Abstract
Globally, several studies have investigated the utilization and efficacy of promising medicinal herbal plants to enhance livestock and poultry production. The most commonly investigated phytobiotics in broiler ration were oregano, garlic, thyme, rosemary, black pepper, hot red pepper (HRP), and sage. Phytobiotics are classified on the basis of the medicinal properties of plants, their essential oil extracts, and their bioactive compounds. The majority of bioactive compounds in plants are secondary metabolites, such as terpenoids, phenolic, glycosides, and alkaloids. The composition and concentrations of these bioactive constitutes vary according to their biological factors and manufacturing and storage conditions. Furthermore, HRP is one of the most important and widely used spices in the human diet. Capsicum annum, that is, HRP, is a species of the plant genus Capsicum (pepper), which is a species native to southern North America and northern South America and is widely grown and utilized for its fresh or cooked fruits. Moreover, these fruits may be used as dried powders or processed forms of oleoresins. Researches have proven that C. annuum is the only plant that produces the alkaloid capsaicinoids. Approximately 48% of its active substances are capsaicin (8-methyl-N-vanillyl-6-nonemide), the main active compound responsible for the intense effects of HRP varieties and the main component inducing the hot flavor. This review aimed to highlight the effects of HRP as a phytobiotic in broiler nutrition and its mode of action as a possible alternative to antibiotics and clarify its impact on broiler and layer productivity.
Key words: antibiotic alternatives, hot red pepper, organic poultry, phytobiotic, poultry diet
INTRODUCTION
Antibiotics have been widely used in animal production for decades to treat and improve animal health, growth rate, and feed conversion ratio (FCR) (Landers et al., 2012; Abd El-Hack et al., 2021a; Swelum et al., 2021). The danger of the continuous emergence of new antibiotic resistance mechanisms in different microorganisms prompted the European Commission to ban antibiotics as growth enhancers in animal feed (European Union, 2005).
However, eliminating antibiotics from the animal diet reflected poorly on growth enhancement, FCR, and feed efficiency (Puvača et al., 2019a; Reda et al., 2020; Sheiha et al., 2020; Reda et al., 2021a). Therefore, the use of natural compounds, such as probiotics (Abd El‐Hack et al., 2020a; Alagawany et al., 2021a; El-Saadony et al., 2021a), prebiotics (Yaqoob et al., 2021; Abd El-Hack et al., 2022), essential oils (Alagawany et al., 2021b; El-Tarabily et al., 2021), herbal extracts (Abou-Kassem et al., 2021; Saad et al., 2021a), amino acids (Abou-Kassem et al., 2021), green synthesized nanoparticles (Abd El-Ghany et al., 2021; El-Saadony et al., 2021b,c,d), bioactive peptides (El-Saadony et al., 2021e,f), safe, natural pigments (Abdelnour et al., 2020a,b; Ashour et al., 2021), bioactive medicinal plants (El-Saadony et al., 2022), plant products (El-Shall et al., 2022), and phytogenic compounds (Abd El-Hack et al., 2020b; Abd El‐Hack et al., 2021b; Abdel-Moneim et al., 2022), for growth promotion and pathogen resistance became a trend and an alternative to antibiotics (Simon et al., 2005; Stanaćev et al., 2011; Puvača et al., 2013; Alagawany et al., 2021c).
One of the most significant spices used in human nutrition is hot red pepper (HRP), often known as “Laal mirch.” They are extremely acceptable as natural feed additives because they are natural, nontoxic, residue-free, and readily available (Munglang and Vidyarthi, 2019).
Capsaicin, found in spicy red pepper, has several biochemical and pharmacological properties, including antioxidant, anti-inflammatory, antiallergenic, and anticarcinogenic effects, and may reduce the risk of cancer (Munglang and Vidyarthi, 2019). Several studies have demonstrated the effect of HRP on the performance of broiler chickens (e.g. Agarwal et al., 2017; Abdelnour et al., 2018; Munglang and Vidyarthi, 2019). HRP can boost pancreatic and intestinal enzyme activity, enhance bile acid secretion, and increase body weight (Bwt) in broiler chickens, as well as reduce heat stress and improve feed digestibility, feed intake (FI), feed conversion efficiency, mortality, carcass features, blood parameters, and production cost (Munglang and Vidyarthi, 2019).
This review article highlights the nutritional value of HRP and its impact on poultry's health, growth, and productive performance and its use as an antibiotic alternative in poultry's feed.
Active Compounds in HRP and Their Medicinal Benefits and Applications in Poultry
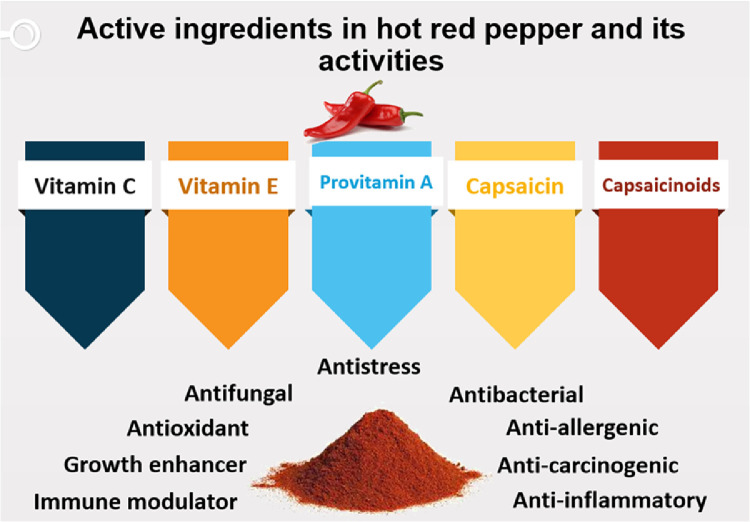
Biologically active constituents and properties of HRP are summarized in Figure 1 and Table 1. Phytogenics are a heterogeneous group of feed additives of plant origin consisting of herbs, spices, fruit, and other plant parts (Gheisar and Kim, 2018; Ashour et al., 2020; Reda et al., 2021b; Saad et al., 2021b,c). The applications of spices and herbs (phytobiotics) in broiler nutrition were previously studied (Horosova et al., 2006; Cross et al., 2007; Al-Kassie et al., 2011a, 2012; Kostadinović and Levic, 2012; Puvača et al., 2013, 2014, 2015a,b).
Figure 1.
Biologically active constituents and properties of hot red pepper.
Table 1.
Various beneficial effects of hot red pepper.
| Effect | Reference |
|---|---|
| Chili pepper (Capsicum annuum L.) shows antioxidant activities. Decreases lipid peroxidation as it is a rich source of carotenoids such as vitamins C and E, and provitamin A | Krinsky, 2001; Kogure et al., 2002; Conforti et al., 2007; Oboh et al., 2007 |
| Acts as an appetizer | Ozer et al., 2005, Al-Kassie et al., 2011b |
| Improves gut integrity | AlKassie et al., 2012 |
| Increases body weight and weight gain | Atapattu and Belpagodagamage, 2011 |
| Improves feed conversion ratio | Eldeeb et al., 2006 |
| Shows antimicrobial and bactericidal effects against intestinal pathogenic bacteria such as Escherichia coli, Clostridium spp., and Salmonella spp. | Zeyrek and Oguz, 2005; Omolo et al., 2014; Agarwal et al., 2017 |
| Darkens the yolk color and improves laying performance | Ozer et al., 2006 |
| Decreases total blood cholesterol concentration | Puvaca et al., 2015b |
| Acts as an important alkaloid owing to its neurotonic activity | Zeyrek and Oguz, 2005; Hayman and Kam, 2008 |
| Shows antiinflammatory, antiallergenic, and anticarcinogenic activities | Lee et al., 2005; Nishino et al., 2009 |
| Capsaicin shows antioxidant, antiinflammatory, antiallergenic, and anticarcinogenic effects and reduces the risk of cancer | Munglang and Vidyarthi, 2019 |
| HRP boosts pancreatic and intestinal enzyme activities, enhances bile acid secretion, and increases body weight growth in broiler chickens. It reduces heat stress and improves feed digestibility, body weight, feed intake, feed conversion efficiency, mortality, carcass characteristics, blood parameters, and production cost | Munglang and Vidyarthi, 2019 |
| Hot or chili pepper (paprika) contains the terpenoid compound capsaicin, which has antibacterial properties | Singletary, 2011 |
| HRP is rich in vitamin C, which significantly mitigates heat stress in birds | Yoshioka et al., 2001; Al-Kassie et al., 2012; Abd El-Hack et al., 2018; Abdelnour et al., 2018 |
| HRP decreases blood cholesterol without affecting triglyceride levels | Eldeeb et al., 2006 |
Abbreviation: HRP, hot red pepper.
Several reports confirmed the activities of these compounds, including antimicrobial, anthelmintic, antioxidant, growth enhancer, and immune modulator activities (Lokaewmanee, 2019). However, their phytogenic effect depends on their herbal characteristics, the knowledge of major and minor constituents, modes of action, and safe concentrations in animals (Abou-Elkhair et al., 2018). The concentration and composition of these active compounds in plants fluctuate based on different biological agents and manufacturing and storage conditions (Zhao et al., 2015).
