Abstract
Introduction:
Severe Acquired Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2) infection represents an unprecedented public health problem and, at present, vaccination is the only weapon available to combat the infection. The simplest and most immediate method to quantify the response of the subject’s immune system to vaccination and / or infection is the serological assessment of the antibody titer. The objective of our study was 1) to evaluate the presence of antibody responses in a sample of healthcare workers subjected to a complete vaccination course as per ministerial provisions (double dose for negatives and single dose for ex-SARS-CoV subjects -2 positive) with Comirnaty vaccine (Pfizer / BioNTech) 2) evaluate the presence of statistically significant associations for sex, age and previous positive swab.
Materials and methods:
the antibody levels of both nucleocapsid antibodies and anti-Sars-CoV2 Spike antibodies of the study subjects were examined with the electrochemiluminescent immunoassay (ECLIA) method developed by Roche®. The cut-off value, as suggested by the manufacturer, for anti-nucleocapsid antibodies was 1 COI, while the Ig Spike value was 0.8 I / mL. The study sample was stratified by age (≤45 years, 46-55, ≥56 years old), previous positive molecular swab, gender and IgG S1 / S2 values at the completed vaccination course (≤200, ≥200 AU / mL ). Statistical analyzes were carried out with the R software.
Results:
almost all of the sample (89.45%) showed IgG Spike values> 200 AU / mL with statistically significant associations in relation to sex (greater in females, p≤0.05), to previous swab positivity in the presence of a vaccine dose (n = 44; p <0.001) and at age (with greater antibody response in subjects under 45; p <0.001).
Discussion and conclusions:
The current study confirms what is reported in the literature. In the light of the results obtained, it could be interesting to promote studies that evaluate the antibody titers trend over time a) in women of childbearing age and postmenopausal age b) in particular categories of subjects with chronic degenerative diseases to assess the actual need for doses booster, it being understood that the immune system response is guaranteed by both cellular and humoral immunity and that the antibody titer does not faithfully reflect the protection obtained. (www.actabiomedica.it)
Keywords: COVID-19, serological evaluation, healthcare workers
Introduction
SARS-CoV-2 represents an unprecedented public health problem. Nevertheless, many other communicable diseases, which have lead to enormous economic and social damage, have affected humanity throughout history, from the bubonic plague, cholera, smallpox, typhus, measles, polio, diphtheria, the Spanish pandemic of 1918 (which killed between 50 and 100 million people, 21 million of which in Europe alone), to the ongoing HIV pandemic and the recent SARS epidemics in 2003, MERS in 2012 and, last but not least Ebola. (1)
In many cases, several efforts were made to obtain an effective vaccination that could reduce the circulation of the pathogen, which in some cases has remained endemic, while in others, treatments have been introduced that have increased the life span of patients.
Only one year has passed since the first known case of Covid-19 was identified in the city of Wuhan in November 2019, leading to the birth of multiple studies beginning in the spring of 2020, and the conditional marketing authorization for a first messenger RNA vaccine: mRNA (Comirnaty, from the company BioNTech / Pfizer) and immediately thereafter, on January 6 2021, the vaccine produced by Moderna. (2)
If in 2020 the news focused on the spread of COVID-19 across the world, in 2021 the main focus was ending the pandemic through vaccine distribution. (3-5)
Covid-19 is caused by a SARS-CoV-2 virus strain which belongs to the Coronaviridae family, and is highly transmissible by air following human contact. The virus causes Severe Acute Respiratory Syndrome type 2 with symptoms including fever, cough and difficulty breathing, and frequent occurrences of bilateral interstitial pneumonia as well as possible extra-pulmonary manifestations (6).
According to a meta-analysis of studies, the severity of the clinical presentation of infected individuals is strongly correlated with advanced age (> 60 years), the male sex and the presence of comorbidities, such as hypertension, diabetes, coronary heart disease, chronic renal failure, cerebrovascular disease and chronic lung diseases (7).
Body response following contact with the virus is both humoral and cellular, mediated by T lymphocytes (8). The SARS-CoV-2 genome encodes approximately 25 proteins. Protein S plays a critical role for fusion and entry into the host cell and comprises an N-terminal S1 receptor binding domain (RBD), the N-terminal domain (NTD) and a C-terminal S2 subunit. It is responsible for interaction with ACE2 receptor (9).
More specifically, humoral immunity is given by the production of antibodies against the Spikes surface glycoproteins and the nucleocapsid, which prevent access of the virus by binding to the cellular ACE2 receptors (angiotensin converting enzyme) (10).
