Abstract
Background and aim:
Vitamin D is known to modulate immune response and its deficiency was associated with respiratory distress in patients hospitalized for pneumonia. Nevertheless, numerous reviews on vitamin D in COVID-19 patients have shown conflicting results, as previously reported also for other respiratory diseases (e.g., influenza).
Methods:
This umbrella review aims to assess whether low serum 25-OHD is associated with susceptibility to COVID 19, their severity, and mortality. A total of 1559 studies were excluded after the title, abstract and full-text articles screening and 9 papers were included in this review: 2 systematic reviews and 7 metanalysis.
Results:
The findings of this review that summarized studies from 5 WHO regions (European Region, Region of the Americas, South-East Asia Region, Eastern Mediterranean Region, Western Pacific Region) to exclusion only African region, show that low serum 25-OHD levels are associated with higher infection risks for COVID-19.
Conclusions:
Although the umbrella findings indicate a potential role of vitamin D deficiency in COVID-19 severity in hospitalized patients and showing an association between Vitamin D supplementation and COVID-19 severity, however, more robust data from randomized controlled trials are further needed to confirm a possible association with the mortality rates. (www.actabiomedica.it)
Keywords: SARS-COV-2, COVID-19, Review, Prevention, Cause of Death, Quality of Life, Vitamin D
Appendix
Introduction
The associations between vitamin D levels and diseases have been assessed in a large and rapidly expanding literature (1-3). Numerous studies have examined the effect of vitamin D supplementation on a range of pathologies and health conditions (3-5). Vitamin D seems linked to skeletal diseases including osteoporosis and involving calcium, phosphorus and bone metabolism or several risk conditions related to fractures, muscle strength, falls; more recently, also cancer, cardiovascular diseases, and metabolic disorders were considered (5-14). Indeed, the vitamin D receptor and the enzyme that activates vitamin D (1α-hydroxylase) have been identified in many organs, leading to claims of its extra-skeletal effects, even if often unconvincing (15). An exception to the weakness of these reports seems be showed by the observation of a more consistent effect of vitamin D on the immune system, and its possible role in increasing the defence against infectious diseases as well as the decrease in autoimmunity conditions (15, 16). Several randomized clinical trials have shown a reduced incidence in acute respiratory infections when a supplementation with vitamin D is present (17-19).
In the 2020s, after the spreading of SARS-CoV-2 pandemic, a growing scientific attention turned to evaluate the association between COVID-19 and vitamin D deficiency (20-23). In this perspective, achieving optimal levels may represent a challenging objective for prevention and treatment of COVID-19 (24). Unravelling these associations might be of great importance for public health, as vitamin D deficiency has been found to be highly prevalent in populations residing at high latitudes or leading an indoors oriented lifestyle (25). The available literature contains contrasting data and is often unclear, leading to still open debates about the optimal concentrations of vitamin D and related guidelines for supplementation (24, 26).
To provide an overview of the breadth and validity of the claimed associations of vitamin D with COVID-19 disease, we have done an umbrella review of the available evidences across existing systematic reviews and meta-analyses focused to address the role and optimal concentrations of vitamin D.
Material and Methods
A detailed protocol for the review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42021255767). The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and the guidelines developed by Aromataris and colleagues were followed to perform this umbrella review (27).
Study design
Systematic reviews and meta-analyses were considered in this study. Studies not following a systematic review approach, narrative reviews and primary studies were excluded. Studies including in this review aimed to investigate the association between Vitamin D and COVID-19 under different social and clinical conditions. Only reviews published in English language were included in this review.
Exclusion Criteria
Reviews not meeting the predefined criteria mentioned above and reviews about other diseases but not including an intervention or with interventions other than Vitamin D supplementation were excluded.
Search Methods for Identification of Studies
Relevant systematic reviews and meta-analyses according to inclusion criteria were identified through systematic searches of the following electronic databases: PubMed, Scopus and the Web of Science. The full search string is [“systematic review” OR “systematic literature review” OR “evidence-based review” OR “meta-analysis” OR “meta-analytic” OR “meta-regression” OR “pooled effect” OR “pooled estimate” OR “scoping review” OR “rapid review” OR “evidence based practice” OR “systematized review” OR “literature review” OR “review of the literature” “Vitamin” AND “D” OR “Cholecalciferol” OR “D3” OR “D2” AND (“COVID” OR “SARS”)]. Studies published from January 2020 until March 2021 were included. Reference lists of identified studies were checked.
Data Collection and Analysis
Titles and abstracts obtained from the search were transferred to a reference site (Covidence – Better systematic review management. https://www.covidence.org/) for relevance assessment. Potentially eligible studies were screened by title and abstract to evaluate if they met the inclusion criteria by three authors (L.M.M., F.V., G.G.) independently. Potentially relevant studies were independently screened through full-text reading by three authors independently (L.M.M., F.V., G.G.) and a decision was made regarding their inclusion. Disagreements were settled by consensus among the authors. Figure 1 shows the review process.
Figure 1.
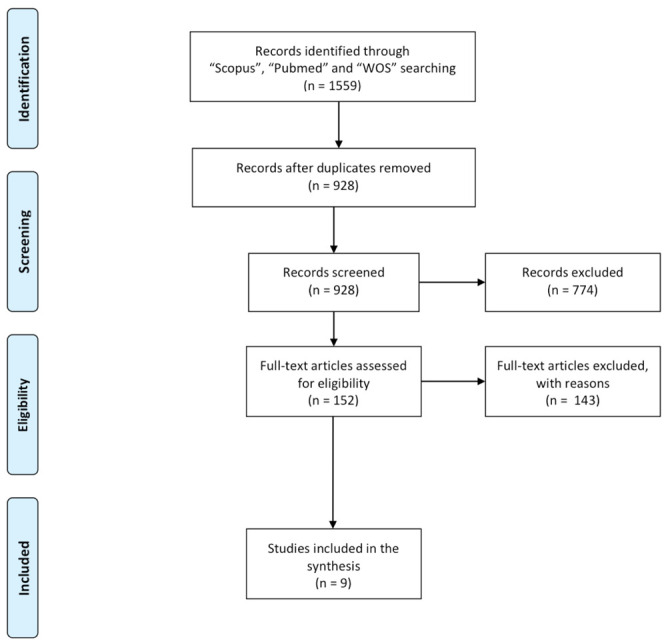
PRISMA flow diagram of the systematic review process.
