Abstract
Background and aim:
The exact COVID-19 severity is still not well defined and it is hotly debated due to a few methodological issues such as the uncertainties about the spread of the SARS-CoV-2 infection.
Methods:
We investigated COVID-19 case-fatality rate and infection-fatality rate in 2020 in Italy, a country severely affected by the pandemic, basing our assessment on publicly available data, and calculating such measures during the first and second waves.
Results:
We found that province-specific crude case-fatality rate in the first wave (February-July 2020) had a median value of 12.0%. Data about infection-fatality rate was more difficult to compute, due to large underestimation of SARS-CoV-2 infection during the first wave when asymptomatic individuals were very rarely tested. However, when using reference population-based seroprevalence data for anti-SARS-CoV-2 antibodies collected in May-July 2020, we computed an infection-fatality rate of 2.2%. During the second wave (Sep-Dec 2020), when SARS-CoV-2 testing was greatly increased and extended to many asymptomatic individuals, we could only compute a ‘hybrid’ case/infection-fatality rate with a value of 2.2%, similar to the infection-fatality rate of the first wave.
Conclusions:
Overall, this study allowed to assess the COVID-19 case- and infection-fatality rates in Italy before of variant spread and vaccine availability, confirming their high values compared with other airborne infections like influenza. Our findings for Italy were similar to those characterizing other Western European countries.
Keywords: COVID-19, case-fatality rate, epidemiology, infection-fatality rate, outbreak, public health, SARS-CoV-2, seroprevalence, wave
Introduction
The COVID-19 pandemic is one of the greatest medical challenges of the last century (1), especially for the possible clinical presentation as a severe and life-threatening disease (2), with limited therapeutic options, and long-term sequelae (3-5). Since the beginning, attempts to control the pandemic spread relied on public health measures such as social distancing, contact tracing, use of face masks and other protective gears (like googles or face shields in health care settings), and lockdowns with limitations of population mobility (6-10). Still in recent months that vaccines are available, the presence of virus variants and the possibility of reinfection are of great concern (11-13).
Italy was the first Western country severely hit by the pandemic, with a widespread population involvement, especially in the North of the country during the first wave (14). Factors associated with increased susceptibility to COVID-19 onset and severity, following the infection with SARS-CoV-2, have been shown to be male sex, and presence of a comorbidity such as hypertension, diabetes, cardiovascular disease, or chronic lung disease (15,16). Also, environmental factors may play a role increasing COVID-19 susceptibility and severity (17-21) as also reported in previous studies carried out in Northern Italy suggesting a positive association between air pollutant levels with both SARS-CoV-2 incidence and COVID-19 mortality (22-24).
During the 2020, Italy experienced two pandemic waves. The first wave started with the first diagnosed case on February 20, 2020 and lasted till end of June 2020, leading to the implementation of a tight lockdown from March 8 to May 4, 2020 (6). After a brief summer period characterized by light restrictions due to the very low number of newly diagnosed cases, from September 2020 cases rapidly increased again, leading to a second, lighter lockdown from November 6, 2020 (25). Since begin of the vaccination campaign in a few subjects in December 27, 2020 and during the 2021, Italy has experienced a subsequent third wave in February-May 2021 and a fourth one in the most recent period, although the last ones have been largely mitigated by the growing number of vaccinated people (26).
Due to lack of a large availability of SARS-CoV-2 swab tests during the first wave, SARS-CoV-2 infection testing was limited to subjects with symptoms potentially related to COVID-19 as well to health professionals (27-29). From the end of the first lockdown, availability of SARS-CoV-2 swab tests greatly increased and, therefore, testing has been extended to asymptomatic and pauci-symptomatic subjects (30). In particular, drive-through facilities have been implemented in several Italian cities with up to 1000 daily tests (31). For this reason, number of SARS-CoV-2 infections was certainly underestimated during the first wave (14), as also confirmed by the nationwide seroprevalence data made available by the National Institute of Statistics based on a population-based survey conducted in May-July 2020 (32). As consequence, there are uncertainties and controversies about the severity of COVID-19 and namely its case-fatality rate (number of COVID-19 deaths divided by COVID-19 cases, i.e. the symptomatic subjects diagnosed with the disease) and infection-fatality rate (number of deaths divided by overall number of cases of SARS-CoV-2 infection, i.e. including both symptomatic and asymptomatic subjects), due to methodological issues (33). Such issues include the censoring when the outcome is still unknown at the time of the investigation, the occurrence of ascertainment biases (34-36), especially the underestimation SARS-CoV-2 infection and COVID-19 incidence during an emergency situation as during the pandemic spread, and heterogeneity in classifying the outcome, i.e. COVID-19 related deaths (37-41).
In this study, we aimed at assessing COVID-19 fatality rates in Italy, focusing on case-fatality and infection-fatality rates during the first and second waves on a provincial level during the first year of the COVID-19 pandemic, when neither virus variants were present in the country nor the vaccination campaign had started yet (42,43).
Methods
We downloaded publicly available COVID-19 data from the website of the Civil Protection Agency (44) and National Institute of Statistics (45), collecting daily data flow that Italian regions had to mandatorily provide with a provincial level of detail. In detail, we used the number of newly diagnosed infections with SARS-CoV-2 (corresponding to the new positive tests of infection based on quantitative reverse transcription polymerase chain reaction) and number of COVID-19 deaths in two time frames: from February 24-June 30, 2020 (first wave), and from September 1-December 31, 2020 (second wave). We also used data about anti-SARS-CoV-2 antibody seroprevalence recently made available at a provincial level by a survey carried out by the National Institute of Statistics in May-July 2020 (32). In order to take into account possible differences in time-frame between the first wave period and the seroprevalence survey, we also considered as alternative first wave period February 24-July 31, 2020.
We calculated the province-specific case-fatality rate, also called ratio (34,46), for the first and second waves by dividing the number of deaths by the number of diagnosed positive cases in the two periods February 24-June 30 and February 24-July 31, 2020. We then calculated the province-specific infection-fatality rate (34) by dividing the number of deaths occurred during the first wave (February 24-June 30, 2020) by the estimated number of seroprevalent subjects using data of the National Institute of Statistics carried out in the period May-July 2020 (32). We eventually computed the rate between deaths and positive molecular tests during the September 1-December 31 period, that we called ‘case/infection-fatality rate’ due to the hybrid nature of such indicator, whose denominator included asymptomatic and symptomatic SARS-CoV-2 infected cases due to nationwide marked changes in testing availability and policy (14). All these estimates were crude, i.e. unadjusted for age and sex.
Using data made publicly available by the European Center for Disease Control (ECDC), we also retrieved COVID-19 cases and deaths occurred in all European countries during the 2020, available on a weekly basis (47). As we did with Italian provinces, we calculated the case-fatality rate and the case/infection- fatality rate for the first and second waves, respectively. For this purpose, we considered as first wave the time from the beginning of the virus spread up to summer period (June 30, 2020) when cases waned in almost all countries (some countries experienced a unique wave in the 2020). The beginning of second wave was considered variable according to the raising of the curve up to the end of the year, generally January 1, 2021 based on the weekly availability of data (47).
We also compared data of fatality rate of COVID-19 with seasonal flu. We retrieved data of flu cases through reports released by the National Institute of Health (48), while we used annual flu deaths available from the National Institute of Statistics (49). We excluded the most recent years, taking into account the influence of the COVID-19 pandemic in the circulation of other airborne infections (50).
To investigate the relation between province-specific estimates, we used linear regression to fit a restricted cubic spline model with three knots at fixed percentiles (10th, 50th and 90th) of first wave distribution and weighted by the provincial population in 2020 (51). We used a multivariable model adjusted for aging index, percentage commuting outside the municipality of residence on a daily basis, and percentage of dwellings occupied by only one resident, available using 2011 census data of the National Institute of Statistics (51). We used the Stata statistical software (Version-17.0 Stata Corp., College Station-TX, 2021) for all analyses.
Results
Table 1 presents detailed information about number of cases and deaths divided by first and second waves along with seroprevalence data in the Italian provinces. In Italy, diagnosed cases and deaths during the first wave were 235,839 and 35,048, respectively. Corresponding values for the second wave were 1,808,260 cases and 40,392 deaths.
Table 1.
