Abstract
Objectives:
Although recent evidence suggests that management of viral bronchiolitis requires something other than guidelines-guided therapy, there is a lack of evidence supporting the economic benefits of phenotypic-guided bronchodilator therapy for treating this disease. The aim of the present study was to compare the cost-effectiveness of phenotypic-guided versus guidelines-guided bronchodilator therapy in infants with viral bronchiolitis.
Methods:
A decision analysis model was developed to compare the cost-effectiveness of phenotypic-guided versus guidelines-guided bronchodilator therapy in infants with viral bronchiolitis. Phenotypic-guided bronchodilator therapy was defined as the administration of albuterol in infants exhibiting a profile of increased likelihood of response to bronchodilators. The effectiveness parameters and costs of the model were obtained from systematic reviews of the literature with meta-analyses and electronic medical records. The main outcome was the avoidance of hospital admission after initial care in the emergency department.
Results:
Compared to guidelines-guided strategy, treating patients with viral bronchiolitis with the phenotypic-guided bronchodilator therapy strategy was associated with lower total costs (US$250.99; 95% uncertainty interval [UI]: US $184.37 to $336.51 vs. US$263.46; 95% UI: US$189.81 to $349.19 average cost per patient) and a higher probability of avoidance of hospital admission (0.7902; 95% UI: 0.7315–0.8356 vs. 0.7638; 95% UI: 0.7062–0.8201), thus leading to dominance. Results were robust to deterministic and probabilistic sensitivity analyses.
Conclusions:
Compared to guidelines-guided strategy, treating infants with viral bronchiolitis using the phenotypic-guided bronchodilator therapy strategy is a more cost-effective strategy, because it involves a lower probability of hospital admission at lower total treatment costs.
Keywords: bronchiolitis, clinical practice guidelines, cost-effectiveness, health economics, phenotype
1 |. INTRODUCTION
Viral bronchiolitis is the most prevalent cause of lower respiratory tract infection in children during the first 2 years of life and is the leading cause of hospitalization among infants younger than 1-year old.1,2 In addition to the significant clinical burden of viral bronchiolitis on patients, the disease is usually associated with substantial direct and indirect costs, not only for healthcare systems but also for families and society as a whole.3
For decades, it has been a well-established position that bronchiolitis treatment is mostly supportive, focusing only on observation, hydration, and oxygen supplementation. Although the 2006 American Academy of Pediatrics (AAP) bronchiolitis guidelines considered it acceptable to use bronchodilators on a trial basis,4 the most recent evidence-based medicine (EBM) clinical practice guidelines (CPGs) on viral bronchiolitis, such as the 2014 AAP5 and the National Institute for Health and Care Excellence guidance on the diagnosis and management of bronchiolitis,6 recommend against the use of medications such as bronchodilators to treat the disease. Although these CPGs have acceptable or even good methodological quality,7 they all labor under the implicit assumption that the affected infants are a homogeneous group of patients with the same clinical condition. However, there is increasing evidence showing that infants with viral bronchiolitis exhibit a high degree of heterogeneity in their clinical presentation, immune responses, and molecular immune signatures, as well as probably distinct responses to different therapeutic options (phenotype-specific treatment).8–11 Specifically, the greatest likelihood of benefit from beta2 adrenor-eceptor (AR) agonist bronchodilators such as albuterol is likely to occur in infants with virus-induced bronchoconstriction manifested as a “wheezing phenotype.”12 Individuals with this phenotype seem to have characteristic features, including older age, proasthmatic type 2 immune responses, atopic dermatitis, or a family history of asthma in a first-degree relative, bronchiolitis caused by rhinovirus (RV) or respiratory syncytial virus (RSV) genotypes ON1 and BA, and infection occurring during nonpeak viral season months or during non-RSV-predominant months.13,14 However, although recent evidence suggests that management of viral bronchiolitis requires something other than guidelines-guided therapy, there is a lack of current randomized clinical trials (RCTs) to fully support the usage of beta2AR agonist bronchodilators in infants with the above-mentioned characteristics, at least on a therapeutic trial basis. In addition, there is also a current lack of evidence supporting the economic benefits of phenotypic-guided bronchodilator therapy in viral bronchiolitis that combines both its cost and its effectiveness. This fact is important because demonstrating not only effectiveness but also cost-effectiveness and cost-savings is important for advocating a new intervention.
Accordingly, the aim of the present study was to compare the cost-effectiveness of phenotypic-guided versus guidelines-guided bronchodilator therapy in infants with viral bronchiolitis.
2 |. METHODS
2.1 |. Model structure
A decision analysis model was developed to estimate the cost-effectiveness of phenotypic-guided versus guidelines-guided bronchodilator therapy in infants with viral bronchiolitis. For this study, we used the definition of viral bronchiolitis by the AAP in children less than 24 months of age: symptoms of a viral upper respiratory infection that then progresses to lower respiratory tract disease with wheezing and increased respiratory effort.5 Although the European guidelines definition of bronchiolitis only include infants less than 12 months,6 we have previously found that the AAP definition is more commonly used among young children hospitalized with viral respiratory infections.15
For each of the two comparators, the model starts with any patient 24 months of age and younger treated in the emergency department (ED) for acute bronchiolitis with a probability (probability node) either of improvement and discharge from the hospital or of being admitted to hospital. Thereafter, for patients admitted to the pediatric ward (PW), the model incorporates the probability (probability node) of staying in the PW or being admitted to the pediatric intermediate care unit (PIMC) or the pediatric intensive care unit (PICU). Thereafter, the model incorporates the probability of readmission for bronchiolitis within 10 days of hospital discharge (Figure 1). The model assumes that no patients died and that all of them were discharged to home. Likewise, the model assumes that all readmitted patients required admission to PW.