HRP (Capsicum annuum L.), one of the main spices widely used in human food, belongs to the family Solanaceae (Singletary, 2011). Hot or chili pepper (paprika) and HRP contains the terpenoid compound capsaicin, which has antibacterial properties (Singletary, 2011). It is rich in vitamin C, which significantly mitigates heat stress in birds (Yoshioka et al., 2001; Al-Kassie et al., 2012; Abd El-Hack et al., 2018; Abdelnour et al., 2018). Chili pepper is a rich source of carotenoids such as vitamins C, E, and provitamin A (β carotene), which have well-known antioxidant functions to fight against the hazardous effects of free radicals, including cell destruction (oxidative stress) in broilers (Krinsky, 2001; Droge, 2002; Tawfeek et al., 2014).
Henkin (1991), Yoshioka et al. (2001) and Al-Kassie et al. (2012) explained that HRP is rich in vitamin C, which has a considerable impact on improving production by alleviating the hazardous effect of heat stress. Kogure et al. (2002) and Luqman and Razvi (2006) reported that capsicum was more effective than vitamin E in inhibiting lipid peroxidation. Capsaicin can potentiate the activities of pancreatic and intestinal enzymes (Platel and Srinivasan, 2004), increase bile acid secretion (Abdel Salam et al., 2005), and increase weight gain (WG) in broiler chickens (Puvača et al., 2014, 2015b).
The active ingredients of HRP have preventive and therapeutic effects (Jancso et al., 1997). The genus Capsicum (C. annuum L.) constitutes a family of flowering plants, including HRP and chili pepper, usually used as appetizers in human diets (Al-Kassie et al., 2011a). The therapeutic properties discovered in the genus Capsicum were attributed to their bioactive components, including capsaicin (Abdelnour et al., 2018; Puvača, 2018). Dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin are among the capsaicinoid compounds found in chili peppers and HRP (Kobata et al., 1998; Fattori et al., 2016; Abou-Elkhair et al., 2018).
Capsaicin is a major ingredient in HRP and other peppers (Fattori et al., 2016; Abou-Elkhair et al., 2018). Capsaicin (8-methyl-N-vanillyl-6-nonemide) represents 50% of the active compounds in this alkaloid; besides some related chemicals, combined capsinoids are the substances that produce the hot sensation associated with peppers (El-Tazi, 2014). In addition, the antioxidant and antistress activities of HRP have been reported previously (Lee et al., 2005; Puvača, 2018; Puvača et al., 2019b; Batiha et al., 2020). The numerous terpenoids in different herbs have a high antioxidant function (Nakatani, 1994).
The chemotherapeutic and chemopreventive properties of HRP have been paid great consideration in poultry nutrition (Tellez et al., 1993; McElroy et al., 1994; Kogure et al., 2002; Zeyrek and Oguz, 2005; Conforti et al., 2007; Oboh et al., 2007; Wahyuni et al., 2013; Puvača et al., 2015a). Furthermore, several studies confirmed the effect of photobiotic feed additives on broiler nutrition (Al-Kassie et al., 2011b, 2012; Puvača et al., 2019a).
Previous studies indicated that the inclusion of HRP into broiler diets improved their FI, Bwt, WG, and FCR (El-Deek et al., 2012 ). The beneficial effects of capsicum in poultry nutrition may be related to capsaicin, which has a bactericidal effect against intestinal pathogens, such as Escherichia coli, Salmonella spp., and Clostridium spp. (Omolo et al., 2014; Agarwal et al., 2017; Salem et al., 2021).
Capsaicin can also protect the gastrointestinal mucosal layer against injuries due to drugs or irritants (Al-Kassie et al., 2012). Poultry cannot sense the pungency of capsicum due to the lack of receptors specific for capsaicin binding, thereby enabling a high concentration of capsicum in broiler diets (Mason and Maruniak, 1983; Geisthovel et al., 1986; Puvača et al., 2015a). Therefore, the addition of small ratios of HRP into experimental diets in previous studies may not be enough to portray the mode of action clearly (Zheng et al., 2017). However, capsaicin increases appetite in poultry, so the addition of HRP to the diet influences broilers' feed consumption (FC) (Yoshioka et al., 2001). The effective compounds in hot peppers are capsaicin, capsisin, and capsantine (Viktorija et al., 2014). Capsinoids are a family of analogs of capsaicin (alkaloids), the pungent component in HRP responsible for the hot taste (Izawa et al., 2010).
The use of chili pepper fruits in broiler and layer diets significantly enhanced FI, darkened the yolk color and improved laying performance (Özer et al., 2005, 2006). Capsaicin is passively absorbed in the intestine (Kawada et al., 1984; Iwai et al., 2003). It efficiently stimulates nutrient and energy metabolism by enhancing the activities of glucose-6-phosphate dehydrogenase, lipoprotein lipase in adipose tissues, and pancreatic and intestinal enzymes (Platel and Srinivasan, 2004; Abdel Salam et al., 2005; AL-Kassie et al., 2011a; Puvača et al., 2014, 2015b).
In addition, Puvača et al. (2015b) confirmed its role in decreasing total blood cholesterol. Small amounts of red pigments and comparatively large amounts of yellow carotenoids are present in red pepper, which pass readily into the yolk, thus enhancing its yellow color intensity (González et al., 1999). Previous results indicated that dietary red pepper had a stimulating effect on intestinal villi and the structure of epithelial cells and improved the egg yolk color (Lokaewmanee et al., 2013).
In quails, Sri Divya (2017) noted that up to 1.0% black pepper could be integrated as a natural feed additive without adversely affecting productive performance. Sayeed et al. (2016) reported that 2% of HRP in quail diets may benefit performance and blood metabolites. Furthermore, El-Ghamry et al. (2004) reported that HRP is a good natural feed additive for improving the performance of Muscovy ducklings.
Capsaicin is an important alkaloid characterized by neurotonic and antimicrobial activities (Zeyrek and Oguz, 2005; Hayman and Kam, 2008). It can decrease lipid peroxidation (Kogure et al., 2002; Conforti et al., 2007; Oboh et al., 2007). Previous reports on the biopharmacological properties of capsaicinoids included antioxidant, antiinflammatory, antiallergenic, and anticarcinogenic activities (Lee et al., 2005; Nishino et al., 2009).
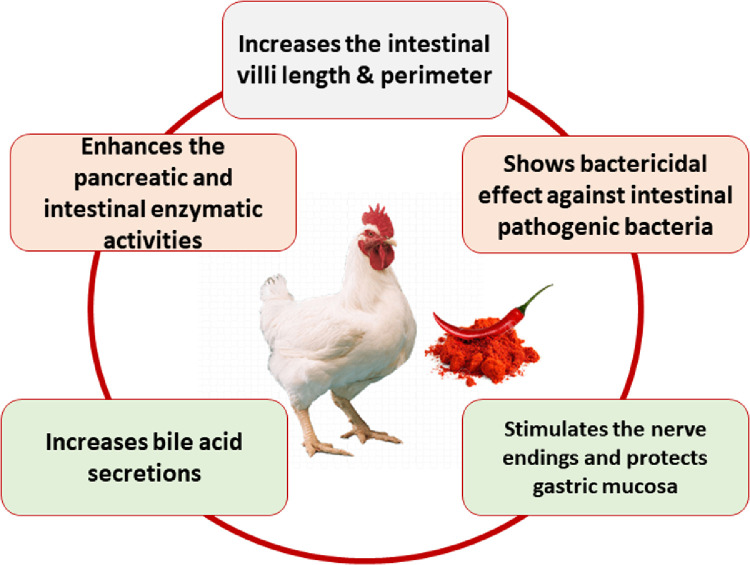
Capsaicin protects the gastric mucosa through the afferent stimulation of nerve endings. Approximately 85% of capsaicin is absorbed by passive diffusion, mainly in the jejunum, which improves the digestibility of feeds in broilers (Figure 2; Kawada et al., 1984; Iwai et al., 2003). However, Hernandez et al. (2004) reported that such ingredients only slightly improved performance and that these differences were not significant. Capsinoids are thought to stimulate certain healing processes in the body (Munglang and Vidyarthi, 2019).
Figure 2.
Effects of hot red pepper on bird digestibility.
Effects of HRP on the Performance of Poultry
Effects of HRP on Productive Performance (Bwt and WG)
HRP improved nutrient digestibility in the whole tract by potentiating the activities of pancreatic and intestinal enzymes (Platel and Srinivasan, 2004) thereby increasing bile acid secretion (Abdel Salam et al., 2005) and maintaining the intestinal mucosa (Al-Kassie et al., 2012). Furthermore, the improvement may be partly related to a reduction in heat stress resulting from a high concentration of vitamin C (Henkin, 1991). Many years ago, Williams and Kienholz (1974) found that several concentrations of chili powder (0, 1.5, 3, 6, and 12%) had little effect on broiler growth and were nontoxic at concentrations up to 2%.
However, Vogt et al. (1989) found that spices such as cayenne (hot) and coriander (white) pepper did not affect WG; however, HRP at 100 mg/kg diet improved FCR by 3.2%. Azouz (2001) stated that adding HRP at concentrations of 1, 1.5, and 2% in the diets significantly improved WG. Soliman (2002) reported that 1.5% and 3% HRP increased the minimum FI by 9.48 and 8.03% compared with the control (P ≤ 0.05), respectively, and improved the viability of the birds, particularly when added to low-energy diets. Moreover, Al-Harthi (2002a,b) noted that a diet supplemented with 0.1% HRP insignificantly improved broiler FCR and growth rate compared with the control diet.