Number of SARS-CoV-2 cases, COVID-19 deaths, COVID-19 case-fatality rate (deaths/cases*100) in the first (1st) wave (February 24-June 30), and case/infection-fatality rate (deaths/cases*100) in the second (2nd) waves (September 30-December 31) in 2020 divided by province and region. SARS-CoV-2 seroprevalence (%) after the 1st wave (period May-July 2020) and infection-fatality rate (deaths/seroprevalents*100).
| Region/Province | Population Jan 1, 2020 | Cases 1st wave | Cases 2nd wave | Seroprev. (%) | Deaths 1st wave | Deaths 2nd wave | Case-fatality rate 1st wave | Infection-fatality rate 1st wave | Case/infection fatality rate 2nd wave |
|---|---|---|---|---|---|---|---|---|---|
| Aosta Valley | 125501 | 1195 | 5771 | 3.72 | 145 | 239 | 12.1 | 3.1 | 4.1 |
| Aosta | 125501 | 1195 | 5771 | 3.72 | 145 | 239 | 12.1 | 3.1 | 4.1 |
| Lombardy | 10103969 | 91813 | 368273 | 7.35 | 16633 | 8321 | 18.1 | 2.2 | 2.3 |
| Bergamo | 1116384 | 14375 | 12873 | 24.3 | 3137 | 193 | 21.8 | 1.2 | 1.5 |
| Brescia | 1268455 | 15626 | 25468 | 7.63 | 2686 | 422 | 17.2 | 2.8 | 1.7 |
| Como | 603828 | 4093 | 29531 | 2.00 | 587 | 794 | 14.3 | 4.8 | 2.7 |
| Cremona | 358347 | 6612 | 7664 | 19.7 | 1130 | 123 | 17.1 | 1.6 | 1.6 |
| Lecco | 337087 | 2831 | 10303 | 6.66 | 481 | 236 | 17.0 | 2.1 | 2.3 |
| Lodi | 230607 | 3570 | 6936 | 7.10 | 679 | 140 | 19.0 | 4.1 | 2.0 |
| Mantua | 411062 | 3496 | 12260 | 6.57 | 684 | 288 | 19.6 | 2.5 | 2.3 |
| Milan | 3279944 | 24379 | 147720 | 3.95 | 4252 | 3197 | 17.4 | 3.3 | 2.2 |
| Monza/Brianza | 878267 | 5772 | 42090 | 4.52 | 979 | 895 | 17.0 | 2.5 | 2.1 |
| Pavia | 546515 | 5568 | 18869 | 5.95 | 1241 | 543 | 22.3 | 3.7 | 2.9 |
| Sondrio | 180941 | 1584 | 6954 | 5.30 | 212 | 201 | 13.4 | 2.2 | 2.9 |
| Varese | 892532 | 3907 | 47605 | 1.71 | 565 | 1289 | 14.5 | 3.7 | 2.7 |
| Veneto | 4907704 | 18937 | 227276 | 1.92 | 2028 | 4960 | 10.7 | 2.1 | 2.2 |
| Belluno | 201972 | 1191 | 13369 | 1.88 | 114 | 330 | 9.6 | 3.0 | 2.5 |
| Padua | 939672 | 3954 | 41651 | 2.32 | 318 | 608 | 8.0 | 1.5 | 1.5 |
| Rovigo | 233386 | 444 | 6932 | 2.39 | 36 | 204 | 8.1 | 0.6 | 2.9 |
| Treviso | 888309 | 2673 | 45715 | 1.89 | 322 | 783 | 12.0 | 1.9 | 1.7 |
| Venice | 851663 | 2682 | 35612 | 1.68 | 299 | 863 | 11.1 | 2.1 | 2.4 |
| Verona | 930339 | 5127 | 44073 | 2.23 | 586 | 1195 | 11.4 | 2.8 | 2.7 |
| Vicenza | 862363 | 2866 | 39924 | 1.33 | 353 | 977 | 12.3 | 3.1 | 2.4 |
| Emilia-Romagna | 4467118 | 28061 | 137052 | 2.90 | 4353 | 3431 | 15.5 | 3.4 | 2.5 |
| Bologna | 1017806 | 5229 | 32314 | 2.33 | 732 | 936 | 14.0 | 3.0 | 2.9 |
| Ferrara | 344840 | 1044 | 7886 | 0.72 | 173 | 222 | 16.6 | 7.0 | 2.8 |
| Forlì-Cesena | 394833 | 1740 | 10213 | 1.04 | 196 | 164 | 11.3 | 4.8 | 1.6 |
| Modena | 707292 | 3873 | 25945 | 1.10 | 480 | 601 | 12.4 | 6.2 | 2.3 |
| Parma | 453930 | 3657 | 8701 | 5.84 | 901 | 215 | 24.6 | 3.4 | 2.5 |
| Piacenza | 287236 | 4428 | 10187 | 9.54 | 956 | 252 | 21.6 | 3.5 | 2.5 |
| Ravenna | 389634 | 1030 | 11337 | 1.18 | 81 | 430 | 7.9 | 1.8 | 3.8 |
| Reggio nell’Emilia | 531751 | 4913 | 18248 | 4.45 | 581 | 306 | 11.8 | 2.5 | 1.7 |
| Rimini | 339796 | 2147 | 12221 | 2.79 | 253 | 305 | 11.8 | 2.9 | 2.5 |
| Piedmont | 4341375 | 30989 | 162730 | 3.45 | 4029 | 3537 | 13.0 | 2.7 | 2.2 |
| Alessandria | 419037 | 4063 | 13240 | 2.08 | 659 | 470 | 16.2 | 7.7 | 3.5 |
| Asti | 213216 | 1874 | 7960 | 2.13 | 249 | 217 | 13.3 | 5.5 | 2.7 |
| Biella | 174384 | 1046 | 5748 | 6.59 | 194 | 112 | 18.5 | 1.7 | 1.9 |
| Cuneo | 586568 | 2862 | 24081 | 0.87 | 373 | 494 | 13.0 | 7.3 | 2.1 |
| Novara | 368040 | 2792 | 12443 | 5.21 | 367 | 272 | 13.1 | 1.9 | 2.2 |
| Turin | 2252379 | 15889 | 87788 | 3.58 | 1844 | 1712 | 11.6 | 2.3 | 2.0 |
| Verbano-Cusio-Ossola | 157455 | 1140 | 5515 | 9.05 | 132 | 122 | 11.6 | 0.9 | 2.2 |
| Vercelli | 170296 | 1323 | 5955 | 3.52 | 211 | 138 | 15.9 | 3.5 | 2.3 |
| Trentino-South Tyrol | 1074819 | 7502 | 43303 | 3.19 | 693 | 1041 | 9.2 | 2.0 | 2.4 |
| Bolzano | 532080 | 2639 | 26559 | 2.95 | 288 | 504 | 10.9 | 1.8 | 1.9 |
| Trento | 542739 | 4863 | 16744 | 3.42 | 405 | 537 | 8.3 | 2.2 | 3.2 |
| Friuli-Venezia Giulia | 1211357 | 3308 | 45651 | 1.02 | 362 | 1426 | 10.9 | 2.9 | 3.1 |
| Gorizia | 139206 | 216 | 5904 | 0.12 | 5 | 104 | 2.3 | -† | 1.8 |
| Pordenone | 312619 | 702 | 9792 | 1.88 | 68 | 291 | 9.7 | 1.2 | 3.0 |
| Trieste | 233276 | 1393 | 9107 | 0.59 | 209 | 270 | 15.0 | -† | 3.0 |
| Udine | 526256 | 997 | 20848 | 0.93 | 80 | 761 | 8.0 | 1.6 | 3.7 |
| Liguria | 1543127 | 9473 | 46958 | 3.24 | 1563 | 1276 | 16.5 | 3.1 | 2.7 |
| Genoa | 835829 | 5573 | 29304 | 3.61 | 943 | 853 | 16.9 | 3.1 | 2.9 |
| Imperia | 213919 | 1494 | 4806 | 2.39 | 231 | 79 | 15.5 | 4.5 | 1.6 |
| La Spezia | 219196 | 860 | 7142 | 1.89 | 159 | 189 | 18.5 | 3.8 | 2.6 |
| Savona | 274183 | 1546 | 5706 | 3.83 | 230 | 155 | 14.9 | 2.2 | 2.7 |
| Tuscany | 3722729 | 9779 | 108429 | 0.90 | 1088 | 2491 | 11.1 | 3.3 | 2.3 |
| Arezzo | 341766 | 676 | 9779 | 1.23 | 47 | 168 | 7.0 | 1.1 | 1.7 |
| Florence | 1004298 | 3192 | 29864 | 0.53 | 401 | 839 | 12.6 | 7.5 | 2.8 |
| Grosseto | 220785 | 396 | 3708 | 1.18 | 28 | 73 | 7.1 | 1.1 | 2.0 |
| Livorno | 333509 | 477 | 8079 | 0.56 | 62 | 195 | 13.0 | 3.3 | 2.4 |
