To the Editor: Umbilical cord blood transplant (UCBT) serves as an alternative option for treating patients with B cell acute lymphoblastic leukemia (ALL), in the absence of a compatible donor. However, disease recurrence presents a difficult challenge. Chimeric antigen receptor (CAR)-T cell therapy is a promising approach for treating patients with relapsed/refractory ALL. Among the intensive investigations of infusion of CAR-T cells after transplant, few focus on patients who were administered CAR-T therapy after they relapsed subsequently to UCBT. Here, we report the response rate, toxicity, and survival of CD19 CAR-T cells administered to 10 children and young adults with relapsed acute B lymphoblastic leukemia (B-ALL) after UCBT.
Between April 2018 and September 2019, 11 patients with relapsed B-ALL after UCBT were enrolled, including three females and eight males, the median age was 10 years (range 7–22 years) and median weight was 35.0 kg (range 21.0–72.0 kg). Patient 10 was excluded because of unsuccessful cell culture. The other ten patients were successfully treated with 1.245 (0.42–3.91) × 106 CD19 CAR-T cells/kg body weight. Flow cytometry (FCM) revealed that all subjects were morphologically unrelieved or minimal residual disease (MRD)-positive after UCBT and that ≥30% of leukemic cells expressed CD19. The endpoints of the study were relapse, death due to primary disease, or loss to follow-up. The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of the University of Science and Technology of China, Hefei, China (No. 2016-101). Patients or their families granted their written informed consent to participate in the study, which is registered on clinicaltrials.gov (No. NCT02851589).
The CAR-T cells in our study were generated by PersonGen-Anke Cellular Therapeutics Co., Ltd., Hefei, China. The CAR is comprised of an FMC63-derived CD19-specific single chain Fv fused to a modified IgG4-hinge spacer, a CD28 costimulatory molecule, and a CD3ζ signaling domain. Patients were administered conditioning chemotherapy comprising fludarabine (30 mg/m2 × 3 days) combined with cyclophosphamide (300 mg/m2 × 3 days) from day 4 to day 15 (median, 7 days) before the infusion of cryopreserved CD19 CAR-T cells. Complete remission (CR) was defined as ≤5% bone marrow blasts according to morphology, without evidence of circulating blasts, and without detectable extramedullary sites. MRD-negative CR was defined as <0.01% marrow blasts according to FCM. Relapsed disease refers to the reappearance of circulating blasts or bone marrow blasts representing ≥5% of the cell population or extramedullary infiltration after CR.
Cytokine release syndrome (CRS) and neurotoxicity were graded according to the American Society for Blood and Marrow Transplantation Consensus.[1] Severe CRS was defined as ≥ grade 3. Acute graft-vs.-host disease (GVHD) was evaluated using the Keystone staging system,[2] and chronic GVHD was evaluated using the National Institutes of Health Consensus.[3]
Patients’ characteristics were evaluated using descriptive statistics. The Kaplan-Meier method was used to analyze progression-free survival (PFS) and overall survival (OS). All analyses were performed using GraphPad Prism 8 (GraphPad Software, LaJolla, CA, USA).
Short tandem repeat-polymerase chain reaction assays showed that 100% of the CD19 CAR-T cells were chimeras. Patients 4, 5, 6, and 9 received an infusion of CD19 CAR-T cell before UCBT. The median disease-free duration after UCBT was 13.3 months (range 5.7–48.3 months). All patients were enrolled within 1 month after relapse. Patient 8, who had central nervous system leukemia (CNSL) before infusion, underwent lumbar punctures combined with intrathecal injection of chemotherapeutic drugs until leukemic cells in the cerebrospinal fluid were undetectable. Patients 1, 2, 6, 8, and 9 harbored gene mutations in BCR/ABL, TEL/AML 1, TLS/ERG, E2A/PBX 1, and BCR/ABL before infusion.
