Highlights
-
•
Lung cancer is the most common and deadliest human malignancies.
-
•
The alterations of PI3K/Akt/mTOR pathway are related to lung cancer progression.
-
•
PI3K axis regulates proliferation, apoptosis, metastasis, and EMT of lung cancer.
-
•
Agents inhibiting components of PI3K axis diminish lung tumor growth and invasion.
-
•
Low efficacy and off-target toxicity could be improved by nanoparticle application.
Keywords: Lung cancer, Phosphatidyl inositol 3-kinase, PI3K/Akt/mTOR pathway, Drug resistance, Adverse event, Nanomedicine
Abstract
Lung cancer is the leading cause of cancer-related mortality worldwide. Although the PI3K/Akt/mTOR signaling pathway has recently been considered as one of the most altered molecular pathways in this malignancy, few articles reviewed the task. In this review, we aim to summarize the original data obtained from international research laboratories on the oncogenic alterations in each component of the PI3K/Akt/mTOR pathway in lung cancer. This review also responds to questions on how aberrant activation in this axis contributes to uncontrolled growth, drug resistance, sustained angiogenesis, as well as tissue invasion and metastatic spread. Besides, we provide a special focus on pharmacologic inhibitors of the PI3K/Akt/mTOR axis, either as monotherapy or in a combined-modal strategy, in the context of lung cancer. Despite promising outcomes achieved by using these agents, however, the presence of drug resistance as well as treatment-related adverse events is the other side of the coin. The last section allocates a general overview of the challenges associated with the inhibitors of the PI3K pathway in lung cancer patients. Finally, we comment on the future research aspects, especially in which nano-based drug delivery strategies might increase the efficacy of the therapy in this malignancy.
Graphical abstract
Introduction
Lung cancer with an 11.6% incidence rate of total cancer cases and an 18.4% death rate of total cancer-related mortality is currently the most common and the deadliest malignancy in the whole population of men and women [2]. Lung cancer could be categorized into two main subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) which are different due to histology, prevalence, biological behavior, prognosis, and response to treatment [20]. NSCLC is the predominant type and accounts for 85% of total cases. The NSCLC per se consists of several subtypes including large cell carcinoma (LCC), squamous cell carcinoma (SCC), adenocarcinoma (ADC), and other less-differentiated variants [39]. The most prevalent type of NSCLC is ADC with an approximately 40% rate [148]; also, SCC and LCC have 25–30% and 5–10% incidence rates, respectively [21]. Lung cancer is profoundly affected by smoking which is the most important risk factor related to this malignancy so that smoking cessation is considered the most valuable arm to struggle with this malignancy. There are also other risk factors such as exposure to tobacco smoke, occupational carcinogens, radon, and pre-existing non-malignant lung disease [8].
Apart from environmental parameters, the significance of molecular abnormalities in the development of lung cancer should not be underestimated. No cancer can develop without the presence of molecular abnormalities, and lung cancer is no exception. Thus far, several signaling axes have been accused of being involved in the pathogenesis of this cancer; however, it seems this is the overlap of the oncogenic pathways that contributes to lung cancer fatality. The first evidence of the tight interplay between signaling networks arose from the failure of RAS-targeted therapies. Although RAS proteins are indeed one of the most important molecules in the pathogenesis of lung cancer, their suppression did not provide outstanding outcomes. With this verdict, investigations went on, and the new wave of studies shed light on the importance of the PI3K/Akt signaling pathway not only in the survival of lung cancer cells but also as an intersection for different oncogenic axes [22,35].
The therapeutic strategies for the treatment of lung cancers are categorized into 4 main groups: surgical resection, radiotherapy, chemotherapy, and immunotherapy [21]. However, the major problem with lung cancer is that a very small percentage of patients show symptoms before the disease progresses too much [172]. Thus, therapeutic approaches demonstrate insufficient efficacy to eliminate the disease. At this stage, treatments mainly focus on controlling the disease and maintaining the quality of life [114]. Nevertheless, targeted therapy has shown promising outcomes [47] and could be the candle in the dark atmosphere of lung cancer treatment. Regarding the oncogenic roles of the PI3K axis, evaluating the inhibitors targeting the components of this pathway in order to achieve favorable responses was of interest in the recent decade. Accordingly, several potential inhibitors of the PI3K/Akt/mTOR signaling pathway have been developed in the context of NSCLC [37]. Notwithstanding the promising outcomes of PI3K axis inhibitors, the application of these agents in lung cancer is commonly associated with some challenges such as drug resistance [15] and the presence of toxicities [27] that should be considered to be managed to increase the efficacy of the treatments in lung cancer cases. This review aims to provide a general overview of alterations in the PI3K/Akt/mTOR pathway and discuss the efficacy and challenges related to the administration of relevant inhibitors in lung cancer cases.
A glance at the PI3K/Akt/mTOR pathway
PI3Ks are lipid kinases that are divided into three classes based on their structure and function [88]. Class I that is mainly involved in human tumors [33,150] is a heterodimer consisting of one of the p85 regulating subunits plus one of the p110 catalytic subunits, and is divided into two subsets: Class IA and IB [66,83]. All p85 regulatory subunits (p85α, p85β, p55α, p55γ, p50α) are encoded by the PIKR1 but the p110 catalytic subunits (p110α, p110β, p110δ) are encoded by the PIK3CA, PIK3B, and PIK3, respectively [96]. Class IA PI3Ks are typically activated by RTKs such as EGFR, IGF1-R, and HER2/neu [48]. Class IB consists of one of two regulatory subunits p101 or p87, and isoform p110γ encoded by PIK3CG [96]. The signaling is started by the interaction of a ligand with the tyrosine receptor kinases (RTKs) which either communicate directly with a regulatory subunit of PI3K (p85) through binding to phosphorylated residues of RTK or indirectly by adapter proteins like the protein-1 insulin receptor substrate [34]. This interaction blocks the p85 inhibitory effect on the p110 catalytic subunit, thereby activating PI3K [173]. Activated kinase binds to the plasma membrane and plays a catalytic role in the conversion of phosphatidylinositol (4,5)–bisphosphate (PIP2) to phosphatidylinositol (3,4,5)–trisphosphate (PIP3). Then, PIP3 activates Akt either directly via phosphorylation or indirectly by adsorption of phosphoinositide-dependent protein kinase (PDK1). Besides, mTORC2 has many known functions in human cells that let it regulate Akt activity by residual phosphorylation of serine 473 in C-terminal hydrophobic residue, which together with PDK1-mediated activation ring phosphorylation results in the complete activation of Akt [42]. The activated Akt induces cell survival and cell growth and by various mechanisms [67]. It inhibits the pro-apoptotic Bcl-2 protein family members such as BCL2 associated agonist of cell death (BAD) and BCL-2-like protein 4 (BAX) [119], BAD, MDM2 proto-oncogene, and also NF-κB transcription factor [17], leading to increased expression of cell survival and anti-apoptotic signals [157]. The phosphorylated Akt (pAkt) is able to activate mTORC1, one of the main downstream components of signaling leading to cell growth, metabolism, and protein synthesis [56].
The PTEN gene is responsible for translating an enzyme found in almost every tissue in the body. With lipid phosphatase activity, PTEN converts PIP3 to PIP2 and prevents the regulation of growth factor signal that is modulated by PI3K/Akt [57,61]. This enzyme acts as a tumor suppressor [76] and blocks the signaling of PI3K by inhibiting PIP3-dependent processes such as membrane uptake and Akt activation, thereby preventing cell survival, growth, and proliferation [140]. Therefore, PTEN plays a very important role in inhibiting carcinogenic transformation [146]. Also, a structural role for PTEN is seen in the maintenance of apical-basal polarity in polar epithelial cells by maintaining an apical pool isolated from PIP2 that cleaves proteins that bind PIP2 to the apical membrane [61,134]. Loss of this function may contribute to epithelial-mesenchymal transmission (EMT) and the progression of epithelial cancers. Lung cancer is generally associated with the aberrant expression in the PI3K pathway; so, in the following section, we aim to take a look at the genetic alterations of this pathway. Fig. 1 is depicted to provide a better understanding of the PI3K axis.
Fig. 1.
An overview of the PI3K/Akt/mTOR signaling pathway. The PI3K axis is initiated by the interaction of a ligand with an RTK that results in the separation of p110α (the catalytic subunit) from p85 (the regulatory subunit). An alternative way is the activation of a GPCR. After the initiation step, p110α catalyzes the conversion of PIP2 to PIP3. Upon PIP3 generation, this molecule per se could phosphorylate Akt directly and/or recruit the PDK1. Then, PDK1 and mTORC1 mediate the phosphorylation and activation of Akt together. By activating mTOR and consequently S6K, Akt could stimulate cell growth. This kinase inhibits FOXO1 and activates NF-κB which therefore suppresses the apoptosis process. Moreover, Akt could inhibit the function of p53 indirectly via activating MDM2. On the other hand, PTEN suppresses the PIP3-dependent processes and consequently restricts cell survival, growth, and proliferation.
Oncogenic alterations of the PI3K/Akt/mTOR pathway in lung cancer
One of the most notable mutations in NSCLC is the mutation of RTKs which are the starting point for the activation of the PI3K/Akt/mTOR axis [9]. The other one includes mutations in the KRAS that can activate this pathway in parallel [64]. The importance of the PI3K/Akt /mTOR pathway also extends to its role in tumors with other known activating mutations, such as EGFR. Studies show that the Akt/mTOR pathway is fundamentally activated in 67% of patients with EGFR mutations [75]. Besides, KRAS mutations are usually associated with LKB1 mutations and play an important role in RAS/RAF/MEK signaling in controlling mTOR activation [97]. Accordingly, it has been reported that the treatment of NSCLC cells with MEK and mTOR inhibitors could significantly decrease cancer cell proliferation [78]. Apart from alterations in the upstream molecules of the PI3K/Akt axis that is extensively reviewed previously [9,97,142], oncogenic alterations in each component of this axis may contribute to lung cancer development and progression. A summary of clinical studies in the context of PI3K/Akt/mTOR alterations is provided in Table 1.
Table 1.
A summary of PI3K/Akt/mTOR pathway alteration in the clinical studies.
| Gene | Alteration | Frequency (%) | St. P. | No. | Female (%) | Age* | Stage | Country | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| PIK3CA | Mutation | 10 | EGFR mutated lung cancer | 20 | NA | NA | Stage IV or recurrent | US | The appearance of concurrent mutations such as PIK3CA mutations is common in EGFR mutated lung cancer which could affect the clinical outcomes. | [151] |
| PIK3CA | Mutation | 9 | NSCLC | 89 | 41.6 | 59.4** | I-IV | China | Patients with concomitant mutations such as PIK3CA mutations showed a higher rate of bone metastasis. | [176] |
| PIK3CA | Mutation | 3.9 of SCC, 2.7 of ADC | NSCLC | 1117 | 42.1 | NA | I-IV | China | Patients with PIK3CA mutations exhibited a significantly worse survival. | [158] |
| PIK3CA | Mutation | 9 of SCC | NSCLC | 112 | 38.3 | 63.9** | NA | US | The identification of biomarkers such as PIK3CA alterations may be required for higher treatment effectiveness. | [137] |
| PIK3CA | Mutation | 4.1 | NSCLC | 166 | 45.2 | 60.2 | III and IV | Italy | PIK3CA mutation had a significant correlation with a shorter median time to progression and a worse OS. | [87] |
| PIK3CA | Mutation | 4.2 | NSCLC (SCC) | 95 | 35 | 68 | I-IV | US | Low frequency of PIK3CA mutations and a low association with tumor size, grade of differentiation, and the stage were observed in SCC NSCLC patients. | [117] |
| PIK3CA | Overexpression | 37 of SCC, 5 of ADC | NSCLC | 112 | 38.3 | 63.9** | NA | US | The identification of biomarkers such as PIK3CA alterations may be required for higher treatment effectiveness. | [137] |
| PIK3CA | Overexpression | NA | NSCLC | 107 | NA | 64 | III-IV | Italy | Overactivation of PI3K pathway is associated with high grade and more advanced disease. | [124] |
| PIK3CA | Increased copy number | 29.2 | NSCLC | 445 | 17.2 | NA | I-IV | Italy | Increased copy number of PIK3CA, SOX2, FGFR1, and BRF2 is likely to occur concurrently. | [147] |
| PIK3CA | Mutation | 11.4 | NSCLC (SCC) | 308 | 33.1 | NA | I-III | Norway | PIK3CA mutations were associated with a significantly longer overall survival and time to relapse. | [98] |
| AKT1 | Overexpression | 18 | NSCLC | 107 | NA | 64 | III-IV | Italy | Overactivation of AKT pathway in NSCLC patients is associated with high grade and more advanced disease. | [124] |
| AKT1 | Mutation | 1.1 | NSCLC (SCC) | 95 | 35 | 68 | I-IV | US | Low frequency of AKT1 mutations and a low association with tumor size, grade of differentiation, and the stage were observed in SCC NSCLC patients | [117] |
| AKT1 | Mutation | 0.47 | NSCLC | 209 | NA | NA | NA | Australia | Akt1 E17K mutation had a very low frequency in NSCLC; however, it could be restricted to SCC with a higher incidence rate. | [25] |
| AKT1 | Mutation | 1.9 | NSCLC | 105 | NA | 68** | NA | Italy | Despite the low frequency of AKT1 mutations in lung cancer the oncogenic properties of E17K-AKT1 may contribute to the development of a subset of SCC | [91] |
| AKT2 | Overexpression | 22 | NSCLC | 107 | NA | 64 | III-IV | Italy | Overactivation of AKT pathway in NSCLC patients is associated with high grade and more advanced disease. | [124] |
| PTEN | Mutation | 5 | EGFR mutated lung cancer | 20 | NA | NA | Stage IV or recurrent | US | The appearance of concurrent mutations such as PTEN mutations is common in EGFR mutated lung cancer which could affect the clinical outcomes. | [151] |
| PTEN | Loss of expression | 39 | NSCLC | 107 | NA | 64 | III-IV | Italy | Loss of PTEN was more commonly reported in SCC than in ADC patients. | [124] |
| PTEN | Loss of expression | 44 | NSCLC | 117 | 42.7 | 70 | I-IV | US | The loss of PTEN was a frequent event in NSCLC cases and thus, this alteration can be a favorable prognostic marker. | [93] |
| PTEN | Loss of expression | 21 of SCC, 4 of ADC | NSCLC | 112 | 38.3 | 63.9** | NA | US | The identification of biomarkers such as loss of PTEN alterations may be required for higher treatment effectiveness. | [137] |
NSCLC: Non-small cell lung cancer; SCC: Squamous cell carcinoma; ADC: Adenocarcinoma; St. P.: Study population; No.: Number of patients; NA: Not available; *: Median (year); **: Mean (year).
PIK3CA
Mutations or increases in the number of PIK3CA (encoding PI3K major catalytic subunit) copies have been observed in patients with lung cancer. It has been shown that an increased number of copies is more common in men, smokers, and patients with SCC, as well as in the early stages of the disease [63]. The mutations in PIK3CA have been also found in advanced NSCLC cases which is responsible for a lower survival rate [74]. Indeed, according to a systematic review and meta-analysis study, the mutated PIK3CA gene is associated with poor overall survival (OS) and progression-free survival (PFS) of NSCLC cases. Also, the mutations in PIK3CA have an association with lymph node metastasis in NSCLC cases [159]. It was shown that PIK3CA mutation has occurred in 4.1% of patients with advanced NSCLC and had a significant correlation with shorter median OS and time to progression. Notably, the authors demonstrated that PIK3CA mutation could be considered an independent prognostic factor for worse OS in NSCLC patients [87,158]. Similarly, the prevalence of elevated PIK3CA copy numbers was determined 29.2% in NSCLC patients [147]. In a study of SCC, PIK3CA mutation was determined in 11.4% of cases, particularly in exon 20. Controversially, the authors demonstrated that the mutations in PIK3CA were associated with a good prognosis and longer OS [98]. However, the majority of studies, as we have reviewed, claimed that PIK3CA mutations have notable roles in the poor prognosis of lung cancer and reduction of survival.
PIK3R1
PIK3R1 is another component of the PI3K pathway that encodes the regulatory subunit (p85). Its mutations are less common than the PIK3CA mutation or the loss of PTEN function in lung cancer [145]. Many PIK3R1 mutations are curtailment or deletion in the SH-2 (iSH2) of the p85 domain that interacts with the C2 domain of p110. This interaction may be disrupted by mutations in p85 that can prevent the p85-dependent inhibition of p110 [54]. The alteration of PIK3R1 was observed in H358 NSCLC cell lines that harbor a KRAS mutation [111]. Similarly, it was shown that PIK3R1 was significantly down-regulated in the tissues of lung ADC cases compared with healthy cases in a Chinese population [164].
