Summary
Background
Phosphorylated tau (p-tau) epitopes in cerebrospinal fluid (CSF) are accurate biomarkers for a pathological and clinical diagnosis of Alzheimer's disease (AD) and are seen to be increased in preclinical stage of the disease. However, it is unknown if these increases transpire earlier, prior to amyloid-beta (Aβ) positivity as determined by position emission tomography (PET), and if an ordinal sequence of p-tau epitopes occurs at this incipient phase
Methods
We measured CSF concentrations of p-tau181, p-tau217 and p-tau231 in 171 participants across the AD continuum who had undergone Aβ ([18F]AZD4694) and tau ([18F]MK6240) position emission tomography (PET) and clinical assessment
Findings
All CSF p-tau biomarkers were accurate predictors of cognitive impairment but CSF p-tau217 demonstrated the largest fold-changes in AD patients in comparison to non-AD dementias and cognitively unimpaired individuals. CSF p-tau231 and p-tau217 predicted Aβ and tau to a similar degree but p-tau231 attained abnormal levels first. P-tau231 was sensitive to the earliest changes of Aβ in the medial orbitofrontal, precuneus and posterior cingulate before global Aβ PET positivity was reached
Interpretation
We demonstrate that CSF p-tau231 increases early in development of AD pathology and is a principal candidate for detecting incipient Aβ pathology for therapeutic trial application
Funding
Canadian Institutes of Health Research (CIHR), Canadian Consortium of Neurodegeneration and Aging, Weston Brain Institute, Brain Canada Foundation, the Fonds de Recherche du Québec.
Keywords: Cerebrospinal fluid, Phosphorylated tau, Alzheimer's disease, Preclinical, Amyloid, Positron emission tomography
Research in context.
Evidence before this study
In the context of dementia, elevated cerebrospinal fluid p-tau is highly indicative of Alzheimer's disease (AD). The residue phosphorylated at threonine 181 (p-tau181) is often the validated biomarker used in routine biochemical assessments. However, recent evidence suggests other p-tau epitopes to have greater diagnostic utility (p-tau217) or, importantly for disease prevention trials, an earlier elevation in the AD continuum (p-tau231).
Added value of this study
Firstly, this is study confirms several aspects already partially described in the current literature; 1) all p-tau epitopes have high diagnostic capability for biomarker defined AD, 2) p-tau epitopes identify Aβ and tau pathologies at all stages of the AD continuum and 3) p-tau217 exhibits larger fold-changes when symptomatic disease is apparent. Our novel finding suggests that CSF p-tau231 levels are raised earliest in preclinical AD. CSF p-tau231 was shown to have markedly stronger focal associations Aβ PET retention in medial orbitofrontal, precuneus and posterior cingulate in CU individuals yet to reach the threshold of positive global Aβ burden. Furthermore, we demonstrated that CSF p-tau231 displays the largest effect size in relation to Aβ deposition, both globally and regionally, and, amongst the biomarkers tested, is the first to achieve abnormal values.
Implications of all the available evidence
While the differences between p-tau epitopes are subtle, the early association of p-tau231 with regional Aβ deposition is of fundamental importance for identifying individuals with subtle AD-related preclinical brain changes for therapeutic trials. It is anticipated that disease-modifying drug candidates have a better opportunity to show effectiveness if initiated before symptom onset or even before overt Aβ plaque load. Thus, our evidence suggests that p-tau231 has an advantage over other p-tau epitope to highlight this early preclinical population.
Alt-text: Unlabelled box
Introduction
Neurofibrillary tangles (NFTs), primarily composed of abnormal hyperphosphorylated tau, are a key pathological hallmark of Alzheimer's disease (AD).1 Increased concentrations of extracellular soluble phosphorylated tau (p-tau) and total tau (t-tau) constitute a reliable index of this intracellular process in mild cognitive impairment due to AD2,3 and AD dementia.2,4 NFTs are present many years after the deposition of extracellular amyloid-beta (Aβ) plaques but are more closely related to symptom onset and longitudinal studies have demonstrated that soluble p-tau and t-tau are increased already in preclinical disease (e.g., no cognitive impairment but evidence of AD pathology).5,6 Specifically, CSF concentrations of t-tau and p-tau are proposed to reflect neurodegeneration and tau pathology in the form of NFTs, respectively.7 However, these biomarkers remain at normal concentrations in other neurodegenerative disorders with substantial neurodegeneration,8, 9, 10 and also those with tau pathology.11, 12, 13 Preclinical studies provide compelling evidence supporting early brain amyloidosis as a driver of tau hyperphosphorylation in AD.14,15 Interestingly, evidence from AD neuronal culture models indicate that soluble Aβ oligomers (AβOs) induce tau hyperphosphorylation in multiple sites.16,17 Indeed, the link between early cerebral Aβ deposition and CSF p-tau elevations has been further supported by the fact that CSF tau species precede tau positron emission tomography (PET) abnormalities by a decade.18,19 Thus, it is suggested that CSF p-tau biomarkers likely indicate an active process of tau secretion, and one that is correlated with cerebral Aβ deposition in early disease.20, 21, 22
CSF p-tau, in the context of AD, is often assumed to be the residue phosphorylated at threonine 181 (p-tau181). A multitude of mid-domain and C-terminal residues have also been described to be abnormally phosphorylated in the AD clinical spectrum11,23, 24, 25, 26 and studies specifically assessing their comparative diagnostic performance remain limited. Recent reports have suggested that CSF p-tau217 might better discriminate AD from other neurodegenerative diseases24,27, 28, 29, 30 than CSF p-tau181. In addition, CSF p-tau217 correlates better with Aβ and tau PET imaging than CSF p-tau181.24,30 These findings support an emerging framework suggesting that certain CSF tau phosphorylation species to predominate across the AD continuum.24,27 We have recently reported that CSF p-tau231 increases early in the AD continuum and, therefore, may detect preclinical AD among cognitively unimpaired (CU) individuals.31 This is of fundamental importance for identifying individuals with subtle AD-related preclinical brain changes for therapeutic trials, as it is anticipated that disease-modifying drug candidates have a better opportunity to show effectiveness if initiated before symptom onset or even before overt Aβ plaque load has been achieved. Indeed, a recent study in transgenic mice suggests that early removal of Aβ seeds, before Aβ deposition becomes detectable, led to a significant reduction of Aβ accumulation and downstream pathologies.32 This emphasizes the need of a biomarker for detecting early Aβ seeds or the “pre-amyloid” phase. Therefore, it is imperative to investigate CSF p-tau epitopes associated with the earliest Aβ measures in CU to determine which of these early tau phosphorylation sites better reflects emerging Aβ pathology.
In order to address this knowledge gap, we investigated whether CSF p-tau epitopes (p-tau181, p-tau217 and p-tau231) are capable of identifying early Aβ pathology at the clinical, preclinical and pre-amyloid phases of AD. We also tested the performance of CSF p-tau181, p-tau217 and p-tau231 as biomarkers to predict clinical outcomes and Aβ and tau PET status. Based on our previous data,31 we hypothesized that CSF p-tau231 associates with early Aβ pathology in brain regions that are affected early in the AD process. For these purposes, we performed voxel-wise analyses of the association between different CSF p-tau biomarkers and Aβ and tau PET in cognitively unimpaired (CU) and impaired (CI) individuals.
Methods
Study design
The main objective of this study was to investigate whether CSF p-tau epitopes can identify early Aβ deposition before Aβ PET positivity. We also aimed at comparing their performance to predict amyloid and tau pathologies indexed by PET in participants ranging within the AD continuum. This cross-sectional study was based on data from the Translational Biomarkers in Aging and Dementia (TRIAD) cohort, which is an observational and longitudinal biomarker-based study. TRIAD participants, mostly ranging in the AD spectrum, are followed yearly with detailed clinical and neuropsychological assessments, as well as with collection of fluid (blood, urine, saliva, and CSF) and acquisition of multiple imaging biomarkers. In addition to meeting standard diagnostic criteria, AD dementia participants had CDR between 1 and 2, subjects with mild cognitive impairment (MCI) had a CDR of 0.5 and CU subjects had a CDR of 0. MCI participants without Aβ pathology are considered as non-AD neurodegenerative disease, together with participants clinically diagnosed with frontotemporal dementia (FTD; clinical diagnosis of behavioral or semantic variant of FTD, CDR score >0.5 and Aβ PET negative). This article includes 171 participants (27 young, <30 years; 82 CU; 20 MCI; 21 AD; 21 non-AD) from which CSF and PET imaging were available on October 2019. For data description purposes, participants were sub-grouped according to their amyloid status as further described. For the imaging analysis, participants were initially evaluated according to their cognitive status as CU and CI.
