To the Editor: Portal vein tumor thrombus (PVTT) is present in 10% to 40% of patients with hepatocellular carcinoma (HCC) at diagnosis and has a profound adverse effect on progno-sis.[1] Sorafenib is recommended as the first-line treatment for patients with advanced HCC, including those who have PVTT. However, its efficacy is modest.[1,2] A combination of transarterial chemoembolization (TACE) and sorafenib (TACE-S) has been reported to be associated with improved outcomes.[1,3] But unfortunately, its efficacy in controlling PVTT remained limited, with an objective response rate (ORR) of only 9.7%.[3] Previous studies have demonstrated that iodine-125 (125I) seed brachytherapy targeting PVTT can lead to a significant reduction in tumor thrombus with few complications.[4,5] We hypothesized that TACE-S combined with 125I seed brachytherapy (TACE-S-I) could improve the control of PVTT and confer a greater survival benefit. Therefore, we conducted this study to evaluate the efficacy and safety of TACE-S-I compared with TACE-S in HCC patients with PVTT.
This study was approved by our institutional review board (No. 2020-hg-ks-03). Written informed consent was obtained from all patients. The medical records of consecutive patients with HCC and PVTT who underwent TACE-S-I or TACE-S from January 2015 to December 2018 at our institution were retrospectively reviewed. The inclusion criteria were as follows: (1) age between 18 and 75 years; (2) Eastern Cooperative Oncology Group performance status of 0 or 1; (3) Child-Pugh class A or B; and (4) the presence of PVTT on images of dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging obtained within 7 days before treatment. The exclusion criteria were as follows: (1) main portal vein obstruction without multiple collateral vessels; (2) PVTT invading the superior mesenteric vein, or hepatic vein and/or vena cava tumor thrombus; (3) previous treatment with sorafenib, systemic chemotherapy, intraarterial chemoinfusion or TACE; (4) malignant tumors in addition to HCC; or (5) severe medical comorbidities including severe heart or kidney dysfunction and severe coagulation disorders (prothrombin time ≥18 s or platelet count of < 50 × 109/L).
Before treatment, either TACE-S-I or TACE-S was recommended by the attending physician. If the patients chose TACE-S-I treatment, sorafenib (400 mg twice a day) was started 3 to 5 days after TACE, and 125I seeds (ZHIBO Bio-Medical Tech., Beijing, China) were implanted into the PVTT (according to the pre-operative planning) under CT guidance 3 to 14 days after TACE. The patients who refused 125I seed brachytherapy received TACE-S only. Follow-up was conducted at 4- to 6-week intervals. TACE or 125I seed implantation was repeated if clinically indicated. Dose reduction or discontinuation of sorafenib was based on the presence of toxicity.[1]
PVTT was classified into three types: (1) type A, PVTT involving the main portal vein; (2) type B, PVTT involving the first-order portal vein branch; and (3) type C, PVTT involving the second- or lower-order portal vein branch. The primary endpoint of this study was overall survival (OS; defined as the time from the initial TACE until death). The secondary endpoints were tumor response, time to tumor progression (TTP; defined as the time from the initial TACE to the first occurrence of disease progression), and adverse events (AEs). Intra-hepatic tumor response was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to the modified Response Evaluation Criteria in Solid Tumors. PVTT response was classified as follows: (1) CR, thrombus disappearance; (2) PR, ≥50% reduction in the product; (3) SD, <50% reduction or ≤25% increase in the product; and (4) PD, >25% increase in the product. AEs were assessed by the Common Terminology Criteria for Adverse Events v4.03.
Statistical analyses were performed using SPSS Statistics 19.0 (IBM, Armonk, NY, USA). Categorical data between groups were compared using the χ2 test or Fisher exact test, as appropriate. Quantitative data were compared using the Mann-Whitney U test. Survival was analyzed using Kaplan-Meier curves and log-rank tests. Univariate and multivariate analyses for OS were performed with the log-rank test and forward stepwise Cox proportional hazard model, respectively. P < 0.050 was considered statistically significant.