Akt
The Akt genes could be altered in lung cancers either in the form of activating mutations or elevated expression of Akt isoforms [108]. The overactivated Akt is often associated with lymph node metastasis and advanced stages of lung cancer [124,143]. With this regard, survival is poor in NCSLC patients with an overactivated Akt profile [79,143]. It was exhibited that 51% of patients with NSCLC had overactivated Akt according to immunohistochemistry analysis [4]. Notably, Akt overactivation in human NSCLC cells might be attributed to induction of activating mutations in EGFR, PIK3CA, and also loss of PTEN [43]. Apart from these alterations, the mutations of Akt1 were observed in a study of NSCLC in which 1 specimen showed E17K mutation out of a total of 219 samples [25]. Similarly, another study of resected NSCLC specimen showed that 2 out of 105 SCC samples had E17K mutation of Akt1 [91]; highlighting the fact that the overactivation of Akt in lung cancer cases is mainly due to alterations in the upstream or regulatory components of the PI3K axis rather than intrinsic aberrancies.
mTOR
The mammalian target of rapamycin (mTOR) is a fully evolutionary serine/threonine kinase belonging to the PI3K kinase family [144]. The PI3K/Akt and the mTOR axes are interconnected in such a way that they can be considered as a single pathway. The onset and development of tumors are significantly associated with the overactivity of this kinase. Accordingly, the modulation of mTOR activity is seen in several types of cancers including lung cancer [167]. It was shown that mTOR activity is regulated by increased PI3K or Akt activity in lung cancer [97]. Besides, overexpression of the downstream of mTOR such as 4E-BP1, S6K, and eIF4E is associated with a poor prognosis in lung cancer [174]. Changes in cancer cell metabolism and intracellular nutrient levels can also contribute to the sustained activation of mTORC1 [30]. Conversely, those changes are regulated by mTOR signaling. Increased mTOR activity affects protein synthesis and increases cell proliferation [102,130]. It also indirectly supports tumor growth through its anti-autophagic activity [81] and increasing the translation of HIF1A causes angiogenesis and oxygenation [26].
PTEN
As mentioned, PTEN is a natural inhibitor for the PI3K/Akt pathway and limits the ability of Akt to bind to membranes by dephosphorylation of PIP3 to PIP2, thereby reducing its activity [86]. The most common mutation in NSCLC is the reduction or elimination of PTEN expression [1]. About 45% of NSCLC cases show loss of PTEN and 29% a decrease in protein expression [73]. In lung cancer, different mechanisms are involved in the regulation of this phosphatase. These include genetic mechanisms that are responsible for the dysfunction of PTEN peptides, epigenetic mechanisms at the transcriptional level and also at the post-transcriptional level [38]. In the oncogenesis of lung cancer, there is evidence of PTEN regulation of apical junction complexes. Smoking (possibly due to a specific immune-mediated mechanism) may reduce the regulation of PTEN expression and thus increase the activation of Akt/mTOR signaling in the respiratory tract epithelium of smokers compared with non-smokers [129,162].
Taken together, all components of the PI3K/Akt/mTOR axis, from the beginning to the end, could undergo some kinds of alterations in lung cancer. Regarding the inevitable roles of this axis in several cellular events such as differentiation, proliferation, growth, survival, intracellular trafficking, and motility, those alterations can consequently induce oncogenic functions and result in lung cancer development. Among PIK3CA, PIK3R1, Akt, mTOR, and PTEN which underwent alterations, loss of PTEN is the main frequent alteration in lung cancers; further highlighting the therapeutic strategies to modulate PTEN deficiency in lung cancer patients.
Cell processes regulated by the PI3K/Akt/mTOR pathway in lung cancer
The PI3K/Akt/mTOR pathway is strongly involved in both tumorigenesis and disease progression in NSCLC [80]. A variety of cellular processes such as survival, proliferation, migration, metastasis, angiogenesis, cellular metabolism, cellular senescence, genomic integrity, and stem cell self-renewal are regulated by the PI3K/Akt/mTOR pathway in lung cancer [84]. As a result, any change in the components of this axis may contribute to lung cancer progression and promotes tumor metastasis. A summary of PI3K/Akt/mTOR oncogenic roles is described in Fig. 2.
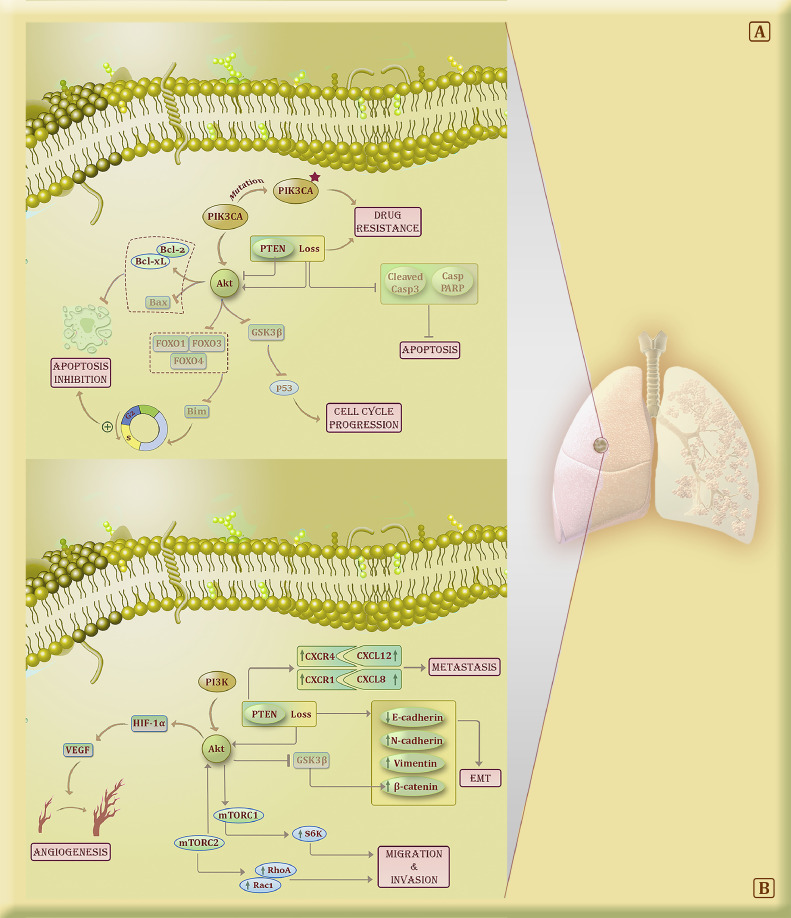
Fig. 2.
The role of PI3K/Akt/mTOR signaling pathway in lung cancer cells. (A) The blockade of FOXO1/3/4 by Akt could lead to the accelerated transition from the G1 phase to S and the inhibition of apoptosis. Also, the inhibition of Akt not only can induce the up-regulation of BAX and down-regulation of Bcl-2/xL but also lead to the lower phosphorylation of GSK3β and the regulation of the lung cancer cell cycle. The loss of PTEN and PIK3CA mutations are the most important alteration related to drug resistance, particularly anti-EGFR treatments in lung cancer cells. Indeed, these alterations could lead to Akt overactivation and consequently, the lung tumor cells become immortal. B) The loss of PTEN up-regulates CXCR4/CXCL12 and CXCR1/CXCL8 and promotes the metastasis process in lung tumor cells. Also, the loss of PTEN and the AKT/GSK3β/β-catenin activation in lung cancer cells can reduce E-cadherin and elevate N-cadherin and vimentin expression which account for the induction of EMT. The blockade of mTORC1/2 via treatments could diminish the activation of S6K, RhoA, and Rac1 which reduces the ability of migration and invasion of lung tumor cells. The stimulation of Akt could up-regulate HIF-1α and consequently VEGF which ultimately cause the induction of the angiogenesis process.
Proliferation, apoptosis, and acquisition of chemoresistant phenotype
It was demonstrated that Akt is involved in apoptosis, proliferation, transcription, and migration. This kinase has a pivotal role in disrupting the inhibition process of cell cycle progression by hindering FOXO1, FOXO3, and FOXO4 which could promote the transition from the G1 phase to S [12]. A lung cancer study showed that cisplatin remarkably elevated the mRNA and protein levels of FOXO3a and moderately the mRNA of FOXO1 in A549 and H460 cell lines. Besides, they showed that Bim, a target of FOXO3a, was up-regulated after cisplatin treatment. Thus, they showed that apoptosis could be induced through blocking PI3K and Akt and up-regulating FOXOs [82]. It was shown that Osthole, an anti-proliferative agent, induced apoptosis in A549 human lung ADC cell lines which are derived by down-regulation of Akt signaling [169]. Similarly, the effects of Bufalin, a toxic steroid, were evaluated in A549 human lung ADC cell lines. They demonstrated that Bufalin is able to modulate the apoptotic proteins by inhibiting the activation of Akt. This phenomenon consequently led to the up-regulation of BAX and down-regulation of Bcl-2 [178]. It was shown in a lung cancer cell line study that the Akt phosphorylation level was dramatically reduced following resveratrol treatment which also decreased the phosphorylation level of GSK3β expression level and increase lung cancer cell apoptosis [77]. The effect of Akt on the apoptosis process in lung tumors was evaluated in a study of human lung cancer cell lines in which a miR-21 inhibitor promoted apoptosis via blocking the expression of Akt. This treatment resulted in a reduction of Bcl-2 expression and an increase in BAX expression [177]. In lung cancer cell lines (A549 and H358) PTEN was shown as a key molecule in the induction of miR-92a oncogenic roles. The inhibition of miR-92a resulted in an elevation of cleaved-caspase-3, caspase-PARP, and BAX as pro-apoptotic proteins [85].
It has been indicated that both PIK3CA mutations and PTEN loss play important roles in conferring resistance to anti-EGFR therapies in lung cancers [105]. In a study of lung cancer cell lines, the presence of PIK3CA mutation was the main cause of gefitinib resistance [29]. Likewise, evaluating the patients with lung cancer who had resistance to EGFR-inhibition demonstrated that 5% of those patients had PIK3CA mutations [127]. Besides, it was shown that PTEN loss induces resistance to erlotinib (an anti-EGFR agent) in EGFR-mutant lung cancer [136]. miR-1269b in lung cancer could inhibit PTEN and induce resistance to cisplatin [171]. In a study of gefitinib-resistant lung cancer cell line (PC-9), the loss of PTEN was associated with elevated Akt phosphorylation [170]. Akt can activate NF-κB and adjust the expression of Akt1 leading to drug resistance of NSCLC cells to chemotherapeutic agents [65].
Metastasis, epithelial to mesenchymal transition (EMT), and angiogenesis
PTEN might promote the invasion of tumors by regulating the tumor microenvironment and affecting the remodeling process of the extracellular matrix [18]. Loss of PTEN leads to ETS2 activation which is the trigger of matrix metalloproteinase 9 (MMP9) and CCL3 activation that consequently affects extracellular matrix remodeling and induces metastasis [11,154]. Lung tumors are not excluded and the alterations in PTEN could aggravate the invasion properties of cancer [106]. It was shown that TOPK, a MAPK-like serine/threonine kinase, facilitates the metastasis of lung cancer in a PTEN-dependent manner by inhibiting PTEN and over-activating Akt [131]. A study evaluated the PTNE knockout in lung cancer cell lines and tumor models. They showed that PTEN loss could increase the expression of CXCR4/CXCL12 and CXCR1/CXCL8 which are related to metastasis induction in tumors [110]. These studies highlighted the role of the PI3K axis and PTEN in the induction of lung cancer metastasis.
The PI3K/Akt/mTOR signaling pathway activates a range of downstream molecules which could down-regulate epithelial proteins such as E-cadherin and up-regulate mesenchymal proteins like N-cadherin and vimentin. These changes are account for EMT induction [168]. Accordingly, it was shown that overexpression of miR-92a in lung cancer cell lines could decrease E-cadherin expression and elevate the expression of N-cadherin, vimentin, and β-catenin through PTEN inhibition [85]. It was shown that metastasis-associated protein 1 (MTA1) overexpression in lung cancer cell lines could induce EMT by reducing the expression of E-cadherin and increasing vimentin levels by activating the signaling of AKT/GSK3β/β-catenin pathway [90]. In A549 human lung ADC, it was demonstrated that TGF-β treatment reduced the expression of E-cadherin while increased the expression of vimentin and fibronectin. Interestingly, when cells were treated with LY294002 (a PI3K inhibitor) before TGF-β treatment, the expression of vimentin and fibronectin was suppressed, and consequently, the EMT is prevented [14]. Another study showed that ginkgolic acid could inhibit the EMT process in A549 and H1299 lung cancer cell lines by suppressing the TGF-β-induced activation of PI3K and Akt [3]. Interestingly, TGF-β has been reported as a vital cytokine to activate the mTOR signaling pathway and thereby an inducer of EMT. In this vein, the inhibition of mTORC1 and mTORC2 could significantly reduce the migration and invasion of lung cancer cells by decreasing the activation of RhoA and Rac1 signaling [132].
The angiogenesis is regulated by several factors downstream of PI3K/Akt such as mTOR, NO synthase, FOXO3, and GSK3 [166]. They could regulate HIF-1α expression, which induces the transcriptional activation of vascular endothelial growth factor (VEGF) which is a potent stimulus in angiogenesis [175]. Therefore, increased activity of the PI3K/Akt pathway could lead to lung tumor development with angiogenic properties [149]. In an experimental mouse model of A549 lung ADC, curcumin was shown to have an inhibitory role in the hepatocyte growth factor (HGF)-stimulated tumor growth. Curcumin could elevate E-cadherin and decrease vimentin, CD34, and VEGF, thus, it prevented EMT and angiogenesis. They reported that curcumin carried out this event by targeting c-Met and inhibiting the c-Met/PI3K/Akt/mTOR/S6 axis [58]. Another study showed that the inhibition of the PI3K/Akt/mTOR axis using specific siRNAs resulted in a reduction of VEGF expression which led to attenuated angiogenesis in A549 lung ADC [16]. It was shown that wortmannin, a specific Akt inhibitor, resulted in a significant reduction of HIF-1α and CD34 expression and the inhibition of angiogenesis and tumor growth in lung cancer tumor models [156]. Similarly, the activity of Akt was demonstrated to be elevated following the up-regulation of RBP2 in NSCLC cell lines. The increased activation of Akt resulted in an elevation of HIF-1α and VEGF expression that accounts for angiogenesis induction [115].
The PI3K/Akt/mTOR pathway inhibitors in lung cancer
Regarding the outstanding role of the PI3K/Akt/mTOR pathway in regulating cell growth in NSCLC, the application of inhibitors that target principal molecules of this pathway seems to be an of-interest area in the context of lung cancer targeted therapy. It is worth noting that the various genetic statuses and the cross-talk of the PI3K/Akt/mTOR pathway in NSCLC might impede the optimal performance of the therapy [138]. Therefore, monotherapy based on the genetic status and/or combination therapy with other inhibitors could be beneficial to overcome the therapeutic resistance.
PI3K inhibitors
The inhibitors of PI3K are generally categorized into the pan- and isoform-selective PI3K inhibitors. It was demonstrated that the inhibition of some major components of the PI3K pathway could suppress cancer growth [24]. The first-generation of PI3K inhibitors like LY294002 and wortmannin showed anti-cancer activities in pre-clinical studies, but their clinical usage was more limited due to lack of selectivity and weak solubility and instability [165]. Nevertheless, their development is the foundation of attaining new clinically applicable PI3K inhibitors.
GDC-0941
GDC-0941 is an oral reversible pan class I PI3K inhibitor which was effective in NSCLC cell lines with RTK activation, PIK3CA mutation, and PTEN loss [137]. The therapeutic effects of this agent in combination with erlotinib (an EGFR inhibitor) or ERK pathway inhibitors were greater in the NSCLC cell line as compared to its effect alone [179]. Also, the efficacy of GDC-0941 plus carboplatin and paclitaxel (with or without bevacizumab) or plus cisplatin, pemetrexed, and bevacizumab was evaluated in a Phase Ib trial in advanced NSCLC patients. It was shown that about 45% of patients showed partial responses to the treatment [7].
BKM120
BKM120 is also one of the pan class I PI3K inhibitors which is effective in the most prevalent PIK3CA mutations (H1047R and E545K) [153]. A Phase I dose-escalation trial assessed the maximum-tolerated dose (MTD), safety, and efficacy of BKM120 in advanced solid tumors (colorectal, breast, lung, and others). It was shown that BKM120 was effective, but the incidence of grade 3 and 4 treatment-related adverse events (AEs) was 63% in all study cases. Notably, the majority of AEs were observed at dose levels of more than 100 mg [6].
SAR245408 (XL147)
SAR245408 (XL147) is a strong ATP competitive oral inhibitor of the class I PI3K family. SAR245408 could significantly inhibit the PI3K tumor pathway in the mouse model of A549 ADC (active KRAS expression and LKB1deficiency) when administered orally. Also, it leads to significant inhibition of tumor growth and shrinkage in models of lung cancer [139]. In a dose-escalation Phase 1 trial of patients with refractory advanced solid malignancies (34% had NSCLC), 13% of patients were reported to show grade 3 and 4 treatment-related AEs. Among all patients, 8 including NSCLC, prostate, and head and neck cancer cases showed PFS for 6 months. The partial response was observed in 1 NSCLC patient [128]. SAR245408 in combination with erlotinib was also examined in another Phase 1 dose-escalation trial in patients who had NSCLC. The MTD was demonstrated to be 400 mg for SAR245408 and 150 mg for erlotinib. The analysis revealed that only 3.7% of patients had a partial response while 51.9% had stable disease. Therefore, the application of this agent in combination with erlotinib didn't show suitable clinical activity in NSCLC cases [135].