Data Extraction and Management
Data were independently extracted by two reviewers (L.M.M., F.V.) and the following information was considered for each article: i) first author and month/year of publication, ii) title, iii) study designs (systematic review or meta-analysis) and number and type of studies included, iv) participants’ age, v) brief description of the study and vi) main finding of study.
Quality Assessment Tools
Since flaws in the design, conduct, analysis, and reporting of studies can cause to be under or overestimated, two independent reviewers (L.M.M., F.V.) extracted data on the quality of evidence as well as on the risk of bias. The Covidence – Better systematic review management was used to evaluate the methodological quality and risk of bias of studies included in the systematic review (28). The overall final rate of each systematic review was judged as high, moderate, low, or critically low. Disagreements between the two reviewers (L.M.M, F.V.) were resolved in a consensus meeting.
Results
The electronic search initially resulted in 1559 citations (Figure 1). A total of 1407 studies were excluded after the title and abstract screening and 152 full-text articles were selected and read. A total of 9 papers were included in this umbrella review (29-37). In table 1, the selected papers for this review were shown and all studies were published in 2021 except for one (33), which was published in 2020.
Table 1.
The list of review studies included in the umbrella review.
| Author (Ref) | Year of Publication | Month of Publication |
|---|---|---|
| Akbar et al. (27) | 2021 | Mar |
| Bassatne et al. (28) | 2021 | Jun |
| Dramè et al. (29) | 2021 | Apr |
| Liu et al. (30) | 2021 | Jan |
| Pereira et al. (31) | 2020 | Nov |
| Petrelli et al. (32) | 2021 | Mar |
| Shah et al. (33) | 2021 | Jan |
| Teshome et al. (34) | 2021 | Mar |
| Yisak et al. (35) | 2021 | May |
Table 2 reported the aims of these studies: principally, the purpose was to assess the Vitamin D level and the association with susceptibility, severity, and mortality related to COVID-19 (29-37). The association of Vitamin D and risk of COVID-19 or adverse outcome in people aged 60 years or over was analysed in only three studies (30, 31, 33). Finally, in this umbrella was included 2 systematic reviews (31, 37) and 7 meta-analyses (29, 30, 31-36). At least three databases were used in all reviews, except Dramè, Shah and Yisak that used only two databases (31, 35, 37). There is a heterogeneity in date range of database searching: 4 of 9 papers had set up the data range until December 2020 (29, 30, 35, 36), 4 reviews until September – November 2020 (31-33, 37), and only a paper until 31 January 2021 (34).
Table 2.
Summary of selected article characteristics for the umbrella review regarding aims of included studies, type of review and list and date range of database searching used.
| Author (REF) | Objective/s of study | Type of review | Types of databases | Date range of database searching |
|---|---|---|---|---|
| Akbar et al. (29) | This systematic review and meta-analysis aimed to assess whether low serum 25-hydroxyvitamin D (25-OHD) level is associated with susceptibility to COVID-19, severity, and mortality related to COVID-19. | A systematic review and meta-analysis | PubMed, Scopus, and Embase database | until 9 December 2020 |
| Bassatne et al. (30) | Vitamin D deficiency is associated with an increased risk of COVID-1 9 related health outcomes and that vitamin D supplementation would decrease these risks. | A systematic review and meta-analysis | Medline (OVID), Embase.com, CINAHL (EBSCO), and Cochrane | until December 18th 2020 |
| Dramè et al. (31) | This systematic review aimed to determine whether there is any available evidence on the association between vitamin D deficiency (compared to non-vitamin D deficiency) or vitamin D supplementation (compared to non-vitamin D supplementation), and risk of COVID-19 or adverse outcome, in people aged 60 years or over. | A systematic review | PubMed and Scopus | All publications up to and, including 5 November, 2020, with no specific start date specified |
| Liu et al. (32) | To assess the relationship between low vitamin D status and COVID-19 risk, authors have performed a meta-analysis of published studies to provide a clinical reference. | A systematic review and meta-analysis | PubMed, Embase, and Cochrane Library databases | from database inception to September 25, 2020, |
| Pereira et al. (33) | Systematic review and meta-analysis, we analyze the association between vitamin D deficiency and COVID-19 severity, via an analysis of the prevalence of vitamin D deficiency and insufficiency in people with the disease | Meta-analysis | Embase, PubMed, Scopus, Web of Science, ScienceDirect and pre-print Medrevix were searched. | studies published up to October 9, 2020 |
| Petrelli et al. (34) | The study’s primary outcome was COVID-19 infection risk in vitamin D-deficient vs non deficient patients. Secondary endpoints were severity (intensive care unit and/or mechanical ventilation), death, and therapeutic effect of vitamin D supplementation in COVID-19-affected patients | A systematic review and meta-analysis | PubMed, the Cochrane Library, and EMBASE | studies published until January 31, 2021 |
| Shah et al. (35) | Meta-analysis aims to understand the effect of oral supplementation of vitamin D on intensive care unit (ICU) requirement and mortality in hospitalized COVID-19 patients. | Meta-analysis | PubMed, preprint servers, and google scholar were searched | From December 2019 to December 2020. |
| Teshome et al. (36) | The present review aimed to summarize the available evidence regarding the association between Vitamin D levels and the risk of COVID-19 infection. | A systematic review and meta-analysis | PUBMED/MEDLINE, Cochrane/Wiley library, Scopus, and SciELO) | from May 15, 2020, to December 20, 2020 |
| Yisak et al. (37) | The purpose of this study was to undertake a systematic review to summarize and determine whether there is a relation between vitamin D status and COVID-19 infection and prognosis | A systematic review | PubMed and Google Scholar | search from August 2020 to September 2020 |
In Table 3 the details of these studies were described. The whole of the studies included in each of the considered reviews, showed a wide coverage of geographical areas: in particular 5 works present investigations from at least 5 regions out of 6 of the World Health Organisation (WHO), including European Region, Region of the Americas, South-East Asia Region, Eastern Mediterranean Region, Western Pacific Region (29, 30, 33, 34, 36), while two papers selected investigations from 2 regions out of 6 (European Region and Region of the Americas or Western Pacific Region) (31, 35) and only one work collected studies from 3 regions out of 6 (European Region, Western Pacific Region, South-East Asia Region) (32). No study analysed works from the African Region. Risk of bias assessment was performed by two independent authors in all papers (29-37).