FIGURE 1.
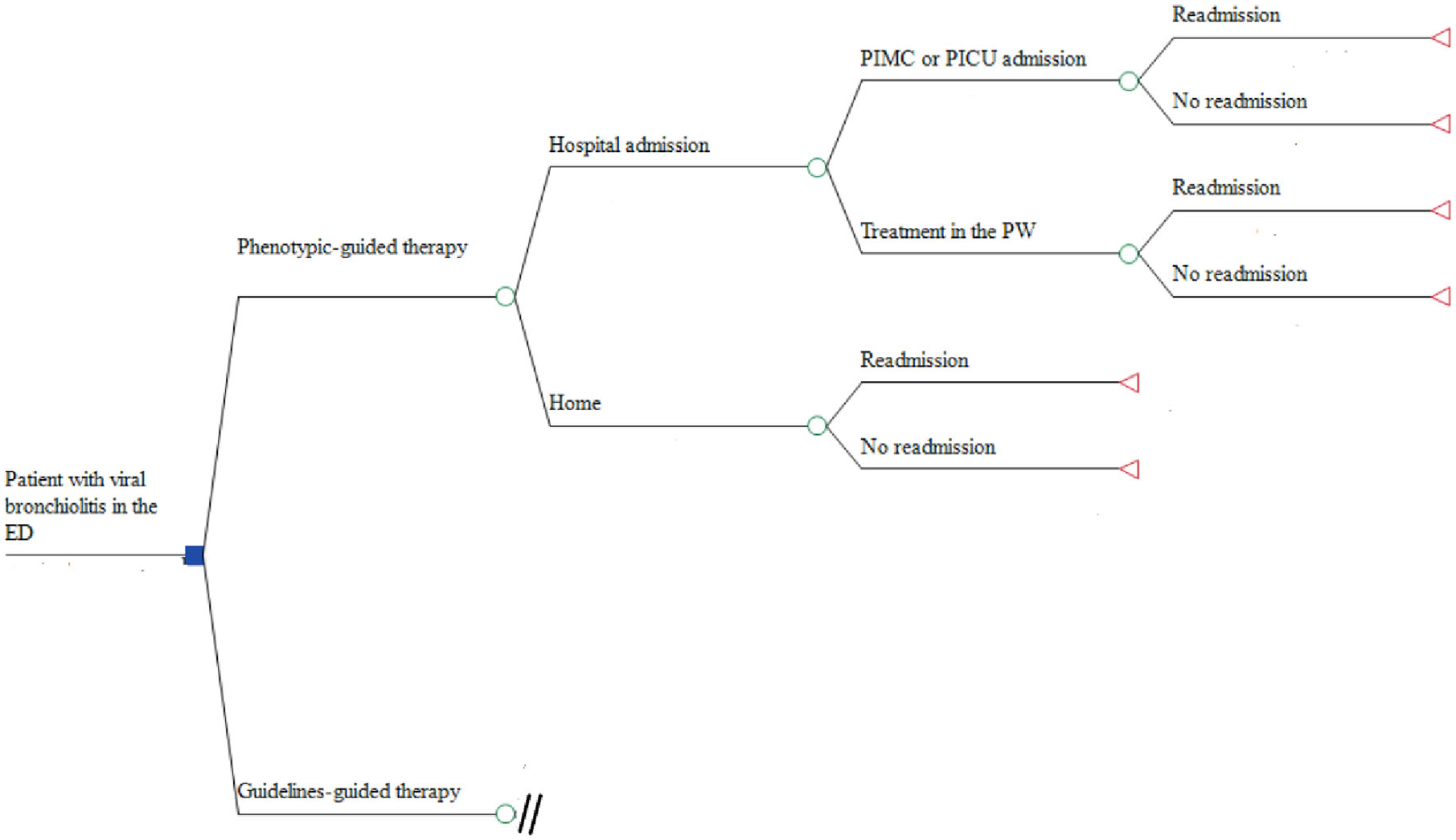
Diagram of a cost-effectiveness model for each treatment option. ED, emergency department; PICU, pediatric intensive care unit; PMIC, pediatric intermediate care unit; PW, pediatric ward
The main outcome of the model is avoidance of hospital admission after initial care in the ED. The time horizon is 10 days after hospital discharge in addition to the duration of hospital care for the acute bronchiolitis episode, without taking into account any sub-sequent respiratory morbidity at follow-up (e.g., recurrent wheezing following acute bronchiolitis). This model compares direct medical costs and disease outcomes from the perspective of the national healthcare system in Colombia.
