A global trend towards greater health awareness with a resulting reduction in smoking has contributed to the improved control and early detection of lung cancer.[1] Furthermore, developments in diagnostic technologies have enhanced the detection of multifocal lung cancer, characterized by multiple cancerous lesions. Multifocal lung cancer can be divided into multiple primary lung cancer (MPLC) and pulmonary metastasis-associated lung cancer intrapulmonary metastases (IM) according to the types of lesions.
The differential diagnosis of MPLC and IM is clinically important because of the direct impacts on tumor-node-metastasis staging and the implications for the treatment of lung cancer. The diagnostic criteria for MPLC were first proposed by Martini and Melamed in 1975[2] and were subsequently revised and supplemented by the American College of Chest Physicians (ACCP) in 2003.[3] These ACCP standards currently provide the main diagnostic criteria for the clinical differential diagnosis of MPLC and IM. MPLC and IM differ in terms of their clonal origins, which could provide a useful basis for multi-gene detection to assist the diagnosis of multifocal lung cancer.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanjing Medical University (No. 2021-SR-134). The study was exempt from the need for informed consent.
The current study reports on the clinical data and lung cancer findings for 50 patients admitted to the First Affiliated Hospital of Nanjing Medical University between 2014 and 2017. All cases were diagnosed as MPLC or IM based on the ACCP criteria. The clinical data including patients’ age, gender, smoking status, histological types, number and location of lesions, and metastasis status were collected. The patients were followed up by telephone to determine their physical condition, whether or not they received targeted treatment and its efficacy, and whether or not they experienced recurrence. Eleven patients (22%) were not contacted.
Formalin-fixed paraffin-embedded (FFPE) blocks were sectioned and each section was evaluated for tumor cell content. Sections with a tumor cell content ≥30% were considered for further analysis. DNA and RNA were extracted using AmoyDx (ADx)-FFPE DNA and ADx-FFPE RNA extraction kits, respectively (AmoyDx, Xiamen, Fujian, China; numbers 8.02.24101X036G and 8.02.23501X036G, respectively). We also screened for epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1), mesenchymal epithelial transition factor (MET), Kirsten rat sarcoma viral oncogene (KRAS), rearranged during transfection proto-oncogene (RET), human epidermal growth factor receptor 2 (HER-2), V-raf murine sarcoma viral oncogene homolog B1 (BRAF), neuroblastoma rat sarcoma (NRAS), and phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic sub-unit alpha (PIK3CA) mutations using ADx-5 gene mutation (detecting nine genes) and ADx-MET gene exon 14 deletion mutation detection kits (AmoyDx, Xiamen, Fujian, China).
Data were expressed as number and percentage for categorical variables. Results were compared between subgroups by χ2 or Fisher exact test for categorical variables, as necessary. Progression-free survival (PFS) was analyzed by the Kaplan–Meier method. Hazard ratios (HR) and 95% confidence intervals (CI) were also calculated by Cox regression model. Differences were considered statistically significant when P value was <0.05. All analyses were carried out using SPSS 21.0 software (IBM, Armonk, NY, USA).
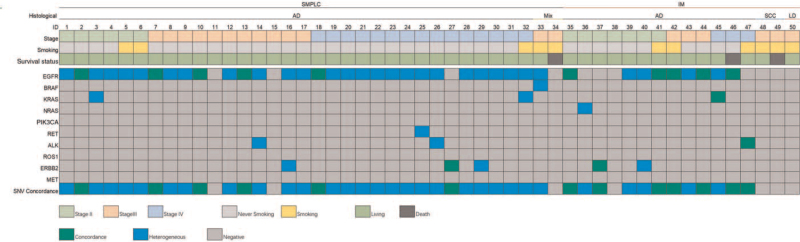
The clinical information of the patients is shown in Supplementary Table 1. Analyses of the clinical diagnoses and gene detection results showed that 13 cases displayed the same gene mutation types, including six patients with MPLC and seven with IM according to ACCP standards, while 18 patients exhibited different gene mutation types, including 16 with MPLC and two with IM. Eleven patients (nine MPLC, two IM, according to ACCP standards) showed a gene mutation in one lesion and the wild-type gene in another lesion; seven patients (three MPLC and four IM) had the wild-type gene in two lesions; and one patient with three lesions had two lesions diagnosed as intra-lung metastatic carcinoma with the same gene mutation, and a third lesion characterized as a primary focal lesion with the wild-type gene. The gene test results were consistent with the differential diagnosis according to the ACCP standard criteria in 33 cases, inconsistent in 10 cases, and the results could not be verified in seven cases. The mutation information of the patient's lesions is shown in Supplementary Table 2. A mutation heat map [Figure 1] based on clinical data and test results indicated that patients with IM had higher mutation consistency rates than those with MPLC (64% [7/11] vs. 19% [6/31], P = 0.019).
