Abstract
Background:
It is uncertain how often patients with autosomal dominant polycystic kidney disease (ADPKD) develop kidney stones.
Objective:
To review English-language studies reporting the incidence and prevalence of stones and stone interventions in adults with ADPKD.
Design:
Systematic review and meta-analysis.
Setting:
Any country of origin.
Patients:
Adult patients with ADPKD.
Measurements:
Incidence or prevalence of kidney stones and stone interventions.
Methods:
We reviewed 1812 citations from bibliographic databases, abstracted data from 49 eligible studies, and assessed methodological quality in duplicate. In some studies, the proportion of adults with ADPKD with the outcome were compared to adults without ADPKD; for these studies, prevalence risk ratios were calculated and pooled using a random effects model.
Results:
We identified 49 articles that met our review criteria. The methodological quality of many studies was limited (scores ranging from 2 to 14 out of 22, with a higher score indicating higher quality). No study clearly reported stone incidence, and in the cross-sectional studies, the definition of stones was often unclear. The prevalence of stones ranged from 3% to 59%, and a prevalence of stone interventions ranged from 1% to 8%; the average patient age at the time of assessment ranged from 26 to 61 years across the studies. Two studies reported a nonstatistically significant higher stone prevalence in patients with ADPKD compared to unaffected family members. Compared to unaffected family members, patients with ADPKD had a higher prevalence of kidney stones (6 cross-sectional studies; unadjusted prevalence ratio: 1.8; 95% confidence interval: 1.3 to 2.6; P = .0007; test for heterogeneity: I2 = 0%, P = .8).
Limitations:
Studies were limited to articles published in English.
Conclusions:
The prevalence of kidney stones and stone interventions in adults with ADPKD remains uncertain. Future studies of higher methodological quality are needed to better characterize the incidence and prevalence of kidney stones in patients with ADPKD.
Trial registration:
We did not register the protocol for this systematic review.
Keywords: polycystic kidney disease, prevalence, kidney stones, stone intervention, epidemiology, observational study, systematic review
Abrégé
Contexte:
La prévalence du développement de calculs rénaux chez les patients atteints de polykystose rénale autosomique dominante (ADPKD) est mal connue.
Objectif:
Examiner les études publiées en anglais portant sur l’incidence et la prévalence des calculs rénaux et des interventions liées à ces derniers chez les adultes atteints d’ADPKD.
Type d’étude:
Revue systématique et méta-analyze.
Cadre:
Tous les pays d’origine.
Sujets:
Des adultes atteints d’ADPKD.
Mesures:
L’incidence ou la prévalence des calculs rénaux et des interventions sur ceux-ci.
Méthodologie:
Nous avons examiné 1 812 citations issues des bases de données bibliographiques, extrait les données des 49 études admissibles et analysé leur qualité méthodologique en duplicata. Dans certaines études, la proportion d’adultes atteints d’ADPKD présentant le résultat d’intérêt avait été comparée à celle de sujets non atteints d’ADPKD; dans ces études, les rapports de risque de la prévalence ont été calculés et regroupés à l’aide d’un modèle à effets aléatoires.
Résultats:
Nous avons repéré 49 articles satisfaisant nos critères, dont plusieurs étaient de qualité méthodologique limitée (scores entre 2 et 14 sur une possibilité de 22, une note élevée indiquant une meilleure qualité). Aucune étude ne faisait clairement état d’une incidence de calculs rénaux. De plus, la définition des calculs rénaux n’était souvent pas très claire dans les études transversales. La prévalence des calculs rénaux variait entre 3 % et 59 % et celle des interventions liées variait de 1 % à 8 %. L’âge moyen des patients au moment de l’évaluation allait de 26 à 61 ans selon les études. Deux études faisaient état d’une prévalence plus élevée, quoique non statistiquement significative, chez les patients atteints d’ADPKD par rapport aux membres de leurs familles non atteints. De même, six études transversales rapportaient une prévalence plus élevée de calculs rénaux chez les patients atteints d’ADPKD comparé aux membres de leurs familles non atteints (rapport de prévalence non corrigé: 1,8; IC 95 %: 1,3 à 2,6; p=0,0007; test d’hétérogénéité: I2=0 %; p=0,8).
Limites:
L’étude ne porte que sur des articles publiés en anglais.
