Abstract
Helicobacter pylori infection is a class I carcinogen that can lead to gastric cancer. Early diagnosis and eradication of H. pylori infection are important to eliminate the risk of gastric cancer. Several invasive diagnostic techniques require biopsy samples, resulting in avoidable injury and medical expense. Furthermore, due to the localized distribution of H. pylori, random biopsies are not always reliable in diagnosing H. pylori infection. This article aimed to review endoscopic findings and new endoscopic options for the diagnosis of H. pylori infection. Using conventional white light imaging (WLI) and image-enhanced endoscopy (IEE), the endoscopic features associated with histological changes have increasingly become apparent. Real-time endoscopy is essential to make a diagnosis of H. pylori infection and allow targeted biopsy. Image-enhanced endoscopy (IEE), such as narrow-band imaging (NBI), linked color imaging (LCI), and blue laser imaging (BLI), enhances visualization of the surface vascular pattern and provides accurate diagnostic performance in H. pylori infection, as well as gastric neoplastic lesions, compared to conventional white light endoscopy. In conclusion, the new endoscopic technologies could be used in current practice with conventional white light endoscopy for accurate and real-time diagnosis of H. pylori infection and pre-cancerous lesions.
Key Words: Endoscopic diagnosis, white light imaging (WLI), image-enhanced endoscopy (IEE)
Introduction
Helicobacter pylori infection is the leading cause of chronic gastritis, and it is classified as a class I carcinogen of gastric cancer by the World Health Organization (WHO) (Marshall, 2008). Early and accurate diagnosis of H. pylori infection is important for eliminating the risk of gastric cancer. Nowadays, there are invasive and non-invasive tests available to diagnose H. pylori infection. However, each technique has some limitations. For example, patients who use proton pump inhibitor PPI), antibiotics, anti-platelet, anti-coagulant, or direct oral anti-coagulant (DOAC) medications. Real-time endoscopy along with conventional white light imaging WLI) and image-enhanced endoscopic (IEE) techniques, such as narrow-band imaging (NBI), linked color imaging (LCI) and blue laser imaging (BLI), appear to have important roles in clinical practice to identify H. pylori-infected status (Malfertheiner et al., 2007). This article aimed to review the endoscopic diagnostic options and findings for H. pylori infection.
Current diagnostic tests for H. pylori infection
Many diagnostic tests are available, including invasive and non-invasive tests. Each method has advantages, disadvantages, and limitations of various clinical situations (Bray et al., 2018; Malfertheiner et al., 2007), which are demonstrated in Table 1. Real-time endoscopy has become an important tool for detecting H. pylori infection. It provides additional endoscopic information on gastric mucosal abnormalities and results in unnecessary mucosal injury and medical costs.
Table 1.
Sensitivity, Specificity, Advantages, and Disadvantages of Diagnostic Tests for Detecting H. pylori Infection
| Diagnostic test | Sensitivity | Specificity | Advantages | Disadvantages |
|---|---|---|---|---|
| Rapid urease test (RUT) | 93 - 97 % | 98% | - Fast | - Invasive - False negative in ATB, PPI usage, and GI bleed |
| Histochemical staining test | 80 - 90 % | 90 - 100 % | - Gold-standard | - Invasive and need pathologist |
| Urea breath test | 90 - 97 % | 95 - 100 % | - Non-invasive - Confirm eradication of treatment |
- Expensive - False negative in ATB, PPI usage, and GI bleed |
| Stool antigen test | 92.20% | 94.40% | - Inexpensive - Confirm eradication of treatment |
- False positive in PPI usage - Difficult to carry specimen |
| H. pylori antibody with current infection (CIM) | 90 - 95% | 90 - 95 % | - Fast | - Not in widespread use |
| H. pylori culture | 85 - 95% | 99 - 100% | - Provide ATB resistance | - Expensive, need expertise, not widespread use |
| PCR for H. pylori | 95% | 95 - 100% | - Fast | - Expensive, false positive result - Risk for contamination |
ATB, antibiotics; GI, gastrointestinal; H. pylori, Helicobacter pylori; PPI, proton pump inhibitor; PCR, polymerase chain reaction
Mechanism and equipment of endoscopic techniques for diagnosis of H. pylori infection
Due to many limitations in the diagnostic tests for H. pylori infection, the development of new endoscopic techniques has provided reliable diagnostic tools for detection of H. pylori infection, pre-cancerous lesions, and gastric cancer.
