Abstract
Background:
Acquired immune deficiency syndrome (AIDS)-related non-Hodgkin lymphoma (AR-NHL) is a high-risk factor for morbidity and mortality in patients with AIDS. This study aimed to determine the prognostic factors associated with overall survival (OS) and to develop a prognostic nomogram incorporating computed tomography imaging features in patients with acquired immune deficiency syndrome-related non-Hodgkin lymphoma (AR-NHL).
Methods:
A total of 121 AR-NHL patients between July 2012 and November 2019 were retrospectively reviewed. Clinical and radiological independent predictors of OS were confirmed using multivariable Cox analysis. A prognostic nomogram was constructed based on the above clinical and radiological factors and then provided optimum accuracy in predicting OS. The predictive accuracy of the nomogram was determined by Harrell C-statistic. Kaplan–Meier survival analysis was used to determine median OS. The prognostic value of adjuvant therapy was evaluated in different subgroups.
Results:
In the multivariate Cox regression analysis, involvement of mediastinal or hilar lymph nodes, liver, necrosis in the lesions, the treatment with chemotherapy, and the CD4 ≤100 cells/μL were independent risk factors for poor OS (all P < 0.050). The predictive nomogram based on Cox regression has good discrimination (Harrell C-index = 0.716) and good calibration (Hosmer–Lemeshow test, P = 0.620) in high- and low-risk groups. Only patients in the high-risk group who received adjuvant chemotherapy had a significantly better survival outcome.
Conclusion:
A survival-predicting nomogram was developed in this study, which was effective in assessing the survival outcomes of patients with AR-NHL. Notably, decision-making of chemotherapy regimens and more frequent follow-up should be considered in the high-risk group determined by this model.
Keywords: Lymphoma, AIDS-related AR-NHL, Computed tomography, Prognosis, Nomogram
Introduction
Acquired immune deficiency syndrome (AIDS)-related non-Hodgkin lymphoma (AR-NHL) is a high-risk factor for morbidity and mortality in patients with AIDS.[1,2] Although the incidence of AIDS-related tumors has decreased with the advent of highly active antiretroviral therapy (HAART), the occurrence rate of AR-NHL appears to be unexpectedly declined.[3,4] In addition to HAART, the use of adjuvant chemotherapy can improve the tolerance and remission rate of patients; however, inappropriate adjuvant therapy may induce adverse effects in patients, including myelosuppression, tissue necrosis, and liver dysfunction. To date, the application of chemotherapies and chemoradiotherapy remains controversial, partially due to the failure of selecting suitable AR-NHL patients.
Additionally, previous studies reported that CD4+ count, human immunodeficiency virus (HIV) ribonucleic acid levels, Ann Arbor stage, lactate dehydrogenase (LDH) levels, international prognostic index (IPI) score, and age are key predictors for survival in AR-NHL patients.[5–7] However, only a few studies investigated the significance of imaging characteristics for predicting prognosis and survival. Novel imaging modalities for assessing lymphoma can provide useful information for treatment regimens and predict patients’ prognoses. With the advancement of imaging techniques such as computed tomography (CT), magnetic resonance, and positron emission computed tomography (PET)/CT, radiological techniques play an increasingly essential role in detecting lesions and evaluating disease.[8–10] CT can detect enlarged lymph nodes, guide biopsy, and observe early relapse through follow-up.[11–13] Magnetic resonance imaging and PET/CT are limited due to various economic and social factors in some developing countries.[14,15] Therefore, a widely applicable nomogram based on clinical and CT characteristics is needed to appropriately predict prognosis in patients.
To address this issue, we integrated clinical and CT-related factors to create a novel nomogram to stratify the low- and high-risk groups. This study aimed to determine the prognostic factors associated with overall survival and to develop a prognostic nomogram incorporating clinical and CT imaging features in patients with AR-NHL.
Methods
Ethics approval
The study was conducted under approval by the Institutional Review Board of You’an Hospital Affiliated of Capital Medical University (No. 2018066). The consent to participate was waived due to the retrospective nature of the study.
Patients
In this multicenter retrospective study, information of 181 patients with AIDS-related lymphoma from three tertiary infectious disease hospitals was retrospectively reviewed and their clinical and imaging data were analyzed between July 2012 and November 2019. The diagnosis of HIV infection was based on the standards of the Centers for Disease Control and prevention of the USA. The diagnosis of lymphoma was based on puncture biopsy (163 patients), endoscopic biopsy (six patients), and operation specimens (12 patients). All intervention and treatment were processed according to National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: non-Hodgkin lymphomas.[16–18] If the patients were in stable conditions, they were followed up once every 3 months in the first year, once every 6 months in the second year, once every year in the third year, and so on. Patients were followed up at any time if disease progression and deterioration occurred. Overall survival (OS) was selected as the endpoint. OS was measured from the lymphoma diagnosis until the last follow-up or death from any cause. Follow-up was continued until November 2020.
Patients eligible for this study were (1) with age >18 years, (2) with a history of HIV-infection, (3) with pathologically confirmed diffuse large B-cell lymphoma (DLBCL) or Burkitt lymphoma (BL),[19] and (4) with available clinical and CT imaging data before any clinical intervention. Patients with Hodgkin lymphoma, indolent B-cell non-Hodgkin lymphoma (NHL), and T-cell NHL or lacking a specific pathological type were excluded. One patient younger than 18 years of age and two patients with severe artifacts in the CT images were also excluded. Patients who were lost during follow-up were also excluded from the study.