Ethics
The TRIAD study was approved by The Research Ethics Board of the Montreal Neurological Institute as well as the Faculty of Medicine Research Ethics Office, McGill University, and all study participants provided written informed consent (Protocols: IUSMD 16-60 and 16-61).
CSF analysis
CSF samples were collected by syringe and transferred to polypropylene tubes for centrifugation at 20 °C, 2200 g for 10 min. Samples were then distributed into 1mL aliquots in polypropylene vials (Fisher Scientific Inc. Catalog # 3741-WP1D-BR) and permanently stored at -80 °C pending analyses.
All samples were analyzed for p-tau181, p-tau217 and p-tau231. P-tau181 and p-tau217 were quantified by novel single molecule array (Simoa) assay that have been previously described.28,31 In brief, rabbit polyclonal antibody specific for p-tau217 (#44-744, Invitrogen) and mouse AT270 mouse monoclonal antibody (MN1050; Invitrogen, Waltham, MA, USA) were used as capture, conjugated to paramagnetic beads (#103207, Quanterix). The mouse monoclonal antibody Tau12 (#806502, BioLegend) raised against the N-terminal epitope 6-18aa was used for detection.33 The assay calibrator was recombinant full-length tau-441 phosphorylated in vitro by glycogen synthase kinase 3β (#TO8-50FN, SignalChem). Calibrators and specimens were diluted with assay diluent (Tau 2.0 buffer; #101556, Quanterix). Analytical validation and assay measurement protocol for both CSF p-tau181 and CSF p-tau217 Simoa assays have been previously described.28 Both methods demonstrated intra and inter assay variation <8%. CSF p-tau231 was quantified using a research enzyme-linked immunosorbent assay (ELISA) assay using cis-conformational selective monoclonal antibody (ADx253, ADx NeuroSciences). Monoclonal mouse antibodies were generated using a 17-mer synthetic peptide, phosphorylated on hTau corresponding Thr231, spanning the tau region K224KVAVVR(pT)PPKSPSSAK240C as a KLH-coupled antigen. Candidate hybridomas were selected on brain extracts of AD and control brain tissue. The final cloned and purified monoclonal antibody, ADx253, was characterized on synthetic peptides spanning the region T217 till S241 for its affinity, its phospho-specificity using both phosphorylated and non-phosphorylated peptides and its preferred selectivity in which proline at position 232 was replaced by a Pip (pipecolinic acid, piperidene-2-carboxylic acid, homoproline), to simulate cis-selectivity of ADx253.34 A pan-tau mouse mAb (ADx205, ADx NeuroSciences) with epitope in the mid tau region was used in biotinylated form as pairing antibody in the p–tau231 ELISA assay. ADx205 was recently fine mapped using overlapping linear synthetic peptides and the ADx205 antibody was found to bind between R194 and G204 of tau441 or 2N4R tau sequence.
Imaging analysis
All participants have acquired 3T T1-weighted images for co-registration purposes. In addition, a Siemens High Resolution Research Tomograph (HRRT) was used for PET imaging acquisitions, which occurred ±80 days from the CSF collection date (median = 53 days). For Aβ PET, images were acquired 40–70 minutes post-injection of [18F]AZD4694 and scans were reconstructed using the ordered subset expectation maximization (OSEM) algorithm on a 4-dimensional volume with 3 frames (3×600s).35 For tau PET, [18F]MK6240 scans were acquired 90–110 minutes post-injection and the OSEM algorithm was also used for reconstruction on a 4D volume with 4 frames (4×300s).36 Additional pre-processing corrections were performed as described elsewhere.37 PET images were meninges and skull stripped, linearly and non-linearly registered to the ADNI template space and then spatially smoothed to achieve a final resolution of 8 mm FWHM.38 The inferior cerebellum and whole cerebellum gray matter were used as the reference regions for [18F]MK6240 and [18F]AZD4694, respectively.
Global Aβ PET used averaged SUVR of the precuneus, cingulate, inferior parietal, medial prefrontal, lateral temporal, and orbitofrontal cortices39 and positivity was visually defined by two neurologists blinded to clinical diagnosis. An additional Aβ PET SUVR cutoff value of 1.55 was estimated as previously described.40 Aβ PET SUVR was also converted to Centiloid units41 as previously described.40,42 The PET SUVR cutoff value of 1.55 corresponds to 24 Centiloids. Tau PET SUVR was globally estimated from a composite area including the transentorhinal (Braak stage I-II) and limbic (Braak III-IV) cortices.43,44 Tau positivity was defined as 2.5 standard deviations (SD) higher than the mean global SUVR of the young participants38 (in this study 2.5SD = 1.03 SUVR).
Statistical analysis
All non-imaging statistical analyses were performed using the R programming language (version 3.4.3). Data distribution was visually inspected using histograms and those variables that did not follow a normal distribution were log10-transformed before parametric analysis when needed. Cross-sectional demographic and clinical data were assessed with linear models and χ2 tests. Spearman rank correlation tests were applied to evaluate the association between biomarkers and correlation coefficients were compared using the R package “Cocor”. Linear regression models also tested the association between log-transformed CSF p-tau and other biomarkers always adjusting by age and gender. Similar models were also applied to evaluate group differences and, when necessary, Tukey honestly significant difference (HSD) test was used in the post hoc analysis. Receiver operating curves (ROC) provided both the area under the curve (AUC), for AD diagnosis or biomarker positivity, and the representative best value for accuracy at an optimal cutoff value. In addition to AUC, sensitivity and specificity, paired Delong's test (pROC R package) was applied to statistically compare biomarker performance. Finally, imaging and CSF cutoffs were used to evaluate concordance between biomarkers.
Voxel-wise analyses were performed using VoxelStats.45 Voxel-based linear regression models evaluated the correlation between log transformed CSF values and PET biomarkers adjusting for age, sex, and diagnosis, when necessary. Random field theory46 was used to correct the resulting t parametric maps for multiple comparisons (one-sided). To compare the effect of CSF biomarkers on the PET biomarkers, the adjusted R-squared values of the models were averaged within ROIs encompassing only the voxels that were significantly associated to all CSF biomarkers simultaneously. The ROIs include precuneus, posterior cingulate, frontal orbital gyri, post central gyri and medial frontal gyri for Aβ PET, whilst for tau PET they were the average of Braak I-II, Braak III-IV and Braak V-VI staging regions.
CSF biomarkers were also plotted as a function of Aβ PET, which served as a proxy of disease progression. For that, Aβ PET values were given in Centiloid units and CSF biomarkers were first adjusted by age and sex and only then transformed in Z-score values based on the average and standard deviation of the CU population. Finally, a locally weighted polynomial regression method was employed, using the lowess function in R, with 0.6 smoother spam.
Role of funding source
This work was supported by the Weston Brain Institute, Canadian Institute of Health Research (CIHR), and Fonds de recherche du Québec – Santé. None of the funders had a role in the study design, data collection, data analyses, interpretation or writing of the report
Results
Participants
This study included 171 participants, stratified as CU (n = 109 [23% Aβ PET+]) and CI (n = 62 [66% Aβ PET+]) groups (Table 1), with cross-sectional CSF (p-tau181, p-tau217, p-tau231) and PET ([18F]AZD4694 and [18F]MK6240). The same participants were also categorized as CU (Aβ+/-), MCI (Aβ+), AD and non-AD (supplementary table 1). The mean age of the population was 62.77 years, with CI participants being older than CU (CU = 59.82; CI = 67.78; P < 0.001) owing to a proportion of the CU being < 30 years. As expected, the CI group had a lower MMSE scores, and higher Aβ PET and tau PET load as compared to the CU group (Table 1). APOE-ε4 carriers and males were also more common in the CI group. Older age was associated with higher levels of all CSF p-tau. There was no association between sex and p-tau biomarkers when adjusting for age and diagnosis.
