Abstract
Keratoconus is the most common form of primary corneal thinning. Different methods have been suggested to deal with the condition, including glasses, contact lenses, and surgical interventions, like penetrating keratoplasty (PKP) and deep anterior lamellar keratoplasty (DALK), well-known methods of the latter. This study was conducted to compare the outcomes and side effects of the two mentioned keratoplasty techniques. First, we systematically reviewed all original articles studies on PubMed, Scopus, Web of Science, and Embase. Then, the extracted data were pooled and meta-analyzed on each of the intended outcomes. A total of 30 studies were included in which PKP was more commonly performed compared to DALK. We found that adverse outcomes consisting of cataracts, graft rejection, graft failure, High-IOP, and corneal infection, were all more common findings in the PKP groups compared to the DALK groups. However, only for the high-IOP, cataracts, and graft rejection, the analysis of the extracted results demonstrated statistical significance. Overall, the DALK groups demonstrated significantly better results when considering the improvement levels by measuring the Endothelial Cell Count (ECC) and Spherical Equivalent (SE). In addition, though statistically insignificant, the Central Corneal Thickness(CCT), Best Corrected Visual Acuity(BCVA), Topographic Cylinder(TC), Refractive Cylinder values were greater in the PKP groups. Based on our study and with its limitations in mind, we can conclude that DALK can be a relatively safer and more effective procedure. Though, a larger number of high-standard randomized clinical trials still need to be conveyed for more definite conclusions.
Keywords: Corneal Transplant, Deep Anterior Lamellar Keratoplasty, Keratoconus, Penetrating Keratoplasty
INTRODUCTION
Keratoconus defined as bilateral[1,2] and asymmetrical[3,4] degeneration of the cornea is the most common form of primary corneal thinning. Local thinning of the cornea caused by keratoconus leads to corneal protrusion and then severe myopia and irregular astigmatism.[2,5]
Different methods have been utilized to treat this condition, including prescribing glasses and contact lenses for the early stages[5,6] and keratoplasty for the advanced stages of the disease.[6] Keratoconus is the most common pathology requiring corneal transplants in most ophthalmology centers worldwide. Similarly, based on available data, keratoconus is also the most common eye pathology requiring corneal transplant in Iran.[8,9] According to one study, approximately 10–20% of keratoconus cases end up requiring standard penetrating keratoplasty (PKP). If the corneal cone's size, the severity of the keratoconus or corneal hydrops limits the possibility of utilizing contact lenses to treat the condition, keratoplasty should be performed.[10] Despite the popular use and high success rates of PKP, there is always a 20% risk that the host develops an immune reaction to the graft, of which 85% is due to the endothelial cell rejection. Approximately 2.5% of graft rejections lead to graft failure.[11,12]
Some studies have also shown that in PKP cases, the number of endothelial cells decreases by 4.2% each year. This decline may continue until 5–10 years after the transplantation.[13] Other PKP complications include expulsive hemorrhage, endophthalmitis, synechiae of the iris to the angle or point of incision of the graft, side effects of long-term corticosteroids use, and predisposition to traumatic injuries.[14]
In the recent decade, the rate of performing the lamellar keratoplasty (LK) procedure has increased.[15] Potential immunological incompatibilities after the insult, leading to complications including graft rejection, are of considerable importance, therefore, the injured corneal layers are removed, and the healthy tissue is preserved.[16] Deep anterior lamellar keratoplasty (DALK) is a type of LK that reconnects the stroma to the Descemet's membrane in cases whose stroma might be in danger of loss.[17] This technique prevents the recipient's endothelium replacement with the donor's and mitigates the risk of endothelial induced rejection. However, the risk of rejection will not be completely eliminated due to the remaining epithelial layer.[18,19]
Although the LK is rapidly becoming the method of choice in corneal transplant, some studies have compared DALK with PKP to determine the more appropriate option for treating keratoconus.[20] Considering the importance of the subject and that very few comprehensive studies have evaluated and compared both techniques in keratoconus cases, our study aims to conduct a systematic review to compare the outcomes and side effects of these two techniques.
METHODS
We completed our systematic review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org/).[21]
For this systematic review, we searched PubMed, Scopus, Embase, Web of Science databases for articles published up to the end of May 2021.
Inclusion and Exclusion Criteria
All English comparative studies on adults including clinical trials, retrospective and prospective cohort studies on keratoconus treated with DALK and PKP were used in the data assessment. We excluded all editorials, conferences, commentaries, letter to editors, and reviews. In addition, non-English studies, case reports, case–controls, noncomparative studies, and those evaluating DALK and PKP effects without a focus on keratoconus were excluded. There were no limitations regarding the sex of the evaluated cases. However, only studies which evaluated adults were included.
Search Strategy
We conducted a thorough manual search on the Web of Science, Embase, PubMed, and Scopus, considering the publications up to May 2021. The searched queries are delineated below:
SCOPUS and Web of Science
TITLE-ABS-KEY (compar* AND (lamell* AND penet*) AND keratocon*) AND (LIMIT-TO (DOCTYPE, "ar")) AND (LIMIT-TO (LANGUAGE, "English"))
Pubmed
compar*[tiab] AND (lamell*[tiab] AND penet*[tiab]) AND keratocon*[tiab] Filters: Clinical Study, Clinical Trial, Comparative Study, Controlled Clinical Trial, Evaluation Study, Multicenter Study, Observational Study, Pragmatic Clinical Trial, Randomized Controlled Trial, English
Embase
compar*:ti,ab,kw AND lamell*:ti,ab,kw AND penet*:ti,ab,kw AND keratocon*:ti,ab,kw AND ('case study'/de OR 'clinical trial'/de OR 'cohort analysis'/de OR 'comparative effectiveness'/de OR 'comparative study'/de OR 'controlled clinical trial'/de OR 'controlled study'/de OR 'cross sectional study'/de OR 'intervention study'/de OR 'major clinical study'/de OR 'observational study'/de OR 'prospective study'/de OR 'randomized controlled trial'/de OR 'randomized controlled trial topic'/de OR 'retrospective study'/de) AND 'article'/it
Evaluating Recovered Evidence
After completing the search, two reviewers separately removed duplicated findings via Endnote version 20. A manual check for duplication was also performed to ensure none existed. Subsequently, two authors performed initial evaluations of titles and abstracts of the recovered evidence. After recovering all the available articles in the second phase, they were then evaluated by the research team.
Data Extraction
Two authors independently extracted data from the articles according to the following criteria:
∙ Included study's first author
∙ The year the study was published
∙ Study type
∙ Country of origin
∙ Patients' age and sex
∙ Duration of follow-up
∙ Corneal infection rates
∙ Graft rejection rates (the rate of the rejection episodes seen in cases)
∙ Graft failure rates
∙ Cataract rates
∙ High-intraocular pressure (IOP; also known as ocular hypertension; an eye pressure of 21 mm Hg) rates
∙ Mean and standard deviation (SD) of best-corrected visual acuity (BCVA) in LogMAR scale (defined as the highest score on the Snellen chart when wearing either a visual aiding device, like glasses or contact lenses)
∙ Refractive (RC) and topographic cylinder (TC; defined as refractive power of the cylindrical lenses and the mapping the anterior curvature of the cornea, respectively)
∙ Central corneal thickness (CCT; defined as the thickness of the cornea measured by optical low coherence reflectometry)
∙ Endothelial cell count (ECC; estimation of corneal endothelial reserve by corneal endothelial photography)
∙ Spherical equivalent (an estimate of the eyes' refractive error calculated by merging the nearsightedness or farsightedness and cylindrical astigmatism components)
All the mentioned values were included based on the last known follow-up of each study. Finally, the extracted data were reviewed and double-checked by the senior author.