PX-866
PX-866 is another pan class I PI3K inhibitor that binds PI3K irreversibly. In a study of A549 NSCLC tumor xenografts, PX-866 suppress the tumor growth and the anti-tumor activities were elevated when it was combined with cisplatin [53]. A Phase 1 trial evaluated PX-866 as a single agent in patients with advanced solid tumors. It was demonstrated that the disease was stabilized in about 22.5% of patients include NSCLC (3.2%). The MTD was determined 12 mg and PX-866 was well-tolerated [59]. Another Phase 2 trial assessed docetaxel with or without PX-866 in advanced NSCLC patients who had a history of receiving systemic treatment regimens. The analysis demonstrated that the differences of PFS, OS, and objective response rates (ORR) were not significant (p-value = 0.65, 0.9, and 0.4, respectively). The prevalence of grade 3 or higher treatment-related AEs was higher in combination therapy compared with monotherapy. Taken together, PX-866 didn't improve the therapeutic efficacy of docetaxel in NSCLC patients.
GSK-2,636,771
GSK2636771 is an orally bioavailable and selective PI3Kβ inhibitor. The outcomes of this agent were evaluated in a first-time in-human study in patients with advanced solid tumors who had PTEN-deficient or PIK3CB alteration. Data showed that GSK2636771 at 400 mg could inhibit the targets sufficiently with a favorable safety profile. Besides, one patient with NSCLC who received GSK2636771 remained on therapy and free of progression for 33 weeks [95].
Akt inhibitors
Akt inhibitors are a broad-spectrum agent that targets the serine/threonine kinase Akt by competing for the ATP-binding site or functioning elsewhere within the protein [68]. Although Akt1-activating mutations are low in NSCLC cases, NSCLC is a sensitive malignancy for Akt inhibitors because of the occurrence of Akt1 and Akt2 overexpression [37].
Perifosine (KRX-0401)
Perifosine is an alkylphosphocholine phospholipid derivative [49]. It is able to induce NSCLC cell death by suppressing Akt and elevating the expression of TRAIL receptors [28]. Furthermore, it was demonstrated that perifosine could block the mTOR axis in human lung cancer by a different method compared with conventional mTOR inhibitors such as rapamycin [36]. A Phase 1 trial of perifosine plus radiation therapy was conducted in patients with advanced solid tumors (81% of patients had NSCLC). It was demonstrated that perifosine plus fractionated radiotherapy was well-tolerated and the toxicity of bone marrow was not observed. Also, the recommended daily dose of perifosine was recommended for the Phase 2 trial at 150 mg, 1 week prior to radiotherapy [152].
MK-2206
MK-2206 is a first-in-class and powerful allosteric inhibitor against all Akt isoforms. It was shown to inhibit tumor cell proliferation in 28 NSCLC cell lines [37]. Furthermore, the suppressive role of MK-2206 on tumor cell growth was enhanced in combination with AZD6244 (a MEK1/2 inhibitor) in NSCLC cell lines [99]. The combination of MK-2206 with erlotinib synergistically suppressed the proliferation of both erlotinib-resistant and erlotinib-sensitive NSCLC cell lines [50]. Accordingly, a Phase 2 trial of erlotinib plus MK-2206 was carried out in metastatic NSCLC patients who were previously treated with erlotinib. Among all patients, 51.2% showed at least one grade 3 treatment-related AEs. Overall, it was demonstrated that Akt pathway inhibition is a feasible therapeutic approach [72]. A Phase 1 trial evaluated the therapeutic outcomes of MK-2206 plus carboplatin/paclitaxel, erlotinib, or docetaxel in patients who had advanced solid tumors (18% of patients had NSCLC). They exhibited that MK-2206 plus the anticancer agents were well-tolerated, with a low rate of grade 3 or 4 AEs. Among all NSCLC patients, only one who received MK-2206 and docetaxel showed a confirmed PR. Taken together, MK-2206 plus the anticancer agents demonstrated early evidence of antitumor activity in patients with advanced NSCLC [101].
AZD5363
AZD5363 is a pan-Akt inhibitor that blocks all isoforms of Akt and inactivates the process of Akt substrates phosphorylation in different cell lines [113]. AZD5363 was evaluated as a single agent in 41 out of 182 tumor cell lines including NSCLC in which it could inhibit the proliferation of tumor cells in vitro and had an antitumor effect in xenografts. The mutations of PIK3CA and/or PTEN in the tumor may increase susceptibility to AZD5363, but RAS mutations may cause AZD5363 resistance [5,41].
mTOR inhibitors
The inhibitors which target mTORC1 are the most developed class of PI3K/Akt/mTOR pathway inhibitors [107]. Rapamycin was the first discovered mTOR inhibitor [37]. Temsirolimus, everolimus, and ridaforolimus which are rapamycin analogs only affect mTORC1 but not mTORC2. These inhibitors of mTORC1 could potentially activate the pathways of Akt and/or ERK as a compensatory mechanism; hence, their combination with MEK inhibitors and PI3K inhibitors could have a wider targeting range and increase anticancer susceptibility [62,139].
Rapamycin
Rapamycin inhibits mTORC1 allosterically by blocking a conserved domain that is unique to mTOR and makes rapamycin act more specifically [123]. In studies of NSCLC, rapamycin shows anti-tumor activities by inhibiting G1 cell cycle progression [10], and inducing apoptosis via the activation of p53-independent pathways [100] or by inhibiting the survivin expression mediated by HIF under hypoxic conditions [13]. Interestingly, by its ability to induce autophagy, this agent bypasses the resistance of NSCLC tumor cells to the EGFR TKI erlotinib [40]. A Phase 1 trial evaluated rapamycin in combination with sunitinib (a multi-targeted RTK inhibitor) in patients with advanced-stage IIIB (with pleural effusion) or IV NSCLC. Among 90 patients who received treatment, no objective responses were observed, and only 6 showed stable disease as the best response. The MTD was determined to be 25 mg daily for sunitinib and 2 mg daily (4 weeks on while 2 weeks off) for rapamycin. Overall, rapamycin in combination with sunitinib was well-tolerated, safe, and feasible with no notable anti-tumor outcome in patients with advanced NSCLC [161].
Temsirolimus
Temsirolimus inhibited the proliferation of NSCLC cell lines (A549, H1299, and H358) was in a dose-dependent manner. Besides, it significantly reduced the growth of the NSCLC mouse model and improved the survival rate in a pleural disseminated tumor-bearing mouse model [104]. In a Phase 2 trial, the outcome of temsirolimus as a single agent was assessed in previously untreated patients with stage IV NSCLC. Among 52 evaluable patients, 4 achieved a confirmed partial response and 14 showed stable disease for 8 weeks or more. Also, the median OS was 6.6 months and the median PFS was 2.3 months. The incidence of grade 3 and 4 AEs was 64% and 23%, respectively. Nevertheless, data showed that the application of temsirolimus as a single agent for the treatment of NSCLC was not optimal enough which might be due to various intracellular pathways and several genetic alterations presented in NSCLC cases [118]. A Phase 1 trial evaluated temsirolimus plus pemetrexed in patients with advanced non-squamous NSCLC. Among only 4 available patients for assessing anti-tumor activities, the best response was observed in 2 patients who had stable disease of 12 weeks and 1 patient who showed stable disease for 4 weeks. Also, 1 patient demonstrated disease progression. Indeed, no objective responses were reported which could be due to the low number of patients in this trial [160].
Everolimus
Everolimus has been examined in several clinical trials in the context of NSCLC. However, it could not generally induce optimal clinical outcomes in monotherapy. Thus, studies have investigated the combination therapy of this agent plus chemotherapy and targeted agents [107]. A Phase 1 trial evaluated the clinical outcomes of everolimus in combination with docetaxel in patients with advanced NSCLC that was progressed following at least one prior chemotherapy. Notably, the combination of everolimus with docetaxel demonstrated promising clinical outcomes with an acceptable safety profile [116]. In another Phase 1 trial, the clinical outcomes of everolimus plus erlotinib were assessed in patients with advanced NSCLC. The combination therapy showed disease control in 47% of patients in which 1% achieved a complete response, 9% achieved a partial response, and 37% achieved stable disease as the best overall response. It could be concluded that the combination of everolimus and erlotinib was well-tolerated with an acceptable disease control ratio in NSCLC cases [109]. In a recent Phase 1 trial in patients with advanced solid tumors (51.6% of patients had NSCLC and KRAS mutations), everolimus plus sorafenib (a multikinase inhibitor) was evaluated for clinical outcomes [103]. Out of 30 patients who received the treatment, 5 achieved partial metabolic response (4 of them were KRAS-mutated), 23 demonstrated stable metabolic disease while 1 had the progressive one. Besides, the median PFS and OS were 3.25 and 5.85 months, respectively. Overall, they showed that everolimus plus sorafenib had acceptable tolerability while they were not able to induce durable responses in KRAS mutant patients with solid tumors including NSCLC [103].
Ridaforolimus
Ridaforolimus inhibits mTOR-dependent activity with a simultaneous effect on cell growth of various tumors including NSCLC in vitro and induced a potent antitumor function in NSCLC xenografts [120,125]. Accordingly, ridaforolimus could inhibit the proliferation of NSCLC cell lines include KRAS-mutant cells [107]. Its combination with erlotinib showed benefits over monotherapy. Ridaforolimus could also suppress the growth of erlotinib-resistant KRAS-mutant NSCLC xenograft models [44]. A Phase I study of ridaforolimus in combination with cetuximab was conducted to assess the toxicity of this combination in advanced NSCLC patients (58.3%), head and neck, and colorectal cancer patients who progressed after at least 1 prior regimen for metastatic disease. Notably, the treatment prolonged the time of stable disease and showed clinical responses in NSCLC cases. The authors indicated that the combination of ridaforolimus and cetuximab in heavily pretreated patients with NSCLC had some promising responses [141].
Dual PI3K/mTORs inhibitors
Regarding the high sequence homology of the mTOR hinge region with PI3K, some second-generation mTOR inhibitors __which termed dual PI3K/mTOR inhibitors__ could hinder PI3K and mTORC1/2, reduce the activation of Akt and lead to more effective inhibition of the signaling pathway compared with the inhibitors of mTOR [92]. Besides, dual PI3K/mTOR inhibitors are more efficient and safer than PI3K inhibitors plus mTOR inhibitors, leading to fewer AEs and reduced feedback loop activation by the feedback loop [23].
NVP-BEZ235
NVP-BEZ235 is an oral imidazoquinolines compound [37]. There is increasing evidence that elucidates the advantages of NVP-BEZ235 application in combination with other strategies to improve its cytotoxic effects [37]. Accordingly, a study demonstrated the impressive role of NVP-BEZ235 in combination with selective blockades of STAT3 signaling in sensitizing NSCLC cell lines to apoptotic cell death, by inducing CHOP (a pro-apoptotic transcription factor) and its target Bim [60]. A study reported that NVP-BEZ235 could remarkably sensitize NSCLC xenografts with oncogenic KRAS to the pro-apoptotic effects of ionizing radiation; however, it could not sufficiently induce apoptosis [45,122]. Although NVP-BEZ235 blocks the PI3K and mTOR, the application of mTOR inhibitors (rapamycin or everolimus) and NVP-BEZ235 produced a synergistic antitumor effect against human lung cancer cells and xenografts [163]. A Phase 1 dose-escalation trial evaluated a special NVP-BEZ235 (NVP-BEZ235 SDS sachet) in patients with advanced solid tumors (3 out of 25 was NSCLC). Overall, they showed that the NVP-BEZ235 SDS sachet exhibited acceptable tolerability with a favorable safety profile [112].
GDC-0980
GDC-0980 is another dual inhibitor of class-I PI3K and mTOR which has been assessed in clinical trials of patients with advanced solid tumors [107]. In a preclinical study of GDC-0980, its anti-tumor activity was investigated in solid tumor cancer cell lines. The evaluation of GDC-0980 activities showed that it had the greatest effectiveness in the prostate, breast, and NSCLC lines. Indeed, GDC-0980 showed broad anti-tumor functions with favorable responses in 124 out of the 167 cell lines. Besides, they demonstrated that GDC-0980 was more effective than GDC-0941 in cell viability experiments [155].
XL765 (SAR245409)
XL765 is another dual inhibitor of class-I PI3K and mTOR. It could delay the growth of the tumor or cause the shrinkage of the tumor in lung xenograft models. Also, it was demonstrated that XL765 was able to suppress angiogenesis and proliferation and induce apoptosis in an NSCLC xenograft model [71]. A Phase 1 trial of XL765 plus erlotinib was conducted in 46 patients with advanced solid tumors (80.4% had lung cancer). This combination regime demonstrated stable disease in 37.5% of patients as the best responses [55].
PF-04691502
PF-04691502 is a potent ATP competitive dual PI3K/mTOR inhibitor with the ability to inhibit cell proliferation in the mutant PIK3CA and deleted PTEN NSCLC cell lines. Its anti-tumor activity has been reported in xenografts, U87 (PTEN-null) and SKOV3 (PIK3CA mutant) NSCLC resistant to gefitinib and erlotinib [89]. Also, PF-04691502 was demonstrated to induce autophagy in NSCLC cell lines in a pre-clinical study [32]. An overview of trials evaluating the outcomes of PI3K/Akt/mTOR inhibitors in lung cancer cases is presented in Table 2. Besides, Fig. 3 demonstrates an overview of the chemical structures of stated drugs and their targets in a lung tumor cell.
Table 2.
An overview of trials utilized PI3K/Akt/mTOR inhibitors in lung cancer cases.
| Description | Cancer type | Phase | Status | Response | Identifier | ||||
|---|---|---|---|---|---|---|---|---|---|
| SD | PR | CR | OR | ||||||
| PI3K inhibitors | |||||||||
| BKM120+Carboplatin+ Disodium pemetrexed | To investigate adverse events and the MTD of BKM120 in combination with carboplatin and disodium pemetrexed | SCLC | I | Completed | NA | NA | NA | NA | NCT01723800 |
| Pilaralisib+Erlotinib | To evaluate safety and tolerability of XL147 in combination with erlotinib | NSCLC | I | Completed | 51% | 3.7% | 0 | 0 | NCT00692640 |
| PX-866+Docetaxel | To assess the safety, MTD, pharmacokinetics, and efficacy of PX-866 combined with docetaxel | NSCLC | I/II | Completed | 68% | 6% | NA | NA | NCT01204099 |
| Taselisib | To evaluate taselisib (GDC-0032) effectiveness in PI3K-overactivated patients | SCC | II | Completed | 61% | 4% | NA | NA | NCT02785913 |
| BYL719 | To investigate the efficacy of single agent BYL719 in patients who carry specific molecular alterations except for EGFR alterations | Advanced NSCLC | II | Completed | NA | NA | NA | NA | NCT02276027 |
| INC280 | To investigate the efficacy of single agent INC280 in patients who carry specific molecular alterations except for EGFR alterations | Advanced NSCLC | II | Completed | NA | NA | NA | NA | NCT02276027 |
| LDK378 | To investigate the efficacy of single agent LDK378 in patients who carry specific molecular alterations except for EGFR alterations | Advanced NSCLC | II | Completed | NA | NA | NA | NA | NCT02276027 |
| Akt inhibitors | |||||||||
| MK2206+Erlotinib | To assess the efficacy and safety profile of MK2206 and erlotinib in patients who have progressed after previous erlotinib therapy | Advanced NSCLC | II | Completed | 9% | NA | NA | NA | NCT01294306 |
| Ipatasertib | To study the effectiveness of Ipatasertib-plus-docetaxel in patients who have failed or are intolerant to first-line immunotherapy | NSCLC | II | Active | NA | NA | NA | NA | NCT04467801 |
| Everolimus+Erlotinib | To evaluate MTD for everolimus-plus-erlotinib in previously chemotherapy-treated patients | NSCLC | I | Completed | 37% | 12% | 12% | NA | NCT00456833 |
| mTOR inhibitors | |||||||||
| Sunitinib+Rapamycin | To determine the optimal dose, MTD, efficacy, and safety of sunitinib-plus-rapamycin | NSCLC | I | Completed | 6.7% | 0 | 0 | 0 | NCT00555256 |
| Neratinib+Temsirolimus | To investigate the efficacy and safety of neratinib monotherapy and neratinib plus temsirolimus in patients harboring HER2-activating mutations | NSCLC | II | Completed | 55% | 12% | 4% | 71% | NCT01827267 |
| Everolimus+Docetaxel | To evaluate responses of everolimus-plus-docetaxel in patients with recurrent disease | Advanced NSCLC | II | Terminated | 63% | 8% | 0 | NA | NCT00406276 |
| Ridaforolimus | To study the dose-limiting toxicity and efficacy of ridaforolimus-plus-cetuximab in patients after at least 1 previous regimen | Advanced NSCLC | I | Terminated | NA | NA | NA | NA | NCT01212627 |
| RAD001+Paclitaxel +Carboplatin | To assess the efficacy and safety of RAD001-plus-paclitaxel and -carboplatin in patients with neuroendocrine differentiation | LCC | IV | Completed | 29% | 45% | 0 | 45% | NCT01317615 |
| BIBW 2992+Rapamycin | To investigate MTD, responses, and safety profile of BIBW 2992-plus-rapamycin in patients carrying an EGFR mutation | NSCLC | Ib | Completed | 46% | 5.1% | 0 | 12% | NCT00993499 |
SD: Steady disease; PR: Partial response; CR: Complete response; OR: Overall response; MTD: Maximum tolerable dose; NSCLC: Non-small cell lung cancer; SCC: Squamous cell carcinoma.