Table 1.
Demographics of the Translational Biomarkers in Aging and Dementia (TRIAD) cohort.
| CU Aβ- (n = 83) | CU Aβ+ (n = 26) | CI Aβ- (n = 21) | CI Aβ+ (n = 41) | |
|---|---|---|---|---|
| Females | 51 (61) | 17 (65) | 11 (52) | 18 (43) |
| Age (yr) | 55.48 (23.25) | 72.20 (7.54)⁎⁎⁎ | 67.24 (9.91)⁎⁎⁎ | 67.86 (7.88)⁎⁎⁎ |
| Education (yr) | 15.56 (3.15) | 14.42 (2.70) | 14.94 (4.92) | 15.42 (2.98) |
| APOE-ε4 carriers | 23 (27) | 8 (30) | 4 (19) | 27 (67)⁎⁎⁎ |
| MMSE | 29.38 (0.90) | 29.23 (0.86) | 26.76 (2.10)⁎⁎ | 23.82 (6.09)⁎⁎⁎ |
| Aβ PET SUVR | 1.23 (0.10) | 1.96 (0.42)⁎⁎⁎ | 1.25 (0.13) | 2.38 (0.42)⁎⁎⁎ |
| Tau PET SUVR | 0.88 (0.10) | 1.00 (0.15) | 0.85 (0.13) | 1.87 (0.61)⁎⁎⁎ |
| p-tau231 (pg/mL) | 8.71 (3.29) | 22.14 (17.67)⁎⁎⁎ | 9.64 (4.19) | 34.32 (19.86)⁎⁎⁎ |
| p-tau217 (pg/mL) | 4.44 (2.88) | 15.64 (17.63)⁎⁎⁎ | 5.04 (2.30) | 26.79 (16.87)⁎⁎⁎ |
| p-tau181 (pg/mL) | 198.83 (122.47) | 404.70 (213.81)⁎⁎⁎ | 219.96 (91.13) | 806.32 (508.79)⁎⁎⁎ |
Data are mean (SD) or n (%); MMSE Mini-Mental State Examination.
*P values were calculated comparing groups against CU Aβ- subjects using linear regression models for continuous variables and Chi-square tests for categorical variables.
*P<0.05; ⁎⁎⁎P<0.001.
CSF p-tau biomarkers biomarker defined groups
CSF p-tau biomarkers were highly correlated between each other, in the whole cohort, and in diagnostic categories groups (supplementary Figure 1a–c) with the strongest association being between CSF p-tau217 and CSF p-tau231 in whole cohort (r = 0.92, P < 0.001) and in the CI group (r = 0.93, P < 0.001). Within the CU group, the strongest association was found between CSF p-tau181 and CSF p-tau231 (r = 0.86, P < 0.001[Spearman correlation]).
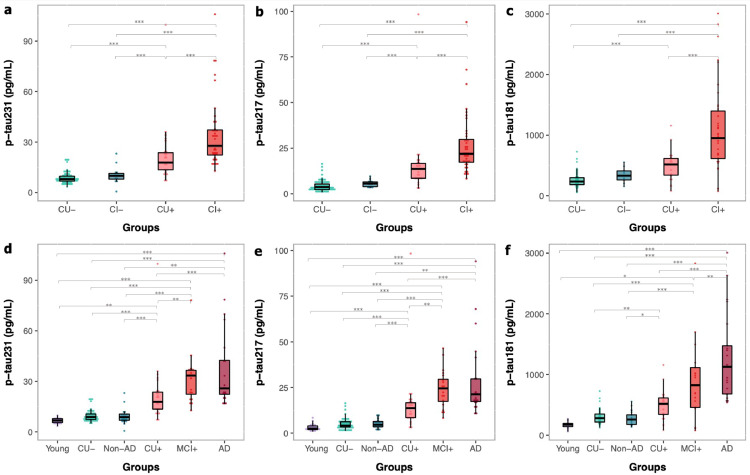
When assessing groups as either CU or CI, all CSF p-tau biomarkers were significantly increased in Aβ+ groups as compared to Aβ- groups. CSF p-tau231 (Figure 1a) and CSF p-tau217 (Figure 1b) were significantly increased in CU Aβ+ as compared to CI Aβ- but this was not observed for CSF p-tau181 (Figure 1c). CSF p-tau217 was 6-fold higher in CI Aβ+ than in CU Aβ-, which was a significantly larger increase than other p-tau biomarkers (Pp-tau231=0.002; Pp-tau181=0.01; supplementary table 2). Similarly, CSF p-tau217 demonstrated the largest fold increase between CI Aβ+ and CI Aβ- (4.7-fold, P < 0.002; supplementary table 2). However, this was not significantly different to other CSF p-tau biomarkers.
Figure 1.
Phosphorylated tau CSF epitopes by group. Distribution of CSF biomarkers concentrations across groups showing higher biomarker levels associated with Aβ positivity. CU-: cognitively unimpaired Aβ negative; CU+: cognitively unimpaired Aβ positive; CI-: cognitively impaired Aβ negative; CI+: cognitively impaired Aβ positive; MCI+: mild cognitively impaired Aβ positive; AD: Alzheimer's disease dementia; Non-AD: mild cognitively impaired Aβ negative and frontotemporal dementia (FTD). ⁎⁎⁎P<0.001; ⁎⁎P<0.01; *P<0.05 (Tukey HSD adjusted).
CSF p-tau biomarkers concentrations were also visualized by specific diagnostic categories (Figure 1d–f) and fold change (supplementary table 2). This analysis further demonstrated that CSF p-tau217 had a superior fold change to other p-tau biomarkers (P < 0.02) when specifically comparing MCI Aβ+ and AD dementia with CU Aβ- (MCI Aβ+, 5.9-fold; AD, 7.1-fold) and non-AD neurodegenerative disorders (MCI Aβ+, 4.8-fold; AD, 5.8-fold). CSF Aβ42/40 groups differences are demonstrated in supplementary Figure 2.
Performance of CSF p-tau in clinically defined groups
Next, we investigated the diagnostic accuracy of CSF p-tau biomarkers in differentiating clinical categories which was predetermined by underlying Aβ and tau PET status (supplementary table 3). In a ROC analysis, CSF p-tau181 was the best performer in distinguishing between CU and AD, when Aβ and tau PET status were unknown (AUC = 0.97; 95% CI, 0.95-0.99). This significantly outperformed CSF p-tau231 (P < 0.01[DeLong's]) but not CSF p-tau217 (P=0.13[DeLong's]). All biomarkers had high accuracies (AUC > 0.96) in separating AD from non-AD and no biomarker was found to be statistically superior in this comparison. A noticeable decline in performance was observed for CSF p-tau181 when distinguishing MCI from non-AD (AUC = 0.83; 95% CI, 0.67-0.99) and MCI from CU (AUC = 0.81; 95% CI, 0.66-0.96) which was significantly different to p-tau231 and p-tau217 (P < 0.05 [DeLong's]). No such changes were observed for other biomarkers demonstrating that CSF p-tau181 is not sensitive to early pathological changes but an accurate biomarker at late-stage disease.