El-Husseiny et al. (2002) found that broiler chicks fed HRP had significantly higher Bwt, dressing percentage, and lower mortality rates than those fed control or fenugreek diets. Eldeeb et al. (2006) reported that the WG of broiler birds was significantly (P < 0.05) higher in chickens fed a diet supplemented with a combination of corn oil and HRP (5% + 150 ppm) and a combination of corn oil, HRP, and botanical extract (5% + 150 ppm + 150 ppm) compared with those fed a diet supplemented with a combination of botanical extract and HRP (150 ppm + 150 ppm).
In contrast, Özer et al. (2006) reported that low-dose HRP added to the cock diet during the developmental period (first 5 mo of age) could reduce WG. An et al. (2007) noticed that the WG of broiler birds was slightly greater on a diet supplemented with HRP than on the control one. This irregularity might result from the variations within the number of spices.
Tollba et al. (2007) found that the supplementation of bioantioxidant HRP to the diet of Egyptian chickens at a concentration of 5 g/kg diet improved (P ≤ 0.05) WG compared with the control. Atapattu and Belpagodagamage (2011) and Al-Kassie et al. (2011a) also observed a significant (P < 0.05) increase in WG. Compared with a basal diet, a diet supplemented with HRP reduced the total cholesterol level in the blood of broiler birds. Paguia et al. (2011) revealed that treated diets with Capsicum frutescens significantly influenced the feed efficiency (P < 0.05) of layers compared with that of the control group.
Al-Kassie et al. (2012) and El-Deek et al. (2012) observed an enhanced FCR due to the inclusion of HRP into broiler diets. Al-Kassie et al. (2012) observed that the live WG of broiler birds was significantly (P < 0.05) higher on a diet supplemented with a mixture of black pepper and HRP at concentrations of 0.75 and 1.0% than on a diet supplemented with the mixture at concentrations of 0, 0.25, and 0.50%. El-Deek et al. (2012) observed that the WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at concentrations of 1.5 g/kg and 3 g/kg of feed as compared to oxytetracycline and control groups. The supplementation of Brazilian red pepper with or without antibiotics improved FCR, with no significant effects (P > 0.05) on broiler weight and relative liver weight at 43 days of age.
Wadasen (2012) observed that the mean final WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at concentrations of 0.5, 1.0, and 1.5% of feed than on a control diet. However, Lokaewmanee et al. (2013) observed no significant difference in feed efficiency among the experimental groups fed a diet containing 0.5% HRP. Conversely, Shahverdi et al. (2013) showed that chicks fed diets with HRP (0.02%), black pepper (0.02%), and mixed powders (0.01% red pepper + 0.01% black pepper powders) had a higher Bwt (P ≤ 0.05) compared with the control chicks.
El-Tazi (2014) observed that, compared with the control group, the final Bwt and WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with a mixture of HRP and black pepper (0.5% + 0.05%) at 1% of feed than at 0.4% and 0.6% of the feed. Thiamhirunsopit et al. (2014) observed that, compared with the control group, the growth performance and average daily WG of broiler chickens were significantly (P < 0.05) better on a diet supplemented with HRP at a concentration of 20 mg/kg of feed.
Valiollahi et al. (2014) fed chicks a basal diet (T1, control group), a basal diet with 0.02% ginger powder (T2), a basal diet with 0.02% black pepper powder (T3), and a basal diet with 0.01% ginger + 0.01% black pepper powder (T4). They showed that Bwt and WG at the end of the experiment were significantly higher in the treated groups than in the control group. El-Tazi (2014) reported that HRP in the diet at concentrations of 0.5, 0.75, and 1% significantly improved broiler chicks' WG and feed efficiency.
However, Dougnon et al. (2014) reported no significant difference in the final Bwt and average daily WG of broiler chicks on a diet supplemented with HRP at 0, 0.5, and 1.0% of the feed for 1 mo and at 0.5 and 1.0% of the feed for 2 mo. El-Amin et al. (2015) observed that the WG of broiler chicks was significantly (P < 0.05) higher on a diet supplemented with HRP at 0.5, 1.0, and 1.5% of feed or a basal diet with 16 mg/kg antibiotic (neomycin) than on the negative control basal diet with no antibiotic/HRP.
Puvača et al. (2015b, 2016) observed that, compared with the control and other treatments, the final Bwt of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with a mixture of garlic (Allium sativum L.), black pepper (Piper nigrum L.), and HRP (C. annuum L.) at 0.5 and 1.0% of the feed. Abo et al. (2016) observed that the WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at 1 g/kg of feed than on a control diet or a diet supplemented with thyme at 1 g or garlic at 1 g/kg of feed.
Moradi et al. (2016) reported that the WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at 200 g/ton and black pepper at 200 g/ton of feed as compared to the control group. Afolabi et al. (2017) observed that the WG of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at 0.1, 0.2, and 0.3% of feed compared with that of the control group.
Afolabi et al. (2017) found that the performance at the end of seventh wk revealed a significantly (P < 0.05) higher daily WG in birds fed diets with different concentrations of HRP (0.1, 0.2, and 0.3%) than in birds fed the control diet. Younis and Abdel-Latif (2017) reported that the WG of broiler birds was significantly (P < 0.05) higher on water supplemented with 1% HRP and a diet supplemented with 2% HRP than on the control diet.
Abou-Elkhair et al. (2018) noticed that the dietary inclusion of HRP significantly (P < 0.05) improved FCR compared with the control. Adegoke et al. (2018) observed that, compared with the control group, the final Bwt and total WG of broiler chickens were significantly (P < 0.05) higher on a diet supplemented with HRP at 100 g/100 kg of feed. Rahimian et al. (2018) noted a significant improvement in Bwt when quail diets were supplemented with ginger, red and black peppers, and protexin at a concentration of 2% compared with the control. This result corroborates the findings of Sayeed et al. (2016), who found that Bwt (g) was significantly higher when quail chicks were fed diets with 2% ginger, red pepper, or black pepper.
Sri Divya (2017) showed that WG increased significantly (P ≤ 0.05) when the amount of black pepper in the diet of quail chicks was increased from 0.0% to 1.0%. Furthermore, the supplementation of garlic, black pepper, and HRP in broiler diets positively impacts productive performance (Puvača et al., 2019a). Adedoyin et al. (2019) noted that adding HRP at 1, 1.25, and 1.5% to Anak broiler diets resulted in a numerically higher average FI and average WG compared with the controls. Soliman and Al-Afifi (2020) reported similar results. They reported that Bwt, WG, and FCR were significantly improved by feeding different graded concentrations of HRP.
Effect of HRP on FC and FCR
In a study in which broiler chicks were fed 10% HRP, Rose et al. (2003) demonstrated a significant increase in FI. Furthermore, compared with the control diet of quails, a diet supplemented with HRP significantly reduced FI (P < 0.01) with an increase in the concentration of HRP up to 4%.
Atapattu and Belpagodagamage (2011) and El-Deek et al. (2012) observed that the FI of the broiler birds was significantly (P < 0.05) higher on a diet supplemented with 3 to 5% chili powder feed than on that supplemented with 1% chili powder feed. Shahverdi et al. (2013) observed that the FI of broiler birds was significantly (P < 0.05) higher on a diet supplemented with a mixture of equal quantities of HRP and black pepper at 0.02% of feed than on the control diet. El-Tazi (2014) observed that the FI of broiler birds was significantly (P < 0.05) higher on a diet supplemented with a mixture of HRP and black pepper (0.5% + 0.5% of feed) than on the control diet.
Abo et al. (2016) observed that the FI of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at 1 g/kg of feed than on the control diet and diets supplemented with 1 g thyme or 1 g garlic per kg of feed. Moradi et al. (2016) reported that, compared to the control group, the FI of broiler birds was significantly (P < 0.05) higher on a diet supplemented with HRP at 200 g/ton and black pepper at 200 g/ton of feed. Afolabi et al. (2017) reported that the FI of broiler birds was significantly (P < 0.05) higher on a diet supplemented with HRP at 0.1% of feed than on a diet supplemented with HRP at 0, 0.2, and 0.3% of the feed.
Adegoke et al. (2018) reported that the FI of broiler birds was significantly (P < 0.05) higher on a diet supplemented with cayenne pepper powder at 100 g/100 kg of feed than on a nonsupplemented diet or a diet supplemented with cayenne pepper powder (200 g) or a combination of turmeric and cayenne pepper powders (200 g + 100 g). However, Wadasen (2012), Dougnon et al. (2014), and El-Amin et al. (2015) reported that there was no difference in the FI of broiler birds on a diet supplemented with HRP at 0, 0.5, 1.0, and 1.5% of the feed. In addition, Azouz (2001) mentioned that the FI of broilers decreased as the concentration of HRP increased to 2%.
Regarding FCR, broilers had a significantly (P < 0.05) better FCR on a diet supplemented with a combination of 5% corn oil and 150 ppm capsicum than on a diet supplemented with 150 ppm botanical extract (Eldeeb et al. 2006). Atapattu and Belpagodagamage (2011) observed that the FCR of broiler chickens significantly improved by 6% on a diet supplemented with 5% HRP that on a diet supplemented with 0, 1, and 3% HRP. Compared with the control group, the FCR of broiler chickens was significantly (P < 0.05) better on a diet supplemented with HRP alone or with black pepper mixture at different concentrations.