2.2 |. Sources of data
2.2.1 |. Disease outcomes and clinical parameters
Effectiveness parameters were obtained from various sources. The probability of hospital admission in patients with guidelines-guided bronchodilator therapy was obtained from data from the placebo branch of the last version of the Cochrane Systematic Review published in 2015 that compared the efficacy of bronchodilators (other than epinephrine) with placebo for bronchiolitis.16 The probability of hospital admission in patients with phenotypic-guided bronchodilator therapy was obtained from data from the metered-dose inhaler (MDI) branch of a systematic review with a meta-analysis aimed at comparing the efficacy of beta-agonists administered by MDI with a valved holding chamber or nebulizer in children under 5 years of age with acute exacerbations of wheezing or asthma in the ED setting.17 There are at least four reasons to have selected the probability from this meta-analysis. First, the range of infants with their first wheezing episode enrolled in the primary studies included in the meta-analysis was from 10% to 83%, suggesting that a significant percentage of them were really suffering from viral bronchiolitis.17 Thus we assumed that the results of the above-mentioned meta-analysis could be considered a good proxy for the probability of hospital admission in patients with phenotypic-guided bronchodilator therapy. Second, there are no recent studies of inhaled beta2AR agonist bronchodilators versus placebo in children with acute asthma presenting to hospitals that evaluate the outcomes of interest, mainly because placebo-controlled trials would be unethical. Third, the great majority of studies assessing the efficacy of bronchodilators for treating bronchiolitis have been based on bronchodilators administered by a nebulizer, and when compared to nebulizers, MDI has been shown to have advantages in outcomes such as admission hospital rates and clinical scores. Consequently, we hypothesized that the reported lack of efficacy of bronchodilators in the treatment of bronchiolitis could be due in part to the method of administration (e.g., by a nebulizer).18,19 Fourth, Rubilar et al.19 compared the efficacy of albuterol delivered via MDI with a spacer versus a nebulizer for the treatment of acute exacerbations of wheezing in children less than 2 years of age, irrespective of whether it was viral bronchiolitis or infantile asthma. When analyses were restricted to first-time wheezing children, the proportion of children treated with MDI and spacer that met the criteria for hospital admission after one hour of treatment was virtually identical to that reported in the above-mentioned systematic review with meta-analysis (10.5% vs. 11.3%, respectively).19
To reflect the proportion of infants with viral bronchitis that potentially could benefit from inhaled beta2AR agonist bronchodilators with the phenotypic-guided strategy, we calculated a weighted average of the proportion of patients with the above-mentioned proasthmatic or Th2-immune characteristics, as reported in the literature (RV isolation,20–23 affection during nonpeak months or during non-RSV-predominant months,20 family history of asthma in a first-degree relative,20,21 age greater than 12 months,21,24 history of atopic dermatitis,21 and wheezing or subcostal retractions as the predominant clinical presentation among affected infants: “wheezing phenotype”).12 All the probabilities of the phenotypic-guided strategy were adjusted based on the above-mentioned weighted average of the proportion of patients with proasthmatic type 2 immune responses.
For patients admitted to the PW, the probability of being admitted to the PIMC or the PICU and the proportion of patients that required readmission for viral bronchiolitis within 10 days of hospital discharge with each of the two comparators were obtained from a review of electronic medical records (EMRs) of 303 patients admitted for acute bronchiolitis (ICD-10 codes J21, J21.0, J21.1, J21.8, and J21.9) from March 2014 to August 2015 to the Fundación Hospital La Misericordia, a tertiary care university-based children’s hospital located in the metropolitan area of Bogota that serves the city of Bogota as well as other cities of the country as a referral center. During that span of time in our institution, infants with bronchiolitis with a family history of asthma in a first-degree relative and/or a history of atopic dermatitis and wheezing as the predominant feature was treated with bronchodilators, considering them, therefore, a good proxy for phenotypic-guided strategy. All probability parameters were validated by clinical experts consisting of a panel of four pediatric pulmonologists using the Delphi technique.25
2.3 |. Resource utilization and costs
Unit costs and resource utilization data were obtained from a recent study aimed at evaluating the direct medical costs associated with bronchiolitis hospitalizations in Bogota, Colombia.26
The costs incorporated in the analyses included only direct costs incurred during the treatment of patients suffering an acute bronchiolitis episode, from the healthcare provider’s perspective. Specifically, medical and therapy services (including respiratory therapy), diagnostics tests and procedures (hemogram, C-reactive protein, viral respiratory panel, and imaging studies), consumables (medications, fluids, supplies, nebulization, and oxygen treatment), and hotel services (hospital stay), were taken into account. While the cost for testing for RV was incorporated in the cost analyses of the totality of patients of the phenotypic-guided strategy, the cost of an MDI of albuterol and the spacer was incorporated only in those that potentially could benefit from inhaled beta2AR agonist bronchodilators. Since albuterol MDI canisters and spacers are not transferable or reusable between patients, the cost of MDI delivery of albuterol was calculated by using the cost of one MDI canister and one spacer per patient. On the other hand, neither the cost for testing for RV nor the cost of MDI of albuterol and the spacer was incorporated in the cost analyses of the patients treated under the guidelines-guided strategy.
All costs considered were as close to reimbursement or true costs as possible. Resource quantities at an individual patient level, including the length of stay, the quantity of medications and supplies utilized, and the number of diagnostic tests and procedures, were determined. Thereafter, we calculated the cost per bronchiolitis episode hospitalization per comparator based on the unit cost of each component multiplied by the resource quantities utilized by each patient. Costs were calculated in Colombian pesos (COP) and converted to dollars (US$) based on the average exchange rate for 2018 (US$1 = COP 2956.55).27 All the costs were adjusted to the 2015 COP before converting them to US$. Given that the time horizon was 10 days after hospital discharge in addition to the duration of hospital care for the acute bronchiolitis episode, costs and effects were not discounted. The study protocol was approved by the local ethics board.