Table 3.
Summary of selected article characteristics for the umbrella review regarding the number and type of included study in each review, WHO Regions and instrument used to appraise the primary studies and the rating of quality.
| Author (REF) | Number of included study | WHO Regions Included in these studies |
Type of studies | Instrument used to appraise the primary studies and the raiting of quality |
|---|---|---|---|---|
| Akbar et al. (29) | 14 | 5/6 (European Region,Region of the Americas, South-East Asia Region,Eastern Mediterranean, Region Western Pacific Region) |
2 Cross-Sectional, 1 Observational 2 Prospective Observational, 1 Restrospective Observational (case-control), 8 Retrospective observational | This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline. Risk of bias assessment was performed by two independent authors using the Newcastle-Ottawa Scale (NOS). |
| Bassatne et al. (30) | 34 | 5/6 (European Region, Region of the Americas, South-East Asia Region, Eastern Mediterranean, Region Western Pacific Region) |
31 obsservatory studies 3 RCT |
The New Castle-Ottawa quality scale for observational studies and the Cochrane Risk of bias tool, version 1 for RCT. |
| Dramè et al. (31) | 11 | 2/6 (European Region, Western Pacific Region) |
4 Prospective cohort 1 Retrospective case control 6 Retrospective cohort | Study selection was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. The quality of included studies was assessed independently by two researchers (LG MD) using the Newcastle–Ottawa scale (NOS) for cohort studies and a modified version of the Newcastle–Ottawa scale for cross-sectional studies. |
| Liu et al. (32) | 10 | 3/6 (European Region, Western Pacific Region, South-East Asia Region) |
Case control studies | The methodological quality of the included study was assessed by the modified Newcastle-Ottawa scale (NOS) |
| Pereira et al. (33) | 27 | 5/6 (European Region Region of the Americas, South-East Asia Region, Eastern Mediterranean, Region Western Pacific Region) |
8 cohort studies 1 Retrospective multicenter 6 retrospective 2 retrospective cross-sectional 4 cross-sectional 2 retrospective cohort 1 population basic 1 case series 1 clinical retrospective 1 prospective cohort |
Methodological quality was assessed according to the Research Triangle Institute Item Bank (RTI–Item Bank) scale, which assesses the risk of bias. |
| Petrelli et al. (34) | 43 | 5/6 (European Region Region of the Americas, South-East Asia Region, Eastern Mediterranean, Region Western Pacific Region) |
2 Case-control 1 Cross-sectional 1 Observational 1 Population-based study 5 Prospective 2 Prospective cohort 2 Prospective observational 1 Randomized 1 Registry data 21 Retrospective 2 Retrospective case-control 1 Retrospective cross-sectional 3 Retrospective observational |
The study adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines. Risk of bias assessment was performed by two independent authors using the Newcastle-Ottawa Scale (NOS). |
| Shah et al. (35) | 3 | 2/6 (European Region, Region of the Americas) |
2 randomized controlled trials 1 retrospective case-control study | Systematic review and meta-analysis were conducted in accordance with the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Cochrane RevMan tool was used for quantitative assessment of the data. For the observational study “Risk of Bias Assessment tool for Nonrandomized Studies (RoBANS)” for the controlled observational studies, as recommended by the Cochrane Handbook for Systematic Reviews of Interventions was used. |
| Teshome et al. (36) | 14 | 5/6 (European Region Region of the Americas, South-East Asia Region, Eastern Mediterranean, Region Western Pacific Region) |
3 Cross-sectional 5 Case-control 5 Cohort studies 1 Interim audit |
JBI tools |
| Yisak et al. (37) | 9 | 2/6 (European Region Region of the Americas) |
4 cross-sectional 1 randomized controlled trial 1 retrospective cohort 1 prospective cohort 1 retrospective, observational study 1 case- controlled survey |
To determine the quality of studies included in this review, the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system was used. |
In table 4, the main findings in all studies were described. Akbar et al. summarized 14 papers, including cross-sectional, prospective, and retrospective studies, and reported that the higher rate of severe COVID-19 was observed in patients with serum 25-OHD level below a cut-off point ranging from 20 to 30 ng/mL (Odd Ratio (OR)= 1.90 (1.24 to 2.93)). Moreover, low serum 25-OHD was associated with higher mortality (OR = 3.08 (1.35 to 7.00)) (29). Bassatne et al. (30) selected 34 papers (31 peer-reviewed observational studies and 3 Randomized Control Trials - RCT). The results of observational studies showed a positive trend between serum 25(OH)D level <20 ng/ml and an increased risk of mortality (Relative Risk (RR) = 2.09 (0.92 to 4.77)), Intensive Care Unit (ICU) admission (RR= 4.89 (0.54 to 44.26)), invasive ventilation (RR= 1.34 (0.64 to 2.79)), non-invasive ventilation (RR= 1.08 (0.30 to 3.80)) or SARS-CoV-2 positivity (RR= 1.35 (0.93 to 1.96)). However, these associations are not statistically significant. In this paper, also three RCTs were investigated. The first trial is a pilot study where the treatment group received 0.532 mg of calcifediol on admission, 0.266 mg on days 3 and 7, and then weekly. The OR ratio of ICU admission in patients with calcifediol treatment v/s those with no treatment was 0.03 (0.003–0.25). The second RCT is small non-registered trial from India, randomized COVID-19 patients with vitamin D deficiency (25(OH)D < 20 ng/ml) to receive either 60,000 IU/day of cholecalciferol or placebo for 7 days. At the 14 days followed up, 62.5% of the participants in the intervention arm became SARS-CoV-2 negative compared to only 20.8% in the control arm. The third trial did not find any effect of vitamin D supplementation on COVID-19 related health outcomes. Although the risk of bias was low in most of the items assessed, the overall risk of bias in this study was unclear due to the lack of description of the allocation concealment method.
