Abstract
Pancreatic cancer is one of the most aggressive malignancies. The poor prognosis of pancreatic cancer patients is mainly attributed to low diagnostic rate at the early stage, highly aggressive nature coupled with the inadequate efficacy of current chemotherapeutic regimens. Novel therapeutic strategies are urgently needed for pancreatic cancer. MicroRNAs (miRNAs) play an important regulatory role in key processes of cancer development. The aberrant expression of miRNAs is often involved in the initiation, progression, and metastasis of pancreatic cancer. The discovery of tumor suppressor miRNAs provides prospects for the development of a novel treatment strategy for pancreatic cancer. We reviewed recent progress on the understanding of the role of miRNAs in pancreatic cancer, highlighted the efficient application of miRNAs-based therapies for pancreatic cancer in animal models and clinical trials, and proposed future prospects. This review focuses on the promise of integrating miRNAs into the treatment of pancreatic cancer and provides guidance for the development of precision medicine for pancreatic cancer.
Keywords: Pancreatic cancer, MicroRNA, MicroRNA carriers, Precision medicine
Introduction
Pancreatic cancer is one of the most devastating digestive malignancies with the worst prognosis, and the incidence is on the rise annually.[1] The poor prognosis of pancreatic cancer patients is mainly attributed to low surgical resection rates, poor sensitivity to chemoradiotherapy, and high recurrence and metastasis rates. Radical surgical resection plus perioperative chemotherapy is the standard treatment for pancreatic cancer, but the efficacy is unsatisfactory. Intravenous chemotherapeutic agents are difficult to reach effective therapeutic concentration within the tumor for maximum bioactivity due to the nature of poor blood supply and dense fibrous stroma and immunosuppressive microenvironment in pancreatic cancer tissue. Improved understanding of the core mechanism related to the initiation and progression of pancreatic cancer will help to develop effective therapeutic agents.
MicroRNAs (miRNAs) are a class of 19 to 23 nucleotides base of non-coding single-stranded endogenous RNAs first discovered by Lee et al[2] in 1993. MiRNAs regulate gene expression at post-transcriptional levels by binding to the 3′ untranslational region of the target messenger RNAs (mRNAs) and inducing mRNAs degradation or translation repression. About 60% of the coding genes are regulated by miRNAs. miRNAs have been reported to be involved in the occurrence, progression, and metastasis of a variety of cancers. Consequently, miRNAs could not only function as candidate diagnostic markers of malignancies but also be identified as potential therapeutic targets of cancers.
Therefore, here, we aim to provide a systematic review on the current status of the understanding of the role of miRNAs in pancreatic cancer and the potential application of miRNAs in pancreatic cancer therapy. In particular, we explore the molecule mechanisms by which miRNAs participate in pancreatic cancer development and how to target miRNAs in pancreatic cancer treatment.
Targeting miRNAs in Pancreatic Cancer Treatment
MiRNAs play an important role in the development and progression of pancreatic cancer. MiRNAs could be divided into two categories: oncogenic miRNAs and tumor suppressor miRNAs, according to the role of target mRNA in tumor progression. The function of oncogenes and tumor suppressor genes comes to an optimal balance under normal conditions. The oncogenic miRNAs inhibit the expression of tumor suppressor genes, while tumor suppressor miRNAs inhibit the expression of oncogenes. The dysregulation of tumor suppressor or oncogenic miRNAs will break the dynamic balance between oncogenes and tumor suppressor genes, and finally promote the occurrence and development of tumors. The introduction of oncogenic miRNA antagonist and tumor suppressor miRNAs can exert anti-cancer effect by inhibiting pancreatic cancer cell proliferation and invasion, promoting apoptosis, and enhancing chemosensitivity, providing the basis for the application of miRNAs in the treatment of pancreatic cancer.
Tumor suppressor miRNAs
Overexpression of tumor suppressor miRNAs could exert anti-tumor effects by inhibiting the progression of pancreatic cancer (summarized in Table 1). Zhan et al[17] found that the overexpression of miR-455-3p suppressed pancreatic cancer progression via inhibiting transcriptional co-activator with PDZ-binding motif (TAZ)-mediated Wnt/catenin signaling pathway, and promoted the apoptosis of pancreatic cancer cells by regulating the expression of apoptosis-related proteins Bcl-2, and Bax. Hu et al[18] reported that miR-373-3p could downregulate the expression of Cycin D2, enhance the chemosensitivity to gemcitabine, and inhibit the growth of gemcitabine-resistant pancreatic cancer cells.
Table 1.
Summary of tumor suppressor miRNAs in pancreatic cancer.
| miRNA | Function | Target gene | Author | Publication year |
| miR-429 | Increase chemosensitivity to gemcitabine | PDCD4 | Yu et al[3] | 2017 |
| miR-205 | Inhibit proliferation, invasion, migration, and increase chemosensitivity to gemcitabine | Not mentioned | Chaudhary et al[4] | 2017 |
| miR-217 | Inhibit proliferation, invasion, and promote apoptosis | E2F3 | Yang et al[5] | 2017 |
| Inhibit invasion and migration | ANLN | Idichi et al[6] | 2017 | |
| Inhibit proliferation, invasion, and migration | Tpd52l2 | Chen et al[7] | 2017 | |
| miR-221 | Induce autophagy and apoptosis | HDAC6 | Yang et al[8] | 2018 |
| Inhibit proliferation | SOCS3 | Xie et al[9] | 2018 | |
| miR-876-3p | Inhibit proliferation, invasion, migration, and promote apoptosis | JAG2 | Yang et al[10] | 2018 |
| miR-675 | Inhibit proliferation | E2F-1 | Ma et al[11] | 2018 |
| miR-98-5p | Inhibit proliferation, invasion, and metastasis | MAP4K4 | Fu et al[12] | 2018 |
| miRNA-339-5p | Inhibit invasion and migration | ZNF689 | Yu et al[13] | 2019 |
| miR-142-5p | Inhibit proliferation and promote apoptosis | RAP1A | Yao et al[14] | 2019 |
| miRNA-33b | Inhibit proliferation, invasion, andmigration | MMP16 | Luo et al[15] | 2020 |
| miR-4516 | Inhibit proliferation, invasion, migration, and promote apoptosis | OTX1 | Chen et al[16] | 2020 |
miRNAs: MicroRNAs; SOCS3: suppressor of cytokine signaling 3.