All examinations were imaged with Philips CT 256 (Philips, Amsterdam, Netherlands), 39 patients accepted contrast-enhanced CT scan via intravenous contrast materials. The CT protocols were: tube voltage, 120 kV; automatic tube current, 30 to 300 mA; rotation time, 0.75 s; collimation, 0.625 mm; pitch, 0.945; matrix, 512 × 512; section thickness, 5 mm; breath-hold at full inspiration. The images were transmitted to the workstation and picture achieving and communication systems for multiplanar reconstruction and post-processing. All images (both axial CT images and multiplanar reconstruction images) were reviewed by three radiologists (Doctor A with 22 years of experience, B with 7 years of experience, and C with 10 years of experience) blinded to clinical and laboratory data. Three statisticians assessed the CT features independently. After separate evaluations, any divergences were resolved by discussion or consultation from a specialist in infectious imaging (Doctor D with 33 years of experience), eventually reviewed by Doctor E for consistency analysis. Baseline data are provided in Supplementary Data.
Statistical analysis
The imaging findings were tested for agreement using the Kappa test. If the Kappa value was <0.400, the consistency of the diagnostic findings was poor. If the Kappa value was >0.750, then the diagnostic findings were considered to be sufficiently consistent. Continuous variables of parameters were tested for normal distribution using the Kolmogorov–Smirnov method. If the data fitted a normal distribution, mean ± standard deviation and the t test were used to check for differences between the two groups. The chi-square test and Fisher exact test were used to compare categorical variables.
The univariate analysis of a Kaplan–Meier (K–M) analysis model was fitted to determine the significant prognostic factors for OS of the patients. If P values of prognostic factors were <0.050, they were tested in a multivariate Cox proportional hazard model for the independence of association. Factors showing significant impact in the multivariate analysis were expressed via forest graph. Proportional hazards assumption was assessed through visual inspection of (log–log) plots of cumulative log hazard against time. A predictive model was developed for AR-NHL using Cox regression and illustrated by nomogram. The accuracy of predictions was assessed by estimating the nomogram discrimination measured by Harrell concordance index (C-index). The C-index is the probability chosen for two patients randomly, the patient who had the event first had a higher probability of having the event according to the model. C-index = 0.500 represents an agreement by chance; C-index = 1.000 represents perfect discrimination.[20] The calibration of the nomogram was evaluated by the Hosmer–Lemeshow test. K–M estimates were used to determine median OS by Log-rank methods, defined as the time period between the date of pathological diagnosis and last follow-up or death. All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The figures were developed using GraphPad Prism 7 (GraphPad Software; San Diego, CA, USA) and R software (version 4.0.1; http://www.r-project.org). All statistical tests were two-sided, the significance level was set at P < 0.050.
Results
Patients’ demographic data
A total of 121 AR-NHL patients was included in the final analysis who were reviewed at multiple centers between 2000 and 2016. The median age at diagnosis was 40 years (range, 35–53 years), and 112 (92.5%) of the eligible patients were male. Among all patients, 83 received chemotherapy, 57 underwent definitive HAART. Table 1 summarizes the demographic data, clinical, and tumor characteristics of laboratory results, such as white blood cell, neutrophils, and lymphocytes. All CT features were defined as a good agreement among the three doctors (Kappa value 0.778–0.998; Supplementary Table S1).
Table 1.
Demographic, clinical, and tumor characteristics of patients with AR-NHL.
| Characteristic | Values |
| Age (years) | 40 (35–53) |
| Sex | |
| Male | 112 (92.5) |
| Female | 9 (7.5) |
| Pathology | |
| DLBCL | 84 (69.4) |
| BL | 37 (30.5) |
| Time of finding HIV (months) | 9 (2–36) |
| Time of finding mass (months) | 3 (1–5) |
| HAART | |
| Administered | 57 (47.1) |
| Not administered | 64 (52.9) |
| Chemotherapy | |
| Administered | 83 (68.5) |
| Not administered | 38 (31.5) |
| Systemic symptoms | |
| Positive | 30 (24.7) |
| Negative | 91 (75.3) |
| CD4 count (cells/μL) | 187 (55–280) |
| WBC, × 109 | 6.17 (3.97–7.44) |
| NEUT, % | 66 (58–75) |
| Lymphocytes, % | 24 (18–32) |
| Ann Arbor stage | |
| 1 | 37 (30.6) |
| 2 | 36 (29.7) |
| 3 | 21 (17.4) |
| 4 | 27 (22.3) |
| Axillary lymph nodes | |
| Involved | 43 (35.5) |
| Uninvolved | 78 (64.5) |
| Cervical lymph nodes | |
| Involved | 17 (14) |
| Uninvolved | 104 (86) |
| Abdominal pelvic and peritoneal lymph nodes | |
| Involved | 15 (12.3) |
| Uninvolved | 106 (87.7) |
| Retroperitoneal lymph nodes | |
| Involved | 14 (11.5) |
| Uninvolved | 107 (88.5) |
| Inguinal lymph nodes | |
| Involved | 8 (6.6) |
| Uninvolved | 113 (93.4) |
| Gastrointestinal tract | |
| Involved | 40 (33) |
| Uninvolved | 81 (67) |
| Urinary system organs | |
| Involved | 11 (9.1) |
| Uninvolved | 110 (90.9) |
| Liver | |
| Involved | 27 (22.3) |
| Uninvolved | 94 (77.7) |
| Lung | |
| Involved | 20 (16.5) |
| Uninvolved | 101 (83.5) |
| Mediastinal/hilar lymph nodes | |
| Involved | 24 (19.8) |
| Uninvolved | 97 (80.1) |
| Pancreas/spleen | |
| Involved | 15 (12.4) |
| Uninvolved | 106 (87.6) |
Data are presented as n (%) or median (inter-quantile range). AR-NHL: AIDS-related non-Hodgkin lymphoma; BL: Burkitt lymphoma; DLBCL: Diffuse large B-cell lymphoma; HAART: Highly active antiretroviral therapy; HIV: Human immunodeficiency virus; IQR: Interquartile range; NEUT: Neutrophil; WBC: White blood cell.