Table 4.
Summary of finding articles selected for the umbrella review.
| Author (REF) | Results/Finding | Vitamin D cut-off (ng/mL) |
|---|---|---|
| Akbar et al. (29) | This meta-analysis indicates that low serum 25-OHD levels were associated with higher infection, severe COVID-19, and mortality rate. | <20-30 ng/ml |
| Bassatne et al. (30) | This systematic review and meta-analysis reveal very uncertain evidence for an association between serum 25(OH)D levels <20 ng/ml and risk of mortality, ICU admission, mechanical ventilation, non-invasive ventilation and testing positive for SARS-CoV-2. However, serum 25(OH)D levels were 6 ng/ml lower in COVID-19 patients as compared to those without COVID-19 infection, this difference was significant. | <20 ng/ml |
| Dramè et al. (31) | This systematic review supports an association between vitamin D deficiency and the risk of COVID-19 in aged people. In addition, vitamin D deficiency appears to expose these subjects to a greater risk of adverse outcomes. Because of its simplicity of administration, and the rarity of side effects, including vitamin D in preventive strategies for certain viral diseases, it appears to be an attractive option. | <20 ng/ml |
| Liu et al. (32) | This systematic review and meta-analysis indicated that low vitamin D status might be associated with an increased risk of COVID-19 infection. | < 25 ng/ml |
| Pereira et al. (33) | This systematic review observes a positive association between vitamin D deficiency and the severity of the disease. | ND |
| Petrelli et al. (34) | Reduced vitamin D values resulted in a higher infection risk, mortality and severity COVID-19 infection. Supplementation may be considered as preventive and therapeutic measure. | <20 ng/ml |
| Shah et al. (35) | Final meta-analysis involved pooled data of 532 hospitalized patients (189 on vitamin D supplementation and 343 on usual care/placebo) of COVID-19 from three studies. Statistically (p<0.0001) lower ICU requirement was observed in patients with vitamin D supplementation as compared to patients without supplementations (odds ratio: 0.36; 95% CI: 0.210-0.626). | ND |
| Teshome et al. (36) | This review showed that improving vitamin D status in the general population has a potential benefit in reducing the risk of acquiring COVID-19 infection. | <20 ng/mL |
| Yisak et al. (37) | This review shows that blood vitamin D status can determine the risk of being infected with COVID-19, seriousness of COVID-19, and mortality from COVID-19. | <20 ng/ml |
Dramè et al. summarized 11 papers, including 7 retrospective and 4 prospective studies (31). 4/11 studies compared vitamin D-supplemented patients to non-supplemented patients, while 7/11 compared patients with vs without vitamin D deficiency. In all four studies, patients with vitamin D supplementation had better rates of primary clinical outcomes (e.g., death, the severity of the disease, oxygen therapy requirement). However, the ideal supplementation regimen (dose, frequency of administration, duration) remains unclear. Moreover, in the seven studies that compared patients with vs without vitamin D deficiency, serum vitamin D level was significantly higher in COVID-19-negative patients compared to COVID-19-positive patients.
Liu et al. selected a total of 10 articles (32). Overall, the pooled OR in the fixed-effect model showed that vitamin D deficiency (< 25 ng/ml) or insufficiency was associated with an increased risk of COVID-19 (OR = 1.43 (1.00–2.05)). In addition, COVID-19-positive individuals had lower vitamin D levels than COVID-19-negative individuals (Standardized Mean Difference (SMD) = -0.37 (-0.52 to -0.21)).
Pereira et al. included in this review 27 papers (33). The main outcome of this papers was the prevalence of vitamin D deficiency in severe cases of COVID-19. Vitamin D deficiency was little associated with a higher chance of infection by COVID-19 (OR = 1.35 (0.80–1.88)) but linked to severe cases of COVID-19 (OR = 1.64 (1.30–2.09)), where il 64% of cases had the vitamin D deficiency compared with mild cases. Moreover, a vitamin D concentration insufficiency increased hospitalization (OR = 1.81 (1.41–2.21)) and mortality from COVID-19 (OR = 1.82 (1.06–2.58)).
Petrelli et al. selected 43 papers for qualitative synthesis and 17 for quantitative synthesis (34). An OR > 1 was associated with the worst outcome in deficient compared with nondeficient patients. Among subjects with deficient vitamin D values, risk of COVID-19 infection was higher compared to those with replete values (OR = 1.26 (1.19–1.34)). Vitamin D deficiency (<20 ng/ml) was also associated with worse severity and higher mortality than in nondeficient patients (OR = 2.6 (1.84–3.67) and OR = 1.22 (1.04–1.43), respectively).
Shah et al. selected for final synthesis 3 papers, including 2 RCTs and 1 retrospective case-control study (35). The results of meta-analysis involved pooled data of 532 hospitalized patients (189 on vitamin D supplementation and 343 on usual care/placebo) of COVID-19 from three studies (two randomized controlled trials, one retrospective case-control study). Statistically (p<0.0001) lower ICU requirement was observed in patients with vitamin D supplementation as compared to patients without supplementations (OR = 0.36 (0.210-0.626)). In case of mortality, vitamin D supplements had comparable findings with placebo treatment/usual care (OR = 0.93 (0.413-2.113)). Subgroup analysis could not be performed due to limited number of studies and hence dose and duration dependent effect of vitamin D could not be evaluated.