Figure 1.
Heat map of patients’ information, including histological classification, lesion stage, smoking history, survival, and type of mutation. ACCP: American College of Chest Physicians; AD: Adenocarcinoma; ALK: Anaplastic lymphoma kinase; BRAF: V-raf murine sarcoma viral oncogene homolog B1; CI: Confidence interval; EGFR: Epidermal growth factor receptor; ERBB2: V-erb-b2 avian erythroblastic leukemia viral oncogene homolog 2; FFPE: Formalin-fixed paraffin-embedded; HER-2: Human epidermal growth factor receptor 2; HR: Hazard ratio; IM: Intrapulmonary metastasis; KRAS: Kirsten rat sarcoma viral oncogene; LD: Low differentiation carcinoma; MET: Mesenchymal epithelial transition factor; NRAS: Neuroblastoma rat sarcoma; PFS: Progression-free survival; PIK3CA: Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic sub-unit alpha; RET: Rearranged during transfection proto-oncogene; ROS1: ROS proto-oncogene 1, receptor tyrosine kinase; SCC: Squamous cell carcinoma; SMPLC: Synchronous multiple primary lung cancers; SNV: Single nucleotide variant.
Kaplan–Meier survival analysis showed that PFS was better in patients with mutations (P = 0.001), those without lymphatic metastasis (P = 0.001), those diagnosed with MPLC according to ACCP standards (P = 0.038), and those with inconsistent mutations (P = 0.002). Cox regression analysis showed that patients with mutations had better PFS than non-carriers (HR, 0.123; 95% CI: 0.030–0.503; P = 0.005), with median PFS of 1579 days (95% CI: 1440–1718 days) and 848 days (95% CI: 444–1253 days), respectively. PFS was also better in patients without lymphatic metastasis compared with those with lymphatic metastasis (HR, 0.132; 95% CI: 0.032–0.555; P = 0.006), with median PFS of 1610 days (95% CI: 1483–1737 days) and 1014 days (95% CI: 597–1430 days), respectively. Patients diagnosed with MPLC according to ACCP standards had better PFS than those with IM (HR, 0.246; 95% CI: 0.059–1.030; P = 0.045), with median PFS of 1594 days (95% CI: 1450–1738 days) and 1233 days (95% CI: 895–1571 days), respectively. Furthermore, patients with inconsistent mutations had higher PFS than those with consistent mutations (HR, 0.098; 95% CI: 0.018–0.542; P = 0.008), with median PFS of 1541 days (95% CI: 1398–1684 days) in patients with inconsistent mutation, 1517 days in those with same-gene mutation types (95% CI: 1241–1793 days). Patients with inconsistent mutations had better PFS than non-carriers (HR, 0.167; 95% CI: 0.030–0.940; P = 0.042), with median PFS of 1541 days (95% CI: 1398–1684 days) and 848 days (95% CI: 444–1253 days), respectively.
Among the 50 cases, 33 (66%) displayed genetic test results that were consistent with ACCP standards, ten (20%) had inconsistent results, and seven (14%) had results that could not be validated. Among the ten inconsistent cases, six were diagnosed with MPLC (although the same mutation type was found in different lesions), two exhibited IM although the mutation types were different, and two exhibited IM but a mutation was only detected in a single lesion, all according to ACCP pathological standards. Similar results were obtained by Girard et al,[4] who found inconsistent results in seven (32%) of 22 cases. Multi-gene detection of multifocal lung cancer is more scientific. The current heat map indicated that the same and different clonal source mutations were related to IM and MPLC, respectively.