Many other researchers reported a significant (P < 0.05) improvement of FCR in broilers using diets supplemented with HRP alone up to 3 kg/ton (Al-Kassie et al., 2011b; El-Deek et al., 2012; Wadasen, 2012; Shahverdi et al., 2013; El-Amin et al., 2015, Afolabi et al., 2017; Younis and Abdel-Latif, 2017; Soliman and Al-Afifi 2020) or mixed with black pepper (El-Tazi, 2014; Moradi et al., 2016). However, Dougnon et al. (2014) reported no difference in the FCR of broiler chicks on a diet supplemented with HRP at 0, 0.5, and 1% of the feed for 1 mo and 0.5 and 1% of the feed for 2 mo.
Effect of HRP on Mortality
Williams and Kienholz (1974) found that HRP concentrations of >2% were nontoxic and had no significant effect on mortality. Atapattu and Belpagodagamage (2011) reported that the mortality (%) of broiler birds on a diet supplemented with HRP at 0, 1, 3, and 5% of feed was 3.3, 3.4, 0, and 0%, respectively, with no significant differences among the treatments. Wadasen (2012), El-Tazi (2014), and Adegoke et al. (2018) showed that there was no mortality or significant difference in the mortality (%) of broiler birds on a diet supplemented with different concentrations of HRP, either alone or mixed with black pepper.
Abo et al. (2016) reported that the mortality of broiler birds was significantly (P < 0.05) higher in the control groups than in the groups fed a diet supplemented with thyme at 1 g, garlic at 1 g, and HRP at 0.1 g/kg of feed.
Effect of HRP on Carcass Traits and Organ Weights
The impacts of HRP supplementation on carcass characteristics are summarized in Figure 3. Compared with the controls, the meat percentage of broiler birds was significantly (P < 0.05) higher when using a diet supplemented with 150 ppm capsicum (Eldeeb et al. 2006). The supplementation of bioantioxidant HRP to the diet of Egyptian chickens at a concentration of 5 g/kg diet significantly enhanced (P ≤ 0.05) the dressing and relative weights of giblets compared with those of the controls, as noted when HRP was added to the Egyptian Golden Montazah chick diet at 0.5% (Tollba et al., 2007).
Figure 3.
Impacts of hot red pepper supplementation on carcass characteristics.
Al-Kassie et al. (2012) reported that the dressing percentage of broiler birds was significantly (P < 0.05) higher on a diet supplemented with a mixture of black pepper and HRP at 0.75 and 1% of feed than that on a diet supplemented with the mixture at 0, 0.25, and 0.5% of the feed. They further reported that the heart, gizzard, and liver weights did not differ significantly (P > 0.05) among the various diet groups compared with those of the control group.
Shahverdi et al. (2013) reported that, compared with the control, the dietary supplementation of HRP (0.02%) alone or in combination with black pepper (0.01% + 0.01%) had significantly (P < 0.05) increased the weights of the liver, drumstick, breast meat, gizzard, heart, and spleen; however, there was a decrease in abdominal fat percentage in the supplemented groups compared with the control group. El-Tazi (2014) observed that the dressing percentage and commercial cut percentage of broiler birds were significantly (P < 0.05) higher on a diet supplemented with a mixture of black pepper and HRP (0.5% + 0.05%) at 1% of feed than on a control diet or a diet supplemented with 0.4% black pepper and 0.6% HRP.
Abo et al. (2016) observed that the carcass quality of broiler chickens was significantly (P < 0.05) higher on a diet supplemented with HRP at 1 g/kg of feed than on a control diet or diets supplemented with thyme or garlic at 1 g/kg of feed. Puvača et al. (2016) observed that the carcass quality of broiler birds was significantly (P < 0.05) better when using a diet supplemented with HRP at 0.5 g and 1 g or mixed with garlic and black pepper (1:1:1) at 0.5 g/100 g of feed than on a control diet. Islam et al. (2018) reported that the highest dressing percentage was recorded in broilers fed a ration with 0.5% red chili.
Many studies recorded no significant differences or decreased dressing percentages and liver, heart, gizzard, and abdominal fat weights, or both of broiler chickens between HRP-treated birds and controls (Azouz 2001; Al-Kassie et al., 2011c; Afolabi et al., 2017; Islam et al., 2018; Soliman and Al-Afifi, 2020). El-Deek et al. (2012) also reported a significant decrease in the abdominal fat percentage of broiler birds fed a diet supplemented with 1.5 g HRP/kg feed. In addition, compared with the control diet, a diet supplemented with 1.5 g and 3 g HRP/kg feed significantly (P < 0.05) decreased the gizzard percentage. However, the spleen and bursa weights did not differ following the addition of HRP into broiler diets.
Moradi et al. (2016) reported that the liver percentage of broiler chickens significantly decreased on a diet supplemented with HRP at 200 g/ton of feed than on a control diet or a diet supplemented with black pepper at the same dose. However, the abdominal fat and gizzard percentage increased on a diet supplemented with 200 g HRP/ton than on the control diet. Afolabi et al. (2017) observed that the dietary supplementation of HRP at the concentrations of 0.1, 0.2, and 0.3% of feed did not affect the weights of the carcass, liver, heart, gizzard, and abdominal fat, but the weight of the kidney significantly (P < 0.05) decreased in the 0.3% group compared with the 0.1%, 0.2%, and control groups.
Younis and Abdel-Latif (2017) reported that the dressing percentage of broiler chickens was significantly (P < 0.05) higher on water supplemented with 1% HRP than on a diet supplemented with HRP at 2% of feed and a control diet; however, the weights of liver, gizzard, abdominal fat, intestine, and heart did not vary, possibly due to variations in the strains of birds or the concentration of HRP supplementation.
Azouz (2001) found that adding HRP to the diet significantly decreased abdominal fat in broilers. The reduction in abdominal fat of chicks fed hot peppers may have been caused by capsaicin, which may possess a lipid-lowering effect (Kawada et al., 1984). However, the mechanisms involved in reducing abdominal fat by herbal feed additives may be dependent on increasing the secretion of lipase and secondary bile acids, which reduce the accumulation of fats in the abdominal cavity (El-Amin et al., 2015).
El-Deek et al. (2012) reported that the taste, flavor, tenderness, color, and acceptability of thigh meat significantly (P < 0.05) increased in broiler birds fed a diet supplemented with 1.5 g HRP/kg feed compared with the controls. However, when the dose was increased to 3 g/kg feed, the flavor, color, and acceptability of breast meat were significantly (P < 0.05) decreased. These results were supported by El-Tazi (2014) and El-Amin et al. (2015).
Effect of HRP on Blood Parameters
Feeding broiler birds with 1% HRP (Atapattu and Belpagodagamage, 2011) and 10% HRP (An et al., 2007) resulted in a significant (P < 0.05) decrease in serum total cholesterol levels compared with the control group. The total protein concentration was significantly (P < 0.05) higher in broiler birds fed a diet supplemented with 150 ppm capsicum; however, there was a decrease in blood cholesterol levels without a difference in triglyceride concentrations (Eldeeb et al., 2006).
Abdul Aziz (2010) noted an improvement in serum total protein concentration with HRP supplementation at 1% and 2% of feed than with the control diet. Al-Kassie et al. (2011a) observed that the levels of cholesterol, hemoglobin (Hb), packed cell volume, white blood cells (WBC), red blood cells (RBC), glucose, and heterophils/lymphocyte (H/L) ratios of broiler birds significantly (P < 0.05) decreased on a diet supplemented with 0.25, 0.50, 0.75, and 1% HRP in comparison with those fed the control diet.
Al-Kassie et al. (2012) observed that the levels of cholesterol, WBC, RBC, Hb, and H/L ratios (indicating a good effect on the immune system of birds) were significantly (P < 0.05) lower in broiler birds fed a diet supplemented with a mixture of black pepper and 0.50, 0.75, and 1% HRP in comparison with those fed the same diet with 0% or 0.25% HRP. These results were supported by Shahverdi et al. (2013).
El-Deek et al. (2012) observed that the plasma triglyceride concentrations significantly (P < 0.05) decreased in broiler birds fed a diet supplemented with HRP at 1.5 g/kg and 3 g/kg as compared with those of the control group. Moreover, an increase in the aspartate aminotransferase (AST) levels and a decrease in alanine aminotransferase levels were observed (Goncalves et al., 2012). Shahverdi et al. (2013) observed a significant (P < 0.05) decrease in the blood cholesterol, triglycerides, and glucose levels; heterophil-to-lymphocyte ratio; and Hb concentration of broiler chicks on a diet supplemented with HRP at 0.02%, black pepper at 0.02%, or their mixture at 0.02% in comparison with those fed the control diet.
Dougnon et al. (2014) reported a reduction in the blood glucose level of broiler chicks fed a diet supplemented with HRP at 0.5 and 1% at either 1 or 2 mo compared with those fed the control diet. El-Amin et al. (2015) observed that the serum cholesterol and AST concentrations were significantly (P < 0.05) lower in broiler birds fed a diet supplemented with HRP at 0.5, 1.0, and 1.5% in comparison with the negative and positive controls. In addition, there was significant difference (P < 0.05) in the total protein and glucose concentrations. In addition, the highest concentrations of triglycerides, total cholesterol, low-density lipoprotein (LDL), and non–high-density lipoprotein (non-HDL) were observed in the control group when compared with the fortified groups (0.75 and 1% HRP) (Puvača et al., 2015a).