| Lucca | 388678 | 1351 | 11010 | 0.42 | 151 | 192 | 11.2 | 9.3 | 1.7 |
| Massa and Carrara | 193934 | 1051 | 6442 | 0.00 | 153 | 179 | 14.6 | -‡ | 2.8 |
| Pisa | 422310 | 930 | 15667 | 1.55 | 91 | 341 | 9.8 | 1.4 | 2.2 |
| Pistoia | 293059 | 747 | 9640 | 0.96 | 76 | 199 | 10.2 | 2.7 | 2.1 |
| Prato | 258152 | 532 | 9790 | 1.02 | 47 | 203 | 8.8 | 1.8 | 2.1 |
| Siena | 266238 | 427 | 4450 | 2.17 | 32 | 102 | 7.5 | 0.6 | 2.3 |
| Umbria | 880285 | 1385 | 26064 | 0.67 | 80 | 530 | 5.8 | 1.4 | 2.0 |
| Perugia | 655403 | 1008 | 19843 | 0.71 | 51 | 369 | 5.1 | 1.1 | 1.9 |
| Terni | 224882 | 377 | 6221 | 0.55 | 29 | 161 | 7.7 | 2.4 | 2.6 |
| Marches | 1518400 | 6549 | 33194 | 2.59 | 987 | 720 | 15.1 | 2.5 | 2.2 |
| Ancona | 469750 | 1875 | 9711 | 2.16 | 218 | 185 | 11.6 | 2.1 | 1.9 |
| Ascoli Piceno | 206363 | 290 | 4790 | 4.95 | 12 | 125 | 4.1 | 0.1 | 2.6 |
| Fermo | 173004 | 473 | 4337 | 2.16 | 67 | 69 | 14.2 | 1.0 | 1.6 |
| Macerata | 312146 | 1154 | 7851 | 2.16 | 145 | 159 | 12.6 | 2.2 | 2.0 |
| Pesaro and Urbino | 357137 | 2757 | 6505 | 4.95 | 545 | 182 | 19.8 | 3.1 | 2.8 |
| Lazio | 5865544 | 8010 | 148533 | 1.00 | 863 | 2815 | 10.8 | 1.4 | 1.9 |
| Frosinone | 485241 | 663 | 12990 | 0.19 | 79 | 162 | 11.9 | 8.6 | 1.2 |
| Latina | 576655 | 607 | 13625 | 0.50 | 44 | 294 | 7.2 | 1.5 | 2.2 |
| Rieti | 154232 | 411 | 4565 | 3.00 | 41 | 149 | 10.0 | 0.9 | 3.3 |
| Rome | 4333274 | 5872 | 108988 | 1.05 | 672 | 2016 | 11.4 | 1.4 | 1.8 |
| Viterbo | 316142 | 457 | 8365 | 1.52 | 27 | 194 | 5.9 | 0.6 | 2.3 |
| Abruzzo | 1305770 | 3261 | 31124 | 1.29 | 461 | 794 | 14.1 | 2.7 | 2.6 |
| Chieti | 383189 | 818 | 6284 | 1.40 | 131 | 136 | 16.0 | 2.4 | 2.2 |
| L’Aquila | 296491 | 225 | 10604 | 0.54 | 11 | 350 | 4.9 | 0.7 | 3.3 |
| Pescara | 318678 | 1586 | 5447 | 1.69 | 239 | 116 | 15.1 | 4.4 | 2.1 |
| Teramo | 307412 | 632 | 8789 | 1.48 | 80 | 192 | 12.7 | 1.8 | 2.2 |
| Molise | 302265 | 426 | 5971 | 0.81 | 28 | 175 | 6.6 | 1.1 | 2.9 |
| Campobasso | 218679 | 364 | 3829 | 0.66 | 22 | 110 | 6.0 | 1.4 | 2.9 |
| Isernia | 83586 | 62 | 2142 | 1.19 | 6 | 65 | 9.7 | 0.6 | 3.0 |
| Campania | 5785861 | 4648 | 182462 | 0.89 | 517 | 2915 | 11.1 | 1.0 | 1.6 |
| Avellino | 413926 | 552 | 8289 | 0.00 | 62 | 143 | 11.2 | -‡ | 1.7 |
| Benevento | 274080 | 209 | 4423 | 0.00 | 19 | 137 | 9.1 | -‡ | 3.1 |
| Caserta | 922171 | 543 | 33741 | 1.48 | 53 | 540 | 9.8 | 0.4 | 1.6 |
| Naples | 3082905 | 2652 | 111294 | 1.04 | 314 | 1811 | 11.8 | 1.0 | 1.6 |
| Salerno | 1092779 | 692 | 24715 | 0.31 | 69 | 284 | 10.0 | 2.1 | 1.1 |
| Apulia | 4008296 | 4502 | 84951 | 0.88 | 566 | 2037 | 12.6 | 1.6 | 2.4 |
| Bari | 1249246 | 1491 | 33237 | 1.50 | 153 | 636 | 10.3 | 0.8 | 1.9 |
| Barletta-Andria-Trani | 388390 | 380 | 10058 | 0.77 | 66 | 295 | 17.4 | 2.2 | 2.9 |
| Brindisi | 390456 | 659 | 5795 | 0.85 | 67 | 100 | 10.2 | 2.0 | 1.7 |
| Foggia | 616310 | 1170 | 18639 | 1.02 | 161 | 655 | 13.8 | 2.6 | 3.5 |
| Lecce | 791122 | 521 | 6420 | 0.01 | 85 | 114 | 16.3 | -† | 1.8 |
| Taranto | 572772 | 281 | 10802 | 0.67 | 34 | 237 | 12.1 | 0.9 | 2.2 |
| Basilicata | 556934 | 400 | 10055 | 0.72 | 36 | 214 | 9.0 | 0.9 | 2.1 |
| Potenza | 360936 | 189 | 6739 | 0.83 | 27 | 156 | 14.3 | 0.9 | 2.3 |
| Matera | 195998 | 211 | 3316 | 0.50 | 9 | 58 | 4.3 | 0.9 | 1.7 |
| Calabria | 1924701 | 1179 | 22191 | 0.51 | 129 | 368 | 10.9 | 1.3 | 1.7 |
| Catanzaro | 354851 | 214 | 3134 | 0.40 | 31 | 49 | 14.5 | 2.2 | 1.6 |
| Cosenza | 700385 | 468 | 6676 | 0.78 | 48 | 176 | 10.3 | 0.9 | 2.6 |
| Crotone | 170718 | 119 | 2065 | 0.11 | 10 | 35 | 8.4 | 5.2 | 1.7 |
| Reggio di Calabria | 541278 | 294 | 8586 | 0.18 | 29 | 81 | 9.9 | 3.1 | 0.9 |
| Vibo Valentia | 157469 | 84 | 1730 | 1.12 | 11 | 27 | 13.1 | 0.6 | 1.6 |
| Sicily | 4968410 | 3056 | 89352 | 0.37 | 342 | 2390 | 11.2 | 1.9 | 2.7 |
| Agrigento | 429611 | 135 | 3651 | 0.19 | 24 | 107 | 17.8 | 3.0 | 2.9 |
| Caltanissetta | 260779 | 186 | 3733 | 0.00 | 18 | 81 | 9.7 | -‡ | 2.2 |
| Catania | 1104974 | 779 | 26464 | 0.26 | 103 | 849 | 13.2 | 3.6 | 3.2 |
| Enna | 162368 | 438 | 2866 | 0.00 | 34 | 76 | 7.8 | -‡ | 2.7 |
| Messina | 620721 | 474 | 10246 | 0.32 | 59 | 136 | 12.4 | 2.9 | 1.3 |
| Palermo | 1243328 | 500 | 24929 | 0.89 | 43 | 665 | 8.6 | 0.4 | 2.7 |
| Ragusa | 321215 | 87 | 6490 | 0.30 | 6 | 161 | 6.9 | 0.6 | 2.5 |
| Siracusa | 397037 | 321 | 5112 | 0.14 | 47 | 162 | 14.6 | 8.8 | 3.2 |
| Trapani | 428377 | 136 | 5861 | 0.00 | 8 | 153 | 5.9 | -‡ | 2.6 |
| Sardinia | 1630474 | 1366 | 28920 | 0.50 | 145 | 712 | 10.6 | 1.8 | 2.5 |
| Cagliari | 430914 | 253 | 6573 | 0.38 | 19 | 161 | 7.5 | 1.2 | 2.4 |
| Nuoro | 206843 | 78 | 6055 | 0.24 | 12 | 123 | 15.4 | 2.4 | 2.0 |
| Oristano | 156078 | 61 | 2416 | 0.43 | 8 | 51 | 13.1 | 1.2 | 2.1 |
| Sassari | 489634 | 875 | 8997 | 0.78 | 90 | 247 | 10.3 | 2.4 | 2.7 |
| South Sardinia | 347005 | 99 | 4879 | 0.42 | 16 | 130 | 16.2 | 1.1 | 2.7 |
| Italy | 60244639 | 235839 | 1808260 | 2.49 | 35048 | 40392 | 14.9 | 2.2 | 2.2 |
†Provinces excluded due to implausible data of seroprevalences since the estimated number of seroprevalent subjects are less than the number of positive cases at the end of the first wave. ‡Provinces excluded due to missing/null data about seroprevalence.