Nine of ten (90.0%) patients achieved MRD-negative CR after the first infusion. Patient 4 did not achieve remission and received a second infusion. He remained unresponsive and died because of a severe pulmonary infection. Upon follow-up (14.2 months; median range, 5.8–32.7 months) of patients who achieved CR, the 6-month and 1-year PFS rates were 44.4% (5/9) and 33.3% (4/9), respectively, and the median PFS was 5.0 months (95% confidence interval [CI]: 2.8–27.9 months). The 6-month and 1-year OS rates were 77.8% (8/9) and 55.6% (6/9), respectively, and the median OS was 17.1 months (95% CI: 5.8–32.7 months). Sustained remission by the end of follow-up was achieved by three of ten (30.0%) patients, and six of ten (60.0%) patients experienced a relapse. Patient 7, who received 100 mg of oral dasatinib daily because his tumor cells harbored the Philadelphia chromosome, achieved sustained remission.
Among the relapsed patients, CD19 was undetectable in patient 6, who was alive when contacted via telephone at the end of follow-up. CD19-positive relapses were confirmed using FCM-analysis of the other five patients who subsequently received a second infusion of CD19 CAR-T cells. Patient 1 again achieved bone marrow remission but developed CNSL 12 days later. Patient 2, who consecutively accepted one infusion of CD19 CAR-T cells and one infusion of CD22 CAR-T cells after recurrence after the first CAR-T cell infusion, failed to achieve remission. Patient 3 suffered an MRD-positive recurrence 1 month after the second CR. Patients 8 and 9 were unresponsive after the second infusion. All patients rejected the second transplant.
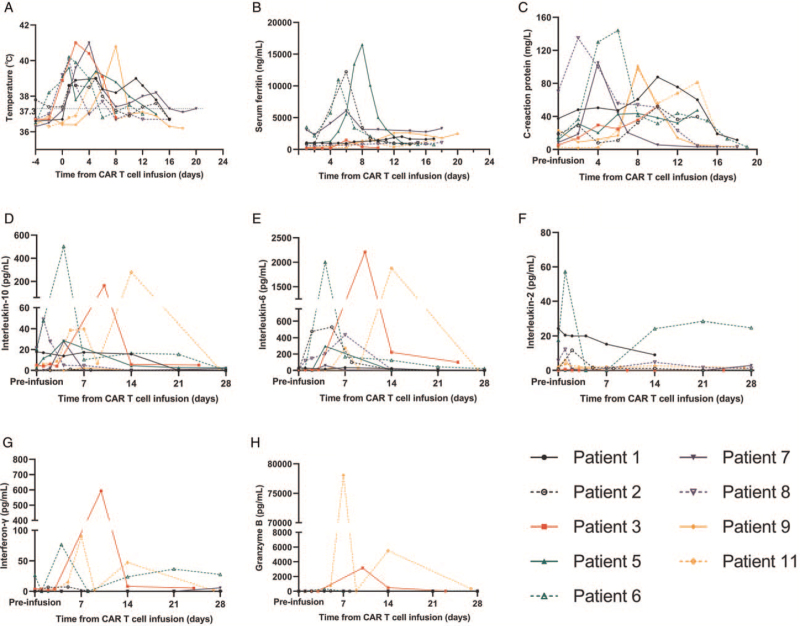
Grades 1, 2, and 3 CRS and neurotoxicity occurred in 40% (4/10), 40% (4/10), 10% (1/10), and 10% (1/10) of patients, respectively. The common manifestations of CRS were hypotension (40%), hypoxemia (10%), pleural effusion (50%), intestinal obstruction (10%), and elevated bilirubin (20%). Patient 11 suffered repeated seizures and was, therefore, diagnosed with grade 4 CAR-T cell-related encephalopathy syndrome. Nine of ten (90%) patients were febrile within 1 day after cell infusion [Figure 1A], and febrility was relieved using a symptomatic treatment. Six of ten patients (60%) developed coagulopathy that required intervention. Tocilizumab was administered to eight patients. Two patients were administered glucocorticoids. All symptoms were reversible. Inflammatory factors (serum ferritin [SF], C-reactive protein [CRP], interleukin (IL)-10, IL-6, IL-2, interferon-γ, and granzyme B) were detected in plasma samples [Figure 1B–1H]. The levels of SF, CRP, IL-6, and IL-10 were significantly elevated after infusion. Patients 3, 6, and 11 had the highest peak levels of cytokines, and Patients 3 and 11 developed more severe adverse events. Acute GVHD or chronic GVHD was not experienced by any patient during hospitalization or follow-up.
Figure 1.