Table 1.
Data related to evaluated studies
|
| ||||||||||||||
| Author | Design | Year | Country | Sample size PKP | Sample size DALK | Mean age in PKP | Mean age in DALK | Follow-up PKP (month) | Follow-up DALK (month) | PKP Sex Male | PKP Sex Female | DALK Sex Male | DALK Sex Female | Main value evaluated |
| Abdelaal et al[23] | Retrospective cohort | 2021 | Saudi Arabia | 15 | 21 | Graft Rejection | ||||||||
| Abu Eta et al[24] | Retrospective cohort | 2020 | Israel | 21 | 32 | High-IOP | ||||||||
| Akdemir et al[25] | Retrospective cohort | 2012 | Turkey | 30 | 30 | 28.07 | 29.67 | 12.58 | 12.58 | 16 | 14 | 14 | 16 | BCVA |
| Alzahrani et al[26] | Retrospective cohort | 2018 | UK | 21 | 16 | 30.14 | 32.56 | 13.95 | 14.87 | 14 | 7 | 11 | 5 | BCVA, CCT |
| Amayem et al[27] | Retrospective cohort | 2012 | Saudi Arabia | 30 | 47 | 26.5 | 24.3 | 24 | 24 | Refractive Cylinder, SE | ||||
| Bahar et al[28] | Retrospective cohort | 2008 | Canada | 20 | 17 | 42.2 | 32.5 | 53.2 | 17 | 11 | 9 | 11 | 6 | Endothelial Cell Count, Infection Rate, Graft Rejection, Topographic Cylinder |
| Cohen et al[29] | Retrospective cohort | 2010 | USA | 30 | 11 | 35.4 | 45.5 | 21.9 | 22.5 | 21 | 9 | 8 | 3 | Cataract Frequency, High-IOP, Graft Rejection, Refractive Cylinder, SE, BCVA |
| Donoso et al[30] | Retrospective cohort | 2015 | Chile | 49 | 90 | 31.7 | 28.3 | 48.6 | 36.8 | High-IOP, ECC, Infection Rate, Graft Rejection, Topographic and Refractive Cylinder, CCT | ||||
| Funnell et al[31] | Cohort | 2006 | UK | 20 | 20 | 32 | 28 | 12 | 12 | 14 | 6 | 11 | 9 | High-IOP, Graft Rejection, Graft Failure, Topographic Cylinder |
| Godefrooij et al[32] | Retrospective cohort | 2016 | Netherlands | 736 | 297 | 38.07 | 35.65 | 499 | 237 | 204 | 93 | BCVA | ||
| Hamdi et al[33] | Cross-sectional | 2017 | Egypt | 12 | 24 | 26.95 | 23.79 | 9 | 3 | 14 | 10 | Topographic and Refractive Cylinder, SE, BCVA | ||
| Huang et al[34] | Retrospective cohort | 2015 | China | 79 | 68 | 24.3 | 24.1 | 93.1 | 93.5 | 50 | 29 | 40 | 28 | Topographic and Refractive Cylinder, SE, BCVA |
| Jafarinasab et al[35] | Cross-sectional | 2011 | Iran | 45 | 23 | 29.8 | 27.2 | 31.4 | 29.2 | Topographic Cylinder, SE, BCVA | ||||
| Janiszewska-Bil et al[36] | Cohort | 2021 | Poland | 40 | 50 | 28.4 | 28.6 | 12 | 12 | 24 | 16 | 28 | 22 | High-IOP, ECC, Topographic Cylinder, CCT, BCVA |
| Javadi et al[37] | Randomized clinical trial | 2010 | Iran | 35 | 42 | 30.89 | 26.91 | 24.6 | 22 | 28 | 7 | 29 | 13 | Graft Rejection, Topographic Cylinder, CCT, SE, BCVA |
| Jones et al[38] | Retrospective cohort | 2009 | UK | 1917 | 455 | Graft Rejection, Graft Failure, SE, BCVA | ||||||||
| Kasbekar et al[39] | Cohort | 2014 | UK | 3124 | 1086 | 60 | 60 | Infection rate, Graft Rejection, Graft Failure | ||||||
| Khattak et al[40] | Retrospective cohort | 2018 | Saudi Arabia | 99 | 108 | 28.9 | 27.8 | 29.3 | 28.1 | 59 | 40 | 67 | 41 | High-IOP, ECC, Cataracts, Infection rate, Graft Rejection, Graft Failure, Topographic and Refractive Cylinder, SE, BCVA |
| Kim et al[41] | Retrospective cohort | 2011 | Korea | 38 | 19 | 26.2 | 25.3 | 51.7 | 22.6 | 25 | 13 | 17 | 2 | SE |
| Koytak et al [42] | Retrospective cohort | 2011 | Turkey | 39 | 44 | 36.24 | 34.54 | 24 | 24 | 24 | 15 | 26 | 18 | ECC, CCT |
| Kubaloglu et al [43] | Cohort | 2011 | Turkey | 24 | 20 | 30.2 | 25.6 | 6 | 6 | 112 | 95 | Topographic and Refractive Cylinder, SE, CCT, BCVA | ||
| Macintyre et al [44] | Retrospective cohort | 2014 | Australia | 42 | 31 | 32.3 | 29.2 | 53.7 | 51.8 | 22 | 20 | 19 | 12 | Cataracts, Graft Rejection, Graft Failure, Refractive Cylinder, BCVA |
| Motlagh et al [45] | Cross-sectional | 2012 | Iran | 57 | 49 | 35 | 30.3 | SE, BCVA | ||||||
| Oh et al [46] | Retrospective cohort | 2013 | Korea | 5 | 11 | 27.4 | 28 | 45 | 30 | 3 | 2 | 7 | 4 | ECC, Refractive and Topographic Cylinder, CCT, SE, BCVA |
| Pedrotti et al [47] | Retrospective cohort | 2016 | Italy | 16 | 16 | 34.1 | 35.9 | 7 | 9 | 7 | 9 | Graft Rejection, Graft Failure, Topographic and REfractive Cylinder, CCT, SE, BCVA | ||
| Sogutlu Sari et al [48] | Cohort | 2012 | Turkey | 75 | 99 | 28.44 | 27.59 | 25.53 | 21.54 | Graft Rejection, Refractive Cylinder, SE, BCVA | ||||
| Watson et al [49] | Retrospective cohort | 2004 | UK | 22 | 25 | 33.9 | 32.6 | 55 | 28 | 14 | 8 | 17 | 8 | Infection, Graft Rejection, Graft Failure, |
| Yüksel et al [50] | Clinical trial | 2017 | Turkey | 38 | 38 | 35.3 | 34.9 | 12 | 12 | 15 | 23 | 10 | 28 | High-IOP, Graft Rejection, Topographic Cylinder, SE, BCVA |
| Zhang et al [51] | Retrospective cohort | 2013 | China | 52 | 75 | 21.9 | 20.6 | 60.2 | 46.9 | 45 | 7 | 55 | 20 | High-IOP, Cataracts, Graft Rejection, Topographic Cylinder, SE |
| Ziaei et al [52] | Retrospective cohort | 2019 | New Zealand | 42 | 27 | 35 | 36.1 | 25 | 17 | 12 | 15 | CCT | ||
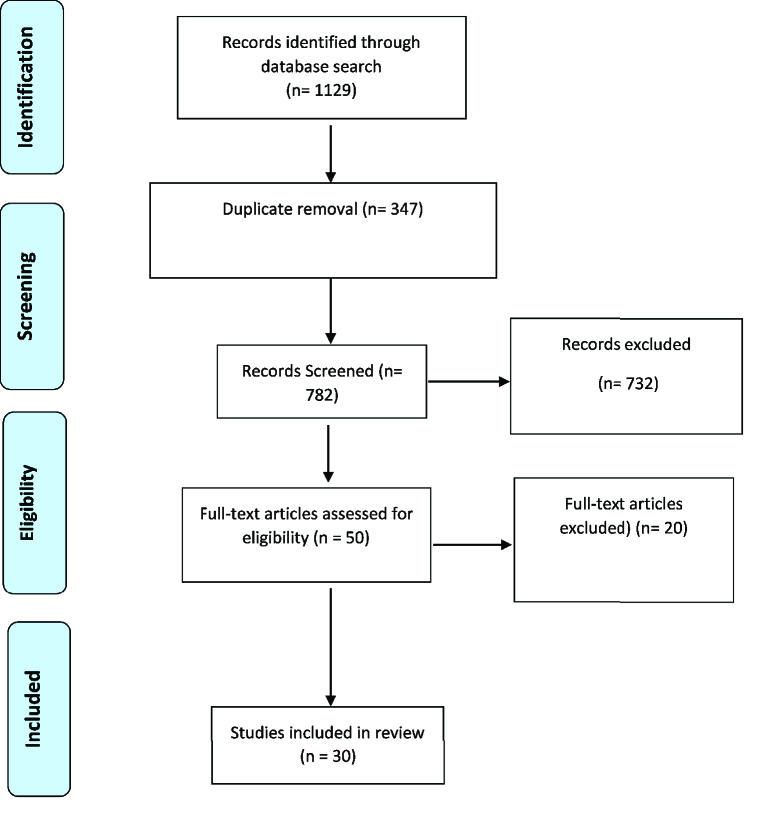
Figure 1.