2.4 |. Sensitivity analyses
To assess the robustness of the model, one-way, two-way, and multi-way deterministic sensitivity analyses (using a tornado diagram) were performed using plausible variations in the main model parameters. Values used in the deterministic sensitivity analyses were based on plausible ranges (for costs, the data ranges were based on the lowest and highest values collected in the review of EMRs, and for probabilities, the data ranges were ±25% of the base value), including 95% confidence intervals (95% CIs) when available. In addition, a probabilistic sensitivity analysis (PSA) using second-order Monte Carlo simulation with 10,000 iterations (assigning uncertainty distributions to input parameters in the model and sampling a random value from each distribution simultaneously), was used to deal with parameter uncertainty. Based on PSA simulations, we calculated 95% uncertainty intervals (UIs) for costs and effects and generated cost-effectiveness scatter plot, plotting incremental costs compared to incremental effectiveness of the 10,000 iterations.28 TreeAgePro 2016 software (TreeAge Software) was used for data analysis.
3 |. RESULTS
3.1 |. Baseline and range values of parameters included in the model
The base-case values and ranges used in the deterministic sensitivity analyses, including the probability of hospital admission after initial care in the ED, probability of admission to the PIMC or PICU, probability of readmission for bronchiolitis within 10 days of hospital discharge, unit costs of resources, and the cost per hospitalization for a bronchiolitis episode according to the hospital setting in which the patients was admitted, along with their respective sources, are presented in Table 1.
TABLE 1.
Baseline and range values of parameters included in the model
| Items | Base-case value | Lower value | Higher value |
|---|---|---|---|
| Probability of hospitalization with phenotypic therapy17 | 0.21 | 0.15 | 0.26 |
| Probability of hospitalization with guidelines therapy16 | 0.23 | 0.17 | 0.29 |
| Probability of PIMC or PICU admission with phenotypic therapy | 0.32 | 0.24 | 0.40 |
| Probability of PIMC or PICU admission with guidelines therapy | 0.36 | 0.32 | 0.39 |
| Probability of readmission with phenotypic therapy | 0.007 | 0.005 | 0.009 |
| Probability of readmission with guidelines therapy | 0.006 | 0.005 | 0.066 |
| Cost of bronchiolitis treated in the ED26 | 38.8 | 21.1 | 64.1 |
| Cost of bronchiolitis treated in the PW26 | 518.0 | 217.0 | 768.9 |
| Cost of bronchiolitis treated in the PIMC26 | 1305.2 | 1051.4 | 1492.2 |
| Cost of bronchiolitis treated in the PICU26 | 2749.7 | 1372.7 | 4156.9 |
| Cost of MDI of albuterol | 9.9 | 7.4 | 12.4 |
| Cost of spacer | 1.9 | 1.4 | 2.4 |
| Cost for testing for RV26 | 17.7 | 10.1 | 25.4 |
Abbreviations: ED, emergency department; guidelines therapy, guidelines-guided therapy; MDI, metered-dose inhaler; phenotypic therapy, phenotypic-guided therapy; PICU, pediatric intensive care unit; PIMC, pediatric intermediate care unit; PW, pediatric ward; RV, rhinovirus.
3.2 |. Base-case analysis
The proportion of infants with viral bronchitis that potentially could benefit from inhaled beta2AR agonist bronchodilators with the phenotypic-guided strategy was calculated as 21.48%. Under the base-case assumptions, the model showed that compared to guidelines-guided strategy, treating patients with viral bronchiolitis with the phenotypic-guided bronchodilator therapy strategy was associated with lower total costs (US$250.99 vs. US$263.46 average cost per patient) and a higher probability of hospital admission avoided (.7902 vs. .7638), thus leading to dominance. A position of dominance eliminated the need to calculate an incremental cost-effectiveness ratio (Table 2).
TABLE 2.
Base-case cost-effectiveness analysis of phenotypic-guided versus guidelines-guided bronchodilator therapy in viral bronchiolitis
| Category | Strategy | Cost (US$) | Incremental cost (US$) | Effectiveness (avoidance of hospital admission) | Incremental effectiveness (avoidance of hospital admission) | Cost/effectiveness |
|---|---|---|---|---|---|---|
| Phenotypic therapy | 250.99 | – | .7902 | – | 317.63 | |
| Absolutely dominated | Guidelines therapy | 263.46 | 12.47 | .7638 | −0.0264 | 344.94 |
Abbreviations: guidelines therapy, guidelines-guided therapy; phenotypic therapy, phenotypic-guided therapy.
3.3 |. Sensitivity analyses
One-way, two-way, and multi-way deterministic sensitivity analyses (using a tornado diagram) showed that the probability of being admitted to the PIMC or the PICU with the phenotypic-guided strategy had the highest impact on the model outcome. However, the phenotypic-guided bronchodilator therapy was the dominant strategy over all of the ranges of the probability of admission to the PIMC or the PICU with the phenotypic-guided strategy analyzed.