Single miRNA could target different mRNAs, and the same mRNA could be regulated by different miRNAs. miR-145 was found to regulate different targets to inhibit the progression of pancreatic cancer. miRNA-145 was reported to suppress the expression of TGF-β receptor and SMAD2, mucin 13, and neural precursor cell expressed, developmentally down–regulated 9 (NEDD9) to inhibit the proliferation, migration, and invasion of pancreatic cancer cells and enhance the chemosensitivity to gemcitabine.[19–21] Wang et al[22] found that miRNA-145 could inhibit cancer cell invasion, growth, and angiogenesis by downregulating angiopoietin-2 (Ang-2). Patel et al[23] found that let-7 could inhibit the progression of pancreatic cancer cells by enhancing the expression of suppressor of cytokine signaling 3 (SOCS3) to inhibit the phosphorylation of STAT3. Liu et al[24] found that miR-708 inhibited the proliferation and chemoresistance of pancreatic cancer cells by suppressing the expression of survivin. Ma et al[25] reported that lncRNA H19 could promote pancreatic ductal adenocarcinoma cell invasion and migration by antagonizing let-7 to increase the expression of high mobility group A2 (HMGA2). miR-34 could act as a tumor suppressor in pancreatic cancer via regulating multiple signaling pathways. miR-34a inhibited the expression of Snail1 and Notch 1 via post-transcriptional regulation, inducing apoptosis and suppressing the migration and invasion of cancer cells. The downregulation of Notch 1 increased the expression of miR-34a, forming a positive feedback loop between miR-34a and Notch 1.[26] miR-34 could also enhance the sensitivity of pancreatic cancer cells to gemcitabine and promote apoptosis by downregulating the expression of Slug.[27] An et al[28] also reported that miR-203a-3p could inhibit the proliferation and epithelial–mesenchymal transition (EMT) of pancreatic cancer cells by downregulating Slug.
Oncogenic miRNAs
Oncogenic miRNAs promote the progression of pancreatic cancer by regulating different targets (summarized in Table 2). The introduction of miRNA antagonists can inhibit carcinogenic miRNA and exert an anti-cancer effect. miRNA antagonists are single-stranded antisense oligodeoxynucleotides (ASO) targeting carcinogenic miRNAs, which could provide high stability, high affinity, and anti-nuclease protection for miRNAs through chemical synthesis and specific modification. Li et al[43] found that exosome miR-5703 derived from pancreatic cancer stellate cells could directly downregulate target gene CKLF (chemokine-like factor)-like MARVEL transmembrane domain containing family member 4, and promote the proliferation of pancreatic cancer cells by activating PI3K/Akt signaling pathway through p21-activated kinase 4. miR-21 could exert an oncogenic role in pancreatic cancer by regulating multiple cancer-associated signaling pathways. Cancer-associated fibroblasts with high miR-21 expression could promote the migration of pancreatic cancer cells and enhance gemcitabine resistance via elevating the expression of matrix metalloproteinase 3, matrix metalloproteinase 9 (MMP-9), and platelet-derived growth factor.[44] Overexpression of miR-21 could increase drug resistance to 5-fluorouracil and promote the proliferation of pancreatic cancer cells via downregulating the expression of target genes PTEN and PDCD4.[45] Zhao et al[46] reported a positive feedback loop between miR-21 and epidermal growth factor (EGF) signaling cascade in pancreatic cancer. EGF promoted the expression of miR-21, while miR-21 could enhance the activity of EGF by inhibiting EGF inhibitors. In addition, miR-21 could activate MAPK/ERK and PI3K/AKT signaling pathways to promote EGF-induced proliferation and suppress the apoptosis via inhibiting targeted gene Sprouty2, which constitutes the self-reinforcing circuit of this pathway. Sun et al[47] proposed that the downregulation of miR-21 could increase the expression of Von Hippel-Lindau tumor suppressor in pancreatic cancer. miR-21 could suppress the proliferation of pancreatic cancer cells via inhibiting the HIF-1α/VEGF signaling pathway and suppressing the expression of matrix metalloproteinase 2 and MMP-9. miR-155 is a key molecule to promote the progression of pancreatic cancer. miR-155 could target Foxo3a to promote the proliferation of pancreatic cancer cells induced by Reactive Oxygen Species generation.[48] miR-155 could also downregulate the expression of suppressor of cytokine signaling 1 and SOCS3 to increase the activation of STAT3 and promote the proliferation and invasion of pancreatic cancer cells.[49,50]
Table 2.
