Abstract
Background:
Pain is common in patients with chronic kidney disease (CKD). Analgesics may be appropriate for some CKD patients.
Objectives:
To determine the prevalence of overall analgesic use and the use of different types of analgesics including acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), adjuvants, and opioids in patients with CKD.
Design:
Systematic review and meta-analysis.
Setting:
Interventional and observational studies presenting data from 2000 or later. Exclusion criteria included acute kidney injury or studies that limited the study population to a specific cause, symptom, and/or comorbidity.
Patients:
Adults with stage 3-5 CKD including dialysis patients and those managed conservatively without dialysis.
Measurements:
Data extracted included title, first author, design, country, year of data collection, publication year, mean age, stage of CKD, prevalence of analgesic use, and the types of analgesics prescribed.
Methods:
Databases searched included MEDLINE, CINAHL, EMBASE, and Cochrane Library. Two reviewers independently screened all titles and abstracts, assessed potentially relevant articles, and extracted data. We estimated pooled prevalence of analgesic use and the I2 statistic was computed to measure heterogeneity. Random-effects models were used to account for variations in study design and sample populations, and a double arcsine transformation of the prevalence variables was used to accommodate potential overweighting of studies with very large or very small prevalence measurements. Sensitivity analyses were performed to determine the magnitude of publication bias and assess possible sources of heterogeneity.
Results:
Forty studies were included in the analysis. The prevalence of overall analgesic use in the random-effects model was 50.8%. The prevalence of acetaminophen, NSAIDs, and adjuvant use was 27.5%, 17.2%, and 23.4%, respectively, while the prevalence of opioid use was 23.8%. Due to the possibility of publication bias, the actual prevalence of acetaminophen use in patients with advanced CKD may be substantially lower than this meta-analysis indicates. A trim-and-fill analysis decreased the pooled prevalence estimate of acetaminophen use to 5.4%. The prevalence rate for opioid use was highly influenced by 2 large US studies. When these were removed, the estimated prevalence decreased to 17.3%.
Limitations:
There was a lack of detailed information regarding the analgesic regimen (such as specific analgesics used within each class and inconsistent accounting for patients on multiple drugs and the use of over-the-counter analgesics such as acetaminophen and NSAIDs), patient characteristics, type of pain being treated, and the outcomes of treatment. Data on adjuvant use were very limited. These results, therefore, must be interpreted with caution.
Conclusions:
There was tremendous variability in the prescribing patterns of both nonopioid and opioid analgesics within and between countries suggesting widespread uncertainty about the optimal pharmacological approach to treating pain. Further research that incorporates robust reporting of analgesic regimens and links prescribing patterns to clinical outcomes is needed to guide optimal clinical practice.
Keywords: chronic kidney disease, dialysis, analgesic use, nonsteroidal anti-inflammatory drugs, acetaminophen, opioids, adjuvants
Abrégé
Contexte:
La douleur est fréquente chez les patients atteints d’insuffisance rénale chronique (IRC). La prise d’analgésiques peut être nécessaire chez certains patients atteints d’IRC.
Objectifs:
Établir la prévalence globale de la prise d’analgésiques chez les patients atteints d’IRC puis de la consommation des différents types d’analgésiques (acétaminophène, anti-inflammatoires non stéroïdiens [AINS], adjuvants, opioïdes).
Type d’étude:
Revue systématique et méta-analyse.
Cadre:
Les études observationnelles et interventionnelles présentant des données depuis l’an 2000. Ont été exclus les cas d’insuffisance rénale aigüe et les études portant sur une population, une cause, un symptôme ou une comorbidité en particulier.
Sujets:
Des adultes atteints d’IRC de stade 3 à 5, incluant des patients dialysés et des patients non dialysés pris en charge de façon conservatrice.
Mesures:
Le titre de l’article, le nom de l’auteur principal, le type d’étude, le pays où s’est tenue l’étude, l’année de collection des données, l’année de publication, l’âge médian des sujets, le stade de l’IRC, la prévalence de la prise d’analgésiques et les types d’analgésiques prescrits.
Méthodologie:
Les données ont été colligées dans MEDLINE, CINAHL, EMBASE et la bibliothèque Cochrane. Deux examinateurs ont trié les titres et les abrégés, évalué les articles potentiellement pertinents et extrait les données de façon indépendante. La prévalence combinée de la prise d’analgésiques a été évaluée et la statistique I2 a été calculée pour mesurer l’hétérogénéité. Des modèles à effets aléatoires ont été employés pour tenir compte des variations entre les différents types d’études et de populations échantillonnées. Les variables de prévalence ont subi une double transformation arc-sinus pour tenir compte d’une potentielle surpondération des études comportant des mesures de prévalence très importantes ou très faibles. Des analyses de sensibilité ont été effectuées pour mesurer l’ampleur des biais de publication et évaluer de possibles sources d’hétérogénéité.
Résultats:
L’analyse porte sur un total de 40 études. Dans les modèles à effets aléatoires, la prévalence globale de prise d’analgésiques était de 50,8 %. Quant à la prévalence selon le type d’analgésique elle s’établissait à 27,5 % pour l’acétaminophène, à 17,2 % pour les AINS, à 23,4 % pour les adjuvants et à 23,8 % pour les opioïdes. Chez les patients atteints d’IRC de stade avancé, de possibles de biais de publication font en sorte que la prévalence réelle de l’acétaminophène pourrait s’avérer nettement inférieure à ce qu’indique cette méta-analyse. Une analyse par la méthode « trim and fill » a réduit à 5,4 % la prévalence groupée estimée pour la prise d’acétaminophène. Le taux de prévalence pour la prise d’opioïdes était fortement influencé par deux vastes études américaines; en les retirant de l’analyse, la prévalence estimée passait à 17,3 %.
Limites:
Ces résultats doivent être interprétés avec prudence puisque des informations détaillées manquaient sur le schéma posologique (analgésiques particuliers utilisés dans chaque classe, comptabilisation incohérente pour les patients prenant plusieurs médicaments, prise d’analgésiques en vente libre tels que l’acétaminophène et les AINS), les caractéristiques des patients, les types de douleurs traitées et les résultats des traitements. De plus, les données sur la prise d’adjuvants étaient très limitées.
Conclusion:
Une très grande variabilité a été observée dans les profils de prescription tant pour les analgésiques opioïdes que pour les non-opioïdes. Une variabilité qui s’observe aussi tant dans un même pays qu’entre les différents pays, ce qui suggère une incertitude généralisée quant à la meilleure approche pharmacologique dans le traitement de la douleur. D’autres recherches intégrant une description rigoureuse du schéma posologique et reliant les profils de prescription aux résultats cliniques sont nécessaires pour guider l’optimisation des pratiques cliniques.
What was known before
Pain is common in patients with chronic kidney disease (CKD), and analgesics may be appropriate for some CKD patients to promote patient-centered care and improve patient outcomes. Very little is known about current analgesic prescribing for patients with CKD.
What this adds
There is tremendous variability in the prescribing of analgesics suggesting widespread uncertainty about the optimal pharmacological approach to treating pain.
Introduction
Pain is common in patients with chronic kidney disease (CKD). It is associated with considerable disability and lower health-related quality of life (QOL) and is a significant burden on the healthcare system.1,2 The treatment for chronic nonmalignant pain may include nonopioids such as acetaminophen, adjuvants (drugs such as gabapentin and amitriptyline that are not primarily indicated to control pain but can be used for this purpose), as well as opioids.3,4
The international nephrology community advocates for routine screening and management of pain as a way to promote patient-centered and outcome-oriented healthcare.5,6 Analgesics may be appropriate for some CKD patients. However, their use, especially that of opioids for chronic pain, is accompanied by significant risks. Patients with advanced CKD are also at added risk of drug-related adverse effects and toxicity due to the altered pharmacokinetics and pharmacodynamics in kidney failure. 7 Given the limited availability of high-quality clinical trials in chronic pain for patients with CKD, current recommendations are based on guidelines for the general population, clinical experience, and best opinion.
A better understanding of the analgesic prescribing patterns for patients with advanced CKD is required to understand care gaps and optimize pain management strategies. The objective of this systematic review was to determine the prevalence of analgesic use in patients with CKD and to determine the types of analgesics being prescribed for these patients.
