Abstract
Depression and trauma are common among women living with HIV. This is the first study to track the longitudinal course of depression and examine the relationship between depression and trauma over time among women in South Africa. HIV-infected and uninfected women (N = 148) were assessed at baseline and one year later. Results of a path analysis show the multi-directional and entwined influence of early life stress, other life-threatening traumas across the lifespan, depression and PTSD over the course of HIV. We also observed higher rates of depressive symptomatology and more persistent cases among infected women compared to uninfected women, as well as a more consistent and enduring relationship between childhood trauma and depression among women living with HIV. The present study is unique in documenting the course of untreated depression and PTSD in women with and without HIV infection with a high prevalence of early childhood trauma.
Keywords: HIV, Depression, PTSD, Childhood trauma
Introduction
Sub-Saharan Africa remains the centre of the HIV epidemic. An estimated 7.03 million South Africans were living with HIV in South Africa by 2016 [1]. People living with HIV (PLHIV) face many challenges, including psychiatric comorbidities. As the life expectancy of PLHIV improves, increased attention has been paid to the impact of comorbid non-communicable diseases such as cardiovascular disease [2] and cancer [3]. However, little attention has been paid to the significant mental health burden associated with HIV [4]. PLHIV show higher rates of depression [5]. Epidemiological data from the US indicate that nearly half of PLHIV are affected by at least one psychiatric disorder, with depression being the most frequent diagnosis [6]. In South Africa, rates of psychopathologies such as mood disorders among PLHIV are higher compared to the general population [7–9]. Worldwide rates of depression ranging from 14 to 26% have been reported [6, 10–15]. A recent study conducted in South Africa showed that mental disorders like major depression are common in individuals presenting for HIV testing, irrespective of the test result, providing tentative evidence that for some PLHIV, receipt of a positive test result was not cause of their mental disorder [16]. Irrespective of whether HIV is the cause of depression in infected individuals, depression in HIV has a negative impact on the course of infection, reducing adherence to treatment [17–21], accelerating disease progression, and increasing mortality [22–25]. Moreover, there is evidence that depressive symptoms adversely affect responsiveness to antiretroviral therapy (ART) [10, 26]. Depression is often unrecognised and untreated in PLHIV [27]. Various interventions for the treatment of depression in PLHIV are available and may lead to improved quality of life and better prognosis [28, 29]. A limitation of this work is that the majority of studies have been cross-sectional in design, with few studies investigating the longitudinal course of depression among PLHIV [10, 13, 18, 19, 24].
Women tend to be particularly affected by the HIV epidemic. In South Africa, the prevalence of HIV was 22.3% among women in 2016, with approximately one-fifth of South African women in their reproductive ages living with HIV [1]. Many of these infected women also have developmental trajectories characterised by trauma. High rates of intimate partner violence, rape and childhood abuse have been reported [30–33]. Traumatic experiences and life adversity may predispose individuals to impulsive and risky behaviours, which increase the risk of HIV infection [34–36]. In South Africa, rates of posttraumatic stress disorder (PTSD) among PLHIV ranging from 5% to 14.8% have been reported [7, 8] and symptoms have been shown to persist, with follow-up rates of 20% reported [8]. There is substantial and consistent evidence that chronic depression, stressful events, and trauma may negatively affect HIV disease progression in terms of decreased CD4 T lymphocytes, increased viral load, and greater risk for clinical decline and mortality [25, 37, 38].
To our knowledge, there are no published studies tracking the longitudinal course of depression or the relationship between depression and trauma over time among women in South Africa. The present study is the first to investigate this. Studies have shown strong evidence for a link between early life trauma, depression and PTSD, with an increased risk for development of depression among women abused in childhood than non-abused women [39]. A substantial influence of multiple childhood trauma on a severe and chronic course of depression in adulthood has been documented [40]. Moreover, in a mouse model, evidence for a mechanism by which early life stress encodes lifelong susceptibility to stress has been shown [41]. In light of these findings, we hypothesised an entwined influence of early life stress, other life-threatening traumas across the lifespan, depression and PTSD over the course of HIV and therefore included these as predictors of depression in our models.
We carried out a longitudinal study with a 12-month follow-up period, collecting demographic, clinical, laboratory and psychiatric data from a cohort of South African women. The study sought to document: (1) rates of incident and persistent depression and posttraumatic stress disorder over time and (2) the relationship between depression, traumatic experiences, posttraumatic stress disorder and HIV infection over time. Previous studies in South Africa investigating the persistence of psychiatric disorders in PLHIV reported baseline rates of depression of 35 and 26% by 6-month follow-up [8]. The incidence of depression was 8% [8]. In the South African Stress and Health (SASH) study, a 12-month and lifetime prevalence study of common mental disorders, depression in HIV-negative women was found to be around 5% at both time points [42]. Therefore, for the present study, we expected to find similar rates of incident and persistent depression as these previous South African studies.
The examination of predictors of depression at follow-up in the present study is an examination of predictors of persistent and emergent depression in a sample of relatively healthy infected women. The primary purpose of this study was to compare the incidence and persistence of depression in HIV-positive compared with HIV-negative women. Documenting differences in incidence and persistence of depression in HIV-positive women has implications for the assessment and management of this comorbid condition in HIV.
Methods
Participants
A total of 148 women were included in a larger longitudinal genetics, cognitive and neuroimaging study at the Department of Psychiatry of Stellenbosch University [43–45]. Sixty-eight women (46%) were living with HIV, of whom 46 (67%) were on ART. Eligibility criteria included: willingness and ability to provide written informed consent, ability to read and write in either English or Afrikaans at 5th grade level, aged between 18 and 65 years, medically well enough to undergo neuropsychological testing and MRI scanning. Exclusions were a current or past history of schizophrenia, bipolar disorder or other psychotic disorders, current substance or alcohol abuse or dependence, significant previous head injury, demonstrated frank dementia on the International HIV Dementia Scale, current seizure disorders of any cause, history of CNS infections of neoplasms, hepatitis B or C positive status, and current use (within the past month) of any psychotropic medication.