The presence of symptoms, younger age and being female were associated with a stronger antibody response, and in particular, a systematic review of virus-specific serum antibody responses in SARS-CoV-2 infected individuals showed that IgM is consistently detected before IgG, peaking between week 2 and week 5 and falling over an additional three to three-five weeks after infection. IgG peaks between the third and seventh week after the onset of symptoms persist for at least eight weeks. Neutralizing antibodies, with the ability to limit virus growth in vitro, are detectable within 7-15 days of disease onset and levels increase until days 14-22, before stabilizing and then decreasing. Lower antibody titers were observed in subjects with asymptomatic or clinically mild disease (11)
To date, the treatments available are limited and the most effective weapon remains vaccination.
The objective of our study was a) to evaluate the antibody response in a sample of healthcare workers undergoing a complete vaccination course as per ministerial provisions (double dose for negatives and single dose for ex-SARS-CoV-2 patients positive) (12) with Comirnaty vaccine (Pfizer / BioNTech) b) evaluate the presence of statistically significant associations for sex, age and previous positive swab
Materials and methods
A cross-sectional observational study was carried out from February 2021 to May 2021.
The subjects enrolled in the study were represented by healthcare workers of the AOU Policlinic “G. Martino” of Messina who, after being vaccinated with a vaccination cycle with Comirnaty vaccine (Pfizer / BioNTech) as product indication (13) presented voluntarily for serological screening to evaluate the efficacy of the vaccine.
The sample was divided into two cohorts:
vaccinated subjects without previous swab positivity;
vaccinated subjects with previous swab positivity.
The vaccination cycle was carried out for the first dose from January 2nd to January 18th and for the second dose, from January 19th to April 2nd 2021.
Serological evaluation
With the consent of the operator concerned, a blood test of the subjects under study was taken to determine the antibody levels of both the nucleocapsid antibodies and the anti-SARS-CoV-2 Spike antibodies with the electrochemiluminescence method (electrochemiluminescent immunoassay - ECLIA) developed by Roche®. The cut-off value, as suggested by the manufacturer, for anti-nucleocapsid antibodies was 1 COI, while the Ig Spike value was 0.8 I / mL.
Blood samples were taken 30 days after the second dose for the vaccinated cohort and for those who tested positive after negativization of the molecular swab.
Statistical data analysis
The study sample was stratified by sex, age (≤45 years, 46-55, ≥56 years) and previous positive molecular swab.
For the statistical analysis, software R rel.4.1.0 was used. Categorical variables were expressed by numbers and percentages, while continuous variables were reported as means±standard deviations.
Shapiro-Wilk test and graphs (histograms and Q-Q plots) will be used to test the assumption of normal distribution. The Chi-square test and Mann-Whitney U tests were performed on categorical and non-parametric continuous data, respectively.
The Kruskal-Wallis test was conducted to compare the stratified age (≤45 years, 46-55, ≥56 years) and analysis post hoc Conover (14) when significance has been verified.
Two groups of patients were identified among the responders, based on IgG S1 / S2 values at the completed vaccination cycle (≤200, and> 200 AU / mL; this cut off was chosen in relation to previous studies (15). The likelihood of committing a type one error was set to 0,05.
Results
The sample under study was represented by 2219 employees, of which 1300 were women and 919 men, aged between 22 and 70 years (45.41± 12.76 SD).
Socio-anagraphical characteristics of the sample are represented in the Table 1.
Table 1.
Socio-anagraphical characteristics of the sample.
| No. | % | |
|---|---|---|
|
Gender Male Female |
919 1,300 |
41.4 58.6 |
| Age (Mean, SD and C.I. 95%) | 45.41 ± 12.76 (44.9 – 45.9) | |
|
Age class <45 46-55 > 55 |
996 619 604 |
44.9 27.9 27.2 |
|
COVID-19 Positive in the past - with one shot of vaccine before withdrawal - never immunized Negative but immunized |
18 26 2.174 |
0.81 1.17 97.97 |
Regarding the Anti-Sars-CoV2 Spike values, almost all of the sample (89.45%) showed values above the cut off we have chosen of 200 AU / mL (Figure 1).
Figure 1.
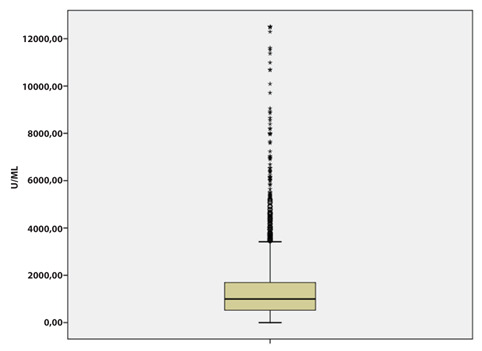
Distribution of Anti-Sars-CoV2 Spike values titers in the sample.
Although no statistically significant differences were found in the sample means in relation to gender, higher values> 200 AU / ml were most observed in females (P<0.05) (Figure 2).
Figure 2.