Prognostic factors for OS
The median follow-up of the entire cohort was 12 months. On univariable analysis, factors associated with poor OS included CD4 ≤100 cells/μL, involvement of mediastinal or hilar lymph nodes, liver, gastrointestinal tract, presence of extracapsular infiltration, necrosis inside the lesions, and treatment without chemotherapy [Table 2]. On multivariable Cox analysis, involvement of mediastinal or hilar lymph nodes, liver, necrosis in the lesions, CD4 ≤100 cells/μL, and treatment without chemotherapy were independent risk factors for short OS. Significant radiological features were shown in Figure 1.
Table 2.
Clinical prognostic factors for survival of total patients using univariate and multivariate analyses.
| Univariate | Multivariate | |||
| Characteristic | Median OS (IQR), months | P | Hazard ratio (95% CI) | P |
| Age (years) | 0.911 | |||
| ≤40 | 18.0 (7.0–29.0) | |||
| >40 | 17.0 (6.0–30.0) | |||
| The period from HIV diagnosis to admission (months) | 0.424 | |||
| ≤6 | 17.0 (7.5–31.5) | |||
| >6 | 12.0 (5.0–27.5) | |||
| The period from finding the mass to admission (months) | 0.314 | |||
| ≤1 | 11.0 (5.0–29.0) | |||
| >1 | 23.0 (9.7–30.0) | |||
| Histology | 0.285 | |||
| DLBCL | 16.0 (6.0–29.0) | |||
| BL | 23.0 (9.0–29.0) | |||
| CD4 cell count (cells/μL) | 0.020 | 2.660 (1.420–4.982) | 0.002 | |
| ≤100 | 8.0 (4.5–29.5) | |||
| >100 | 21.5 (8.6–29.7) | |||
| HAART | 0.869 | |||
| Administered | 20.0 (5.2–29.8) | |||
| Not administered | 16.0 (7.0–29.0) | |||
| Chemotherapy | <0.001 | 0.482 (0.261–0.892) | 0.020 | |
| Administered | 23.0 (10.0–30.0) | |||
| Not administered | 7.0 (3.0–27.5) | |||
| Axillary lymph nodes | 0.120 | |||
| Involved | 10.0 (6.0–28.0) | |||
| Uninvolved | 21.5 (7.0–29.2) | |||
| Cervical lymph nodes | 0.280 | |||
| Involved | 12.0 (4.0–27.0) | |||
| Uninvolved | 19.0 (7.0–29.0) | |||
| Mediastinal/hilar lymph nodes | 0.022 | 1.699 (1.019–2.833) | 0.042 | |
| Involved | 9.5 (5.0–24.5) | |||
| Uninvolved | 21.0 (7.0–30.0) | |||
| Abdominal pelvic and peritoneal lymph nodes | 0.710 | |||
| Involved | 10.0 (5.0–28.0) | |||
| Uninvolved | 18.0 (6.7–29.0) | |||
| Retroperitoneal lymph nodes | 0.560 | |||
| Involved | 11.0 (5.3–23.5) | |||
| Uninvolved | 19.0 (6.0–30.0) | |||
| Inguinal lymph nodes | 0.050 | |||
| Involved | 26.5 (20.7–34.5) | |||
| Uninvolved | 16.0 (6.0–29.0) | |||
| Gastrointestinal tract | 0.015 | 1.860 (1.120–3.100) | 0.210 | |
| Involved | 11.0 (5.6–26.0) | |||
| Uninvolved | 23.0 (7.0–30.0) | |||
| Urinary system organs | 0.550 | |||
| Involved | 16 (3–27) | |||
| Uninvolved | 18 (7–30) | |||
| Liver | <0.001 | 2.484 (1.453–4.247) | 0.001 | |
| Involved | 7.0 (4.0–17.0) | |||
| Uninvolved | 22.5 (9.0–30.5) | |||
| Lung | 0.680 | |||
| Involved | 22.5 (6.2–30.5) | |||
| Uninvolved | 17.0 (6.5–29.0) | |||
| Diameter of max. focus (mm) | 0.534 | |||
| <5 | 16.0 (5.5–29.0) | |||
| ≥5 | 19.5 (7.2–29.7) | |||
| Shape | 0.458 | |||
| Irregular | 14.5 (6.2–30.0) | |||
| Circular | 21.0 (7.0–28.0) | |||
| Fusion tendency | 0.796 | |||
| With | 17 (6–30) | |||
| Without | 19 (7–29) | |||
| Extracapsular infiltration | 0.032 | 1.750 (1.050–2.910) | 0.090 | |
| With | 12 (6–27) | |||
| Without | 24 (7–30) | |||
| Necrosis | 0.005 | 2.020 (1.214–3.360) | 0.007 | |
| With | 8.7 (3.6–27.0) | |||
| Without | 22.0 (9.0–30.0) | |||
| Texture | 0.139 | |||
| Heterogeneous | 13.0 (6.0–29.0) | |||
| Homogeneous | 22.0 (6.5–29.5) | |||
| Attenuation | 0.059 | |||
| Hypoattenuation | 8.0 (4.5–23.0) | |||
| Isoattenuation | 21.0 (7.0–30.0) | |||
| Hyperattenuation | 12.0 (3.0–30.0) | |||
| Enhancement scan texture | 0.793 | |||
| Heterogeneous | 13.0 (5.0–27.5) | |||
| Homogeneous | 15.0 (3.7–31.5) | |||
| Enhancement degree | 0.568 | |||
| Mild | 13.0 (5.0–29.0) | |||
| Moderate | 15.0 (5.5–28.5) | |||
BL: Burkitt's lymphoma; DLBCL: Diffuse large B-cell lymphoma; HAART: Highly active antiretroviral therapy; HIV: Human immunodeficiency virus; IQR: Interquartile range; OS: Overall survival. CI: confidence interval.