Conventional WLI
The first case of flexible gastrointestinal (GI) endoscopy was performed in the 1960s (East et al., 2016), then advances in endoscopic technology have continued with high resolutions. Conventional white light endoscopy is the current standard for evaluating the mucosa of the GI tract due to accessibility, short endoscopic time, and low cost. In white light imaging, the normal gastric body is surrounded with folds, called rugae, which vary in size depending on the degree of insufflation. The mucosa of the fundus and antrum is normally smooth, and the color is velvety and red with regular arrangement of collecting venules (RAC). The RAC, mainly in the lesser curvature, were observed to be associated with H. pylori-negative gastric mucosa and a decreased risk of gastric cancer (Dohi et al., 2020). A magnifying (zoom) endoscopic technique shows normal fundic gland mucosa, including pit patterns and vascular details. There are consistent round or oval crypt openings in which pin-like dark spots are at the center of the gastric gland. The subepithelial capillary networks (SECNs) that surround the crypts have a honeycomb-like appearance (Sugano et al., 2015).
NBI
The first commercial narrow-spectrum technology, narrow-band imaging (NBI) (Olympus Medical Systems, Tokyo, Japan), was established in 2004. The narrow illumination is filtered by the function of NBI. The standard red, green, and blue (RGB) filters discard the red component, while the width of the spectral bands of the green and blue light is decreased from 50-70 nm to 20-30 nm. Narrow-band illumination is absorbed by hemoglobin, and the shortened wavelength penetrates the surface tissue. This technique results in enhanced contrast of superficial microvessels and mucosal surface (East et al., 2016). Magnifying narrow-band imaging (M-NBI) has widespread use in Asian countries but not in Western countries.
LCI
LCI (Lasereo; FUJIFILM Co., Tokyo, Japan) was launched in 2015. LCI is a color enhancement technology. The information on three colors (RBG) is used unlike the technique of WLI. The output of LCI provides the image with color enhancement in its range, enhancing the differences of mucosal color and helping to detect sufficient brightness (East et al., 2016).
BLI
BLI (Lasereo; FUJIFILM Co., Tokyo, Japan) was first introduced in 2014. BLI functions with two types of lasers with wavelengths of 410 and 450 nm. The 450 nm laser conducts illumination light, which is similarly obtained with a xenon lamp. In BLI mode, the ratio of the BLI laser provides enhanced microvessels on the mucosal surface (Kato, 2016). Thus, its main role is observing the target at a short distance, which is called magnifying endoscopy. BLI-bright mode is a brighter BLI, consisting of BLI and white light mode laser illumination, and is mainly used for observing the target at middle and short distances. The high-intensity contrast imaging produced by magnifying blue laser imaging (M-BLI) provides clear visualization of microvascular and microsurface patterns like M-NBI.
Histological findings and endoscopic findings of H. pylori infection
H. pylori is a gram-negative microaerophilic spiral bacterium. H. pylori infection causes neutrophils and mononuclear cells to infiltrate the mucus neck region of the gastric mucosa and aggregate in the lumen of the pit. Chronic H. pylori gastritis results in continuous destruction and regeneration of pits and vessels. These ongoing processes can cause atrophic gastritis, intestinal metaplasia, dysplasia, and eventually gastric cancer (Wang et al., 2015).
According to the development of endoscopic technologies, many studies have demonstrated that endoscopic features were associated with histological findings (Toyoshima et al., 2020). Table 2 describes the findings (Kato, 2016). Endoscopic techniques, such as conventional white light imaging (WLI) and image-enhanced endoscopy (IEE), have become reliable diagnostic modalities for H. pylori infection.
Table 2.
Relationship between Histological and Endoscopic Findings of H. pylori Infection (Suzuki et al., 2016).
| Histological findings | Endoscopic findings |
|---|---|
| Mucosal hyperemia | Erythema |
| Mucosal edema | Mucosal swelling |
| Mucosal epithelial defect | Erosions and ulcers |
| Mucosal hemorrhage | Bleeding spot |
| Infiltration of polymorphonuclear cells and mononuclear cells | Diffuse redness and disappearance of RAC |
| Visibility of vascular pattern and rugal atrophy | |
| Mucosal atrophy | Whitish elevated lesion (specific type) |
| Intestinal metaplasia | Light blue crest (by IEE) |
| Marginal turbid band | |
| White opaque substance (by IEE) |
RAC, regular arrangement of collecting venules; IEE, image-enhanced endoscopy
Diagnostic performance of H. pylori infection in various endoscopic techniques and clinical applicability
Each endoscopic technique could be used for identifying H. pylori infection by using the specific endoscopic features. Meanwhile, some endoscopic features could be used for excluding H. pylori infection. A summary of the endoscopic features of H. pylori-positive and H. pylori-negative gastric mucosa in various techniques are demonstrated in Table 3 and Table 4, respectively.