Methods
Search Strategy and Eligibility Criteria
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for systematic reviews were used. The literature search was developed and conducted by an experienced librarian. The detailed protocol is outlined in Supplemental Table S1. The predetermined inclusion and exclusion criteria are outlined in Table 1. We included all interventional and observational studies that presented original data of the use of analgesics (including nonsteroidal anti-inflammatory drugs [NSAIDs], acetaminophen, opioids, and adjuvants) in adult patients with glomerular filtration rate (GFR) category (G) 3-5 CKD. Kidney transplant patients were included if they also had reduced kidney function presenting as G3-5 CKD. We included studies presenting data from 2000 or later, given the change in clinical practice around pain management with increased focus on prescribing analgesics since that time. Single-case studies or case series were excluded, as were studies that were presented only as abstracts, posters, or Letters to the Editor. Articles published in a language other than English were translated and included. An online Neural Machine Translation tool was utilized to provide a general translation of the non-English articles and native speakers were consulted as needed. Studies that only enrolled patients with a primary diagnosis of acute kidney injury or kidney transplant patients with preserved kidney graft function were excluded as were studies that limited the study population to a specific cause, symptom, and/or comorbidity (with the exception of chronic pain) of CKD as these studies were outside the scope of our study objectives. Other exclusion criteria included studies that were limited to acute or intradialytic pain.
Table 1.
Inclusion and Exclusion Criteria.
| PICOs | Description |
|---|---|
| Populationa | • CKD of stage 3, 4, 5 (predialysis, dialysis, or CKM) • ≥18 years of age • Any treatment type (peritoneal dialysis, hemodialysis, or CKM) • Must be identified as having CKD prior to enrollment in study |
| Outcome | • Any analgesic use (quantified) • Opioid use • NSAID use • Acetaminophen use • Adjuvant use |
| Study Design | • Cross-sectional studies • Observational studies • Case-control studies • Cross-over trials • Clinical trials • Chart reviews |
| Exclusion Criteria | • <18 years of age • Case series, abstracts, posters, reviews, opinions • Acute kidney injury • Kidney transplant, unless clearly identified as having CKD (stage 3-5 or eGFR lower than 60) • Data (initial assessment) prior to 2000 • Population limited to a specific cause of ESKD or selected based on specific symptom/comorbidity (with the exception of chronic pain) • Acute pain or pain related to dialysis treatment • Missing raw data, numerator, or denominator • Palliative study populations |
Note. CKD = chronic kidney disease; CKM = conservative kidney management; NSAID = nonsteroidal anti-inflammatory drug; eGFR = estimated glomerular filtration rate; ESKD = end stage kidney disease; PICOs = population, outcome, study design, and exclusion criteria.
Information Sources
Information sources included electronic databases, reference lists of relevant literature, and websites of relevant networks, organizations, and societies. The electronic databases searched included MEDLINE, CINAHL, EMBASE, and Cochrane Library databases. These were last searched on February 19, 2019.
Study Selection and Data Collection
Two reviewers independently screened all titles and abstracts to identify potentially relevant articles. Full texts of potentially relevant articles were retrieved and assessed independently by two reviewers for possible inclusion based on the predetermined selection criteria. The reference lists of reviews, systematic reviews, and guidelines were also reviewed to ensure all relevant studies were identified. The two reviewers compared individually recorded decisions for inclusion and exclusion and any disagreements were resolved based on discussion and consensus with a third reviewer.
Data Items
The outcomes of interest were the prevalence of analgesic use in patients with G 3-5 CKD and the types of analgesics prescribed. The research team developed a standardized data extraction table using Microsoft Excel. The two reviewers populated the table independently from the selected full text articles. The information collected included study location (geographical area and time), study objectives, study design, population demographics, analgesics used, and numerical data on the prevalence of analgesic use. The two data extraction tables were subsequently compared and cross-checked for accuracy and then merged into a single unified table for data analysis and presentation in the article.
Statistical Methods
All analyses were conducted using Microsoft R Open version 3.4.1 using R package meta.8,9 A meta-analysis was conducted to estimate pooled prevalence of analgesic use. Random-effects models were used to account for variations in study design and sample populations. The results were plotted using forest plots. A double arcsine transformation of the prevalence variables was used in the model to accommodate possible issues with overweighting studies with either very large or very small prevalence measurements. 10
The I2 statistic was computed to measure heterogeneity. 11 The I2 value is the percentage of total observed variation across studies due to real heterogeneity rather than chance; a value of greater than 75% is indicative of high heterogeneity. To assess the possibility of publication bias, a meta-regression testing funnel plot asymmetry was conducted using the Peters’ method.8,9,12 Sensitivity analyses using trim and fill algorithms were performed to determine the magnitude of publication bias. 13 Meta-regressions of various continuous and categorical grouping variables on prevalence were conducted to assess possible sources of heterogeneity. Bubble plots were used to illustrate the regression of transformed prevalence onto continuous covariates, and stratified forest plots were constructed to visualize the effects of grouping categorical covariates on both random-effects estimates and heterogeneity.
Results
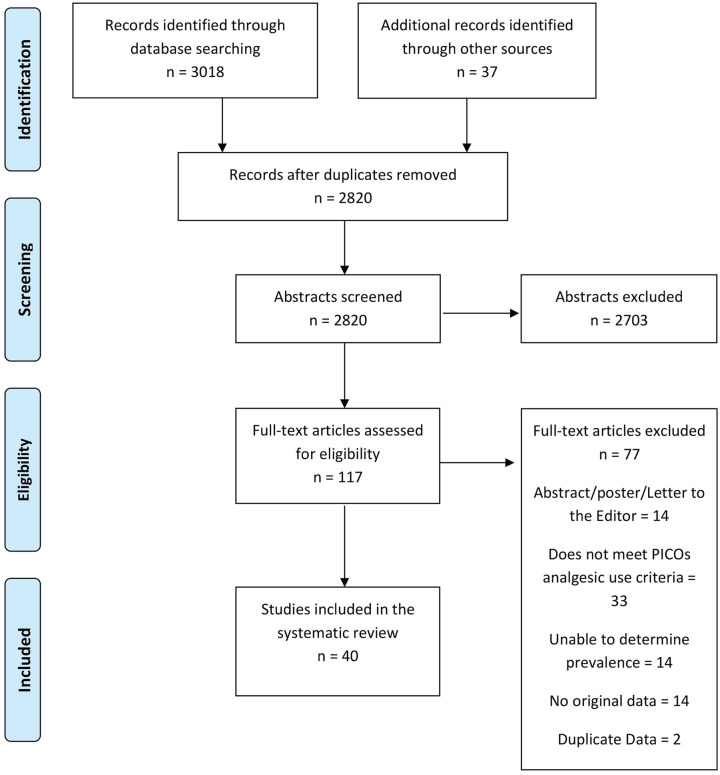
The literature review yielded 3055 citations of which 117 were deemed eligible for full text review. Of these, 40 studies were included in the analysis. The flow chart in Figure 1 outlines this process, including reasons for exclusion. Supplemental Table S2 provides a list of excluded studies with reasons for exclusion.
Figure 1.
Analgesic prevalence systematic review Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Note. PICOs = population, outcome, study design, and exclusion criteria.
Details of Included Studies
Details of the 40 included studies14-53 are reported in Table 2 and include data from 963 269 patients from 21 countries. Out of the 40 studies, 17 included a prevalence measure of overall analgesic use,14,16-18,20,23,25,29,33,36,37,43,45,46,48,49,52 33 included a prevalence measure of one or more specific analgesic drug class, defined as opioid, NSAID, adjuvant, and nonopioid,14-24,26-28,30-47,50 and 18 measured the prevalence of one or more specific analgesic drug, including acetaminophen.14-16,19,20,22,30,33,36-40,43,48,50,51,53 Three studies14,38,44 contained repeated measures; the final prevalence data were used in these cases.
Table 2.