CRS-related indicators in patients who achieved a CR. (A) The body temperature of patients increased after infusion. (B) SF levels of four patients were significantly elevated. (C) CRP levels were elevated. (D–H) ELISAs of the concentrations of serum cytokines, which were variably elevated. CAR: Chimeric antigen receptor; CR: Complete remission; CRP: C-reactive protein; CRS: Cytokine release syndrome; ELISAs: Enzyme-linked immunosorbent assays; SF: Serum ferritin.
CAR-T cells which were analyzed using the quantitative polymerase chain reaction and FCM reached their peak numbers in vivo after an average of 7.4 days. FCM showed that 1 month after infusion, the percentage of CD19+ B cells in peripheral blood of nine (90%) patients who achieved CR was nearly 0%.
The treatment of patients who relapsed after transplantation is highly patient-specific and depends on the time of relapse, and these treatments have their limitations. For example, donor lymphocytes are unavailable for UCBT patients. The response to chemotherapy is limited (CR rates of mild chemotherapy:17% and intensive chemotherapy: 27%).[4] Secondary transplantation is costly and risky, and it requires achieving complete morphological remission, preferably with a negative or low level of MRD. Here, we found a high rate of CR and a low incidence of adverse events among ten patients who received CD19 targeted CAR-T cells for ALL that was relapsed after UCBT. Acute or chronic GVHD was not observed during follow-up.
CD19 CAR-T cell therapy may be a safe and effective treatment for patients who relapse after UCBT. Possible reasons for the low incidence of GVHD in our present study are as follows: (1) The doses of CD19 CAR-T cells were lower than those using donor lymphocyte infusion, which may not induce strong GVHD. (2) The limited persistence of the CD19 CAR-T cells may explain the absence of GVHD, because acute GVHD induced by allogeneic cell infusion typically develops after 4 weeks (median),[5] whereas the proliferation of CD19 CAR-T cells in our study was generally diminished after 4 weeks.
Despite the high remission rate and mild adverse events encountered here, the problem of recurrence was intractable. The mechanisms of relapse may include the following components: (1) CD19-negative relapse: This relapse may be explained by CD19 mutations, selection through immune pressure, lineage switches induced by immune pressure, and eventual loss of CD19 expression. (2) CD19-positive relapse: This relapse occurs in a large proportion of patients and is usually associated with limited persistence of CARs, low potency of CARs, lower response to CARs, and transient B-cell aplasia. The limited duration of remission presents a challenge to CAR-T cell therapy. Proposals to reduce recurrence after CAR-T cell therapy include preventive measures such as novel CARs that target multiple antigens, optimization of CAR design, new manufacturing technologies, or consolidated hematopoietic stem cell transplantation for patients who achieve remission.
Furthermore, the efficacy of repeated infusions of CAR-T cells was poor in our study. Here, five patients accepted repeated infusion with the same CAR-T cells, written informed consent was obtained from each patient, and the response was insufficient. Reinfusion of CAR-T cells targeting different antigens may represent a better approach.
In conclusion, CD19-targeted CAR-T cell therapy may serve as an alternative for patients with B-ALL who relapse after UCBT. However, the long-term efficacy of CD19-targeted CAR-T cell therapy must be improved. Our findings must be confirmed by a multicenter clinical trial with a larger sample size.
Funding
The work was supported by grants from the Science and Technology Planning Project of Anhui Province, China (No. 1604a0802071) and the Major Science and Technology Projects of Anhui Province, China (No. 18030801126).
Conflicts of interest
None.
Footnotes
How to cite this article: Xu Q, Xu H, Xue L, Wang M, Pan G, Zhang X, Song K, Yao W, Wan X, Tong J, Liu H, Xu H, Liu X, Zhu X, Sun Z, Yang L, Wang X. CD19-targeted chimeric antigen receptor-modified T cells induce remission in patients with relapsed acute B lymphoblastic leukemia after umbilical cord blood transplantation. Chin Med J 2022;135:98–100. doi: 10.1097/CM9.0000000000001491
References
- 1.Lee DW, Santomasso BD, Locke FL, Ghobodi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019; 25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 2.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant 1995; 15:825–828. [PubMed] [Google Scholar]
- 3.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant 2015; 21:389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci 2019; 20:228.doi: 10.3390/ijms20010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]