Lesions are generally considered metastatic if the gene detection results for both lesions in the lungs are the same, while both lesions may be considered as primaries if their gene detection results are different. However, two primary lung lesions may have the same gene detection results under certain circumstances, depending on the detected mutation type. If the proportion of a mutation in a population is very low, both lesions have a very low probability of showing that mutation, and two lesions with the same mutation are thus likely to be IM. One patient in the present study exhibited two lesions carrying a HER-2 mutation. The HER-2 gene has a 1% to 2% mutation rate in Asian patients with lung cancer, and our patient was therefore diagnosed with IM. However, if the mutation is more common in the population, it may occur coincidentally in multiple primary lesions. Among the patients with inconsistent results, two patients in the current study exhibited two lesions with simultaneous EGFR L858R mutations and three had simultaneous EGFR exon 19 deletions. However, the EGFR mutation rate in Asian patients with lung cancer is 30%,[5] of which 85% to 90% are exon 19 deletions and L858R mutations. Two lesions might thus have identical but independent mutations. It is necessary to consider the patient's combined clinical information, including the similarity of histological type, the lobe in which the lesion is located, and the presence of infiltration or lymph node metastasis, in order to make a comprehensive judgment.
Moreover, previous studies showed that primary lung cancers and metastases might have different genotypes. IM therefore cannot be ruled out, even if two lesions display different mutation types, depending on the mutation type of the detected genes. If the two mutation types differ, the possibility of metastasis is low and the lesions are likely to be independent of each other; however, if one lesion is a non-carrier and the other carries a mutation, metastasis is possible.[6] Some researchers have suggested that this may occur if the primary tumor includes a small (undetectable) number of mutant cells with strong metastatic ability. In contrast, the proportion of these cells is much higher in metastatic tumors, and the mutations can therefore be detected.[7] One patient in the current study exhibited two histologically consistent lesions in the right lower lung, and was diagnosed with intrapulmonary metastatic carcinoma according to the ACCP criteria. However, genetic tests revealed that one of the two lesions carried an NRAS mutation and the other was a non-carrier. In accordance with the above research results, the patient was diagnosed with IM.
Three patients had cancer foci with different histological types but the same gene mutation types. Tumors with the same gene mutation type might originate from the same cancer stem cells; however, different histological types might arise as a result of differentiation of the cancer stem cells during the development process. According to clinical diagnostic criteria, multifocal lung cancers with different histological morphologies are usually diagnosed as MPLCs, irrespective of the existence of homology between the primary tumor and metastasis. This might have contributed to the clinical diagnosis results in this study, and might also explain the inconsistency with the polygenic test results.
In summary, the differential diagnosis of MPLC and IM should take account of genetic, as well as clinical data. The differential diagnosis of multifocal lung cancer using traditional multi-gene detection methods, including amplification refractory mutation system-polymerase chain reaction, has the advantages of low cost, simple process, easy interpretation of results, and high sensitivity.
Funding
This study was supported by a grant from the National Natural Science Foundation of China (No. 81773109).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Xu L, Chen J, Zeng Y, Li X, Zhang Z. Differential diagnosis of multiple primary lung cancers and intra-lung metastasis of lung cancer by multiple gene detection. Chin Med J 2022;135:86–88. doi: 10.1097/CM9.0000000000001739
Liuyang Xu and Jin Chen contributed equally to the work.
Supplemental digital content is available for this article.
References
- 1.Khaltaev N, Axelrod S. Global lung cancer mortality trends and lifestyle modifications: preliminary analysis. Chin Med J 2020; 133:1526–1532. doi: 10.1097/CM9.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975; 70:606–612. [PubMed] [Google Scholar]
- 3.Detterbeck FC, Jones DR, Kernstine KH, Naunheim KS. American College of Physicians. Lung cancer. Special treatment issues. Chest 2003; 123: (1 Suppl): 244S–258S. doi: 10.1378/chest.123.1_suppl.244s. [DOI] [PubMed] [Google Scholar]
- 4.Girard N, Ostrovnaya I, Lau C, Park B, Ladanyi M, Finley D, et al. Genomic and Mutational Profiling to Assess Clonal Relationships Between Multiple Non-Small Cell Lung Cancers. Clin Cancer Res 2009; 15:5184–5190. doi: 10.1158/1078-0432.CCR-09-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Zhang Q, Luan H, Zhan Z, Wang C, Sun B. Comparison of KRAS and EGFR gene status between primary non-small cell lung cancer and local lymph node metastases: implications for clinical practice. J Exp Clin Cancer Res 2011; 30:30.doi: 10.1186/1756-9966-30-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun LN, Zhang Q, Luan HL, Zhan ZL, Sun BC. Comparison of KRAS and EGFR gene statuses between primary non-small cell lung cancer and local lymph node metastases and their clinical significance. Chin J Clin Oncol 2012; 39:970–973. doi: 10.3969/j.issn.l000-8179.2012.14.008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.