A total of 194 patients were assessed for eligibility. Twenty-three of them met the exclusion criteria. Finally, 171 patients were included in this study (74 in the TACE-S-I group and 97 in the TACE-S group) [Supplementary Figure 1]. The baseline characteristics of the patients were shown in Supplementary Table 1. There was no significant difference in the characteristics between the two groups. The median follow-up was 16.0 (range 4.0– 36.0) and 10.0 (range 2.5–25.5) months for the TACE-S-I group and TACE-S group, respectively. The mean number of TACE procedures was 5.6 (range 2.0–10.0) and 3.9 (range 2.0–8.0) in the TACE-S-I group and TACE-S group, respectively. Forty-seven patients in the TACE-S-I group underwent repeated I seed implantation, with a mean of 1.7 (range 1.0–4.0) procedures per patient. The median duration of sorafenib administration was 15.5 (range 4.0– 36.0) and 9.5 (range 2.5–25.0) months for the TACE-S-I group and TACE-S group, respectively.
The tumor responses in the TACE-S-I group were markedly better than those in the TACE-S group (ORR of PVTT: 58.1% vs. 11.3%, P < 0.001; ORR of intrahepatic tumor: 59.5% vs. 30.9%, P < 0.001). Subgroup analyses demonstrated higher ORRs of both PVTT and intra-hepatic tumor for type B/C PVTT patients in the TACE-S-I group [Supplementary Table 2]. The median TTP was 12.0 (95% confidence interval [CI] 10.2–13.8) and 5.0 (95% CI 4.1–5.9) months for the TACE-S-I group and TACE-S group, respectively (P < 0.001). Subgroup analyses demonstrated a significantly longer TTP for type B/C PVTT patients in the TACE-S-I group [Supplementary Table 3].
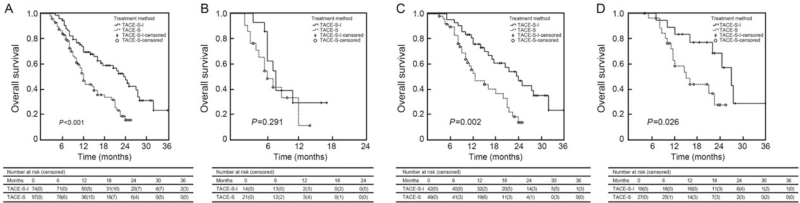
During the follow-up, 40 patients in the TACE-S-I group and 60 patients in the TACE-S group died. The median OS was 23.5 (95% CI 19.1–27.9) and 12.0 (95% CI 10.7– 13.3) months in the TACE-S-I group and TACE-S group, respectively (P < 0.001) [Figure 1A]. The 1-, 2-, and 3-year survival rates were 73%, 50%, and 24% in the TACE-S-I group and 54%, 20%, and 14% in the TACE-S group, respectively. Subgroup analyses demonstrated no significant difference in survival for type A PVTT patients between the two groups but a remarkably longer OS for type B/C PVTT patients in the TACE-S-I group [Figure 1B–D]. Uni- and multivariate analyses showed that treatment modality (TACE-S-I/TACE-S), type of PVTT (A/B + C), and ɑ-fetoprotein level (≥400/ < 400 ng/mL) were independent prognostic factors for OS [Supplementary Table 4]. The median OS was 7.5 (95% CI 6.3–8.7) and 20.5 (95% CI 16.0–25.0) months for patients with type A and type B + C PVTT, respectively (P < 0.001).
Figure 1.
Kaplan-Meier analyses of survival according to treatment group. (A) For the entire study population, the median OS was 23.5 (95% CI 19.1–27.9) and 12.0 (95% CI 10.7–13.3) months in the TACE-S-I group and TACE-S group, respectively. (B) For patients with type A PVTT, the median OS was 8.0 (95% CI 6.3–9.7) and 6.5 (95% CI 4.1–8.9) months in the TACE-S-I group and TACE-S group, respectively. (C) For patients with type B PVTT, the median OS was 24.0 (95% CI 18.7–29.3) and 12.5 (95% CI 7.8–17.2) months in the TACE-S-I group and TACE-S group, respectively. (D) For patients with type C PVTT, the median OS was 27.0 (95% CI 21.3–32.7) and 15.0 (95% CI 9.3–20.7) months in the TACE-S-I group and TACE-S group, respectively. CI: Confidence interval; OS: Overall survival; PVTT: Portal vein tumor thrombus; TACE-S: Transarterial chemoembolization combined with sorafenib; TACE-S-I: Transarterial chemoembolization combined with sorafenib and 125I seed brachytherapy.
