticoagulant monitoring services or structured pathways of care with a coordinated patient follow-up, utilization of technological aids and mobile health applications, and the optimization of the prescription of oral anticoagulants by HCPs through education, digital clinical decision support systems and electronic health records may constitute strategies for adherence and persistence improvement. Patient-reported outcomes could help to better understand the patient’s experience of their condition or treatment and therefore assist in prescription choices.
The integrated management of AF patients including a comprehensive treatment approach as the ABC pathway may lead to improved adherence, reduced treatment burden, better clinical outcomes, and a more cost-effective care. However, clinical management adherent to the ABC pathway remains suboptimal, meaning that more efforts are needed to implement and ensure adherence with this strategy.
Funding
This paper was published as part of a supplement financially supported by Daiichi Sankyo Europe GmbH.
Conflict of interest: J.M.F.: none declared. I.D.J.: received research grant funding from BMS. G.Y.H.L.: consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally.
Contributor Information
José Maria Farinha, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK.
Ian D Jones, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; School of Nursing and Allied Health, Liverpool John Moores University, Liverpool, UK.
Gregory Y H Lip, Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool John Moores University and Liverpool Heart & Chest Hospital, Liverpool, UK; Department of Clinical Medicine, Aalborg University, Aalborg, Denmark.
References
- 1. Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes 2020;doi:10.1093/ehjqcco/qcaa093. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 3. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–867. [DOI] [PubMed] [Google Scholar]
- 4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau J-P, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 5. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand J-P, Berge E, Cools F, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Kayani G, Koretsune Y, Mantovani LG, Misselwitz F, Oh S, Turpie AGG, Verheugt FWA, Kakkar AK; GARFIELD-AF Investigators. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Apenteng PN, Gao H, Hobbs FR, Fitzmaurice DA. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open 2018;8:e018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kozieł M, Teutsch C, Bayer V, Lu S, Gurusamy VK, Halperin JL, Rothman KJ, Diener H-C, Ma C-S, Huisman MV, Lip GYH; GLORIA-AF Investigators. Changes in anticoagulant prescription patterns over time for patients with atrial fibrillation around the world. J Arrhythmia 2021;37:990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hohnloser SH, Basic E, Nabauer M. Changes in oral anticoagulation therapy over one year in 51,000 atrial fibrillation patients at risk for stroke: a practice-derived study. Thromb Haemost 2019;119:882–893. [DOI] [PubMed] [Google Scholar]
- 9. De Caterina R, Ageno W, Agnelli G, Chan NC, Diener H-C, Hylek E, Raskob GE, Siegal DM, Verheugt FWA, Lip GYH, Weitz JI. The non-vitamin K antagonist oral anticoagulants in heart disease: section V—special situations. Thromb Haemost 2019;119:14–38. [DOI] [PubMed] [Google Scholar]
- 10. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KAA, Califf RM; the ROCKET AF Steering Committee. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 11. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 12. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FWA, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 13. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener H-C, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 14. Raparelli V, Proietti M, Cangemi R, Lip GYH, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation. Thromb Haemost 2017;117:209–218. [DOI] [PubMed] [Google Scholar]
- 15. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 16. Rivera-Caravaca JM, Roldán V, Esteve-Pastor MA, Valdés M, Vicente V, Lip GYH, Marín F. Cessation of oral anticoagulation is an important risk factor for stroke and mortality in atrial fibrillation patients. Thromb Haemost 2017;117:1448–1454. [DOI] [PubMed] [Google Scholar]
- 17. Ozaki AF, Choi AS, Le QT, Ko DT, Han JK, Park SS, Jackevicius CA. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes 2020;13:e005969. [DOI] [PubMed] [Google Scholar]
- 18. Orlowski A, Gale CP, Ashton R, Petrungaro B, Slater R, Nadarajah R, Cowan JC, Buck J, Smith W, Wu J. Clinical and budget impacts of changes in oral anticoagulation prescribing for atrial fibrillation. Heart 2021;107:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshpande CG, Kogut S, Willey C. Real-world health care costs based on medication adherence and risk of stroke and bleeding in patients treated with novel anticoagulant therapy. J Manag Care Spec Pharm 2018;24:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Vanassche T, Potpara T, Camm AJ, Heidbüchel H; External reviewers. 2021 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–1676. [DOI] [PubMed] [Google Scholar]
- 21. Adcock DM, Gosselin R. Direct oral anticoagulants (DOACs) in the laboratory: 2015 review. Thromb Res 2015;136:7–12. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Adherence to Long Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization. 2003.