Summary of oncogenic miRNAs in pancreatic cancer.
| miRNA | Function | Target gene | Author | Publication year |
| miR-196a | Promote proliferation, invasion, and migration, and inhibit apoptosis | ING5 | Liu et al[29] | 2013 |
| miR-221 | Promote proliferation | PTEN, p27kip1, p57kip2, and PUMA | Sarkar et al[30] Yang et al[31] | 2013 2016 |
| miR-371-5p | Promote proliferation | ING1 | He et al[32] | 2014 |
| miR-301b | Promote invasion, migration, and resistance to gemcitabine | TP63 | Funamizu et al[33] | 2014 |
| miR-221-3p | Promote proliferation, invasion, migration, and resistance to 5-FU | RB1 | Zhao et al[34] | 2016 |
| miR-451 | Promote proliferation and migration | CAB39 | Guo et al[35] | 2017 |
| miR-301a-3p | Increase chemoresistance to gemcitabine | PTEN | Xia et al[36] | 2017 |
| miR-106b | Increase chemoresistance to gemcitabine | TP53INP1 | Fang et al[37] | 2019 |
| miR-302a-3p | Promote invasion and migration | SOCS5 | Zhang et al[38] | 2019 |
| miR-132 | Promote proliferation and inhibit apoptosis | Shh | Zhao et al[39] | 2019 |
| miR-132 | Promote proliferation, invasion, and migration | PTEN | Zhang et al[40] | 2019 |
| miR-210 | Increase chemoresistance to gemcitabine | Not mentioned | Yang et al[41] | 2020 |
| miR-193a-5p | Promote invasion and migration | SRSF6 | Li et al[42] | 2020 |
miRNAs: MicroRNAs; 5-FU: 5-fluorouracil.
Different studies demonstrated the opposite role of miR-203 in pancreatic cancer, which implies the differential and individualized gene expression among pancreatic cancers. miR-203 was reported to promote the proliferation, migration, and invasion of pancreatic cancer cells by inhibiting the expression of salt-inducible kinase 1 and SOCS3.[51,52] On the other hand, miR-203 was reported to inhibit pancreatic cancer cell proliferation and induce apoptosis and G1 phase cell cycle arrest by targeting Survivin.[53] Du et al[54] reported that miR-203 could inhibit the expression of DJ-1 and increase the expression of PTEN to inhibit the proliferation, induce apoptosis, and reduce cisplatin resistance of pancreatic cancer cells. Miao et al[55] also found that miR-203 inhibited tumor cell migration and invasion by upregulating the expression of caveolin-1 in pancreatic cancer cells.
Tumor suppressor miRNAs could degrade targeted mRNAs to inhibit the progression of pancreatic cancer, and miRNA antagonists could reduce tumor dissemination by blocking the function of oncogenic miRNAs, providing the basis of integrating miRNAs into the treatment of pancreatic cancer.[56–65] However, the introduction of a single miRNA mainly targets one target, and the effect might be generally temporary. In addition, miRNAs have some disadvantages such as high hydrophilicity, poor membrane penetration, and the susceptibility to be cleared by the kidney, which limit their clinical application.
MiRNA Vectors for Pancreatic Cancer Therapy
MiRNA could induce post-transcriptional downregulation by binding to target mRNA in a sequence-specific way, but it is difficult to enter the cells due to the negative charge repulsion of the cell membrane. Therefore, it is urgent to choose and design effective carrier to deliver miRNA through the dense fibrous stroma to achieve effective concentration in the tumor tissue, and enhance intracellular uptake to maximize the bioactivity.
Viral vector
The virus could be used to deliver miRNA as a carrier after detoxifying treatment. Hu et al[66] reported that intratumoral injection of an adenovirus vector to deliver miR-143 could significantly inhibit tumor progression in a xenograft tumor model. The liver metastatic lesions of mice were significantly suppressed after inoculating ad-miR-143 infected cells in the liver metastasis model. Sicard et al[56] constructed lentivirus vector (LV) (a/miR-21) by using LVs to deliver anti-miRNA, and they confirmed that the proliferation of pancreatic cancer cells was inhibited in a dose-dependent manner in mice. Chaudhary et al[4] reported that the overexpression of miR-205 mediated by LVs could enhance the chemosensitivity of pancreatic cancer stem cells and inhibit the proliferation of tumor cells. HIV-1-based LVs were more effective than other vectors such as adenovirus and SV40 and did not affect the production of endogenous miRNA. However, LVs-based vectors might lead to genotoxicity, unpredictable risk of insertion mutations, activation of proto-oncogenes, and even aberrant transcripts.
Nanoparticles and liposome carriers
Although the miRNA delivery system based on viral vectors is efficient, the toxicity and immunogenicity of viruses limit their further applications compared with non-viral vectors such as nanoparticles and exosomes. Nanoparticle packaged oligonucleotides are considered to be safer and more efficient. Gilles et al[57] designed targeted nanoparticulate carriers coated with oligonucleotide analogs iRGD-TPN-21, which could selectively deliver anti-miR-21 to the tumor site and inhibit tumor progression in a dose- and time-dependent manner. Passadouro et al[58] constructed a novel liposome nano-system by coating cationic liposomes with albumin, which could effectively transfer ASO to pancreatic cancer cells and suppress the expression of miR-21, miR-10b, miR-221, and miR-222. Ferino et al[59] designed a novel vector based on palmityl-oleoyl-phosphatidylcholine liposomes conjugated with lipid-modified cell- penetrating peptide and coated with single-stranded miR-216b mimic. The target KRAS protein was reduced by about 70% and colony formation was inhibited by about 40% in vitro. miRNAs packaged by nano-systems could precisely target pancreatic cancer, avoid the risk of genotoxicity and insertion mutation caused by LVs, and increase significantly the efficiency of chemotherapeutic drugs. The nano-systems provide promising prospects for clinical application.