Teshome et al. have selected 14 papers that met the inclusion criteria (36). The qualitative synthesis indicated that vitamin D deficient individuals were at higher risk of COVID-19 infection as compared to vitamin D sufficient patients. The pooled analysis showed that individuals with Vitamin-D deficiency were 80% more likely to acquire COVID-19 infection as compared to those who had sufficient Vitamin D levels (OR = 1.80 (1.72 - 1.88)).
Yisak et al. included 9 studies in the review (37). Risk Factors of COVID-19 Infection, Severity, and Mortality data from COVID-19 patients were associated with vitamin D low level (<20 ng/ml) (OR = 1.77 (1.07– 2.93)). In addition, disease severity was associated with vitamin D deficiency (OR = 1.95 (1.07– 3.56)), and odds of admission to ICU were higher in vitamin D–deficient individuals (OR = 2.55 (1.28–5.08)).
Finally, 7 papers out of 9 defined Vitamin D cut-off, expressed in ng/mL, below which the vitamin D level can be considered insufficient (29-32, 34, 36, 37). In 5 reviews the cut off is <20 ng/ml (28, 29, 32, 34, 35), in one <20-30 ng/ml (29), while in another is < 25 ng/ml (32).
The methodological quality of included reviews was generally high. Six studies were judged to be of high quality (29-32, 36), 2 of moderate quality (35, 37) and 1 studies of low quality (34). The systematic review and metanalysis conducted by Petrelli and colleagues (34) failed to meet nearly 5, 6 and 7 items of AMSTAR, perhaps due to a lack of clarifications in the description of the article. Findings of the quality assessment were presented in Supplemental Table S1.
Supplemental Table S1.
Quality assessment by AMSTAR2
| Author | Values* of the items of AMSTAR 2** | Final rate*** | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Ref) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Akbar et al. (29) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | High |
| Bassatne et al. (30) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | High |
| Dramè et al. (31) | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | NA | NA | NA | NA | NA | NA | NA | High |
| Liu et al. (32) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | High |
| Pereira et al. (33) | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | High |
| Petrelli et al. (34) | 1 | 2 | 2 | 2 | / | / | / | 2 | 1 | NA | 1 | 1 | 2 | 2 | 2 | NA | Low |
| Shah et al. (35) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | Moderate |
| Teshome et al. (36) | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | NA | 2 | 2 | 2 | 2 | 2 | NA | High |
| Yisak et al. (37) | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | NA | NA | NA | NA | NA | NA | NA | Moderate |
* Quantitative value of each item: “NA” non applicable, “/” no, “1” partial yes, ”2” yes
- Did the research questions and inclusion criteria for the review include the components of PICO?
- Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any
- Did the review authors explain their selection of the study designs for inclusion in the review?
- Did the review authors use a comprehensive literature search strategy?
- Did the review authors perform study selection in duplicate?
- Did the review authors perform data extraction in duplicate?
- Did the review authors provide a list of excluded studies and justify the exclusions?
- Did the review authors describe the included studies in adequate detail?
- Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review?
- Did the review authors report on the sources of funding for the studies included in the review?
- If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results?
- If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?
- Did the review authors account for RoB in individual studies when interpreting/ discussing the results of the review?
- Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?
- If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?
- Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
*** Final rate evaluation
High - No or one non-critical weakness: the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest
Moderate - More than one non-critical weakness*: the systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review
Low - One critical flaw with or without non-critical weaknesses: the review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest
Critically low - More than one critical flaw with or without non-critical weaknesses: the review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
Discussion
The different approaches to cope with COVID-19 pandemic raised several discussions on the optimal strategies to apply for contrasting infection disease spreading in the population and provide appropriate treatments and effective prevention measures. Although interpersonal distancing, masks, contact tracing and vaccination remain the main tools to protect the population from SARS-CoV-2 infection, additional approaches were considered (42-46). Within this context several studies highlighted also the potential benefits of vitamin D supplementation in preventing and treating infection diseases and in particular COVID-19 (20-23). Vitamin D is a lipid-soluble vitamin that is essential for maintaining good health, growth, and strong bones (5, 7-9). The effect of vitamin D on infections is less clear, however, several studies have underlined that the sufficient blood vitamin D levels seems to play a helpful role in immune system functioning, as cellular response, and protection against the severity of infections caused by microorganisms (38-46). Moreover, several investigations have highlighted the role of Vitamin D levels versus severe COVID-19 raising discussions about the benefits of supplementation of this vitamin when treating the illness caused by SARS-CoV-2, as well as in their prevention (47). However, the studies on vitamin D in COVID-19 patients have shown conflicted results and even if several systematic reviews and metanalysis have been published they are affected by heterogeneity regarding methodologies, criteria, and the different population target (48). Therefore, the purpose of this umbrella review was to summarize and comprehensively review systematic reviews and meta-analyses on whether low levels of serum 25-OHD are associated with susceptibility to COVID 19, severity, and mortality related to COVID-19. This is the first umbrella review applying the principles of evidence-based medicine to find out a possible role of vitamin D in prevention of COVID-19. A total of 9 studies were selected including investigations in different countries. Here, we summarized investigations covering all WHO regions, with the only exception of the African region, to have a wider view of the world population and reduce possible bias related to local features. Indeed, the levels of vitamin D in serum and their absorption and production may depend by several variables, including genetic determinants (49, 50), demographic factors, as age or gender (51-53), lifestyles (54-59), seasonal and geographical variations (60-62). The results of this study support the observation that vitamin D deficiency can present an association with COVID-19 severity, independently from the WHO macro-regions where the data were collected. The findings of metanalysis that include populations from 5 WHO regions show that low serum 25-OHD levels are associated with higher infection risks, more severe COVID-19 outcomes, and mortality rates. Moreover, the low levels of Vitamin D are also correlated with the need of ICU admission and mechanical ventilation. (30, 34, 36). The whole of these results supports the role of vitamin D in contrasting COVID-19 infection and the need of supplementation and adequate diets to prevent the disease and its consequences. Our final results are in agreement with previous reports that suggest how vitamin D may play a role in SARS-CoV-2 infection (63, 64). From a mechanistic viewpoint, the hypothesis that vitamin D can modulate host responses to SARS-CoV-2 has relevant plausibility, as many potential links can be considered between respiratory viral infections such as COVID-19 and vitamin D status (65, 66).