Moreover, Moradi et al. (2016) reported a significant (P < 0.05) decrease in triglycerides, cholesterol, and LDL concentrations, with an increase in HDL concentration, in broiler birds, fed a diet supplemented with HRP at 200 g/ton feed compared with those fed the control diet. Afolabi et al. (2017) reported that the leucocyte counts of broiler birds fed a diet supplemented with HRP at 0.2 or 0.3% were significantly (P < 0.05) higher than those of broiler birds fed the control diet or a diet supplemented with HRP at 0.1%.
The levels of plasma total protein and albumin increased by 2%. Conversely, the plasma triglyceride levels reduced significantly, which may be related to uninvestigated intestinal irritation or hepatic disorder due to the inclusion of HRP at high concentrations (Afolabi et al., 2017). However, the data on this aspect is still lacking in poultry research (Soliman and Al-Afifi, 2020). These results corroborate those reported by Traesel et al. (2011) and Corduk et al. (2013), who did not observe any significant effects on the serum levels of total protein, albumin, and globulins following the addition of HRP in broiler diets.
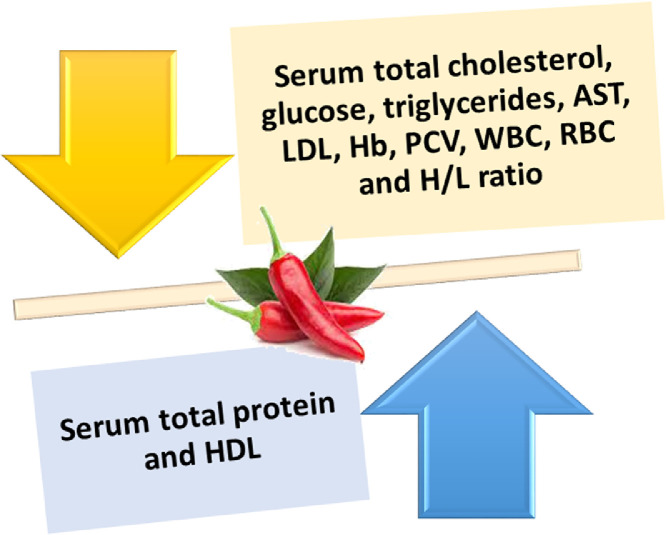
Puvača et al. (2015b) reported that triglycerides, total cholesterol, LDL, and HDL concentrations showed significant (P < 0.05) differences in broiler birds fed a diet supplemented with HRP at 0.5 and 1.0% and a mixture of garlic, black pepper, and HRP (1:1:1) at 0.5% when compared with those fed the control diet. This could be attributed to the inhibitory effects of the bioactive components of HRP on hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity (a critical enzyme in cholesterol biosynthesis), thereby reducing cholesterol synthesis (Crowell, 1999) and the intestinal resorption of cholesterol (Brunton, 1990; Figure 4).
Figure 4.
Effects of hot red pepper on blood parameters. Abbreviations: AST, aspartate aminotransferase; Hb, Hemoglobin; H/L, heterophils and lymphocytes ratio; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PCV, packed cell volume; RBC, red blood cells; WBC, white blood cells.
Several reports have established the effect of capsaicin and HRP in reducing blood cholesterol levels in broilers. Adegoke et al. (2018) reported that feeding the broiler birds with a mixture of 2 kg turmeric plus 1 kg HRP per ton of feed resulted in better blood profiles than broiler birds in the control group. The hypocholesterolemic effect of HRP may be related to its role in stimulating the hepatic cholesterol-7-hydroxylase enzyme needed for converting cholesterol to bile acids and subsequently depleting blood cholesterol levels (Adedoyin et al., 2019).
The reduction in plasma triglycerides was reported previously and is related to a decrease in lipid absorption (El-Deek et al., 2012) or the inhibition of acetyl CoA synthetase, an enzyme necessary for fatty acid biosynthesis (Puvača et al., 2015a). Blood protein derivative values were not affected significantly (P < 0.05) by adding HRP at 0.5 or 1%.
Antimicrobial and Intestinal Histomorphology Enhancement Effects of HRP
All the previous beneficial effects of HRP on Bwt, WG, FI, FCR, blood parameters, and carcass characteristics are related to improvements in intestinal histology and gut health (integrity), which increase nutrient utilization via increased villi length and reduce the abundance of harmful bacterial strains (Soliman and Al-Afifi, 2020).
While demonstrating the alterations in the morphology of the small intestine of broiler chicks, Azouz (2001) showed that the best results for villi length were obtained in hot pepper groups, especially in birds receiving 2% hot pepper. Prakash and Srinivasan (2010) and Madhupriya et al. (2018) reported that Wistar rats fed black pepper, HRP, or ginger diets generally showed an increase in microvilli length (villi height and crypt depth, especially in the jejunum of chicks).
Soliman and Al-Afifi (2020) showed that villi length significantly (P < 0.05) improved due to the addition of HRP at 0.5 and 1% in diets. The increase in villi length was associated with a significant reduction in crypt depth. It is generally believed that red pepper is used to increase the bioavailability of corn gluten meal by two pathways: a) modification of the morphology of the small intestine by reducing the growth of pathogenic intestinal bacteria (Shahverdi et al., 2013); b) these reductions in pathogenic bacteria reduce the inflammatory reactions in the intestinal mucosa, thereby leading to a significant (P ≤ 0.05) increase in villus area and improvement in the functions of secretion, digestion, and nutrient absorption (Cardoso et al., 2012; Shahverdi et al., 2013).
Scanning electron microscopy of the intestinal villi in HRP treatment groups revealed alterations in the ultrastructure, especially an increase in the microvilli length and perimeter, which would mean a beneficial increase in the absorptive surface of the small intestine, thereby increasing the bioavailability of micronutrients (Srinivasan, 2015).
The inclusion of HRP into the broiler diet reduced the growth of the gram-negative pathogenic bacteria E. coli and other members of the family Enterobacteriaceae. The reduction was significant (P < 0.05) for E. coli. Furthermore, HRP supplementation significantly inhibited the growth of gram-positive lactobacilli. It has been reported that HRP has broad-spectrum bactericidal activity against gram-positive and gram-negative pathogenic and nonpathogenic bacteria (Soliman and Al-Afifi, 2020). This finding agrees with the results of Corduk et al. (2013), who stated that capsaicinoids of HRP possess antimicrobial activities against pathogenic E. coli and other genera of the family Enterobacteriaceae.
Abdul Aziz (2010) suggested that the capsicum pepper has a broad-spectrum effect on isolated bacterial strains due to the bacteriostatic and bactericidal activities of the capsaicin derivatives t-cinnamic and caffeic acids, respectively.
Effect of HRP on Egg Production
Significant (P < 0.05) improvements in laying performance (egg production, egg weight, and egg mass by 10 to 30 g/kg) as well as egg quality traits were observed through the dietary inclusion of HRP compared with the control group; however, there were no significant effects on the yolk weight percentage (Abou-Elkhair et al., 2018).
Lokaewmanee et al. (2011) showed no significant difference in hen-day production and egg mass among the experimental groups fed 0.5% HRP but reported that capsanthin improved eggs color and was responsible for the deep red color of the egg yolk. The fruits of HRP are also used to darken the color and improve the nutritive value of egg yolk (Özer et al., 2006). Furthermore, HRP contains small amounts of red pigments and comparatively large amounts of yellow carotenoids, which pass readily into the yolk, enhancing the yellow color's intensity (González et al., 1999).
Paguia et al. (2011) stated that laying hens fed diets containing C. frutescens were not affected in terms of yolk color intensity. Lokaewmanee et al. (2013) found that the Haugh unit was not influenced by the dietary supplementation of 0.5% HRP in laying hen diets. Paguia et al. (2011) noted that egg production and egg weights were similar between control birds and layers fed diets supplemented with C. frutescens from 54 to 70 wk of age.
Other Biopharmacological Properties (Antioxidant, Antiinflammatory, and Anticarcinogenic Activities)
Very few animal and cell line studies investigating the chemopreventive potential of capsaicin have been performed. Antioxidant, antiinflammatory, and anticarcinogenic properties of capsaicin were examined in a few in vitro and in vivo studies (mostly in rats) (Lee et al., 2005; Sim and Sil, 2008; Nishino et al., 2009; Srinivasan, 2015).
CONCLUSIONS
Several economic benefits (cost-benefit ratio) were reported with the supplementation of HRP to broiler bird diet, including improved performance, Bwt, WG, FI, FCR, and blood parameters, as well as antimicrobial effect and intestinal histomorphology enhancement, thus resulting in the highest profitability ratio (economic efficiency). However, further studies are warranted to investigate the various effects of HRP supplementation in poultry diets, particularly immunomodulation, antioxidant, and antiinflammatory activities.
ACKNOWLEDGMENTS
Prof. Khaled A. El-Tarabily thanks the library at Murdoch University, Australia, for the valuable online resources and comprehensive databases.