Characteristics and Results of Included Studies.
| Study | Country | Patient population | Study design | Number of patients | Mean age (Years) | Analgesic use prevalence |
|---|---|---|---|---|---|---|
| Bailie et al 14 | United States | Prevalent HD | Prospective observational study (United States DOPPS data) |
3749 | 61 | In 2000 the prevalence of analgesic prescription in the study population was 24.3%. 4.9% of the entire study population was prescribed COX-2 agents in 2000. The total prevalence of patients prescribed a narcotic was 14.9% in 2000. Prevalence of combination use of a narcotic and COX-2 agent was 1.2%. The total prevalence of any NSAID prescription in 2000 was 2.3%. Prevalence of prescription combining a narcotic and a NSAID was 0.6% while the prevalence of combination use of a COX-2 agent and a NSAID was 0.2%. The total prevalence of acetaminophen use in the study population was 6.3%. The prevalence of prescriptions for combination use of a narcotic and acetaminophen was 1.7% and 0.5% for a combination of acetaminophen and a COX-2 agent. In 2000, 0.3% of the study population had a prescription for Acetaminophen combined with a NSAID. |
| Bailie et al 15 | France, Germany, Italy, Spain, United Kingdom, Japan, United States | Prevalent HD | Cross-sectional, prospective, observation study (DOPPS I data) |
Total: 8628 N for analgesic prevalence calculation: 8455 |
60 (Calculated with n = 8542) |
In 2000, the prevalence of NSAID use was 5.3%, 4.4% for acetaminophen, and narcotic use had a prevalence of 9.5% |
| Barrantes et al 16 | United States | Transplant | Single center retrospective study | Total: 1064 Chronic opioid usage: 108 No chronic opioid usage: 956 |
Chronic opioid usage: 51 No chronic opioid usage: 49 |
Of the total study population 10.2% had a history of chronic opioid usage prior transplantation. Of those that used opioid medications the most prevalent were hydrocodone (43.1%), propoxyphene (18.1%), oxycodone (16.4%), tramadol (13.8%), others (8.6%). Prevalence of a nonopioid analgesic was 10.1% of the total study population. |
| Battistella et al 40 | Canada | Prevalent HD | Descriptive, retrospective, cross-sectional study | 3134 | 77 | The total prevalence of prescriptions for opioid medication was 25.5%, 5.5% for NSAID and 3.5% for aspirin. |
| Bouattar et al 17 | Morocco | Prevalent HD | Cross-sectional study | 67 | 44 | The total prevalence of analgesic use 50.7%. Of those that used analgesics, 52.9% used a weak opioid and 47.1% used nonopioid analgesics. |
| Carreon et al 18 | United States | Prevalent HD | Cross-sectional analysis of a cohort of HD patients | Total: 75 With bone/joint pain: 27 (Analgesic use data unavailable for 1 patient that reported pain) |
59 | Of the patients reporting bone/joint pain (that had analgesics data available) 48.1% were receiving analgesic treatment. Six patients were taking both a narcotic and over-the-counter agent, 4 were taking a narcotic only, and 3 were using over-the-counter NSAIDs or acetaminophen. There were 22 other patients on analgesic therapy who did not report bone/joint pain. 73% of these patients without bone/joint pain were receiving NSAIDs and/or acetaminophen, while 27% were prescribed narcotics. |
| Chan et al 43 | Hong Kong | CKM | Intervention | 253 | Patients with Pain: 79 Patients without Pain: 80 |
At baseline of those patients with significant pain (Edmonton Symptom Assessment System pain score ≥ 4) 56.5% were on a regularly scheduled analgesic: 43.5% patients received regular acetaminophen, 2% were on weak opioid (tramadol), and 11% on other alternatives (gabapentin/pregabalin/sodium valproate). |
| Claxton et al 19 | United States | Prevalent HD | Prospective observational, cross-sectional study | 62 | 59 | Opioids and gabapentin were the most frequently prescribed for pain (32% for both). The prevalence of prescription acetaminophen was 26%, 11% for tramadol, and 16% for NSAIDs. |
| Daubresse et al 44 | United States | Prevalent HD | Retrospective cohort study | 2007-2014: 484 745 2007: 163 558 2014: 208 807 |
Not available | The percentage of patients undergoing hemodialysis who received an opioid prescription slightly increased from 62.4% in 2007, 62.5% in 2008, 62.8% in 2009 and 63.2% in 2010. The prevalence of opioid usage declined to 62.4% in 2011, 60.9% in 2012, and 60.2% in 2013. By 2014, the proportion of patients received an opioid declined to 53.7%. |
| Davison 20 | Canada | Prevalent HD | Prospective cohort study | Total: 205 With pain: 103 No pain:102 |
60 | Of the patients reporting pain 35% were administered no analgesics, 29.1% were administered nonopioid analgesics, 26.2% were administered weak opioids, and 9.7% were administered strong opioids. Two types of weak opioids were administered concurrently in 6 patients and 2 types of strong opioids were administered concurrently in 3 patients. The prevalence of nonopioid medication was 21.4% for acetaminophen, 5.8% for NSAIDs and 1.9% for Adjuvant only. Adjuvant therapy was used in combination with analgesics for 22 patients (21.4%). The prevalence of weak opioid medication was 20.4% for codeine, 3.9% for propoxyphene, 1.0% for pethidine, and 6.8% for oxycodone. The prevalence of strong opioid medication was 5.8% for hydromorphone, 2.9% for methadone, 1.9% for fentanyl, and 1.9% for morphine. |
| Desmet et al 21 | Belgium | Prevalent HD | Prospective multicenter cohort study | Total: 308 Falls: 39 No falls: 269 |
Falls: 73 No fall: 66 |
The prevalence of opioid derivative medication use was 28.2% patients with falls used and 9.7% patients without falls. |
| Dorks et al 22 | Germany | Nursing home residents (Categorized by renal function) |
Multicenter cross-sectional study | Total: 685 CCR ≥ 90: 84 CCR 60-89: 165 CCR 30-59: 330 CCR < 30: 106 |
83 | In the subgroup of residents with moderate renal failure (CCR 30-59), 48.2% were treated with NSAIDs. In the subgroup of residents with severe renal failure (CCR < 30), 15.8% were treated with NSAIDs. The most commonly used NSAIDs was ibuprofen (92.0 %), diclofenac (4.0 %), and acemetacin (4.0 %). |
| El Harraqui et al 23 | Morocco | Prevalent HD | Cross-sectional study | 93 | 52 | The total prevalence of analgesic use in the study population was 53.8%. Of the patients using analgesics 68.0% were using nonopioid medication and 44% were using an opioid. |
| Elder et al 24 | France, Germany, Italy, Spain, United Kingdom, Japan, United States | Prevalent HD | Prospective observational study (DOPPS data) |
Total: 6321 Good sleepers: 3189 Poor Sleepers: 3132 |
Good sleepers: 59 Poor sleepers: 59 |
The total prevalence of prescribed narcotic medications was 34.1% in the poor sleep quality group and 22.4% in the good sleep quality group. |
| Finkelstein et al 25 | United States | Prevalent HD (At home short daily HD patients) |
Prospective, observational cohort study (FREEDOM Study) |
291 | 53 | The total prevalence of analgesic use in the study population was 36.4%. |
| Fleishman et al 45 | Israel | Prevalent HD | Cross-sectional survey study | 336 | 63 | Of the 277 patients with pain, 66.1% reported being regularly treated with pain medication. Based on purchase data, 63% of patients purchased some analgesics over the year before study interview: 31% of patients purchased nonopioid medications, 21% weak opioids, and 11% strong opioids. |
| Gamondi et al 26 | Switzerland | Prevalent HD | Cross-sectional, observational multicenter study | 123 | 71 | Of the 81 patients reporting pain. 80.2% were using NSAIDs, 16.0% were treated with weak opioids, and 4.9% with strong opioids. Other pain specific pharmacotherapy prevalence for patients reporting pain was and 11.1% for neuropathic pain medication. |
| Gómez Pozo et al 46 | Spain | Prevalent HD | Observational, descriptive, transversal study | 134 | 68 | The overall prevalence of analgesic use was 60%. Of those using an analgesic medication, 82% were using a nonopioid, 12% were using a weak opioid, and 6% were using a strong opioid. The prevalence of analgesic use for musculoskeletal pain was 9.1%. |
| Guirguis-Blake et al 47 | United States | Predialysis CKD Stage 3-5 | Cross-sectional descriptive study | 373 | Not Available | The prevalence of a NSAID prescription was 34.0%. |
| Heleniak et al 27 | Poland | Predialysis CKD Stage 1-4 Prevalent HD or PD Transplant |
Cross-sectional survey study | Total: 972 CKD 1-4: 574 PD: 44 HD: 40 Transplant: 314 |
Total: 55 CKD 1-4: 57 PD: 56 HD: 61 Transplant: 50 |
In the hemodialysis patient group 42.5% reported they did not use NSAIDs, 10% reported they used NSAIDs a few times a year, 12.5% reported few times a month, 17.5% reported using NSAIDs a few times a week, and 17.5% reported daily use. In the peritoneal dialysis patient group, 43.2% reported they did not use NSAIDs, 29.5% reported they used NSAIDs a few times a year, 18.2% reported few times a month, 4.5% reported using NSAIDs a few times a week, and 4.5% reported daily use. |