Table 3.
Summary of Endoscopic Features of H. pylori-Positive Gastric Mucosa in Various Techniques (Chatrangsun et al., 2021; Mao et al., 2016; Tomomitsu Tahara et al., 2017).
| Endoscopic technique | H. pylori-positive gastric mucosa | ||
|---|---|---|---|
| Endoscopic features | Sensitivity | Specificity | |
| WLI | Diffuse redness | 57.50% | 95.80% |
| Antral nodularity | 100% | 100% | |
| Spotty hemorrhage at fundus | 61.00% | 95.80% | |
| Enlarged gastric folds | 60.10% | 92.20% | |
| Sticky tenacious mucus | 53.30% | 95.10% | |
| Xanthoma | 11.20% | 98.00% | |
| LCI | Diffuse redness (deep red color) | 93.30% | 78.30% |
| Antral nodularity | 25% | 100% | |
| Spotty hemorrhage at fundus | 50% | 100% | |
| Enlarged gastric folds | 15% | 100% | |
| Sticky tenacious mucus | 5% | 100% | |
| Xanthoma | 5% | 100% | |
| NBI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 97.00% | 81.00% | |
| BLI | Elongated pits, variable sizes and shapes | N/A | N/A |
| Obliterated collecting venules | 98.00% | 92.00% | |
BLI, blue laser imaging; H. pylori, Helicobacter pylori; LCI, linked color imaging; NBI, narrow-band imaging; WLI, white light imaging
Table 4.
Summary of Endoscopic Features of H. pylori-Negative Gastric Mucosa in Various Techniques
| Endoscopic technique | H. pylori-negative gastric mucosa | ||
|---|---|---|---|
| Endoscopic features | Sensitivity | Specificity | |
| WLI | RAC | 92.40% | 94.50% |
| Fundic gland polyp | 14.60% | 95.50% | |
| Hematin spots | 12.80% | 93.80% | |
| Red streaks | 100% | 2.80% | |
| Raised erosion | 2.80% | 99.10% | |
| LCI | Light orange/white apricot mucosa | 96.70% | 50% |
| RAC | 76.70% | 90% | |
| Fundic gland polyp | 13.30% | 100% | |
| Hematin spots | 16.70% | 100% | |
| Red streaks | 16.70% | 100% | |
| Raised erosion | 10% | 100% | |
| NBI | Round homogenous sized pits and presence of RAC | 80% | 85% |
| BLI | Round homogenous sized pits and presence of RAC | 80% | 95% |
BLI, blue laser imaging; H. pylori, Helicobacter pylori; LCI, linked color imaging; N/A, not available; NBI, narrow-band imaging; RAC, regular arrangement of collecting venules; WLI, white light imaging.
Conventional WLI
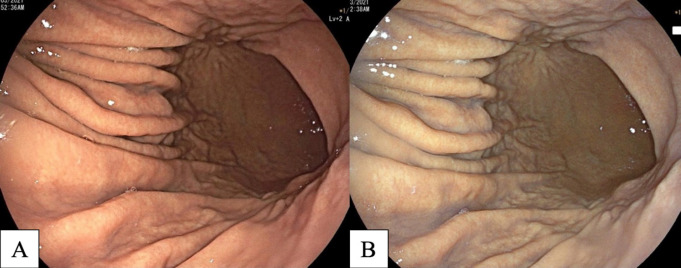
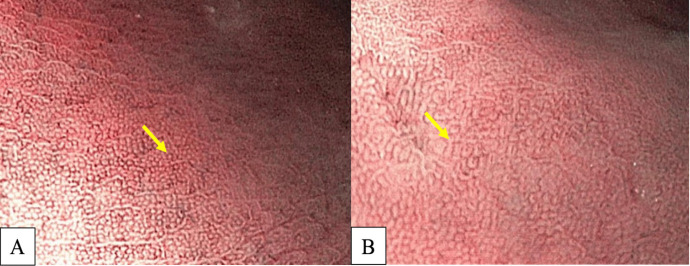
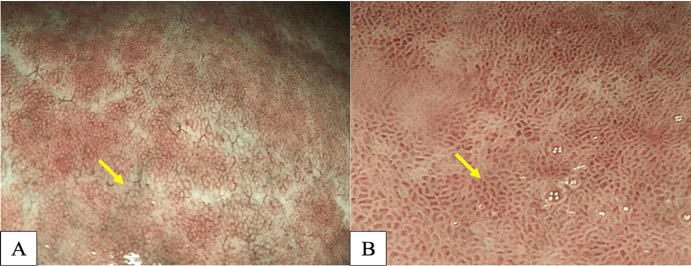
Many studies have reported diffuse redness of the gastric mucosa, spotty hemorrhage at the fundus, enlarged gastric folds, and sticky mucus and antral nodularity in conventional white light imaging (WLI) were associated with H. pylori-positive gastric mucosa with a sensitivity/specificity of 57.52%/95.8%, 61.06%/95.8%, 60.18%/92.25%, 53.33%/95.1%, and 100%/100%, respectively (Nishizawa et al., 2020; Ono et al., 2020). Figure 1 demonstrates patterns of H. pylori-positive gastric mucosae. On the other hand, the presence of RAC in the corpus10 (Figure 2), fundic gland polyps, and red streaks (Figure 3) were associated with H. pylori-negative gastric mucosa with a sensitivity/specificity of 92.4%/94.5%, 20.4%/96.9%, and 19.5%/95.4%, respectively (Zhao et al., 2020).