- 23. Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, Wong PK. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47. [DOI] [PubMed] [Google Scholar]
- 24. Chakrabarti S. What’s in a name? Compliance, adherence and concordance in chronic psychiatric disorders. World J Psychiatry 2014;4:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De las Cuevas C. Towards a clarification of terminology in medicine taking behavior: compliance, adherence and concordance are related although different terms with different uses. Curr Clin Pharmacol 2011;6:74–77. [DOI] [PubMed] [Google Scholar]
- 26. Pandya EY, Bajorek B. Factors affecting patients’ perception on, and adherence to, anticoagulant therapy: anticipating the role of direct oral anticoagulants. Patient 2017;10:163–185. [DOI] [PubMed] [Google Scholar]
- 27. Garkina SV, Vavilova TV, Lebedev DS, Mikhaylov EN. Compliance and adherence to oral anticoagulation therapy in elderly patients with atrial fibrillation in the era of direct oral anticoagulants. J Geriatr Cardiol 2016;13:807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaikh F, Pasch LB, Newton PJ, Bajorek BV, Ferguson C. Addressing multimorbidity and polypharmacy in individuals with atrial fibrillation. Curr Cardiol Rep 2018;20:32. [DOI] [PubMed] [Google Scholar]
- 29. Buck J, Fromings Hill J, Martin A, Springate C, Ghosh B, Ashton R, Lee G, Orlowski A. Reasons for discontinuing oral anticoagulation therapy for atrial fibrillation: a systematic review. Age Ageing 2021;50:1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Binding C, Olesen JB, Lee CJ-Y, Lip G, Sindet-Petersen C, Gislason G, Bonde AN. Discontinuation of direct oral anticoagulants among patients with atrial fibrillation according to gender and cohabitation status: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother 2021;doi:10.1093/ehjcvp/pvab065. [DOI] [PubMed] [Google Scholar]
- 31. Gebreyohannes EA, Salter S, Chalmers L, Bereznicki L, Lee K. Non-adherence to thromboprophylaxis guidelines in atrial fibrillation: a narrative review of the extent of and factors in guideline non-adherence. Am J Cardiovasc Drugs 2021;21:419–433. [DOI] [PubMed] [Google Scholar]
- 32. Lane D, Meyerhoff J, Rohner U, Lip G. Patients’ perceptions of atrial fibrillation, stroke risk, and oral anticoagulation treatment: an international survey. TH Open 2018;2:e233–e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reading SR, Black MH, Singer DE, Go AS, Fang MC, Udaltsova N, Harrison TN, Wei RX, Liu I-LA, Reynolds K; for the ATRIA-CVRN Investigators. Risk factors for medication non-adherence among atrial fibrillation patients. BMC Cardiovasc Disord 2019;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki T, Shiga T, Omori H, Tatsumi F, Nishimura K, Hagiwara N. Adherence to medication and characteristics of Japanese patients with non-valvular atrial fibrillation. J Cardiol 2017;70:238–243. [DOI] [PubMed] [Google Scholar]
- 35. Platt AB, Localio AR, Brensinger CM, Cruess DG, Christie JD, Gross R, Parker CS, Price M, Metlay JP, Cohen A, Newcomb CW, Strom BL, Laskin MS, Kimmel SE. Risk factors for nonadherence to warfarin: results from the IN-RANGE study. Pharmacoepidemiol Drug Saf 2008;17:853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Potpara TS, Mihajlovic M, Zec N, Marinkovic M, Kovacevic V, Simic J, Kocijancic A, Vajagic L, Jotic A, Mujovic N, Stankovic GR. Self-reported treatment burden in patients with atrial fibrillation: quantification, major determinants, and implications for integrated holistic management of the arrhythmia. Europace 2020;22:1788–1797. [DOI] [PubMed] [Google Scholar]