Procedure
All participants were recruited by a team comprising a researcher, a research assistant and a professional nurse, with the help of doctors and adherence counselors from community health care facilities in and around the Cape Town area. All participants who consented were screened for eligibility either in person at the clinic or telephonically. Those who met initial screening criteria subsequently underwent behavioural, neuromedical, neurocognitive and neuroimaging assessments at Stellenbosch University at baseline and one year later. Participants were reimbursed for their travel costs to the University for all study visits and provided with refreshments at each visit. All participants screening positive for depression at baseline or at follow-up were referred to their local health care facility for further care.
Measures
Demographic and Clinical Characteristics
A comprehensive history was obtained from, and a general physical examination conducted in, all participants. Virologic markers of HIV disease progression (CD4 T cell count and viral loads) were obtained from blood samples. Age, gender, marital status, ethnicity, years of education and employment status were captured.
Psychiatric Morbidity
Current and lifetime psychiatric disorders were assessed using the MINI-International Neuropsychiatric Interview-Plus (M.I.N.I.-Plus) [46].
Depression
Participants were assessed for depressive symptomatology using the Center for Epidemiologic Studies Depression Scale (CES-D) [47]. The CES-D is a 20-item widely used self-report instrument designed to measure depressive symptomatology. The scale was specifically designed to measure depressive symptomatology in the general population, unlike previous depression scales which have been predominantly used in clinical populations. The CES-D emphasises the affective component of depressive symptomatology, namely depressed mood. Each item comprises a Likert scale ranging from 0 to 3. A total score for the 20 items is obtained, with the lowest possible score being 0 and the highest possible score being 60. Higher scores are indicative of more severe depression.
Traumatic Life Events
Exposure to traumatic life events were assessed for using the life events checklist (LEC) [48]. The LEC is a widely used measure of exposure to potentially traumatic events and was developed to facilitate the diagnosis of PTSD. One of the LEC’s unique features is that it enquires about various types of exposure to each potentially traumatic event. Therefore, the LEC elicits whether the participant experienced, witnessed, or learned of the traumatic event, a feature that other traumatic event measures do not possess. Participants rate their experience of each traumatic event listed on a Likert scale. A total score is derived from the sum of experiencing and/or witnessing the event. Higher scores are indicative of the experience of more traumatic life events.
Posttraumatic Stress Symptomatology
Posttraumatic stress symptomatology was assessed using the Davidson trauma scale (DTS) [49]. The DTS is a widely used 17-item self-report and measures symptoms of PTSD on frequency and severity scales. The items are categorised according to the criteria set out in the DSM-IV: criteria B (intrusive re-experiencing) criteria C (avoidance and numbness) and criteria D (hyper-arousal). For each item, the participant rates the frequency and severity during the previous week on 5-point (0–4) scales, with the lowest possible score being 0 and the highest possible score being 136. Higher scores are indicative of more PTSD symptoms.
Childhood Trauma
The childhood trauma questionnaire short form (CTQ-SF) [50], a 28-item self-report inventory that provides valid screening for histories of abuse and neglect was administered. It assesses five types of maltreatment including, emotional, physical, and sexual abuse, and emotional and physical neglect. These five subscales each consist of 5 items with scores ranging from 5 to 25. The overall trauma score ranges from 25 to 125 with higher scores indicating higher levels of childhood trauma (score of 25–31 = no trauma, score of 41–51 = low to moderate, 56–68 = moderate to severe, and 73–125 = severe to extreme).
Data Analysis
Data were analysed using the Statistical Package for the Social Sciences (SPSS) for Windows, version 24.0 and Statistica, version 13.0. Basic statistical analyses including descriptive statistics were performed. To ensure that the present study was adequately powered for the regression and PLS SEM path analyses performed, power calculations were determined using G*Power [51]. The power calculations confirmed that the study was adequately powered to perform the multivariate modelling. The number of baseline, emergent and persistent cases of depression and PTSD were computed using cut-off scores on the CES-D and DTS self-report measures. A conceptual framework guided all analyses (see Fig. 1). Multiple linear regression analyses were carried out to assess the relationship between the variables of interest. Predictor variables were entered simultaneously into the model using the enter method. Lastly, a PLS SEM path analysis was performed to confirm the results of the aforementioned analyses.