| Hull et al 28 | East London, United Kingdom | Stage 3-5 CKD (GFR < 60) predialysis | Cross-sectional, database review study | 12 011 | 71 | The prevalence of a NSAID prescription for patients with Stage 3 CKD was 11.5%, 5.5% for Stage 4, and 3.0% for Stage 5. |
| Iacono 29 | United States | Prevalent HD | Cross-sectional observational study | 45 | Not Available | 30% of patients reported taking prescription medication to control pain. |
| Ingrasciotta et al 30 | Italy | CKD (Stage not specified; Identified by CKD-related ICD 9 code among cause for hospitalizations, procedures in hospital & drug prescriptions) |
Chart review/ cross-sectional study | Incident CKD: 1989 Patients with Incident CKD receiving dialysis during follow-up: 112 |
Not Available | Data from 112 patients 1 year after starting dialysis showed the prevalence of at least one prescription NSAID was 29.5%. The most commonly most commonly reported NSAID use was nimesulide (7.1%), diclofenac (6.3%), ketoprofen (8.0%), coxib (6.3%), piroxicam (0.9%), and ketorolac (7.1%) The combined prevalence of use for other NSAIDs was 4.5%. |
| Ishida et al 48 | United States | Prevalent HD | Observational cohort study | 140 899 | Median age: 61 (51-72) | The prevalence of an opioid prescription opioid was 64% in 2011. The prevalence of individual opioid medications was as follows: hydrocodone (43%), oxycodone (22%), tramadol (15%), codeine (7%), hydromorphone (3%), fentanyl (3%), morphine (2%), and methadone (1%). |
| Iwagami et al 53 | United Kingdom | CKD (GFR < 60) predialysis CKM |
Matched cohort study | Patients with CKD: 242 379 Patients without CKD: 242 349 |
Not available | Of the 202 291 CKD patients followed up, 1.7% were on tricyclic antidepressants and had neuropathic pain. |
| Jadoul et al 31 | Australia, New Zealand, Belgium, Canada, France, Germany, Italy, Spain, Sweden, United Kingdom, United States | Prevalent HD | Prospective observational study (DOPPS II data) |
12 782 | Not available | The prevalence of narcotic combinations (eg, acetaminophen-codeine, acetaminophen-hydrocodone, and acetaminophen-oxycodone) use was 5.6% while the prevalence of narcotic only use was 3.0%. |
| Jhamb et al 49 | United States | CKD Stage 4-5 (not on dialysis) Prevalent dialysis (does not specify) |
Post hoc analysis of a prospective cohort study | Predialysis CKD Stage 4-5: 82 Dialysis: 149 |
CKD Stage 4-5 (not on dialysis): 52 Dialysis: 56 |
The prevalence of analgesic use was 50.0% in the nondialysis group. Of those reporting significant pain, visual analog scale (VAS) score ≥ 5/10, 80.0% reported pain medication use. The prevalence of analgesic use was 35.9% in the dialysis group. Of those reporting significant pain (VAS score ≥ 5/10), 48.6% reported pain medication use. |
| Keohane et al 50 | United Kingdom | CKD (GFR <60) | Post hoc analysis of a prospective cohort study | Total: 158 Stage 3: 146 Stage 4: 10 Stage 5: 2 |
76 | The prevalence of a NSAID prescription was 10.12% for the CKD stage 3-5 cohort. The prevalence of individual NSAID was as follows: Cox-2 (5.7%), dexketoprofen (1.9%), ibuprofen (1.3%), and diclofenac (0.6%). |
| Kimmel et al 38 | United States | Prevalent HD or PD | Cohort study | 153 758 | Not available | In 2010, 64% of the study population had a prescription for opioid medication. Of those, 41% were short-term (<90 days) and 23% chronic prescription (≥90 days). Of the chronic opioid prescription, 11.7% had a prescription for hydrocodone, 5.4% for oxycodone, 1.4% propoxyphene, 2.5% tramadol, 0.6% codeine, 0.7% morphine, 0.6% hydromorphone, 1.3% fentanyl. In 2010, 14.2% of the study population had a prescription for a nonopioid analgesic. |
| Kristensen et al 39 | Denmark | Prevalent dialysis (does not specify) |
Cross-sectional study | 6663 | 62 | The total prevalence of NSAID use in the study population was 18.4% 1 year prior to starting renal replacement therapy. Of the NSAIDs reported, the prevalence of use was 7.9% for ibuprofen, 4.6% for diclofenac, 1.1% for rofecoxib, 0.8% for celecoxib, 1.3% for naproxen, and 5.3% for other NSAIDs. |
| Mahmoud et al 32 | Tunisia | Stage 3-5 CKD (Admitted with superimposed AKI) |
Prospective case-control study | Case patients:58 Stage 3: 19 Stage 4: 34 Stage 5: 5 Control: 114 |
67 | Nine case patients out of 58 (15.5%) had used NSAIDs within 30 days of AKI episode, whereas 5 control patients out of 114 (4.4%) had used NSAIDs in the last 30 days. |
| Masajtis-Zagajewska et al 33 | Poland | Prevalent HD Transplant |
Cross-sectional study | HD: 164 Transplant: 114 |
HD: 61 Transplant: 47 |
Of the 120 hemodialysis patients with chronic pain 15% reported no analgesic use, 50% used paracetamol, 49.1% used metamizol, 36.6% used ketoprofen, 11.7% used diclofenac, 28.3% used ibuprofen, 13.3% reported Other NSAID use, 16.7% reported use of tramadol, and 1.7% reported using opioid medication. |
| Mina et al 51 | United States | Prevalent HD | Retrospective cohort study | 140 899 | Muscle relaxant use: 56 No muscle relaxant use: 61 |
The prevalence of muscle relaxant use was 10.2%. The prevalence of use for individual agents was as follows: cyclobenzaprine (6.8%), carisoprodol (1.4%), methocarbamol (1.2%), baclofen (0.7%), tizanidine (0.7%), orphenadrine citrate (0.2%), metaxalone (0.2%), chlorzoxazone (0.08%), and dantrolene sodium (0.02%). |
| Otsuki et al 34 | Japan | Prevalent HD (with neuropathic pain) |
Prospective, open-label, single-arm, multicenter trial | Included: 45 Analyzed: 35 |
72 (68-76) | The total prevalence of NSAID use in the study population was 20%. |
| Ou et al 35 | Taiwan | Prevalent HD | Nationwide, population-based case-control study | Total: 55 742 Before matching: 12 486 NSAID users and 43 256 nonusers Matched cohort pairs: 11 699 patients using NSAIDs and 11 699 controls |
Not using NSAID: 59 Using NSAID: 58 |
The total prevalence of NSAID use in the study population was 22.4%. |
| Plantinga et al 41 | United States | CKD (GFR ≥ 15) Predialysis | Cross-sectional study | Total: 12 065 No CKD: 9604 Mild CKD (Stage 1-2): 1137 Moderate-severe CKD (Stage 3-4): 1324 |
Total: 51 No CKD: 47 Mild CKD: 58 Moderate-severe CKD: 73 |
The prevalence of reported NSAID use in the moderate to severe CKD group was 5.7%. |
| Rodriguez Calero et al 36 | Spain | Prevalent HD | Descriptive transversal cohort study | 32 | 67 | 34.3% of patients did not report analgesics use. The total prevalence of paracetamol use in the study population was 65.6%. The prevalence of weak opioid use was 25% and strong opioid use was reported by 15.6%. The prevalence of adjuvant use was 31.2% if the study population. |
| Wu et al 37 | United States | CKD (GFR < 60) predialysis | Descriptive cohort study (Use baseline data from the safe kidney cohort study) |
Total: 308 No chronic pain: 121 Mild pain: 97 Severe pain: 90 |
No chronic pain: 66 Mild: 67 Severe: 65 |
Of the NSAIDs reported the prevalence of use was 2.3% for aspirin (dosage > 325mg), 1.9% for ibuprofen, 1.6% for naproxen, 0.9% for indomethacin, 0.3% for diclofenac, 0.3% for etodolac, and 0.3% for salsalate. The prevalence of opioid use was 15.3% for tramadol, 8.1% for oxycodone, 3.2% for codeine, 2.6% for hydrocodone with acetaminophen, 0.9% for morphine, and 0.3% for methadone. Of the other analgesics recorded, the prevalence of use was 33.8% for acetaminophen, 0.6% for butalbital with acetaminophen and caffeine, and 0.3% for sulfasalazine. |
| Yesil et al 52 | Turkey | Prevalent HD | Cross-sectional survey study | 70 | 45 | Of the 53 patients with pain the prevalence of analgesic use was 54.7% |
| Zhan et al 42 | United States | CKD (GFR 20-70) predialysis | Observational cohort study (Chronic Renal Insufficiency Cohort Study) |
Total: 3872 Stage 1/2: 389 Stage 3A: 1242 Stage 3B: 1486 Stage 4/5: 755 |
21-74 | At baseline the prevalence of reported NSAID use in the stage 3A group was 31.1%. The stage 3B group had a NSAID use prevalence of 20.2%. The prevalence of NSAID used declined to 8.9% in the stage 4/5 group. |
Note. HD = hemodialysis; DOPPS = Dialysis Outcomes and Practice Patterns Study; COX-2 = cyclooxygenase-2; NSAID = nonsteroidal anti-inflammatory drug; CCR = creatinine clearance; FREEDOM = Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease; CKD = chronic kidney disease; GFR = glomerular filtration rate; CKM = conservative kidney management; PD = peritoneal dialysis; ICD = International Statistical Classification of Diseases and Related Health Problems; AKI = acute kidney injury.