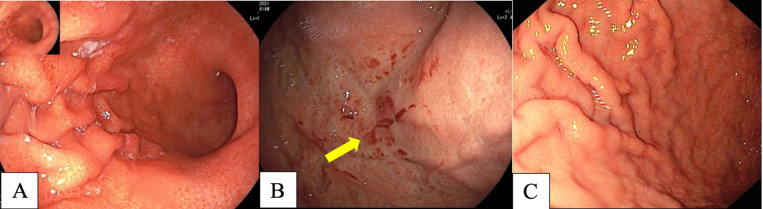
Figure 1.
H. pylori-Positive Gastritis in Conventional White Light Imaging (WLI). A, diffuse redness of gastric mucosa; B, spotty hemorrhage at fundus (arrow); C, enlarged gastric folds
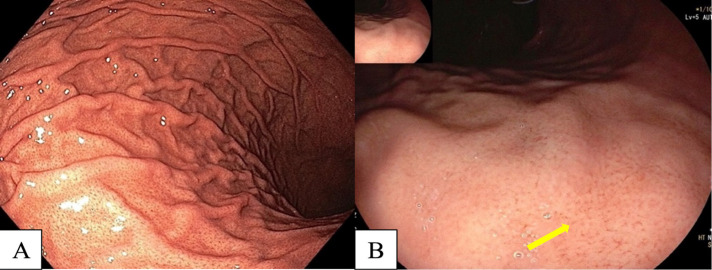
Figure 2.
A, Regular Arrangement of Collecting Venules (RAC) at Body; B, Near Focus of RAC (arrow).
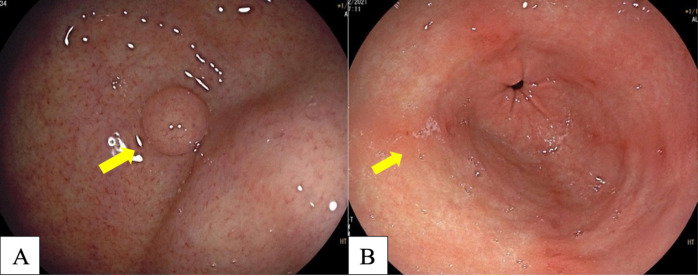
Figure 3.
A, Fundic Gland Polyp (arrow); B, Red Streak (arrow).
According to the inconsistent results of many studies, the Kyoto consensus meeting focused on endoscopic findings to accurately determine H. pylori infection using summation of the scores for endoscopic findings, such as gastric atrophy, intestinal metaplasia, enlarged gastric folds, antral nodularity, and RAC.
The Kyoto classification has been defined as follows: a score of 0 indicates H. pylori-negative gastritis and a score ≥2 indicates H. pylori-positive gastritis, with an accuracy of 90% (Toyoshima et al., 2020).
IEE
Advanced endoscopic imaging can improve mucosal and vascular visualization, especially in magnifying mode. Magnifying endoscopy, especially NBI and BLI, could enhance fine structural and microvascular detail (pit plus vascular pattern) (Qi et al., 2016). Many clinical studies have reported IEE could help identification of mucosal changes and be used for precise targeted biopsies. Limitations of using IEE include needing more training and learning curve for experiences, as well as it being time consuming.
