The coronavirus disease 2019 (COVID-19) has been declared as a pandemic by the World Health Organization.[1] Most COVID-19 patients exhibit mild to moderate symptoms, while approximately 15% progress rapidly to severe pneumonia, and about 5% eventually develop acute respiratory distress syndrome (ARDS),[2] which requires mechanical ventilation (MV) and even extracorporeal membrane oxygenation. The mortality of COVID-19 patients who received MV was reported to be as high as 66%.[3] Therefore, the treatments aiming to improve mortality should focus on two aspects: first, prevention of the aggravation of the disease in mild and moderate COVID-19 patients; second, the rescue therapy for patients in serious conditions.
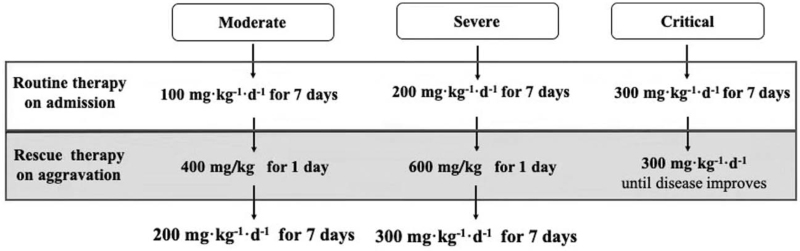
We have been applying high-dose intravenous vitamin C (HDIVC) in the treatment of critical illnesses for almost 10 years in our center. Our previous in vivo research showed that HDIVC protected hemorrhagic shock-related multiple organ failure (MOF) by inhibiting inflammatory cytokines and oxidative indicators through activating Sirtuin1 pathway.[4] Two randomized controlled trials are now being conducted to evaluate the efficiency and safety of HDIVC in sepsis (ChiCTR1800017633) and severe acute pancreatitis (ChiCTR1900022022). Based on that, we started to apply HDIVC in COVID-19 pneumonia since February 2, 2020 in Shanghai Public Health Clinical Center. By summarizing the experience with these patients, a HDIVC protocol [Figure 1] was proposed by the Shanghai COVID-19 Clinical Treatment Expert Group. The application of HDIVC protocol varied according to the disease severity which was classified as mild, moderate, severe, and critical.[5] The mild type did not require HDIVC treatment. The HDIVC protocol for moderate, severe, and critical type mainly consisted of two parts: the routine usage of HDIVC at admission and then for seven consecutive days, which might be beneficial for the prevention of disease aggravation. The other part is about the rescue therapy, which might be essential for live saving when disease aggravation occurs. Our studies showed the inflammatory response, immune and organ function improved after HDIVC application in a retrospective case series study,[6] and the number of moderate COVID-19 patients transferring to severe type was reduced after HDIVC protocol application.[7]
Figure 1.
Flowchart of HDIVC protocol. Aggravation means disease severity transfers to the next level within 24 to 48 hours. HDIVC: High-dose intravenous vitamin C.
The rationale for HDIVC in the treatment of COVID-19 is based on the following aspects: (1) Rapid scavenging of reactive oxygen species (ROS) and relieving ROS related inflammatory response, endothelial dysfunction, coagulopathy, ARDS, and MOF. Under the condition of hypoxemia induced by COVID-19, ROS are produced in mitochondria in a huge amount.[8] ROS induce release of cytokines and chemokines via certain mechanisms such as nuclear factor kappa B (NF-κB) signaling pathway,[8] resulting in so-called “cytokine storm.” ROS directly damages the vascular endothelial cells and causes the pulmonary interstitial edema, which represents the main pathophysiology of ARDS. Furthermore, ROS can induce the occurrence of coagulopathy by triggering platelet aggregation and activating the coagulation system. Coagulopathy is a common feature of COVID-19 characterized by an increase in D-dimer, fibrin degradation product levels and longer activated partial thromboplastin time.[9] The extensive microvascular clot formation further augments tissue hypoxia due to increased diffusion distance for oxygen, thereby leading to MOF. Vitamin C, eliminating ROS in direct and indirect way, has been shown to be beneficial for severe sepsis patient. The vitamin C infusion for treatment in sepsis induced acute lung injury trial,[10] the largest randomized clinical trial on HDIVC, reported that the 28-day all-cause mortality (29.8% vs. 46.3%) was reduced by HDIVC in a cohort of septic patients with ARDS. (2) Potential improving effect on lymphopenia. Lymphopenia was correlated with the development of ARDS and disease severity.[3] The mechanisms of lymphopenia mainly include growth inhibition and apoptosis of hematopoietic cells and T lymphocytes induced by the severe acute respiratory coronavirus 2 through the promotion of autoimmune antibody and production of certain cytokine.[11] Vitamin C is essential for the development, maturation, and proliferation of functional T-lymphocytes.[12] Although there are limited studies on the effect of HDIVC on lymphocytes in sepsis, it is speculated that HDIVC might be beneficial for the lymphopenia occurring in COVID-19 patients as we found the number of CD4+ T lymphocyte increased after HDIVC application.[6,7] (3) Maintaining circulation function stability. Vitamin C is an important co-factor for the synthesis of the endogenous hormone, including catecholamine, corticosteroid, and vasopressin.[13] A previous clinical study[14] showed less need for vasopressor and lower 28-day mortality in septic shock patients after HDIVC application. For COVID-19 patients with septic shock, HDIVC, especially a high bolus dose over a short time, might help the recovery of circulation failure. (4) Attenuating the COVID-19 related scurvy rapidly. According to the latest report,[15] the level of vitamin C is almost undetectable in severe COVID-19 patients. The “scurvy” state is correlated with MOF in critically ill patients.[16] Giving vitamin C intravenously can quickly increase the serum levels of vitamin C from scurvy (10–20 μmol/L) to mmol/L level.[17] Therefore, patients might draw benefits from HDIVC because of its quick supplemental effect compared to oral pathway. (5) Safety of HDIVC. One major concern of HIDVC is its potential adverse effects including oxalate nephropathy and formation of urine stone. No confirmed evidence supported the high-dose vitamin C related adverse events mentioned above in critically illness.[18] Similarly, we did not observe any potential adverse events either.