Author contribution: All authors were equally contributed in writing this review article. All authors reviewed and approved the final version of the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
References
- Abd El-Ghany W.A., Shaalan M., Salem H.M. Nanoparticles applications in poultry production: an updated review. Worlds Poult. Sci. J. 2021;77:1–25. [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Abdelnour S. Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter. J. Anim. Physiol. Anim. Nutr. 2018;103:509–517. doi: 10.1111/jpn.13045. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2022 doi: 10.1080/10495398.2021.1883637. In Press. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E.-S., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abdel Salam O.M.E., Heikal O.A., EL-Shenawy S.M. Effect of capsaicin on bile secretion in the rat. Pharmacology. 2005;73:121–128. doi: 10.1159/000081954. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdel-Moneim A.M.E., El-Saadony M.T., Shehata A.M., Saad A.M., Aldhumri S.A., Ouda S.M., Mesalam N.M. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022 doi: 10.1016/j.sjbs.2021.09.046. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnour S., Alagawany M., Abd El-Hack M.E., Mohamed E., Sheiha A.M., Saadeldin I.M., Swelum A.A. Growth, carcass traits, blood hematology, serum metabolites, immunity, and oxidative indices of growing rabbits fed diets supplemented with red or black pepper oils. Animals. 2018;8:168. doi: 10.3390/ani8100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul Aziz A. The effect of the Capsicum annuum in the diet of broilers on the isolation and shedding rate of Salmonella paratyphoid. Kufa J. Vet. Sci. 2010;1:28–38. [Google Scholar]
- Abo N.E.P., Djakalia B., Guichard B.L. Effect of Capsicum annum, Allium sativum and Thymus vulgaris on the zootechnic performance of broiler in growth phase. Glob. J. Adv. Res. 2016;3:1070–1077. [Google Scholar]
- Abou-Elkhair R., Selim S., Hussein E. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018;4:394–400. doi: 10.1016/j.aninu.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Adedoyin A., Mosobalaje M.A., Bamimore A.I. Performance, immuno-stimulatory and blood biochemical indices of broiler chickens fed hot red pepper (Capsicum annuum L.) supplemented diets. J. Exp. Agric. Int. 2019;36:1–8. [Google Scholar]
- Adegoke A.V., Abimbola M.A., Sanwo K.A., Egbeyale L.T., Abiona J.A., Oso A.O., Iposu S.O. Performance and blood biochemistry profile of broiler chickens fed dietary turmeric (Curcuma longa) powder and cayenne pepper (Capsicum frutescens) powders as antioxidants. Vet. Anim. Sci. 2018;6:95–102. doi: 10.1016/j.vas.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolabi K.D., Ndelekwute E.K., Alabi O.M., Olajide R. Hot red pepper (Capsicum annum L.) meal enhanced the immunity, performance and economy of broilers fed in phases. J. Biol. Agric. Healthc. 2017;7:1–7. [Google Scholar]
- Agarwal P., Das C., Dias O., Shanbhag T. Antimicrobial property of capsaicin. Int. Res. J. Biol. Sci. 2017;6:7–11. [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., Qattan S.Y.A., Attia Y.A., El-Saadony M.T., Elnesr S.S., Mahmoud M.A., Madkour M., Abd El-Hack M.E., Reda F.M. Use of chemical nano-selenium as an antibacterial and antifungal agent in quail diets and its effect on growth, carcasses, antioxidant, immunity and caecal microbes. Animals. 2021;11:3027. doi: 10.3390/ani11113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harthi M.A. Performance and carcass characteristics of broiler chicks as affected by different dietary types and levels of herbs and spices as no classical growth promoters. Egypt. Poult. Sci. 2002;22:325–343. [Google Scholar]
- Al-Harthi M.A. Efficacy of vegetable diets with antibiotics and different type of spices or their mixtures on performance, economic efficiency and carcass traits of broilers. J. Agri. Sci. Mansoura Univ. 2002;27:819–831. [Google Scholar]
- Al-Kassie G.A.M., Al-Nasrawi M.A.M., Ajeena S.J. The effects of using hot red pepper as a diet supplement on some performance traits in broiler. Pak. J. Nutr. 2011;10:842–845. [Google Scholar]
- AL-Kassie G.A.M., AL-Nasrawi M.A.M., Ajeena S.J. Use of black pepper (Piper nigrum) as feed additive in broilers diet. Res. Opin. Anim. Vet. Sci. 2011;1:169–173. [Google Scholar]
- Al-Kassie G.A., Butris G.Y., Ajeena S.J. The potency of feed supplemented mixture of hot red pepper and black pepper on the performance and some hematological blood traits in broiler diet. Int. J. Adv. Res. Biol. Sci. 2012;2:53–57. [Google Scholar]
- AL-Kassie G.A., Mohseen A.M., Abd-Aljaleel R.A. Modification of productive performance and physiological aspects of broilers on the addition of a mixture of cumin and turmeric to the diet. Res. Opin. Anim. Vet. Sci. 2011;1:31–34. [Google Scholar]
- An B.K., Im H.J., Kang C.W. Nutritional values of red pepper seed oil meal and effects of its supplementation on performances and physiological responses of broiler chicks. Asian-Aust. J. Anim. Sci. 2007;20:971–997. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Ashour E.A., Farsi R.M., Alaidaroos B.A., Abdel-Moneim A.M.E., El-Saadony M.T., Osman A.O., Abou Sayed-Ahmed E.T., Albaqami N.M., Shafi M.E., Taha A.E., Abd El-Hack M.E. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital. J. Anim. Sci. 2021;20:1357–1372. [Google Scholar]
- Atapattu N.S.B.N., Belpagodagamage U.D. Effect of dietary chilli powder on growth performance and serum cholesterol contents of broiler chicken. Trop. Agr. Res. Ext. 2011;13:106–109. [Google Scholar]
- Azouz H.M.M. Cairo University; Cairo, Egypt: 2001. Effect of hot pepper and fenugreek seeds supplementation on broiler diets. PhD Thesis. [Google Scholar]
- Batiha G.E., Alqahtani A., Ojo O.A., Shaheen H.M., Wasef L., Elzeiny M., Ismail M., Shalaby M., Murata T., Zaragoza-Bastida A., Rivero-Perez N., Beshbishy A.M., Kasozi K.I., Jeandet P., Hetta H.F. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020;21:5179. doi: 10.3390/ijms21155179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton LL. In: Pages 914–924 in The Pharmacological Basis of Therapeutics. 8th ed. Gilman A.G., Rall T.W., Nies A.S., Taylor P, editors. Pergamon Press; New York: 1990. Agents affecting gastrointestinal water flux and motility, digestants, and bile acids. [Google Scholar]
- Cardoso V.S., de Lima C.A.R., de Lima M.E.F., Dorneles L.E.G., Danelli M.G.M. Piperine as a phytogenic additive in broiler diets. Pesq. Agropec. Bras. Brasília. 2012;47:489–496. [Google Scholar]
- Conforti F., Statti G.A., Menichini F. Chemical and biological variability of hot pepper fruits (Capsicum annuum var. acuminatum L.) in relation to maturity stage. Food Chem. 2007;102:1096–1104. [Google Scholar]
- Corduk M., Sarica S., Yarim G.F. Effects of oregano or red pepper essential oil supplementation to diets for broiler chicks with delayed feeding after hatching. 1. performance and microbial population. J. Appl. Poult. Res. 2013;22:738–749. [Google Scholar]
- Cross D.E., Mcdevith R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Crowell P.L. Prevention and therapy of cancer by dietary monoterpenes. J. Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- Dougnon T.J., Kiki P., Dougnon T.V., Youssao I. Evaluation of Capsicum frutescens powder effects on the growth performances, biochemical and hematological parameters in Hubbard broiler. J. App. Pharm. Sci. 2014;4:038–043. [Google Scholar]
- Droge W. Free radicals in the physiological control of cellular function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- El-Amin H.M.S., Mohamed K.A., Mukhtar M.A. Effect of hot red pepper (Capsicum frutescens) on performance abdominal fat and blood serum parameters of broiler. J. Glob. Biosci. 2015;4:2251–2257. [Google Scholar]
- Eldeeb M.A., Metwally M.A., Galal A.E. The impact of botanical extract, capsicum (Capsicum frutescence L.), oil supplementation and their interactions on the productive performance of broiler chicks. Egypt. J. Anim. Prod. 2006;12:243–247. [Google Scholar]
- El-Deek A.A., Al-Harthil M.A., Osman M., Al-Jassas F., Nassar R. Hot pepper (Capsicum annum) as an alternative to oxytetracycline in broiler diets and effects on productive traits, meat quality, immunological responses and plasma lipids. Arch. Geflügelk. 2012;76:73–80. [Google Scholar]
- El-Ghamry A., Azouz H., Elyamny A.T. Effect of hot pepper and fenugreek seed supplementation to low energy diets on Muscovy duckling performance. Egypt. Poult. Sci. J. 2004;24:613–627. [Google Scholar]
- El-Husseiny O., Shalash S.M., Azouz H.M. Response of broiler performance to diets containing hot pepper and/or fenugreek at different metabolizable energy level. Egypt. Poult. Sci. 2002;22:387–406. [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.M., Fouda S.E.E., El-Tahan A.M., Hassan M.A.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany N. Yehia M., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2022 doi: 10.1080/87559129.2021.1944183. In Press. [DOI] [Google Scholar]
- El-Shall N.A., Abd El-Hack M.E., Albaqami N.M., Khafaga A.F., Taha A.E., Swelum A.A., El-Saadony M.T., Salem H.M., El-Tahan A.M., AbuQamar S.F., El-Tarabily K.A., Elbestawy A.R. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 2022;101:101542. doi: 10.1016/j.psj.2021.101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tazi S.M.A. Response of broiler chicken to diets containing different mixture powder levels of red pepper and black pepper as natural feed additive. Ani. Vet. Sci. 2014;2:81–86. [Google Scholar]
- European Union. 2005. Ban on antibiotics as growth promoters in animal feed enters into effect. European Commission. Accessed Nov. 2021. https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687.