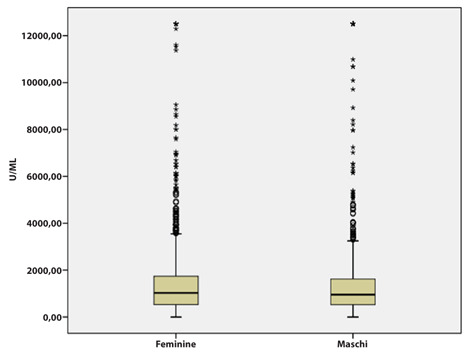
Distribution of Anti-Sars-CoV2 Spike values by gender
The Table 2 shows the distribution of antibody titers by gender, age group and Covid 19 history and immunization. As regards gender, no significant statistical differences were observed. The anti-SARS-CoV2 Nucleocapsid total Ig values were, in subjects with previous positivity, ranging from a minimum of 0 to a value of 241.2 (6.94±28.48).
Table 2.
Difference in level of Anti-Sars-CoV2 Spike Ig stratified by gender, age class and Covid-19 history and immunization
| ≤ 200 | > 200 | p value | |
|---|---|---|---|
| N (%) | N (%) | ||
|
Gender Male Female |
83 151 |
836 1149 |
0.05 |
|
Age class <45 46-55 > 55 |
78 86 70 |
918 533 534 |
0.001 |
|
CoViD-19 history and immunization Positive in the past -with one shot of vaccine before withdrawal - never immunized Negative but immunized |
1 11 222 |
26 7 1952 |
0.001 |
Furthermore, age stratification showed higher levels of antibody response in subjects under 45 (p <0.001) (Figure 3).
Figure 3.
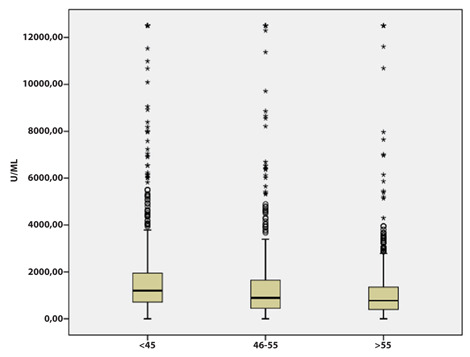
Distribution of Anti-Sars-CoV2 Spike values by age
Through Post Hoc analysis it is possible to highlight that there are significant differences in the three age groups. This significance is to be attributed to all three age groups analyzed (Table 3).
Table 3.
Analysis of age groups analyzed
| Age classes | <45 | 46-55 | >55 | p value |
|---|---|---|---|---|
| Spike | 1763,21 ± 2113,51 | 1426,27 ± 1942,43 | 1159,93 ± 1582,56 | 0.001 |
In relation to the previous positivity to the swab (n = 44), a reduction in the antibody titer of the subjects was observed below of 200 AU / ml in 61.1% of cases; moreover, in 96.3% of cases the value was higher than 200 AU / ml after a single dose of vaccine. In particular, a statistically significant difference was observed between vaccinated with two doses, vaccinated with one dose and not vaccinated resulting from antibody titers> 200 AU / ml found in the first and second cohort. There were no differences in sample means for Ig spike levels in the three cohorts.
Discussion
In this study we analyzed two different patient cohorts in order estimate the antibody response in a sample of healthcare workers undergoing a complete vaccination course as per ministerial provisions (double dose for negatives and single dose for ex - SARS-CoV-2 positive patients) with Comirnaty vaccine (Pfizer / BioNTech); and to estimate the presence of statistically significant associations for sex, age and previous positive swab
First, we found that all subjects immunized with the BNT162b2 m-RNA COVID-19 vaccine achieved an immunological response with higher-than-cut IgG concentrations. Furthermore, infected subjects also had a serological response with an increase after a single vaccine shot above the level of 200 AU / ml, as reported in other studies (15). Considering that about 60% of patients had IgG concentrations below 200 AU / mL, and that in about 96% of patients after a single dose the value was higher than this, we can state that immunization, even with one administration, increases the level of antibodies. This may be important in some categories of patients given the decrease in persistence of antibodies after natural immunity that has been reported in the literature.
These results were in line with other studies in the literature reporting that in a COVID 19 positive subject, a single dose of mRNA vaccine elicited post-vaccination antibody concentrations similar to or higher than the concentrations found in seronegative participants who received two doses of the vaccine (15).
Furthermore, we observed that younger age and female gender appear to be associated with higher antibody titers, as previously described in the literature (16-18).
Some possible limitations of the study are that only humoral and non-cellular immune responses are considered; furthermore, as reported in the literature, we did not identify a protection cut-off.
However, on the other hand, higher antibody titers may be related to protective immunity, and we cannot fail to state that a lower titer is indicative of a higher risk of infection.
As reported by previous studies, identifying a protective cut-off could be useful in some categories of patients (19).