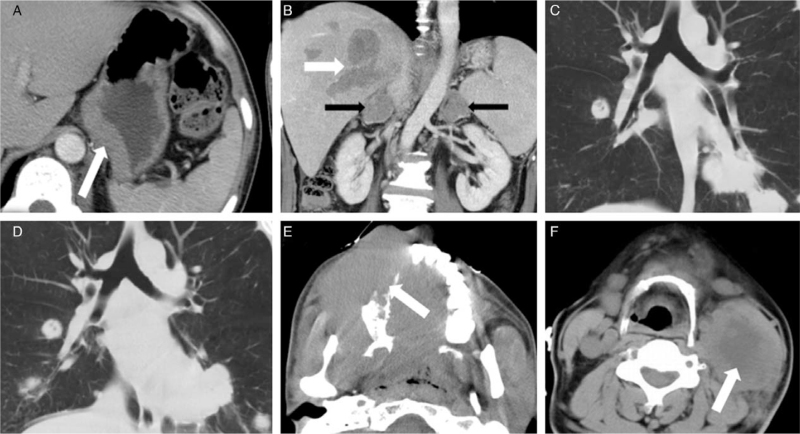
Figure 1.
(A) A 37-year-old man with DLBCL was found positive HIV-antibodies for 1 month and with nausea and fever for 1 day. The thickening wall of the stomach was detected using CT (white arrow). The OS time of this patient was 11 months. (B) A 31-year-old man with BL was found positive HIV-antibodies for 1 month, fever for 5 days. CT showed multiple, irregular, heterogeneous lesions of the liver (white arrow) and bilateral adrenal glands (black arrows). The OS of this patient was 5 months. (C, D) A 29-year-old man with BL was found positive HIV-antibodies for 2 months and dyspnea and fever for 3 days. CT lung window showed multiple irregular lesions in bilateral lungs; enlarged lymph nodes were found on mediastinal and hilum. Progression was found on a 1-month follow-up. (D) The OS time of this patient was 3 months. (E) A 25-year-old man with DLBCL was found positive HIV-antibodies for 3 months. A large irregular, homogeneous, isoattenuation mass that infiltrated adjacent bone and muscles was found in the right cervical area IX (white arrow). (F) A 40-year-old man with DLBCL was found HIV-antibodies for 5 months. A large circular, heterogeneous mass with necrosis was found inside the left cervical area II (white arrow). BL: Burkitt lymphoma; CT: Computed tomography; DLBCL: Diffuse large B-cell lymphoma; HIV: Human immunodeficiency virus; OS: Overall survival.
Development of nomogram
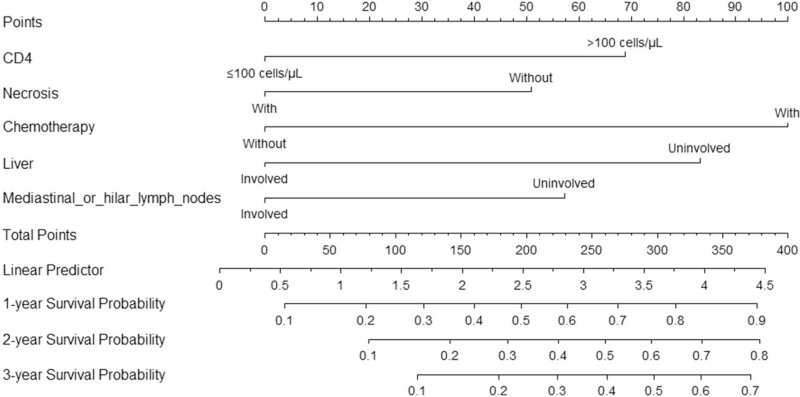
The predictive models were based on Cox regression and illustrated by nomogram [Figure 2], indicating the probability of 1-, 2-, and 3-year OS in patients with AR-NHL. Harrell C-index was 0.716, suggesting relatively good discrimination. The Hosmer–Lemeshow test demonstrated a P = 0.620 > 0.050, indicating no departure from a good fit. The probability of survival at 1, 2, and 3 years was obtained by drawing a vertical line from the “total points” axis straight down to the outcome axes. The total number of points for each patient was obtained by summing the points for each of the individual factors in the nomogram. In the predictive model, treated with chemotherapy can increase 100 points for total points, then followed by liver involved free (add 82 points), CD4 counts >100 cells/μL (add 69 points), mediastinal or hilar lymph nodes involved free (add 58 points), and without necrosis (add 50 points). For instance, an AR-NHL patient with liver involved (0 points), without mediastinal or hilar lymph nodes involved (58 points), CD4 counts 125 cells/μL (69 points), with tumor necrosis (0 points), treated with chemotherapy (100 points), and the total points were 227 points. This patient was predicted to have a 58% probability of surviving for 1 year, 40% probability of surviving for 2 years, and 31% probability of surviving for 3 years [Figure 3].