Three prevalence groupings were extracted that had sufficient data to complete full meta-analyses: overall analgesic use prevalence (17 studies),14,16-18,20,23,25,29,33,36,37,43,45,46,48,49,52 opioid use prevalence (13 studies),14-16,18,19,21,24,26,31,37,38,40,44 and NSAID use prevalence (19 studies).14,15,19,20,22,26-28,30,32,34,35,37,39-42,47,50
A meta-analysis was also done for acetaminophen use, which included 8 studies,14,15,19,20,33,36,37,43 and adjuvant use (5 studies).19,20,26,36,43 Analyses were also conducted using data from the studies that characterized analgesics as nonopioids (8 studies),16-18,20,23,37,38,46 weak opioids (8 studies),17,20,23,26,36,43,45,46 and strong opioids (6 studies).20,26,33,36,45,46
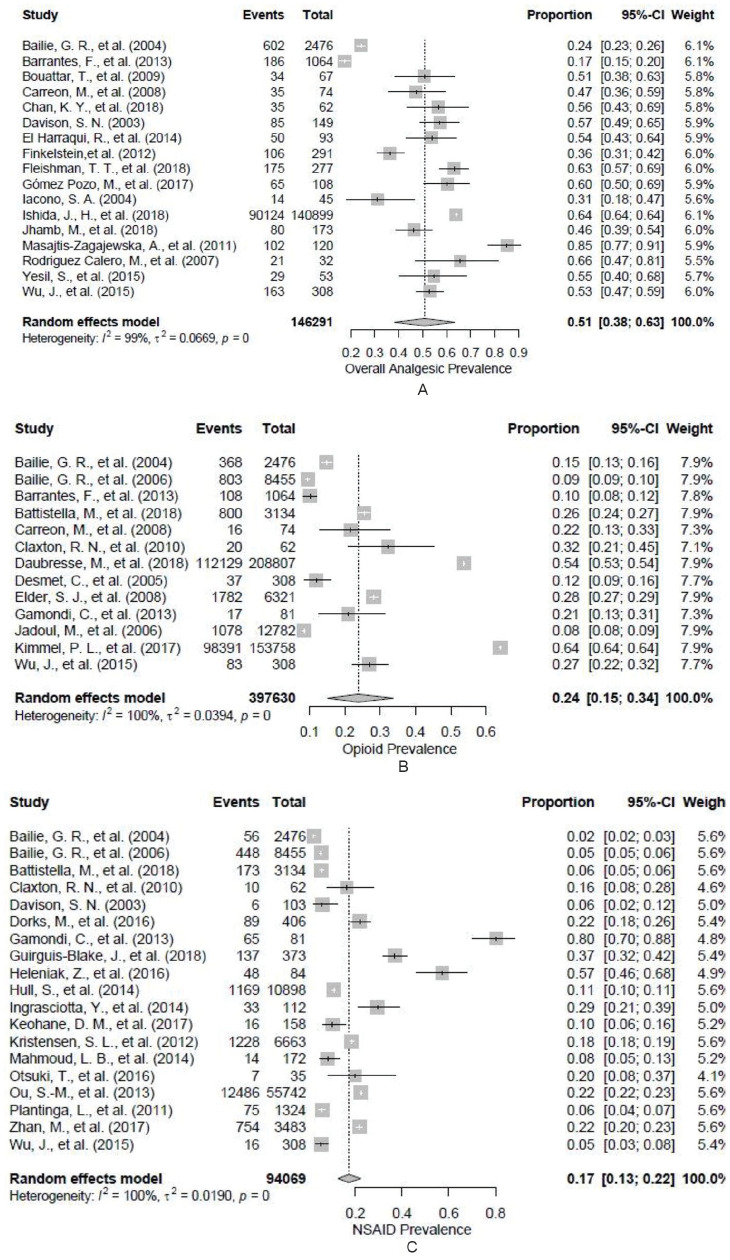
Figure 2 displays the results from random-effects meta-analyses on the 3 main prevalence groupings. The prevalence of overall analgesic use was 50.8% (38.8%-63.3%). The prevalence for use of opioids and NSAIDs was 23.8% (15.2%-33.7%) and 17.2% (12.6%-22.3%), respectively. In all cases, heterogeneity was extremely high (I2 > 98%). The prevalence rate for opioid use was highly influenced by 2 large US studies.31,38,44 When these were removed, the estimated prevalence decreased to 17.3% (13.0%-22.7%), although the heterogeneity remained high.
Figure 2.
Forest plot of random-effects model with pooled estimate and 95% confidence interval on (A) overall analgesic use prevalence, (B) opioid use prevalence, and (C) NSAID use prevalence.
Note. Double arcsine transformation used. NSAID = nonsteroidal anti-inflammatory drug.
Prevalence categories with number of articles, estimated pooled prevalence, and I2 are shown in Table 3, with a further breakdown by specific analgesic in each analgesic category in Supplemental Table S3. The prevalence for use of acetaminophen and adjuvants was 27.5% (17.6%-38.5%) and 23.4% (16.5%-31.0%), respectively. In studies where analgesics were characterized as either nonopioid, weak opioid, or strong opioid, the prevalence for use was 26.8% (19.2%-35.2%), 17.1% (10.8%-24.5%), and 6.7% (3.2%-11.2%), respectively. Heterogeneity was moderate in reported adjuvant use (I2 = 58.4%), high in strong opioid use (I2 = 74.8%), and extremely high (I2 > 98%) in acetaminophen, nonopioid, and weak opioid use.
Table 3.
Prevalence Categories With Number of Articles, Estimated Pooled Prevalence, and I. 2
| Category | Number of articles | Pooled prevalence (95% CI) | I 2 |
|---|---|---|---|
| Overall analgesic14,16-18,20,23,25,29,33,36,37,43,45,46,48,49,52 | 17 | 50.8% (38.3%-63.3%) | 99.4% |
| Opioid14-16,18,19,21,24,26,31,37,38,40,44 | 13 | 23.8% (15.2%-33.7%) | 100.0% |
| Weak opioida,17,20,23,26,36,43,45,46 | 8 | 17.1% (10.8%-24.5%) | 83.8% |
| Strong opioida,20,26,33,36,45,46 | 6 | 6.7% (3.2%-11.2%) | 74.8% |
| NSAID14,15,19,20,22,26-28,30,32,34,35,37,39-42,47,50 | 19 | 17.2% (12.6%-22.3%) | 99.6% |
| Acetaminophen14,15,19,20,33,36,37,43 | 8 | 27.5% (17.6%-38.5%) | 98.6% |
| Adjuvants19,20,26,36,43 | 5 | 23.4% (16.5%-31.0%) | 58.4% |
| Nonopioid16-18,20,23,37,38,46 | 8 | 26.8% (19.2%-35.2%) | 96.9% |
Note. CI = confidence interval; NSAID = nonsteroidal anti-inflammatory drug.
Weak opioids are typically codeine or tramadol. All other opioids are considered strong opioids.
Many studies limited analgesic information to analgesic class without specifying the specific drug used. However, in studies where specifics were provided, diclofenac and ibuprofen were the most commonly reported NSAIDs, with 622,30,33,37,39,50 and 522,30,37,39,50 studies giving prevalence information, respectively (Supplemental Table S3). The most commonly reported weak opioid was tramadol16,19,33,37,38,43,48; the most commonly reported strong opioid was oxycodone.16,20,37,38,48 In the United States, however, hydrocodone was the most commonly reported opioid used.