Parameter distributions used in the PSA are presented in Table 3. Beta distribution was used to model probabilities, and gamma distribution was used to model costs. The results of the PSA are graphically represented as a scatter plot in Figure 2. This scatter plot shows that compared to guidelines-guided strategy, treating patients with viral bronchiolitis with the phenotypic-guided bronchodilator therapy strategy tends to be associated with lower overall treatment costs and lower probability of hospital admission. Based on the results from these simulations, the 95% UI for cost per patient treated with phenotypic-guided strategy and with guidelines-guided bronchodilator therapy strategy were US$184.37–336.51 and US $189.81–349.19, respectively. The 95% UI for the avoidance of hospital admission was 0.7315–0.8356 and 0.7062–0.8201, respectively. In 59.4% of the iterations, the phenotypic-guided strategy was associated with both a lower probability of hospital admission and lower costs, compared with treatment administered under the guidelines-guided bronchodilator therapy strategy.
TABLE 3.
Parameter distributions used in the probabilistic sensitivity analysis
| Probability distribution | Distribution parameters | Distribution parameters |
|---|---|---|
| Beta distribution | Alpha | Beta |
| Probability of hospitalization with phenotypic therapy | 50.363 | 189.689 |
| Probability of hospitalization with guidelines therapy | 48.647 | 157.309 |
| Probability of PIMC or PICU admission with phenotypic therapy | 43.125 | 91.021 |
| Probability of PIMC or PICU admission with guidelines therapy | 254.437 | 446.491 |
| Probability of readmission with phenotypic therapy | 48.65 | 6901.35 |
| Probability of readmission with guidelines therapy | 397.594 | 65,868.07 |
| Gamma distribution | Alpha | Lambda |
| Cost of bronchiolitis treated in the ED | 13.027 | 0.335 |
| Cost of bronchiolitis treated in the PW | 14.094 | 0.027 |
| Cost of bronchiolitis treated in the PIMC | 140.278 | 0.107 |
| Cost of bronchiolitis treated in the PICU | 15.605 | 0.005 |
| Cost of MDI of albuterol | 62.726 | 6.336 |
| Cost of spacer | 60.760 | 31.978 |
| Cost for testing for RV | 21.534 | 1.213 |
Abbreviations: ED, emergency department; guidelines therapy, guidelines-guided therapy; MDI, metered-dose inhaler; phenotypic therapy, phenotypic-guided therapy; PICU, pediatric intensive care unit; PIMC, pediatric intermediate care unit; PW, pediatric ward; RV, rhinovirus.
FIGURE 2.
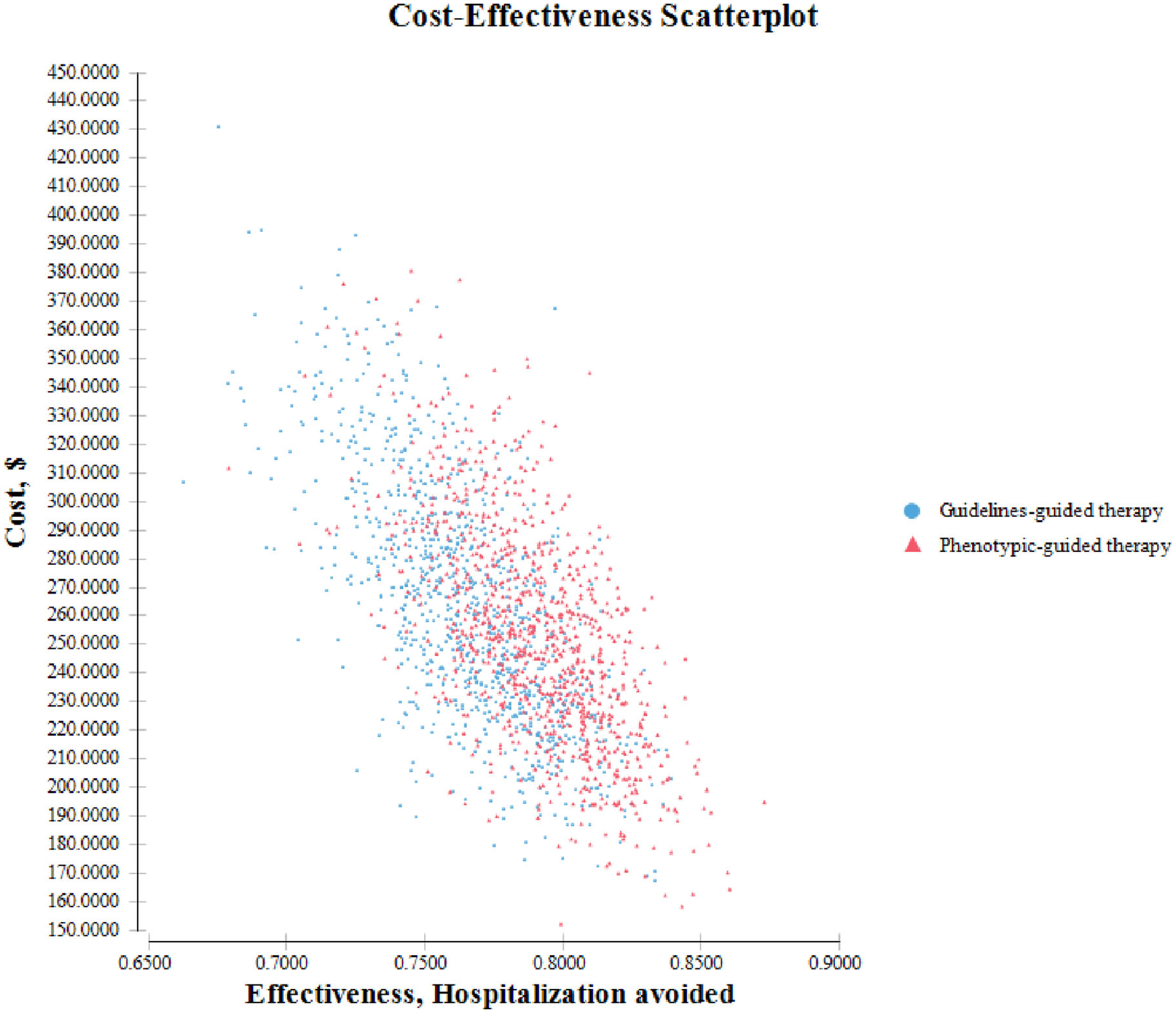
Scatter plot of each iteration’s cost and effectiveness values for each strategy in viral bronchiolitis. The X-axis shows the effectiveness, measured as hospitalizations avoided; the Y-axis shows the costs measured in dollars (US$, 2018). Each point represents one of the 10,000 trial runs, where each input was assigned a random value according to its probability density function
4 |. DISCUSSION
The present study shows that compared to guidelines-guided strategy, treating infants with viral bronchiolitis using the phenotypic-guided bronchodilator therapy strategy is a more cost-effective strategy, because it involves a lower probability of hospital admission at lower total treatment costs. Although the variable that exhibited the highest impact on these results was the probability of being admitted to the PIMC or the PICU with the phenotypic-guided strategy, the phenotypic-guided bronchodilator therapy was the dominant strategy over all the ranges of the probability of admission to the PIMC or the PICU analyzed. Furthermore, the PSA results were consistent with the base-case results.