Fig. 1.
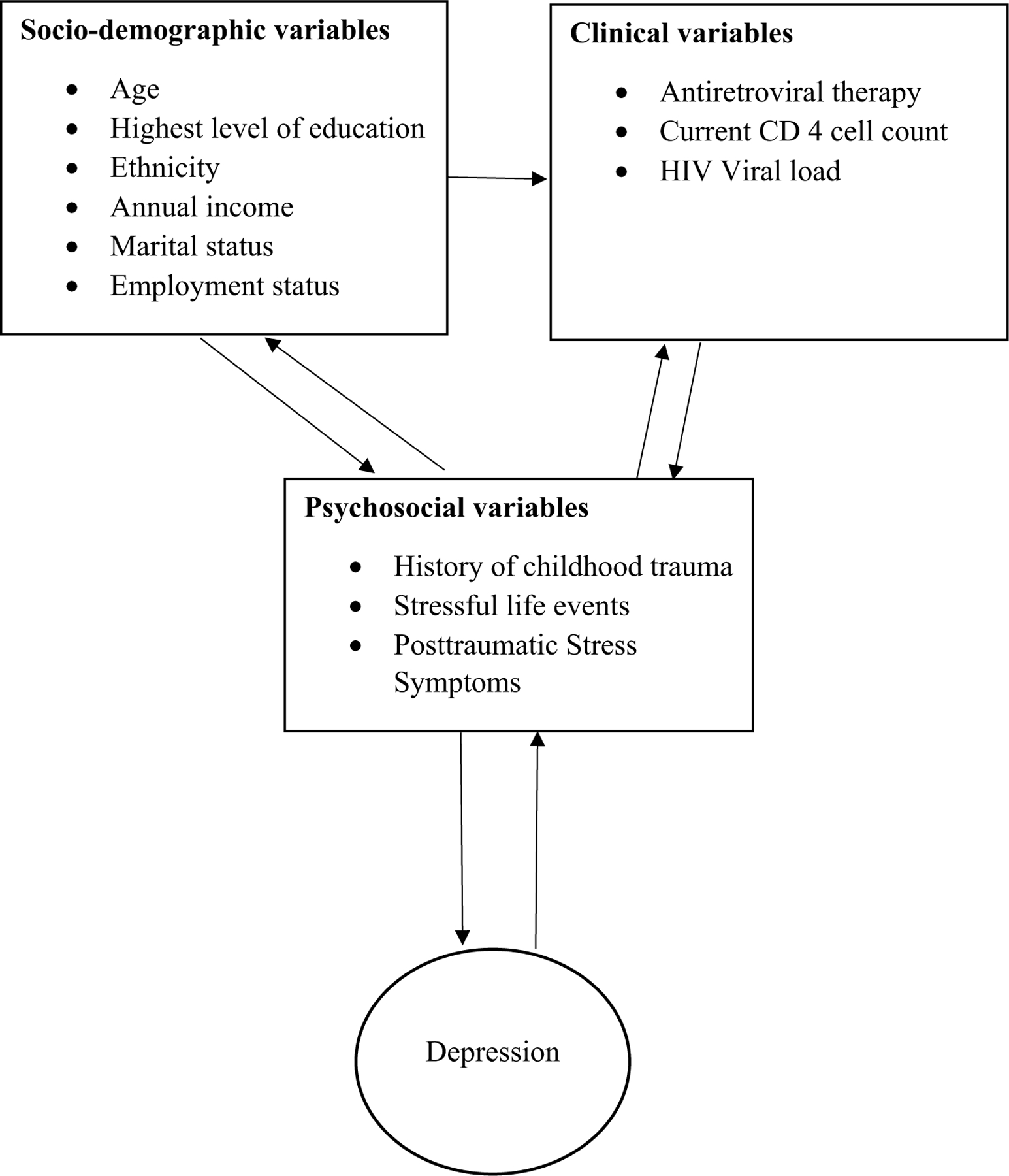
Conceptual framework
Results
Demographic Characteristics of the Sample
All participants were African black, Xhosa (African indigenous language) speaking (98%) women, with a mean age of 32.5 years, SD = 7.9 (range 18–50). The mean highest level of education was 10.5 years, SD = 1.7, ranging from five to 14 years. The majority were single (68%), unemployed (70%), and reported a combined annual household income of less than ZAR10 000 ($730) per year (89%).
Clinical Characteristics of the Sample
The mean CD4 T-cell count was 436 cells/mm3, SD = 244, (range 25–1053 cells/mm3). The mean HIV viral load was 65740.55 copies/ml, SD = 175212, and ranged from below the detectable limit to 1,052,380 copies/ml. The lower limit for detection was 40 copies/ml. The predominant HIV clade was subtype C. Forty-six (67%) of the 68 women living with HIV were on ART. Five cases seroconverted between baseline and follow-up. None of the women were on treatment for depression and/or PTSD at baseline or at follow-up.
Depression
At baseline, the mean CES-D score was 10.8, with a minimum of 0 and a maximum of 60. A year later, the mean CES-D scores was 8.7, with a minimum of 0 and a maximum of 60. At baseline, the mean CES-D scores for women living with HIV was 14 compared to a CES-D score of 8 for uninfected women. A t-test revealed a significant difference between groups on the CES-D score at baseline (p = 0.04). Based on a cut-off score of 16 on the CES-D, the number of baseline, emergent and persistent cases of depression was calculated (see Table 1). Baseline cases refer to the number of women with depression at baseline only (i.e. remitted at the follow-up visit). Emergent cases refer to the number of women with new onset depression at follow-up (i.e. these women did not have depression at baseline). Persistent cases refers to the number of women with depression at baseline persisting through to follow-up.
Table 1.
Baseline, persistent and emergent cases of depression and PTSD
| Total sample (N = 148) | HIV + (n = 68) | HIV − (n = 80) | |
|---|---|---|---|
| Baseline depression | 28 (19%) | 13 | 15 |
| Persistent depression | 10 (7%) | 8 | 2 |
| Emergent depression | 20 (13.5%) | 9 | 11 |
| Baseline PTSD | 15 (10%) | 10 | 5 |
| Emergent PTSD | 9 (6%) | 5 | 4 |
| Persistent PTSD | 6 (4%) | 2 | 4 |
PTSD
At baseline, the mean DTS score was 13.8, with a minimum of 0 and a maximum of 136. A year later, the mean DTS scores was 12, with a minimum of 0 and a maximum of 93. The mean DTS scores for women living with HIV was 17 compared to a DTS score of 11.5 for uninfected women. There were no significant differences between groups on the DTS score (p > 0.31). Based on a cut-off score of 40 on the DTS, the number of baseline, emergent and persistent cases of PTSD was calculated (see Table 1).
Trauma Exposure
Childhood trauma was determined with a score of > 40 on the CTQ-SF. In total, 79 women (53%) reported experiencing childhood trauma. The mean score on the CTQ-SF was 50, with a minimum of 25 and a maximum of 114. Of the 68 women living with HIV, 53 (78%) reported experiencing childhood trauma, with the majority of these cases reporting trauma in the severe to extreme range (n = 28). Of 80 uninfected women, 26 (32%) reported experiencing childhood trauma, with the majority of these cases in the mild to moderate range (n = 17). These women were also exposed to other discrete lifetime traumas regarded as index events for the development of PTSD. At baseline, the mean number of traumatic life events experienced was five with a maximum of 18 life events reported.
Regression
Socio-demographic and clinical characteristics shown in the conceptual framework (Fig. 1) were not significantly associated with baseline, follow-up or persistent depression in univariate analyses and were therefore not included in multivariate regression models.
For the group as a whole (N = 148), multiple linear regression revealed that childhood trauma (CTQ) and posttraumatic stress symptomatology (DTS) significantly predicted depression at baseline (Table 2). This model explained 28% of the variance of depressive symptomatology at baseline (R2 = 0.28). Multiple linear regression revealed that for the group as a whole (N = 148), childhood trauma and PTSD symptoms at follow-up significantly predicted depression at follow-up and that this model explained 34% of the variance of depressive symptomatology at follow-up (R2 = 0.34). See Table 3.
Table 2.
Regression summary for depression at baseline (N = 148)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.275 | 0.260 |
| Childhood trauma | 0.260 | 0.051 | 0.000 | ||
| PTSD (baseline) | 0.171 | 0.039 | 0.000 | ||
| Life events (baseline) | −0.502 | 0.343 | 0.146 |
Table 3.