National average seroprevalence was 2.49%, with the highest values in Bergamo (24.3%), Cremona (19.7%), and Piacenza (9.5%). Six provinces (Avellino, Benevento, Caltanissetta, Enna, Massa-Carrara and Trapani) reported null seroprevalence, while three provinces, Lecce, Gorizia and Trieste, showed extremely low and implausible seroprevalence rates. We considered these latter provinces as unwarranted outliers arising from a low and potentially highly biased participation in the survey since the estimated number of seroprevalent subjects is lower than the ascertained cases during the first wave. For this reason, we removed these provinces from the analyses concerning the infection-fatality rate.
Figure 1 shows the case-fatality rate (from swab testing) and the infection-fatality rate (from seroprevalence data) in the first wave, and the case/infection-fatality rate (from swab testing) in the second wave across the Italian provinces. Overall in Italy, crude case-fatality rate was 14.9% for the first wave and 2.2% for the second wave, while the crude infection-fatality rate based on seroprevalence after the first wave was 2.2%. Province-specific values of case-fatality rate showed a median value of 12% (ranging from 2.3% in Gorizia to 24.6% in Parma), while the infection-fatality rate using seroprevalence data was much lower with a median value of 2.2%. During the second wave, SARS-CoV-2 testing greatly increased and was extended also to asymptomatic subjects, leading to a ‘mixed’ case/infection-fatality rate with median value of 2.2%, comparable to the infection fatality rate of 2.2% (Table 1).
Figure 1.
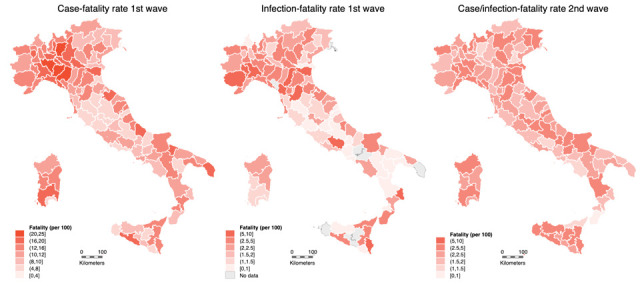
Crude case-fatality rate (deaths per 100 cases) for first wave (February 24-June 30, 2020), infection-fatality rate (deaths per 100 cases) after the first wave, and case/infection-fatality rate for the second (September 1-December 31, 2020) wave.
In Table 2 we report SARS-CoV-2 cases and COVID-19 deaths occurred in European countries in 2020 divided in the two pandemic waves, and the case-fatality and case/infection-fatality rates in European countries for the first and second waves, respectively. Overall, Italy showed one of the highest first-wave case-fatality rate (14.43%) along with other severely hit countries such as France (18.22%), Belgium (15.48), and UK (14.15%) compared to the value of EE/EEA area (10.59%) (Table 2 and Figure 2). In the majority of European countries, the second wave began from August to mid-September 2020, with some exceptions reporting an earlier onset in July, namely France, Spain, Malta, and Ukraine, with consequent difficulties in the comparison. Overall in 2020, the EE/EEA area showed a fatality rate of 2.38%, with the highest values reported by Bulgaria (3.78%), Greece (3.54%), and Italy (3.49%).
Table 2.
Crude case-fatality (deaths per 100 cases) for the first (1st) wave for all EU/EEA countries (+ Switzerland and United Kingdom) from the beginning of the pandemic to June 30 if not differently specified, and case/infection-fatality rate (deaths per 100 cases) for the second (2nd) wave. Time-frame is different with the begin of the second wave indicated for each country, while the end was January 3, 2021 for all countries.
| Country | Total population 2020† | Cases 1st wave | Deaths 1st wave | Case-fatality 1st wave | 2nd wave time-frame | Cases 2nd wave | Deaths 2nd wave | Case/infection fatality 2nd wave | Total 2020 cases | Total 2020 deaths | 2020 fatality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Andorra | 76177 | 855 | 52 | 6.08 | 14 Sep | 6848 | 31 | 0.45 | 8192 | 84 | 1.03 |
| Austria | 8901064 | 18269 | 706 | 3.86 | 14 Sep | 331239 | 5497 | 1.66 | 364574 | 6253 | 1.72 |
| Belgium | 11522440 | 62394 | 9660 | 15.48 | 14 Sep | 556759 | 9927 | 1.78 | 651968 | 19876 | 3.05 |
| Bulgaria | 6951482 | 5740 | 246 | 4.29 | 5 Oct | 181464 | 6834 | 3.77 | 203051 | 7678 | 3.78 |
| Croatia | 4058165 | 3151 | 113 | 3.59 | 5 Oct | 195299 | 3774 | 1.93 | 212958 | 4072 | 1.91 |
| Cyprus | 888005 | 1003 | 19 | 1.89 | 12 Oct | 21988 | 106 | 0.48 | 23974 | 131 | 0.55 |
| Czech Republic | 10693939 | 12556 | 352 | 2.80 | 31 Aug | 722615 | 12005 | 1.66 | 747003 | 12431 | 1.66 |
| Denmark | 5822763 | 12832 | 606 | 4.72 | 7 Sep | 151164 | 747 | 0.49 | 168711 | 1374 | 0.81 |
| Estonia | 1328976 | 1993 | 63 | 3.16 | 23 Oct | 25110 | 178 | 0.71 | 29521 | 251 | 0.85 |
| Finland | 5525292 | 7272 | 309 | 4.25 | 7 Sep | 28628 | 289 | 1.01 | 36919 | 607 | 1.64 |
| France | 67320216 | 164068 | 29893 | 18.22 | 27 Jul | 2457579 | 34845 | 1.42 | 2636772 | 65037 | 2.47 |
| Germany | 83166711 | 196554 | 9016 | 4.59 | 7 Sep | 1524714 | 25249 | 1.66 | 1775513 | 34574 | 1.95 |
| Greece | 10718565 | 3519 | 192 | 5.46 | 10 Aug | 134476 | 4745 | 3.53 | 140099 | 4957 | 3.54 |
| Hungary | 9769526 | 4183 | 589 | 14.08 | 31 Aug | 322890 | 9363 | 2.90 | 328851 | 9977 | 3.03 |
| Iceland | 364134 | 1830 | 10 | 0.55 | 14 Sep | 3589 | 19 | 0.53 | 5754 | 29 | 0.50 |
| Ireland | 4964440 | 25527 | 1741 | 6.82 | 7 Sep | 72215 | 482 | 0.67 | 101887 | 2259 | 2.22 |
| Italy | 59641488 | 241611 | 34861 | 14.43 | 31 Aug | 1887228 | 39855 | 2.11 | 2155446 | 75332 | 3.49 |
| Latvia | 1907675 | 1124 | 30 | 2.67 | 21 Sep | 40972 | 644 | 1.57 | 42497 | 680 | 1.60 |
| Liechtenstein | 38747 | 84 | 1 | 1.19 | 5 Oct | 2096 | 34 | 1.62 | 2222 | 35 | 1.58 |
| Lithuania | 2794090 | 1836 | 79 | 4.30 | 5 Oct | 142802 | 1856 | 1.30 | 147987 | 1950 | 1.32 |
| Luxembourg | 626108 | 4522 | 110 | 2.43 | 14 Sep | 39725 | 382 | 0.96 | 46919 | 506 | 1.08 |
| Malta | 514564 | 671 | 9 | 1.34 | 27 Jul | 12520 | 208 | 1.66 | 13219 | 217 | 1.64 |
| Monaco | 39244 | 75 | 1 | 1.33 | 5 Oct | 685 | 2 | 0.29 | 907 | 3 | 0.33 |
| The Netherlands | 17407585 | 50621 | 6127 | 12.10 | 31 Aug | 750122 | 5383 | 0.72 | 820193 | 11598 | 1.41 |
| Norway | 5367580 | 8895 | 251 | 2.82 | 19 Oct | 34579 | 171 | 0.49 | 50715 | 449 | 0.89 |
| Poland | 37958138 | 35950 | 1517 | 4.22 | 28 Sep | 1235617 | 26729 | 2.16 | 1322947 | 29161 | 2.20 |
| Portugal | 10295909 | 43897 | 1614 | 3.68 | 7 Sep | 371365 | 5356 | 1.44 | 431623 | 7196 | 1.67 |
| Romania | 19328838 | 28973 | 1750 | 6.04 | 21 Sep | 527648 | 11544 | 2.19 | 640429 | 15979 | 2.50 |
| San Marino | 34453 | 698 | 42 | 6.02 | 12 Oct | 1741 | 20 | 1.15 | 2493 | 62 | 2.49 |
| Slovakia | 5457873 | 1798 | 28 | 1.56 | 21 Sep | 306848 | 2616 | 0.85 | 314117 | 2657 | 0.85 |
| Slovenia | 2095861 | 1700 | 111 | 6.53 | 7 Sep | 122684 | 2761 | 2.25 | 125858 | 2891 | 2.30 |
| Spain | 47332614 | 251789 | 28388 | 11.27 | 6 Jul | 1702891 | 22672 | 1.33 | 1958844 | 51078 | 2.61 |
| Sweden | 10327589 | 70612 | 5576 | 7.90 | 5 Oct | 366858 | 4232 | 1.15 | 462661 | 10125 | 2.19 |
| Switzerland | 8606033 | 32184 | 1685 | 5.24 | 5 Oct | 405397 | 5455 | 1.35 | 459660 | 7238 | 1.57 |
| Ukraine | 43733759 | 48500 | 1249 | 2.58 | 20 Jul | 1015251 | 17369 | 1.71 | 1074093 | 18854 | 1.76 |
| United Kingdom | 68059863 | 287121 | 40632 | 14.15 | 7 Sep | 2307627 | 33473 | 1.45 | 2654779 | 75024 | 2.83 |
| EU/EEA countries | 45309377 | 1264974 | 133967 | 10.59 | - | - | - | - | 15963232 | 379360 | 2.38 |
† Population data from Eurostat (47).