Associations between CSF p-tau biomarkers with tau PET
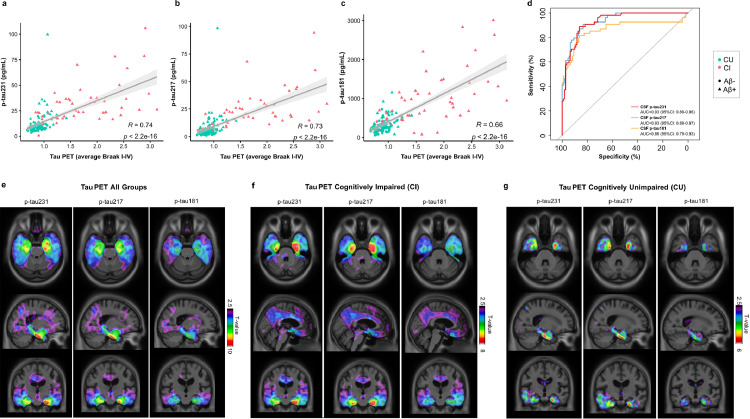
Tau PET was performed in all 171 participants included in this study. High concentrations of all CSF p-tau biomarkers were associated with increased retention of [18F]MK6240 composite Braak stage I-IV. A similar correlation coefficient was observed between CSF p-tau231 (r = 0.74, P < 0.001, Figure 2a) and CSF p-tau217 (r = 0.73, P < 0.001, Figure 2b) with Braak stage I-IV. In contrast, significantly inferior correlations (Pp-tau231 vs p-tau181= 0.004; Pp-tau217 vs p-tau181= 0.02) were observed for CSF p-tau181 (r = 0.66, P < 0.001, Figure 2c). No significant differences were found between the CSF biomarkers when predicting tau PET positivity and all demonstrated comparable AUC values (AUC = 0.86-0.93, Figure 2d). This included CSF Aβ42/40 which also had high accuracy to distinguish tau PET status (AUC 0.91; 95% CI, 86-97%).
Figure 2.
Phosphorylated tau CSF epitopes and Tau PET associations. Spearman rank correlations between CSF biomarkers and tau PET ([18F]MK6240) across all groups show p-tau231 as having the highest correlation coefficient, followed by p-tau217 (a–c). The accuracy of CSF biomarkers in distinguishing tau PET status (positive vs negative) is evidenced by AUCs as shown in (d). T-parametric maps show the voxel-wise association between CSF biomarkers and [18F]MK6240 in all participants (e) as well as within CI (f) and CU (g). Models were adjusted for age and sex and RFT was used to account for multiple comparisons (significant t-values > 3.1). CU: cognitively unimpaired; CI: cognitively impaired.
At the voxel level, CSF biomarkers were associated with higher [18F]MK6240 uptake in the inferior, medial and lateral temporal regions (Tall>3.14; Pall<0.05, Figure 2E), with associated areas overlapping between CSF biomarkers. No marked difference was observed between CSF p-tau231 and CSF p-tau217, whilst CSF p-tau181 indicated weaker associations narrowed to reduced regions if compared to the other biomarkers (Figure 2e). When groups were evaluated as CI (Figure 2f) and CU (Figure 2g) separately, broad associations were found in the CI group (TCI>3.25; PCI<0.05, Figure 2f), where high concentrations of CSF biomarkers were associated with high [18F]MK6240 binding in the temporal lobes, cingulate regions, and orbitofrontal cortices, encompassing Braak stage regions I-VI. A stronger relationship between [18F]MK6240 and CSF p-tau231 and CSF p-tau217 (TCU>3.16; PCU<0.05) were found in CU participants, with significant associations confined to temporal regions corresponding to Braak stage regions I-IV (Figure 2g).
Associations between CSF p-tau biomarkers with Aβ PET
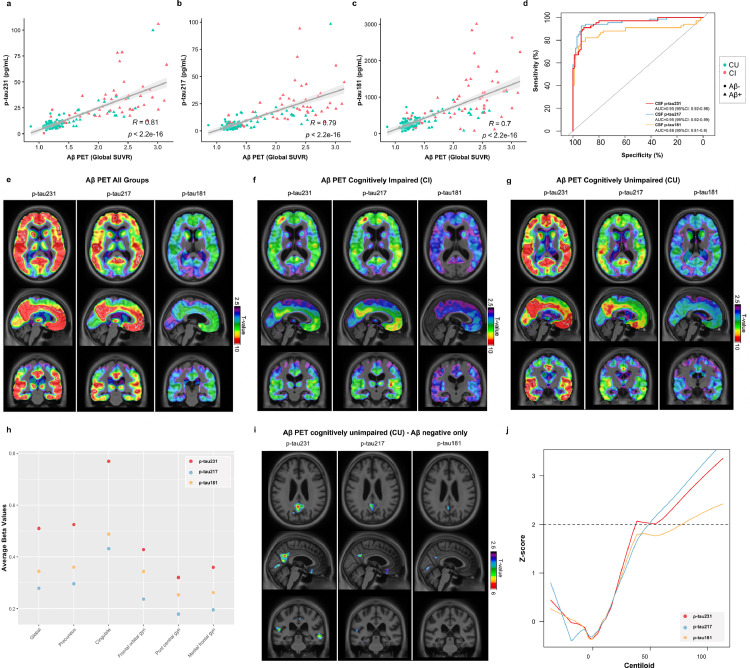
Aβ PET was performed in all 171 participants included this study. In a similar findings to tau PET, correlation coefficients where strongest between [18F]AZD4694 global retention and CSF p-tau231 (r = 0.81, P < 0.001, Figure 3a) and CSF p-tau217 (r = 0.79, P < 0.001, Figure 3b). Once more, correlations were inferior for CSF p-tau181 (r = 0.70, P < 0.001, Figure 3c) which were significantly different to other p-tau biomarkers (Pp-tau231 vs p-tau181< 0.001; Pp-tau217 vs p-tau181= 0.01). CSF P-tau231 (AUC = 0.95; 95% CI, 0.92-0.98) and CSF p-tau217 (AUC = 0.95; 95% CI, 0.92-0.99) were significantly better predictors of Aβ PET status than CSF p-tau181 (AUC = 0.88; 95% CI, 0.81-0.94; both P < 0.01, Figure 3d). CSF Aβ42/40 was also a significantly better predictor of Aβ PET status than p-tau181 (AUC= 94%; 95% CI: 90-99%; PAβ42/40 vs p-tau181= 0.003). No significant difference was found between CSF p-tau231, CSF p-tau217 and CSF Aβ42/40 in the prediction of Aβ PET status. To further investigate these associations, biomarker accuracy to detect Aβ positivity was evaluated within CU and CI groups separately (supplementary Figure 3). In CU individuals, CSF p-tau231 (AUC = 0.91; 95% CI, 0.84-0.98) was significantly superior to CSF p-tau181 (AUC = 0.82; 95% CI, 0.71-0.93; P = 0.02) but not CSF p-tau217 (AUC = 0.90; 95% CI, 0.83-0.98; P=0.75). Conversely, in CI individuals, no CSF p-tau was found to be significantly superior, although CSF p-tau217 had numerically the highest AUC (0.99; 95% CI, 0.99-1.00). Performing a ratio of p-tau231 and p-tau217 with CSF Aβ42 showed no statistically significance difference than p-tau alone. However, a numerical advantage was observed in CU participants (p-tau231/Aβ42, AUC = 0.96; 95% CI, 92-99%; p-tau217/Aβ42, AUC=95%; 95% CI, 91-99%). A ratio of CSF Aβ42 with p-tau181 improved the prediction of amyloid status in CU participants (p-tau181/Aβ42, AUC=90%; 95% CI, 82-99%; Pp-tau181/Aβ42 vs p-tau181= 0.03).
Figure 3.
Phosphorylated tau CSF epitopes and Aβ PET associations. Spearman rank correlations between CSF biomarkers and Aβ PET ([18F]AZD4694) across all groups show p-tau231 as having the highest correlation coefficient (a-c). The accuracy of CSF biomarkers in distinguishing Aβ PET status (positive vs negative) is evidenced by AUCs as shown in (d). T-parametric maps show the voxel-wise association between CSF biomarkers and [18F]AZD4694 in all participants (e) as well as within CI (f) and CU (g). Models were adjusted by age and sex and RFT was used to account for multiple comparisons (significant t-values > 3.1). The dot plot (h) shows the average slope (beta) values by ROI for each of the CSF biomarkers. The beta value was derived from linear models that had Aβ PET as the outcome measure, the CSF biomarkers were the predictors and the covariates were sex and age. These models were performed at the voxel level and the CSF p-tau beta values of each voxel were averaged within ROIs. Were included in the ROIs only the voxels that had a significant association between Aβ PET and all of the CSF biomarkers evaluated here. T-parametric maps (i) show the voxel-wise association between CSF biomarkers (p-tau231, p-tau217 and p-tau181) and [18F]AZD4694 in Aβ-negative CU subjects. Models were adjusted for age and sex and RFT was used to account for multiple comparisons (significant t-values > 3.1). In addition, CSF p-tau biomarkers were plotted (j) as a function of Aβ PET deposition (in Centiloids) in the CU group, which was used to infer AD pathology progression. Dashed line indicates the biomarker cut off for positivity. CU: cognitively unimpaired; CI: cognitively impaired.