Some studies in this umbrella have investigated the association between vitamin D3 and COVID-19 infection risk and severity (30, 34, 37). Although the priority of some systematic review approaches was to include a higher number of studies and enhance the stringency of the analysis by excluding preprint articles that had not been peer reviewed yet and systematically avoiding those investigations with major confounders or a higher risk of bias, studies that have investigated the association between severity and Vitamin D supplementation does not seem conclusive. Particularly, we found low certainty of evidence from RCTs on the use of vitamin D supplementation and reduction of severity of COVID-19, suggesting that further studies are needed to extend this conclusion to COVID-19 patients and its reduction in mortality rate. This observation can be explained from the limitations in several study designs that collected patients with different degree of severity of the disease, age or other demographic and medical conditions that lead some troubles and complications in the interpretations.
The present study selected, with rigorous methodological appraisal, 9 systematic reviews with several study design and collection of different primary papers. Among the results, a critical point seems to be the definition of vitamin D deficiency or of insufficient level and vitamin D cut-off. In the literature, this question is often discussed, but there are still disagreements about the optimal serum level for 25-hydroxyvitamin D (25(OH)D) and the appropriate supplemental dose (67). We have further searched among the analysed reviews the optimal cut off below which we can say that the level is insufficient. In five of the reviews the cut off is <20 ng/ml (30, 31, 34, 36, 37), while in two is slightly higher different <20-30 ng/ml (29), and < 25 ng/ml (32). This finding is in accordance with the considerable evidence that is available to support a final threshold for sufficiency at least over 20 ng/ml or even 50 nmol/L as indicated by some consensus documents and health authorities (68-73).
With respect to the methodological quality assessment, more than 77 % of the included studies were classified as having a high methodological quality. Most studies missed only item number one, which concerns the inclusion of the components of the PICO process. Some systematic reviews, in fact, have not established some of the specified inclusion criteria, especially the target population (29, 32, 34-37). However, this can, also, be considered as a choice of the study design in order to acquire just a general representation of the population. Furthermore, most of the reviewed studies did not explain or only partially explain the risk of bias (items number 5-7, 9, 11 and 12) or did not consider the impact of bias in the paper discussion. These aspects are fundamental for the quality of a review since they strongly depend on the quality of the primary evidence and most papers have comprehended this aspect, further supporting the stringent selection and elevated level of studies included in this umbrella analysis. However, our study has both strengths and limitations that should be acknowledged when interpreting the results. The main strength of this umbrella review is that it provides a systematic synthesis of studies that involved population on a global scale, within the 5 WHO regions. Moreover, a rigorous quality assessment was performed using the latest version of an effective and recognised tool to assess systematic reviews, namely the AMSTAR 2. Main limits of the work involve the considerable heterogeneity in the study designs included in each single review, and the types of strategies for the interventions, including duration, outcomes, methodology, and other measures whose difference may confound an homogeneous synthesis and comparison of collected data. This umbrella review summarized available evidences for a possible role of Vitamin D as a tool in prevention of COVID-19 and its severity. Recent in vitro study further confirms this hypothesis showing how vitamin D3 is effective in slowing SARS-CoV-2 replication in human cells (74). The whole of the observed results strongly welcomes further epidemiological studies and randomized clinical trials, to confirm potentials for prevention, unravel mechanisms and optimize Vitamin D supplementation as an additional and promising tool in coping with SARS-COV-2 pandemic.
Conclusion
The recent literature contains contrasting data opening debates about the optimal concentrations of vitamin D and related guidelines for application in dietary supplementation. Within this context, this umbrella study overviews evidences related to a potential role of vitamin D deficiency in COVID-19 severity. Collected data support the association between Vitamin D supplementation and the reduction in the COVID-19 gravity outcomes. Additional and more robust data from randomized controlled trials are needed to substantiate also possible effects on a decrease in COVID-19 mortality. The overall results support the need for further epidemiological studies and clinical trials, in order to strengthen knowledge about possible therapeutic uses, biological mechanisms and optimization of Vitamin D posology. In addition, innovative public health programs and health education campaigns, are desirable to optimize the widespread deficiency of vitamin D in the populations. This umbrella review approached the state of the art summarizing evidences and supporting a possible role of Vitamin D in prevention of COVID-19 and its severity.
Acknowledgements:
The authors would like to thank Named srl for supporting publication costs.
Conflict of Interest:
Each author declares no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Funding:
Not applicable: only internal resources of University of Rome “Foro Italico” and no external funds were used for this umbrella review.