These results indicated that TACE-S-I could significantly improve the OS in HCC patients with type B/C PVTT compared with TACE-S, which might be attributed to the potent effects of additional brachytherapy with 125I seeds for controlling PVTT and intra-hepatic tumor. Moreover, our data confirmed that the extent of PVTT was an important prognostic factor for survival.[1] In this study, patients with type A PVTT had worse outcomes regardless of which treatment was used. Owing to the tumor thrombus extending into the main trunk of the portal vein, it was difficult to implant 125I seeds to obtain full radiation coverage of the main PVTT under CT guidance. In these cases, irradiation stent placement may be an effective alternative.[2,5]
The AEs were detailed in Supplementary Table 5. Most of the AEs were sorafenib-related and both the incidence of overall AEs (94.6% vs. 91.8%, P = 0.471) and ≥grade 3 AEs (33.8% vs. 28.9%, P = 0.490) in the TACE-S-I group were comparable to those in the TACE-S group. These results indicated that TACE-S-I did not significantly increase the risk of AEs compared with TACE-S.
Our study did have limitations: (1) the nature of the retrospective study and the treatment preferences might lead to selection bias; (2) blinded assessment of the tumor response was impossible because the 125I seeds could be seen on the images, which might introduce bias into the response assessment. Thus, our findings should be validated in randomized clinical trials.
To conclude, TACE-S-I achieved a promising outcome in HCC patients with first- or lower-order branch PVTT. The patients who underwent TACE-S-I had markedly better treatment responses, a longer TTP, and a significantly improved OS than those who received TACE-S.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81873920, 81571774), the High-Level University Clinical Research Promotion Program of Guangzhou Medical University (No. B185004019), the Science and Technology Project of Guangzhou (No. 202002030135), and the Medical Science and Technology Research Foundation of Guangdong Province (Nos. A2019187, B2019055, and B2019089).
Conflicts of interest
None.
Supplementary Material
Supplementary Material
Footnotes
How to cite this article: Huang J, Cai M, Huang W, Guo Y, Zhou J, Liang L, Lin L, Zhou Z, Lian H, He M, Zhu K. Transarterial chemoembolization combined with sorafenib and iodine-125 seed brachytherapy for hepatocellular carcinoma with portal vein tumor thrombus: a retrospective controlled study. Chin Med J 2022;135:113–115. doi: 10.1097/CM9.0000000000001537
Jingjun Huang and Mingyue Cai contributed equally to this work.
Supplemental digital content is available for this article.
References
- 1.Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib – a retrospective controlled study. Radiology 2014; 272:284–293. doi: 10.1148/radiol.14131946. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Guo JH, Zhu HD, Zhu GY, Chen L, Teng GJ. Safety and efficacy of irradiation stent placement for malignant portal vein thrombus combined with transarterial chemoembolization for hepatocellular carcinoma: a single-center experience. J Vasc Interv Radiol 2017; 28:786–794. doi: 10.1016/j.jvir.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Pan T, Li XS, Xie QK, Wang JP, Li W, Wu PH, et al. Safety and efficacy of transarterial chemoembolization plus sorafenib for hepatocellular carcinoma with portal venous tumour thrombus. Clin Radiol 2014; 69:e553–e561. doi: 10.1016/j.crad.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang FJ, Li CX, Jiao DC, Zhang NH, Wu PH, Duan GF, et al. CT guided 125iodine seed implantation for portal vein tumor thrombus in primary hepatocellular carcinoma. Chin Med J 2008; 121:2410–2414. [PubMed] [Google Scholar]
- 5.Zhang L, Hu B, Li W, Huang P, Zhang S, Zhong BY, et al. 125I irradiation stent for hepatocellular carcinoma with main portal vein tumor thrombosis: a systematic review. Cardiovasc Intervent Radiol 2020; 43:196–203. doi: 10.1007/s00270-019-02346-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.