Diagram of the studies evaluation.
Quality Assessment
The quality of the included studies was evaluated independently by two authors using the critical appraisal tool, provided by The Joanna Briggs Institute, containing 13, 12, and 8 items for assessing RCTs, cohort, and cross-sectional studies, respectively.
Data Analysis
For quantitative outcomes, the mean differences and SD, and the risk-ratio for qualitative outcomes were determined and analyzed. Methods of meta-analysis and random-effects models were used to combine the results using the 14 edition of the STATA software. Furthermore, heterogeneity between the studies was determined by employing the I2 test. The p-value was set at 0.05 for the significance level.
RESULTS
We identified 1129 articles in the systematic search of resources. After reviewing the titles and abstracts, 1079 articles were excluded from the study of which 347 were duplications. After reviewing the full-text of the articles, 20 were put aside again. Finally, 30 articles were included into this meta-analysis. The information of the selected articles is shown in Figure 1.
Among the 30 articles chosen, 25 were cohort (retrospective or prospective), 3 were cross-sectionals, and 2 were randomized-clinical trials. Information about each study is shown in Table 1.
Quality Assessment
Based on the Joanna Briggs Institute's critical appraisal tool, the three cross-sectional studies scored within a range of 6–8 out of a possible 8. The two randomized-clinical trials scored 10 out of a possible 13. In addition, the 25 cohort studies within a range of 9–11 out of a possible 12.
Meta-Analysis Results
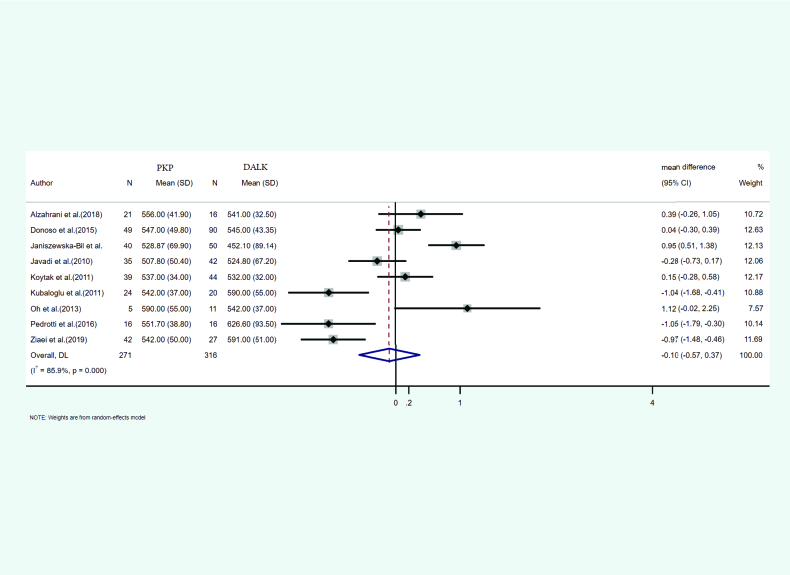
Central corneal thickness (CCT)
In nine of the studies, the mean and SD of central corneal thickness after PKP and DALK was reported. A total of 271 eyes were treated with PKP and 316 eyes with DALK. The mean age of the cases treated with PKP and DALK was 31.56 and 30.72, respectively. Furthermore, the follow-up duration was 24.87 and 20.81 months for PKP and DALK, respectively. Heterogeneity between the studies was significant (I2 = 85.9%, p-value < 0.001). According to the meta-analysis results with the help of the random-effects model, integrated mean differences (mean PKP – mean DALK) for the central corneal thickness were measured as –0.10 (pooled MD = –0.10, 95% CI: –0.57 – 0.37, p-value = 0.671). Figure 2 shows the forest plot of the meta-analysis.
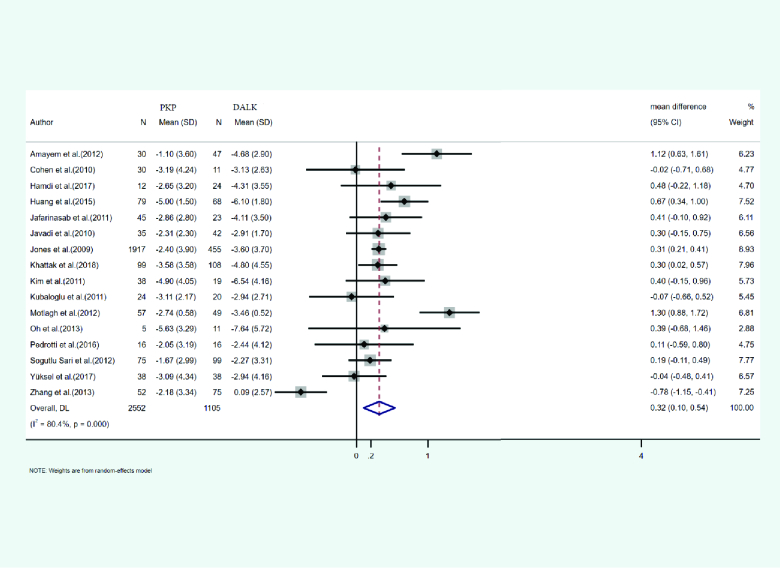
Spherical equivalent (SE)
In 16 studies, the mean and SD of the spherical equivalent identified after PKP and DALK was reported. A total of 2552 eyes were treated with PKP, and 1105 eyes were treated with DALK. The mean age of cases treated with PKP and DALK was 29.2 and 28.39 years, respectively. The duration of the follow-up was 35.36 months for PKP and 27.33 months for DALK cases. Heterogeneity was statistically significant (I2 = 80.4%, p-value < 0.001). Integrated mean differences (mean PKP – mean DALK) of PKP and DALK for the spherical equivalent was 0.32 (pooled MD = 0.32, 95% CI; 0.10 – 0.54, p-value = 0.004). Figure 3 illustrates the forest plot of the meta-analysis results.