- 37. Buffel Du Vaure C, Ravaud P, Baron G, Barnes C, Gilberg S, Boutron I. Potential workload in applying clinical practice guidelines for patients with chronic conditions and multimorbidity: a systematic analysis. BMJ Open 2016;6:e010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tran VT, Montori VM, Eton DT, Baruch D, Falissard B, Ravaud P. Development and description of measurement properties of an instrument to assess treatment burden among patients with multiple chronic conditions. BMC Med 2012;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eton DT, Ramalho de Oliveira D, Egginton JS, Ridgeway JL, Odell L, May CR, Montori VM. Building a measurement framework of burden of treatment in complex patients with chronic conditions: a qualitative study. Patient Relat Outcome Meas 2012;3:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noseworthy PA, Brito JP, Kunneman M, Hargraves IG, Zeballos-Palacios C, Montori VM, Ting HH. Shared decision-making in atrial fibrillation: navigating complex issues in partnership with the patient. J Interv Card Electrophysiol 2019;56:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient preferences for oral anticoagulation therapy in atrial fibrillation: a systematic literature review. Patient 2017;10:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potpara TS, Pison L, Larsen TB, Estner H, Madrid A, Blomström-Lundqvist C; Scientific Initiatives Committee, and European Heart Rhythm Association. How are patients with atrial fibrillation approached and informed about their risk profile and available therapies in Europe? Results of the European Heart Rhythm Association Survey. Europace 2015;17:468–472. [DOI] [PubMed] [Google Scholar]
- 43. Huisman MV, Lip GYH, Diener HC, Dubner SJ, Halperin JL, Ma CS, Rothman KJ, Teutsch C, Zint K, Ackermann D, Clemens A, Bartels DB. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 2014;167:329–334. [DOI] [PubMed] [Google Scholar]
- 44. Kozieł M, Mazurek M, Teutsch C, Diener H-C, Dubner SJ, Halperin JL, Ma C-S, Rothman KJ, Brandes A, Paquette M, Zint K, França LR, Lu S, Bartels DB, Huisman MV, Lip GYH. Persistence with anticoagulation for atrial fibrillation: report from the GLORIA-AF phase III 1-year follow-up. J Clin Med 2020;9:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vrijens B, Heidbuchel H. Non-vitamin K antagonist oral anticoagulants: considerations on once- vs. twice-daily regimens and their potential impact on medication adherence. Europace 2015;17:514–523. [DOI] [PubMed] [Google Scholar]
- 46. Weeda ER, Coleman CI, McHorney CA, Crivera C, Schein JR, Sobieraj DM. Impact of once- or twice-daily dosing frequency on adherence to chronic cardiovascular disease medications: a meta-regression analysis. Int J Cardiol 2016;216:104–109. [DOI] [PubMed] [Google Scholar]
- 47. Alberts MJ, Peacock WF, Fields LE, Bunz TJ, Nguyen E, Milentijevic D, Schein JR, Coleman CI. Association between once- and twice-daily direct oral anticoagulant adherence in nonvalvular atrial fibrillation patients and rates of ischemic stroke. Int J Cardiol 2016;215:11–13. [DOI] [PubMed] [Google Scholar]
- 48. Loewen PS, Ji AT, Kapanen A, McClean A. Patient values and preferences for antithrombotic therapy in atrial fibrillation. Thromb Haemost 2017;117:1007–1022. [DOI] [PubMed] [Google Scholar]
- 49. Li G, Lip GYH, Holbrook A, Chang Y, Larsen TB, Sun X, Tang J, Mbuagbaw L, Witt DM, Crowther M, Thabane L, Levine MAH. Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies. Eur J Epidemiol 2019;34:173–190. [DOI] [PubMed] [Google Scholar]
- 50. Levy JH, Douketis J, Weitz JI. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol 2018;15:273–281. [DOI] [PubMed] [Google Scholar]