Peters’ regression testing funnel plot asymmetry did not give evidence for publication bias in overall analgesic, opioid, or NSAID prevalence reporting (P = .42, P = .54, and P = .55, respectively). A similar analysis of the additional groupings suggested possible bias leading to over reporting in the literature for acetaminophen (P = .02) and nonopioid (P = .03) use. A trim-and-fill analysis decreased the pooled prevalence estimates to 5.4% (1.1%-12.2%) for acetaminophen use and 15.0% (9.4%-21.8%) for nonopioid use. However, the I2 value was not improved in either case.
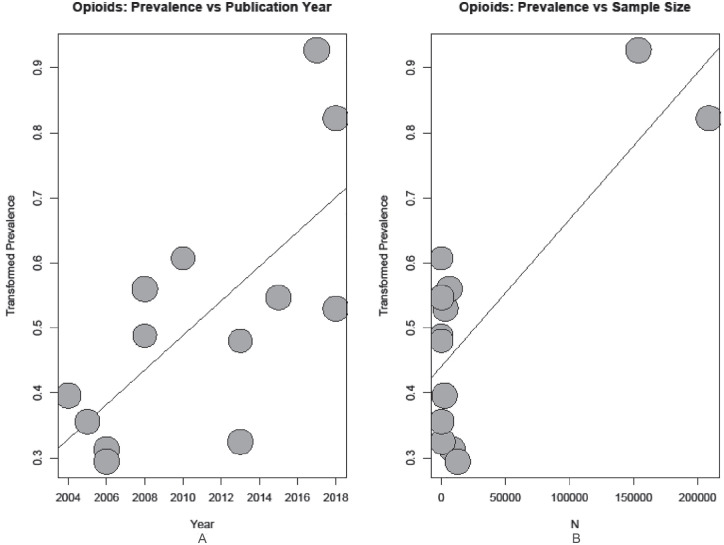
Seven covariates were tested in the meta-analyses: sample size, publication year, study region, patient population, whether or not the study used prescription/insurance data vs patient reported analgesic use, and whether or not analgesics were the primary focus of the study. Results of the meta-regression with respect to overall analgesic use returned no evidence for a relationship between use and any of the covariates tested. In testing overall opioid use, the meta-regression found evidence for a relationship between prevalence and both publication year (P < .001) and sample size (P < .001); in both cases, bubble plots show an increase in prevalence reporting (Figure 3).
Figure 3.
Bubble plot of reported opioid use prevalence by (A) publication year and (B) sample size.
Note. Regression line from meta-regression plotted: (A) P value < .001; (B) P value < .001.
In the case of sample size, this appears to be the result of 2 very large studies heavily influencing the results.31,44 For NSAID use, study year and region were found to be significant (P = .004 and P = .01, respectively); a bubble plot of the study year regression shows an increase in prevalence reporting by year (Figure 4). A stratified analysis of NSAID use prevalence returned 2 large regional groupings which appear to differ in reported prevalence: North America with a pooled prevalence of 10.5% (4.4%-18.8%), and Europe with a pooled prevalence estimate of 29.2% (21.5%-37.5%) (Supplemental Figure S1). Three additional regions were present (Africa, Asia, and 1 multiregion group), with pooled prevalence estimates of 8.1% (4.5%-12.8%), 22.0% (21.7%-22.4%), and 5.3% (4.8%-5.8%), respectively. However, in all cases heterogeneity remained extremely high.
Figure 4.
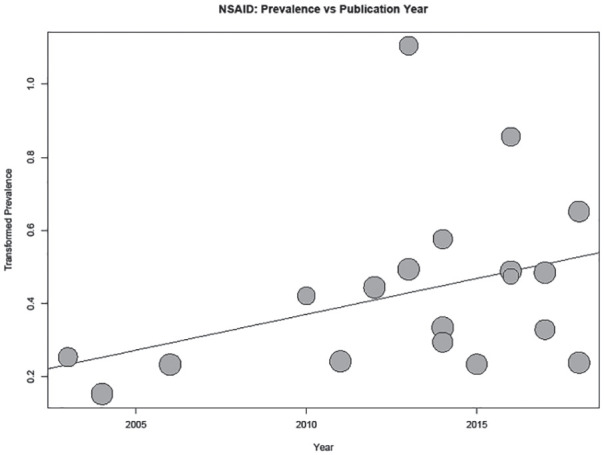
Bubble plot of reported NSAID use prevalence by publication year.
Note. Regression line from meta-regression plotted: P value = .004. NSAID = nonsteroidal anti-inflammatory drug.
Due to the smaller number of studies available to analyze acetaminophen and adjuvant use, as well as the data characterized as nonopioid, weak opioid, or strong opioid, many covariates left the data too sparse to properly interpret using regression. However, a decrease in heterogeneity was present in some models. Heterogeneity in strong opioid use decreased to I2 = 19.5% when stratified by whether prescription/insurance data were used versus self-report, with prescription data showing higher prevalence use. Stratification by region also decreased heterogeneity in strong opioid use to I2 = 63.0%, and adjuvant use to I2 = 13.7%. The European studies report lower use of strong opioids compared with North American and a single Asian study. The high heterogeneity for adjuvant use was driven by a single Asian study with low prevalence use of 11%. Reported adjuvant use in the 2 European studies of 18% to 34% was similar to the reported use in the 2 North American studies of 19% to 36%.
Discussion
The prevalence of overall analgesic use was 50.8%. Our findings highlight tremendous variability in the prescribing of both nonopioid and opioid analgesics for patients with advanced CKD within and between countries, even with the removal of some highly influential studies. This variability in the context of consistently high pain prevalence across international studies suggests that factors other than patient characteristics account for differences and that there is widespread uncertainty regarding the optimal pharmacologic management of pain in patients with CKD. The prevalence of acetaminophen use was 27.5%, but was extremely low at 5.4% once adjusted for publication bias. NSAID and adjuvant use were 17.2% and 23.4%, respectively. Opioid use was 23.8%, although decreased to 17.3% when 2 highly influential US studies were removed. 31,38,44
Pain is experienced by approximately 60% of patients with advanced CKD whether they are treated with dialysis or managed conservatively2,54 These patients have serious medical illness with complex comorbidities that present numerous potential etiologies for ongoing pain. Pain is a highly complex, multidimensional phenomenon with physical and psychosocial components; a simple, 1-dimensional approach to pain management, especially one that relies exclusively on analgesics, is unlikely to be successful. This is particularly relevant for patients with chronic pain. A multimodal therapy approach that integrates nonpharmacological therapies is considered vital for successfully managing chronic pain. Analgesics, however, play an important role in the management of chronic nonmalignant pain management for some patients. 3 The overall prevalence of analgesic use of 50.8% suggests that pain is being addressed pharmacologically in a large proportion of patients experiencing pain. Unfortunately, existing data for optimizing pharmacological approaches to chronic nonmalignant pain are highly variable. While there is evidence that long-term opioid use may be beneficial for some patients in terms of improving pain control, functional status, and QOL, 55 and there appears to be low incidences of substance abuse and serious adverse effects when analgesic doses are titrated slowly and carefully against pain, 55 the increase in opioid prescribing over the last 10 years has been accompanied by significant risks, including addiction and opioid-related hospitalizations and deaths. Quality clinical trials for pain management in patients with CKD are extremely limited and predate the opioid crisis. 56 Current recommendations for analgesic use in CKD are based on recommendations for the general population considering pharmacologic data in CKD, clinical experience, and best opinion. These recommendations have been reviewed recently elsewhere. 7 The variability in the prevalence of analgesic use and the types of analgesics prescribed likely reflects uncertainty about how best to manage pain in CKD patients given the lack of evidence.
Current recommendations advocate for the judicious use of adjuvant and nonopioid analgesics.3,7 Acetaminophen is the mainstay of treatment for mild to moderate pain in patients with CKD. The range of acetaminophen use from 4% to 6% in 2 large cohorts from the international Dialysis Outcomes and Practice Patterns Study (DOPPS)14,15 to 50% to 66% in 2 small European studies33,36 is difficult to explain outside of differing approaches to pain management. The pooled prevalence of acetaminophen use of 27.5% may represent underuse of acetaminophen. The meta-analysis was potentially subject to publication bias; the adjusted prevalence was extremely low at 5.4% indicating actual rates of acetaminophen use may be substantially lower than our pooled results indicate. Conversely, our findings may underestimate the actual use of acetaminophen as data on over-the-counter medications were limited and it is unclear in many studies whether patients had trialed acetaminophen before proceeding to another analgesic.