Results of the voxel-wise analysis showed high CSF biomarker levels being associated with high [18F]AZD4694 binding in frontal, precuneus, posterior cingulate and temporal cortices (Tall>3.14; Pall<0.05, Figure 3e). Even though there was a topographical overlap of the significantly associated regions between the CSF biomarkers evaluated, a markedly stronger association was observed for CSF p-tau231 when the whole sample was analyzed (Figure 3e). In the CI group analyses, there was no difference between the associations of [18F]AZD4694 with CSF p-tau231 and CSF p-tau-217 (TCI>3.25; PCI<0.05; Figure 3f). In contrast, in the CU group, there was an evident difference between CSF p-tau231 and the other biomarkers (Figure 3g), despite there not being an evident difference in the whole set of participants (TCU>3.16; PCU<0.05). Voxel-wise analysis including CSF Aβ42/40 as a comparator in CI and CU demonstrated in supplementary figure 4 and 5. As an additional comparison between biomarkers in the CU group, the average beta values from the linear models were computed at the voxel-level and then averaged within ROIs. For all the regions evaluated, CSF p-tau231 had the highest beta values as compared to the other biomarkers globally and in all ROIs (Figure 3h). To support these initial findings, we used Aβ PET (in Centiloids) as a proxy of AD progression and we estimated at which point the relationship between the CSF p-tau231, CSF p-tau217 and CSF p-tau181 changes in relation to the disease in the whole study population (supplementary Figure 6). To perform this, we used locally-weighted polynomial regression analysis in which CSF biomarker levels were transformed into Z-scores and 2 Z-scores was defined as the cut-off value for biomarker positivity. No difference between the inflection point of these biomarkers was identified. Contrarily, CSF biomarkers showed to become abnormal at different pathological stages, with CSF p-tau231 status being positive at approximately Centiloid 37, followed by CSF p-tau181 at Centiloid 47 and CSF p-tau217 at Centiloid 50.
CSF p-tau231 in emerging amyloid-beta pathology
Considering the visually stronger association between CSF p-tau231 and [18F]AZD4694 within the CU group (Figure 3g), as compared to the other CSF biomarkers, we further investigated how these associations would be within the subjects in the earliest possible process of Aβ accumulation. Therefore, we repeated the voxel-wise analysis only in individuals classified as CU Aβ- (Figure 3i; supplementary Figure 7). CSF p-tau231 was the biomarker that best associated with [18F]AZD4694 uptake (TCU->3.19; PCU-<0.05, Figure 3i) which was significantly superior to CSF p-tau217 and CSF p-tau181 biomarkers in this analysis. These associated areas were very focal and included the medial orbitofrontal, precuneus and posterior/isthmus cingulate cortices. In order to further support the findings suggesting that CSF p-tau231 best reflects the earliest Aβ dysmetabolism, we repeated locally-weighted polynomial regression analysis, and re-estimated at which point the relationship between the CSF p-tau231, CSF p-tau217 and CSF p-tau181 changes in relation to the disease within the CU group (Figure 3j). Again, no significant difference was observed between the inflection point of these biomarkers however, abnormality of CSF p-tau231 were observed far earlier (Centiloid 38.3) than whilst CSF p-tau217 and CSF p-tau181 (Centiloids >50). Thus, this analysis suggests CSF p-tau231 as the biomarker with the fastest preclinical increases which is the first to present abnormal levels as Aβ accumulates in the brain of emerging AD pathology.
Discussion
The present study examined the relationship between CSF p-tau biomarkers (p-tau231, p-tau217 and p-tau181) with amyloid and tau PET in 171 individuals across the AD continuum. We demonstrate that CSF p-tau231 and CSF p-tau217 biomarkers have a similar relationship with [18F]MK6240 and [18F]AZD4694, being both better predictors of PET status in comparison with CSF p-tau181. At the voxel level, however, CSF p-tau231 had a markedly stronger relationship with Aβ pathology in CU individuals. Our main finding suggests that CSF p-tau231 abnormality arises during the lag phase of Aβ protein aggregation in the brain as shown by markedly stronger focal associations of CSF p-tau231 and Aβ PET in CU individuals without positive global Aβ burden. Furthermore, we demonstrated that CSF p-tau231 displays the largest effect size in relation to Aβ deposition, both globally and regionally, and, amongst the biomarkers tested, is the first to achieve abnormal values.
The formation of NFT by the aggregation of tau is a fundamental hallmark of AD pathogenesis. However, in what way the differential release of soluble phosphorylated tau into the CSF relates to the development of Aβ and NFT pathologies, and consequently neurodegeneration, remains to be clarified. It is also unclear whether extracellular soluble phosphorylated tau is a driver of tau propagation in AD.47,48 Our data, like prior studies,18,28,31 shows an increase of CSF p-tau epitopes in the preclinical phase of the disease, prior to symptom onset and before substantial tau PET ligand retention. We also confirm that CSF p-tau do not increase in non-AD dementias or cognitive impairment with absent or minimal Aβ burden. Thus, in relation to sporadic AD, this study adds further support to the hypothesis that CSF p-tau is closely related to Aβ-mediated tau release from neurons19 and challenges the notion of being simply a state marker of NFT. However, while it is presumed that p-tau secretion, and subsequent increase in CSF, occurs after globally aggregated Aβ, our data shows that CSF p-tau already changes with subtle Aβ pathology and therefore occurs considerably earlier than previously anticipated. This is seemingly more apparent for CSF p-tau231, which changes with Aβ deposition in the medial orbitofrontal, precuneus and posterior cingulate cortices before Aβ PET positivity is achieved. Thus, one could propose that increases in CSF p-tau231 are related to early Aβ seeds and could act as a key biomarker for the recently described “pre-amyloid phase” of AD, which occurs before Aβ deposition threshold.32
CSF p-tau231 has been widely reported as a biomarker to detect AD at both the MCI and dementia stages of disease11,49, 50, 51, 52, 53 but often it has been concluded these changes are not different from CSF p-tau181 when directly compared.54 Yet, most of these studies did not evaluate the cognitively unimpaired phases of the AD continuum. Previous neuropathological findings, which motivated the CSF assay development of p-tau231, highlighted that a key component of pre-tangle pathology was phosphorylation at threonine 23155 and that CSF p-tau231 is an important discriminant of Braak 0-I from more advanced Braak stages.56 Tau phosphorylation at threonine 231 is one of the earliest events in the cascade of phosphorylation,57 that modulates tubulin assembly.58 Interestingly, soluble AβOs are known to enhance kinase activity triggering tau hyperphosphorylation at threonine 231 in primary neuronal cultures.16 Therefore, now that we can monitor the in vivo development of Aβ and tau pathologies by PET, it is not surprising that our analysis concludes that CSF p-tau231 is an early marker of emerging AD pathology in the phase when only subtle brain amyloidosis is apparent. Although CSF p-tau231 demonstrated numerically identical AUC's to CSF p-tau217 in the prediction of Aβ PET at CU stage of the AD continuum, only CSF p-tau231 and not CSF p-tau217 was significantly superior to CSF p-tau181. Furthermore, at the voxel-wise level, CSF p-tau231 demonstrated substantially higher associations with [18F]AZD4694 retention than CSF p-tau217 in CU individuals. We further performed a voxel-wise analysis in CU participants without prominent Aβ pathology (Aβ-) and demonstrated focal associations of CSF p-tau231 with emerging Aβ pathology in the medial orbitofrontal, precuneus and posterior/isthmus cingulate cortices which were stronger than what was observed for p-tau217 and absent for p-tau181. Previously, Palmqvist et al.59 showed that Aβ fibrils begin to accumulate early in core regions of default mode network (DMN), namely precuneus, posterior cingulate cortex, and orbitofrontal cortex. This finding was even reported in individuals with seemingly normal levels of Aβ in both PET and CSF biomarkers (PET-/CSF-) but who later progressed to exhibiting abnormal levels of CSF Aβ (PET-/CSF+). This demonstrates that Aβ fibrils start to accumulate predominantly within certain parts of the DMN in preclinical AD and our data reveals that CSF p-tau231 is a stronger correlate to these early accumulating regions. Consequently, CSF p-tau231 could be a useful indicator of early Aβ deposition, which can have practical implications for highlighting early AD risk and enrich the enrollment of individuals in the pre-amyloid phase in clinical trials. This was further substantiated by CSF p-tau231 having the largest beta values in relation to Aβ deposition and seemingly becoming abnormal before CSF p-tau217 and CSF p-tau181 when the pathophysiological progression was delineated by Aβ PET Centiloids. P-tau231 levels in blood have also recently been demonstrated to increase earlier than p-tau18160 (Tissot et al., 2022), however, a direct comparison with p-tau217 in early preclinical disease is not currently available.