LCI
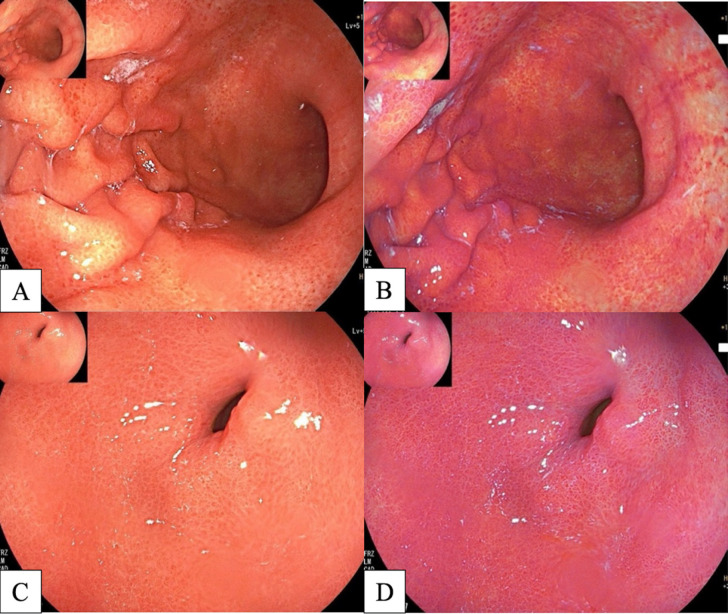
LCI (FUJIFILM Co., Tokyo, Japan) is an IEE technique using a laser light source. LCI provides an approximate color difference twice as high as in WLI. Recent studies have reported LCI produces three times greater amplification to distinguish abnormal lesions from normal mucosa. As is demonstrated in Figure 4, WLI shows diffuse redness over the entirety of the gastric mucosa, while LCI shows deep reddish mucosa over the entirety of the stomach, implying H. pylori-positive mucosa. In the case of H. pylori-negative mucosa, WLI shows yellowish mucosa over the entirety of the gastric mucosa, while LCI shows a light orange (white apricot) hue over the entirety of the gastric mucosa, as is demonstrated in Figure 5. Retrospective studies in Japan (Yagi, Aruga, Nakamura, & Sekine, 2005) showed LCI was more accurate at identifying H. pylori-positive mucosa than WLI, with a sensitivity of 93.3.8% and a specificity of 78.3% (Dohi et al., 2016; Sun et al., 2016).
Figure 4.
A, Diffuse Redness in Gastric Body in WLI; B, Diffuse Redness in Gastric Body (Deep Reddish Color) in LCI; C, Diffuse Redness in Gastric Antrum in WLI; D, Diffuse Redness in Gastric Antrum (Deep Red Color) in LCI
Figure 5.
A, H. pylori-Negative Infection Gastric Mucosa in WLI; B, Light Orange/White Apricot Gastric Mucosa in LCI
NBI
Generally, NBI is used in combination with magnifying mode (M-NBI). A normal gastric corpus mucosal surface is composed of round or oval crypt openings. Dark brownish spots in the crypt openings are at the center of the gastric gland. The subepithelial collecting networks (SECNs) surrounding the crypts have a honeycomb-like appearance with RAC.
In H. pylori-related gastritis, the edematous mucosa results from infiltration of neutrophils and mononuclear cells. Pits are enlarged or elongated due to destruction of the vessels and increased density of irregular microvessels (Horiguchi et al., 2017). The collecting venules are obliterated due to inflammation. The sensitivity and specificity of magnifying NBI (M-NBI) endoscopy for detecting H. pylori infection is high with 97% and 81%, respectively (Tahara et al., 2019; Tahara et al., 2009). Figure 6 demonstrates H. pylori-negative and H. pylori-positive gastric mucosae.
Figure 6.
A, H. pylori-Negative Gastric Mucosa is Characterized by Homogeneous, Round Pits with Regular Honeycomb-Like SECNs in NBI; B, H. pylori-Positive Gastric Mucosa is Characterized by Enlarged or Elongated, Varies in Sized and Shaped of Pits with Unclear SECNs in NBI
BLI
The NBI technologies can be limited by a dark field of view. However, the BLI system provides laser light system shows a clearer view with high contrast of the gastric mucosa and vascular structures. Similarly, in NBI, M-BLI patterns of gastric mucosa are associated with histological findings of H. pylori infection. Moreover, it is also useful for distinguishing H. pylori-related gastritis, as is magnifying NBI (M-NBI). M-BLI endoscopy has potential diagnostic performance for H. pylori-related gastritis with a sensitivity of 98% and a specificity of 92% (Tomomitsu Tahara et al., 2017), as is demonstrated in Figure 7.