The prevalence for NSAIDs use was 17.2%. As with overall analgesic and acetaminophen use, NSAID use in patients with CKD was higher in European studies compared with North America. The prevalence of NSAID use appeared to increase with publication year, particularly after 2010. We speculate this might reflect the desire of care providers to avoid using opioids for pain management. However, NSAIDs are also associated with higher risks for death and hospitalization in patients with advanced CKD. 57 While NSAIDs have a role for specific indications of acute pain, their use in patients with CKD should be limited to the lowest effective dose and shortest duration, especially in the elderly.3,7
Many pains experienced by patients with CKD have a neuropathic component that is poorly responsive to NSAIDs and opioids. For pain that has a neuropathic component, adjuvant therapy such as gabapentin is typically recommended to prevent inappropriate opioid use. Data on adjuvant use were limited but with a reported use of 23.4%. Unfortunately, several studies combined all nonopioids into a single category. Some of these studies specified this to be acetaminophen and NSAIDs, while others gave no further information and theoretically could have included adjuvants. However, use of “nonopioids” appeared similar with a pooled prevalence of 26.8% and like acetaminophen, use may have been subjected to publication bias, with actual rates as low as 15%.
Current guidelines for chronic pain management only recommend opioid therapy when nonpharmacologic therapies and nonopioid analgesics have failed to control pain adequately. Recommendations suggest that opioids should be added to acetaminophen and/or the adjuvant, rather than being prescribed alone. The prevalence of opioid use in this meta-analysis was 23.8%. However, our findings indicate the potential for both publication and sample size biases; prevalence rates were influenced by 2 large studies and our sensitivity analysis indicated that actual prevalence rates might be substantially lower if there are missing studies due to publication bias. When the 2 highly influential US studies were removed,31,38,44 the estimated prevalence decreased to 17.3%. Regardless, variability in opioid use remained high, again suggesting clinical uncertainty around opioid use. While this may not represent an excessively high rate of opioid use, there are exceptions such as the large Kimmel et al 38 study that reported that 64% of 153 758 dialysis patients were prescribed an opioid in 2010 and that 23% of those patients were using opioids long term (defined as receiving an opioid prescription for ≥90 days duration). Again, there was tremendous regional variation with chronic opioid prescription rates ranging 9.5% in Hawaii to 40.6% in West Virginia. This study was one of the few that provided information regarding the specific analgesics prescribed. Opioids were prescribed without the concurrent use of an adjuvant and/or nonopioid in over 51% of patients. In addition, only 1.9% of patients received a prescription for an opioid that is considered safer for use in patients with advanced CKD, raising concerns about inappropriate opioid use.
There are several limitations that need to be considered when interpreting these results. Data available for meta-analyses were limited by inconsistent characterization of analgesics. There were very few data regarding the specific analgesics used within each class and poor characterization of over-the-counter analgesic use including acetaminophen and NSAIDs, potentially explaining some of the heterogeneity in the meta-regression and underestimating their actual use. Studies that reported use of more than 1 specific drug or class of drug often presented results grouped by the various categories without providing a measurement for overall opioid or analgesic use to account for patients on multiple drugs. If research in pain management and analgesic use is to be advanced, greater attention to the characterization of the analgesic regimen is required. In addition, publication bias cannot be ruled out, particularly for nonopioid and acetaminophen use. Furthermore, given the lack of clinical data regarding indications for analgesic use, pain characteristics (severity and nature, eg, neuropathic or nociceptive), dosing or duration of use, efficacy, or safety, we can only speculate regarding appropriate use. This is an area that requires greater clinical focus and research if we are to change clinical practice and improve patient outcomes.
Our results suggest widespread uncertainty regarding the optimal pharmacologic management of pain in patients with CKD. Safe and effective pain management that includes adequate prescribing and oversight of treatment requires a targeted clinical focus. Clinical care would benefit from increased evidence and education with collaboration across specialties such as nephrology, pain medicine, and palliative care.
Supplemental Material
Supplemental material, Figure-S1-NSAID-Region-080120 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, TableS1_Search_Strategy_211019 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, Table_S2_Excluded_Studies_170519 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, Table_S3-Detailed_Prevalence_Revised_191219 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Footnotes
Ethics Approval and Consent to Participate: Not applicable.
Consent for Publication: All authors consent to the publication of this manuscript.
Availability of Data and Materials: All data available on request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Sara N. Davison  https://orcid.org/0000-0003-4513-6449
https://orcid.org/0000-0003-4513-6449
Sarah Rathwell  https://orcid.org/0000-0003-3176-994X
https://orcid.org/0000-0003-3176-994X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Davison SN, Jhangri GS. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J Pain Symptom Manage. 2010;39:477-485. [DOI] [PubMed] [Google Scholar]
- 2. Davison SN, Koncicki H, Brennan F. Pain in chronic kidney disease: a scoping review. Semin Dial. 2014;27:188-204. [DOI] [PubMed] [Google Scholar]
- 3. National Pain Centre. The 2017 Canadian Guideline for Opioids for Chronic Non-Cancer Pain (J Busse, ed.), 2018, http://nationalpaincentre.mcmaster.ca/documents/Opioid%20GL%20for%20CMAJ_01may2017.pdf. Accessed February 24, 2020.
- 4. World Health Organization. Scoping Document for WHO Treatment Guidelines on Chronic Non-Malignant Pain in Adults, https://www.who.int/medicines/areas/quality_safety/Scoping_WHOGuide_non-malignant_pain_adults.pdf, 2008. Accessed February 24, 2020.
- 5. Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO controversies conference on supportive care in chronic kidney disease: developing a roadmap to improving quality care. Kidney Int. 2015;88:447-459. [DOI] [PubMed] [Google Scholar]
- 6. Moss AH, Davison SN. How the ESRD quality incentive program could potentially improve quality of life for patients on dialysis. Clin J Am Soc Nephrol. 2015;10:888-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davison SN. Clinical pharmacology considerations in pain management. Clin J Am Soc Nephrol. 2019;14:917-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Microsoft. Microsoft R Open 3.4.1 (The enhanced R distribution from Microsoft). Redmond, WA: Microsoft Corporation; 2017. [Google Scholar]
- 9. Schwarzer G. meta: an R package for meta-analysis. R News, 2007:40-45. [Google Scholar]
- 10. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. 2013;67:974-978. [DOI] [PubMed] [Google Scholar]
- 11. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676-680. [DOI] [PubMed] [Google Scholar]
- 13. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [DOI] [PubMed] [Google Scholar]
- 14. Bailie GR, Mason NA, Bragg-Gresham JL, Gillespie BW, Young EW. Analgesic prescription patterns among hemodialysis patients in the DOPPS: potential for under prescription. Kidney Int. 2004;65:2419-2425. [DOI] [PubMed] [Google Scholar]
- 15. Bailie GR, Mason NA, Elder SJ, et al. Large variations in prescriptions of gastrointestinal medications in hemodialysis patients on three continents: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Hemodial Int. 2006;10:180-188. [DOI] [PubMed] [Google Scholar]
- 16. Barrantes F, Luan FL, Kommareddi M, et al. A history of chronic opioid usage prior to kidney transplantation may be associated with increased mortality risk. Kidney Int. 2013;84:390-396. [DOI] [PubMed] [Google Scholar]
- 17. Bouattar T, Skalli Z, Rhou H, et al. [The evaluation and analysis of chronic pain in chronic hemodialysis patients]. Nephrol Ther. 2009;5:637-641. [DOI] [PubMed] [Google Scholar]
- 18. Carreon M, Fried LF, Palevsky PM, Kimmel PL, Arnold RM, Weisbord SD. Clinical correlates and treatment of bone/joint pain and difficulty with sexual arousal in patients on maintenance hemodialysis. Hemodial Int. 2008;12:268-274. [DOI] [PubMed] [Google Scholar]