Regression summary for depression at follow-up (N = 148)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.311 | 0.335 |
| Childhood trauma | 0.138 | 0.046 | 0.003 | ||
| PTSD (baseline) | 0.035 | 0.034 | 0.308 | ||
| Life events (baseline) | 0.054 | 0.314 | 0.861 | ||
| PTSD (follow-up) | 0.353 | 0.047 | 0.000 |
For women living with HIV (n = 68), multiple linear regression revealed that childhood trauma and posttraumatic stress symptomatology significantly predicted depression at baseline (Table 4). This model explained 26% of the variance of depressive symptomatology at baseline (R2 = 0.26). Childhood trauma, traumatic life events at baseline and PTSD symptoms at follow-up significantly predicted depression at follow-up and that this model explained 44% of the variance of depressive symptomatology at follow-up (R2 = 0.44). See Table 5.
Table 4.
Regression summary for depression at baseline among women living with HIV (n = 68)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.257 | 0.222 |
| Childhood trauma | 0.274 | 0.093 | 0.004 | ||
| PTSD (baseline) | 0.173 | 0.060 | 0.005 | ||
| Life events (baseline) | 0.080 | 0.722 | 0.911 |
Table 5.
Regression summary for depression at follow-up among women living with HIV (n = 68)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.442 | 0.397 |
| Childhood trauma | 0.144 | 0.069 | 0.041 | ||
| PTSD (baseline) | 0.057 | 0.044 | 0.203 | ||
| Life events (baseline) | 0.131 | 0.549 | 0.043 | ||
| PTSD (follow-up) | 0.395 | 0.069 | 0.000 |
For uninfected women (n = 80), multiple linear regression revealed that childhood trauma, traumatic life events at baseline and posttraumatic stress symptomatology at baseline significantly predicted depression at baseline (Table 6). This model explained 30% of the variance of depressive symptomatology at baseline (R2 = 0.30). PTSD symptoms at follow-up significantly predicted depression at follow-up with this model explaining 28% of the variance of depressive symptomatology at follow-up (R2 = 0.28). See Table 7.
Table 6.
Regression summary for depression at baseline among women without HIV (n = 80)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.301 | 0.273 |
| Childhood trauma | 0.353 | 0.090 | 0.000 | ||
| PTSD (baseline) | 0.169 | 0.048 | 0.000 | ||
| Life events (baseline) | −0.920 | 0.336 | 0.007 |
Table 7.
Regression summary for depression at follow-up among women without HIV (n = 80)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.278 | 0.228 |
| Childhood trauma | 0.079 | 0.105 | 0.455 | ||
| PTSD (baseline) | 0.020 | 0.058 | 0.732 | ||
| Life events (baseline) | −0.429 | 0.392 | 0.277 | ||
| PTSD (follow-up) | 0.328 | 0.068 | 0.000 |
For the group as a whole (N = 148), multiple linear regression revealed that childhood trauma and posttraumatic stress symptomatology at follow-up significantly predicted persistent depression (Table 8). This model explained 24% of the variance of persistent depression (R2 = 0.24).
Table 8.
Regression summary for persistent depression (N = 148)
| Variable | β | SE of β | p | R 2 | Adjusted R2 |
|---|---|---|---|---|---|
| Model | – | – | 0.000 | 0.241 | 0.220 |
| Childhood trauma | 0.004 | 0.001 | 0.000 | ||
| PTSD (baseline) | 0.001 | 0.006 | 0.161 | ||
| Life events (baseline) | −0.001 | 0.001 | 0.816 | ||
| PTSD (follow-up) | 0.004 | 0.001 | 0.000 |
PLS SEM Path Analysis
For the group as a whole (N = 148), path analysis revealed significant effects between depression at baseline and depression at follow-up (p = 0.03), childhood trauma and depression at baseline (p < 0.01), posttraumatic stress symptoms at baseline and depression at baseline (p < 0.01), and posttraumatic stress symptoms at follow-up with depression at follow-up (p < 0.01).
For women living with HIV, there was a significant relationship between childhood trauma and depression at baseline (p = 0.002), posttraumatic stress symptoms at baseline and depression at baseline (p = 0.014), and posttraumatic stress symptoms at follow-up with depression at follow-up (p < 0.001).
For uninfected women, there was a significant relationship between childhood trauma and depression at baseline (p < 0.001), posttraumatic stress symptoms at baseline and depression at baseline (p = 0.005), posttraumatic stress symptoms at follow-up with depression at follow-up (p < 0.001), posttraumatic stress symptoms at baseline and posttraumatic stress symptoms at follow-up (p = 0.002), and traumatic life events at baseline with depression at baseline (p = 0.04).
No significant differences (p > 0.05) were found when testing for differences in path coefficients between HIV-positive and HIV-negative women. However, there was a trend between life events at baseline and depression at both baseline and follow-up, with these coefficients approaching significance. See Fig. 2.
Fig. 2.