The findings of the present study support and build on previous reports on the concept of phenotype-specific responses to different therapeutic options in viral bronchiolitis, in this case suggesting the cost-effectiveness of beta2AR agonist bronchodilators in infants of the phenotype of virus-induced wheezing and proasthmatic type 2 immune responses.12 Our study results are in line with previous studies that have shown significant benefits in clinically important outcomes (and therefore presumptively also in unmeasured economic outcomes) in infants with viral bronchiolitis with some of the above-mentioned phenotypical features treated with medications used for pediatric asthma.29–31 At this point, it is worth mentioning that Seumois et al.32 examined the correlation between the expression of asthma-specific genes and lung physiological measures such as forced expiratory volume in 1 s, bronchodilator reversibility (BDR) following albuterol treatment, and methacholine challenge, and found a moderately good correlation (Spearman correlation coefficient ranging from .351 to .40, p < .05) between BDR and transcriptional profiling of Th2 cells (ZBTB10, SGK1, and GABARAPL1), suggesting that the molecular program in circulating Th2 cells may influence BDR.32 The findings of our current cost-effectiveness analysis lend support to the use of albuterol and other beta2AR agonist bronchodilators in infants with certain clinical characteristics (e.g., atopic dermatitis),33 a treatment employed by clinicians despite the fact that the current CPGs on viral bronchiolitis advocate against the use of beta2AR bronchodilators.5,6 This apparently non-conforming behavior could be due to the fact that while the EBM approach focuses on using RCTs to establish the best treatment for the average patient and ignores the outliers, the approach based on personalized medicine focuses on the outliers.34
Although at first glance studies evaluating the impact of CPGs on bronchiolitis that discourage the of bronchodilators and show positive impacts on clinical and economic outcomes do not appear to corroborate our findings, it is important to take into account two factors: first, the great majority of these CPGs encourage the use of a monitored trial of bronchodilators instead of completely discouraging their use, thus increasing the probability of administering bronchodilators to patients who will benefit the most from them.35–42 Second, the uncontrolled before and after the design of most of these studies poses threats to internal validity, such as the history (i.e., some other influential event that could affect the outcome occurs during the intervention), instrumentation/reporting (i.e., the validity of the measurement method changes over the course of the intervention), regression to the mean, maturation, and Hawthorne threats.43 Additional methodological challenges that need to be considered are lack of a causal relationship, residual confounding bias, misclassification bias, comparisons of populations that are not concurrent in time, availability of quality metrics for bronchodilator use, setting where the study was performed, inclusion/exclusion of patients with comorbidities, times of outcomes assessment, and method and duration of CPG implementation.