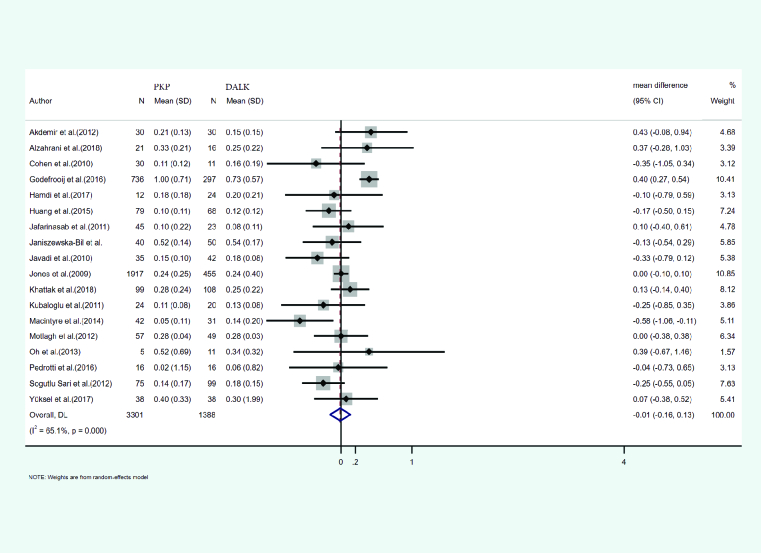
Best-corrected visual acuity (BCVA)
Eighteen studies reported the mean and SD of BCVA after both PKP and DALK. A total of 3301 eyes were treated with PKP and 1388 eyes with DALK. The mean age of cases treated with PKP and DALK was 30.54 and 28.14 years, respectively. Duration of follow-up was 29.71 months for PKP and 27.60 months for DALK cases. Heterogeneity was statistically significant (I2 = 65.1%, p-value < 0.001). Integrated mean differences (mean PKP – mean DALK) for the BCVA was measured as –0.01 (pooled MD = –0.01, 95% CI; –0. 61 – 0.13, p-value = 0.869). Figure 4 illustrates the forest plot of the meta-analysis.
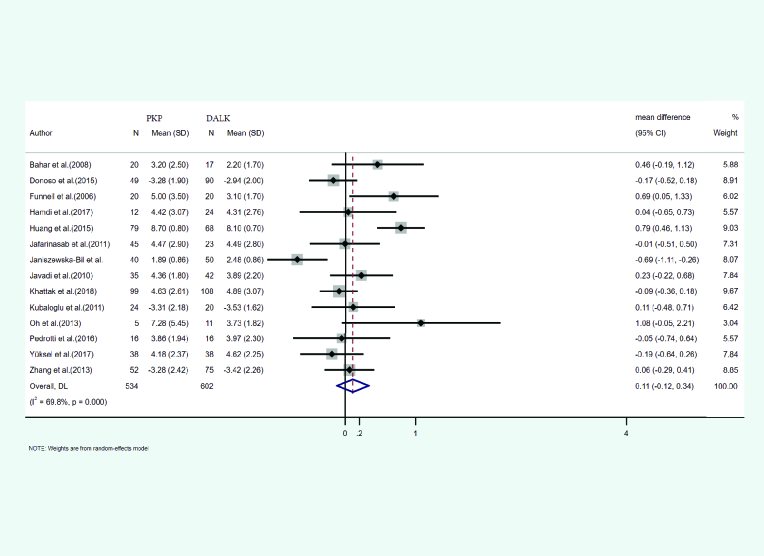
Topographic cylinder
The mean and SD of the topographic cylinder occurring after PKP and DALK was reported in 14 studies. A total of 534 eyes underwent PKP and 602 underwent DALK. The mean age of cases treated with PKP and DALK was 30.00 and 28.01 years, respectively. Follow-up for PKP and DALK groups was 35.61 and 28.79 months, respectively. Heterogeneity was statistically significant (I2 = 69.8%, p-value < 0.001). Integrated mean differences (mean PKP – mean DALK) for the topographic cylinder was 0.11 (pooled MD = 0.11.95% CI; –0.12 – 0.34, p-value = 0.359). Figure 5 shows the forest plot of the meta-analysis.
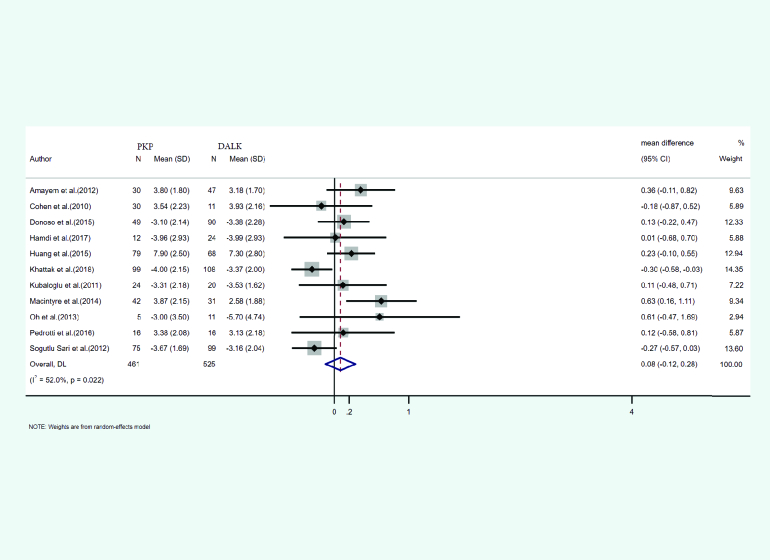
Refractive cylinder
The mean and SD of refractive cylinder occurring after PKP and DALK was reported in 11 studies. A total of 439 eyes underwent PKP and 525 underwent DALK. The mean age of cases treated with PKP and DALK was 29.65 and 29.09 years, respectively. Follow-up for PKP and DALK groups was 38.57 and 34.91 months, respectively. Heterogeneity was statistically significant (I2 = 52%, p-value = 0.022). Integrated mean differences (mean PKP – mean DALK) for the refractive cylinder was 0.08 (pooled MD = 0.27, 95% CI; –0.12 – 0.28, p-value = 0.428). Figure 6 shows the forest plot of the meta-analysis.