Exosome carrier
The exosome is composed of unique lipids and proteins as a kind of endogenous nanoparticles, with several advantages such as natural stability, better immunocompatibility, specific targeting ability, and abundant drug loading. Many proteins on the surface of the exosome could be modified and exosomes could be used as new drug delivery carriers. The hucMSC-derived exosome vector constructed by Ding et al[60] could transfer miR-145-5p through endocytosis. Intratumoral injection of exo-miR-145-5p into nude mice bearing human pancreatic cancer cells could significantly inhibit the proliferation and invasion of pancreatic cancer cells and promote apoptosis and cell cycle arrest. Zuo et al[61] isolated exosomes from HEK293 cells and synthesized exosomes coated with miR-34a by ultrasound, which could significantly inhibit the progression of pancreatic cancer in vivo and in vitro. The unmodified exosomes could be easily cleared by the liver intravenously and showed minimal tumor accumulation.[67]
Application of miRNA-Based Drugs in Pancreatic Cancer
Many mechanisms are involved in the development of pancreatic cancer. Therefore, the combination of two or more drugs with different mechanisms, such as targeted drugs based on different miRNAs or the combination of miRNA-based drugs and chemotherapeutic agents, might significantly inhibit tumor progression through synergistic effects. Passadouro et al[58] treated pancreatic cancer cells with anti-miRNA oligonucleotides or chemotherapeutic drug sunitinib, and the cell survival rate decreased by about 21% compared with the control group, while the combination of oligonucleotide anti-miR-21 and sunitinib resulted in a decrease of 45% in cell viability. Uz et al[62] developed a dual drug delivery nano-device to deliver miR-345 and gemcitabine, which could stably release miR-345 and gemcitabine to effectively suppress tumor progression and metastasis. Li et al[63] developed a novel nanoparticle for targeted co-delivery of miRNA-21 (ASO-miR-21) and gemcitabine, and it could achieve active and targeted delivery and protect ASO from enzymatic degradation. The nanoparticle could significantly inhibit the EMT of pancreatic cancer cells and reduce liver metastasis.
Based on the overexpression of epidermal growth factor receptor (EGFR) in nearly 95% of pancreatic cancer patients, Mondal et al[64] prepared mixed micelle for the co-delivery of gemcitabine and miR-205, and it was decorated with EGFR-targeting cetuximab (C225) monoclonal antibody to achieve targeted delivery. The micelle significantly reversed gemcitabine resistance and inhibited the progression of advanced pancreatic cancer. Kumar and colleagues synthesized self-assembled micromicelles targeting tumor suppressor miR-let7b with Hedgehog pathway inhibitor GDC-0449.[68] Nearly 80% of GDC-0449 was continuously released from the polymer within 2 days, and miRNA could be stable for 24 h in the presence of serum.
The combination of multiple drugs could achieve a synergistic anti-tumor effect at a lower dose. In most studies, the two drugs were given separately by different drug delivery systems.[58,69] However, there are some disadvantages to using two different carriers, including different biocompatibility and biological distribution, increased drug toxicity, and decreased bioactivity of the drug combinations. The design of a single nano-device as an effective co-delivery carrier could promote drug targeting and internalization, achieve stable and continuous co-release of drugs, improve the therapeutic effect and reduce side effects. Although these targeted miRNA therapeutic delivery systems enhanced bioactivity in tissue culture and mouse models, it is unclear whether they can achieve the same effects in humans.
Clinical Trials of miRNAs for Pancreatic Cancer Therapy
Nucleic acid therapeutics based on RNA therapy could induce target-specific inhibition. Compared with traditional targeted therapy, the regulation of mRNA might exert faster and more lasting effects. Tumor suppressor miRNA is introduced to restore its expression in cancer to induce the sequence-specific degradation of target mRNA and has a promising application prospect. Beg et al[70] reported the first phase I clinical trial of miRNA-based cancer therapy (MRX34) (serial number: NCT01829971). MRX34 is a liposomal formulation based on tumor suppressor miR-34a. A total of 47 adult patients with refractory advanced solid tumors were included in this clinical trial, including five cases of pancreatic cancer. They received MRX34 therapy twice a week for 3 weeks, with a cycle of 4 weeks. The most common adverse reactions included fever, fatigue, and back pain. Therefore, miRNA-based therapeutics with MRX34 were effective, tolerable, and feasible. However, Hong et al[71] reported that four patients died due to severe immune-mediated adverse reactions in the follow-up study and the trial ended prematurely. In this trial, among 66 patients who could be assessed,16 patients achieved clinically significant stable disease for ≥ 4 cycles and three patients reached partial responses (PRs). Golan et al[72] reported an open-label phase 1/2a clinical trial (serial number: NCT01188785) in inoperable locally advanced pancreatic cancer. In this trial, miniature biodegradable implant siG12D-LODER™ which could release siRNA drug against KRAS(G12D) was inserted into pancreatic tumors combined with gemcitabine chemotherapy. Among 12 patients who were evaluated, ten patients were in stable condition and two patients reached PR. The most common adverse reactions were grade one or two (89%), such as transient abdominal pain, diarrhea, and nausea. Intratumoral injection of siRNA combined with chemotherapy was confirmed to be well tolerated with good safety and potential efficacy. Therefore, in future studies, we should design an effective miRNA carrier to avoid systemic immune activation and predict the toxic side effects of these drugs, especially the immune-associated adverse effects in the subsequent clinical trials.