We are aware that our study may have some limitations. The first is that the probability of hospital admission in patients with phenotypic-guided bronchodilator therapy was obtained from a systematic review with a meta-analysis that did not limit data exclusively to patients with viral bronchiolitis. This fact could have resulted in the overestimation of the cost-effectiveness of the phenotypic-guided strategy. However, this parameter uncertainty was handled using several accepted methods to deal with uncertainly in cost-effectiveness models, such as the Delphi technique and deterministic and probabilistic sensitivity analyses,44–46 our base-case results being robust to variations in values of this probability. In addition, this probability value was virtually identical to that reported in an RCT when the analysis was restricted to first-time wheezing children.19 Second, cost data were obtained from a single clinical center and may not be representative of the whole country. However, these data were obtained from a pediatric clinic that receives patients from the most important and representative medical insurance companies in the city. Moreover, the costs were subjected to wide sensitivity analyses. Third, the fact of having used the definition of viral bronchiolitis by the AAP in our analyses (i.e., including children <24 months of age) instead of the European definition (i.e., using a more restricted age definition) could have caused upward bias in the cost-effectiveness of the phenotypic-guided strategy. This is because greater age is one of the characteristics that could predict a higher probability of response to albuterol in infants with viral bronchiolitis,47 and in fact, it was included in our analysis of the phenotypic-guided strategy. Finally, the differences in efficacy and costs associated with the two strategies could be due to the imprecision of their estimates, because of their 95% UIs overlap. Therefore, we cannot be 95% confident or more about which is the better spacer with regard to their economic value.
In conclusion, the results of our analysis suggest that using beta2AR agonist bronchodilators based on specific patient characteristics or biomarkers (e.g., infants with proasthmatic immune responses), that is, the use of personalized precision medicine instead of a “one-size-fits-all” treatment strategy, could contribute to the treatment of viral bronchiolitis in a more cost-effective way. Future RCTs using biomarkers to stratify patients most likely to respond to treatment with beta2AR bronchodilators are necessary to provide more accurate data input in forthcoming economic evaluations on the topic.
ACKNOWLEDGMENT
The authors would like to thank Mr. Charlie Barret for his editorial assistance.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1.Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5 suppl):S127–S132. [DOI] [PubMed] [Google Scholar]
- 2.Scheltema NM, Gentile A, Lucion F, et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): a retrospective case series. Lancet Glob Health. 2017;5(10):e984–e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. 2004;22(5):275–284. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics. 2006;118(4):1774–1793. [DOI] [PubMed] [Google Scholar]
- 5.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–e1502. [DOI] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence. Bronchiolitis in children: diagnosis and management. NICE guideline [NG9]. 2015. https://www.nice.org.uk/guidance/ng9/resources/bronchiolitis-inchildren-diagnosis-and-management-pdf-51048523717. Accessed May 22, 2020. [PubMed] [Google Scholar]
- 7.Rodriguez-Martinez CE, Sossa-Briceño MP, Acuña-Cordero R. Quality assessment of acute viral bronchiolitis clinical practice guidelines. J Eval Clin Pract. 2017;23(1):37–43. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez-Martínez CE, Castro-Rodriguez JA, Nino G, Midulla F. The impact of viral bronchiolitis phenotyping: is it time to consider phenotype-specific responses to individualize pharmacological management? Paediatr Respir Rev. 2020;34:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellick LB, Gonzalez J. The problematic 2014 American Academy of Pediatrics bronchiolitis guidelines. Pediatr Emerg Care. 2019;35(9): 654–658. [DOI] [PubMed] [Google Scholar]
- 10.Paggi DA, Polack FP. Toward personalized medicine in bronchiolitis. Am J Respir Crit Care Med. 2019;199(12):1456–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack FP, Stein RT, Custovic A. The syndrome we agreed to call bronchiolitis. J Infect Dis. 2019;220(2):184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arroyo M, Salka K, Perez GF, et al. Phenotypical sub-setting of the first episode of severe viral respiratory infection based on clinical assessment and underlying airway disease: a pilot study. Front Pediatr. 2020;8:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nino G, Rodriguez-Martinez CE, Castro-Rodriguez JA. The use of β2-AR agonist in viral bronchiolitis: scientific rationale beyond evidence-based guidelines. ERJ Open Res. 2020;6(4):00135–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Martinez CE, Nino G, Castro-Rodriguez JA, Acuna-Cordero R, Sossa-Briceno M, Midulla F. For which infants with viral bronchiolitis could it be deemed appropriate to usealbuterol, at least on a therapeutic trial basis? Allergol Immunopathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Megalaa R, Perez GF, Kilaikode-Cheruveettara S, Kotwal N, Rodriguez-Martinez CE, Nino G. Clinical definition of respiratory viral infections in young children and potential bronchiolitis misclassification. J Investig Med. 2018;66(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;2014(6):CD001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Rodriguez JA, Rodrigo GJ. β-Agonists through metered-dose inhaler with valved holding chamber versus nebulizer for acute exacerbation of wheezing or asthma in children under 5 years of age: a systematic review with meta-analysis. J Pediatr. 2004;145(2):172–177. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Martinez CE, Castro-Rodriguez JA. Bronchodilators should be considered for all patients with acute bronchiolitis, but closely monitored for objectively measured clinical benefits. Acta Paediatr. 2015;104(9):858–860. [DOI] [PubMed] [Google Scholar]