Fig. 3.
The chemical structure and the target of PI3K/Akt/mTOR inhibitors in a lung tumor cell. Several types of PI3K/Akt/mTOR inhibitors target the components of this pathway from PI3K (GDC-0941, PX-866, and BKM120) to Akt (perifosine and MK-2206), and mTORC1 (rapamycin, temsirolimus, and everolimus) in order to disrupt the overactivated signaling.
Challenges associated with treatments targeting the PI3K/Akt/mTOR pathway in lung cancer
Despite promising results of the therapeutic agents targeting the PI3K/Akt/mTOR pathway in cancer targeted therapy, there are also some challenges that could impede the application of these inhibitors. Investigations showed that single agent often resulted in stable disease as the best response [15]. As discussed, the PI3K/Akt/mTOR pathway has a complex association with other parallel cascades such as the RAS/RAF/MEK/ERK pathway. Hence, studies hypothesized that the functions of these compensatory pathways and also the releases of feedback loops are the main reasons for resistance to PI3K/Akt/mTOR inhibitors. However, the understanding of the mechanism associated with the resistance to these agents is still a novel research area and needed further investigation [15,46]. It was demonstrated that multi-targeting of different pathways is able to improve the outcome of the treatment. Therefore, one approach to increase the therapeutic efficacy of PI3K/Akt/mTOR inhibitors is combination therapy. In this context, the application of PI3K inhibitors and MEK inhibitors exhibited promising outcomes in pre-clinical studies of lung cancer [46].
Alongside the drug resistance, the incidence of treatment-related AEs poses new challenges in the treatment of various cancers [27]. Despite favorable outcomes of PI3K/Akt/mTOR in combination with the inhibitors of parallel pathways, the incidence of AEs is higher with greater toxicities [133]. Studies demonstrated that metabolic derangements such as hyperlipidemia and hyperglycemia are frequent AEs for patients receiving PI3K/Akt/mTOR inhibitors [19]. Indeed, the existence of these adverse events might be due to the effective roles of PI3K/Akt/mTOR components in the insulin signaling pathway [69]. The evaluation of BKM120 (PI3K inhibitor) in a study of solid tumors (include NSCLC) showed that the most frequent treatment-related AEs were rash, hyperglycemia, diarrhea, anorexia, mood alteration, and nausea. Moreover, 63% of patients demonstrated grade 3 and 4 treatment-related AEs [6]. Another PI3K inhibitor, XL147 was evaluated in patients with refractory advanced NSCLC. Maculopapular rash and hypersensitivity reactions were considered as the DLTs. The incidence of grade 3 and 4 treatment-related AEs was 13% and the most frequent treatment-related AEs were dermatologic toxicities, diarrhea, nausea, and decreased appetite [135]. MK-2206 (Akt inhibitor) plus erlotinib resulted in the presence of rash, diarrhea, fatigue, and mucositis in patients with NSCLC. Among all patients, 51.2% showed at least one grade 3 treatment-related AEs [72]. In a study of Everolimus in combination with erlotinib in patients with metastatic or unresectable NSCLC, the most frequent dose-limiting toxicities were stomatitis, rash, and diarrhea. Of all examined patients, 43% experienced grade 3 or 4 treatment-related AEs. Also, the incidence of serious AEs was 54%, most frequently gastrointestinal disorders, infections and infestations, and respiratory disorders [109].
Taken together, many components of the PI3K/Akt/mTOR axis have been investigated as the targets of drugs in the treatment of lung cancers. Drugs targeting PI3K, Akt, and mTOR have demonstrated the most promising outcomes with a wide range of inhibitors; nevertheless, there is still a lack of data about how the response of those drugs is enough to control the development and growth of lung tumors. Notably, it is worthy to mention that mTOR inhibitors such as rapamycin, temsirolimus, and everolimus and drugs targeting mTOR as one of the targets like NVP-BEZ235 showed much more promising results with a more satisfying response profile compared with PI3K and Akt inhibitors. Thus, it could be inferred that the optimal efficacy of PI3K/Akt/mTOR inhibitors was achieved when other therapeutic agents accompany them in the context of combination therapy that could have some reasons to be elucidated. One of them is the presence of complex signaling in cells that may cause the single component targeting of a pathway such as the PI3K axis not to yield satisfying outcomes because of negative feedbacks which could induce the activation of the upstream pathway and also the interconnection of PI3K axis with other pathways like MAPK. Besides, the detection of the exact alteration of the PI3K/Akt/mTOR pathway in lung cancer patients was not performed routinely in the clinic, and hence, anti-PI3K/Akt/mTOR could be associated with lower effectiveness. Although the evaluation of PI3K/Akt/mTOR inhibitors was typically in Phase III/IV clinical trials, in the lung cancer context the status of those drugs is yet in Phase I/II level which affirms why there is still a gap to be filled.
Moreover, another concern about PI3K/Akt/mTOR inhibition is the incidence of treatment-related AEs which is, unfortunately, more severe in combination treatment regimes in lung cancer cases. Regarding the cross-reactions of the PI3K axis with other signaling pathways and numerous targets of this axis, it is rational that blocking some components led to a pathological condition; indeed, AEs related to PI3K/Akt/mTOR inhibition range from metabolic to immunological disorders. The most frequent grade 3 or higher AEs were decreased leukocytes (lymphopenia, neutropenia), elevated AST, ALT, and CPK, diarrhea, and nausea. These side effects could be life-threatening that need acute intervention; however, monitoring toxicities, medical actions, and also patients’ education are some strategies that can be utilized to manage AEs. The details of PI3K/Akt/mTOR inhibitors-related AEs in lung cancer trials are summarized in Table 3.
Table 3.
Details of reported treatment-related AEs following the administration of PI3K/Akt/mTOR inhibitors in lung cancer cases.
| Phase | Treatment-related AEs * | Any grade AEs * | High frequent treatment-related AEs | Trial identifier | ||||
|---|---|---|---|---|---|---|---|---|
| Any G. | ≥ Grade 3 | Serious | Dis-cont. | Any grade | ≥ Grade 3 | |||
| PI3K inhibitors | ||||||||
| GDC-0941+Erlotinib | I | 100 | 66.7 | 33.3 | 19.3 | Diarrhea, Nausea, Fatigue | Rash, Elevated ALT | NCT00975182 |
| GDC-0941+Paclitaxel+ Carboplatin+/-Bevacizumab | I | 100 | 86.4 | 84.8 | 33.3 | Nausea, Vomiting, Fatigue | Neutropenia, Dyspnea, Anemia | NCT00974584 |
| BKM120 | II | NA | NA | NA | NA | Hyperglycemia, Pruritus, Diarrhea | Hyperglycemia, Asthenia, Increased ALT | NCT01820325 |
| BKM120+MEK162 | Ib | 93.3 | 64 | NA | 29.2 | Increased CPK, Diarrhea, Increased AST | Increased CPK, Increased ALT, Increased AST | NCT01363232 |
| BKM120+Trametinib | Ib | 97 | 65 | 19 | 31 | Dermatitis acneiform, Diarrhea | Increased CPK, Increased ALT, Stomatitis | NCT01155453 |
| BKM120+Everolimus | I | NA | NA | NA | NA | Hyperglycemia, Increased AST/ALT | Fatigue, Hypokalemia, Pneumonia | NCT01470209 |
| XL147 | I | 63.8 | 13 | 4.3 | 2.9 | Skin toxicities, Nausea, Diarrhea | Rash, Diarrhea | NCT00486135 |
| PX-866+Docetaxel | II | NA | NA | NA | NA | Diarrhea, Nausea, Vomiting | Neutropenia | NCT01204099 |
| Akt inhibitors | ||||||||
| Perifosine+Radiotherapy | I | NA | NA | NA | NA | Nausea, Fatigue, Diarrhea | NA | [152] |
| MK-2206+Erlotinib | II | NA | 55 | NA | NA | NA | Rash, Diarrhea, Fatigue | NCT01294306 |
| MK-2206+AZD6244+Sorafenib | II | NA | NA | NA | NA | Fatigue, Diarrhea, Rash | Fatigue, Diarrhea, Rash | NCT01248247 |
| MK-2206+Gefitinib | I | NA | NA | NA | NA | Increased eosinophil, Rash, Diarrhea | NA | NCT01147211 |
| mTOR inhibitors | ||||||||
| Rapamycin+Pemetrexed | I/II | NA | NA | NA | NA | Lymphopenia, Anemia, Fatigue | Lymphopenia, Hypophosphatemia, Neutropenia | NCT00923273 |
| Rapamycin+Afatinib | Ib | 100 | 66.7 | 56.4 | 23.1 | Diarrhea, Mucosal inflammation, Rash | Diarrhea, Mucosal inflammation, Asthenia, Rash | NCT00993499 |
| Temsirolimus | II | NA | 64 | NA | NA | NA | Dyspnea, Fatigue, Hyperglycemia, Hypoxia, Nausea | [118] |
| Everolimus+Gefitinib | II | NA | 22.5 | 4.76 | 3.2 | Rash, Diarrhea, Oral ulcerations | Lymphopenia, Hyponatremia, Fatigue, Diarrhea | NCT00096486 |
| Everolimus+Erlotinib | II | 100 | 72.7 | NA | 10.6 | Diarrhea, Stomatitis, Rash | Stomatitis, Asthenia, Diarrhea | NCT00456833 |
| Ridaforolimus | I | NA | NA | NA | 0 | Mouth sores, Rash, Anemia | Mouth sores, Hyperglycemia, Thrombocytopenia | NCT00704054 |
| Dual PI3K/mTORs inhibitors | ||||||||
| NVP-BEZ235+Everolimus | Ib | NA | NA | 17.3 | NA | Fatigue, Anorexia, Nausea, Diarrhea | Increased AST, Increased ALP, Anemia | NCT01508104 |
| XL765+Erlotinib | I | 89.1 | 15 | 8.7 | 4.3 | Diarrhea, Rash, Nausea | Increased AST, Nausea, Vomiting | NCT00777699 |
AEs: Adverse events; Any G.: Any grade; ALT: Alanine aminotransferase; CPK: Creatine phosphokinase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; Dis-cont: Discontinued; NA: Not available.*: % of all patients.
The application of nano-based strategies
One way to manage the PI3K/Akt/mTOR inhibition toxicities could be the application of nano-based drug delivery strategies. Studies showed that the combination of molecular targeted agents and nano-carriers result in local delivery of the payload and the controlled release of the drugs; an event that may prevent the toxicities due to systemic exposure to the inhibitors [31,126]. Nanoparticles can be a favorable strategy to enhance the efficacy of treatments by crossing various barriers in the body, being able to carry the drugs to the target site, and having the capacity to co-deliver more than one drug [94]. Therefore, the administration of nano-based inhibitors could enhance the pharmacokinetics and safety profiles, efficacy, and also bioavailability of those therapeutic agents [70].
Despite the potential capacity of nanoparticles to improve the efficacy of PI3K/Akt/mTOR inhibitors in the treatment of several cancers such as colorectal [121] and gastric cancer [66], there are limited numbers of studies evaluating the outcomes of those inhibitors in conjugation with nanoparticles in lung cancer, and consequently, this area is in the initial steps. In lung cancer mice models, it was shown that a sorbitol diacrylate-polyethylenimine (SDA-PEI)-based Akt1 inhibition resulted in a potent antitumor effect against lung tumors while increased the efficacy of therapy compared with naked DNA treatments [52]. Similarly, it was shown that the conjugation of shRNA targeting Akt1 with a nanoparticle carrier, glycerol triacrylate-spermine (GT-SPE) polyspermine, inhibited the tumorigenesis of the lung cancer mice model. Indeed, this approach protected the shRNA from being degraded by nuclease successfully [51]. The comparison of nano-based PI3K/Akt/mTOR inhibitors and inhibitors alone is depicted in Fig. 4.
Fig. 4.
The application of nanotechnology in drug delivery of PI3K/Akt/mTOR inhibitors in lung cancer. The administration of PI3K/Akt/mTOR inhibitors in lung cancer could result in tumor regression; however, due to low local concentration, those treatments need to be applied in high dosages and/or in a combination form which consequently leads to higher AEs. Another challenge is the bio-degradation of drugs that diminishes their efficacy in the tumor microenvironment. The conjugation of inhibitors with nanoparticles is a novel approach to deliver drugs more efficiently to the tumor microenvironment. Nanoparticles not only elevate the local concentration of inhibitors that results in higher tumor regression but also reduce the off-target toxicities and drug bio-degradation.
Conclusion and future perspectives
Cancers in the lung are considered malignancies with a dismal prognosis and a high rate of mortality. The progression in illustrating the molecular changes of lung cancer has resulted in determining the potential pathways which are associated with the initiation and advancement of this malignancy. The PI3K/Akt/mTOR signaling pathway is one of those which has a pivotal role in the proliferation and cell growth events and therefore, the presence of alterations in this axis could initiate and enhance lung cancer progression. Regarding the development of genomic analysis methods, several alterations in the PI3K/Akt/mTOR axis have been identified in lung cancer from RTKs (at the top of the PI3K axis) to mTOR (at the bottom of this axis). Accordingly, alterations frequently occur in PIK3CA and PTEN, but PIK3R1, Akt, and mTOR are also altered. The presence of those molecular changes consequently leads to the induction of some oncogenic events such as the acquisition of chemoresistant, metastasis, tumor invasion, and EMT. Therefore, studies have focused on blocking the components of the PI3K axis due to their oncogenic role in the context of lung cancer. Also, investigating the genetic alteration and interactive signaling networks seems to be necessary to develop an efficient approach to maximize the anti-tumor responses. With this regard, the inhibition of PI3K, Akt, and mTOR results in the induction of anti-tumor responses, but at the cost of some challenges. Typically, the application of PI3K/Akt/mTOR inhibitors as monotherapy exhibits unsatisfactory anti-tumor activity that might be related to the drug-resistance phenomenon. Despite the unknown aspect of this challenge, researchers believe that utilizing the combination therapy either with another targeted therapy or chemotherapy is a promising approach to overcome drug resistance and enhance therapeutic efficacy. However, the inhibition of the PI3K axis whether as monotherapy or combination therapy is also associated with the presence of treatment-related AEs which is another challenge. The application of the nanoparticle-based tool to carry and deliver inhibitors to the targeted site could be a promising strategy to improve the effectiveness of the PI3K/Akt/mTOR inhibitors. Nonetheless, there is a big gap for lung cancer context treatment using nanoparticle-based inhibitors; however, few studies which have been performed showed a potent capacity.
In a word, it seems that there is a lack of data for the application of nano-based approaches in the context of PI3K/Akt/mTOR inhibitors and lung cancer, and we propose this area as an untouchable field for future researchers in lung cancer treatment. Taken together, it is essential to understand the costs and benefits of PI3K/Akt/mTOR inhibitors for future treatment approaches to gain the most reliable therapy with a favorable efficacy and safety profile in the context of lung cancer.
CRediT authorship contribution statement
Mohammad-Javad Sanaei: Investigation, Writing – original draft, Writing – review & editing. Sara Razi: Investigation, Writing – original draft, Writing – review & editing. Atieh Pourbagheri-Sigaroodi: Visualization. Davood Bashash: Conceptualization, Supervision, Writing – review & editing, Project administration.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Acknowledgment
The authors would like to express their gratitude to Shahid Beheshti University of Medical Sciences (Tehran, Iran) for supporting this study.
Data availability statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Dear editor-in-chief,
Our manuscript entitled "The gravity of the PI3K/Akt/mTOR signaling pathway alterations in lung cancer and challenges toward targeting it; Where are we now?" was transferred to your reputable journal from “European Journal of Pharmacology”. It is our pleasure to submit our article to your journal; however, regarding the open-access condition we do not afford to pay the charges. In this case, Prof. Justin Lathia and Prof. Megan Monachino kindly accepted our article for a waiver as part of our Article Advance program.
I would like to submit our manuscript for review and, hopefully, publication in your prestigious journal. Neither the article nor any part of it has been or will be published or submitted elsewhere.
Author's expertise in the subject of the PI3K pathway in cancer:
Bashash D, Delshad M, Riyahi N, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Momeny M. Inhibition of PI3K signaling pathway enhances the chemosensitivity of APL cells to ATO: Proposing novel therapeutic potential for BKM120. Eur J Pharmacol. 2018 Dec 15;841:10–18. doi: 10.1016/j.ejphar.2018.10.007.