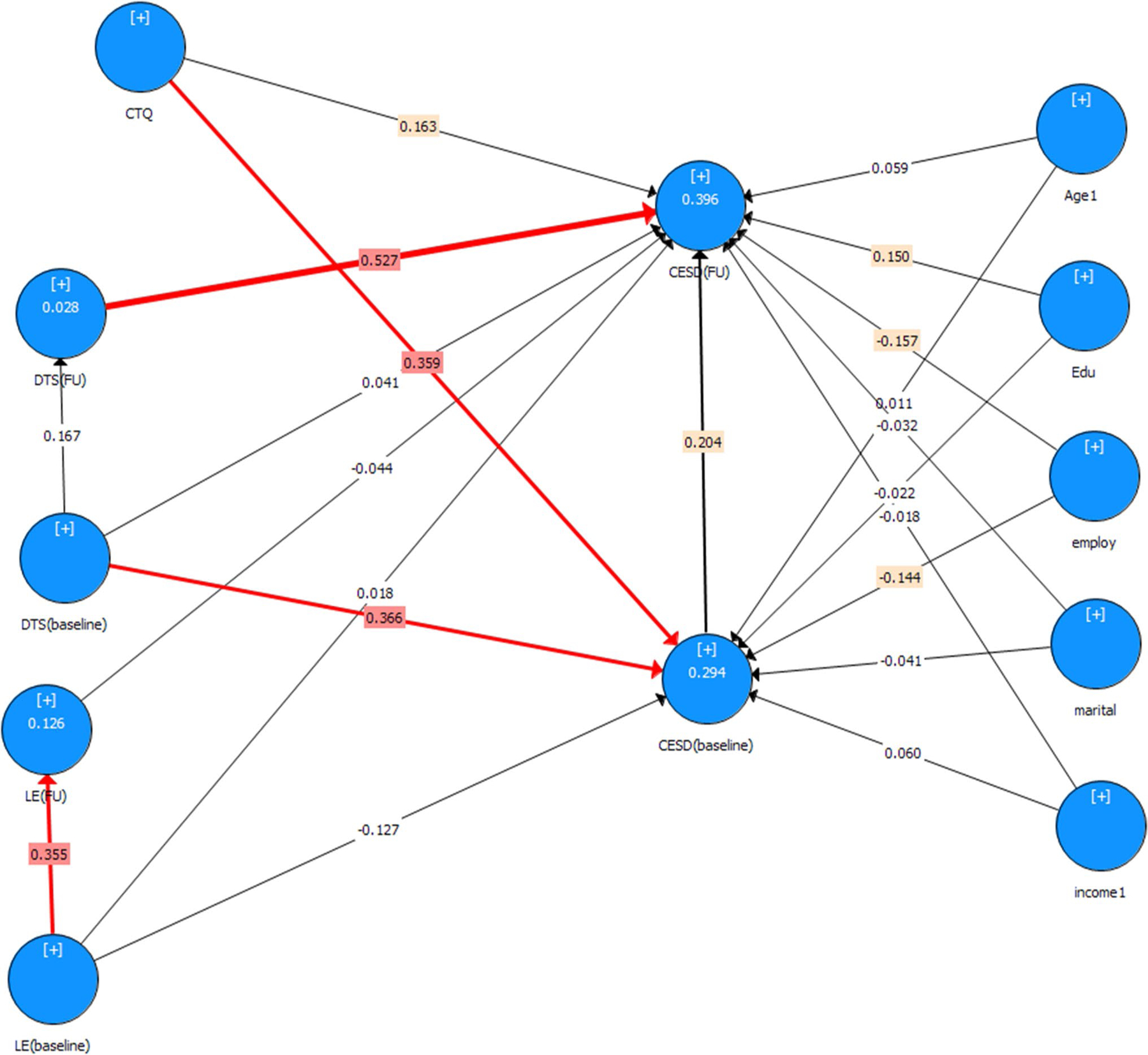
PLS SEM. CTQ childhood trauma, DTS (baseline) PTSD at baseline, DTS (FU) PTSD at follow-up, CESD (baseline) depression at baseline, CESD (FU) depression at follow-up, LE (baseline) traumatic life experiences at baseline, LE (FU) traumatic life experiences at follow-up, Edu highest level of education, employ current employment status, marital marital status, income1 combined annual household income
Discussion
The present study provides prevalence data on depression and PTSD, over time, in a cohort of South African women allowing us to investigate the dynamics of these disorders and their relation to biographical and socioeconomic factors (traumatic life events and childhood trauma) and HIV status. Results of the path analysis in this sample over a 12-month period shows the multi-directional and entwined influence of early life stress, other life-threatening traumas across the lifespan, depression and PTSD over the course of HIV.
This sample represents South African women who are socioeconomically disadvantaged and face several challenges such as increased rates of childhood and adult traumatic experiences. There were no significant demographic differences between HIV infected and uninfected women in this sample. The women were Black African, poor (mean annual combined household income of less than 750 dollars), and mostly single and unemployed. Given that poverty and low education correlate with lower access to information and appropriate health care, and with greater exposure to violence, these factors can be broadly considered as contributors to disease and poor quality of life [52]. As proposed by the ‘relative income hypothesis’ (psychosocial hypothesis), income inequality has a greater impact on health than absolute low income as such, because social comparisons lead to stress and shame, which, in turn, enhance the propensity for several diseases [53].
As expected, and in line with existing literature, traumatic life events, childhood trauma and PTSD were associated with depression [54–56]. It is widely recognised that adversity arising from long-lasting social hardship, neglect, violence and sexual abuse, present risk factors for various neuropsychiatric disorders [55, 57–59]. The autonomic stress response system and resulting HPA axis changes are the most frequently understood underlying mechanisms [56, 60, 61]. Even though the stress response provides acute benefits for survival, when long lasting, it may create enduring neuroplastic changes that predispose to maladaptive neurobiological and emotional reactions to future negative events, such as socioeconomic challenges, poverty, low education and stigma [61, 62].
Childhood trauma and traumatic life events at baseline predicted depression at follow-up only for women living with HIV and not uninfected women. This finding suggests that there may be a more consistent and enduring relationship between childhood trauma and depression for PLHIV than for those uninfected by HIV. Because adverse early life experiences may control patterns of gene expression in adolescence and adulthood that modulate neurodevelopmental and neuroplastic changes [63–65], victims of adversity may be more vulnerable to additional adversity. Early life stress may induce a vulnerability to stress later in life, resulting in an increased risk for stress-related disorders [39]. Individuals with depression who have experienced early life adversity have a poorer response to antidepressant treatment [66] and increased relapse rates [67]. This highlights the need for screening and early detection of childhood trauma and the management of the effects thereof.
Considering the difficult life circumstances reported by the women in the present study, high rates of depression in these women at baseline could be expected. Indeed, with rates of 19% at baseline, 13.5% for emergent cases and 7% for persistent cases, these women have rates that are comparable to the general population of the US and South Africa [9, 68]. However, it should be noted that the depression rates were based on CES-D scores and not on clinical diagnosis. The CES-D was developed to identify populations at risk of developing depressive disorders but is not a clinical diagnostic tool [47]. Previous studies have found that the majority of depression cases resolved within six months [13, 69]. However, the aforementioned studies included non-traumatised individuals and the course of depression in individuals with early life stress is often different and characterised by chronicity [39]. Early life stress influences both response to treatment for depression as well as relapse rate [39, 67]. Early life stress and PTSD may be associated with treatment resistance and increased relapse rates of depression. Depression in HIV-infected women can emerge at any time following a diagnosis of HIV or initiation of ART. While there may be natural recovery of incident depressive episodes within a 12-month time frame, emergence and natural resolution of a major depressive episode may occur even within a 6-month time window as the course of major depressive disorder is highly variable. In the present study, the M.I.N.I.-Plus was administered at both baseline and 12-month follow up and the MDD module assessed for lifetime depression (which included the past 12 months). Item A6 in the M.I.N.I. module specifically asks “How many episodes of depression did you have in your lifetime? At the 12 month assessment, we were therefore able to capture any depressive episodes in the past year that may have occurred and had a natural recovery between the baseline and 12-month intervals. In this sample, no such episodes were reported. Providing some contrast, the rates of PTSD (10% at baseline, 6% for emergent cases and 4% for persistent cases) are below what we expected in this sample, with a 53% rate of reported childhood traumatic experiences. This suggests that mechanisms of resilience providing protection against the development of clinical depression and PTSD may be at play. This deserves further investigation. Notably, we previously reported that higher levels of resilience were associated with lower levels of self-reported depression in this sample of women [70].