Figure 7.
A, H. pylori-Negative Gastric Mucosa is Characterized by Homogeneous, Round Pits with Regular Honeycomb-Like SECNs in BLI; B, H. pylori-Positive Gastric Mucosa is Characterized by Enlarged or Elongated, Varied in Sized and Shaped of Pits with Unclear SECNs in BLI
Clinical study in Thailand
Our randomized prospective study conducted at Thammasat University Hospital, Thailand, during 2020-2021, is the first study comparing each endoscopic technique, simultaneous EGD using WLI, LCI, NBI and BLI, for the diagnosis of H. pylori infection. We found that the endoscopic features associated with H. pylori infection were diffuse redness, enlarged gastric folds and sticky mucus (positive predictive value [PPV]: 83.3%, 100% and 100%, respectively). RAC had a high negative predictive value (NPV) (88%) for excluding H. pylori infection. The sensitivity, specificity, PPV, NPV and accuracy for diagnosis of H. pylori infection using WLI, LCI, NBI, and BLI are demonstrated in Table 5. Moreover, additional IEE to conventional WLI could improve the diagnostic performance of H. pylori infection in our study.
Table 5.
Sensitivity, Specificity, PPV, NPV, and Accuracy of Each Endoscopic Technique for Diagnosis of H. pylori Infection (Chatrangsun et al., 2021)
| Endoscopic technique | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| WLI | 90.00% (68.3-98.8) |
70.00% (50.6-85.3) |
66.70% (53.2-77.9) |
91.30% (73.4-97.6) |
78.00% (64.0-88.5) |
| LCI | 95.00% (75.1-99.9) |
76.70% (57.7-90.1) |
73.10% (58.5-84.0) |
95.80% (77.1-99.4) |
84.00% (70.9-92.8) |
| BLI | 95.00% (75.1-99.9) |
80.00% (61.4-92.3) |
76.00% (60.6-86.7) |
96.00% (77.9-99.4) |
86.00% (73.3-94.2) |
| NBI | 85.00% (62.1-96.8) |
80.00% (61.4-92.3) |
73.90% (57.5-85.6) |
88.90% (73.5-95.8) |
82.00% (68.6-91.4) |
BLI, blue light imaging; CI, confidence interval; LCI, linked color imaging; NBI, narrow-band imaging; NPV, negative predictive value; PPV, positive predictive value; WLI, white light imaging
New innovative tool (EndoFaster)
EndoFaster (NISO Biomed S.r.l., Turin, Italy) was first introduced in 2005. EndoFaster is a real-time analysis machine using gastric juice that provides information on ammonium concentration and gastric pH (Sánchez Rodríguez et al., 2020). Because H. pylori can produce the urease enzyme, which breaks down urea into carbon dioxide and ammonia, this machine could diagnose H. pylori infection through a urease test on gastric juice. A total of 2-4 ml of gastric juice was aspirated during EGD and analyzed by the EndoFaster within 1 minute (Costamagna et al., 2016). Many studies about using the EndoFaster for the real-time diagnosis of H. pylori infection have reported a high accuracy, which is comparable to the urea breath test (UBT). One large prospective study conducted in Italy, which compared the EndoFaster and urea breath test (UBT) with histological examination as the gold standard for diagnosis of H. pylori infection, demonstrated a sensitivity of 90.3% and a specificity of 85.5%. Moreover, the overall benefits of this device include being less invasive, not requiring proton pump inhibitor (PPI) discontinuation before testing, and less costs. Recent studies have demonstrated the EndoFaster has advantages in detection of hypochlorhydric conditions, neoplastic risk conditions, and as an adjunct to gastroesophageal reflux (GERD) treatment (Zullo et al., 2021).
Artificial intelligence for predicting H. pylori infection in endoscopic images
Artificial intelligence (AI) has been recently introduced and increasingly used in clinical practice. The diagnostic performance of AI is used in endoscopic images to detect pre-cancerous and cancer lesions. The application of AI in H. pylori infection is to decrease interobserver disagreement and time consumption (Pannala et al., 2020). The development of AI potentially detects H. pylori infection by integrating data into endoscopic images. The innovation of AI is to mimic human neural networks in the brain. AI could analyze images for many features, including sizes, shapes, colors, and even textures. Most of the studies on the application of AI in endoscopic practices are in Japan because of the high incidence of H. pylori infection and burden of gastric cancer screening (Bang et al.,, 2020).