Figure 2.
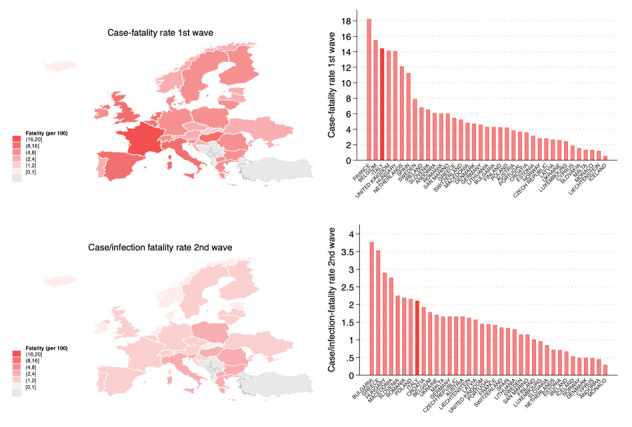
Map and histograms of case-fatality and case/infection-fatality during the first and second waves in EU/EEA countries (+ Switzerland and United Kingdom).
In Table 3, we report data about cases of seasonal flu epidemics, and we computed an average case-fatality rate from past seasons of 0.01%, which is orders of magnitude lower of COVID-19 disease.
Table 3.
Number of influenza cases and deaths in Italy during the most recent seasonal flu epidemics.
| Season | Flu cases | ISTAT report | Flu deaths |
|---|---|---|---|
| 2012/2013 | 5995000 | 2013 | 417 |
| 2013/2014 | 4542000 | 2014 | 272 |
| 2014/2015 | 6299000 | 2015 | 675 |
| 2015/2016 | 4876900 | 2016 | 316 |
| 2016/2017 | 5440900 | 2017 | 663 |
| 2017/2018 | 8677300 | 2018 | 745 |
When we compared the case-fatality rate of the first wave with the infection-fatality rate after the first wave using seroprevalence data in the Italian provinces using the spline analysis (Figure 3), we found a substantially linear positive association up to approximately 12% of case-fatality rate in the first wave corresponding to 3.6 infection-fatality rate, while the curve flattened at higher values.
Figure 3.
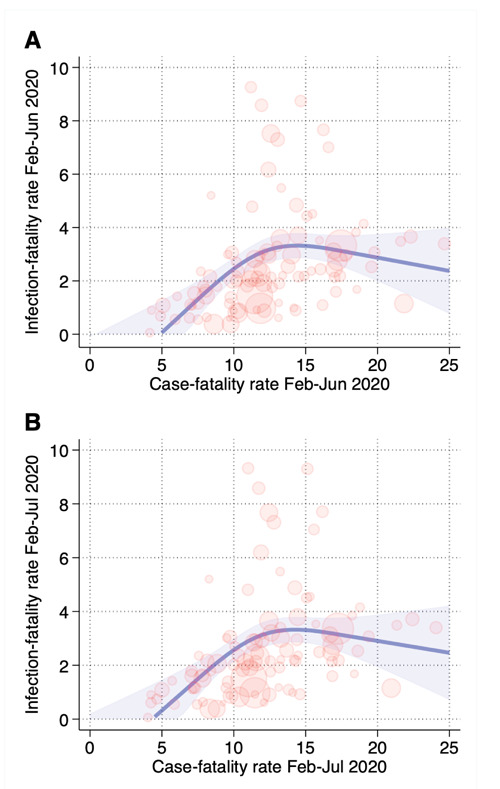
Comparison of first wave case-fatality rate (using positive swab data to estimate COVID-19 cases) and infection-fatality rate (using May-June seroprevalence data to estimate infected cases) considering the time frames February 24-June 30, 2020 (A) and February 24-July 31, 2020 (B). Spline regression model adjusted for aging index, percentage commuting outside the municipality of residence on a daily basis, and percentage of dwellings occupied by only one resident.
In the spline regression model comparing case-fatality rate in the first wave with the case/infection-fatality rate in the second wave, we did not find any relation between the two variables. On average, the case-fatality rate was 5.6 times 95% CIs (95% CI 5.2-6.1) higher in the first compared to the second wave (Figure 4).
Figure 4.
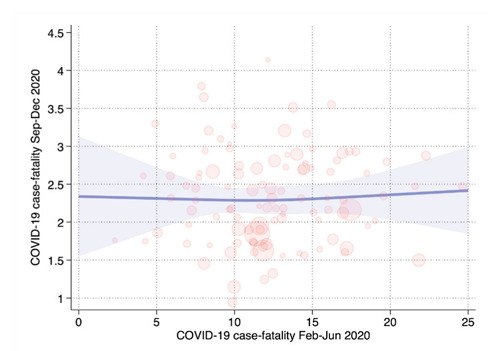
Comparison of first and second wave case-fatality rate in a spline regression model adjusted for aging index, percentage commuting outside the municipality of residence on a daily basis, and percentage of dwellings occupied by only one resident.
Discussion
At the end 2020, Italy was one of the countries reporting the highest number of confirmed positive cases as well as COVID-19 deaths (52). Since many uncertainties still exist about the real impact and severity of COVID-19 pandemic (33,37,53,54), in the present investigation we provided an assessment of the COVID-19 case-fatality and infection-fatality rates during the 2020 in Italian provinces.
Overall, our data confirmed that during the first wave, when almost all subjects underwent SARS-CoV-2 testing due to presence of symptoms related to COVID-19, the Italian case-fatality rate was as high as around 15%, being much higher than the infection-fatality rate. Conversely, during the second wave, the case/infection fatality rate we could compute waned to a much lower value of 2.2%. The most plausible explanation for this discrepancy is the hybrid nature of the latter estimate, due to the different policy for SARS-CoV-2 infection assessment. In fact, during the first wave only suspected cases due to travelling from high risk countries or with symptoms suggesting of COVID-19 were tested (55), while during the second waves also asymptomatic cases underwent swab testing. These findings appear to be confirmed by the assessment of the infection-fatality rate estimated through seroprevalence data, almost identical to COVID-19 fatality during the second wave, with the same overall national value of 2.2%. In addition, the comparison of the COVID-19 fatality rates in other European countries demonstrated generally a higher case-fatality rate for Italy during the first wave, and a marked decrease during the second wave that could have been at least partially due to the increase of population screening with SARS-CoV-2 swab testing of a large proportion of asymptomatic individuals (55). However, since the availability of SARS-CoV-2 molecular testing increased all over Europe during the second wave, our results may also indicate that the severity of the disease and the spread of the infection decreased in Italy with time during 2020, as compared with the other European countries, for reasons possibly related to the higher severity of the first wave, such as a larger prevalence of immunity in the population, or the increased depletion of highly susceptible individuals due to the high first wave COVID-19 mortality (56-59).