- 51. Bhat A, Khanna S, Chen HHL, Gupta A, Gan GCH, Denniss AR, MacIntyre CR, Tan TC. Integrated care in atrial fibrillation. Circ Cardiovasc Qual Outcomes 2021;14:347–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wan D, Healey JS, Simpson CS. The guideline-policy gap in direct-acting oral anticoagulants usage in atrial fibrillation: evidence, practice, and public policy considerations. Can J Cardiol 2018;34:1412–1425. [DOI] [PubMed] [Google Scholar]
- 53. Pritchett RV, Bem D, Turner GM, Thomas GN, Clarke JL, Fellows R, Lane DA, Jolly K. Improving the prescription of oral anticoagulants in atrial fibrillation: a systematic review. Thromb Haemost 2019;119:294–307. [DOI] [PubMed] [Google Scholar]
- 54. Choudhry NK, Anderson GM, Laupacis A, Ross-Degnan D, Normand S-LT, Soumerai SB. Impact of adverse events on prescribing warfarin in patients with atrial fibrillation: matched pair analysis. BMJ 2006;332:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lane DA, Lip GYH. Use of the CHA2DS2-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation 2012;126:860–865. [DOI] [PubMed] [Google Scholar]
- 56. Olesen JB, Lip GYH, Lindhardsen J, Lane DA, Ahlehoff O, Hansen ML, Raunsø J, Tolstrup JS, Hansen PR, Gislason GH, Torp-Pedersen C. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost 2011;106:739–749. [DOI] [PubMed] [Google Scholar]
- 57. Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin in patients with atrial fibrillation. Circulation 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 58. Lip GYH, Laroche C, Popescu MI, Rasmussen LH, Vitali-Serdoz L, Dan G-A, Kalarus Z, Crijns HJGM, Oliveira MM, Tavazzi L, Maggioni AP, Boriani G. Improved outcomes with European Society of Cardiology guideline-adherent antithrombotic treatment in high-risk patients with atrial fibrillation: a report from the EORP-AF General Pilot Registry. Europace 2015;17:1777–1786. [DOI] [PubMed] [Google Scholar]
- 59. Marzec LN, Wang J, Shah ND, Chan PS, Ting HH, Gosch KL, Hsu JC, Maddox TM. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
- 60. Steinberg BA, Shrader P, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Hylek E, Kowey PR, Mahaffey KW, O'Brien EC, Singer DE, Peterson ED, Piccini JP. Factors associated with non–vitamin K antagonist oral anticoagulants for stroke prevention in patients with new-onset atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II (ORBIT-AF II). Am Heart J 2017;189:40–47. [DOI] [PubMed] [Google Scholar]
- 61. Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010-2017. Pharmacotherapy 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Murray S, Lazure P, Pullen C, Maltais P, Dorian P. Atrial fibrillation care: challenges in clinical practice and educational needs assessment. Can J Cardiol 2011;27:98–104. [DOI] [PubMed] [Google Scholar]
- 63. Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, Phibbs CS, Than CT, Wang PJ, Heidenreich PA. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: the Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J 2013;165:93–101.e1. [DOI] [PubMed] [Google Scholar]
- 64. Raptis S, Chen JN, Saposnik F, Pelyavskyy R, Liuni A, Saposnik G. Aversion to ambiguity and willingness to take risks affect therapeutic decisions in managing atrial fibrillation for stroke prevention: results of a pilot study in family physicians. Patient Prefer Adherence 2017;11:1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Saposnik G, Maurino J, Sempere AP, Ruff CC, Tobler PN. Herding: a new phenomenon affecting medical decision-making in multiple sclerosis care? Lessons learned from DIScUTIR MS. Patient Prefer Adherence 2017;11:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sposato LA, Stirling D, Saposnik G. Therapeutic decisions in atrial fibrillation for stroke prevention: the role of aversion to ambiguity and physicians’ risk preferences. J Stroke Cerebrovasc Dis 2018;27:2088–2095. [DOI] [PubMed] [Google Scholar]