References
- Daneshkhah A, Agrawal V, Eshein A, et al. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin Exp Res. 2020;32:2141–2158. doi: 10.1007/s40520-020-01677-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips P. Vitamin D to prevent acute respiratory infections. Lancet Diabetes Endocrinol. 2021;9(5):249–251. doi: 10.1016/S2213-8587(21)00075-9. [DOI] [PubMed] [Google Scholar]
- Mandal AKJ, Baktash V, Hosack T, Missouris CG. Vitamin D status and COVID-19 in older adults. Aging Clin Exp Res. 2020;32:2425–2426. doi: 10.1007/s40520-020-01716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino S, Gallo C, Zippi M, et al. Cytokine storm in aged people with CoV-2: possible role of vitamins as therapy or preventive strategy. Aging Clin Exp Res. 2020;32:2115–2131. doi: 10.1007/s40520-020-01669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzke F, Bechthold A, Egert S, et al. Role of vitamin D in preventing and treating selected extraskeletal diseases—an umbrella review. Nutrients. 2020;12:969. doi: 10.3390/nu12040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NC, Biver E, Kaufman JM, et al. The role of calcium supplementation in healthy musculoskeletal ageing: an expert consensus meeting of the European Society for Clinical and Economic Aspects of Osteoporosis. Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Foundation for Osteoporosis (IOF) Osteoporos Int. 2017;28:447–462. doi: 10.1007/s00198-016-3773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrobot A, Demkow U, Wachowska M. Immunomodulatory role of vitamin D: a review. Adv Exp Med Biol. 2018;1108:13–23. doi: 10.1007/5584_2018_246. [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH Randomized Clinical Trial. JAMA J Am Med Assoc. 2020;324:1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimaleswaran KS, Berry DJ, Lu C, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnik T, Czarnik A, Gawda R, et al. Vitamin D kinetics in the acute phase of critical illness: a prospective observational study. J Crit Care. 2018;43:294–299. doi: 10.1016/j.jcrc.2017.09.179. [DOI] [PubMed] [Google Scholar]
- Harvey NC, Cooper C. Vitamin D: Some perspective please. BMJ. 2012;345:4695. doi: 10.1136/bmj.e4695. [DOI] [PubMed] [Google Scholar]
- Vuichard Gysin D, Dao D, Gysin CM, et al. Effect of vitamin D3 supplementation on respiratory tract infections in healthy individuals: a systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2016;11:0162996. doi: 10.1371/journal.pone.0162996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and metaanalysis of individual participant data. BMJ. 2017;356:6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:120–30. doi: 10.1016/S2213-2600(14)70255-3. [DOI] [PubMed] [Google Scholar]
- Camargo CA Jr, Toop L, Sluyter J, Lawes CMM, Waayer D, Khaw KT, Martineau AR, Scragg R. Effect of Monthly Vitamin D Supplementation on Preventing Exacerbations of Asthma or Chronic Obstructive Pulmonary Disease in Older Adults: Post Hoc Analysis of a Randomized Controlled Trial. Nutrients. 2021;13(2):521. doi: 10.3390/nu13020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham-New SA, Webb AR, Cashman KD, et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutr Prev Health. 2020;13:106–110. doi: 10.1136/bmjnph-2020-000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok CK, Ng YL, Ahidjo BA, et al. Calcitriol, the active form of vitamin D, is a promising candidate for COVID-19 prophylaxis. bioRxiv. 2000 2020.06.21.162396. [Google Scholar]
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195–1198. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avolio A, Avataneo V, Manca A, et al. 25-hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients. 2020;12:1359. doi: 10.3390/nu12051359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli R. Vitamin D supplementation: upper limit for safety revisited? Aging Clin Exp Res. 2021;33:19–24. doi: 10.1007/s40520-020-01678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrand S. Autumn COVID-19 surge dates in Europe correlated to latitudes, not to temperature-humidity, pointing to vitamin D as contributing factor. Sci Rep. 2021;11:1981. doi: 10.1038/s41598-021-81419-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Hewison M, Hopkin J, Kenny RA, Quinton R, Rhodes J, Subramanian S, Thickett D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clin Med (Lond) 2021;21(1):48–51. doi: 10.7861/clinmed.2020-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid. Based Health. 2015;13:132–140. doi: 10.1097/XEB.0000000000000055. [DOI] [PubMed] [Google Scholar]
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front Nutr. 2021;8:660420. doi: 10.3389/fnut.2021.660420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, El-Hajj Fuleihan G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. 2021;119:154753. doi: 10.1016/j.metabol.2021.154753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramé M, Cofais C, Hentzien M, Proye E, Coulibaly PS, Demoustier-Tampère D, Destailleur MH, Lotin M, Cantagrit E, Cebille A, Desprez A, Blondiau F, Kanagaratnam L, Godaert L. Relation between Vitamin D and COVID-19 in Aged People: A Systematic Review. Nutrients. 2021;13(4):1339. doi: 10.3390/nu13041339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Sun J, Wang X, Zhang T, Zhao M, Li H. Low vitamin D status is associated with coronavirus disease 2019 outcomes: a systematic review and meta-analysis. Int J Infect Dis. 2021;104:58–64. doi: 10.1016/j.ijid.2020.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Dantas Damascena A, Galvão Azevedo LM, de Almeida Oliveira T, da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;4:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: A systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol. 2021;211:105883. doi: 10.1016/j.jsbmb.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K, Saxena D, Mavalankar D. Vitamin D supplementation, COVID-19 and disease severity: a meta-analysis. QJM. 2021;114(3):175–181. doi: 10.1093/qjmed/hcab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshome A, Adane A, Girma B, Mekonnen ZA. The Impact of Vitamin D Level on COVID-19 Infection: Systematic Review and Meta-Analysis. Front Public Health. 2021;9:624559. doi: 10.3389/fpubh.2021.624559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yisak H, Ewunetei A, Kefale B, Mamuye M, Teshome F, Ambaw B, Yideg Yitbarek G. Effects of Vitamin D on COVID-19 Infection and Prognosis: A Systematic Review. Risk Manag Healthc Policy. 2021;14:31–38. doi: 10.2147/RMHP.S291584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. Journal of Infection and Public Health. 2020;13(10):1373–80. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dini C, Bianchi A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Ann Ist Super Sanità. 2012;48(3):319–327. doi: 10.4415/ANN_12_03_13. [DOI] [PubMed] [Google Scholar]