Bashash D, Delshad M, Safaroghli-Azar A, Safa M, Momeny M, Ghaffari SH. Novel pan PI3K inhibitor-induced apoptosis in APL cells correlates with suppression of telomerase: An emerging mechanism of action of BKM120. Int J Biochem Cell Biol. 2017 Oct;91(Pt A):1–8. doi: 10.1016/j.biocel.2017.08.009.
Bashash D, Safaroghli-Azar A, Dadashi M, Safa M, Momeny M, Ghaffari SH. Anti-tumor activity of PI3K-δ inhibitor in hematologic malignant cells: Shedding new light on resistance to Idelalisib. Int J Biochem Cell Biol. 2017 Apr;85:149–158. doi: 10.1016/j.biocel.2017.02.007.
Bashash D, Safaroghli-Azar A, Delshad M, Bayati S, Nooshinfar E, Ghaffari SH. Inhibitor of pan class-I PI3K induces differentially apoptotic pathways in acute leukemia cells: Shedding new light on NVP-BKM120 mechanism of action. Int J Biochem Cell Biol. 2016 Oct;79:308–317. doi: 10.1016/j.biocel.2016.09.004.
Safaroghli-Azar A, Bashash D, Sadreazami P, Momeny M, Ghaffari SH. PI3K-δ inhibition using CAL-101 exerts apoptotic effects and increases doxorubicin-induced cell death in pre-B-acute lymphoblastic leukemia cells. Anticancer Drugs. 2017 Apr;28(4):436–445. doi: 10.1097/CAD.0000000000000477.
Riyahi N, Safaroghli-Azar A, Sheikh-Zeineddini N, Sayyadi M, Bashash D. Synergistic Effects of PI3K and c-Myc Co-targeting in Acute Leukemia: Shedding New Light on Resistance to Selective PI3K-δ Inhibitor CAL-101. Cancer Invest. 2019;37(7):311–324. doi: 10.1080/07357907.2019.1651328.
Safaroghli-Azar A, Bashash D, Kazemi A, Pourbagheri-Sigaroodi A, Momeny M. Anticancer effect of pan-PI3K inhibitor on multiple myeloma cells: Shedding new light on the mechanisms involved in BKM120 resistance. Eur J Pharmacol. 2019 Jan 5;842:89–98. doi: 10.1016/j.ejphar.2018.10.036.
Shiri Heris R, Safaroghli-Azar A, Yousefi AM, Hamidpour M, Bashash D. Anti-leukemic effect of PI3K inhibition on chronic myeloid leukemia (CML) cells: shedding new light on the mitigating effect of c-Myc and autophagy on BKM120 cytotoxicity. Cell Biol Int. 2020 May;44(5):1212–1223. doi: 10.1002/cbin.11322.
Alipour F, Riyahi N, Safaroghli-Azar A, Sari S, Zandi Z, Bashash D. Inhibition of PI3K pathway using BKM120 intensified the chemo-sensitivity of breast cancer cells to arsenic trioxide (ATO). Int J Biochem Cell Biol. 2019 Nov;116:105615. doi: 10.1016/j.biocel.2019.105615.
Sadeghi S, Esmaeili S, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, Bashash D. PI3K Abrogation Using Pan-PI3K Inhibitor BKM120 Gives Rise to a Significant Anticancer Effect on AML-Derived KG-1 Cells by Inducing Apoptosis and G2/M Arrest. Turk J Haematol. 2020 Aug 28;37(3):167–176. doi: 10.4274/tjh.galenos.2020.2019.0440.
Mosleh M, Safaroghli-Azar A, Bashash D. Pan-HDAC inhibitor panobinostat, as a single agent or in combination with PI3K inhibitor, induces apoptosis in APL cells: An emerging approach to overcome MSC-induced resistance. Int J Biochem Cell Biol. 2020 May;122:105734. doi: 10.1016/j.biocel.2020.105734.
Zehtabcheh S, Yousefi AM, Salari S, Safa M, Momeny M, Ghaffari SH, Bashash D. Abrogation of histone deacetylases (HDACs) decreases survival of chronic myeloid leukemia cells: New insight into attenuating effects of the PI3K/c-Myc axis on panobinostat cytotoxicity. Cell Biol Int. 2021 May;45(5):1111–1121. doi: 10.1002/cbin.11557. Epub 2021 Feb 4. PMID: 33501756.
Sayyadi M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Abolghasemi H, Bashash D. c-Myc Inhibition Using 10058-F4 Increased the Sensitivity of Acute Promyelocytic Leukemia Cells to Arsenic Trioxide Via Blunting PI3K/NF-κB Axis. Arch Med Res. 2020 Oct;51(7):636–644. doi: 10.1016/j.arcmed.2020.06.002.
Esmaeili S, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Salari S, Gharehbaghian A, Hamidpour M, Bashash D. Activation of PPARγ intensified the effects of arsenic trioxide in acute promyelocytic leukemia through the suppression of PI3K/Akt pathway: Proposing a novel anticancer effect for pioglitazone. Int J Biochem Cell Biol. 2020 May;122:105739. doi: 10.1016/j.biocel.2020.105739.
Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, Safaroghli-Azar A, Zali MR, Bashash D. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021 May 5;898:173983. doi:10.1016/j.ejphar.2021.173983.
Akbari Dilmaghani N, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/Akt/mTORC signaling axis in head and neck squamous cell carcinoma: Possibilities for therapeutic interventions either as single agents or in combination with conventional therapies. IUBMB Life. 2021 Apr;73(4):618–642. doi: 10.1002/iub.2446.
Sanaei M-J, Pourbagheri-Sigaroodi A, Kaveh V, Sheikholeslami SA, Salari S, Bashash D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit Rev Oncol Hematol. 2020:103160. doi: 10.1016/j.critrevonc.2020.103160
Sanaei MJ, Pourbagheri-Sigaroodi A, Kaveh V, Abolghasemi H, Ghaffari SH, Momeny M, Bashash D. Recent advances in immune checkpoint therapy in non-small cell lung cancer and opportunities for nanoparticle-based therapy. European journal of pharmacology. 2021;909:174404. doi: 10.1016/j.ejphar.2021.174404.
References
- 1.Aisner D.L., Sholl L.M., Berry L.D., Rossi M.R., Chen H., Fujimoto J., Moreira A.L., Ramalingam S.S., Villaruz L.C., Otterson G.A. The impact of smoking and TP53 mutations in lung adenocarcinoma patients with targetable mutations-the Lung Cancer mutation consortium (LCMC2) Clin. Cancer Res. 2018;24:1038–1047. doi: 10.1158/1078-0432.CCR-17-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bade B.C., Dela Cruz C.S. Lung Cancer 2020: epidemiology, etiology, and prevention. Clin. Chest Med. 2020;41:1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Baek S.H., Ko J.H., Lee J.H., Kim C., Lee H., Nam D., Lee J., Lee S.G., Yang W.M., Um J.Y., Sethi G., Ahn K.S. Ginkgolic acid inhibits invasion and migration and TGF-β-induced EMT of Lung Cancer cells through PI3K/Akt/mTOR inactivation. J. Cell. Physiol. 2017;232:346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 4.Balsara B.R., Pei J., Mitsuuchi Y., Page R., Klein-Szanto A., Wang H., Unger M., Testa J.R. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 5.Banerji U., Dean E.J., Pérez-Fidalgo J.A., Batist G., Bedard P.L., You B., Westin S.N., Kabos P., Garrett M.D., Tall M. A phase I open-label study to identify a dosing regimen of the Pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers. Clin. Cancer Res. 2018;24:2050–2059. doi: 10.1158/1078-0432.CCR-17-2260. [DOI] [PubMed] [Google Scholar]
- 6.Bendell J.C., Rodon J., Burris H.A., de Jonge M., Verweij J., Birle D., Demanse D., De Buck S.S., Ru Q.C., Peters M., Goldbrunner M., Baselga J. Phase I, dose-escalation study of BKM120, an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:282–290. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 7.Besse B., Soria J., Gomez-Roca C., Ware J.A., Adjei A.A., Dy G.K., Shankar G., Brachmann R.K., Groen H.J. A phase Ib study to evaluate the PI3-kinase inhibitor GDC-0941 with paclitaxel (P) and carboplatin (C), with and without bevacizumab (BEV), in patients with advanced non-small cell lung cancer (NSCLC) J. Clin. Oncol. 2011;29:3044. -3044. [Google Scholar]
- 8.Bilello K.S., Murin S., Matthay R.A. Epidemiology, etiology, and prevention of lung cancer. Clin. Chest Med. 2002;23:1–25. doi: 10.1016/s0272-5231(03)00057-1. [DOI] [PubMed] [Google Scholar]
- 9.Bockorny B., Rusan M., Chen W., Liao R.G., Li Y., Piccioni F., Wang J., Tan L., Thorner A.R., Li T. RAS–MAPK reactivation facilitates acquired resistance in FGFR1-amplified lung cancer and underlies a rationale for upfront FGFR–MEK blockade. Mol. Cancer Ther. 2018;17:1526–1539. doi: 10.1158/1535-7163.MCT-17-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boffa D.J., Luan F., Thomas D., Yang H., Sharma V.K., Lagman M., Suthanthiran M. Rapamycin inhibits the growth and metastatic progression of non-small cell lung cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2004;10:293–300. doi: 10.1158/1078-0432.ccr-0629-3. [DOI] [PubMed] [Google Scholar]
- 11.Bronisz A., Godlewski J., Wallace J.A., Merchant A.S., Nowicki M.O., Mathsyaraja H., Srinivasan R., Trimboli A.J., Martin C.K., Li F., Yu L., Fernandez S.A., Pécot T., Rosol T.J., Cory S., Hallett M., Park M., Piper M.G., Marsh C.B., Yee L.D., Jimenez R.E., Nuovo G., Lawler S.E., Chiocca E.A., Leone G., Ostrowski M.C. Reprogramming of the tumour microenvironment by stromal PTEN-regulated miR-320. Nat. Cell Biol. 2011;14:159–167. doi: 10.1038/ncb2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunet A., Bonni A., Zigmond M.J., Lin M.Z., Juo P., Hu L.S., Anderson M.J., Arden K.C., Blenis J., Greenberg M.E. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen B., Yuping S., Ni J. Rapamycin decreases survivin expression to induce NSCLC cell apoptosis under hypoxia through inhibiting HIF-1α induction. Mol. Biol. Rep. 2012;39:185–191. doi: 10.1007/s11033-011-0724-3. [DOI] [PubMed] [Google Scholar]
- 14.Chen X.F., Zhang H.J., Wang H.B., Zhu J., Zhou W.Y., Zhang H., Zhao M.C., Su J.M., Gao W., Zhang L., Fei K., Zhang H.T., Wang H.Y. Transforming growth factor-β1 induces epithelial-to-mesenchymal transition in human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling pathways. Mol. Biol. Rep. 2012;39:3549–3556. doi: 10.1007/s11033-011-1128-0. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H., Shcherba M., Pendurti G., Liang Y., Piperdi B., Perez-Soler R. Targeting the PI3K/AKT/mTOR pathway: potential for lung cancer treatment. Lung Cancer Manag. 2014;3:67–75. doi: 10.2217/lmt.13.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetty C., Lakka S.S., Bhoopathi P., Rao J.S. MMP-2 alters VEGF expression via alphaVbeta3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer. 2010;127:1081–1095. doi: 10.1002/ijc.25134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuffa L.G.D.A., Reiter R.J., Lupi L.A. Melatonin as a promising agent to treat ovarian cancer: molecular mechanisms. Carcinogenesis. 2017;38:945–952. doi: 10.1093/carcin/bgx054. [DOI] [PubMed] [Google Scholar]
- 18.Cox T.R., Erler J.T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis. Models Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crouthamel M.C., Kahana J.A., Korenchuk S., Zhang S.Y., Sundaresan G., Eberwein D.J., Brown K.K., Kumar R. Mechanism and management of AKT inhibitor-induced hyperglycemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009;15:217–225. doi: 10.1158/1078-0432.CCR-08-1253. [DOI] [PubMed] [Google Scholar]
- 20.Cruz C.S.D., Tanoue L.T., Matthay R.A. Lung cancer: epidemiology, etiology, and prevention. Clin. Chest Med. 2011;32:605–644. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cryer A.M., Thorley A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019;198:189–205. doi: 10.1016/j.pharmthera.2019.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Cully M., You H., Levine A.J., Mak T.W. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 23.Di Leo A., Johnston S., Lee K.S., Ciruelos E., Lønning P.E., Janni W., O'Regan R., Mouret-Reynier M.A., Kalev D., Egle D. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 24.Dimri M., Humphries A., Laknaur A., Elattar S., Lee T.J., Sharma A., Kolhe R., Satyanarayana A. NAD (P) H quinone dehydrogenase 1 ablation inhibits activation of the phosphoinositide 3-kinase/Akt serine/threonine kinase and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways and blocks metabolic adaptation in hepatocellular carcinoma. Hepatology. 2020;71:549–568. doi: 10.1002/hep.30818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Do H., Solomon B., Mitchell P.L., Fox S.B., Dobrovic A. Detection of the transforming AKT1 mutation E17K in non-small cell lung cancer by high resolution melting. BMC Res. Notes. 2008;1:14. doi: 10.1186/1756-0500-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dodd K.M., Yang J., Shen M.H., Sampson J.R., Tee A.R. mTORC1 drives HIF-1α and VEGF-A signalling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3. Oncogene. 2015;34:2239–2250. doi: 10.1038/onc.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donahue S., Santos Fulgencio G. PI3K inhibitors and adverse events: optimizing patient care for the treatment of advanced breast cancer. Clin. J. Oncol. Nurs. 2020;24:673–680. doi: 10.1188/20.CJON.673-680. [DOI] [PubMed] [Google Scholar]
- 28.Elrod H.A., Lin Y.D., Yue P., Wang X., Lonial S., Khuri F.R., Sun S.Y. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol. Cancer Ther. 2007;6:2029–2038. doi: 10.1158/1535-7163.MCT-07-0004. [DOI] [PubMed] [Google Scholar]
- 29.Engelman J.A., Mukohara T., Zejnullahu K., Lifshits E., Borrás A.M., Gale C.M., Naumov G.N., Yeap B.Y., Jarrell E., Sun J., Tracy S., Zhao X., Heymach J.V., Johnson B.E., Cantley L.C., Jänne P.A. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J. Clin. Invest. 2006;116:2695–2706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ericksen R.E., Lim S.L., McDonnell E., Shuen W.H., Vadiveloo M., White P.J., Ding Z., Kwok R., Lee P., Radda G.K. Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 2019;29:1151–1165. doi: 10.1016/j.cmet.2018.12.020. e1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang J., Nakamura H., Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug. Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Fei H.R., Tian H., Zhou X.L., Yang M.F., Sun B.L., Yang X.Y., Jiao P., Wang F.Z. Inhibition of autophagy enhances effects of PF-04691502 on apoptosis and DNA damage of lung cancer cells. Int. J. Biochem. Cell Biol. 2016;78:52–62. doi: 10.1016/j.biocel.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 33.Foukas L.C., Berenjeno I.M., Gray A., Khwaja A., Vanhaesebroeck B. Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc. Nat. Acad. Sci. USA. 2010;107:11381–11386. doi: 10.1073/pnas.0906461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox M., Mott H.R., Owen D. Class IA PI3K regulatory subunits: p110-independent roles and structures. Biochem. Soc. Trans. 2020 doi: 10.1042/BST20190845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fresno Vara J.A., Casado E., de Castro J., Cejas P., Belda-Iniesta C., González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Fu L., Kim Y.A., Wang X., Wu X., Yue P., Lonial S., Khuri F.R., Sun S.Y. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fumarola C., Bonelli M.A., Petronini P.G., Alfieri R.R. Targeting PI3K/AKT/mTOR pathway in non small cell lung cancer. Biochem. Pharmacol. 2014;90:197–207. doi: 10.1016/j.bcp.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Gkountakos A., Sartori G., Falcone I., Piro G., Ciuffreda L., Carbone C., Tortora G., Scarpa A., Bria E., Milella M. PTEN in lung cancer: dealing with the problem, building on new knowledge and turning the game around. Cancers. 2019;11:1141. doi: 10.3390/cancers11081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldstraw P., Ball D., Jett J.R., Le Chevalier T., Lim E., Nicholson A.G., Shepherd F.A. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 40.Gorzalczany Y., Gilad Y., Amihai D., Hammel I., Sagi-Eisenberg R., Merimsky O. Combining an EGFR directed tyrosine kinase inhibitor with autophagy-inducing drugs: a beneficial strategy to combat non-small cell lung cancer. Cancer Lett. 2011;310:207–215. doi: 10.1016/j.canlet.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Gris-Oliver A., Palafox M., Monserrat L., Brasó-Maristany F., Òdena A., Sánchez-Guixé M., Ibrahim Y.H., Villacampa G., Grueso J., Parés M. Genetic alterations in the PI3K/AKT pathway and baseline AKT activity define AKT inhibitor sensitivity in breast cancer patient-derived xenografts. Clin. Cancer Res. 2020;26(14):3720–3731. doi: 10.1158/1078-0432.CCR-19-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo, S. T. (2017). THE ROLE OF INOSITOL POLYPHOSPHATE 4-PHOSPHATASE II (INPP4B) IN THE PATHOGENESIS OF COLON CANCER (Doctoral dissertation, The University of Newcastle Australia).
- 43.Guo Y., Du J., Kwiatkowski D.J. Molecular dissection of AKT activation in lung cancer cell lines. Mol. Cancer Res. MCR. 2013;11:282–293. doi: 10.1158/1541-7786.MCR-12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haines Brian, Bittinger Mark, Chenard Melissa, Marlene Artime, Guertin Amy, Zhang Weisheng, et al. The mTOR inhibitor deforolimus is efficacious in models of mutant KRAS lung cancer. Am. Assoc. Cancer. Res. 2009;69:615. [Google Scholar]
- 45.He H., Xu C., Cheng Z., Qian X., Zheng L. Drug combinatorial therapies for the treatment of KRAS mutated lung cancers. Curr. Top. Med. Chem. 2019;19:2128–2142. doi: 10.2174/1568026619666190902150555. [DOI] [PubMed] [Google Scholar]
- 46.Heavey S., O'Byrne K.J., Gately K. Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC. Cancer Treat. Rev. 2014;40:445–456. doi: 10.1016/j.ctrv.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 48.Higgins G.S., Krause M., McKenna W.G., Baumann M. Personalized radiation oncology: epidermal growth factor receptor and other receptor tyrosine kinase inhibitors. Mol. Radio Oncol. 2016;198:107–122. doi: 10.1007/978-3-662-49651-0_5. Springerpp. [DOI] [PubMed] [Google Scholar]
- 49.Hilgard P., Klenner T., Stekar J., Nössner G., Kutscher B., Engel J. D-21266, a new heterocyclic alkylphospholipid with antitumour activity. Eur. J. Cancer. 1997;33:442–446. doi: 10.1016/s0959-8049(97)89020-x. (Oxford, England : 1990) [DOI] [PubMed] [Google Scholar]
- 50.Hirai H., Sootome H., Nakatsuru Y., Miyama K., Taguchi S., Tsujioka K., Ueno Y., Hatch H., Majumder P.K., Pan B.S., Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 51.Hong S.H., Kim J.E., Kim Y.K., Minai-Tehrani A., Shin J.Y., Kang B., Kim H.J., Cho C.S., Chae C., Jiang H.L., Cho M.H. Suppression of lung cancer progression by biocompatible glycerol triacrylate-spermine-mediated delivery of shAkt1. Int. J. Nanomed. 2012;7:2293–2306. doi: 10.2147/IJN.S29152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hong S.H., Lee J.H., Jiang H.L., Kim J.E., Lee A.Y., Kim S., Cho C.S., Cho M.H. Dual expression of shAkt1 and Pdcd4 suppresses lung tumorigenesis in K-rasLA1 mice. Anticancer Res. 2015;35:2015–2019. [PubMed] [Google Scholar]
- 53.Ihle N.T., Williams R., Chow S., Chew W., Berggren M.I., Paine-Murrieta G., Minion D.J., Halter R.J., Wipf P., Abraham R., Kirkpatrick L., Powis G. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol. Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 54.Jaiswal B.S., Janakiraman V., Kljavin N.M., Chaudhuri S., Stern H.M., Wang W., Kan Z., Dbouk H.A., Peters B.A., Waring P., Dela Vega T., Kenski D.M., Bowman K.K., Lorenzo M., Li H., Wu J., Modrusan Z., Stinson J., Eby M., Yue P., Kaminker J.S., de Sauvage F.J., Backer J.M., Seshagiri S. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jänne P.A., Cohen R.B., Laird A.D., Macé S., Engelman J.A., Ruiz-Soto R., Rockich K., Xu J., Shapiro G.I., Martinez P., Felip E. Phase I safety and pharmacokinetic study of the PI3K/mTOR inhibitor SAR245409 (XL765) in combination with erlotinib in patients with advanced solid tumors. J. Thorac. Oncol. 2014;9:316–323. doi: 10.1097/JTO.0000000000000088. official publication of the International Association for the Study of Lung Cancer. [DOI] [PubMed] [Google Scholar]
- 56.Jhanwar-Uniyal M., Wainwright J.V., Mohan A.L., Tobias M.E., Murali R., Gandhi C.D., Schmidt M.H. Diverse signaling mechanisms of mTOR complexes: mTORC1 and mTORC2 in forming a formidable relationship. Adv. Biol. Regul. 2019;72:51–62. doi: 10.1016/j.jbior.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Jiang B.H., Liu L.Z. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv. Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiao D., Wang J., Lu W., Tang X., Chen J., Mou H., Chen Q.Y. Curcumin inhibited HGF-induced EMT and angiogenesis through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways in lung cancer. Mol. Ther. Oncolytics. 2016;3:16018. doi: 10.1038/mto.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jimeno A., Herbst R.S., Falchook G.S., Messersmith W.A., Hecker S., Peterson S., Hausman D.F., Kurzrock R., Eckhardt S.G., Hong D.S. Final results from a phase I, dose-escalation study of PX-866, an irreversible, pan-isoform inhibitor of PI3 kinase. J. Clin. Oncol. 2010;28:3089. -3089. [Google Scholar]
- 60.Jin H.O., Lee Y.H., Park J.A., Kim J.H., Hong S.E., Kim H.A., Kim E.K., Noh W.C., Kim B.H., Ye S.K., Chang Y.H., Hong S.I., Hong Y.J., Park I.C., Lee J.K. Blockage of stat3 enhances the sensitivity of NSCLC cells to PI3K/mTOR inhibition. Biochem. Biophys. Res. Commun. 2014;444:502–508. doi: 10.1016/j.bbrc.2014.01.086. [DOI] [PubMed] [Google Scholar]
- 61.Jung H.Y., Fattet L., Tsai J.H., Kajimoto T., Chang Q., Newton A.C., Yang J. Apical–basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat. Cell Biol. 2019;21:359–371. doi: 10.1038/s41556-019-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kauko O., O'Connor C.M., Kulesskiy E., Sangodkar J., Aakula A., Izadmehr S., Yetukuri L., Yadav B., Padzik A., Laajala T.D. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018;10:eaaq1093. doi: 10.1126/scitranslmed.aaq1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawano O., Sasaki H., Okuda K., Yukiue H., Yokoyama T., Yano M., Fujii Y. PIK3CA gene amplification in Japanese non-small cell lung cancer. Lung Cancer. 2007;58:159–160. doi: 10.1016/j.lungcan.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 64.Kempf E., Rousseau B., Besse B., Paz-Ares L. KRAS oncogene in lung cancer: focus on molecularly driven clinical trials. Eur. Respir. Rev. 2016;25:71–76. doi: 10.1183/16000617.0071-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy S.G., Kandel E.S., Cross T.K., Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 1999;19:5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Khorasani A.B.S., Pourbagheri-Sigaroodi A., Pirsalehi A., Safaroghli-Azar A., Zali M.R., Bashash D. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur. J. Pharmacol. 2021 doi: 10.1016/j.ejphar.2021.173983. [DOI] [PubMed] [Google Scholar]
- 67.Kim J., Guan K.L. mTOR as a central hub of nutrient signalling and cell growth. Nat. Cell Biol. 2019;21:63–71. doi: 10.1038/s41556-018-0205-1. [DOI] [PubMed] [Google Scholar]
- 68.Kondapaka S.B., Singh S.S., Dasmahapatra G.P., Sausville E.A., Roy K.K. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol. Cancer Ther. 2003;2:1093–1103. [PubMed] [Google Scholar]
- 69.Kraemer F.B., Takeda D., Natu V., Sztalryd C. Insulin regulates lipoprotein lipase activity in rat adipose cells via wortmannin- and rapamycin-sensitive pathways. Metabolism. 1998;47:555–559. doi: 10.1016/s0026-0495(98)90239-6. [DOI] [PubMed] [Google Scholar]
- 70.Kumari P., Ghosh B., Biswas S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016;24:179–191. doi: 10.3109/1061186X.2015.1051049. [DOI] [PubMed] [Google Scholar]
- 71.Laird A.D. AACR; 2007. XL765 Targets Tumor growth, survival, and Angiogenesis in Preclinical Models By Dual Inhibition of PI3K and mTOR. [Google Scholar]
- 72.Lara P.N., Longmate J., Mack P.C., Kelly K., Socinski M.A., Salgia R., Gitlitz B., Li T., Koczywas M., Reckamp K.L., Gandara D.R. Phase II study of the AKT inhibitor MK-2206 plus erlotinib in patients with advanced non-small cell lung cancer who previously progressed on erlotinib. Clin. Cancer Res. 2015;21:4321–4326. doi: 10.1158/1078-0432.CCR-14-3281. : an official journal of the American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lastwika K.J., Wilson W., Li Q.K., Norris J., Xu H., Ghazarian S.R., Kitagawa H., Kawabata S., Taube J.M., Yao S. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non–small cell lung cancer. Cancer Res. 2016;76:227–238. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 74.Le X., Puri S., Negrao M.V., Nilsson M.B., Robichaux J., Boyle T., Hicks J.K., Lovinger K.L., Roarty E., Rinsurongkawong W. Landscape of EGFR-dependent and-independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin. Cancer Res. 2018;24:6195–6203. doi: 10.1158/1078-0432.CCR-18-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee B., Lee T., Lee S.H., Choi Y.L., Han J. Clinicopathologic characteristics of EGFR, KRAS, and ALK alterations in 6,595 lung cancers. Oncotarget. 2016;7:23874. doi: 10.18632/oncotarget.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee Y.R., Chen M., Pandolfi P.P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat. Rev. Mol. Cell Biol. 2018;19:547–562. doi: 10.1038/s41580-018-0015-0. [DOI] [PubMed] [Google Scholar]
- 77.Li Y., Yang Y., Liu X., Long Y., Zheng Y. PRMT5 promotes human lung cancer cell apoptosis via Akt/Gsk3β signaling induced by resveratrol. Cell Transplant. 2019;28:1664–1673. doi: 10.1177/0963689719885083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang S.-Q., Bührer E.D., Berezowska S., Marti T.M., Xu D., Froment L., Yang H., Hall S.R., Vassella E., Yang Z. mTOR mediates a mechanism of resistance to chemotherapy and defines a rational combination strategy to treat KRAS-mutant lung cancer. Oncogene. 2019;38:622–636. doi: 10.1038/s41388-018-0479-6. [DOI] [PubMed] [Google Scholar]
- 79.Lim W.T., Zhang W.H., Miller C.R., Watters J.W., Gao F., Viswanathan A., Govindan R., McLeod H.L. PTEN and phosphorylated AKT expression and prognosis in early- and late-stage non-small cell lung cancer. Oncol. Rep. 2007;17:853–857. [PubMed] [Google Scholar]
- 80.Liu F., Gao S., Yang Y., Zhao X., Fan Y., Ma W., Yang D., Yang A., Yu Y. Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol. Rep. 2018;39:1523–1531. doi: 10.3892/or.2018.6188. [DOI] [PubMed] [Google Scholar]
- 81.Liu G., Pei F., Yang F., Li L., Amin A.D., Liu S., Buchan J.R., Cho W.C. Role of autophagy and apoptosis in non-small-cell lung cancer. Int. J. Mol. Sci. 2017;18:367. doi: 10.3390/ijms18020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu H., Yin J., Wang C., Gu Y., Deng M., He Z. FOXO3a mediates the cytotoxic effects of cisplatin in lung cancer cells. Anticancer Drugs. 2014;25:898–907. doi: 10.1097/CAD.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 83.Liu X., Xu Y., Zhou Q., Chen M., Zhang Y., Liang H., Zhao J., Zhong W., Wang M. PI3K in cancer: Its structure, activation modes and role in shaping tumor microenvironment. Future Oncol. 2018;14:665–674. doi: 10.2217/fon-2017-0588. [DOI] [PubMed] [Google Scholar]
- 84.Losuwannarak N., Maiuthed A., Kitkumthorn N., Leelahavanichkul A., Roytrakul S., Chanvorachote P. Gigantol targets cancer stem cells and destabilizes tumors via the suppression of the PI3K/AKT and JAK/STAT pathways in ectopic lung cancer xenografts. Cancers. 2019;11:2032. doi: 10.3390/cancers11122032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu C., Shan Z., Hong J., Yang L. MicroRNA-92a promotes epithelial-mesenchymal transition through activation of PTEN/PI3K/AKT signaling pathway in non-small cell lung cancer metastasis. Int. J. Oncol. 2017;51:235–244. doi: 10.3892/ijo.2017.3999. [DOI] [PubMed] [Google Scholar]
- 86.Lu X.X., Cao L.Y., Chen X., Xiao J., Zou Y., Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed. Res. Int. 2016;2016 doi: 10.1155/2016/2476842. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ludovini V., Bianconi F., Pistola L., Chiari R., Minotti V., Colella R., Giuffrida D., Tofanetti F.R., Siggillino A., Flacco A., Baldelli E., Iacono D., Mameli M.G., Cavaliere A., Crinò L. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2011;6:707–715. doi: 10.1097/JTO.0b013e31820a3a6b. : official publication of the International Association for the Study of Lung Cancer. [DOI] [PubMed] [Google Scholar]
- 88.Luk S.K., Piekorz R.P., Nürnberg B., Tony To S.S. The catalytic phosphoinositol 3-kinase isoform p110δ is required for glioma cell migration and invasion. Eur. J. Cancer. 2012;48:149–157. doi: 10.1016/j.ejca.2011.09.006. (Oxford, England : 1990) [DOI] [PubMed] [Google Scholar]
- 89.Lv Y., Du T., Ji M., Wang C., Lin S., Xue N., Jin J., Xu H., Chen X. A novel PI3K/mTOR dual inhibitor XH002 exhibited robust antitumor activity in NSCLC. J. Drug Target. 2019;27:451–459. doi: 10.1080/1061186X.2018.1542533. [DOI] [PubMed] [Google Scholar]
- 90.Ma K., Fan Y., Dong X., Dong D., Guo Y., Wei X., Ning J., Geng Q., Wang C., Hu Y., Li M., Niu W., Li E., Wu Y. MTA1 promotes epithelial to mesenchymal transition and metastasis in non-small-cell lung cancer. Oncotarget. 2017;8:38825–38840. doi: 10.18632/oncotarget.16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malanga D., Scrima M., De Marco C., Fabiani F., De Rosa N., De Gisi S., Malara N., Savino R., Rocco G., Chiappetta G., Franco R., Tirino V., Pirozzi G., Viglietto G. Activating E17K mutation in the gene encoding the protein kinase AKT1 in a subset of squamous cell carcinoma of the lung. Cell Cycle. 2008;7:665–669. doi: 10.4161/cc.7.5.5485. [DOI] [PubMed] [Google Scholar]
- 92.Marquard F.E., Jücker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020;172 doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- 93.Marsit C.J., Zheng S., Aldape K., Hinds P.W., Nelson H.H., Wiencke J.K., Kelsey K.T. PTEN expression in non-small-cell lung cancer: evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum. Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 94.Masoumi E., Tahaghoghi-Hajghorbani S., Jafarzadeh L., Sanaei M.-J., Pourbagheri-Sigaroodi A., Bashash D. The application of immune checkpoint blockade in breast cancer and the emerging role of nanoparticle. J. Controll. Release. 2021;340:168–187. doi: 10.1016/j.jconrel.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 95.Mateo J., Ganji G., Lemech C., Burris H.A., Han S.W., Swales K., Decordova S., DeYoung M.P., Smith D.A., Kalyana-Sundaram S., Wu J., Motwani M., Kumar R., Tolson J.M., Rha S.Y., Chung H.C., Eder J.P., Sharma S., Bang Y.J., Infante J.R., Yan L., de Bono J.S., Arkenau H.T. A first-time-in-human study of GSK2636771, a phosphoinositide 3 kinase beta-selective inhibitor, in patients with advanced solid tumors. Clin. Can. Res. 2017;23:5981–5992. doi: 10.1158/1078-0432.CCR-17-0725. : an official journal of the American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 96.Mazloumi Gavgani F., Smith Arnesen V., Jacobsen R.G., Krakstad C., Hoivik E.A., Lewis A.E. Class I phosphoinositide 3-kinase PIK3CA/p110α and PIK3CB/p110β isoforms in endometrial cancer. Int. J. Mol. Sci. 2018;19:3931. doi: 10.3390/ijms19123931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McCubrey J.A., Rakus D., Gizak A., Steelman L.S., Abrams S.L., Lertpiriyapong K., Fitzgerald T.L., Yang L.V., Montalto G., Cervello M. Effects of mutations in Wnt/β-catenin, hedgehog, Notch and PI3K pathways on GSK-3 activity-diverse effects on cell growth, metabolism and cancer. Biochim. Biophys. Acta. 2016;1863:2942–2976. doi: 10.1016/j.bbamcr.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 98.McGowan M., Hoven A.S., Lund-Iversen M., Solberg S., Helland Å., Hirsch F.R., Brustugun O.T. PIK3CA mutations as prognostic factor in squamous cell lung carcinoma. Lung Cancer. 2017;103:52–57. doi: 10.1016/j.lungcan.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 99.Meng J., Dai B., Fang B., Bekele B.N., Bornmann W.G., Sun D., Peng Z., Herbst R.S., Papadimitrakopoulou V., Minna J.D., Peyton M., Roth J.A. Combination treatment with MEK and AKT inhibitors is more effective than each drug alone in human non-small cell lung cancer in vitro and in vivo. PLoS One. 2010;5:e14124. doi: 10.1371/journal.pone.0014124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyake N., Chikumi H., Takata M., Nakamoto M., Igishi T., Shimizu E. Rapamycin induces p53-independent apoptosis through the mitochondrial pathway in non-small cell lung cancer cells. Oncol. Rep. 2012;28:848–854. doi: 10.3892/or.2012.1855. [DOI] [PubMed] [Google Scholar]
- 101.Molife L.R., Yan L., Vitfell-Rasmussen J., Zernhelt A.M., Sullivan D.M., Cassier P.A., Chen E., Biondo A., Tetteh E., Siu L.L., Patnaik A., Papadopoulos K.P., de Bono J.S., Tolcher A.W., Minton S. Phase 1 trial of the oral AKT inhibitor MK-2206 plus carboplatin/paclitaxel, docetaxel, or erlotinib in patients with advanced solid tumors. J. Hematol. Oncol. 2014;7:1. doi: 10.1186/1756-8722-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moore J., Megaly M., MacNeil A.J., Klentrou P., Tsiani E. Rosemary extract reduces Akt/mTOR/p70S6K activation and inhibits proliferation and survival of A549 human lung cancer cells. Biomed. Pharmacother. 2016;83:725–732. doi: 10.1016/j.biopha.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 103.Nogova L., Mattonet C., Scheffler M., Taubert M., Gardizi M., Sos M.L., Michels S., Fischer R.N., Limburg M., Abdulla D.S.Y., Persigehl T., Kobe C., Merkelbach-Bruse S., Franklin J., Backes H., Schnell R., Behringer D., Kaminsky B., Eichstaedt M., Stelzer C., Kinzig M., Sörgel F., Tian Y., Junge L., Suleiman A.A., Frechen S., Rokitta D., Ouyang D., Fuhr U., Buettner R., Wolf J. Sorafenib and everolimus in patients with advanced solid tumors and KRAS-mutated NSCLC: A phase I trial with early pharmacodynamic FDG-PET assessment. Cancer Med. 2020;9:4991–5007. doi: 10.1002/cam4.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ohara T., Takaoka M., Toyooka S., Tomono Y., Nishikawa T., Shirakawa Y., Yamatsuji T., Tanaka N., Fujiwara T., Naomoto Y. Inhibition of mTOR by temsirolimus contributes to prolonged survival of mice with pleural dissemination of non-small-cell lung cancer cells. Cancer Sci. 2011;102:1344–1349. doi: 10.1111/j.1349-7006.2011.01967.x. [DOI] [PubMed] [Google Scholar]
- 105.Ohashi K., Sequist L.V., Arcila M.E., Moran T., Chmielecki J., Lin Y.L., Pan Y., Wang L., de Stanchina E., Shien K., Aoe K., Toyooka S., Kiura K., Fernandez-Cuesta L., Fidias P., Yang J.C., Miller V.A., Riely G.J., Kris M.G., Engelman J.A., Vnencak-Jones C.L., Dias-Santagata D., Ladanyi M., Pao W. Lung cancers with acquired resistance to EGFR inhibitors occasionally harbor BRAF gene mutations but lack mutations in KRAS, NRAS, or MEK1. Proc. Nat. Acad. Sci. USA. 2012;109:E2127–E2133. doi: 10.1073/pnas.1203530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pan C., Yao G., Liu B., Ma T., Xia Y., Wei K., Wang J., Xu J., Chen L., Chen Y. Long noncoding RNA FAL1 promotes cell proliferation, invasion and epithelial-mesenchymal transition through the PTEN/AKT signaling axis in non-small cell lung cancer. Cell. Physiol. Biochem. 2017;43:339–352. doi: 10.1159/000480414. [DOI] [PubMed] [Google Scholar]
- 107.Papadimitrakopoulou V. Development of PI3K/AKT/mTOR pathway inhibitors and their application in personalized therapy for non-small-cell lung cancer. J. Thorac. Oncol. 2012;7:1315–1326. doi: 10.1097/JTO.0b013e31825493eb. : official publication of the International Association for the Study of Lung Cancer. [DOI] [PubMed] [Google Scholar]
- 108.Papadimitrakopoulou V., Adjei A.A. The Akt/mTOR and mitogen-activated protein kinase pathways in lung cancer therapy. J. Thorac. Oncol. 2006;1:749–751. : official publication of the International Association for the Study of Lung Cancer. [PubMed] [Google Scholar]
- 109.Papadimitrakopoulou V.A., Soria J.C., Jappe A., Jehl V., Klimovsky J., Johnson B.E. Everolimus and erlotinib as second- or third-line therapy in patients with advanced non-small-cell lung cancer. J. Thorac. Oncol. 2012;7:1594–1601. doi: 10.1097/JTO.0b013e3182614835. : official publication of the International Association for the Study of Lung Cancer. [DOI] [PubMed] [Google Scholar]
- 110.Perumal E., So Youn K., Sun S., Seung-Hyun J., Suji M., Jieying L., Yeun-Jun C. PTEN inactivation induces epithelial-mesenchymal transition and metastasis by intranuclear translocation of β-catenin and snail/slug in non-small cell lung carcinoma cells. Lung Cancer. 2019;130:25–34. doi: 10.1016/j.lungcan.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 111.Pesson M., Eymin B., De La Grange P., Simon B., Corcos L. A dedicated microarray for in-depth analysis of pre-mRNA splicing events: application to the study of genes involved in the response to targeted anticancer therapies. Mol. Cancer. 2014;13:9. doi: 10.1186/1476-4598-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Peyton J.D., Ahnert J.R., Burris H., Britten C., Chen L.C., Tabernero J., Duval V., Rouyrre N., Silva A.P., Quadt C., Baselga J. A dose-escalation study with the novel formulation of the oral pan-class I PI3K inhibitor BEZ235, solid dispersion system (SDS) sachet, in patients with advanced solid tumors. J. Clin. Oncol. 2011;29:3066. -3066. [Google Scholar]
- 113.Politz O., Siegel F., Bärfacker L., Bömer U., Hägebarth A., Scott W.J., Michels M., Ince S., Neuhaus R., Meyer K. BAY 1125976, a selective allosteric AKT1/2 inhibitor, exhibits high efficacy on AKT signaling-dependent tumor growth in mouse models. Int. J. Cancer. 2017;140:449–459. doi: 10.1002/ijc.30457. [DOI] [PubMed] [Google Scholar]
- 114.Postmus P., Kerr K., Oudkerk M., Senan S., Waller D., Vansteenkiste J., Escriu C., Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 115.Qi L., Zhu F., Li S.H., Si L.B., Hu L.K., Tian H. Retinoblastoma binding protein 2 (RBP2) promotes HIF-1α-VEGF-induced angiogenesis of non-small cell lung cancer via the Akt pathway. PLoS One. 2014;9 doi: 10.1371/journal.pone.0106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramalingam S.S., Harvey R.D., Saba N., Owonikoko T.K., Kauh J., Shin D.M., Sun S.Y., Strychor S., Tighiouart M., Egorin M.J., Fu H., Khuri F.R. Phase 1 and pharmacokinetic study of everolimus, a mammalian target of rapamycin inhibitor, in combination with docetaxel for recurrent/refractory nonsmall cell lung cancer. Cancer. 2010;116:3903–3909. doi: 10.1002/cncr.25264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rekhtman N., Paik P.K., Arcila M.E., Tafe L.J., Oxnard G.R., Moreira A.L., Travis W.D., Zakowski M.F., Kris M.G., Ladanyi M. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin. Cancer Res. 2012;18:1167–1176. doi: 10.1158/1078-0432.CCR-11-2109. : an official journal of the American Association for Cancer Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reungwetwattana T., Molina J.R., Mandrekar S.J., Allen-Ziegler K., Rowland K.M., Reuter N.F., Luyun R.F., Dy G.K., Marks R.S., Schild S.E., Jett J.R., Adjei A.A. Brief report: a phase II "window-of-opportunity" frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. J. Thorac. Oncol. 2012;7:919–922. doi: 10.1097/JTO.0b013e31824de0d6. : official publication of the International Association for the Study of Lung Cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rice S.J., Liu X., Wang H.G., Belani C.P. EGFR mutations and AKT phosphorylation are markers for sensitivity to combined MCL-1 and BCL-2/xL inhibition in non-small cell lung cancer. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rivera V.M., Squillace R.M., Miller D., Berk L., Wardwell S.D., Ning Y., Pollock R., Narasimhan N.I., Iuliucci J.D., Wang F. Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol. Cancer Ther. 2011;10:1059–1071. doi: 10.1158/1535-7163.MCT-10-0792. [DOI] [PubMed] [Google Scholar]
- 121.Sanaei M.J., Baghery Saghchy Khorasani A., Pourbagheri-Sigaroodi A., Shahrokh S., Zali M.R., Bashash D. The PI3K/Akt/mTOR axis in colorectal cancer: Oncogenic alterations, non-coding RNAs, therapeutic opportunities, and the emerging role of nanoparticles. J. Cell. Physiol. 2021;237:1–33. doi: 10.1002/jcp.30655. [DOI] [PubMed] [Google Scholar]
- 122.Santarpia M., Gil N., Rosell R. Strategies to overcome resistance to tyrosine kinase inhibitors in non-small-cell lung cancer. Expert Rev. Clin. Pharmacol. 2015;8:461–477. doi: 10.1586/17512433.2015.1055252. [DOI] [PubMed] [Google Scholar]
- 123.Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.H., Hsu P.P., Bagley A.F., Markhard A.L., Sabatini D.M. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 124.Scrima M., De Marco C., Fabiani F., Franco R., Pirozzi G., Rocco G., Ravo M., Weisz A., Zoppoli P., Ceccarelli M., Botti G., Malanga D., Viglietto G. Signaling networks associated with AKT activation in non-small cell lung cancer (NSCLC): new insights on the role of phosphatydil-inositol-3 kinase. PLoS One. 2012;7:e30427. doi: 10.1371/journal.pone.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seki Y., Yamamoto N., Tamura Y., Goto Y., Shibata T., Tanioka M., Asahina H., Nokihara H., Yamada Y., Shimamoto T. Phase I study for ridaforolimus, an oral mTOR inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012;69:1099–1105. doi: 10.1007/s00280-011-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sengupta S., Eavarone D., Capila I., Zhao G., Watson N., Kiziltepe T., Sasisekharan R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 127.Sequist L.V., Waltman B.A., Dias-Santagata D., Digumarthy S., Turke A.B., Fidias P., Bergethon K., Shaw A.T., Gettinger S., Cosper A.K., Akhavanfard S., Heist R.S., Temel J., Christensen J.G., Wain J.C., Lynch T.J., Vernovsky K., Mark E.J., Lanuti M., Iafrate A.J., Mino-Kenudson M., Engelman J.A. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shapiro G.I., Rodon J., Bedell C., Kwak E.L., Baselga J., Braña I., Pandya S.S., Scheffold C., Laird A.D., Nguyen L.T., Xu Y., Egile C., Edelman G. Phase I safety, pharmacokinetic, and pharmacodynamic study of SAR245408 (XL147), an oral pan-class I PI3K inhibitor, in patients with advanced solid tumors. Clin. Cancer Res. 2014;20:233–245. doi: 10.1158/1078-0432.CCR-13-1777. : an official journal of the American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 129.Shaykhiev R., Otaki F., Bonsu P., Dang D.T., Teater M., Strulovici-Barel Y., Salit J., Harvey B.G., Crystal R.G. Cigarette smoking reprograms apical junctional complex molecular architecture in the human airway epithelium in vivo. Cell. Mol. Life Sci. 2011;68:877–892. doi: 10.1007/s00018-010-0500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi H., Pu J., Zhou X.L., Ning Y.Y., Bai C. Silencing long non-coding RNA ROR improves sensitivity of non-small-cell lung cancer to cisplatin resistance by inhibiting PI3K/Akt/mTOR signaling pathway. Tumor Biol. 2017;39 doi: 10.1177/1010428317697568. [DOI] [PubMed] [Google Scholar]
- 131.Shih M.C., Chen J.Y., Wu Y.C., Jan Y.H., Yang B.M., Lu P.J., Cheng H.C., Huang M.S., Yang C.J., Hsiao M., Lai J.M. TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene. 2012;31:2389–2400. doi: 10.1038/onc.2011.419. [DOI] [PubMed] [Google Scholar]
- 132.Shimizu M., Tanaka N. IL-8-induced O-GlcNAc modification via GLUT3 and GFAT regulates cancer stem cell-like properties in colon and lung cancer cells. Oncogene. 2019;38:1520–1533. doi: 10.1038/s41388-018-0533-4. [DOI] [PubMed] [Google Scholar]
- 133.Shimizu T., Tolcher A.W., Papadopoulos K.P., Beeram M., Rasco D.W., Smith L.S., Gunn S., Smetzer L., Mays T.A., Kaiser B., Wick M.J., Alvarez C., Cavazos A., Mangold G.L., Patnaik A. The clinical effect of the dual-targeting strategy involving PI3K/AKT/mTOR and RAS/MEK/ERK pathways in patients with advanced cancer. Clin. Cancer Res. 2012;18:2316–2325. doi: 10.1158/1078-0432.CCR-11-2381. : an official journal of the American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 134.Skwarek L.C., Boulianne G.L. Great expectations for PIP: phosphoinositides as regulators of signaling during development and disease. Dev. Cell. 2009;16:12–20. doi: 10.1016/j.devcel.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 135.Soria J.C., LoRusso P., Bahleda R., Lager J., Liu L., Jiang J., Martini J.F., Macé S., Burris H. Phase I dose-escalation study of pilaralisib (SAR245408, XL147), a pan-class I PI3K inhibitor, in combination with erlotinib in patients with solid tumors. Oncologist. 2015;20:245–246. doi: 10.1634/theoncologist.2014-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sos M.L., Koker M., Weir B.A., Heynck S., Rabinovsky R., Zander T., Seeger J.M., Weiss J., Fischer F., Frommolt P., Michel K., Peifer M., Mermel C., Girard L., Peyton M., Gazdar A.F., Minna J.D., Garraway L.A., Kashkar H., Pao W., Meyerson M., Thomas R.K. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spoerke J.M., O'Brien C., Huw L., Koeppen H., Fridlyand J., Brachmann R.K., Haverty P.M., Pandita A., Mohan S., Sampath D., Friedman L.S., Ross L., Hampton G.M., Amler L.C., Shames D.S., Lackner M.R. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin. Cancer Res. 2012;18:6771–6783. doi: 10.1158/1078-0432.CCR-12-2347. : an official journal of the American Association for Cancer Research. [DOI] [PubMed] [Google Scholar]
- 138.Sun Z., Wang Z., Liu X., Wang D. New development of inhibitors targeting the PI3K/AKT/mTOR pathway in personalized treatment of non-small-cell lung cancer. Anticancer Drugs. 2015;26:1–14. doi: 10.1097/CAD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 139.Sun Z., Wang Z., Liu X., Wang D. New development of inhibitors targeting the PI3K/AKT/mTOR pathway in personalized treatment of non-small-cell lung cancer. Anticancer Drugs. 2015;26:1–14. doi: 10.1097/CAD.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 140.Świderska E., Strycharz J., Wróblewski A., Szemraj J., Drzewoski J., Śliwińska A. Blood Glucose Levels. IntechOpen; 2018. Role of PI3K/AKT pathway in insulin-mediated glucose uptake. [Google Scholar]
- 141.Taber A.M., Khurshid H., Perez K., Birnbaum A.E., Olszewski A.J., Luppe D., Jean M., Rosati K., Safran H. Phase I study of ridaforolimus with cetuximab for patients with advanced non-small cell lung cancer (NSCLC), colorectal cancer, and head and neck cancer. J. Clin. Oncol. 2013;31:8075. -8075. [Google Scholar]
- 142.Tan A.C. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC) Thorac. Cancer. 2020;11:511–518. doi: 10.1111/1759-7714.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang J.M., He Q.Y., Guo R.X., Chang X.J. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 144.Tao Y.F., Li Z.H., Du W.W., Xu L.X., Ren J.L., Li X.L., Fang F., Xie Y., Li M., Qian G.H. Inhibiting PLK1 induces autophagy of acute myeloid leukemia cells via mammalian target of rapamycin pathway dephosphorylation. Oncol. Rep. 2017;37:1419–1429. doi: 10.3892/or.2017.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tian F., Ouyang T., Lu N., Shen Y., Bai Y., Xie X., Wang J., Lu J., Ge Q. MiR-486-5p serves as a good biomarker in NSCLC and suppresses cell growth with the involvement of a target-PIK3R1. Front. Genet. 2019;10:688. doi: 10.3389/fgene.2019.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tian J., Yuan L. Sirtuin 6 inhibits colon cancer progression by modulating PTEN/AKT signaling. Biomed. Pharmacother. 2018;106:109–116. doi: 10.1016/j.biopha.2018.06.070. [DOI] [PubMed] [Google Scholar]
- 147.Toschi L., Finocchiaro G., Nguyen T.T., Skokan M.C., Giordano L., Gianoncelli L., Perrino M., Siracusano L., Di Tommaso L., Infante M., Alloisio M., Roncalli M., Scorsetti M., Jänne P.A., Santoro A., Varella-Garcia M. Increased SOX2 gene copy number is associated with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and predicts improved survival in early stage disease. PLoS One. 2014;9:e95303. doi: 10.1371/journal.pone.0095303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Travis W.D., Brambilla E., Burke A.P., Marx A., Nicholson A.G. Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J. Thorac. Oncol. 2015;10:1240–1242. doi: 10.1097/JTO.0000000000000663. [DOI] [PubMed] [Google Scholar]
- 149.Troncone M., Cargnelli S.M., Villani L.A., Isfahanian N., Broadfield L.A., Zychla L., Wright J., Pond G., Steinberg G.R., Tsakiridis T. Targeting metabolism and AMP-activated kinase with metformin to sensitize non-small cell lung cancer (NSCLC) to cytotoxic therapy: translational biology and rationale for current clinical trials. Oncotarget. 2017;8:57733. doi: 10.18632/oncotarget.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Utermark T., Rao T., Cheng H., Wang Q., Lee S.H., Wang Z.C., Iglehart J.D., Roberts T.M., Muller W.J., Zhao J.J. The p110α and p110β isoforms of PI3K play divergent roles in mammary gland development and tumorigenesis. Genes Dev. 2012;26:1573–1586. doi: 10.1101/gad.191973.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.VanderLaan P.A., Rangachari D., Mockus S.M., Spotlow V., Reddi H.V., Malcolm J., Huberman M.S., Joseph L.J., Kobayashi S.S., Costa D.B. Mutations in TP53, PIK3CA, PTEN and other genes in EGFR mutated lung cancers: correlation with clinical outcomes. Lung Cancer. 2017;106:17–21. doi: 10.1016/j.lungcan.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vink S.R., Schellens J.H., Beijnen J.H., Sindermann H., Engel J., Dubbelman R., Moppi G., Hillebrand M.J., Bartelink H., Verheij M. Phase I and pharmacokinetic study of combined treatment with perifosine and radiation in patients with advanced solid tumours. Radiother. Oncol. 2006;80:207–213. doi: 10.1016/j.radonc.2006.07.032. : journal of the European Society for Therapeutic Radiology and Oncology. [DOI] [PubMed] [Google Scholar]
- 153.Voliva C., Pecchi S., Burger M. Proceedings of the American Association for Cancer Research Annual Meeting. 2010. Biological characterization of NVP-BKM120, a novel inhibitor of phosphoinositide-3-kinase in phase I/II trials. Washington, DC. [Google Scholar]
- 154.Wallace J.A., Li F., Balakrishnan S., Cantemir-Stone C.Z., Pecot T., Martin C., Kladney R.D., Sharma S.M., Trimboli A.J., Fernandez S.A., Yu L., Rosol T.J., Stromberg P.C., Lesurf R., Hallett M., Park M., Leone G., Ostrowski M.C. Ets2 in tumor fibroblasts promotes angiogenesis in breast cancer. PLoS One. 2013;8:e71533. doi: 10.1371/journal.pone.0071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wallin J.J., Edgar K.A., Guan J., Berry M., Prior W.W., Lee L., Lesnick J.D., Lewis C., Nonomiya J., Pang J., Salphati L., Olivero A.G., Sutherlin D.P., O'Brien C., Spoerke J.M., Patel S., Lensun L., Kassees R., Ross L., Lackner M.R., Sampath D., Belvin M., Friedman L.S. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol. Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 156.Wan J., Wu W. Hyperthermia induced HIF-1a expression of lung cancer through AKT and ERK signaling pathways. J. Exp. Clin. Cancer Res. 2016;35:119. doi: 10.1186/s13046-016-0399-7. CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H., Cai J., Du S., Guo Z., Xin B., Wang J., Wei W., Shen X. Fractalkine/CX3CR1 induces apoptosis resistance and proliferation through the activation of the AKT/NF-κB cascade in pancreatic cancer cells. Cell Biochem. Funct. 2017;35:315–326. doi: 10.1002/cbf.3278. [DOI] [PubMed] [Google Scholar]
- 158.Wang L., Hu H., Pan Y., Wang R., Li Y., Shen L., Yu Y., Li H., Cai D., Sun Y., Chen H. PIK3CA mutations frequently coexist with EGFR/KRAS mutations in non-small cell lung cancer and suggest poor prognosis in EGFR/KRAS wildtype subgroup. PLoS One. 2014;9:e88291. doi: 10.1371/journal.pone.0088291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y., Wang Y., Li J., Li J., Che G. Clinical significance of PIK3CA gene in non-small-cell lung cancer: a systematic review and meta-analysis. Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/3608241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Waqar S.N., Baggstrom M.Q., Morgensztern D., Williams K., Rigden C., Govindan R. A phase I trial of temsirolimus and pemetrexed in patients with advanced non-small cell lung cancer. Chemotherapy. 2016;61:144–147. doi: 10.1159/000442147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Waqar S.N., Gopalan P.K., Williams K., Devarakonda S., Govindan R. A phase I trial of sunitinib and rapamycin in patients with advanced non-small cell lung cancer. Chemotherapy. 2013;59:8–13. doi: 10.1159/000348584. [DOI] [PubMed] [Google Scholar]
- 162.West K.A., Brognard J., Clark A.S., Linnoila I.R., Yang X., Swain S.M., Harris C., Belinsky S., Dennis P.A. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wise-Draper T.M., Moorthy G., Salkeni M.A., Karim N.A., Thomas H.E., Mercer C.A., Beg M.S., O'Gara S., Olowokure O., Fathallah H. A phase Ib study of the dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target. Oncol. 2017;12:323–332. doi: 10.1007/s11523-017-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu H., Meng S., Xu Q., Wang X., Wang J., Gong R., Song Y., Duan Y., Zhang Y. Gene expression profiling of lung adenocarcinoma in Xuanwei, China. Eur. J. Cancer Prev. 2016;25:508–517. doi: 10.1097/CEJ.0000000000000214. : the official journal of the European Cancer Prevention Organisation (ECP) [DOI] [PubMed] [Google Scholar]
- 165.Wymann M.P., Bulgarelli-Leva G., Zvelebil M.J., Pirola L., Vanhaesebroeck B., Waterfield M.D., Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xu F., Na L., Li Y., Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10:1–12. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 167.Xu K., Liu P., Wei W. mTOR signaling in tumorigenesis. Biochim. Biophys. Acta (BBA) Rev. Cancer. 2014;1846:638–654. doi: 10.1016/j.bbcan.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu W., Yang Z., Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adhes. Migr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xu X., Zhang Y., Qu D., Jiang T., Li S. Osthole induces G2/M arrest and apoptosis in lung cancer A549 cells by modulating PI3K/Akt pathway. J. Exp. Clin. Cancer Res. 2011;30:33. doi: 10.1186/1756-9966-30-33. : CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamamoto C., Basaki Y., Kawahara A., Nakashima K., Kage M., Izumi H., Kohno K., Uramoto H., Yasumoto K., Kuwano M., Ono M. Loss of PTEN expression by blocking nuclear translocation of EGR1 in gefitinib-resistant lung cancer cells harboring epidermal growth factor receptor-activating mutations. Cancer Res. 2010;70:8715–8725. doi: 10.1158/0008-5472.CAN-10-0043. [DOI] [PubMed] [Google Scholar]
- 171.Yang W., Xiao W., Cai Z., Jin S., Li T. miR-1269b drives cisplatin resistance of human non-small cell lung cancer via modulating the PTEN/PI3K/AKT signaling pathway. OncoTarget. Ther. 2020;13:109–118. doi: 10.2147/OTT.S225010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zappa C., Mousa S.A. Non-small cell lung cancer: current treatment and future advances. Transl. Lung Cancer Res. 2016;5:288. doi: 10.21037/tlcr.2016.06.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang M., Jang H., Gaponenko V., Nussinov R. Phosphorylated calmodulin promotes PI3K activation by binding to the SH2 domains. Biophys. J. 2017;113:1956–1967. doi: 10.1016/j.bpj.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang X., Cheng D., Liu Y., Wu Y., He Z. Gephyrin suppresses lung squamous cell carcinoma development by reducing mTOR pathway activation. Cancer Manag. Res. 2019;11:5333. doi: 10.2147/CMAR.S204358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao H., Wang Y., Ren X. Nicotine promotes the development of non-small cell lung cancer through activating LINC00460 and PI3K/Akt signaling. Biosci. Rep. 2019;39 doi: 10.1042/BSR20182443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao J., Han Y., Li J., Chai R., Bai C. Prognostic value of KRAS/TP53/PIK3CA in non-small cell lung cancer. Oncol. Lett. 2019;17:3233–3240. doi: 10.3892/ol.2019.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou B., Wang D., Sun G., Mei F., Cui Y., Xu H. Effect of miR-21 on apoptosis in lung cancer cell through inhibiting the PI3K/Akt/NF-κB signaling pathway in Vitro and in Vivo. Cell. Physiol. Biochem. 2018;46:999–1008. doi: 10.1159/000488831. : international journal of experimental cellular physiology, biochemistry, and pharmacology. [DOI] [PubMed] [Google Scholar]
- 178.Zhu Z., Sun H., Ma G., Wang Z., Li E., Liu Y., Liu Y. Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway. Int. J. Mol. Sci. 2012;13:2025–2035. doi: 10.3390/ijms13022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zou Z.Q., Zhang L.N., Wang F., Bellenger J., Shen Y.Z., Zhang X.H. The novel dual PI3K/mTOR inhibitor GDC-0941 synergizes with the MEK inhibitor U0126 in non-small cell lung cancer cells. Mol. Med. Rep. 2012;5:503–508. doi: 10.3892/mmr.2011.682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Dear editor-in-chief,
Our manuscript entitled "The gravity of the PI3K/Akt/mTOR signaling pathway alterations in lung cancer and challenges toward targeting it; Where are we now?" was transferred to your reputable journal from “European Journal of Pharmacology”. It is our pleasure to submit our article to your journal; however, regarding the open-access condition we do not afford to pay the charges. In this case, Prof. Justin Lathia and Prof. Megan Monachino kindly accepted our article for a waiver as part of our Article Advance program.
I would like to submit our manuscript for review and, hopefully, publication in your prestigious journal. Neither the article nor any part of it has been or will be published or submitted elsewhere.
Author's expertise in the subject of the PI3K pathway in cancer:
Bashash D, Delshad M, Riyahi N, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Momeny M. Inhibition of PI3K signaling pathway enhances the chemosensitivity of APL cells to ATO: Proposing novel therapeutic potential for BKM120. Eur J Pharmacol. 2018 Dec 15;841:10–18. doi: 10.1016/j.ejphar.2018.10.007.
Bashash D, Delshad M, Safaroghli-Azar A, Safa M, Momeny M, Ghaffari SH. Novel pan PI3K inhibitor-induced apoptosis in APL cells correlates with suppression of telomerase: An emerging mechanism of action of BKM120. Int J Biochem Cell Biol. 2017 Oct;91(Pt A):1–8. doi: 10.1016/j.biocel.2017.08.009.
Bashash D, Safaroghli-Azar A, Dadashi M, Safa M, Momeny M, Ghaffari SH. Anti-tumor activity of PI3K-δ inhibitor in hematologic malignant cells: Shedding new light on resistance to Idelalisib. Int J Biochem Cell Biol. 2017 Apr;85:149–158. doi: 10.1016/j.biocel.2017.02.007.
Bashash D, Safaroghli-Azar A, Delshad M, Bayati S, Nooshinfar E, Ghaffari SH. Inhibitor of pan class-I PI3K induces differentially apoptotic pathways in acute leukemia cells: Shedding new light on NVP-BKM120 mechanism of action. Int J Biochem Cell Biol. 2016 Oct;79:308–317. doi: 10.1016/j.biocel.2016.09.004.
Safaroghli-Azar A, Bashash D, Sadreazami P, Momeny M, Ghaffari SH. PI3K-δ inhibition using CAL-101 exerts apoptotic effects and increases doxorubicin-induced cell death in pre-B-acute lymphoblastic leukemia cells. Anticancer Drugs. 2017 Apr;28(4):436–445. doi: 10.1097/CAD.0000000000000477.
Riyahi N, Safaroghli-Azar A, Sheikh-Zeineddini N, Sayyadi M, Bashash D. Synergistic Effects of PI3K and c-Myc Co-targeting in Acute Leukemia: Shedding New Light on Resistance to Selective PI3K-δ Inhibitor CAL-101. Cancer Invest. 2019;37(7):311–324. doi: 10.1080/07357907.2019.1651328.
Safaroghli-Azar A, Bashash D, Kazemi A, Pourbagheri-Sigaroodi A, Momeny M. Anticancer effect of pan-PI3K inhibitor on multiple myeloma cells: Shedding new light on the mechanisms involved in BKM120 resistance. Eur J Pharmacol. 2019 Jan 5;842:89–98. doi: 10.1016/j.ejphar.2018.10.036.
Shiri Heris R, Safaroghli-Azar A, Yousefi AM, Hamidpour M, Bashash D. Anti-leukemic effect of PI3K inhibition on chronic myeloid leukemia (CML) cells: shedding new light on the mitigating effect of c-Myc and autophagy on BKM120 cytotoxicity. Cell Biol Int. 2020 May;44(5):1212–1223. doi: 10.1002/cbin.11322.
Alipour F, Riyahi N, Safaroghli-Azar A, Sari S, Zandi Z, Bashash D. Inhibition of PI3K pathway using BKM120 intensified the chemo-sensitivity of breast cancer cells to arsenic trioxide (ATO). Int J Biochem Cell Biol. 2019 Nov;116:105615. doi: 10.1016/j.biocel.2019.105615.
Sadeghi S, Esmaeili S, Pourbagheri-Sigaroodi A, Safaroghli-Azar A, Bashash D. PI3K Abrogation Using Pan-PI3K Inhibitor BKM120 Gives Rise to a Significant Anticancer Effect on AML-Derived KG-1 Cells by Inducing Apoptosis and G2/M Arrest. Turk J Haematol. 2020 Aug 28;37(3):167–176. doi: 10.4274/tjh.galenos.2020.2019.0440.
Mosleh M, Safaroghli-Azar A, Bashash D. Pan-HDAC inhibitor panobinostat, as a single agent or in combination with PI3K inhibitor, induces apoptosis in APL cells: An emerging approach to overcome MSC-induced resistance. Int J Biochem Cell Biol. 2020 May;122:105734. doi: 10.1016/j.biocel.2020.105734.
Zehtabcheh S, Yousefi AM, Salari S, Safa M, Momeny M, Ghaffari SH, Bashash D. Abrogation of histone deacetylases (HDACs) decreases survival of chronic myeloid leukemia cells: New insight into attenuating effects of the PI3K/c-Myc axis on panobinostat cytotoxicity. Cell Biol Int. 2021 May;45(5):1111–1121. doi: 10.1002/cbin.11557. Epub 2021 Feb 4. PMID: 33501756.
Sayyadi M, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Abolghasemi H, Bashash D. c-Myc Inhibition Using 10058-F4 Increased the Sensitivity of Acute Promyelocytic Leukemia Cells to Arsenic Trioxide Via Blunting PI3K/NF-κB Axis. Arch Med Res. 2020 Oct;51(7):636–644. doi: 10.1016/j.arcmed.2020.06.002.
Esmaeili S, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Salari S, Gharehbaghian A, Hamidpour M, Bashash D. Activation of PPARγ intensified the effects of arsenic trioxide in acute promyelocytic leukemia through the suppression of PI3K/Akt pathway: Proposing a novel anticancer effect for pioglitazone. Int J Biochem Cell Biol. 2020 May;122:105739. doi: 10.1016/j.biocel.2020.105739.
Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, Safaroghli-Azar A, Zali MR, Bashash D. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021 May 5;898:173983. doi:10.1016/j.ejphar.2021.173983.
Akbari Dilmaghani N, Safaroghli-Azar A, Pourbagheri-Sigaroodi A, Bashash D. The PI3K/Akt/mTORC signaling axis in head and neck squamous cell carcinoma: Possibilities for therapeutic interventions either as single agents or in combination with conventional therapies. IUBMB Life. 2021 Apr;73(4):618–642. doi: 10.1002/iub.2446.
Sanaei M-J, Pourbagheri-Sigaroodi A, Kaveh V, Sheikholeslami SA, Salari S, Bashash D. The application of nano-medicine to overcome the challenges related to immune checkpoint blockades in cancer immunotherapy: Recent advances and opportunities. Crit Rev Oncol Hematol. 2020:103160. doi: 10.1016/j.critrevonc.2020.103160
Sanaei MJ, Pourbagheri-Sigaroodi A, Kaveh V, Abolghasemi H, Ghaffari SH, Momeny M, Bashash D. Recent advances in immune checkpoint therapy in non-small cell lung cancer and opportunities for nanoparticle-based therapy. European journal of pharmacology. 2021;909:174404. doi: 10.1016/j.ejphar.2021.174404.