One large prospective randomized controlled study in Japan compared accuracy in the diagnosis of H. pylori infection between experienced endoscopists and AI. A total of 32,208 endoscopic images in eight important areas of stomach were categorized as having H. pylori-positive or H. pylori-negative status. The results of this study found that AI has greater sensitivity in the diagnosis of H. pylori infection than experienced endoscopists, with a sensitivity and specificity of 81.9%/83.4% and 79%/83.2%, respectively (Nakashima et al., 2018). On the other hand, studies of AI in IEE have been increasing. Another prospective pilot study conducted in Japan, compared AI-assisted BLI-bright, LCI and WLI in the diagnosis H. pylori infection. The area under the curve (AUC) of AI-BLI-bright and AI-LCI were 0.96 and 0.95, respectively, whereas AI-WLI had and AUC of 0.66 (Nakashima et al., 2018). Nowadays, AI technology has become a useful diagnostic tool for endoscopists, especially when using it with IEE. AI provides a second opinion, some important findings during endoscopy, decreased time consumption, and less of a learning-experience requirement. AI might be an excellent future diagnostic modality for the diagnosis of H. pylori infection.
In conclusion, developments of endoscopic techniques contribute to the real-time diagnosis of H. pylori infection during endoscopy. Endoscopic imaging can reflect histological features of the gastric mucosa. WLI seems to be a good modality for the diagnosis of H. pylori infection because of its widespread use, short endoscopic time, and requirements of less experience (Glover et al., 2020). Endoscopic findings, including diffused redness of gastric mucosa, and spotty hemorrhage at fundus, were strongly suggestive of H. pylori-positive status. On the other hand, RAC is associated with an absence of H. pylori infections by WLI with high sensitivity and specificity. Using IEE can improve mucosal, fine structural, and microvascular visualization, especially use with M-BLI endoscopy, which could provide a high potential diagnostic performance for H. pylori-related gastritis. The aforementioned techniques could accurately diagnose H. pylori infection and pre-cancerous lesions (targeted biopsy) better than the current practice with conventional WLI.
Author Contribution Statement
The contributions of all authors must be described in the following manner: The authors confirm contribution to the paper as follows: study conception and design: X. Author, Y. Author; data collection: Y. Author; analysis and interpretation of results: X. Author, Y. Author. Z. Author; draft manuscript preparation: Y. Author. Z. Author. All authors reviewed the results and approved the final version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was also supported by a grant from Chulabhorn International College of Medicine (CICM), Thammasat University, Thailand Science Research and Innovation Fundamental Fund, Bualuang ASEAN Chair Professorship at Thammasat University, and Center of Excellence in Digestive Diseases, Thammasat University, Thailand and Gastroenterology Association of Thailand.
References
- Bang CS, Lee JJ, Baik GH. Artificial intelligence for the prediction of Helicobacter pylori infection in endoscopic images: systematic review and meta-analysis of diagnostic test accuracy. J Med Internet Res. 2020;22:e21983. doi: 10.2196/21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Chatrangsun B, Pornthisarn B, Chonprasertsuk S, et al. Accuracy of Helicobacter pylori detection under white light imaging and image-enhanced endoscopy. Poster presented at: Digestive Disease Week; Virtual meeting. 2021 [Google Scholar]
- Costamagna G, Zullo A, Bizzotto A, et al. Real-time diagnosis of H pylori infection during endoscopy: accuracy of an innovative tool (EndoFaster) United European Gastroenterol J. 2016;4:339–42. doi: 10.1177/2050640615610021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi O, Majima A, Naito Y, et al. Can image-enhanced endoscopy improve the diagnosis of Kyoto classification of gastritis in the clinical setting? Dig Endosc. 2020;32:191–203. doi: 10.1111/den.13540. [DOI] [PubMed] [Google Scholar]
- Dohi O, Yagi N, Onozawa Y, et al. Linked color imaging improves endoscopic diagnosis of active Helicobacter pylori infection. Endosc Int Open. 2016;4:E800–5. doi: 10.1055/s-0042-109049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East JE, Vleugels JL, Roelandt P, et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029–45. doi: 10.1055/s-0042-118087. [DOI] [PubMed] [Google Scholar]