Conclusion and Future Prospect
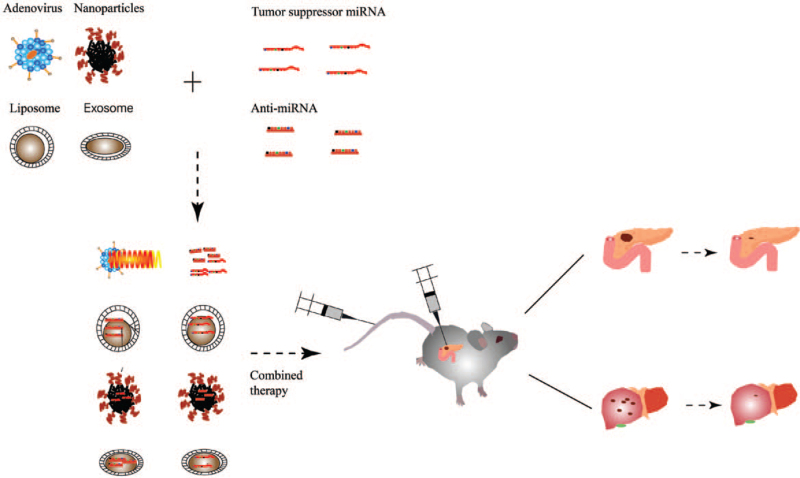
MiRNAs play an important regulatory role in the progression of pancreatic cancer. The latest advances in the delivery vector and therapeutic application of miRNAs in pancreatic cancer provide new ideas and directions for pancreatic cancer treatment [Figure 1]. The function and expression level of miRNAs differ not only in normal and diseased tissues and organs but also in different stages of the disease and different patients.[9,23,27,28,51–55] The identification of cancer-specific and core miRNAs of pancreatic cancer is the key to achieve the clinical application of miRNAs-based therapy. The first clinical trial of miRNA-based therapeutic strategy for cancer treatment confirmed the potential application of miRNA in oncology. However, the efficiency of a single miRNA might be limited, and combination therapy should be considered. The novel drug delivery carriers should be designed to cross the interstitial barrier, increase local drug concentration, improve therapeutic effect, and reduce side effects. However, miRNAs could regulate gene networks involved in multiple signaling pathways, and the following risks should be considered for clinical application of miRNA-based therapy: (1) whether other mechanisms may lead to anti-tumor activity or toxic side effects, (2) possible immunostimulatory effects, (3) off-target effects, (4) non-specific inflammatory effects of miRNAs. Therefore, further understanding of the mechanisms of miRNAs in pancreatic cancer and the optimization of miRNA vectors provide great promise for developing new treatment strategies against pancreatic cancer.
Figure 1.
Current targeting of miRNAs with different vectors for inhibiting the progression and metastasis of pancreatic cancer. miRNAs: MicroRNAs.
Conflicts of interest
None.
Footnotes
How to cite this article: Chu X, Wei D, Liu X, Long D, Tian X, Yang Y. MicroRNAs as potential therapeutic targets for pancreatic cancer: recent progress and future prospects. Chin Med J 2022;135:4–10. doi: 10.1097/CM9.0000000000001826
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin 2021; 71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 3.Yu G, Jia B, Cheng Y, Zhou L, Qian B, Liu Z, et al. MicroRNA-429 sensitizes pancreatic cancer cells to gemcitabine through regulation of PDCD4. Am J Transl Res 2017; 9:5048–5055. [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary AK, Mondal G, Kumar V, Kattel K, Mahato RI. Chemosensitization and inhibition of pancreatic cancer stem cell proliferation by overexpression of microRNA-205. Cancer Lett 2017; 402:1–8. doi: 10.1016/j.canlet.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Zhang HF, Qin CF. MicroRNA-217 functions as a prognosis predictor and inhibits pancreatic cancer cell proliferation and invasion via targeting E2F3. Eur Rev Med Pharmacol Sci 2017; 21:4050–4057. [PubMed] [Google Scholar]
- 6.Idichi T, Seki N, Kurahara H, Yonemori K, Osako Y, Arai T, et al. Regulation of actin-binding protein ANLN by antitumor miR-217 inhibits cancer cell aggressiveness in pancreatic ductal adenocarcinoma. Oncotarget 2017; 8:53180–53193. doi: 10.18632/oncotarget.18261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Q, Wang P, Fu Y, Liu X, Xu W, Wei J, et al. MicroRNA-217 inhibits cell proliferation, invasion and migration by targeting Tpd52l2 in human pancreatic adenocarcinoma. Oncol Rep 2017; 38:3567–3573. doi: 10.3892/or.2017.6036. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Sun Y, Wang H, Li H, Zhang M, Zhou L, et al. MicroRNA-221 induces autophagy through suppressing HDAC6 expression and promoting apoptosis in pancreatic cancer. Oncol Lett 2018; 16:7295–7301. doi: 10.3892/ol.2018.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie J, Wen JT, Xue XJ, Zhang KP, Wang XZ, Cheng HH. MiR-221 inhibits proliferation of pancreatic cancer cells via down regulation of SOCS3. Eur Rev Med Pharmacol Sci 2018; 22:1914–1921. doi: 10.26355/eurrev_201804_14714. [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Zhao WJ, Jia CL, Li XK, Wang Q, Chen ZL, et al. MicroRNA-876-3p functions as a tumor suppressor gene and correlates with cell metastasis in pancreatic adenocarcinoma via targeting JAG2. Am J Cancer Res 2018; 8:636–649. [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Tian X, Guo H, Zhang Z, Du C, Wang F, et al. Long noncoding RNA H19 derived miR-675 regulates cell proliferation by down-regulating E2F-1 in human pancreatic ductal adenocarcinoma. J Cancer 2018; 9:389–399. doi: 10.7150/jca.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Y, Liu X, Chen Q, Liu T, Lu C, Yu J, et al. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J Exp Clin Cancer Res 2018; 37:130.doi: 10.1186/s13046-018-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Z, Zhao S, Wang L, Wang J, Zhou J. miRNA-339-5p plays an important role in invasion and migration of pancreatic cancer cells. Med Sci Monit 2019; 25:7509–7517. doi: 10.12659/MSM.917038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao R, Xu L, Wei B, Qian Z, Wang J, Hui H, et al. miR-142-5p regulates pancreatic cancer cell proliferation and apoptosis by regulation of RAP1A. Pathol Res Pract 2019; 215:152416.doi: 10.1016/j.prp.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Wang Q, Teng L, Zhang J, Song J, Bo W, et al. LncRNA DANCR promotes proliferation and metastasis in pancreatic cancer by regulating miRNA-33b. FEBS Open Bio 2020; 10:18–27. doi: 10.1002/2211-5463.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Xu M, Zhao J, Shen J, Li J, Liu Y, et al. MicroRNA-4516 suppresses pancreatic cancer development via negatively regulating orthodenticle homeobox 1. Int J Biol Sci 2020; 16:2159–2169. doi: 10.7150/ijbs.45933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan T, Zhu Q, Han Z, Tan J, Liu M, Liu W, et al. miR-455-3p functions as a tumor suppressor by restraining Wnt/β-Catenin signaling via TAZ in pancreatic cancer. Cancer Manag Res 2020; 12:1483–1492. doi: 10.2147/CMAR.S235794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Liu Q, Pan J, Sui Z. MiR-373-3p enhances the chemosensitivity of gemcitabine through cell cycle pathway by targeting CCND2 in pancreatic carcinoma cells. Biomed Pharmacother 2018; 105:887–898. doi: 10.1016/j.biopha.2018.05.091. [DOI] [PubMed] [Google Scholar]
- 19.Chen S, Xu J, Su Y, Hua L, Feng C, Lin Z, et al. MicroRNA-145 suppresses epithelial to mesenchymal transition in pancreatic cancer cells by inhibiting TGF-β signaling pathway. J Cancer 2020; 11:2716–2723. doi: 10.7150/jca.34902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan S, Ebeling MC, Zaman MS, Sikander M, Yallapu MM, Chauhan N, et al. MicroRNA-145 targets MUC13 and suppresses growth and invasion of pancreatic cancer. Oncotarget 2014; 5:7599–7609. doi: 10.18632/oncotarget.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han T, Yi XP, Liu B, Ke MJ, Li YX. MicroRNA-145 suppresses cell proliferation, invasion and migration in pancreatic cancer cells by targeting NEDD9. Mol Med Rep 2015; 11:4115–4120. doi: 10.3892/mmr.2015.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Hang C, Ou XL, Nie JS, Ding YT, Xue SG, et al. MiR-145 functions as a tumor suppressor via regulating angiopoietin-2 in pancreatic cancer cells. Cancer Cell Int 2016; 16:65.doi: 10.1186/s12935-016-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel K, Kollory A, Takashima A, Sarkar S, Faller DV, Ghosh SK. MicroRNA let-7 downregulates STAT3 phosphorylation in pancreatic cancer cells by increasing SOCS3 expression. Cancer Lett 2014; 347:54–64. doi: 10.1016/j.canlet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Lu Y, Zhang D, Shi L, Zu G, Yan H, et al. MicroRNA-708 inhibits the proliferation and chemoresistance of pancreatic cancer cells. Biocell 2020; 44:73–80. doi: 10.32604/biocell.2020.08613. [Google Scholar]
- 25.Ma C, Nong K, Zhu H, Wang W, Huang X, Yuan Z, et al. H19 promotes pancreatic cancer metastasis by derepressing let-7's suppression on its target HMGA2-mediated EMT. Tumour Biol 2014; 35:9163–9169. doi: 10.1007/s13277-014-2185-5. [DOI] [PubMed] [Google Scholar]
- 26.Tang Y, Tang Y, Cheng YS. miR-34a inhibits pancreatic cancer progression through Snail1-mediated epithelial-mesenchymal transition and the Notch signaling pathway. Sci Rep 2017; 7:38232.doi: 10.1038/srep38232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang QA, Yang XH, Chen D, Yan X, Jing FC, Liu HQ, et al. miR-34 increases in vitro PANC-1 cell sensitivity to gemcitabine via targeting Slug/PUMA. Cancer Biomark 2018; 21:755–762. doi: 10.3233/CBM-170289. [DOI] [PubMed] [Google Scholar]
- 28.An N, Zheng B. MiR-203a-3p inhibits pancreatic cancer cell proliferation, EMT, and apoptosis by regulating SLUG. Technol Cancer Res Treat 2020; 19:1533033819898729.doi: 10.1177/1533033819898729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Du Y, Gao J, Liu J, Kong X, Gong Y, et al. Aberrant expression miR-196a is associated with abnormal apoptosis, invasion, and proliferation of pancreatic cancer cells. Pancreas 2013; 42:1169–1181. doi: 10.1097/MPA.0b013e3182962acb. [DOI] [PubMed] [Google Scholar]
- 30.Sarkar S, Dubaybo H, Ali S, Goncalves P, Kollepara SL, Sethi S, et al. Down-regulation of miR-221 inhibits proliferation of pancreatic cancer cells through up-regulation of PTEN, p27(kip1), p57(kip2), and PUMA. Am J Cancer Res 2013; 3:465–477. [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Yang Y, Xia L, Yang Y, Wang F, Song M, et al. MiR-221 promotes Capan-2 pancreatic ductal adenocarcinoma cells proliferation by targeting PTEN-Akt. Cell Physiol Biochem 2016; 38:2366–2374. doi: 10.1159/000445589. [DOI] [PubMed] [Google Scholar]