Interestingly, our results are partially conflicting with data from a recent meta-analysis suggesting much lower value (2.7%) during the first wave in European region (33) but higher estimates for Italy with a mean value of 7.8% (median=8.58%) and range from 1.7% up to 14.5%. This high heterogeneity could be explained by the modality of case-fatality assessment among different studies. In particular, the lower value was reported from a study implementing modelling techniques, e.g. SEIR (Susceptible-Exposed-Infective-Recovered) model (60), as well as when based on incomplete data when the first wave was still ongoing (61). Conversely, studies using real and comprehensive data demonstrated similar or even higher estimates compared with the present study (62-64). Interestingly, a comparable pattern of discrepancies in the estimation of case-fatality rate can be noted also for other countries severely hit by the pandemic such as United Kingdom and France (33). For these reasons, despite such modelling demonstrated a high reliability in the prediction of pandemic tend/curves (65,66), the estimation of disease case-fatality was not so effective and reliable, also since that the number of infections and deaths may be affected by other determinants, in particular the advances in SARS-CoV-2 infection as well as COVID-19 diagnosis (67), and especially treatment (68-70).
The occurrence of a high case-fatality rate in Italy was not entirely unexpected, being explained by the demographic and health characteristics of the Italian population. Also, at the very beginning of the pandemic in Italy, a case-fatality rate of 7.2% was estimated by the National Institute of Health (71), much higher compared with the one reported in China (72). Indeed, COVID-19 demonstrated to be more severe and deadly in vulnerable individuals due to older age and/or comorbidities (73,74), leading to a higher mortality in older subjects (75). Similarly, our findings are consistent with the recent report of the National Institute of Statistics, as they found a slightly higher (sex and age-adjusted) case-fatality rate of 4.3% in the entire 2020 (52). Consistently with our findings, such analysis yielded a higher value in the first pandemic period (although based on a slightly different timeframe, February-May 2020), i.e. 6.6%, a lower value in June-September (1.5%), and again a slightly higher value in October (2.4%).
Results of the seroprevalence nationwide survey confirmed that some Northern Italy areas were heavily affected during the first wave (76), especially the provinces of Bergamo, Brescia, Lodi, Cremona in Lombardy region, and Piacenza and Parma in Emilia-Romagna region (14). Such provinces were those that experienced the highest decrease in the hybrid case/infection fatality rate we could compute for the second wave, consistently with a pattern we have documented for COVID-19 incidence (14).
Our results indicated that the case-fatality rate of COVID-19 was much higher as compared with influenza through 2020 and independently from the time period, indicating that COVID-19 should not be considered a simply flu-like syndrome (77,78), with much larger implications in terms of population and public health burden. This further confirms how relevant is the implementation of effective preventive medicine measures against SARS-CoV-2 infection and COVID-19 including but not limited to vaccination, also in the absence of most effective therapy for this disease (79). Finally, our findings are particularly relevant from a public health perspective since they highlight how different was the impact of the COVID-19 pandemic compared to the seasonal flu and other outbreaks, taking into account the number of affected people and deaths, the health care systems overload, and the psychological and economic burden (80-82).
Our study has some limitations. First, we used aggregated data at a provincial level, showing much higher level of geographical detail than the previous ‘regional’ analyses but still not entirely homogeneous in terms of population size characteristics, despite we tried to control for some potential confounders. In addition, we could not calculate sex- and age-standardized estimates, and therefore the comparison across different geographical areas must be made with caution (83).
Strengths of our analysis include the assessment of the COVID-19 severity during the first two pandemic waves when there was no circulation of virus variants (43), making unlikely this possible confounding related to differences in virus transmission and severity (84,85). Similarly, the vaccination campaign effectively began in January 2021 (86), thus not affecting the susceptibility of subjects and the reliability of our analysis.
Conclusions
Our findings demonstrate that COVID-19 severity in Italy, as assessed through either case-fatality or infection-fatality rates, has been much higher compared with other airborne infections like influenza, while being substantially similar to a few other Western European countries. They also indicate that COVID-19 case-fatality rate and infection fatality rate substantially differ, though such measures are difficult to assess, due to methodological issues and potential biases that can affect these estimates. An adequate assessment of COVID-19 severity may also be of major relevance to plan and test public health interventions aimed at curbing the spread of SARS-CoV-2 infection.
Conflict of interest:
Each author declares that he/she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
Funding:
This study was supported by grants UNIMORE FAR 2020 Interdisciplinare Linea FCRMO - Fondazione Cassa di Risparmio di Modena to Dr. Filippini, and FISR 2020-COVID19 by the Italian Ministry of the University and the Research to Dr. Vinceti.
References
- Neagu M, Calina D, Docea AO, et al. Back to basics in COVID-19: Antigens and antibodies-Completing the puzzle. J Cell Mol Med. 2021;25(10):4523–33. doi: 10.1111/jcmm.16462. https://doi.org/10.1111/jcmm.16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docea AO, Tsatsakis A, Albulescu D, et al. A new threat from an old enemy: Reemergence of coronavirus (Review) Int J Mol Med. 2020;45(6):1631–43. doi: 10.3892/ijmm.2020.4555. https://doi.org/10.3892/ijmm.2020.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanna F, Veronesi F, Martini L, et al. Post-COVID-19 syndrome: The persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne) 2021;8:653516. doi: 10.3389/fmed.2021.653516. https://doi.org/10.3389/fmed.2021.653516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Antinori A, et al. Lopinavir/ritonavir and darunavir/cobicistat in hospitalized COVID-19 patients: Findings from the multicenter Italian CORIST study. Front Med (Lausanne) 2021;8:639970. doi: 10.3389/fmed.2021.639970. https://doi.org/10.3389/fmed.2021.639970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsatsakis A, Calina D, Falzone L, et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem Toxicol. 2020;146:111769. doi: 10.1016/j.fct.2020.111769. https://doi.org/10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Rothman KJ, et al. Lockdown timing and efficacy in controlling COVID-19 using mobile phone tracking. EClinicalMedicine. 2020;25:100457. doi: 10.1016/j.eclinm.2020.100457. https://doi.org/10.1016/j.eclinm.2020.100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ma N, Witt C, et al. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science. 2021:eabg6296. doi: 10.1126/science.abg6296. https://doi.org/10.1126/science.abg6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Garcia JA, Ruiz-Marin M, Carceles-Alvarez A, et al. Social distancing at health care centers early in the pandemic helps to protect population from COVID-19. Environ Res. 2020;189:109957. doi: 10.1016/j.envres.2020.109957. https://doi.org/10.1016/j.envres.2020.109957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez M, Tobias A, Varga D, et al. Effectiveness of the measures to flatten the epidemic curve of COVID-19. The case of Spain. Sci Total Environ. 2020;727:138761. doi: 10.1016/j.scitotenv.2020.138761. https://doi.org/10.1016/j.scitotenv.2020.138761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli C, Scognamiglio T, Odone A. COVID-19 in Italy: impact of containment measures and prevalence estimates of infection in the general population. Acta Biomed. 2020;91(3-S):175–9. doi: 10.23750/abm.v91i3-S.9511. https://doi.org/10.23750/abm.v91i3-S.9511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad LJ, Chemaitelly H, Coyle P, et al. SARS-CoV-2 antibody-positivity protects against reinfection for at least seven months with 95% efficacy. EClinicalMedicine. 2021;35:100861. doi: 10.1016/j.eclinm.2021.100861. https://doi.org/10.1016/j.eclinm.2021.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E. Could new COVID variants undermine vaccines? Labs scramble to find out. Nature. 2021;589(7841):177–8. doi: 10.1038/d41586-021-00031-0. https://doi.org/10.1038/d41586-021-00031-0. [DOI] [PubMed] [Google Scholar]