On the other hand, as reported by Westmeier et al. in infected elderly patients we had a decrease in CD8 T cells, which are responsable for cellular immunity, and this could also be related to the lower titer response to immunization (20).
The current study has confirmed what was reported in the literature and, in the light of the results obtained, it could be interesting to promote studies that evaluate the antibody titer trend over time a) in women of childbearing age and postmenopausal age b) in particular categories of subjects with chronic degenerative diseases, in order to estimate the actual need for booster doses, it being understood that the immune system response is guaranteed by both cellular and humoral immunity and that the antibody titer does not faithfully reflect the protection obtained.
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Morens DM, Fauci AS. Emerging Pandemic Diseases: How We Got to COVID-19 [published correction appears in Cell. 2020 Oct 29;183(3):837] Cell. 2020;182(5):1077–1092. doi: 10.1016/j.cell.2020.08.021. doi: 10.1016/j.cell.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.who.int/news/item/27-04-2020-who-timeline---covid-1. [Google Scholar]
- https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-in-2021. [Google Scholar]
- Coronavirus disease (COVID-19) pandemic. Available on line on https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Last access 12.10.2020. [Google Scholar]
- COVID-19 pandemic. Available on line on https://www.ecdc.europa.eu/en/novel-coronavirus-china. last access 12.10.2020. [Google Scholar]
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. https://doi.org/10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhong X, Wang Y, Zeng X, Luo T, Liu Q. Clinical determinants of the severity of COVID-19: A systematic review and meta-analysis. PLoS One. 2021 May 3;16(5):e0250602. doi: 10.1371/journal.pone.0250602. doi: 10.1371/journal.pone.0250602. PMID: 33939733; PMCID: PMC8092779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, John Wherry E. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. https://doi.org/10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Gao G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82. doi: 10.1038/s41577-020-00480-0. https://doi.org/10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liang B, Chen C, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun 12. 2021;1813 doi: 10.1038/s41467-021-22034-1. https://doi.org/10.1038/s41467-021-22034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European center of disease control. Immune responses and correlates of protective immunity against SARS-CoV-2. Available on https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses#:~:text=Immune%20responses%20and%20correlates%20of,transmission%20risk%2C%20or%20disease%20outcome . Last access on 8 September 2021. [Google Scholar]
- Ministry of Health’s Decree, 3 March 2021, n. 8284 [Google Scholar]
- Summary of product characteristics. Comirnaty concentrate for injectable dispersion COVID-19 mRNA vaccine (nucleoside modified) Available on https://farmaci.agenziafarmaco.gov.it/aifa/servlet/PdfDownloadServlet?pdfFileName=footer_005389_049269_RCP.pdf&retry=0&sys=m0b1l3 . [Google Scholar]
- Conover WJ. Practical nonparametric statistics. New York: John Wiley & Sons; 1999. [Google Scholar]
- Amodio E, Capra G, Casuccio A, et al. Antibodies Responses to SARS-CoV-2 in a Large Cohort of Vaccinated Subjects and Seropositive Patients. Vaccines (Basel) 2021;9(7):714. doi: 10.3390/vaccines9070714. Published 2021 Jul 1. doi: 10.3390/vaccines9070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner A, Watschinger C, Rössler A, Farcet MR, Penner A, Böhm V, Kiechl SJ, Stampfel G, Hintenberger R, Tilg H, Koch R, Antlanger M, Kreil TR, Kimpel J, Moschen AR. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine. 2021 Aug;70:103539. doi: 10.1016/j.ebiom.2021.103539. doi: 10.1016/j.ebiom.2021.103539. Epub 2021 Aug 12. PMID: 34391087; PMCID: PMC8358275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S.L, Morgan R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex Differ. 2020;11:24. doi: 10.1186/s13293-020-00301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Ellingson M.K, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh J.E, Tokuyama M, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. 2020;588:315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg S.P, Connors T.J, Zhu Y, Baldwin M.R, Lin W.-H, Wontakal S, Szabo P.A, Wells S.B, Dogra P, Gray J, et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2020;22:25–31. doi: 10.1038/s41590-020-00826-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmeier J, Paniskaki K, Karaköse Z, Werner T, Sutter K, Dolff S, Overbeck M, Limmer A, Liu J, Zheng X, Brenner T, Berger MM, Witzke O, Trilling M, Lu M, Yang D, Babel N, Westhoff T, Dittmer U, Zelinskyy G. Impaired Cytotoxic CD8+ T Cell Response in Elderly COVID-19 Patients. mBio. 2020 Sep 18;11(5):e02243–20. doi: 10.1128/mBio.02243-20. doi: 10.1128/mBio.02243-20. Erratum in: mBio. 2020 Nov 10;11(6): PMID: 32948688; PMCID: PMC7502863. [DOI] [PMC free article] [PubMed] [Google Scholar]