- Glover B, Teare J, Patel N. A systematic review of the role of non-magnified endoscopy for the assessment of H. pylori infection. Endosc Int Open. 2020;8:E105–14. doi: 10.1055/a-0999-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi N, Tahara T, Kawamura T, et al. A comparative study of white light endoscopy, chromoendoscopy and magnifying endoscopy with narrow band imaging in the diagnosis of early gastric cancer after Helicobacter pylori eradication. J Gastrointestin Liver Dis. 2017;26:357–62. doi: 10.15403/jgld.2014.1121.264.hpy. [DOI] [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao T, Wang Y, Yin F, et al. Association of endoscopic features of gastric mucosa with Helicobacter pylori infection in Chinese patients. Gastroenterol Res Pract. 2016;2016:6539639. doi: 10.1155/2016/6539639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. Helicobacter pylori--a Nobel pursuit? Can J Gastroenterol. 2008;22:8956. doi: 10.1155/2008/459810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H, Kawahira H, Kawachi H, et al. Artificial intelligence diagnosis of Helicobacter pylori infection using blue laser imaging-bright and linked color imaging: a single-center prospective study. Ann Gastroenterol. 2018;31:462–8. doi: 10.20524/aog.2018.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T, Sakitani K, Suzuki H, et al. Clinical features of cardiac nodularity-like appearance induced by Helicobacter pylori infection. World J Gastroenterol. 2020;26:5354–61. doi: 10.3748/wjg.v26.i35.5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Dohi O, Yagi N, et al. Accuracies of endoscopic diagnosis of Helicobacter pylori-gastritis: multicenter prospective study using white light imaging and linked color imaging. Digestion. 2020;101:624–30. doi: 10.1159/000501634. [DOI] [PubMed] [Google Scholar]
- Pannala R, Krishnan K, Melson J, et al. Artificial intelligence in gastrointestinal endoscopy. VideoGIE. 2020;5:598–613. doi: 10.1016/j.vgie.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, Guo C, Ji R, et al. Diagnostic performance of magnifying endoscopy for Helicobacter pylori infection: a meta-analysis. PLoS One. 2016;11:e0168201. doi: 10.1371/journal.pone.0168201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Rodríguez E, Sánchez Aldehuelo R, Ríos León R, et al. Clinical validation of Endofaster® for a rapid diagnosis of Helicobacter pylori infection. Rev Esp Enferm Dig. 2020;112:23–6. doi: 10.17235/reed.2019.6441/2019. [DOI] [PubMed] [Google Scholar]
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–67. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Dong T, Bi Y, et al. Linked color imaging application for improving the endoscopic diagnosis accuracy: a pilot study. Sci Rep. 2016;6:33473. doi: 10.1038/srep33473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Marshall BJ, Hibi T. Helicobacter pylori . 2016;1:157–67. [Google Scholar]
- Tahara T, Horiguchi N, Yamada H, et al. Comparative study of magnifying narrow-band imaging and conventional white light endoscopy in the diagnosis of Helicobacter pylori status after eradication therapy. Medicine (Baltimore) 2019;98:e17697. doi: 10.1097/MD.0000000000017697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara T, Shibata T, Nakamura M, et al. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246–53. doi: 10.1016/j.gie.2008.11.046. [DOI] [PubMed] [Google Scholar]
- Tahara T, Takahama K, Horiguchi N, et al. A comparative study of magnifying blue laser imaging and magnifying narrow-band imaging system for endoscopic diagnosis of Helicobacter pylori infection. Biomed Rep. 2017;7:236–40. [Google Scholar]
- Toyoshima O, Nishizawa T, Koike K. Endoscopic Kyoto classification of Helicobacter pylori infection and gastric cancer risk diagnosis. World J Gastroenterol. 2020;26:466–77. doi: 10.3748/wjg.v26.i5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YK, Kuo FC, Liu CJ, et al. Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. 2015;21:11221–35. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K, Aruga Y, Nakamura A, et al. Regular arrangement of collecting venules (RAC): a characteristic endoscopic feature of Helicobacter pylori-negative normal stomach and its relationship with esophago-gastric adenocarcinoma. J Gastroenterol. 2005;40:443–52. doi: 10.1007/s00535-005-1605-0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Xu S, Gao Y, et al. Accuracy of endoscopic diagnosis of Helicobacter pylori based on the Kyoto classification of gastritis: a multicenter study. Front Oncol. 2020;10:599218. doi: 10.3389/fonc.2020.599218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo A, Germanà B, Galliani E, et al. Optimizing the searching for H pylori in clinical practice with EndoFaster- Dig Liver Dis. 2021;53:772–5. doi: 10.1016/j.dld.2021.02.004. [DOI] [PubMed] [Google Scholar]