- Calina D, Hernández AF, Hartung T, et al. Challenges and scientific prospects of the newest generation of mRNA-based vaccines against SARS-CoV-2. Life. 2021;11(9):907. doi: 10.3390/life11090907. https://doi.org/10.3390/life11090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Rothman KJ, et al. SARS-CoV-2 infection incidence during the first and second COVID-19 waves in Italy. Environ Res. 2021;197:111097. doi: 10.1016/j.envres.2021.111097. https://doi.org/10.1016/j.envres.2021.111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Bonaccio M, Costanzo S, et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30(11):1899–913. doi: 10.1016/j.numecd.2020.07.031. https://doi.org/10.1016/j.numecd.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciceri F, Castagna A, Rovere-Querini P, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. https://doi.org/10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone G. The determinants of COVID-19 case fatality rate (CFR) in the Italian regions and provinces: An analysis of environmental, demographic, and healthcare factors. Sci Total Environ. 2021;755(Pt 1):142523. doi: 10.1016/j.scitotenv.2020.142523. https://doi.org/10.1016/j.scitotenv.2020.142523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copat C, Cristaldi A, Fiore M, et al. The role of air pollution (PM and NO2) in COVID-19 spread and lethality: A systematic review. Environ Res. 2020;191:110129. doi: 10.1016/j.envres.2020.110129. https://doi.org/10.1016/j.envres.2020.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M, Domingo JL. Positive association between outdoor air pollution and the incidence and severity of COVID-19. A review of the recent scientific evidences. Environ Res. 2021:111930. doi: 10.1016/j.envres.2021.111930. https://doi.org/10.1016/j.envres.2021.111930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi A, Barnett-Itzhaki Z. Effects of chronic exposure to ambient air pollutants, demographic, and socioeconomic factors on COVID-19 morbidity: The Israeli case study. Environ Res. 2021;202:111673. doi: 10.1016/j.envres.2021.111673. https://doi.org/10.1016/j.envres.2021.111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Parliament. Air pollution and COVID-19. 2021 https://www.europarl.europa.eu/RegData/etudes/STUD/2021/658216/IPOL_STU(2021)658216_EN.pdf . [Accessed September 8, 2021] [Google Scholar]
- Filippini T, Rothman KJ, Cocchio S, et al. Associations between mortality from COVID-19 in two Italian regions and outdoor air pollution as assessed through tropospheric nitrogen dioxide. Sci Total Environ. 2021;760:143355. doi: 10.1016/j.scitotenv.2020.143355. https://doi.org/10.1016/j.scitotenv.2020.143355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini T, Rothman KJ, Goffi A, et al. Satellite-detected tropospheric nitrogen dioxide and spread of SARS-CoV-2 infection in Northern Italy. Sci Total Environ. 2020;739:140278. doi: 10.1016/j.scitotenv.2020.140278. https://doi.org/10.1016/j.scitotenv.2020.140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugno M, Fedrizzi L, Borroni E, et al. Air pollution exposure increases the probability of SARS-CoV-2 infection and influences the immune response of healthcare workers affected by COVID-19. Environ Health Perspect 2021. 2021;(1):P-272. https://ehp.niehs.nih.gov/doi/abs/10.1289/isee.2021.P-272. [Google Scholar]
- Decreto del Presidente del Consiglio dei Ministri. Ulteriori disposizioni attuative del decreto-legge 25 marzo 2020, n. 19, convertito, con modificazioni, dalla legge 25 maggio 2020, n. 35, recante «Misure urgenti per fronteggiare l’emergenza epidemiologica da COVID-19», e del decreto-legge 16 maggio 2020, n. 33, convertito, con modificazioni, dalla legge 14 luglio 2020, n. 74, recante «Ulteriori misure urgenti per fronteggiare l’emergenza epidemiologica da COVID-19». https://www.gazzettaufficiale.it/eli/id/2020/11/04/20A06109/sg. [Accessed August 30, 2021]. GU Serie Generale n.275 2020; 275 04 Nov 2020 - Suppl. Ordinario n. 41. [Google Scholar]
- Ministery of Health. Anti COVID-19 vaccine report. 2021 https://www.governo.it/it/cscovid19/report-vaccini/ [Accessed August 30, 2021] [Google Scholar]
- Modenese A, Gobba F. Increased risk of COVID-19-related deaths among general practitioners in Italy. Healthcare (Basel) 2020;8(2):155. doi: 10.3390/healthcare8020155. https://doi.org/10.3390/healthcare8020155. [Accessed August 30, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berselli N, Filippini T, Paduano S, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in the Northern Italy population before the COVID-19 second wave. Int J Occup Med Environ Health. 2021;35(1) doi: 10.13075/ijomeh.1896.01826. https://doi.org/10.13075/ijomeh.1896.01826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Valle P, Fabbri M, Madotto F, et al. Occupational exposure in the Lombardy Region (Italy) to SARS-CoV-2 infection: Results from the MUSTANG-OCCUPATION-COVID-19 study. Int J Environ Res Public Health. 2021;18(5):2567. doi: 10.3390/ijerph18052567. https://doi.org/10.3390/ijerph18052567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiston R. COVID-19: Prima e seconda ondata a confronto. Scienzainrete. 2020 [Google Scholar]
- NATO Rapid Deployable Corps - Italy. Two new COVID-19 drive-through testing centres in Milan and Solbiate Olona. 2020 http://www.nrdc-ita.nato.int/956/two-new-covid-19-drive-through-testing-centres-in-milan-and-solbiate-olona. [Accessed Ausgust 30, 2021] [Google Scholar]
- ISTAT - Italian National Institute of Statisics. SARS-CoV-2 seroprevalence survey: Data tables. 2021 https://www.istat.it/it/archivio/256536. [Accessed August 31, 2021] [Google Scholar]
- Ahammed T, Anjum A, Rahman MM, et al. Estimation of novel coronavirus (COVID-19) reproduction number and case fatality rate: A systematic review and meta-analysis. Health Sci Rep. 2021;4(2):e274. doi: 10.1002/hsr2.274. https://doi.org/10.1002/hsr2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Estimating mortality from COVID-19. 2020 https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19. [Accessed September 8, 2021] [Google Scholar]
- Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect Dis. 2020;20(6):669–77. doi: 10.1016/S1473-3099(20)30243-7. https://doi.org/10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A, Delmonte D, Gaetti G, et al. Doubled mortality rate during the COVID-19 pandemic in Italy: Quantifying what is not captured by surveillance. Public Health. 2021;190:108–15. doi: 10.1016/j.puhe.2020.11.016. https://doi.org/10.1016/j.puhe.2020.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay M, Kuehl R, Tschudin-Sutter S, et al. 2019-novel Coronavirus (2019-nCoV): Estimating the case fatality rate - A word of caution. Swiss Med Wkly. 2020;150:w20203. doi: 10.4414/smw.2020.20203. https://doi.org/10.4414/smw.2020.20203. [DOI] [PubMed] [Google Scholar]
- Chirico F, Nucera G, Magnavita N. Estimating case fatality ratio during COVID-19 epidemics: Pitfalls and alternatives. J Infect Dev Ctries. 2020;14(5):438–9. doi: 10.3855/jidc.12787. https://doi.org/10.3855/jidc.12787. [DOI] [PubMed] [Google Scholar]
- Ioannidis JPA. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur J Clin Invest. 2020;50(12):e13423. doi: 10.1111/eci.13423. https://doi.org/10.1111/eci.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA. Over-and under-estimation of COVID-19 deaths. Eur J Epidemiol. 2021;36(6):581–8. doi: 10.1007/s10654-021-00787-9. https://doi.org/10.1007/s10654-021-00787-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A, Delmonte D, Scognamiglio T, et al. COVID-19 deaths in Lombardy, Italy: Data in context. Lancet Public Health. 2020;5(6):e310. doi: 10.1016/S2468-2667(20)30099-2. https://doi.org/10.1016/S2468-2667(20)30099-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health. Anti-COVID vaccination campaign. www.salute.gov.it. [Accessed August 30, 2021] [Google Scholar]
- ISS - Italian National Health Institute. Italian National Health Institute first report on SARS-CoV-2 variants. 2021 www.covid19dataportal.it/highlights/highlight6/ [Accessed August 30, 2021] [Google Scholar]
- CPD - Italian Civil Protection Department. COVID-19 data. doi: 10.1016/j.dib.2020.105526. https://github.com/pcm-dpc/COVID-19. [Accessed August 30, 2021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT - Italian National Institute of Statisics. COVID-19 deaths in Italy. Report March 5, 2021. https://www.istat.it/it/files//2020/03/tabella-decessi-provinciali_fonte_istat_decessi_provinciali_per_covid_fonte_ISS_5marzo.xlsx2021. [Accessed August 30, 2021] [Google Scholar]
- Lash TL, Vander Weele TJ, Haneuse S, et al. Modern Epidemiology. 4th Edition ed. Wolters Kluwer; 2020. [Google Scholar]
- ECDC - The European Centre for Disease Prevention and Control. Data on 14-day notification rate of new COVID-19 cases and deaths. https://www.ecdc.europa.eu/en/publications-data/data-national-14-day-notification-rate-covid-19. [Accessed August 30, 2021] [Google Scholar]
- ISS - Italian National Health Institute. FluNews - Italy. Report of integrated seasons flu serveillance. https://www.epicentro.iss.it/influenza/FluNews. [Accessed August 30, 2021] [Google Scholar]