- 67. Moulson N, McIntyre WF, Oqab Z, Yazdan-Ashoori P, Quinn KL, van Oosten E, Hopman WM, Baranchuk A. The anticoagulation choices of internal medicine residents for stroke prevention in non-valvular atrial fibrillation. Postgrad Med J 2017;93:308–312. [DOI] [PubMed] [Google Scholar]
- 68. Yazdan-Ashoori P, Oqab Z, McIntyre WF, Quinn KL, Oosten E. V, Hopman WM, Baranchuk A. How do family medicine residents choose an anticoagulation regimen for patients with nonvalvular atrial fibrillation? Prim Health Care Res Dev 2017;18:472–481. [DOI] [PubMed] [Google Scholar]
- 69. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, Davies PA, Bodalia PN, Bryden PA, Welton NJ, Hollingworth W, Caldwell DM, Savović J, Dias S, Salisbury C, Eaton D, Stephens-Boal A, Sofat R. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lorenzoni V, Pirri S, Turchetti G. Cost-effectiveness of direct non-vitamin K oral anticoagulants versus vitamin K antagonists for the management of patients with non-valvular atrial fibrillation based on available “real-world” evidence: the Italian National Health System Perspective. Clin Drug Investig 2021;41:255–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hylek E. Treatment persistence in atrial fibrillation: the next major hurdle. Thromb Haemost 2018;118:2018–2019. [DOI] [PubMed] [Google Scholar]
- 72. Yong CM, Liu Y, Apruzzese P, Doros G, Cannon CP, Maddox TM, Gehi A, Hsu JC, Lubitz SA, Virani S, Turakhia MP. Association of insurance type with receipt of oral anticoagulation in insured patients with atrial fibrillation: a report from the American College of Cardiology NCDR PINNACLE registry. Am Heart J 2018;195:50–59. [DOI] [PubMed] [Google Scholar]
- 73. Geng Y-P, Lan D-H, Liu N, Du X, Zheng D, Tang R-B, Long D-Y, Yu R-H, Sang C-H, Bai R, Jiang C-X, Li S-N, Guo X-Y, Wang W, Xia S-J, Chang S-S, Dong J-Z, Chen A-H, Ma C-S. Patient-reported treatment satisfaction with dabigatran versus warfarin in patients with non-valvular atrial fibrillation in China. Thromb Haemost 2018;118:1815–1822. [DOI] [PubMed] [Google Scholar]
- 74. Proietti M, Lip GYH. Simple decision-making between a vitamin K antagonist and a non-vitamin K antagonist oral anticoagulant: using the SAMe-TT2R2 score. Eur Heart J Cardiovasc Pharmacother 2015;1:150–152. [DOI] [PubMed] [Google Scholar]
- 75. Diener H-C, Aisenberg J, Ansell J, Atar D, Breithardt G, Eikelboom J, Ezekowitz MD, Granger CB, Halperin JL, Hohnloser SH, Hylek EM, Kirchhof P, Lane DA, Verheugt FWA, Veltkamp R, Lip GYH. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J 2017;38:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Clarkesmith DE, Pattison HM, Khaing PH, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database Syst Rev 2017;4:CD008600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Vinereanu D, Lopes RD, Bahit MC, Xavier D, Jiang J, Al-Khalidi HR, He W, Xian Y, Ciobanu AO, Kamath DY, Fox KA, Rao MP, Pokorney SD, Berwanger O, Tajer C, de Barros E Silva PGM, Roettig ML, Huo Y, Granger CB; IMPACT-AF investigators. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 2017;390:1737–1746. [DOI] [PubMed] [Google Scholar]
- 78. Montalescot G, Brotons C, Cosyns B, Crijns HJ, D'Angelo A, Drouet L, Eberli F, Lane DA, Besse B, Chan A, Vicaut E, Darius H; AEGEAN Study Investigators. Educational impact on apixaban adherence in atrial fibrillation (the AEGEAN STUDY): a randomized clinical trial. Am J Cardiovasc Drugs 2020;20:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gallagher C, Hendriks JM, Nyfort-Hansen K, Sanders P, Lau DH. Integrated care for atrial fibrillation: the heart of the matter. Eur J Prev Cardiol 2020;zwaa065.doi: 10.1093/eurjpc/zwaa065. [DOI] [PubMed] [Google Scholar]