- 19. Claxton RN, Blackhall L, Weisbord SD, Holley JL. Undertreatment of symptoms in patients on maintenance hemodialysis. J Pain Symptom Manage. 2010;39:211-218. [DOI] [PubMed] [Google Scholar]
- 20. Davison SN. Pain in hemodialysis patients: prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239-1247. [DOI] [PubMed] [Google Scholar]
- 21. Desmet C, Beguin C, Swine C, Jadoul M. Falls in hemodialysis patients: prospective study of incidence, risk factors, and complications. Am J Kidney Dis. 2005;45:148-153. [DOI] [PubMed] [Google Scholar]
- 22. Dorks M, Herget-Rosenthal S, Schmiemann G, Hoffmann F. Use of nonsteroidal anti-inflammatory drugs and renal failure in nursing home residents-results of the study “Inappropriate Medication in Patients with Renal Insufficiency in Nursing Homes.” Wien Klin Wochenschr. 2016;128:287-290. [DOI] [PubMed] [Google Scholar]
- 23. El Harraqui R, Abda N, Bentata Y, Haddiya I. [Evaluation and analysis of pain in chronic hemodialysis]. Nephrol Ther. 2014;10:500-506. [DOI] [PubMed] [Google Scholar]
- 24. Elder SJ, Pisoni RL, Akizawa T, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2008;23:998-1004. [DOI] [PubMed] [Google Scholar]
- 25. Finkelstein FO, Schiller B, Daoui R, et al. At-home short daily hemodialysis improves the long-term health-related quality of life. Kidney Int. 2012;82:561-569. [DOI] [PubMed] [Google Scholar]
- 26. Gamondi C, Galli N, Schonholzer C, et al. Frequency and severity of pain and symptom distress among patients with chronic kidney disease receiving dialysis. Swiss Med Wkly. 2013;143:w13750. [DOI] [PubMed] [Google Scholar]
- 27. Heleniak Z, Cieplinska M, Szychlinski T, et al. Nonsteroidal anti-inflammatory drug use in patients with chronic kidney disease. J Nephrol. 2017;30:781-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hull S, Mathur R, Dreyer G, Yaqoob MM. Evaluating ethnic differences in the prescription of NSAIDs for chronic kidney disease: a cross-sectional survey of patients in general practice. Br J Gen Pract. 2014;64:e448-e455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iacono SA. Chronic pain in the hemodialysis patient population. Dialysis Transplant. 2004;33:92-101. [Google Scholar]
- 30. Ingrasciotta Y, Sultana J, Giorgianni F, et al. The burden of nephrotoxic drug prescriptions in patients with chronic kidney disease: a retrospective population-based study in Southern Italy. PLoS ONE. 2014;9(2):e89072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358-1366. [DOI] [PubMed] [Google Scholar]
- 32. Mahmoud LB, Pariente A, Kammoun K, et al. Risk factors for acute decompensation of chronic kidney disease in hospitalized patients in the nephrology department: a case-control study. Clin Nephrol. 2014;81:86-92. [DOI] [PubMed] [Google Scholar]
- 33. Masajtis-Zagajewska A, Pietrasik P, Krawczyk J, et al. Similar prevalence but different characteristics of pain in kidney transplant recipients and chronic hemodialysis patients. Clin Transplant. 2011;25:E144-E151. [DOI] [PubMed] [Google Scholar]
- 34. Otsuki T, Higuchi T, Yamazaki T, Okawa E, Okada K, Abe M. Efficacy and safety of pregabalin for the treatment of neuropathic pain in patients undergoing hemodialysis. Clin Drug Investig. 2016;37:95-102. [DOI] [PubMed] [Google Scholar]
- 35. Ou S-M, Chen Y-T, Chao P-W, et al. Nonsteroidal anti-inflammatory drug use is associated with cancer risk reduction in chronic dialysis patients. Kidney Int. 2013;84:198-205. [DOI] [PubMed] [Google Scholar]
- 36. Rodriguez Calero M, Hernández Sánchez D, Gutiérrez Navarro MJ, Juan Amer F, Calls Ginesta J. Evaluation of chronic pain in a population of patients on haemodialysis. Rev Soc Esp Enferm Nefrol. 2007;10:65-71. [Google Scholar]
- 37. Wu J, Ginsberg JS, Zhan M, et al. Chronic pain and analgesic use in CKD: implications for patient safety. Clin J Am Soc Nephrol. 2015;10:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kimmel PL, Fwu CW, Abbott KC, Eggers AW, Kline PP, Eggers PW. Opioid prescription, morbidity, and mortality in United States dialysis patients. J Am Soc Nephrol. 2017;28:3658-3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kristensen SL, Fosbøl EL, Kamper AL, et al. Use of nonsteroidal anti-inflammatory drugs prior to chronic renal replacement therapy initiation: a nationwide study. Pharmacoepidemiol Drug Saf. 2012;21:428-434. [DOI] [PubMed] [Google Scholar]
- 40. Battistella M, Jandoc R, Ng JY, McArthur E, Garg AX. A province-wide, cross-sectional study of demographics and medication use of patients in hemodialysis units across Ontario. Can J Kidney Health Dis. 2018;5:2054358118760832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Plantinga L, Grubbs V, Sarkar U, et al. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9:423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhan M, St Peter WL, Doerfler RM, et al. Patterns of NSAIDs use and their association with other analgesic use in CKD. Clin J Am Soc Nephrol. 2017;12:1778-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan KY, Yap DYH, Yip T, Sham MK, Lui SL, Chan TM. Palliative care consultation in advanced chronic kidney disease with pain. J Palliat Med. 2018;21:809-814. [DOI] [PubMed] [Google Scholar]
- 44. Daubresse M, Alexander GC, Crews DC, Segev DL, McAdams-DeMarco MA. Trends in opioid prescribing among hemodialysis patients, 2007-2014. Am J Nephrol. 2019;49:20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleishman TT, Dreiher J, Shvartzman P. Pain in maintenance hemodialysis patients: a multicenter study. J Pain Symptom Manage. 2018;56:178-184. [DOI] [PubMed] [Google Scholar]
- 46. Gómez Pozo M, del Carmen Ruiz Parrado M, Crespo Garrido M, Gómez López VE, Crespo Montero R. Caracterización del dolor en el paciente en hemodiálisis. Enfermería Nefrológica. 2017;20:295-304. [Google Scholar]
- 47. Guirguis-Blake J, Keppel GA, Holmes J, Force RW, Kriegsman W, Baldwin LM. Prescription of high-risk medications among patients with chronic kidney disease: a cross-sectional study from the Washington, Wyoming, Alaska, Montana and Idaho region Practice and Research Network. Fam Pract. 2018;35:589-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL. Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol. 2018;13:746-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jhamb M, Abdel-Kader K, Yabes J, et al. Comparison of fatigue, pain, and depression in patients with advanced kidney disease and cancer—symptom burden and clusters. J Pain Symptom Manage. 2019;57:566-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Keohane DM, Dennehy T, Keohane KP, Shanahan E. Reducing inappropriate non-steroidal anti-inflammatory prescription in primary care patients with chronic kidney disease. Int J Health Care Qual Assur. 2017;30:638-644. [DOI] [PubMed] [Google Scholar]
- 51. Mina D, Johansen KL, McCulloch CE, Steinman MA, Grimes BA, Ishida JH. Muscle relaxant use among hemodialysis patients: prevalence, clinical indications, and adverse outcomes. Am J Kidney Dis. 2019;73:525-532. [DOI] [PubMed] [Google Scholar]
- 52. Yesil S, Karsli B, Kayacan N, Suleymanlar G, Ersoy F. [Pain evaluation in patients with chronical renal failure undergoing hemodialysis]. Agri. 2015;27:197-204. [DOI] [PubMed] [Google Scholar]
- 53. Iwagami M, Tomlinson LA, Mansfield KE, McDonald HI, Smeeth L, Nitsch D. Prevalence, incidence, indication, and choice of antidepressants in patients with and without chronic kidney disease: a matched cohort study in UK Clinical Practice Research Datalink. Pharmacoepidemiol Drug Saf. 2017;26:792-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Brkovic T, Burilovic E, Puljak L. Prevalence and severity of pain in adult end-stage renal disease patients on chronic intermittent hemodialysis: a systematic review. Patient Prefer Adherence. 2016;10:1131-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hauser W, Bernardy K, Maier C. [Long-term opioid therapy in chronic noncancer pain: a systematic review and meta-analysis of efficacy, tolerability and safety in open-label extension trials with study duration of at least 26 weeks.]. Schmerz. 2014;29:96-108. [DOI] [PubMed] [Google Scholar]
- 56. Barakzoy AS, Moss AH. Efficacy of the world health organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17:3198-3203. [DOI] [PubMed] [Google Scholar]
- 57. Novick TK, Surapaneni A, Shin JI, et al. Associations of opioid prescriptions with death and hospitalization across the spectrum of estimated GFR. Clin J Am Soc Nephrol. 2019;14:1581-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Figure-S1-NSAID-Region-080120 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, TableS1_Search_Strategy_211019 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, Table_S2_Excluded_Studies_170519 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease
Supplemental material, Table_S3-Detailed_Prevalence_Revised_191219 for Analgesic Use in Patients With Advanced Chronic Kidney Disease: A Systematic Review and Meta-Analysis by Sara N. Davison, Sarah Rathwell, Chelsy George, Syed T. Hussain, Kate Grundy and Liz Dennett in Canadian Journal of Kidney Health and Disease