[18F]MK6240 detects paired helical filament (PHF) tau and has shown a high binding affinity for brain homogenate rich in NFTs.61 CSF p-tau, regardless of phosphorylation site, has been considered as a biomarker of NFT pathology, while other studies propose CSF p-tau as a state marker for brain tau phosphorylation,62 yet the direct association between CSF p-tau and tau PET has been inconsistent. Increases in CSF p-tau occur before both tau PET20,63,64 and established measures of neuronal death (e.g., neurofilament light, magnetic resonance imaging or [18F]FDG PET), thus CSF p-tau cannot merely reflect NFT leakage from dying neurons. Instead, evidence now documents active secretion of tau in normal and disease conditions.19,65 In this study, we found that CSF p-tau231 and CSF p-tau217 associated with [18F]MK6240 to the same degree in the whole group and at both CI and CU stages, whereas CSF p-tau181 had weaker associations. In agreement to this, Janelidze et al. previously described that CSF p-tau217 had a consistently greater regional association to tau PET than CSF p-tau181 but CSF p-tau231 was not analyzed in this particular study. Our results are also in line with data suggesting that p-tau217 is a superior clinical biomarker. While no statistical superiority was demonstrated for p-tau217 over p-tau231, we did observe higher fold changes between AD and all other clinical groups for p-tau217 which is consistent with more recent findings.30
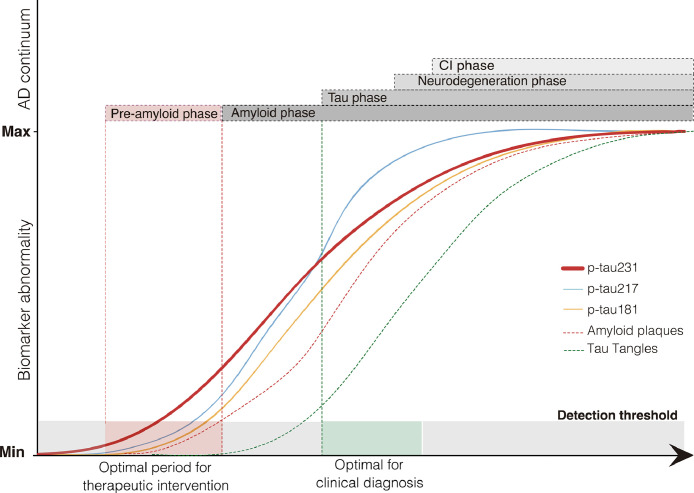
On the basis of the evidence presented above, we propose a theoretical framework in which CSF p-tau epitopes become abnormal in a temporally ordered manner as the disease progresses (Figure 4). Specifically, CSF p-tau231 becomes abnormal first and seems the principal p-tau candidate to identify early Aβ seeds in the recently proposed “pre-amyloid phase”,32 which is currently seen as the optimal period for therapeutic intervention. As Aβ accumulates toward Aβ positivity, other p-tau epitopes (p-tau217 and p-tau181) then become abnormal with p-tau217 changing at quicker rate. At the time of tau positivity, during neurodegeneration and cognitive abnormalities phases, CSF p-tau epitopes have largely reached a plateau.66
Figure 4.
P-tau231 as an early biomarker of Aβ pathology. The theoretical framework proposed presents a temporarily ordered progression of CSF p-tau epitopes in the context of the AD continuum, in which we hypothesize CSF p-tau231 as the first biomarker to become altered in the pre-amyloid phase, being then followed by p-tau217 and p-tau181 at pre-clinical and clinical stages.
Limitations
We are aware of limitations to this study. Firstly, to definitively describe an ordinal sequence of CSF p-tau biomarkers, from pre-amyloid to symptomatic phases of the disease, longitudinal studies are required. Further to this, our main finding of early of pre-amyloid changes of p-tau231 is based on 83 CU Aβ- individuals and should be replicated in an independent cohort which is not enriched for AD with exclusion criteria's. Using the ratio of Aβ42 to p-tau did not largely change our results but produced higher AUC's, which should be further investigated in larger cohort sizes or utilized if the optimal p-tau is not available, as highlighted in this study. Furthermore, the use of cross‐sectional Aβ PET Centiloids as a proxy of time in the disease was employed and while a gradual Aβ accumulation is observed though AD development, it is not certain that a greater Aβ PET SUVR is indicative of more advanced disease state. CSF p-tau biomarkers in this study are compared on two different platforms, with differing detection and capture antibody configurations: N-terminally derived CSF p-tau181 and CSF p-tau217 on the Simoa platform, Mid-terminal CSF p-tau231 by ELISA. Thus, it cannot be ruled out that subtle changes in biomarkers performances are determined by platform differences or antibody superiority. Yet, we believe this is unlikely given that our most promising finding is derived from a traditionally less sensitive technique. In addition, the ADx253 antibody utilized in the p-tau231 ELISA was determined to simulate cis-selectivity however it cannot be determined the specific abundance of the toxic cis p-tau231 species in this assay format.
In conclusion, this study documents the pronounced preclinical increases of CSF p-tau biomarkers but specifically highlights CSF p-tau231 which begins to associate with Aβ deposition before the threshold of Aβ PET positivity has occurred and strongly associates with known early Aβ accumulating regions in the DMN. This finding further supports a model of active soluble tau release by cells into the CSF which is related to early Aβ deposition, likely induced by early Aβ seeds. Thus, CSF p-tau231 is a novel candidate for detecting early and emerging Aβ pathology in individuals for the recruitment into therapeutic trials, act as a target engagement biomarker to monitor the effect of Aβ therapeutics or supports the notion of therapeutically targeting tau epitopes in preclinical disease before tau aggregation can be visualized by tau PET.
Contributors
NJA and ALB are equal 1st co-authors. KB and PR-N are equal senior co-authors.
NJA, ALB, TAP, TKK, HK, OH, KB and PR-N conceived the study. Data acquisition was achieved NJA, TKK, JLR. NJA, ALB and WSB performed statistical analysis. KB and PR-N verified the underlying data. ALB, TAP, SJ, JT, MS, MC PR-N designed and implemented MRI and PET acquisition protocols, as well as performed image processing and quality control. SG, HZ, KB and PR-N recruited participants, and collected clinical data. NJA, ALB, TAP, TKK, ERZ, SG, HZ, KB and PR-N interpreted the data. NJA, ALB, ERZ, KB and PR-N drafted the initial manuscript. All authors contributed to revision and editing of the manuscript.
Declaration of interests
EVM is the Chief scientific officer in ADx Neurosciences. ES and CF are employees of ADx NeuroSciences. SG has served as a consultant for TauRx, Biogen Canada Member and Roche Canada. HZ has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, and has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen. Alzheimer's Association Global Biomarker Standardization Consortium chair and cofounder of Brain Biomarker Solutions in gothenburg AB (BBS) which is a part of the GU ventures Incubator program. KB has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. CF, and ES and are employee of ADx NeuroSciences, Gent, Belgium, EVM is a co-founder of ADx NeuroSciences. The other authors declare no competing interest.
Acknowledgments
Acknowledgements
The authors thank all participants of the study and staff at University of Gothenburg, Sahlgrenska University Hospital, McGill University Research Centre for Studies, and Montreal Neurological Institute who supported this project. We thank Cerveau Technologies for MK-6240, and to GE Healthcare for providing the precursor of flutemetamol, and Roche for providing the precursor of RO948. We also thank Kimberley Mauroo for her assistance for the ADx205 epitope mapping. NJA was supported by the Wallenberg Centre for Molecular and Translational Medicine, Swedish Alzheimer Foundation, Hjärnfonden, the Swedish Dementia Foundation (Demensfonden) and Gamla Tjänarinnor. TAP was supported by the Alzheimer Society Research Program and the Canadian Consortium on Neurodegeneration in Aging. TKK is supported by the BrightFocus Foundation (#A2020812F), the Swedish Alzheimer Foundation (Alzheimerfonden; #AF-930627), the Swedish Brain Foundation (Hjärnfonden; #FO2020-0240), the Swedish Parkinson Foundation (Parkinsonfonden; #1252/20), the Swedish Dementia Foundation (Demensförbundet), Gamla Tjänarinnor Foundation, the Aina (Ann) Wallströms and Mary-Ann Sjöbloms Foundation, the Agneta Prytz-Folkes & Gösta Folkes Foundation (#2020-00124), the Gun and Bertil Stohnes Foundation, and the Anna Lisa and Brother Björnsson's Foundation. HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018-02532), the European Research Council (#681712), Swedish State Support for Clinical Research (#ALFGBG-720931), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), and the UK Dementia Research Institute at UCL. KB was supported by the Alzheimer Drug Discovery Foundation (ADDF; #RDAPB-201809-2016615), the Swedish Research Council (#2017-00915), the Swedish Alzheimer Foundation (#AF-742881), Hjärnfonden, Sweden (#FO2017-0243), and a grant (#ALFGBG-715986) from the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement. TRIAD is supported by the Canadian Institutes of Health Research (CIHR) [MOP-11-51-31; RFN 152985, 159815, 162303], Canadian Consortium of Neurodegeneration and Aging (CCNA; MOP-11-51-31 -team 1), Weston Brain Institute, Brain Canada Foundation (CFI Project 34874; 33397), the Fonds de Recherche du Québec – Santé (FRQS; Chercheur Boursier, 2020-VICO-279314). TAP, P.R-N and SG are members of the CIHR-CCNA Canadian Consortium of Neurodegeneration in Aging.
Data sharing statement
All data presented in this study is available upon request to the corresponding author. Data is not publicly available as it contains information that could compromise the privacy of research participants.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.103836.
Appendix. Supplementary materials
References
- 1.Scheltens P., Blennow K., Breteler M.M., et al. Alzheimer's disease. Lancet. 2016;388(10043):505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 2.Olsson B., Lautner R., Andreasson U., et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673–684. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 3.Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 4.Shaw L.M., Vanderstichele H., Knapik-Czajka M., et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vos S.J., Xiong C., Visser P.J., et al. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12(10):957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schindler S.E., Li Y., Todd K.W., et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer's disease. Alzheimers Dement. 2019;15(5):655–665. doi: 10.1016/j.jalz.2018.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riemenschneider M., Wagenpfeil S., Vanderstichele H., et al. Phospho-tau/total tau ratio in cerebrospinal fluid discriminates Creutzfeldt-Jakob disease from other dementias. Mol Psychiatry. 2003;8(3):343–347. doi: 10.1038/sj.mp.4001220. [DOI] [PubMed] [Google Scholar]
- 9.Skillback T., Rosen C., Asztely F., Mattsson N., Blennow K., Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish mortality registry. JAMA Neurol. 2014;71(4):476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- 10.Schoonenboom N.S., Reesink F.E., Verwey N.A., et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology. 2012;78(1):47–54. doi: 10.1212/WNL.0b013e31823ed0f0. [DOI] [PubMed] [Google Scholar]
- 11.Hampel H., Buerger K., Zinkowski R., et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61(1):95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Leuzy A., Smith R., Ossenkoppele R., et al. Diagnostic performance of RO948 F 18 tau positron emission tomography in the differentiation of Alzheimer disease from other neurodegenerative disorders. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.0989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Joie R., Bejanin A., Fagan A.M., et al. Associations between [(18)F]AV1451 tau PET and CSF measures of tau pathology in a clinical sample. Neurology. 2018;90(4):e282–e290. doi: 10.1212/WNL.0000000000004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maia L.F., Kaeser S.A., Reichwald J., et al. Changes in amyloid-beta and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci Transl Med. 2013;5(194):194re2. doi: 10.1126/scitranslmed.3006446. [DOI] [PubMed] [Google Scholar]
- 15.Schelle J., Hasler L.M., Gopfert J.C., et al. Prevention of tau increase in cerebrospinal fluid of APP transgenic mice suggests downstream effect of BACE1 inhibition. Alzheimers Dement. 2017;13(6):701–709. doi: 10.1016/j.jalz.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 16.De Felice F.G., Wu D., Lambert M.P., et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;29(9):1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sackmann C., Hallbeck M. Oligomeric amyloid-beta induces early and widespread changes to the proteome in human iPSC-derived neurons. Sci Rep. 2020;10(1):6538. doi: 10.1038/s41598-020-63398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barthelemy N.R., Li Y., Joseph-Mathurin N., et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease. Nat Med. 2020;26(3):398–407. doi: 10.1038/s41591-020-0781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato C., Barthelemy N.R., Mawuenyega K.G., et al. Tau kinetics in neurons and the human central nervous system. Neuron. 2018;97(6):1284–1298. doi: 10.1016/j.neuron.2018.02.015. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateman R.J., Xiong C., Benzinger T.L., et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toledo J.B., Xie S.X., Trojanowski J.Q., Shaw LM. Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in ADNI. Acta Neuropathol. 2013;126(5):659–670. doi: 10.1007/s00401-013-1151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunello C.A., Merezhko M., Uronen R.L., Huttunen HJ. Mechanisms of secretion and spreading of pathological tau protein. Cell Mol Life Sci. 2020;77(9):1721–1744. doi: 10.1007/s00018-019-03349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishiguro K., Ohno H., Arai H., et al. Phosphorylated tau in human cerebrospinal fluid is a diagnostic marker for Alzheimer's disease. Neurosci Lett. 1999;270(2):91–94. doi: 10.1016/s0304-3940(99)00476-0. [DOI] [PubMed] [Google Scholar]
- 24.Janelidze S., Stomrud E., Smith R., et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer's disease. Nat Commun. 2020;11(1):1683. doi: 10.1038/s41467-020-15436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell C.L., Mitra V., Hansson K., et al. Comprehensive quantitative profiling of tau and phosphorylated tau peptides in cerebrospinal fluid by mass spectrometry provides new biomarker candidates. J Alzheimers Dis. 2017;55(1):303–313. doi: 10.3233/JAD-160633. [DOI] [PubMed] [Google Scholar]