- Jiménez-Sousa M, Martínez I, Medrano LM, Fernández-Rodríguez A, Resino S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front Immunol. 2018;9:458. doi: 10.3389/fimmu.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23(2) doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DK, Akl EA, Duda S, Solo K, Yaacoub S, Schünemann HJ. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet (London, England) 2020;395(10242):1973–1987. doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8:434–436. doi: 10.1016/S2213-2600(20)30134-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Infection prevention and control guidance - (COVID-19) Available online: https://www.who.int/westernpacific/emergencies/covid-19/technical-guidance/infection-prevention-control. (accessed on 8 August 2021) [Google Scholar]
- Ford N, Holmer HK, Chou R, Villeneuve PJ, Baller A, Van Kerkhove M, Allegranzi B. Mask use in community settings in the context of COVID-19: A systematic review of ecological data. EClinicalMedicine. 2021;38:101024. doi: 10.1016/j.eclinm.2021.101024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girum T, Lentiro K, Geremew M, Migora B, Shewamare S, Shimbre MS. Optimal strategies for COVID-19 prevention from global evidence achieved through social distancing, stay at home, travel restriction and lockdown: a systematic review. Archives of public health - Archives belges de sante publique. 2021;79(1):150. doi: 10.1186/s13690-021-00663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speeckaert MM, Delanghe JR. Association between low vitamin D and COVID-19: Don’t forget the vitamin D binding Pprotein. Aging Clinical and Experimental Research. 2020;32(7):1207–8. doi: 10.1007/s40520-020-01607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha AP, Atallah AN, Aldrighi JM, Pires A, Dos Santos Puga ME, Pinto A. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. International journal of clinical practice. 2021:14649. doi: 10.1111/ijcp.14649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Chen X, Hou L, Yue J, Liu X, Wang Y, Xia X, Dong B. The Relationship between Sarcopenia and Vitamin D Levels in Adults of Different Ethnicities: Findings from the West China Health and Aging Trend Study. The journal of nutrition, health & aging. 2021;25(7):909–913. doi: 10.1007/s12603-021-1645-z. [DOI] [PubMed] [Google Scholar]
- Arabi A, Baddoura R, El-Rassi R, El-Hajj FG. Age but not gender modulates the relationship between PTH and vitamin D. Bone. 2010;47:408–412. doi: 10.1016/j.bone.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, Vestergaard P. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009;20:133–140. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- Gharaibeh MA, Stoecker BJ. Assessment of serum 25(OH)D concentration in women of childbearing age and their preschool children in Northern Jordan during summer. Eur J Clin Nutr. 2009;63:1320–1326. doi: 10.1038/ejcn.2009.99. [DOI] [PubMed] [Google Scholar]
- Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra SH, van BA, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Arch Dis Child. 2007;92:750–753. doi: 10.1136/adc.2006.105577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): National Academies Press (US); 2011. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D. [PubMed] [Google Scholar]
- van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- Scully H, Laird E, Healy M, Walsh JB, Crowley V, McCarroll K. Geomapping Vitamin D Status in a Large City and Surrounding Population-Exploring the Impact of Location and Demographics. Nutrients. 12(9):2663. doi: 10.3390/nu12092663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Costa MF, Mambrini J, de Souza-Junior P, de Andrade FB, Peixoto SV, Vidigal CM, de Oliveira C, Vidigal PG. Nationwide vitamin D status in older Brazilian adults and its determinants: The Brazilian Longitudinal Study of Aging (ELSI) Scientific reports. 2020;10(1):13521. doi: 10.1038/s41598-020-70329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedhain A, Bhattarai GR, Yadav SR, Pandey BR, Pant TP. Geographic and Seasonal Variation of Vitamin D: A Retrospective Study in Two Centers of Nepal. Journal of Nepal Health Research Council. 2020;18(1):103–107. doi: 10.33314/jnhrc.v18i1.1873. [DOI] [PubMed] [Google Scholar]
- Quesada-Gomez JM, Entrenas-Castillo M, Bouillon R. Vitamin D receptor stimulation to reduce acute respiratory distress syndrome (ARDS) in patients with coronavirus SARS-CoV-2 infections: Revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol. 2020;202:105719. doi: 10.1016/j.jsbmb.2020.105719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardizzone S, Cassinotti A, Trabattoni D, et al. Immunomodulatory effects of 1,25-dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol. 2009;22:63–71. doi: 10.1177/039463200902200108. [DOI] [PubMed] [Google Scholar]
- Isaia G, Medico E. Associations between hypovitaminosis D and COVID-19: a narrative review. Aging Clin Exp Res. 2020;32(9):1879–1881. doi: 10.1007/s40520-020-01650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Hewison M, Hopkin J, Kenny RA, Quinton R, Rhodes J, Subramanian S, Thickett D. Preventing vitamin D deficiency during the COVID-19 pandemic: UK definitions of vitamin D sufficiency and recommended supplement dose are set too low. Clinical medicine (London, England) 2021;21(1):48–e51. doi: 10.7861/clinmed.2020-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466–79. doi: 10.1038/nrendo.2017.31. [DOI] [PubMed] [Google Scholar]
- Giustina A, Adler RA, Binkley N, et al. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev Endocr Metab Disord. 2020;21:89–116. doi: 10.1007/s11154-019-09532-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Advisory Committee on Nutrition Vitamin D and Health. SACN, 2016. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf. (accessed on 8 August 2021) [Google Scholar]
- London: The Stationery Office; 1998. Department of Health Nutrition and Bone health: with particular reference to calcium and vitamin D. Report on the Subgroup on Bone Health, Working Group on the Nutritional Status of the Population of the Committee on Medical Aspects of Food and Nutrition Policy. [PubMed] [Google Scholar]
- London: The Stationery Office; 1991. Department of Health Dietary reference values for food energy and nutrients for the United Kingdom. [Google Scholar]
- Wakeman M. A Literature Review of the Potential Impact of Medication on Vitamin D Status. Risk Manag Healthc Policy. 2021;14:3357–3381. doi: 10.2147/RMHP.S316897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard A, Calverley BC, Chang J, Garva R, Gago S, Lu Y, Kadler KE. Discovery of re-purposed drugs that slow SARS-CoV-2 replication in human cells. PLoS Pathog. 2021;17(9):1009840. doi: 10.1371/journal.ppat.1009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix