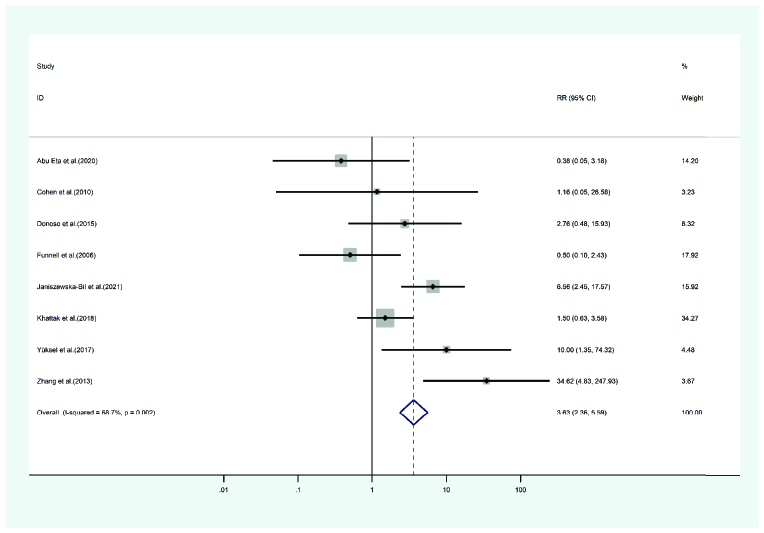
High IOP
High IOP occurring after PKP and DALK appeared in eight studies. A total of 349 eyes were treated with PKP, and 424 were treated with DALK. The mean age of cases treated with PKP and DALK was
Figure 2.
Results of meta-analysis for central corneal thickness.
Figure 3.
Results of the meta-analysis of the spherical equivalent.
Figure 4.
Results of the meta-analysis of the best-corrected visual acuity.
Figure 5.
Results of the meta-analysis of the topographic cylinder.
Figure 6.
Results of the meta-analysis of the refractive cylinder.
Figure 7.
Results of the meta-analysis of the high IOP.
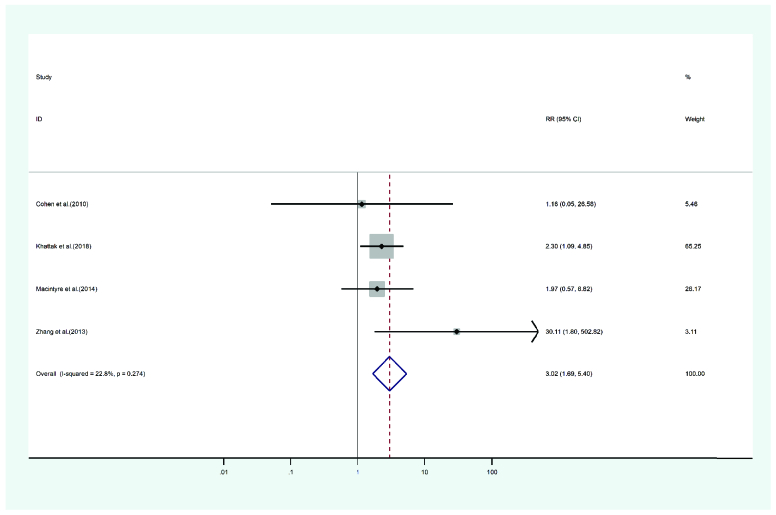
Figure 8.
Results of the meta-analysis of the cataract.
Figure 9.
Results of the meta-analysis of the corneal infection.
Figure 10.
Results of the meta-analysis of the graft rejection.
Figure 11.
Graft failure meta-analysis results.
Figure 12.
Endothelial cell count meta-analysis results.
30.51 and 30.52 years, respectively. The duration of follow-up for the PKP cases was 28 and 24.32 months for the DALK cases. Heterogeneity was statistically significant (I2 = 68.7, p-value = 0.002). The random-effects model showed the PKP group's high IOP risk ratio as 3.63 times that of the DALK group (pooled HR = 3.63, 95% CI; 2.36 – 5.59, p-value = 0.018). Forest's plot is presented in Figure 7.
Cataract
In six studies reporting cataract occurring after PKP and DALK, 223 eyes were treated with PKP and 225 eyes with DALK. The mean age of cases was 29.62 years for PKP and 30.77 years for DALK. Follow-up period was 41.27 months for PKP and 37.32 months for DALK cases. Heterogeneity was not statistically significant (I2 = 22.8, p-value = 0.274). The risk ratio of cataract incidence in the PKP group was 3.02 times that of the DALK group (pooled HR = 3.02, 95% CI; 1.69 – 5.40, p-value = 0.62). Figure 8 presents the forest plot of the meta-analysis.
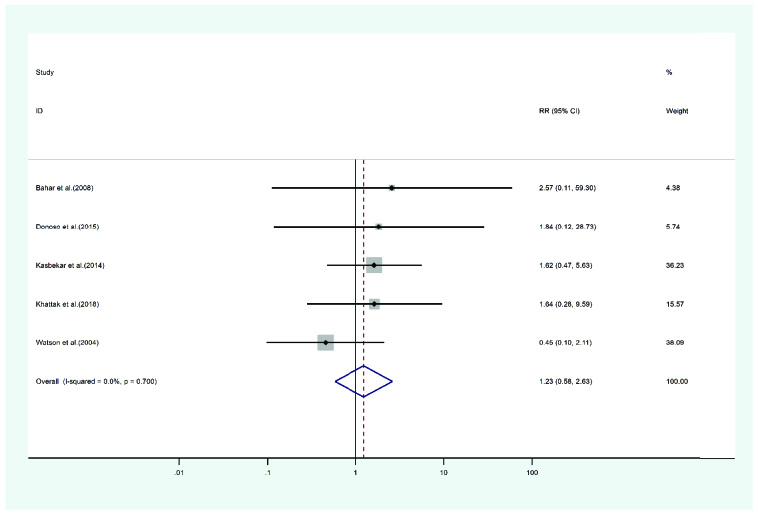
Corneal infection
Corneal infection manifesting after PKP and DALK was reported in five studies. A total of 3314 eyes were treated with PKP and 1326 eyes were treated with DALK. The mean age of cases treated with the PKP and DALK method was 34.17 and 30.3 years, respectively. The duration of the cases' follow-up in the PKP and the DALK groups was 49.22 and 33.98 months. Heterogeneity was not statistically significant (Q-value = 0.24, df = 2, (I2 = 0.000, p-value = 0.7). The risk ratio of corneal infection incidence in the PKP group was measured at 1.23 times that of the DALK group (pooled HR = 1.23, 95% CI; 0.58 – 2.63, p-value = 0.700). Figure 9 illustrates the forest plot of the meta-analysis.
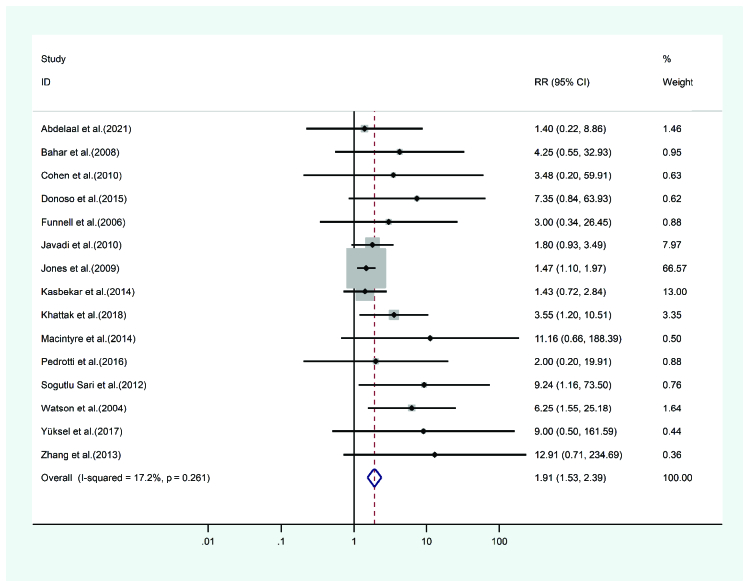
Graft rejection
Graft rejection occurring after PKP and DALK was observed in 15 studies. A total of 5554 eyes were treated with PKP and 2134 eyes with DALK. The mean age of cases undergoing PKP was 32.25 and 30.81 years for DALK. PKP cases were followed-up for 38 months and the DALK cases for 29.88 months. Heterogeneity was not statistically significant (I2 = 17.2, p-value = 0.261). The graft rejection risk ratio of PKP to DALK was 1.91 (pooled HR = 2.33, 95% CI; 1.69 – 3.22, p-value = 0.179). Figure 10 presents the forest plot.