- 32.He D, Miao H, Xu Y, Xiong L, Wang Y, Xiang H, et al. MiR-371-5p facilitates pancreatic cancer cell proliferation and decreases patient survival. PLoS One 2014; 9:e112930.doi: 10.1371/journal.pone.0112930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funamizu N, Lacy CR, Parpart ST, Takai A, Hiyoshi Y, Yanaga K. MicroRNA-301b promotes cell invasiveness through targeting TP63 in pancreatic carcinoma cells. Int J Oncol 2014; 44:725–734. doi: 10.3892/ijo.2014.2243. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L, Zou D, Wei X, Wang L, Zhang Y, Liu S, et al. MiRNA-221-3p desensitizes pancreatic cancer cells to 5-fluorouracil by targeting RB1. Tumour Biol 2016; doi: 10.1007/s13277-016-5445-8. [DOI] [PubMed] [Google Scholar]
- 35.Guo R, Gu J, Zhang Z, Wang Y, Gu C. MiR-451 promotes cell proliferation and metastasis in pancreatic cancer through targeting CAB39. Biomed Res Int 2017; 2017:2381482.doi: 10.1155/2017/2381482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Xia X, Zhang K, Luo G, Cen G, Cao J, Huang K, et al. Downregulation of miR-301a-3p sensitizes pancreatic cancer cells to gemcitabine treatment via PTEN. Am J Transl Res 2017; 9:1886–1895. [PMC free article] [PubMed] [Google Scholar]
- 37.Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu W, et al. Exosomal miRNA-106b from cancer-associated fibroblast promotes gemcitabine resistance in pancreatic cancer. Exp Cell Res 2019; 383:111543.doi: 10.1016/j.yexcr.2019.111543. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z, Li J, Guo H, Wang F, Ma L, Du C, et al. BRM transcriptionally regulates miR-302a-3p to target SOCS5/STAT3 signaling axis to potentiate pancreatic cancer metastasis. Cancer Lett 2019; 449:215–225. doi: 10.1016/j.canlet.2019.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Zhao DW, Hou YS, Sun FB, Han B, Li SJ. Effects of miR-132 on proliferation and apoptosis of pancreatic cancer cells via Hedgehog signaling pathway. Eur Rev Med Pharmacol Sci 2019; 23:1978–1985. doi: 10.26355/eurrev_201903_17236. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Liu A, Feng X, Tian L, Bo W, Wang H, et al. MiR-132 promotes the proliferation, invasion and migration of human pancreatic carcinoma by inhibition of the tumor suppressor gene PTEN. Prog Biophys Mol Biol 2019; 148:65–72. doi: 10.1016/j.pbiomolbio.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr) 2020; 43:123–136. doi: 10.1007/s13402-019-00476-6. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Wu P, Yang Z, Deng S, Ni L, Zhang Y, et al. miR-193a-5p promotes pancreatic cancer cell metastasis through SRSF6-mediated alternative splicing of OGDHL and ECM1. Am J Cancer Res 2020; 10:38–59. [PMC free article] [PubMed] [Google Scholar]
- 43.Li M, Guo H, Wang Q, Chen K, Marko K, Tian X, et al. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer Lett 2020; 490:20–30. doi: 10.1016/j.canlet.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, Yao J, Li W, Zhang C. Micro-RNA-21 regulates cancer-associated fibroblast-mediated drug resistance in pancreatic cancer. Oncol Res 2018; 26:827–835. doi: 10.3727/096504017X14934840662335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei X, Wang W, Wang L, Zhang Y, Zhang X, Chen M, et al. MicroRNA-21 induces 5-fluorouracil resistance in human pancreatic cancer cells by regulating PTEN and PDCD4. Cancer Med 2016; 5:693–702. doi: 10.1002/cam4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Q, Chen S, Zhu Z, Yu L, Ren Y, Jiang M, et al. miR-21 promotes EGF-induced pancreatic cancer cell proliferation by targeting Spry2. Cell Death Dis 2018; 9:1157.doi: 10.1038/s41419-018-1182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun J, Jiang Z, Li Y, Wang K, Chen X, Liu G. Downregulation of miR-21 inhibits the malignant phenotype of pancreatic cancer cells by targeting VHL. Onco Targets Ther 2019; 12:7215–7226. doi: 10.2147/OTT.S211535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang P, Zhu CF, Ma MZ, Chen G, Song M, Zeng ZL, et al. Micro-RNA-155 is induced by K-Ras oncogenic signal and promotes ROS stress in pancreatic cancer. Oncotarget 2015; 6:21148–21158. doi: 10.18632/oncotarget.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C, Li H, Wu W, Jiang T, Qiu Z. Regulation of miR-155 affects pancreatic cancer cell invasiveness and migration by modulating the STAT3 signaling pathway through SOCS1. Oncol Rep 2013; 30:1223–1230. doi: 10.3892/or.2013.2576. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Guo J, Fan H. MiR-155 regulates the proliferation and apoptosis of pancreatic cancer cells through targeting SOCS3. Eur Rev Med Pharmacol Sci 2020; 24:12625.doi: 10.26355/eurrev_202012_24143. [DOI] [PubMed] [Google Scholar]