Results of the present study revealed differences in depression between infected and uninfected women, with a higher endorsement of depressive symptomatology (CES-D score) among infected women at baseline compared to uninfected women. The finding of higher levels of depressive symptomatology among South African women living with HIV is consistent with studies documenting higher rates of psychiatric disorders among PLHIV in South Africa [8, 9, 71]. Stigma, fear of disclosure and lack of social support represent severe social and cultural problems [72]. HIV-related symptoms present another challenge, whether they arise directly from HIV or opportunistic infections, are due to comorbid illness, or manifest as side effects of cART [73]. Interestingly, among the ten cases of persistent depression, eight were women living with HIV. This finding may suggest that depression associated with HIV may be singular in some respect, and more resistant to remission. Longitudinal studies have been scarce but the few studies conducted report persistence of depression in HIV-infected individuals [8, 13, 74]. Substantial evidence indicates that among PLHIV, depression arises at least in part from virally mediated chronic neuroinflammation and neurotoxicity [75–77].
Despite not statistically differing in the rate of PTSD, infected and uninfected women in the present study have differential rates and severity of reported childhood trauma. Among uninfected women, 32% reported having experienced trauma during childhood, with the majority of these cases in the mild to moderate range, whereas 78% of women living with HIV experienced childhood trauma, with the majority of these cases reporting trauma in the severe to extreme range. These results point to a possible link between early life trauma and HIV infection. The HIV epidemic occurs largely within a context of considerable social inequality, which leads people with a history of childhood abuse and adversity to be overrepresented among PLHIV [6, 26]. As a result of these prenatal and early life experiences, neuroplastic and epigenetic changes with lifelong and persisting effects emerge, in turn predisposing PLHIV to wide-ranging neuropsychiatric conditions [63, 65, 78–80].
A limitation of the present study was the retrospective assessment of childhood trauma and its contribution to recall bias. Moreover, the CTQ-SF does not include any questions around whether the abuse reported was a single isolated event(s) or repeated exposures. Moreover, depression and PTSD were self-reported and not based on a clinical diagnosis. Another limitation of the present study is that the factors associated with emergent depression in HIV are likely to differ from the factors associated with persistent depression. Ideally, the regressions for persistent and emergent depression should be parsed out and should be a consideration for other longitudinal studies of depression in HIV. Sub-analyses by HIV group may also be underpowered and should be considered as exploratory. Future studies are needed.
Conclusion
The present study, which is unique in documenting the course of untreated depression and PTSD in women with and without HIV infection with a high prevalence of early childhood trauma, provides evidence for increased depressive symptomatology among women living with HIV and persistence over time. Findings also suggest that there may be a more consistent and enduring relationship between childhood trauma and depression among women living with HIV compared to uninfected counterparts, highlighting the need to detect childhood trauma and intervene early.
Acknowledgements
This work is supported by the South African Research Chair in PTSD awarded to S Seedat and hosted by Stellenbosch University, funded by the DST and administered by NRF and the Faculty of Medicine and Health Sciences, Stellenbosch University (Deputy Dean’s strategic fund for postdoctoral fellows and sub-committee C postdoctoral fellowship). Additional research support was provided by a CFAR grant awarded to S Seedat [P30-AI036214] Professor Martin Kidd from the department of Statistics and Actuarial Sciences of Stellenbosch University provided statistical assistance.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflicts of interest.
Ethical Approval All procedures involving human participants were in accordance with the ethical standards for the institutional review board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Health Research Ethics Committee (HREC) of Stellenbosch University, South Africa (HREC #: N07/07/153).
Informed Consent Informed consent was obtained from all individual participants included in the study. No personal identifying information is included in the manuscript.
References
- 1.Statistics South Africa. Mid-year population estimates Vol. 2017. 2016. [Google Scholar]
- 2.Bloomfield GS, Khazanie P, Morris A, Rabadan-Diehl C, Benjamin LA, Murdoch D, et al. HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr 2014;67(Suppl 1):S40–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adebamowo CA, Casper C, Bhatia K, Mbulaiteye SM, Sasco AJ, Phipps W, et al. Challenges in the detection, prevention, and treatment of HIV-associated malignancies in low- and middle-income countries in Africa. J Acquir Immune Defic Syndr 2014;67(Suppl 1):S17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abas M, Ali GC, Nakimuli-Mpungu E, Chibanda D. Depression in people living with HIV in sub-Saharan Africa: time to act. Trop Med Int Health 2014;19(12):1392–6. [DOI] [PubMed] [Google Scholar]
- 5.Colibazzi T, Hsu TT, Gilmer WS. Human immunodeficiency virus and depression in primary care: a clinical review. Prim Care Companion J Clin Psychiatry 2006;8(4):201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 7.Myer L, Smit J, Roux L Le, Parker S, Stein DJ, Seedat S. Common mental disorders among HIV-infected individuals in South Africa: prevalence, predictors, and validation of brief psychiatric rating scales. AIDS Patient Care STDs 2008;22(2):147–58. http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=29344623&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 8.Olley BO, Seedat S, Stein DJ. Persistence of psychiatric disorders in a cohort of HIV/AIDS patients in South Africa: a 6-month follow-up study. J Psychosom Res 2006;61(4):479–84. [DOI] [PubMed] [Google Scholar]