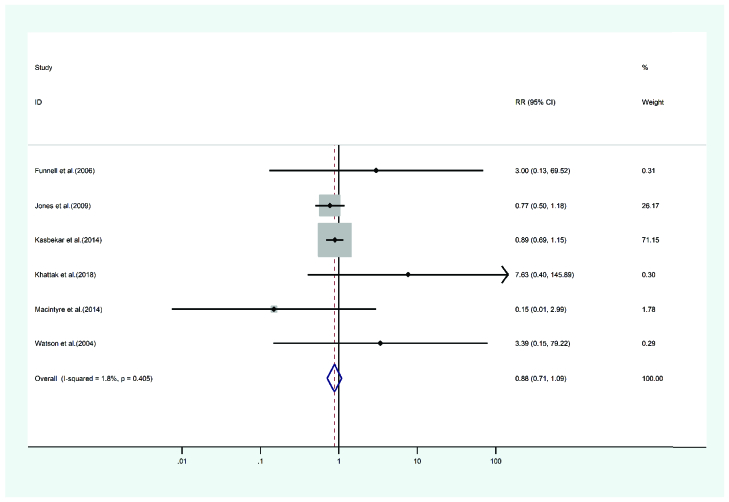
Graft failure
Six studies discussed graft failure presenting after PKP and DALK. A total of 5224 eyes were treated with PKP and 1725 were treated with DALK. The mean age of cases treated with PKP and DALK was 31.77 and 29.4 years, respectively. Follow-up for the PKP group was 42 months and 35.98 months for the DALK group. Heterogeneity was not statistically significant (I2 = 1.8, p-value = 0.405). The risk ratio of the graft failure incidence in the PKP group was 0.88 of the DALK group (pooled HR = 0.88, 95% CI; 0.71 – 1.09, p-value = 0.98). In Figure 11, a related forest plot is illustrated.
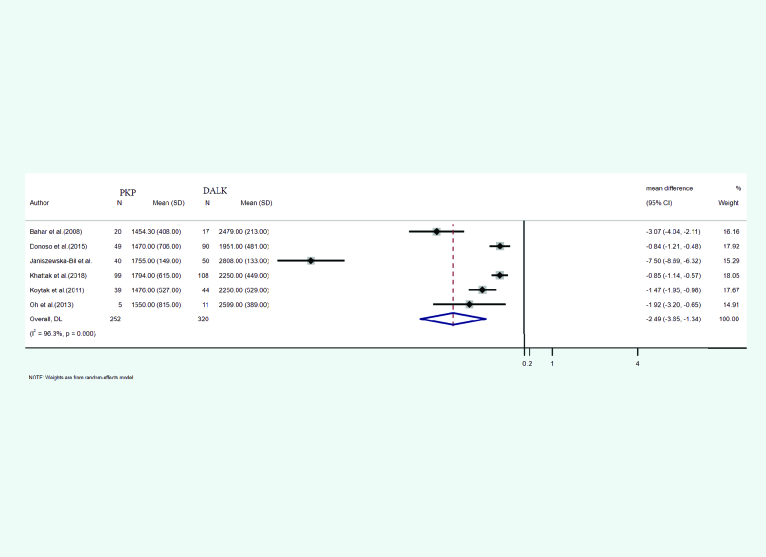
Endothelial cell count
The mean and SD of endothelial cell count presenting after PKP and DALK was reported in six studies. A total of 252 eyes underwent PKP and 320 underwent DALK. The mean age of cases treated with PKP and DALK was 32.47 and 29.95 years, respectively. Follow-up for PKP and DALK groups was 35.35 and 24.65 months, respectively. Heterogeneity was statistically significant (I2 = 96.3%, p-value < 0.001). Integrated mean differences (mean PKP – mean DALK) for the endothelial cell count was –2.49 (pooled MD = –2.49 95% CI; –3.65 – –1.34, p-value = 0.000). Figure 12 shows the forest plot of the meta-analysis.
DISCUSSION
In this study, a total of 6773 cases of PKP and 2891 cases of DALK were documented and reviewed. In those studies, where differences in sex were evaluated, the male cases were subjected to more interventions in both the PKP and DALK procedures than the female cases. The mean age for PKP and DALK categories was 30.97 and 29.80 years, respectively. Furthermore, the cases were followed-up in the PKP and DALK groups for 34.99 and 28.59 months, respectively.
CCT in the DALK group was 10% greater overall than registered in the PKP group, which was not statistically significant. The study by Liu et al[53] could not find statistical significance regarding the differences in this value when comparing the two groups.
The SE value in the PKP group was 32% higher overall, which was also statistically significant. This finding may be due to the tighter suturing in DALK. Henein et al and Liu et al[53] did not find any statistical significance when comparing the value between the two groups. However, the study by Song et al[55] found this value to be significantly more improved in the DALK group.
BCVA measured in the DALK group insignificantly demonstrated to be 1% better as compared to the PKP group. However, the study by Henein et al[54] revealed statistical significance in favor of the PKP group. In Song et al[55] and Liu et al[53], this value was not statistically significant.
Regarding the topographic cylinder, the PKP group showed insignificantly greater results (about 11%). Furthermore, neither of the studies by Henein et al[54] nor Song et al[55] demonstrated statistical significance.
The PKP group demonstrated insignificantly greater results on the refractive cylinder values than the DALK group (8% overall). Henein et al[54] demonstrated significance in the improvement of the RC in the DALK group. Song et al,[55] however, found no statistical significance in the differences of RC between the two groups.
The ECC was 2.49 score unique higher in the DALK group as compared to the PKP group. Consistent with the study by Liu et al,[53] this finding also turned out to be statistically significant. In the study by Henein et al,[54] the ECC values were not statistically significant between the two groups. DALK involved the inner portion of the cornea less often than PKP. The procedure is also less invasive.
Cataracts (38 vs 12 cases), graft rejection (413 vs 80 cases), graft failure (286 vs 105 cases), High-IOP (73 vs 30 cases), and corneal infection (22 vs 11 cases) were all more common findings in the PKP groups as compared to the DALK groups. Except for the high IOP, cataract and graft rejection the remaining complications were not statistically significant when we compared the results of the two groups.
Graft failure, consistent with our study, was not statistically significant in the studies by Henein et al[54] and Liu et al.[53] In addition, High IOP was significantly more common in the PKP group when compared in the study by Liu et al.[53] Furthermore, in prior review studies by Henein et al,[54] Song et al,[55] and Liu et al,[53] the DALK groups suffered significantly fewer graft rejection episodes. Furthermore, consistent with our study, Liu et al[53] found statistical significance regarding the number of post-op cataracts occurring in the PKP group.