- 51.Ren ZG, Dong SX, Han P, Qi J. miR-203 promotes proliferation, migration and invasion by degrading SIK1 in pancreatic cancer. Oncol Rep 2016; 35:1365–1374. doi: 10.3892/or.2015.4534. [DOI] [PubMed] [Google Scholar]
- 52.Lin XM, Chen H, Zhan XL. MiR-203 regulates JAK-STAT pathway in affecting pancreatic cancer cells proliferation and apoptosis by targeting SOCS3. Eur Rev Med Pharmacol Sci 2019; 23:6906–6913. doi: 10.26355/eurrev_201908_18730. [DOI] [PubMed] [Google Scholar]
- 53.Xu D, Wang Q, An Y, Xu L. MiR203 regulates the proliferation, apoptosis and cell cycle progression of pancreatic cancer cells by targeting Survivin. Mol Med Rep 2013; 8:379–384. doi: 10.3892/mmr.2013.1504. [DOI] [PubMed] [Google Scholar]
- 54.Du SL, Xu LY, Gao P, Liu QS, Lu FF, Mo ZH, et al. MiR-203 regulates DJ-1 expression and affects proliferation, apoptosis and DDP resistance of pancreatic cancer cells. Eur Rev Med Pharmacol Sci 2019; 23:8833–8840. doi: 10.26355/eurrev_201910_19278. [DOI] [PubMed] [Google Scholar]
- 55.Miao L, Xiong X, Lin Y, Cheng Y, Lu J, Zhang J, et al. miR-203 inhibits tumor cell migration and invasion via caveolin-1 in pancreatic cancer cells. Oncol Lett 2014; 7:658–662. doi: 10.3892/ol.2014.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sicard F, Gayral M, Lulka H, Buscail L, Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol Ther 2013; 21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilles ME, Hao L, Huang L, Rupaimoole R, Lopez-Casas PP, Pulver E, et al. Personalized RNA medicine for pancreatic cancer. Clin Cancer Res 2018; 24:1734–1747. doi: 10.1158/1078-0432.CCR-17-2733. [DOI] [PubMed] [Google Scholar]
- 58.Passadouro M, de LimaMCP, Faneca H. MicroRNA modulation combined with sunitinib as a novel therapeutic strategy for pancreatic cancer. Int J Nanomedicine 2014; 9:3203–3217. doi: 10.2147/IJN.S64456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferino A, Miglietta G, Picco R, Vogel S, Wengel J, Xodo LE. MicroRNA therapeutics: design of single-stranded miR-216b mimics to target KRAS in pancreatic cancer cells. RNA Biol 2018; 15:1273–1285. doi: 10.1080/15476286.2018.1526536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett 2019; 442:351–361. doi: 10.1016/j.canlet.2018.10.039. [DOI] [PubMed] [Google Scholar]
- 61.Zuo L, Tao H, Xu H, Li C, Qiao G, Guo M, et al. Exosomes-coated miR-34a displays potent antitumor activity in pancreatic cancer both in vitro and in vivo. Drug Des Devel Ther 2020; 14:3495–3507. doi: 10.2147/DDDT.S265423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uz M, Kalaga M, Pothuraju R, Ju J, Junker WM, Batra SK, et al. Dual delivery nanoscale device for miR-345 and gemcitabine co-delivery to treat pancreatic cancer. J Control Release 2019; 294:237–246. doi: 10.1016/j.jconrel.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Chen Y, Li J, Zhang Z, Huang C, Lian G, et al. Co-delivery of microRNA-21 antisense oligonucleotides and gemcitabine using nanomedicine for pancreatic cancer therapy. Cancer Sci 2017; 108:1493–1503. doi: 10.1111/cas.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mondal G, Almawash S, Chaudhary AK, Mahato RI. EGFR-targeted cationic polymeric mixed micelles for codelivery of gemcitabine and miR-205 for treating advanced pancreatic cancer. Mol Pharm 2017; 14:3121–3133. doi: 10.1021/acs.molpharmaceut.7b00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie Y, Hang Y, Wang Y, Sleightholm R, Prajapati DR, Bader J, et al. Stromal modulation and treatment of metastatic pancreatic cancer with local intraperitoneal triple miRNA/siRNA nanotherapy. ACS Nano 2020; 14:255–271. doi: 10.1021/acsnano.9b03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, Ou Y, Wu K, Chen Y, Sun W. miR-143 inhibits the metastasis of pancreatic cancer and an associated signaling pathway. Tumour Biol 2012; 33:1863–1870. doi: 10.1007/s13277-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 67.Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 2015; 199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar V, Mondal G, Slavik P, Rachagani S, Batra SK, Mahato RI. Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol Pharm 2015; 12:1289–1298. doi: 10.1021/mp500847s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhayat SA, Abdeen B, Kohler G, Senninger N, Haier J, Mardin WA. MicroRNA-100 and microRNA-21 as markers of survival and chemotherapy response in pancreatic ductal adenocarcinoma UICC stage II. Clin Epigenetics 2015; 7:132.doi: 10.1186/s13148-015-0166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs 2017; 35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer 2020; 122:1630–1637. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golan T, Khvalevsky EZ, Hubert A, Gabai RM, Hen N, Segal A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015; 6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]