- 9.Stein DJ, Seedat S, Herman A, Moomal H, Heeringa SG, Kessler RC, et al. Lifetime prevalence of psychiatric disorders in South Africa. Br J Psychiatry 2008;192(2):112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbie T, Mijch A, Ellen S, Hoy J, Hutchison C, Wright E, et al. Depression and neurocognitive performance in individuals with HIV/AIDS: 2-year follow-up. HIV Med 2006;7(2):112–21. http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=19438753&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 11.Judd F, Komiti A, Chua P, Mijch A, Hoy J, Grech P, et al. Nature of depression in patients with HIV/AIDS. Aust N Z J Psychiatry 2005;39(9):826–32. [DOI] [PubMed] [Google Scholar]
- 12.Kinyanda E, Nakasujja N, Levin J, Birabwa H, Mpango R, Grosskurth H, et al. Major depressive disorder and suicidality in early HIV infection and its association with risk factors and negative outcomes as seen in semi-urban and rural Uganda. J Affect Disord 2017;212:117–27. [DOI] [PubMed] [Google Scholar]
- 13.Kinyanda E, Weiss HA, Levin J, Nakasujja N, Birabwa H, Nakku J, et al. Incidence and persistence of major depressive disorder among people living with HIV in Uganda. AIDS Behav 2017;21(6):1641–54. [DOI] [PubMed] [Google Scholar]
- 14.Olatunji BO, Mimiaga MJ, O’Cleirigh C, Safren SA. Review of treatment studies of depression in HIV. Top HIV Med 2006;14(3):112–24. [PubMed] [Google Scholar]
- 15.Wright E, Brew B, Arayawichanont A, Robertson K, Samintharapanya K, Kongsaengdao S, et al. Neurologic disorders are prevalent in HIV-positive outpatients in the Asia-Pacific region. Neurology 2008;71(1):50–6. [DOI] [PubMed] [Google Scholar]
- 16.Kagee A, Saal W, De Villiers L, Sefatsa M, Bantjes J. The prevalence of common mental disorders among South Africans seeking HIV testing. AIDS Behav 2017;21(6):1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 2004;45(5):394–402. [DOI] [PubMed] [Google Scholar]
- 18.Kacanek D, Jacobson DL, Spiegelman D, Wanke C, Isaac R, Wilson IB. Incident depression symptoms are associated with poorer HAART adherence: a longitudinal analysis from the Nutrition for Healthy Living study. J Acquir Immune Defic Syndr 2010;53(2):266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS 2007;21(9):1175–83. [DOI] [PubMed] [Google Scholar]
- 20.Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS 2012;26(Suppl 2):S117–35. [DOI] [PubMed] [Google Scholar]
- 21.Tegger MK, Crane HM, Tapia KA, Uldall KK, Holte SE, Kitahata MM. The effect of mental illness, substance use, and treatment for depression on the initiation of highly active antiretroviral therapy among HIV-infected individuals. AIDS Patient Care STDS 2008;22(3):233–43. [DOI] [PubMed] [Google Scholar]
- 22.Burack JH, Barrett DC, Stall RD, Chesney MA, Ekstrand ML, Coates TJ. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA 1993;270(21):2568–73. [PubMed] [Google Scholar]
- 23.Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health 2004;94(7):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA 2001;285(11):1466–74. [DOI] [PubMed] [Google Scholar]
- 25.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008;70(5):539–45. [DOI] [PubMed] [Google Scholar]
- 26.Vlassova N, Angelino AF, Treisman GJ. Update on mental health issues in patients with HIV infection. Curr Infect Dis Rep 2009;11(2):163–9. [DOI] [PubMed] [Google Scholar]
- 27.Leserman J HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry 2003;54(3):295–306. [DOI] [PubMed] [Google Scholar]
- 28.Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci 2014;68(2):96–109. [DOI] [PubMed] [Google Scholar]
- 29.Sherr L, Clucas C, Harding R, Sibley E, Catalan J. HIV and depression—a systematic review of interventions. Psychol Health Med 2011;16(5):493–527. [DOI] [PubMed] [Google Scholar]
- 30.Andersson N, Cockcroft A, Shea B. Gender-based violence and HIV: relevance for HIV prevention in hyperendemic countries of southern Africa. AIDS 2008;22(Suppl. 4):73–86. [DOI] [PubMed] [Google Scholar]
- 31.Jewkes R, Penn-Kekana L, Levin J, Ratsaka M, Schrieber M. Prevalence of emotional, physical and sexual abuse of women in three South African provinces. S Afr Med J 2001;91(5):421–8. [PubMed] [Google Scholar]
- 32.Jewkes R, Levin J, Mbananga N, Bradshaw D. Rape of girls in South Africa. Lancet 2002;359(9303):319–20. [DOI] [PubMed] [Google Scholar]
- 33.Kalichman SC, Simbayi LC. Sexual assault history and risks for sexually transmitted infections among women in an African township in Cape Town, South Africa. AIDS Care 2004;16(6):681–9. [DOI] [PubMed] [Google Scholar]
- 34.Davis SK. The relationship between HIV, maternal childhood sexual abuse survival, and parental sexual communication among African American women 18–24. J Health Care Poor Underserved 2017;28(2S):24–32. [DOI] [PubMed] [Google Scholar]
- 35.Fang L, Chuang D-M, Lee Y. Adverse childhood experiences, gender, and HIV risk behaviors: results from a population-based sample. Prev Med Rep 2016;4:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whetten K, Reif S, Toth M, Jain E, Leserman J, Pence BW. Relationship between trauma and high-risk behavior among HIV-positive men who do not have sex with men (MDSM). AIDS Care 2012;24(11):1453–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the South. Psychosom Med 2005;67(3):500–7. [DOI] [PubMed] [Google Scholar]
- 38.Leserman J, Pence B, Whetten K, Mugavero M, Thielman N, Swartz M, et al. Relation of lifetime trauma and depressive symptoms to mortality in HIV. Am J Psychiatry 2007;164(11):1707–13. http://proquest.umi.com/pqdweb?did=1378064611&Fmt=7&clientId=57290&RQT=309&VName=PQD. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie CF, Nemeroff CB. Early life stress and depression. Childhood trauma may lead to neurobiologically unique mood disorders. Curr Psychiatr 2005;4(10):15–30. [Google Scholar]
- 40.Negele A, Kaufhold J, Kallenbach L, Leuzinger-Bohleber M. Childhood trauma and its relation to chronic depression in adulthood. Depress Res Treat 2015;2015:650804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pena CJ, Kronman HG, Walker DM, Cates HM, Bagot RC, Purushothaman I, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017;356(6343):1185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herman AA, Stein DJ, Seedat S, Heeringa SG, Moomal H, Williams DR. The South African Stress and Health (SASH) study: 12-month and lifetime prevalence of common mental disorders. S Afr Med J 2009;99(5):339–44. [PMC free article] [PubMed] [Google Scholar]