Figure 2.
Nomogram indicates the probability of 1-, 2-, and 3-year OS in patients with AR-NHL. CD4 was determined by drawing a line straight up to the point axis for clinical. Then, this process was repeated for other four variables (necrosis, chemotherapy, liver involved, mediastinal or hilar lymph nodes involved). Each variable had a corresponding value (points), which were marked in the superior toolbar. Total adding up scores of all variables could predict OS probability. AR-NHL: AIDS-related non-Hodgkin lymphoma; NHL: Non-Hodgkin lymphoma; OS: Overall survival.
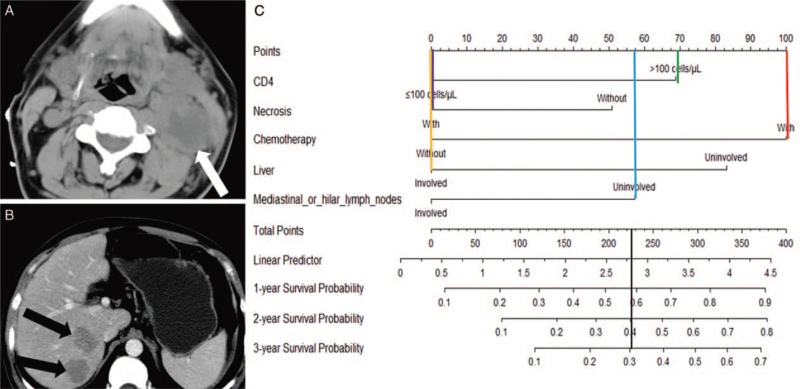
Figure 3.
Examples of using the nomogram to predict the individual survival probability by manually placing straight lines across the diagram. A 32-year-old man was found positive HIV-antibodies for 7 months and with the treatment of HAART. (A) A large circular, heterogeneous mass with necrosis was found inside the left cervical area II (white arrow). (B) Two irregular, heterogeneous lesions were found in the liver (black arrow). DLBCL was confirmed by biopsy. The patient received a chemotherapy regimen of CHOP and OS time was 14 months. The total points are 227 points. This patient is predicted to have a 58% probability of surviving for 1 year, 40% probability of surviving for 2 years, and 31% probability of surviving for 3 years (black line). CHOP: Cyclophosphamide, doxorubicin, vincristine and prednisolone; DLBCL: Diffuse large B-cell lymphoma; HAART: Highly active antiretroviral therapy; HIV: Human immunodeficiency virus; OS: Overall survival.
Clinical value of the novel classification system
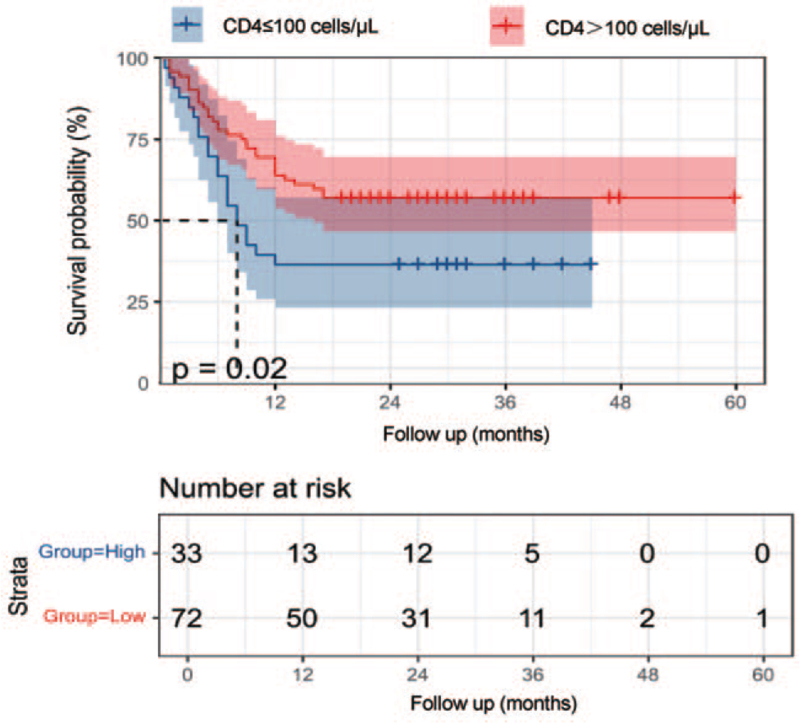
Compared with the patients with levels of CD4 < 100 cells/μL, the patients with levels of CD4 over 100 cells/μL had a good prognosis (P = 0.020) [Figure 4]. K–M analysis and the log-rank test showed that patients who did not receive chemotherapy had poor survival outcomes than those receiving chemotherapy (P < 0.050) [Supplementary Figure S1]. The patients with involvement of mediastinal or hilar lymph nodes or involvement of the liver, or the lesions with necrosis had a worse prognosis (P < 0.050) [Supplementary Figure S1].
Figure 4.