In conclusion, HDIVC is an efficient and safe treatment for patients with COVID-19. It might be applied in prevention of disease aggravation in moderate types, as well as rescue therapy for the severe and critical type. Anyway, high-quality randomized clinical trials are warranted.
Acknowledgements
The authors thank all the staff of the Shanghai Public Health Clinical Center for their great efforts in the treatment of COVID-19 patients.
Funding
This study was supported by the grants from the second batch of the emergency key scientific and technological project of Shanghai Municipal Committee of Science and Technology (Nos. 20411950300 and 20411950301).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhao B, Li M, Ling Y, Peng Y, Huang J, Qu H, Gao Y, Li Y, Hu B, Lu S, Lu H, Zhang W, Mao E. Potential benefit of high-dose intravenous vitamin C for coronavirus disease 2019 pneumonia. Chin Med J 2022;135:23–25. doi: 10.1097/CM9.0000000000001746
References
- 1.Dyer O. Covid-19: hospitals brace for disaster as US surpasses China in number of cases. BMJ 2020; 368:m1278.doi: 10.1136/bmj.m1278. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qi MZ, Yao Y, Xie RL, Sun SL, Sun WW, Wang JL, et al. Intravenous vitamin C attenuates hemorrhagic shock-related renal injury through the induction of SIRT1 in rats. Biochem Biophys Res Commun 2018; 501:358–364. doi: 10.1016/j.bbrc.2018.04.111. [DOI] [PubMed] [Google Scholar]
- 5.National Health Commission & National Administration of Traditional Chinese Medicine. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J 2020; 133:1087–1095. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao B, Ling Y, Li J, Peng Y, Huang J, Wang Y, et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: a retrospective case series study. Ann Palliat Med 2021; 10:1599–1609. doi: 10.21037/apm-20-1387. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Liu M, Liu P, Peng Y, Huang J, Li M, et al. High dose intravenous vitamin C for preventing the disease aggravation of moderate COVID-19 pneumonia. A retrospective propensity matched before-after study. Front Pharmacol 2021; 12:638556.doi: 10.3389/fphar.2021.638556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, Shimizu S, Kiyonaka S, Takahashi N, Wajima T, Hara Y, et al. TRPM2-mediated Ca2+influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat Med 2008; 14:738–747. doi: 10.1038/nm1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID-19 coagulopathy. Crit Care 2020; 24:360.doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowler AA, 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 2019; 322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res 2014; 59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning J, Mitchell B, Appadurai DA, Shakya A, Pierce LJ, Wang H, et al. Vitamin C promotes maturation of T-cells. Antioxid Redox Signal 2013; 19:2054–2067. doi: 10.1089/ars.2012.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr AC, Shaw GM, Fowler AA, Natarajan R. Ascorbate-dependent vasopressor synthesis: a rationale for vitamin C administration in severe sepsis and septic shock. Crit Care 2015; 19:418.doi: 10.1186/s13054-015-1131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zabet MH, Mohammadi M, Ramezani M, Khalili H. Effect of high-dose ascorbic acid on vasopressor's requirement in septic shock. J Res Pharm Pract 2016; 5:94–100. doi: 10.4103/2279-042X.179569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiscano-Camón L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Roca O, Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care 2020; 24:522.doi: 10.1186/s13054-020-03249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996; 24:392–397. doi: 10.1097/00003246-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Marik PE. Vitamin C for the treatment of sepsis: the scientific rationale. Pharmacol Ther 2018; 189:63–70. doi: 10.1016/j.pharmthera.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Yanase F, Fujii T, Naorungroj T, Belletti A, Luethi N, Carr AC, et al. Harm of IV high-dose vitamin C therapy in adult patients: a scoping review. Crit Care Med 2020; 48:e620–e628. doi: 10.1097/CCM.0000000000004396. [DOI] [PubMed] [Google Scholar]