- 43.Malan-Muller S, Hemmings SM, Spies G, Kidd M, Fennema-Notestine C, Seedat S. Shorter telomere length—a potential susceptibility factor for HIV-associated neurocognitive impairments in South African women. PLoS ONE 2013;8(3):e58351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spies G, Ahmed-Leitao F, Fennema-Notestine C, Cherner M, Seedat S. Effects of HIV and childhood trauma on brain morphometry and neurocognitive function. J Neurovirol 2016;22(2):149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spies G, Fennema-Notestine C, Cherner M, Seedat S. Changes in cognitive function in women with HIV infection and early life stress. AIDS Care 2017;29(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- 47.Radloff SF. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 48.Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the life events checklist. Assessment 2004;11(4):330–41. [DOI] [PubMed] [Google Scholar]
- 49.Davidson JRT, Book SW, Colket JT, Tupler LA, Roth S, David D, et al. Assessment of a new self-rating scale for post-traumatic stress disorder. Psychol Med 1997;27(1):153–60. [DOI] [PubMed] [Google Scholar]
- 50.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl 2003;27(2):169–90. [DOI] [PubMed] [Google Scholar]
- 51.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41(4):1149–60. [DOI] [PubMed] [Google Scholar]
- 52.Pickett KE, Wilkinson RG. Inequality: an underacknowledged source of mental illness and distress. Br J Psychiatry 2010;197(6):426–8. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson RG. The impact of inequality: how to make sick societies healthier New York: New York Press; 2005. [Google Scholar]
- 54.Shea A, Walsh C, Macmillan H, Steiner M. Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 2005;30(2):162–78. [DOI] [PubMed] [Google Scholar]
- 55.Meyer-Lindenberg A Social neuroscience and mechanisms of risk for mental disorders. World Psychiatry 2014;13(2):143–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Syed SA, Nemeroff CB. Early life stress, mood, and anxiety disorders Chronic Stress: Thousand Oaks; 2017. p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel V, Kleinman A. Poverty and common mental disorders in developing countries. Bull World Health Organ 2003;81(8):609–15. [PMC free article] [PubMed] [Google Scholar]
- 58.van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature 2010;468(7321):203–12. [DOI] [PubMed] [Google Scholar]
- 59.Yang BZ, Zhang H, Ge W, Weder N, Douglas-Palumberi H, Perepletchikova F, et al. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med 2013;44(2):101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson PO, Goodyer IM. Childhood adversity and allostatic overload of the hypothalamic-pituitary-adrenal axis: a vulnerability model for depressive disorders. Dev Psychopathol 2011;23(4):1017–37. [DOI] [PubMed] [Google Scholar]
- 61.Duman RS. Neural plasticity: consequences of stress and actions of antidepressant treatment. Dialogues Clin Neurosci 2004;6(2):157–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plast 2017;2017:6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clark US, Cohen RA, Sweet LH, Gongvatana A, Devlin KN, Hana GN, et al. Effects of HIV and early life stress on amygdala morphometry and neurocognitive function. J Int Neuropsychol Soc 2012;18(4):657–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, et al. Childhood trauma and health outcomes in HIV-infected patients: an exploration of causal pathways. J Acquir Immune Defic Syndr 2012;59(4):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vaiserman AM. Epigenetic programming by early-life stress: evidence from human populations. Dev Dyn 2015;244(3):254–65. [DOI] [PubMed] [Google Scholar]
- 66.Williams LM, Debattista C, Duchemin AM, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 2016;6:e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kaplan MJ, Klinetob NA. Childhood emotional trauma and chronic posttraumatic stress disorder in adult outpatients with treatment-resistant depression. J Nerv Ment Dis 2000;188(9):596–601. [DOI] [PubMed] [Google Scholar]
- 68.National Institute of Mental Health. Major depression among adults Vol. 2017. 2015. [Google Scholar]
- 69.Spijker J, de Graaf R, Bijl RV, Beekman ATF, Ormel J, Nolen WA. Determinants of persistence of major depressive episodes in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). J Affect Disord 2004;81(3):231–40. [DOI] [PubMed] [Google Scholar]
- 70.Spies G, Seedat S. Depression and resilience in women with HIV and early life stress: does trauma play a mediating role? A cross-sectional study. BMJ Open 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freeman M, Nkomo N, Kafaar Z, Kelly K. Mental disorder in people living with HIV/AIDS in South Africa. S Afr J Psychol 2008;38(3):489–500. http://search.ebscohost.com/login.aspx?direct=true&db=aph&AN=34993548&site=ehost-live. [DOI] [PubMed] [Google Scholar]
- 72.De Santis JP, Gonzalez-Guarda RM, Vasquez EP. Psychosocial and cultural correlates of depression among Hispanic men with HIV infection: a pilot study. J Psychiatr Ment Health Nurs 2012;19(10):860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fonseca MG, Bastos FI. Twenty-five years of the AIDS epidemic in Brazil: principal epidemiological findings, 1980–2005. Cad Saude Publica 2007;23(Suppl 3):S333–44. [DOI] [PubMed] [Google Scholar]
- 74.Malee KM, Mellins CA, Huo Y, Tassiopoulos K, Smith R, Sirois PA, et al. Prevalence, incidence, and persistence of psychiatric and substance use disorders among mothers living with HIV. J Acquir Immune Defic Syndr 2014;65(5):526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barreto IC, Viegas P, Ziff EB, Konkiewitz EC. Animal models for depression associated with HIV-1 infection. J Neuroimmune Pharmacol 2014;9(2):195–208. [DOI] [PubMed] [Google Scholar]
- 76.Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol 2013;19(4):314–27. [DOI] [PubMed] [Google Scholar]
- 77.Rivera-Rivera Y, Vazquez-Santiago FJ, Albino E, Sanchez MD, Rivera-Amill V. Impact of depression and inflammation on the progression of HIV disease. J Clin Cell Immunol 2016;7(3):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci 2008;20(3):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown AS, Susser ES. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr Bull 2008;34(6):1054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 2008;105(44):17046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