Kaplan–Meier.curves of CD4 for the whole patient population. Compared with the patients with levels of CD4 <100 cells/μL, the patients with levels of CD4 over 100 cells/μL had a good prognosis (P = 0.020).
Next, we further compared the survival outcomes between patients with and without chemotherapy in each subgroup based on our novel survival-predicting model. Notably, patients in the high-risk group could benefit from chemotherapy treatment (P = 0.040).
Discussion
To the best of our knowledge, this is the largest study from triple institutions in Asia to evaluate prognostic factors in patients with AR-NHL. We developed a nomogram based on patients’ demographics, CT imaging features, and laboratory data, which can be effectively stratified patients into low- and high-risk groups. Involvement of mediastinal or hilar lymph nodes, liver, necrosis in lesions, CD4 ≤100 cells/μL, and treatment without chemotherapy were independent risk factors for shorter OS. Notably, only patients in the high-risk group in our study were found to be significantly benefited from chemotherapy, so we strongly recommend patients in the high-risk group as candidates for chemotherapy.
Imaging plays an important role in the detection and evaluation of AR-NHL lesions.[8,10,12] CT of the head and neck, chest, abdomen, and pelvis is a critical staging modality recommended by the NCCN guidelines.[18–20] The potential mechanism of necrosis in lymphoma is the occlusion of the supplying hilar artery by the tumor (compression or invasion) in addition to lymphatic flow obstruction.[21,22]A previous study reported that HIV (−) NHL patients with necrosis had significantly higher Ann Arbor stages, greater IPI, and higher serum LDH levels than those without necrosis. However, in their K–M survival analysis, no statistically significant difference was noted for necrosis.[23] Our study focused on patients with AR-NHL, and necrosis in the lesions was an independent risk factor for shorter OS. Necrosis of the lesion indicated the aggressive behavior of the tumor and the tendency for treatment resistance. The presence of extracapsular infiltration is also correlated with the OS in the current study. The invasion of tumor cells may be a potential mechanism of extracapsular infiltration and may correlate with a poor prognosis.[24] Natural killer (NK) cells play an important role in the growth and infiltration of lymphoma cells and activated NK cells could be a promising immunotherapeutic tool against lymphoma cells either alone or in combination with conventional therapy.[25]
AR-NHLs are usually B-cell, high-grade, and poorly differentiated lymphomas.[26] Extranodal sites involvement is common. The liver is the second most common site with an incidence ranging from 26% to 45%. HIV (+) patients have a higher incidence of NHL than HIV (−) patients.[27,28] A previous study indicated that primary mediastinal large B-cell lymphoma (PMBCL), representing 10% of all DLBCL, was predictive of poor OS and progression-free survival.[29] Compared with cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) regimen, rituximab and its use with intensified chemotherapy such as R-Hyper-cyclophosphamide, vincristine, doxorubicin, and dexamethasone and R-EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) might improve the response rate and survival outcome for patients with mediastinal NHL, especially for PMBCL.[29–31]
Although Guech-Ongey et al[32,33] found acquired immune deficiency syndrome-related Burkitt lymphoma incidence declined at low CD4 counts, suggesting functional CD4 lymphocytes may be required for BL to develop, we consider lower CD4 counts to reflect more severe immunodeficiency, which is likely to cause opportunistic infections and other malignant tumors for patients with AR-NHL.
For patients who have factors correlated with shorter OS time, intensive chemotherapy should be considered. Intensive chemotherapy is relatively safe and effective in AR-NHL.[34] Notably, only patients in the high-risk group in our study were found to be significantly benefited from chemotherapy, so we strongly recommend patients in the high-risk group as candidates for chemotherapy. Chemotherapy and concomitant HAART for AR-NHL does not cause prolonged suppression of lymphocyt-e subsets. On the contrary, chemotherapy can increase the counts of CD4, CD8, CD19, and CD56 cell populations, which provide reassurance regarding the long-term consequences of chemotherapy in these individuals.[35] While for patients in the lower-risk group, the survival difference was not statistically significant, so HAART alone and regular examination are recommended, because adverse characteristics, such as severe bone marrow toxicity chemotherapy, should be considered.[36]
There are several limitations to the current study. First, it is a retrospective study with a limited number of patients, and patients were diagnosed in different hospitals. Therefore, the quality of chemotherapy and the methods employed by pathologists for diagnosing metastatic lymph nodes were uniform, which might bias our results. Second, although univariate analysis showed that pathology classifications (acquired immune deficiency syndrome-related diffuse large B-cell lymphoma and acquired immune deficiency syndrome-related Burkitt lymphoma) were not an independent prognostic factor for patients, other studies have reported that pathology classifications correspond to a different OS. Prospective investigations with larger samples focus on certain pathological types should be designed to find more predictive factors for prognoses of AR-NHL patients. Importantly, external validation of the proposed staging system in an independent cohort is required to determine whether it can be generalized to other institutions. Despite the current limitations, our study still has a high value because CT lesions necrosis characteristics and organ involvement in clinical work have a high degree of recognition.
In conclusion, a survival-predicting nomogram integrating CT features was developed in this study, which was promising for assessing the survival outcomes of patients with AR-NHL. Notably, decision-making of chemotherapy regimens and more frequent follow-up should be considered in the high-risk group determined by this model.
Acknowledgements
We thank all the patients, investigators, co-investigators, and study teams at each participating site.