- Fattori V., Hohmann M.S., Rossaneis A.C., Pinho-Ribeiro F.A., Verri W.A. Capsaicin: current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules. 2016;21:844. doi: 10.3390/molecules21070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisthovel E., Ludwig O., Simon E. Capsaicin fails to produce disturbances of autonomic heat and cold defense in avian species (Anas platyrhynchos) Pflug. Arch. Eur. J. Phy. 1986;406:343–350. doi: 10.1007/BF00590934. [DOI] [PubMed] [Google Scholar]
- Gheisar M.M., Kim I.H. Phytobiotics in poultry and swine nutrition - a review. Ital. J. Anim. Sci. 2018;17:92–99. [Google Scholar]
- Goncalves F.G., Zanini S.F., Feitosa M.L., Goncalves E.P.M., Colnago G.L. Effect of Brazilian red pepper meal associated with different levels of antibiotics on broilers chickens. Ciencia Rural. 2012;42:1503–1509. [Google Scholar]
- González M., Castano E., Avila E., de Mejıá E.G. Effect of capsaicin from red pepper (Capsicum sp.) on the deposition of carotenoids in egg yolk. J. Sci. Food Agric. 1999;79:1904–1908. [Google Scholar]
- Hayman M., Kam P.C.A. Capsaicin: a review of its pharmacology and clinical applications. Curr. Anaesth. Crit. Care. 2008;19:338–343. [Google Scholar]
- Henkin R. Cooling the burn from hot peppers. JAMA. 1991;266:2766–2770. doi: 10.1001/jama.266.19.2766b. [DOI] [PubMed] [Google Scholar]
- Hernandez F., Madrid J., Garcia V., Orengo J., Megias M.D. Influence of two plant extract on broiler performance digestibility and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Horosova K., Bujnakova D., Kmet V. Effect of oregano essential oil on chicken lactobacilli and E. coli. Folia Microbiol. 2006;51:278–280. doi: 10.1007/BF02931812. [DOI] [PubMed] [Google Scholar]
- Islam M.A., Haque M.E., Shikhauu M.S.A., Uddin M.N., Uddin M.J., Islam M.T., Islam M.S. Effect of red chili and garlic nutrition as feed additives on growth performance of broiler chicken. Int. J. Nat. Soc. Sci. 2018;5:16–24. [Google Scholar]
- Iwai K., Yazawa A., Watanabe T. Roles as metabolic regulators of the non-nutrients, capsaicin and capsiate, supplemented to diets. Proc. Jpn. Acad. 2003;79:207–212. [Google Scholar]
- Izawa K., Amino Y., Kohmura M., Ueda Y., Kuroda M. In: Pages 631-671 in Comprehensive Natural Products II. Liu H.-W., Mander L., editors. Elsevier; Oxford. UK: 2010. Human–environment interactions – taste. [Google Scholar]
- Jancso G., Kiraly E., Jansco-Gabor A. Pharmacologically induced selective degeneration of chemo sensitive primary sensory neurons. Nature. 1997;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Kawada T., Suzuki T., Takahashi M., Iwai K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol. Appl. Pharmacol. 1984;72:449–456. doi: 10.1016/0041-008x(84)90121-2. [DOI] [PubMed] [Google Scholar]
- Kobata K., Todo T., Yazawa S., Iwa K., Watanabe T. Novel capsaicinoid - like substances, capsiate and dihydrocapsiate, from the fruits of a non-pungentcultivar, CH-19 sweet of pepper (Capsicum annuum L.) J. Agric. Food Chem. 1998;46:1695–1697. [Google Scholar]
- Kogure K., Goto S., Nishimura M., Yasumoto M., Abe K., Ohiwa C., Sassa H., Kusumi T., Terada H. Mechanism of potent antiperoxidative effect of capsaicin. Biochim. Biophys. Acta. 2002;10:84–92. doi: 10.1016/s0304-4165(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Kostadinovic L., Levic J. Pages 64–74 in Proceedings of 15th International Feed Technology Symposium. University of Novi Sad, Institute of Food Technology; Novi Sad, Serbia: 2012. Use of phytogenic products for pig and broiler diseases. [Google Scholar]
- Krinsky N.I. Carotenoids as antioxidants. Nutrition. 2001;17:815–817. doi: 10.1016/s0899-9007(01)00651-7. [DOI] [PubMed] [Google Scholar]
- Landers T.F., Cohen B., Wittum T.E., Larson E.L. A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep. 2012;127:4–22. doi: 10.1177/003335491212700103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.J., Crosby K.M., Pike L.M., Yoo K.S., Leskovar D.I. Impact of genetic and environmental variation of development of flavonoids and carotenoids in pepper (Capsicum spp.) Sci. Hortic. 2005;106:341–352. [Google Scholar]
- Lokaewmanee K. Effect of chili leaf powder on laying hen performance, egg quality and egg yolk cholesterol levels. Int. J. Poult. Sci. 2019;18:168–173. [Google Scholar]
- Lokaewmanee K., Yamauchi K., Komori T., Saito K. Enhancement of yolk color in raw and boiled egg yolk with lutein from marigold flower meal and marigold flower extract. J. Poult. Sci. 2011;48:25–32. [Google Scholar]
- Lokaewmanee K., Yamauchi K., Okuda N. Effects of dietary red pepper on egg yolk colour and histological intestinal morphology in laying hens. J. Anim. Physiol. Anim. Nutr. 2013;97:986–995. doi: 10.1111/jpn.12011. [DOI] [PubMed] [Google Scholar]
- Luqman S., Razvi S.I. Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother. Res. 2006;20:303–306. doi: 10.1002/ptr.1861. [DOI] [PubMed] [Google Scholar]
- Madhupriya V., Shamsudeen P., Manohar G.R., Senthilkumar S., Soundarapandiyan V., Moorthy M. Phytofeed additives in poultry nutrition – a review. Int. J. Environ. Sci. Technol. 2018;7:815–822. [Google Scholar]
- Mason J.R., Marunaik J.A. Behavioural and physiological effects of capsaicin in red wing black birds. Pharmacol. Biochem. Behav. 1983;19:857–862. doi: 10.1016/0091-3057(83)90093-x. [DOI] [PubMed] [Google Scholar]
- McElroy A.P., Manning J.G., Jaeger L.A., Taub M., Williams J.D., Hargis B.M. Effect of prolonged administration of dietary capsaicin on broiler growth and Salmonella enteritidis susceptibility. Avian Dis. 1994;38:329–333. [PubMed] [Google Scholar]
- Moradi S., Mousavinia M., Galeh B.A. Effect of use black and red pepper powder as feed additive on performance and some immune parameters of Cobb 500 broiler chicks. CIBTech J. Zool. 2016;5:45–50. [Google Scholar]
- Munglang N., Vidyarthi V.K. Hot red pepper powder supplementation diet of broiler chicken - a review. Int. J. Livest. Res. 2019;7:159–167. [Google Scholar]
- Nakatani N. In: Pages 251–271 in Spices, Herbs and Edible Fungi Developments in Food Science. Charalambous G., editor. Elsevier Science; The Netherlands: 1994. Antioxidative and antimicrobial constituents of herbs and spices. [Google Scholar]
- Nishino H., Murakosh M., Tokuda H., Satomi Y. Cancer prevention by carotenoids. Arch. Biochem. Biophys. 2009;483:165–168. doi: 10.1016/j.abb.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Oboh G., Puntel R.L., Rocha J.B.T. Hot pepper (Capsicum annuum, Tepin and Capsicum Chinese, Habanero) prevents Fe2+-induced lipid peroxidation in brain in vitro. Food Chem. 2007;102:178–185. [Google Scholar]
- Omolo M.A., Wong Z., Mergen A.K., Hastings J.C., Le N.C., Reiland H.A., Case K.A., Baumler D.J. Antimicrobial properties of chili peppers. J. Infect. Dis. Ther. 2014;2:145. [Google Scholar]
- Özer A., Erdost H., Zik B. Histological investigations on the effects of feeding a diet containing red hot pepper on the reproductive organs of the chicken. Photother. Res. 2005;19:501–505. doi: 10.1002/ptr.1690. [DOI] [PubMed] [Google Scholar]
- Özer A., Zik B., Erdost H., Ozfiliz N. Histological investigations on the effects of feeding with diet containing red hot pepper on the reproductive system organs of the cock. Turk. J. Vet. Anim. Sci. 2006;30:7–15. [Google Scholar]
- Paguia H.M., Magpantay D.O., Paguia R.Q. Laying performance of chicken (Gallus domesticus L.) fed diets supplemented with Capsicum frutescens. Pages 44–49 in International Conference on Asia Agriculture and Animal; IACSIT Press, Singapore; Singapore: IACSIT Press; 2011. [Google Scholar]
- Platel K., Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J. Med. Res. 2004;119:167–179. [PubMed] [Google Scholar]
- Prakash U.N.S., Srinivasan K. Beneficial influence of dietary spices on the ultrastructure and fluidity of intestinal brush border in experimental rats. Br. J. Nutr. 2010;104:31–39. doi: 10.1017/S0007114510000334. [DOI] [PubMed] [Google Scholar]
- Puvača N.M. Bioactive compounds in selected hot spices and medicinal plants. J. Agron. Technol. Eng. Manag. 2018;1:8–17. [Google Scholar]
- Puvača N.M., Kostadinovic L.J., Ljubojevic D., Lukac D., Levic J., Popovic S., Novakov N., Vidovic B., Đuragic O. Effect of garlic, black pepper and hot red pepper on productive performances and blood lipid profile of broiler chickens. Eur. Poult. Sci. 2015;79:1–13. [Google Scholar]