- 26.Buerger K., Zinkowski R., Teipel S.J., et al. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59(8):1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 27.Barthelemy N.R., Bateman R.J., Hirtz C., et al. Cerebrospinal fluid phospho-tau T217 outperforms T181 as a biomarker for the differential diagnosis of Alzheimer's disease and PET amyloid-positive patient identification. Alzheimers Res Ther. 2020;12(1):26. doi: 10.1186/s13195-020-00596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karikari T.K., Emersic A., Vrillon A., et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer's disease diagnosis. Alzheimers Dement. 2020 doi: 10.1002/alz.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mielke M.M., Aakre J.A., Algeciras-Schimnich A., et al. Comparison of CSF phosphorylated tau 181 and 217 for cognitive decline. Alzheimers Dement. 2021 doi: 10.1002/alz.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuzy A., Janelidze S., Mattsson-Carlgren N., et al. Comparing the clinical utility and diagnostic performance of CSF P-Tau181, P-Tau217, and P-Tau231 Assays. Neurology. 2021;97(17):e1681–e1694. doi: 10.1212/WNL.0000000000012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suarez-Calvet M., Karikari T.K., Ashton N.J., et al. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer's continuum when only subtle changes in Abeta pathology are detected. EMBO Mol Med. 2020:e12921. doi: 10.15252/emmm.202012921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlmann R.E., Rother C., Rasmussen J., et al. Acute targeting of pre-amyloid seeds in transgenic mice reduces Alzheimer-like pathology later in life. Nat Neurosci. 2020;23(12):1580–1588. doi: 10.1038/s41593-020-00737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horowitz P.M., Patterson K.R., Guillozet-Bongaarts A.L., et al. Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer's disease. J Neurosci. 2004;24(36):7895–7902. doi: 10.1523/JNEUROSCI.1988-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Vos A. A novel conformational, phospho-Threonine 231 specific assay for CSF protein tau. J Prev Alzheimers Dis. 2016 [Google Scholar]
- 35.Cselenyi Z., Jonhagen M.E., Forsberg A., et al. Clinical validation of 18F-AZD4694, an amyloid-beta-specific PET radioligand. J Nucl Med. 2012;53(3):415–424. doi: 10.2967/jnumed.111.094029. [DOI] [PubMed] [Google Scholar]
- 36.Pascoal T.A., Shin M., Kang M.S., et al. In vivo quantification of neurofibrillary tangles with [(18)F]MK-6240. Alzheimers Res Ther. 2018;10(1):74. doi: 10.1186/s13195-018-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Therriault J., Benedet A.L., Pascoal T.A., et al. Association of Apolipoprotein E epsilon4 With Medial Temporal Tau Independent of Amyloid-beta. JAMA Neurol. 2020;77(4):470–479. doi: 10.1001/jamaneurol.2019.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascoal T.A., Therriault J., Benedet A.L., et al. 18F-MK-6240 PET for early and late detection of neurofibrillary tangles. Brain. 2020;143(9):2818–2830. doi: 10.1093/brain/awaa180. [DOI] [PubMed] [Google Scholar]
- 39.Pascoal T.A., Mathotaarachchi S., Shin M., et al. Amyloid and tau signatures of brain metabolic decline in preclinical Alzheimer's disease. Eur J Nucl Med Mol Imaging. 2018;45(6):1021–1030. doi: 10.1007/s00259-018-3933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Therriault J., Benedet A., Pascoal T.A., et al. Determining Amyloid-beta positivity using [(18)F]AZD4694 PET imaging. J Nucl Med. 2020 doi: 10.2967/jnumed.120.245209. [DOI] [PubMed] [Google Scholar]
- 41.Klunk W.E., Koeppe R.A., Price J.C., et al. The Centiloid Project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1–15. doi: 10.1016/j.jalz.2014.07.003. e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowe C.C., Jones G., Dore V., et al. Standardized expression of 18F-NAV4694 and 11C-PiB beta-amyloid PET results with the centiloid scale. J Nucl Med. 2016;57(8):1233–1237. doi: 10.2967/jnumed.115.171595. [DOI] [PubMed] [Google Scholar]
- 43.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 44.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112(4):389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathotaarachchi S., Wang S., Shin M., et al. VoxelStats: a MATLAB package for multi-modal voxel-wise brain image analysis. Front Neuroinform. 2016;10:20. doi: 10.3389/fninf.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Worsley K.J., Taylor J.E., Tomaiuolo F., Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23(Suppl 1):S189–S195. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Wu J.W., Herman M., Liu L., et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288(3):1856–1870. doi: 10.1074/jbc.M112.394528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirbaha H., Holmes B.B., Sanders D.W., Bieschke J., Diamond MI. Tau trimers are the minimal propagation unit spontaneously internalized to seed intracellular aggregation. J Biol Chem. 2015;290(24):14893–14903. doi: 10.1074/jbc.M115.652693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos J.R.F., Bauer C., Schuchhardt J., et al. Validation of a prototype tau Thr231 phosphorylation CSF ELISA as a potential biomarker for Alzheimer's disease. J Neural Transm. 2019;126(3):339–348. doi: 10.1007/s00702-019-01982-5. (Vienna) [DOI] [PubMed] [Google Scholar]
- 50.Spiegel J., Pirraglia E., Osorio R.S., et al. Greater specificity for cerebrospinal fluid P-tau231 over P-tau181 in the differentiation of healthy controls from Alzheimer's disease. J Alzheimers Dis. 2016;49(1):93–100. doi: 10.3233/JAD-150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blennow K., Vanmechelen E., Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol Neurobiol. 2001;24(1-3):87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 52.Glodzik-Sobanska L., Pirraglia E., Brys M., et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer's disease. Neurobiol Aging. 2009;30(5):672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kidemet-Piskac S., Babic Leko M., Blazekovic A., et al. Evaluation of cerebrospinal fluid phosphorylated tau231 as a biomarker in the differential diagnosis of Alzheimer's disease and vascular dementia. CNS Neurosci Ther. 2018;24(8):734–740. doi: 10.1111/cns.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell AJ. CSF phosphorylated tau in the diagnosis and prognosis of mild cognitive impairment and Alzheimer's disease: a meta-analysis of 51 studies. J Neurol Neurosurg Psychiatry. 2009;80(9):966–975. doi: 10.1136/jnnp.2008.167791. [DOI] [PubMed] [Google Scholar]
- 55.Augustinack J.C., Schneider A., Mandelkow E.M., Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol. 2002;103(1):26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 56.Ercan-Herbst E., Ehrig J., Schondorf D.C., et al. A post-translational modification signature defines changes in soluble tau correlating with oligomerization in early stage Alzheimer's disease brain. Acta Neuropathol Commun. 2019;7(1):192. doi: 10.1186/s40478-019-0823-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castellani R.J., Perry G. Tau biology, tauopathy, traumatic brain injury, and diagnostic challenges. J Alzheimers Dis. 2019;67(2):447–467. doi: 10.3233/JAD-180721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amniai L., Barbier P., Sillen A., et al. Alzheimer disease specific phosphoepitopes of Tau interfere with assembly of tubulin but not binding to microtubules. FASEB J. 2009;23(4):1146–1152. doi: 10.1096/fj.08-121590. [DOI] [PubMed] [Google Scholar]
- 59.Palmqvist S., Scholl M., Strandberg O., et al. Earliest accumulation of beta-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun. 2017;8(1):1214. doi: 10.1038/s41467-017-01150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashton N.J., Pascoal T.A., Karikari T.K., et al. Plasma p-tau231: a new biomarker for incipient Alzheimer's disease pathology. Acta Neuropathol. 2021;141(5):709–724. doi: 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malarte M.L., Nordberg A., Lemoine L. Characterization of MK6240, a tau PET tracer, in autopsy brain tissue from Alzheimer's disease cases. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-05035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blennow K., Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2(10):605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 63.Mattsson-Carlgren N., Andersson E., Janelidze S., et al. Abeta deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease. Sci Adv. 2020;6(16):eaaz2387. doi: 10.1126/sciadv.aaz2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gordon B.A., Blazey T.M., Christensen J., et al. Tau PET in autosomal dominant Alzheimer's disease: relationship with cognition, dementia and other biomarkers. Brain. 2019;142(4):1063–1076. doi: 10.1093/brain/awz019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pooler A.M., Phillips E.C., Lau D.H., Noble W., Hanger D.P. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14(4):389–394. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutphen C.L., McCue L., Herries E.M., et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer's disease. Alzheimers Dement. 2018;14(7):869–879. doi: 10.1016/j.jalz.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.