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Li X, Pan Z, Wang X, Hu T, Ye W, Jiang D, Shen W, Liu J, Shi Y, Xia S, Li H. Prognostic nomogram incorporating radiological features for predicting overall survival in patients with AIDS-related non-Hodgkin lymphoma. Chin Med J 2022;135:70–78. doi: 10.1097/CM9.0000000000001785
Xueqin Li and Ziang Pan contributed equally to the article.
Supplemental digital content is available for this article.
References
- 1.Vangipuram R, Tyring SK. AIDS-associated malignancies. Cancer Treat Res 2019; 177:1–21. doi: 10.1007/978-3-030-03502-0_1. [DOI] [PubMed] [Google Scholar]
- 2.Re A, Cattaneo C, Rossi G. HIV and lymphoma: from epidemiology to clinical management. Mediterr J Hematol Infect Dis 2019; 11:e2019004.doi: 10.4084/mjhid.2019.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanik EL, Achenbach CJ, Gopal S, Coghill AE, Cole SR, Eron JJ, et al. Changes in clinical context for kaposi's sarcoma and non-Hodgkin lymphoma among people with HIV infection in the United States. J Clin Oncol 2016; 34:3276–3283. doi: 10.1200/jco.2016.67.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond C, Taylor TH, Aboumrad T, Anton-Culver H. Changes in acquired immunodeficiency syndrome-related non-Hodgkin lymphoma in the era of highly active antiretroviral therapy: incidence, presentation, treatment, and survival. Cancer 2006; 106:128–135. doi: 10.1002/cncr.21562. [DOI] [PubMed] [Google Scholar]
- 5.Bower M, Gazzard B, Mandalia S, Newsom-Davis T, Thirlwell C, Dhillon T, et al. A prognostic index for systemic AIDS-related non-Hodgkin lymphoma treated in the era of highly active antiretroviral therapy. Ann Intern Med 2005; 143:265–273. doi: 10.7326/0003-4819-143-4-200508160-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cuellar LE, Anampa-Guzmán A, Holguín AM, Velarde J, Portillo-Alvarez D, Zuñiga-Ninaquispe MA, et al. Prognostic factors in HIV-positive patients with non-Hodgkin lymphoma: a Peruvian experience. Infect Agent Cancer 2018; 13:27.doi: 10.1186/s13027-018-0200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miralles P, Berenguer J, Ribera JM, Rubio R, Mahillo B, Téllez MJ, et al. Prognosis of AIDS-related systemic non-Hodgkin lymphoma treated with chemotherapy and highly active antiretroviral therapy depends exclusively on tumor-related factors. J Acquir Immune Defic Syndr 2007; 44:167–173. doi: 10.1097/QAI.0b013e31802bb5d0. [DOI] [PubMed] [Google Scholar]
- 8.Kwee TC, Nievelstein RAJ, Torigian DA. Role of structural imaging in lymphoma. PET Clin 2012; 7:1–19. doi: 10.1016/j.cpet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Evans WC, Gilmore D, English J. The role of PET and PET/CT in managing the care of lymphoma patients. J Nucl Med Technol 2011; 39:190–194. doi: 10.2967/jnmt.110.082982. [DOI] [PubMed] [Google Scholar]
- 10.Javadi S, Menias CO, Karbasian N, Shaaban A, Shah K, Osman A, et al. HIV-related malignancies and mimics: imaging findings and management. Radiographics 2018; 38:2051–2068. doi: 10.1148/rg.2018180149. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Chen A, Jiang H, Zhang Y, Zhu L, Xia C, et al. HRCT in primary pulmonary lymphoma: can CT imaging phenotypes differentiate histological subtypes between mucosa-associated lymphoid tissue (MALT) lymphoma and non-MALT lymphoma? J Thorac Dis 2018; 10:6040–6049. doi: 10.21037/jtd.2018.10.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah HJ, Keraliya AR, Jagannathan JP, Tirumani SH, Lele VR, Dipiro PJ. Diffuse large B-cell lymphoma in the era of precision oncology: how imaging is helpful. Korean J Radiol 2017; 18:54–70. doi: 10.3348/kjr.2017.18.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thurnher MM, Rieger A, Kleibl-Popov C, Settinek U, Henk C, Haberler C, et al. Primary central nervous system lymphoma in AIDS: a wider spectrum of CT and MRI findings. Neuroradiology 2001; 43:29–35. doi: 10.1007/s002340000480. [DOI] [PubMed] [Google Scholar]
- 14.Mhlanga JC, Durand D, Tsai HL, Durand CM, Leal JP, Wang H, et al. Differentiation of HIV-associated lymphoma from HIV-associated reactive adenopathy using quantitative FDG PET and symmetry. Eur J Nucl Med Mol Imaging 2014; 41:596–604. doi: 10.1007/s00259-013-2671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warwick JM, Sathekge MM. PET/CT scanning with a high HIV/AIDS prevalence. Transfus Apher Sci 2011; 44:167–172. doi: 10.1016/j.transci.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Zelenetz AD, Abramson JS, Advani RH, Andreadis CB, Byrd JC, Czuczman MS, et al. NCCN clinical practice guidelines in oncology: non-Hodgkin's lymphomas. J Natl Compr Canc Netw 2010; 8:288–334. doi: 10.6004/jnccn.2010.0021. [DOI] [PubMed] [Google Scholar]