- Puvača N.M., Kostadinović L.M., Đuragić O.M., Ljubojević D.B., Miščević B.M., Könyves T.L., Popović S.J., Lević J.D., Nikolova N.B. Influence of herbal drugs in broiler chicken nutrition on primal carcass cuts quality assessements. Food Feed Res. 2016;43:43–49. [Google Scholar]
- Puvača N.M., Ljubojević D., Čabarkapa I., Popović S., Tomičić Z., Nikolova N., Lević J. Quality of broiler chickens carcass fed dietary addition of garlic, black pepper and hot red pepper. J. Agron. Technol. Eng. Manag. 2019;2:218–227. [Google Scholar]
- Puvača N.M., Ljubojevic D., Kostadinovic L.J., Levic L., Nikolova N., Miscevic B., Konyves T., Lukac D., Popovic S. Species and herbs in broiler nutrition: Hot red pepper (Capsicum annuum L.) and its mode of action. Worlds Poult. Sci. J. 2015;71:683–688. [Google Scholar]
- Puvača N.M., Ljubojević D., Lukač D., Kostadinović L.J., Stanaćev V., Popović S., Baloš M.Živkov, Nikolova N. Digestibility of fat in broiler chickens influenced by dietary addition of spice herbs. Maced. J. Ani. Sci. 2014;4:61–67. [Google Scholar]
- Puvača N.M., Pelić D.L., Popović S., Ikonić P., Đuragić O., Peulić T., Lević J. Evaluation of broiler chickens lipid profile influenced by dietary chili pepper addition. J. Agron. Technol. Eng. Manag. 2019;2:318–324. [Google Scholar]
- Puvača N.M., Stanaćev V., Glamočić D., Lević J., Perić L., Stanaćev V., Milić D. Beneficial effects of phytoadditives in broiler nutrition. Worlds Poult. Sci. J. 2013;69:27–34. [Google Scholar]
- Rahimian Y., Kheiri F., Moghaddam M. Effect of using ginger, red and black pepper powder as phytobiotics with Protexin® probiotic on performance, carcass characteristics and some blood biochemical on Japanese quails (Coturnix japonica) Vet. Sci. Dev. 2018;8:4–7. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T.K., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S.P., Pirgozliev V.R., Countney J., Hare S.D. Pages 227–230 in EAAP Publication No. 109. Rostock-Warnemünde; Germany: 2003. Dietary protein source and lysine balance on the efficiency of energy utilization in broiler chickens in Progress in research on energy and protein metabolism. 13-18 September. [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56:3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148 [Google Scholar]
- Salem H.M., Ismael E., Shaalan M. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int. J. Nanomed. 2021;16:6783–6796. doi: 10.2147/IJN.S319708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed M.D, Yaser R., Esfandiar R., Hamzeh M., Mehrdad Y., Abbas D.P. Effect of using ginger, red and black pepper powder as phytobiotics with protexin® probiotic on performance, carcass characteristics and some blood biochemical on Japanese quails. J. Agric. Sci. 2016;6:120–125. [Google Scholar]
- Shahverdi A., Kheiri F., Faghani M., Rahimian Y., Rafiee A. The effect of use red pepper (Capsicum annum L.) and black pepper (Piper nigrum L.) on performance and hematological parameters of broiler chicks. Eur. J. Zool. Res. 2013;2:44–48. [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim K.H., Sil H.Y. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int. J. Food Sci. Technol. 2008;43:1813–1823. [Google Scholar]
- Simon O., Vahjen W., Scharek L. Microorganisms as feed additives-probiotics. Adv. Pork Prod. 2005;16:161–167. [Google Scholar]
- Singletary K. Red pepper: overview of potential health benefits. Nutr. Today. 2011;46:33–47. [Google Scholar]
- Soliman A.Z.M. The bioefficacy of hot red pepper in practical layer diets varying in their energy content. Egypt. Poult. Sci. 2002;22:1047–1062. [Google Scholar]
- Soliman N.K., AlAfifi Sh.F. The productive performance, intestinal bacteria and histomorphology of broiler chicks fed diets containing hot red pepper. Egypt. Poult. Sci. 2020;40:345–357. [Google Scholar]
- Sri Divya, V. E. 2017. Effect of black pepper (Piper nigrum) as natural feed additive on the performance of Japanese quail. MSc. Thesis, Sri Venkateswara Veterinary University, India.
- Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit. Rev. Food Sci. Nutr. 2015;56:1488–1500. doi: 10.1080/10408398.2013.772090. [DOI] [PubMed] [Google Scholar]
- Stanaćev V., glamočić D., Milošević N., Puvača N., Stanaćev V., Plavša N. Effect of garlic (Allium sativum L.) in fattening chicks nutrition. Afr. J. Agric. Res. 2011;6:943–948. [Google Scholar]
- Swelum A.A., Elbestawy A.R., El-Saadony M.T., Hussein E.O., Alhotan R., Suliman G.M., Taha A.E., Ba-Awdah H., El-Tarabily K.A., Abd El-Hack M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: an updated overview. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfeek S.S., Hassanin K.M.A., Youssef I.M.I. The effect of dietary supplementation of some antioxidants on performance, oxidative stress, and blood parameters in broilers under natural summer conditions. J. Worlds Poult. Res. 2014;4:10–19. [Google Scholar]
- Tellez G.I., Jaeger L., Dean C.E., Corrier D.E., Deloach J.R., Williams J.D., Hargis B.M. Effect of prolonged administration of dietary capsaicin on Salmonella enteritidis infection in leghorn chicks. Avian Dis. 1993;37:143–148. [PubMed] [Google Scholar]
- Thiamhirunsopit K., Phisalaphong C., Boonkird S., Kilparkorn S. Effect of chilli meal (Capsicum frutescens LINN.) on growth performance, stress index, lipid peroxidation and ileal nutrient digestibility in broilers reared under high stocking density condition. Anim. Feed Sci. Technol. 2014;192:90–100. [Google Scholar]
- Tollba A.A., Azouz H.M., AbdSamad M.H. Antioxidants supplementation to diet of Egyptian chicken under different environmental condition: 2- The growth during cold winter stress. Egypt Poult. Sci. 2007;27:727–748. [Google Scholar]
- Traesel C.K., Wolkmer P., Schmidt C., Silva C.B., Paim F.C., Rosa A.P., Alves S.H., Santurio J.M., Lopes S.T. Serum biochemical profile and performance of broiler chickens fed diets containing essential oils and pepper. Comp. Clin. Pathol. 2011;20:453–460. [Google Scholar]
- Valiollahi M.R., Rahimian Y., Miri Y., Asgarian F., Rafiee A. Effect of ginger (Zingiber officinale) and black pepper (Piper nigrum L.) powder on performance, hematological parameters and antibody titre in broiler chicks. Res. Opin. Anim. Vet. Sci. 2014;4:128–132. [Google Scholar]
- Viktorija M., Liljana K.G., Tatjana R., Ana C., Rubin G. Antioxidative effect of Capsicum oleoresins compared with pure capsaicin. IOSR J. Pharm. 2014;4:44–48. [Google Scholar]
- Vogt H., Harnisch S., Rauch H.W., Heil G.H. Dried natural spices in broiler rations. Arch. Geflugelk. 1989;53:144–150. [Google Scholar]
- Wadasen M.L.P. Benguet State University; Philippines: 2012. Study on the effect of hot red pepper (Capsicum annuum) supplementation on the growth performance of broilers. MSc. Thesis. [Google Scholar]
- Wahyuni Y., Ballester A.R., Tikunov Y., De Vos R.C., Pelgrom K.T., Maharijaya A., Sudarmonowati E., Bino R.J., Bovy A.G. Metabolomics and molecular marker analysis to explore pepper (Capsicum sp.) biodiversity. Metabolomics. 2013;9:130–144. doi: 10.1007/s11306-012-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Klenholz E.W. The effect of chilli, curry, and black pepper powder in diet for broiler chicks. Poult. Sci. 1974;53:2232–2234. [Google Scholar]
- Yaqoob M.U., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A.F., Batiha G.E., Yehia N., Elnesr S.S., Alagawany M., El-Tarabily K.A., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Doucet E., Drapeau V., Dionne I., Tremblay A. Combined effects of red pepper and caffeine consumption on 24 h energy balance in subjects given free access to foods. Br. J. Nutr. 2001;85:203–211. doi: 10.1079/bjn2000224. [DOI] [PubMed] [Google Scholar]
- Younis M.E., Abdel-Latif M.A. Influence of breed and route of hot pepper supplementation on productive performance, carcass traits and Immune response of two breeds of broilers. Alex. J. Vet. Sci. 2017;52:181–189. [Google Scholar]
- Zeyrek F.Y., Oguz E. In vitro activity of capsaicin against Helicobacter pylori. Ann. Microbiol. 2005;55:125–127. [Google Scholar]
- Zhao Y., Wu Y., Wang M. In: Pages 967–1008 in Handbook of Food Chemistry. Cheung P.C.K., Mehta B.M., editors. Springer Berlin Heidelberg; Germany: 2015. Bioactive substances of plant origin. [Google Scholar]
- Zheng J., Zheng S., Feng Q., Zhang Q., Xiao X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170286. [DOI] [PMC free article] [PubMed] [Google Scholar]