- ISTAT - Italian National Institute of Statisics. Deaths and causes. 2021 https://www.istat.it/it/archivio/240401. [Accessed August 30, 2021] [Google Scholar]
- Gianfredi V, Santangelo OE, Provenzano S. The effects of COVID-19 pandemic on the trend of measles and influenza in Europe. Acta Biomed. 2021;92(4):e2021318. doi: 10.23750/abm.v92i4.11558. https://doi.org/10.23750/abm.v92i4.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT - Italian National Institute of Statisics. Populations and households. 2020 https://www.istat.it/en/population-and-households. [Accessed August 30, 2021] [Google Scholar]
- Fabiani M, Onder G, Boros S, et al. Roma: Istituto Superiore di Sanità; Version of January 20, 2021. Case fatality rate of SARS-CoV-2 infection at regional level and across different phases of the epidemic in Italy; p. 2021. [Google Scholar]
- He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID-19: Meta-analysis and sensitivity analysis. J Med Virol. 2020;92(11):2543–50. doi: 10.1002/jmv.26041. https://doi.org/10.1002/jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AT, Alyafei F, Elalaily R. COVID-19 virus case fatality rate: How to avoid errors in calculation of data during the outbreak? Acta Biomed. 2020;91(2):222–3. doi: 10.23750/abm.v91i2.9530. https://doi.org/10.23750/abm.v91i2.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECDC - The European Centre for Disease Prevention and Control. Technical Report: Novel coronavirus (SARS-CoV-2). Discharge criteria for confirmed COVID-19 cases: When is it safe to discharge COVID-19 cases from the hospital or end home isolation? https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-Discharge-criteria.pdf. [Accessed August 30, 2021] [Google Scholar]
- Kault D. Superspreaders, asymptomatics and COVID-19 elimination. Med J Aust. 2020;213(10):447–8.e1. doi: 10.5694/mja2.50835. https://doi.org/10.5694/mja2.50835. [DOI] [PubMed] [Google Scholar]
- Okell LC, Verity R, Watson OJ, et al. Have deaths from COVID-19 in Europe plateaued due to herd immunity? Lancet. 2020;395(10241):e110–e1. doi: 10.1016/S0140-6736(20)31357-X. https://doi.org/10.1016/S0140-6736(20)31357-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papachristodoulou E, Kakoullis L, Parperis K, et al. Long-term and herd immunity against SARS-CoV-2: Implications from current and past knowledge. Pathog Dis. 2020;78(3):ftaa025. doi: 10.1093/femspd/ftaa025. https://doi.org/10.1093/femspd/ftaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID-19 in the second wave: A study in 53 countries or regions. Transbound Emerg Dis. 2021;68(2):213–5. doi: 10.1111/tbed.13819. https://doi.org/10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- Hauser A, Counotte MJ, Margossian CC, et al. Estimation of SARS-CoV-2 mortality during the early stages of an epidemic: A modeling study in Hubei, China, and six regions in Europe. PLoS Med. 2020;17(7):e1003189. doi: 10.1371/journal.pmed.1003189. https://doi.org/10.1371/journal.pmed.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcheddu R, Serra C, Kelvin D, et al. Similarity in case fatality rates (CFR) of COVID-19/SARS-CoV-2 in Italy and China. J Infect Dev Ctries. 2020;14(2):125–8. doi: 10.3855/jidc.12600. https://doi.org/10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- Hoseinpour Dehkordi A, Alizadeh M, Derakhshan P, et al. Understanding epidemic data and statistics: A case study of COVID-19. J Med Virol. 2020;92(7):868–82. doi: 10.1002/jmv.25885. https://doi.org/10.1002/jmv.25885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Kumar Patel K, Sivaraman S, et al. Global epidemiology of first 90 days into COVID-19 pandemic: Disease incidence, prevalence, case fatality rate and their association with population density, urbanisation and elderly population. J Health Manag. 2020;22(2):117–28. https://doi.org/10.1177/0972063420932762. [Google Scholar]
- Balasco N, D’Alessandro V, Ferrara P, et al. Analysis of the time evolution of COVID-19 lethality during the first epidemic wave in Italy. Acta Biomed. 2021;92(2):e2021171. doi: 10.23750/abm.v92i2.11149. https://doi.org/10.23750/abm.v92i2.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Parr T, Zeidman P, et al. Dynamic causal modelling of COVID-19. Wellcome Open Res. 2020;5:89. doi: 10.12688/wellcomeopenres.15881.1. https://doi.org/10.12688/wellcomeopenres.15881.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfi D, Pagnoni G, Filippini T, et al. Dynamic causal modeling of the COVID-19 pandemic in Northern Italy predicts possible scenarios for the second wave. medRxiv. 2020 2020.08.20.20178798. https://doi.org/10.1101/2020.08.20.20178798 . [Google Scholar]
- Lippi G, Henry BM, Sanchis-Gomar F, et al. Updates on laboratory investigations in coronavirus disease 2019 (COVID-19) Acta Biomed. 2020;91(3):e2020030. doi: 10.23750/abm.v91i3.10187. https://doi.org/10.23750/abm.v91i3.10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castelnuovo A, Costanzo S, Antinori A, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: The multicenter Italian CORIST study. Thromb Haemost. 2021;121(8):1054–65. doi: 10.1055/a-1347-6070. https://doi.org/10.1055/a-1347-6070. [DOI] [PubMed] [Google Scholar]
- Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020;2(8):e474–e84. doi: 10.1016/S2665-9913(20)30173-9. https://doi.org/10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, Demurtas J, Yang L, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: A systematic review of the literature. Front Med (Lausanne) 2020;7:170. doi: 10.3389/fmed.2020.00170. https://doi.org/10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–6. doi: 10.1001/jama.2020.4683. https://doi.org/10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42. doi: 10.1001/jama.2020.2648. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66. doi: 10.1136/annrheumdis-2020-217871. https://doi.org/10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo A, Balestrino R, Imbalzano G, et al. Neurological comorbidity and severity of COVID-19. J Neurol. 2021;268(3):762–9. doi: 10.1007/s00415-020-10123-y. https://doi.org/10.1007/s00415-020-10123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISTAT-ISS. National Institute of Statistics and National Institute of Health. Impact of COVID-19 epidemic on total mortality in the resident population. 2021 https://www.istat.it/it/archivio/254507. [Accessed August 30, 2021] [Google Scholar]
- Signorelli C, Odone A, Gianfredi V, et al. COVID-19 mortality rate in nine high-income metropolitan regions. Acta Biomed. 2020;91(9-S):7–18. doi: 10.23750/abm.v91i9-S.10134. https://doi.org/10.23750/abm.v91i9-S.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty K, Hamilton V, Kavanagh PM. Just a bad flu? Tackling the “infodemic” in Ireland through a comparative analysis of hospitalised cases of COVID-19 and influenza. Public Health. 2021;194:19–24. doi: 10.1016/j.puhe.2021.02.019. https://doi.org/10.1016/j.puhe.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic J, Boucher VG, Boyle J, et al. COVID-19 is not the flu: Four graphs from four countries. Front Public Health. 2021;9:628479. doi: 10.3389/fpubh.2021.628479. https://doi.org/10.3389/fpubh.2021.628479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calina D, Docea AO, Petrakis D, et al. Towards effective COVID19 vaccines: Updates, perspectives and challenges (Review) Int J Mol Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. https://doi.org/10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A, Vitale M, Signorelli C. The identity of public health in COVID-19 times. Acta Biomed. 2020;91(9-S):5–6. doi: 10.23750/abm.v91i9-S.10200. https://doi.org/10.23750/abm.v91i9-S.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odone A, Lugo A, Amerio A, et al. COVID-19 lockdown impact on lifestyle habits of Italian adults. Acta Biomed. 2020;91(9-S):87–9. doi: 10.23750/abm.v91i9-S.10122. https://doi.org/10.23750/abm.v91i9-S.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirparo G, Oradini-Alacreu A, Migliori M, et al. Public health impact of the COVID-19 pandemic on the emergency healthcare system. J Public Health (Oxf) 2021:fdab212. doi: 10.1093/pubmed/fdab212. https://doi.org/10.1093/pubmed/fdab212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MS, Peer V, Schwartz N, et al. The confounded crude case-fatality rates (CFR) for COVID-19 hide more than they reveal-a comparison of age-specific and age-adjusted CFRs between seven countries. PLoS One. 2020;15(10):e0241031. doi: 10.1371/journal.pone.0241031. https://doi.org/10.1371/journal.pone.0241031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NG, Abbott S, Barnard RC, et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. https://doi.org/10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino S, Kelvin N, Bermejo-Martin JF, et al. As COVID-19 cases, deaths and fatality rates surge in Italy, underlying causes require investigation. J Infect Dev Ctries. 2020;14(3):265–7. doi: 10.3855/jidc.12734. https://doi.org/10.3855/jidc.12734. [DOI] [PubMed] [Google Scholar]
- CPD - Italian Civil Protection Department. Anti COVID-19 Vaccine Report. https://italia.github.io/covid19-dashboard-vaccini/ [Accessed August 30, 2021] [Google Scholar]