- 17.Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, Andreadis CB, et al. Non-Hodgkin's lymphomas, version 4.2014. J Natl Compr Cancer Netw 2014; 12:1282–1303. doi: 10.6004/jnccn.2014.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horwitz SM, Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, et al. NCCN guidelines insights: non-Hodgkin's lymphomas, Version 3.2016. J Natl Compr Cancer Netw 2016; 14:1067–1079. doi: 10.6004/jnccn.2016.0117. [DOI] [PubMed] [Google Scholar]
- 19.Agostini A, Borgheresi A, Bruno F, Natella R, Floridi C, Carotti M, et al. New advances in CT imaging of pancreas diseases: a narrative review. Gland Surg 2020; 9:2283–2294. doi: 10.21037/gs-20-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15:361–387. doi: 10.1002/(sici)1097-0258(19960229)15:4<361::Aid-sim168>3.0.Co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Saito A, Takashima S, Takayama F, Kawakami S, Momose M, Matsushita T. Spontaneous extensive necrosis in non-Hodgkin lymphoma: prevalence and clinical significance. J Comput Assist Tomogr 2001; 25:482–486. doi: 10.1097/00004728-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 22.You SH, Kim B, Yang KS, Kim BK. Cervical necrotic lymphadenopathy: a diagnostic tree analysis model based on CT and clinical findings. Eur J Radiol 2019; 29:5635–5645. doi: 10.1007/s00330-019-06155-2. [DOI] [PubMed] [Google Scholar]
- 23.Adams HJA, De Klerk JMH, Fijnheer R, Dubois SV, Nievelstein RAJ, Kwee TC. Prognostic value of tumor necrosis at CT in diffuse large B-cell lymphoma. Eur J Radiol 2015; 84:372–377. doi: 10.1016/j.ejrad.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Wu Q, Zheng D, Shi L, Liu M, Wang M, Shi D. Differentiating metastatic from nonmetastatic lymph nodes in cervical cancer patients using monoexponential, biexponential, and stretched exponential diffusion-weighted MR imaging. Eur Radiol 2017; 27:5272–5279. doi: 10.1007/s00330-017-4873-1. [DOI] [PubMed] [Google Scholar]
- 25.Dewan MZ, Terunuma H, Toi M, Tanaka Y, Katano H, Deng X, et al. Potential role of natural killer cells in controlling growth and infiltration of AIDS-associated primary effusion lymphoma cells. Cancer Sci 2006; 97:1381–1387. doi: 10.1111/j.1349-7006.2006.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maso LD, Franceschi S. Epidemiology of non-Hodgkin lymphomas and other haemolymphopoietic neoplasms in people with AIDS. Lancet Oncol 2003; 4:110–119. doi: 10.1016/s1470-2045(03)00983-5. [DOI] [PubMed] [Google Scholar]
- 27.Rizzi EB, Schinina V, Cristofaro M, David V, Bibbolino C. Non-Hodgkin's lymphoma of the liver in patients with AIDS: sonographic, CT, and MRI findings. J Clin Ultrasound 2001; 29:125–129. doi: 10.1002/1097-0096(200103/04)29:3<125::aid-jcu1011>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 28.Mcginnis KA, Fultz SL, Skanderson M, Conigliaro J, Bryant K, Justice AC. Hepatocellular carcinoma and non-Hodgkin's lymphoma: the roles of HIV, hepatitis C infection, and alcohol abuse. J Clin Oncol 2006; 24:5005–5009. doi: 10.1200/jco.2006.05.7984. [DOI] [PubMed] [Google Scholar]
- 29.Roschewski M, Phelan JD, Wilson WH. Molecular classification and treatment of diffuse large B-cell lymphoma and primary mediastinal B-cell lymphoma. Cancer J 2020; 26:195–205. doi: 10.1097/ppo.0000000000000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27:v91–v102. doi: 10.1093/annonc/mdw175. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Zhao J, An T, Wang Y, Zhuo M, Wu M, et al. Clinical characteristics and outcomes of patients with primary mediastinal germ cell tumors: a single-center experience. Front Oncol 2020; 10:1137.doi: 10.3389/fonc.2020.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piriou E, Van Dort K, Nanlohy NM, Van Oers MH, Miedema F, Van Baarle D. Loss of EBNA1-specific memory CD4+ and CD8+ T cells in HIV-infected patients progressing to AIDS-related non-Hodgkin lymphoma. Blood 2005; 106:3166–3174. doi: 10.1182/blood-2005-01-0432. [DOI] [PubMed] [Google Scholar]
- 33.Guech-Ongey M, Simard EP, Anderson WF, Engels EA, Bhatia K, Devesa SS, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010; 116:5600–5604. doi: 10.1182/blood-2010-03-275917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rey J, Charbonnier A, De Colella JMS, Stoppa AM, Poizot-Martin I, Gastaut JA, et al. Intensive chemotherapy with rituximab is safe and effective in AIDS non-Hodgkin's lymphoma. AIDS (London, England) 2003; 17:2006–2007. doi: 10.1097/00002030-200309050-00030. [DOI] [PubMed] [Google Scholar]
- 35.Bower M, Stebbing J, Tuthill M, Campbell V, Krell J, Holmes P, et al. Immunologic recovery in survivors following chemotherapy for AIDS-related non-Hodgkin lymphoma. Blood 2008; 111:3986–3990. doi: 10.1182/blood-2007-10-115659. [DOI] [PubMed] [Google Scholar]
- 36.Galicier L, Fieschi C, Borie R, Meignin V, Daniel MT, Gérard L, et al. Intensive chemotherapy regimen (LMB86) for St Jude stage IV AIDS-related Burkitt lymphoma/leukemia: a prospective study. Blood 2007; 110:2846–2854. doi: 10.1182/blood-2006-10-051771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.