SUMMARY
Despite the results favoring the DALK procedure and its utility in most of the evaluated outcomes, we cannot definitively conclude that the procedure is more eventful compared to PKP. This remark is primarily due to the small sample size, study design variability, and mismatched follow-up durations leading to, in some incidences, significant heterogeneity that could not be addressed via met-regression or sensitivity analyses. Therefore, we believe that any conclusions from the comparisons must be taken with a grain of salt. Ultimately, we believe that to increase the validity of a possible meta-analysis, future randomized controlled trials need to be conducted with consistently matching follow-up durations and timing between the sessions.
Limitations
Our study's limitation was that we did not include other types of lamellar keratoplasty techniques due to the low number of available cases. Furthermore, formulas used to convert the domains to the SD might not be as accurate as desired, which can be due to statistical limitations.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
The authors do not have any conflicts of interest.
References
- Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group Cornea. 1996;15:139–146. doi: 10.1097/00003226-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–273. doi: 10.1016/0002-9394(86)90817-2. [DOI] [PubMed] [Google Scholar]
- Zadnik K, Steger-May K, Fink BA, Joslin CE, Nichols JJ, Rosenstiel CE, et al. Between-eye asymmetry in keratoconus. Cornea. 2002;21:671–679. doi: 10.1097/00003226-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Chopra I, Jain AK. Between eye asymmetry in keratoconus in an Indian population. Clin Exp Optom. 2005;88:146–152. doi: 10.1111/j.1444-0938.2005.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Bilgin LK, Yilmaz S, Araz B, Yüksel SB, Sezen T. 30 years of contact lens prescribing for keratoconic patients in Turkey. Cont Lens Anterior Eye. 2009;32:16–21. doi: 10.1016/j.clae.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Steger-May K, Szczotka-Flynn L, Riley C, Joslin CE, Weissman BA, et al. Baseline factors predictive of incident penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2006;142:923–930. doi: 10.1016/j.ajo.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Fallahi Motlagh B, Javadi MA, Jafari Nasab MR, Rabbanikhah Z, Anisian A, Souri H. Corneal transplantation in patients with keratoconus. Iran J Ophthalmol. 2003;16:9–19. [Google Scholar]
- Javadi MA, Mohammadi MJ, Mirdehghan SA, Sajjadi SH. A comparison between donor-recipient corneal size and its effect on the ultimate refractive error induced in keratoconus. Cornea. 1993;12:401–405. doi: 10.1097/00003226-199309000-00006. [DOI] [PubMed] [Google Scholar]
- Brierly SC, Izquierdo Jr L, Mannis MJ. Penetrating keratoplasty for keratoconus. Cornea. 2000;19:329–332. doi: 10.1097/00003226-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Kirkness CM, Ficker LA, Steele AD, Rice NS. The success of penetrating keratoplasty for keratoconus. Eye. 1990;4:673–688. doi: 10.1038/eye.1990.95. [DOI] [PubMed] [Google Scholar]
- Lim L, Pesudovs K, Coster DJ. Penetrating keratoplasty for keratoconus: visual outcome and success. Ophthalmology. 2000;107:1125–1131. doi: 10.1016/s0161-6420(00)00112-3. [DOI] [PubMed] [Google Scholar]
- Colin J, Velou S. Current surgical options for keratoconus. J Cataract Refract Surg. 2003;29:379–386. doi: 10.1016/s0886-3350(02)01968-5. [DOI] [PubMed] [Google Scholar]
- Agarwal Rk Deep lamellar keratoplasty, an alternative to penetrating keratoplasty. Br J Ophthalmol. 1997;81:178–179. doi: 10.1136/bjo.81.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croasdale CR, Barney E, Warner EJ. Eye bank tissue utilization between endothelial keratoplasty and penetrating keratoplasty. Cornea. 2013;32:280–284. doi: 10.1097/ICO.0b013e3182561305. [DOI] [PubMed] [Google Scholar]
- Cassidy D, Beltz J, Jhanji V, Loughnan MS. Recent advances in corneal transplantation for keratoconus. Clin Exp Optom. 2013;96:165–172. doi: 10.1111/cxo.12047. [DOI] [PubMed] [Google Scholar]
- Weed KH, McGhee CN, MacEwen CJ. Atypical unilateral superior keratoconus in young males. Cont Lens Anterior Eye. 2005;28:177–179. doi: 10.1016/j.clae.2005.10.002. [DOI] [PubMed] [Google Scholar]
- MacIntyre R, Chow SP, Chan E, Poon A. Long-term outcomes of deep anterior lamellar keratoplasty versus penetrating keratoplasty in Australian keratoconus patients. Cornea. 2014;33:6–9. doi: 10.1097/ICO.0b013e3182a9fbfd. [DOI] [PubMed] [Google Scholar]
- Van Dooren B, Mulder PG, Nieuwendaal CP, Beekhuis WH, Melles GR. Endothelial cell density after posterior lamellar keratoplasty (Melles techniques): 3 years follow-up. Am J Ophthalmol. 2004;138:211–217. doi: 10.1016/j.ajo.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Shimazaki J, Shimmura S, Ishioka M, Tsubota K. Randomized clinical trial of deep lamellar keratoplasty vs penetrating keratoplasty. Am J Ophthalmol. 2002;134:159–165. doi: 10.1016/s0002-9394(02)01523-4. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanna Briggs Institute The Joanna Briggs Institute Critical Appraisal tools for use in JBI systematic reviews checklist for prevalence studies [Internet]; Joanna Briggs Institute; Available, from. 2017. [Google Scholar]
- Abdelaal AM, Alqassimi AH, Malak M, Hijazi HT, Hadrawi M, Khan MA. Indications of keratoplasty and outcomes of deep anterior lamellar keratoplasty compared to penetrating keratoplasty. Cureus. 2021;13:e13825. doi: 10.7759/cureus.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busool Abu Eta Y, Tomkins-Netzer O, Mimouni M, Hamed Azzam S, Shehadeh Mashour R. Predicting factors of ocular hypertension following keratoplasty: Indications versus the procedure. Eur J Ophthalmol. 2021;31:1749–1753. doi: 10.1177/1120672120948757. [DOI] [PubMed] [Google Scholar]
- Akdemir MO, Kandemir B, Sayman IB, Selvi C, Dogan OK. Comparison of contrast sensitivity and visual acuity between deep anterior lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Int J Ophthalmol. 2012;5:737–741. doi: 10.3980/j.issn.2222-3959.2012.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani K, Dardin SF, Carley F, Brahma A, Morley D, Hillarby MC. Corneal clarity measurements in patients with keratoconus undergoing either penetrating or deep anterior lamellar keratoplasty. Clin Ophthalmol. 2018;12:577–585. doi: 10.2147/OPTH.S157286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amayem AF, Hamdi IM, Hamdi MM. doi: 10.1097/ICO.0b013e31825ca70b. Refractive and visual outcomes of penetrating keratoplasty versus deep anterior lamellar keratoplasty with hydrodissection for treatment of keratoconus. Cornea 2013;32:e2–e5. [DOI] [PubMed]