- 19.Rubilar L, Castro-Rodriguez JA, Girardi G. Randomized trial of salbutamol via metered-dose inhaler with spacer versus nebulizer for acute wheezing in children less than 2 years of age. Pediatr Pulmonol. 2000;29(4):264–269. [DOI] [PubMed] [Google Scholar]
- 20.Cangiano G, Nenna R, Frassanito A, et al. Bronchiolitis: analysis of 10 consecutive epidemic seasons. Pediatr Pulmonol. 2016;51(12):1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansbach JM, Clark S, Teach SJ, et al. Children Hospitalized with rhinovirus bronchiolitis have asthma-like characteristics. J Pediatr. 2016;172:202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Midulla F, Scagnolari C, Bonci E, et al. Respiratory syncytial virus, human bocavirus and rhinovirus bronchiolitis in infants. Arch Dis Child. 2010;95(1):35–41. [DOI] [PubMed] [Google Scholar]
- 24.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linstone H, Turoff M. The Delphi Method: Techniques and Applications. Los Angeles, CA: University of Southern California; 2002. [Google Scholar]
- 26.Rodriguez-Martinez CE, Sossa-Briceño MP, Castro-Rodriguez JA. Direct medical costs of RSV-related bronchiolitis hospitalizations in a middle-income tropical country. Allergol Immunopathol. 2020;48(1):56–61. [DOI] [PubMed] [Google Scholar]
- 27.Banco de la República, Colombia. Series estadísticas. Tasas de cambio. Santa Fe de Bogotá: Banco de la República; 2011. http://www.banrep.gov.co/series-estadisticas/see_ts_cam.htm. Accessed July 20, 2020. [Google Scholar]
- 28.O’Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care. 1994;32(2):150–163. [DOI] [PubMed] [Google Scholar]
- 29.Alansari K, Sakran M, Davidson BL, Ibrahim K, Alrefai M, Zakaria I. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics. 2013;132(4):e810–e816. [DOI] [PubMed] [Google Scholar]
- 30.Al-Shawwa B, Al-Huniti N, Weinberger M, Abu-Hasan M. Clinical and therapeutic variables influencing hospitalisation for bronchiolitis in a community-based paediatric group practice. Prim Care Respir J. 2007; 16(2):93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuh S, Coates AL, Binnie R, et al. Efficacy of oral dexamethasone in outpatients with acute bronchiolitis. J Pediatr. 2002;140(1):27–32. [DOI] [PubMed] [Google Scholar]
- 32.Seumois G, Zapardiel-Gonzalo J, White B, et al. Transcriptional profiling of Th2 cells identifies pathogenic features associated with asthma. J Immunol. 2016;197(2):655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarmiento L, Rojas-Soto GE, Rodríguez-Martínez CE. Predictors of inappropriate use of diagnostic tests and management of bronchiolitis. BioMed Res Int. 2017;2017:9730696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Leon J Evidence-based medicine versus personalized medicine: are they enemies? J Clin Psychopharmacol. 2012;32(2):153–164. [DOI] [PubMed] [Google Scholar]
- 35.Akenroye AT, Baskin MN, Samnaliev M, Stack AM. Impact of a bronchiolitis guideline on ED resource use and cost: a segmented time-series analysis. Pediatrics. 2014;133(1):e227–e234. [DOI] [PubMed] [Google Scholar]
- 36.Florin TA, Byczkowski T, Ruddy RM, Zorc JJ, Test M, Shah SS. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 American Academy of Pediatrics bronchiolitis guidelines. J Pediatr. 2014;165(4):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henao-Villada R, Sossa-Briceño MP, Rodríguez-Martínez CE. Impact of the implementation of an evidence-based guideline on diagnostic testing, management, and clinical outcomes for infants with bronchiolitis. Ther Adv Respir Dis. 2016;10(5):425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCulloh RJ, Smitherman SE, Koehn KL, Alverson BK. Assessing the impact of national guidelines on the management of children hospitalized for acute bronchiolitis. Pediatr Pulmonol. 2014;49(7): 688–694. [DOI] [PubMed] [Google Scholar]
- 39.Mittal V, Darnell C, Walsh B, et al. Inpatient bronchiolitis guideline implementation and resource utilization. Pediatrics. 2014;133(3): e730–e737. [DOI] [PubMed] [Google Scholar]
- 40.Mittal V, Hall M, Morse R, et al. Impact of inpatient bronchiolitis clinical practice guideline implementation on testing and treatment. J Pediatr. 2014;165(3):570–576. [DOI] [PubMed] [Google Scholar]
- 41.Parikh K, Hall M, Teach SJ. Bronchiolitis management before and after the AAP guidelines. Pediatrics. 2014;133(1):e1–e7. [DOI] [PubMed] [Google Scholar]
- 42.Perlstein PH, Kotagal UR, Bolling C, et al. Evaluation of an evidence-based guideline for bronchiolitis. Pediatrics. 1999;104(6):1334–1341. [DOI] [PubMed] [Google Scholar]
- 43.Before-and-after design: A simple evaluation design. http://158.132.155.107/posh97/private/research/evaluation/Chp_3.pdf. Accessed September 10, 2020.
- 44.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Russell LB, Sinha A. Handling parameter uncertainty in cost-effectiveness models simply and responsibly. Med Decis Making. 2015; 35(5):567–569. [DOI] [PubMed] [Google Scholar]
- 46.Simoens S Using the Delphi technique in economic evaluation: time to revisit the oracle? J Clin Pharm Ther. 2006;31(6):519–522. [DOI] [PubMed] [Google Scholar]
- 47.Dumas O, Mansbach JM, Jartti T, et al. A clustering approach to identify severe bronchiolitis profiles in children. Thorax. 2016;71(8): 712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]