- 80. Tran V-T, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med 2014;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lane D, Lip G. Patient’s values and preferences for stroke prevention in atrial fibrillation: balancing stroke and bleeding risk with oral anticoagulation. Thromb Haemost 2014;111:381–383. [DOI] [PubMed] [Google Scholar]
- 82. Lane DA, Barker RV, Lip GYH. Best practice for atrial fibrillation patient education. Curr Pharm Des 2015;21:533–543. [DOI] [PubMed] [Google Scholar]
- 83. Clarkesmith DE, Pattison HM, Lip GYH, Lane DA. Educational intervention improves anticoagulation control in atrial fibrillation patients: the TREAT randomised trial. PLoS One 2013;8:e74037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball D, Francis K. Evaluation of patients’ attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost 2014;111:465–473. [DOI] [PubMed] [Google Scholar]
- 85. Desteghe L, Engelhard L, Raymaekers Z, Kluts K, Vijgen J, Dilling-Boer D, Koopman P, Schurmans J, Dendale P, Heidbuchel H. Knowledge gaps in patients with atrial fibrillation revealed by a new validated knowledge questionnaire. Int J Cardiol 2016;223:906–914. [DOI] [PubMed] [Google Scholar]
- 86. Tam W, Woo B, Lim TW. Questionnaires designed to assess knowledge of atrial fibrillation. J Cardiovasc Nurs 2019;34:E14–E21. [DOI] [PubMed] [Google Scholar]
- 87. Baker WL, Cios DA, Sander SD, Coleman CI. Meta-analysis to assess the quality of warfarin control in atrial fibrillation patients in the United States. J Manag Care Pharm 2009;15:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lip GYH, Lane DA, Potpara TS. Innovative strategies to improve adherence to non-vitamin K antagonist oral anticoagulants for stroke prevention in atrial fibrillation. Eur Heart J 2018;39:1404–1406. [DOI] [PubMed] [Google Scholar]
- 89. Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, De Geest S, Dobbels F, Lewek P, Urquhart J, Vrijens B; for the ABC project team. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs 2013;73:545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Desteghe L, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, Dendale P, Heidbuchel H. Telemonitoring-based feedback improves adherence to non-vitamin K antagonist oral anticoagulants intake in patients with atrial fibrillation. Eur Heart J 2018;39:1394–1403. [DOI] [PubMed] [Google Scholar]
- 91. Lane DA, McMahon N, Gibson J, Weldon JC, Farkowski MM, Lenarczyk R, Watkins CL, Dilaveris P, Caiani EG, Potpara TS. Mobile health applications for managing atrial fibrillation for healthcare professionals and patients: a systematic review. Europace 2020;22:1567–1578. [DOI] [PubMed] [Google Scholar]
- 92. Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GYH. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF app trial. Am J Med 2017;130:1388–1396.e6. [DOI] [PubMed] [Google Scholar]
- 93. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, Wen J, Xing Y, Wu F, Xia Y, Liu T, Wu F, Liang Z, Liu F, Zhao Y, Li R, Li X, Zhang L, Guo J, Burnside G, Chen Y, Lip GYH, Guo Y, Lip GYH, Lane DA, Chen Y, Wang L, Eckstein J, Thomas GN, Tong L, Mei F, Xuejun L, Xiaoming L, Zhaoliang S, Xiangming S, Wei Z, Yunli X, Jing W, Fan W, Sitong Y, Xiaoqing J, Bo Y, Xiaojuan B, Yuting J, Yangxia L, Yingying S, Zhongju T, Li Y, Tianzhu L, Chunfeng N, Lili Z, Shuyan L, Zulu W, Bing X, Liming L, Yuanzhe J, Yunlong X, Xiaohong C, Fang W, Lina Z, Yihong S, Shujie J, Jing L, Nan L, Shijun L, Huixia L, Rong L, Fan L, Qingfeng G, Tianyun G, Yuan W, Xin L, Yan R, Xiaoping C, Ronghua C, Yun S, Yulan Z, Haili S, Yujie Z, Quanchun W, Weidong S, Lin W, Chan E, Guangliang S, Chen Y, Wei Z, Dandi C, Xiang H, Anding X, Xiaohan F, Ziqiang Y, Xiang G, Fulin G. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol 2020;75:1523–1534. [DOI] [PubMed] [Google Scholar]