- Bahar I, Kaiserman I, Srinivasan S, Ya-Ping J, Slomovic AR, Rootman DS. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146:905–912. doi: 10.1016/j.ajo.2008.06.034. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Goins KM, Sutphin JE, Wandling GR, Wagoner MD. Penetrating keratoplasty versus deep anterior lamellar keratoplasty for the treatment of keratoconus. Int Ophthalmol. 2010;30:675–681. doi: 10.1007/s10792-010-9393-9. [DOI] [PubMed] [Google Scholar]
- Donoso R, DÃaz C, Villavicencio P. Comparative study of keratoconus between Anwar’s deep anterior lamellar keratoplasty versus converted penetrating keratoplasty. Arch Soc Esp Oftalmol. 2015;90:257–263. doi: 10.1016/j.oftal.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Funnell CL, Ball J, Noble BA. Comparative cohort study of the outcomes of deep lamellar keratoplasty and penetrating keratoplasty for keratoconus. Eye. 2006;20:527–532. doi: 10.1038/sj.eye.6701903. [DOI] [PubMed] [Google Scholar]
- Godefrooij DA, Gans R, Imhof SM, Wisse RP. Trends in penetrating and anterior lamellar corneal grafting techniques for keratoconus: a national registry study. Acta Ophthalmol. 2016;94:489–493. doi: 10.1111/aos.13041. [DOI] [PubMed] [Google Scholar]
- Hamdi IM, Hamdi MM. Quality of vision after deep anterior lamellar keratoplasty (fluid dissection) compared to penetrating keratoplasty for the treatment of keratoconus. J Ophthalmol. 2017;2017:4507989. doi: 10.1155/2017/4507989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Hu Y, Gui M, Hou C, Zhang H. Comparison of refractive outcomes in three corneal transplantation techniques for keratoconus. Graefes Arch Clin Exp Ophthalmol. 2015;253:1947–1953. doi: 10.1007/s00417-015-3091-2. [DOI] [PubMed] [Google Scholar]
- Jafarinasab MR, Feizi S, Javadi MA, Hashemloo A. Graft biomechanical properties after penetrating keratoplasty versus deep anterior lamellar keratoplasty. Curr Eye Res. 2011;36:417–421. doi: 10.3109/02713683.2011.556303. [DOI] [PubMed] [Google Scholar]
- Janiszewska-Bil D, Czarnota-Nowakowska B, Krysik K, Lyssek-Boron A, Dobrowolski D, Grabarek BO, et al. Comparison of long-term outcomes of the lamellar and penetrating keratoplasty approaches in patients with keratoconus. J Clin Med. 2021;10:2421. doi: 10.3390/jcm10112421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi MA, Feizi S, Yazdani S, Mirbabaee F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: a clinical trial. Cornea. 2010;29:365–371. doi: 10.1097/ICO.0b013e3181b81b71. [DOI] [PubMed] [Google Scholar]
- Jones MN, Armitage WJ, Ayliffe W, Larkin DF, Kaye SB. Penetrating and deep anterior lamellar keratoplasty for keratoconus: a comparison of graft outcomes in the United kingdom. Invest Ophthalmol Vis Sci. 2009;50:5625–5629. doi: 10.1167/iovs.09-3994. [DOI] [PubMed] [Google Scholar]
- Kasbekar SA, Jones MN, Ahmad S, Larkin DF, Kaye SB. Corneal transplant surgery for keratoconus and the effect of surgeon experience on deep anterior lamellar keratoplasty outcomes. Am J Ophthalmol. 2014;158:1239–1246. doi: 10.1016/j.ajo.2014.08.029. [DOI] [PubMed] [Google Scholar]
- Khattak A, Nakhli FR, Al-Arfaj KM, Cheema AA. Comparison of outcomes and complications of deep anterior lamellar keratoplasty and penetrating keratoplasty performed in a large group of patients with keratoconus. Int Ophthalmol. 2018;38:985–992. doi: 10.1007/s10792-017-0548-9. [DOI] [PubMed] [Google Scholar]
- Kim KH, Choi SH, Ahn K, Chung ES, Chung TY. Comparison of refractive changes after deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Jpn J Ophthalmol. 2011;55:93–97. doi: 10.1007/s10384-010-0914-x. [DOI] [PubMed] [Google Scholar]
- Koytak A, Kubaloglu A, Sari ES, Atakan M, Culfa S, Ozerturk Y. Changes in central macular thickness after uncomplicated corneal transplantation for keratoconus: penetrating keratoplasty versus deep anterior lamellar keratoplasty. Cornea. 2011;30:1318–1321. doi: 10.1097/ICO.0b013e31821eeaad. [DOI] [PubMed] [Google Scholar]
- Kubaloglu A, Coskun E, Sari ES, Gunes AS, Cinar Y, Piñero DP, et al. Comparison of astigmatic keratotomy results in deep anterior lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2011;151:637–643. doi: 10.1016/j.ajo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Macintyre R, Chow SP, Chan E, Poon A. Long-term outcomes of deep anterior lamellar keratoplasty versus penetrating keratoplasty in Australian keratoconus patients. Cornea. 2014;33:6–9. doi: 10.1097/ICO.0b013e3182a9fbfd. [DOI] [PubMed] [Google Scholar]
- Motlagh BF, Sedghipoor MR, Abroon G, Sadigh AL. Outcomes of penetrating keratoplasty and deep anterior lamellar keratoplasty for keratoconus in a university teaching hospital. Iran J Ophthalmol. 2012;24:52–56. [Google Scholar]
- Oh BL, Kim MK, Wee WR. Comparison of clinical outcomes of same-size grafting between deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Korean J Ophthalmol. 2013;27:322–330. doi: 10.3341/kjo.2013.27.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrotti E, Passilongo M, Fasolo A, Ficial S, Ferrari S, Marchini G. Refractive outcomes of penetrating keratoplasty and deep anterior lamellar keratoplasty in fellow eyes for keratoconus. Int Ophthalmol. 2017;37:911–919. doi: 10.1007/s10792-016-0350-0. [DOI] [PubMed] [Google Scholar]
- Sögütlü Sari E, Kubaloglu A, Ãœnal M, Piñero Llorens D, Koytak A, Ofluoglu AN, et al. Penetrating keratoplasty versus deep anterior lamellar keratoplasty: comparison of optical and visual quality outcomes. Br J Ophthalmol. 2012;96:1063–1067. doi: 10.1136/bjophthalmol-2011-301349. [DOI] [PubMed] [Google Scholar]
- Watson SL, Ramsay A, Dart JK, Bunce C, Craig E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology. 2004;111:1676–1682. doi: 10.1016/j.ophtha.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Yüksel B, Kandemir B, Uzunel UD, Çelik O, Ceylan S, Küsbeci T. Comparison of visual and topographic outcomes of deep-anterior lamellar keratoplasty and penetrating keratoplasty in keratoconus. Int J Ophthalmol. 2017;10:385–390. doi: 10.18240/ijo.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14:438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei M, Vellara HR, Gokul A, Ali NQ, McGhee CNJ, Patel DV. Comparison of corneal biomechanical properties following penetrating keratoplasty and deep anterior lamellar keratoplasty for keratoconus. Clin Exp Ophthalmol. 2020;48:174–182. doi: 10.1111/ceo.13677. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen Y, Wang P, Li B, Wang W, Su Y, et al. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: a meta-analysis PLoS ONE. 2015;10:e0113332. doi: 10.1371/journal.pone.0113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henein C, Nanavaty MA. Systematic review comparing penetrating keratoplasty and deep anterior lamellar keratoplasty for management of keratoconus. Cont Lens Anterior Eye. 2017;40:3–14. doi: 10.1016/j.clae.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhang J, Pan Z. Systematic review and meta-analysis of clinical outcomes of penetrating keratoplasty versus deep anterior lamellar keratoplasty for keratoconus. Exp Clin Transplant. 2020;18:417–428. doi: 10.6002/ect.2019.0123. [DOI] [PubMed] [Google Scholar]