- 94. Guo Y, Guo J, Shi X, Yao Y, Sun Y, Xia Y, Yu B, Liu T, Chen Y, Lip GYH. Mobile health technology-supported atrial fibrillation screening and integrated care: a report from the mAFA-II trial Long-term Extension Cohort. Eur J Intern Med 2020;82:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Afzal SK, Hasan SS, Babar ZU. A systematic review of patient-reported outcomes associated with the use of direct-acting oral anticoagulants. Br J Clin Pharmacol 2019;85:2652–2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seligman WH, Das-Gupta Z, Jobi-Odeneye AO, Arbelo E, Banerjee A, Bollmann A, Caffrey-Armstrong B, Cehic DA, Corbalan R, Collins M, Dandamudi G, Dorairaj P, Fay M, Van Gelder IC, Goto S, Granger CB, Gyorgy B, Healey JS, Hendriks JM, Hills MT, Hobbs FDR, Huisman MV, Koplan KE, Lane DA, Lewis WR, Lobban T, Steinberg BA, McLeod CJ, Moseley S, Timmis A, Yutao G, Camm AJ. Development of an international standard set of outcome measures for patients with atrial fibrillation: a report of the International Consortium for Health Outcomes Measurement (ICHOM) atrial fibrillation working group. Eur Heart J 2020;41:1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barnes GD, Nallamothu BK, Sales AE, Froehlich JB. Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes 2016;9:182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rush KL, Burton L, Schaab K, Lukey A. The impact of nurse-led atrial fibrillation clinics on patient and healthcare outcomes: a systematic mixed studies review. Eur J Cardiovasc Nurs 2019;18:526–533. [DOI] [PubMed] [Google Scholar]
- 99. Khalil V, Blackley S, Subramaniam A. Evaluation of a pharmacist-led shared decision-making in atrial fibrillation and patients’ satisfaction—a before and after pilot study. Ir J Med Sci 2021;190:819–824. [DOI] [PubMed] [Google Scholar]
- 100. Clark NP. Role of the anticoagulant monitoring service in 2018: beyond warfarin. Hematology 2018;2018:348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shore S, Ho PM, Lambert-Kerzner A, Glorioso TJ, Carey EP, Cunningham F, Longo L, Jackevicius C, Rose A, Turakhia MP. Site-level variation in and practices associated with dabigatran adherence. JAMA 2015;313:1443–1450. [DOI] [PubMed] [Google Scholar]
- 102. Hendriks JML, de Wit R, Crijns HJGM, Vrijhoef HJM, Prins MH, Pisters R, Pison LAFG, Blaauw Y, Tieleman RG. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J 2012;33:2692–2699. [DOI] [PubMed] [Google Scholar]
- 103. Wijtvliet EPJP, Tieleman RG, van Gelder IC. Nurse-led vs. usual-care for atrial fibrillation. Eur Heart J 2020;41:634–641. [DOI] [PubMed] [Google Scholar]
- 104. Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P, Hendriks JML. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017;103:1947–1953. [DOI] [PubMed] [Google Scholar]
- 105. Generalova D, Cunningham S, Leslie SJ, Rushworth GF, McIver L, Stewart D. A systematic review of clinicians’ views and experiences of direct-acting oral anticoagulants in the management of nonvalvular atrial fibrillation. Br J Clin Pharmacol 2018;84:2692–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Torres Roldan VD, Brand-McCarthy SR, Ponce OJ, Belluzzo T, Urtecho M, Espinoza Suarez NR, Toloza FJK, Thota AD, Organick PW, Barrera F, Liu-Sanchez C, Jaladi S, Prokop L, Ozanne EM, Fagerlin A, Hargraves IG, Noseworthy PA, Montori VM, Brito JP. Shared decision making tools for people facing stroke prevention strategies in atrial fibrillation: a systematic review and environmental scan. Med Decis Mak 2021;41:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang SV, Rogers JR, Jin Y, Bates DW, Fischer MA. Use of electronic healthcare records to identify complex patients with atrial fibrillation for targeted intervention. J Am Med Informatics Assoc 2017;24:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hulme OL, Khurshid S, Weng L-C, Anderson CD, Wang EY, Ashburner JM, Ko D, McManus DD, Benjamin EJ, Ellinor PT, Trinquart L, Lubitz SA. Development and validation of a prediction model for atrial fibrillation using electronic health records. JACC Clin Electrophysiol 2019;5:1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Murphy A, Banerjee A, Breithardt G, Camm AJ, Commerford P, Freedman B, Gonzalez-Hermosillo JA, Halperin JL, Lau C-P, Perel P, Xavier D, Wood D, Jouven X, Morillo CA. The World Heart Federation roadmap for nonvalvular atrial fibrillation. Glob Heart 2017;12:273–284. [DOI] [PubMed] [Google Scholar]
- 110. Gulpen AJW, van Dijk JK, Damen NL, ten Cate H, Schalla S, ten Cate-Hoek AJ. Organisation of care for patients using direct oral anticoagulants. Neth Heart J 2020;28:452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Romiti GF, Pastori D, Rivera-Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Kozieł M, Yang P-S, Guo Y, Lip GYH, Proietti M. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes—a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2021;doi:10.1055/a-1515-9630. [DOI] [PubMed] [Google Scholar]
- 112. Field M, Kuduvalli M, Torella F, McKay V, Khalatbari A, Lip GY. Integrated care pathways and the aortovascular hub. Thromb Haemost 2021;doi:10.1055/a-1591-8033. [DOI] [PubMed] [Google Scholar]
- 113. Lip GY, Ntaios G. Integrated care for stroke management: easy as ABC. Thromb Haemost 2021;doi:10.1055/a-1632-1777. [DOI] [PubMed] [Google Scholar]
- 114. Wagner EH, Austin BT, Korff MV. Organizing care for patients with chronic illness. Milbank Q 1996;74:511–544. [PubMed] [Google Scholar]
- 115. Stevens D, Harrison SL, Kolamunnage-Dona R, Lip GYH, Lane DA. The atrial fibrillation better care pathway for managing atrial fibrillation: a review. Europace 2021;23:1511–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cowie MR, Bax J, Bruining N, Cleland JGF, Koehler F, Malik M, Pinto F, van der Velde E, Vardas P. e-Health: a position statement of the European Society of Cardiology. Eur Heart J 2016;37:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol 2017;14:627–628. [DOI] [PubMed] [Google Scholar]
- 118. Joung B, Lee JM, Lee KH, Kim TH, Choi EK, Lim WH, Kang KW, Shim J, Lim HE, Park J, Lee SR, Lee YS, Kim JB; KHRS Atrial Fibrillation Guideline Working Group. 2018 Korean Guideline of atrial fibrillation management. Korean Circ J 2018;48:1033–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, Lane DA, Ruff CT, Turakhia M, Werring D, Patel S, Moores L. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest 2018;154:1121–1201. [DOI] [PubMed] [Google Scholar]
- 120. Saraswat MK, Carter L, Berrigan P, Sapp JL, Gray C, Fearon A, Gardner M, Parkash R. Integrated management approach to atrial fibrillation care: a cost utility analysis. Can J Cardiol 2019;35:1142–1148. [DOI] [PubMed] [Google Scholar]
- 121. Gallagher C, Hendriks JM, Middeldorp ME, Elliott AD, Lau DH, Sanders P. Reducing the burden of atrial fibrillation cost: is integrated care the answer? Can J Cardiol 2019;35:1094–1096. [DOI] [PubMed] [Google Scholar]
- 122. Pastori D, Farcomeni A, Pignatelli P, Violi F, Lip GY. ABC (Atrial fibrillation Better Care) pathway and healthcare costs in atrial fibrillation: the ATHERO-AF study. Am J Med 2019;132:856–861. [DOI] [PubMed] [Google Scholar]