Conclusion:
La prévalence des calculs rénaux et des interventions relatives à ces derniers demeure mal connue chez les adultes atteints d’ADPKD. Des études supplémentaires et de meilleure qualité méthodologique sont nécessaires afin de mieux caractériser l’incidence et la prévalence des calculs rénaux dans cette population.
Enregistrement de l’essai:
Le protocole de cette revue systématique n’a pas été enregistré.
What was known before
It is uncertain how often patients with autosomal dominant polycystic kidney disease (ADPKD) develop kidney stones.
What this adds
This review summarized the results of 49 studies. The prevalence of kidney stones reported in the literature ranged between 3 and 59%, and the prevalence of stone intervention ranged from 1 to 8% in patients with ADPKD. The quality of published literature was poor, and no study clearly reported stone incidence in ADPKD. This review calls for better studies to be conducted in the future.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most commonly inherited kidney disease and is characterized by focal cyst development in both kidneys. 1 In early stages of ADPKD, the cysts cause structural deformation to the kidney and damage adjacent nephrons, but overall kidney function is maintained by compensatory hyperfiltration of functioning nephrons.2,3 As the number and size of cysts increase progressively, more nephrons become damaged, and overall kidney function starts to decline. 4 By the age of 55 years, about half of the patients reach end-stage kidney disease (ESKD) and require kidney transplantation or dialysis to sustain life.5,6
End-stage kidney disease is not the only kidney manifestation of ADPKD. Previous studies suggest that kidney stones are more prevalent in patients with ADPKD compared to the general population; however, there remains uncertainty about the incidence and prevalence of kidney stone in patients with ADPKD.7-12 Kidney stones in patients with ADPKD are associated with significant morbidity. For example, stones are a significant determinant of pain and may accelerate disease progression to ESKD in patients with ADPKD.13,14
We conducted this systematic review to critically appraise and summarize studies which reported the incidence and prevalence of kidney stones and stone interventions in patients with ADPKD. This encompassed studies which also included patients without ADPKD as a comparator.
Methods
Design and Study Selection
We conducted this systematic review using a pre-specified protocol not previously published but detailed below and report this review according to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Supplementary Table S1). 15
The following studies met our eligibility criteria for review: (1) published English full-text articles and conference proceedings; (2) any study design (eg, cross-sectional or cohort study); (3) mean age of studied population 18 years or older; (4) study populations not solely restricted to patients with ESKD; (5) reported prevalence or incidence of stones; and (6) studies published any time after 1970 (the resolution of imaging modalities in older studies would be different from current ones). In some studies, patients without ADPKD were included as a comparator to patients with ADPKD, and in such cases, we abstracted information on both groups of patients.
Identifying Relevant Articles
We performed a comprehensive search of bibliographic databases from 1970 to February 2019 (MEDLINE, EMBASE, Web of Science, BIOSIS Preview, and CINAHL) to identify all relevant journal articles and conference proceedings (detailed in Supplementary Table S2). To identify further relevant articles, we also used the “cited by” function on Web of Science and Google Scholar and “related article” function on Google Scholar and “similar article” function on PubMed to identify other relevant articles. We also reviewed the reference lists of all relevant articles.
Two reviewers (V.K. and G.G.) independently removed duplicates and rated the title and abstract of each citation as “relevant,” “possibly relevant” or “not relevant.” We then retrieved the full text of “relevant” and “possibly relevant” articles to assess study eligibility. The 2 reviewers resolved any disagreement through discussion and consensus.
Data Abstraction
Two reviewers (V.K. and G.G.) independently abstracted data from all included articles, recorded the data on the standardized abstraction form (Supplementary Table S3), and resolved any disagreements through discussion, or with the help of a third reviewer (D.M.N.). We collected data on study characteristics, patient characteristics, incidence or prevalence of stones, and stone characteristics. We abstracted the prevalence of stone intervention from the included studies that reported it.
We assessed the methodological quality of included studies using a modified Downs and Black checklist (Supplementary Table S4). We assigned all included studies a score between 0 and 22 based on our modified checklist with a higher score indicating a greater quality. 16
Data Analysis
We used a Fischer Exact test for studies with controls that did not statistically compare the prevalence of stones between patients with ADPKD and controls. We also calculated the prevalence ratio of kidney stones for each of the studies with controls using Cochrane Review Manager 5.3. We assessed for heterogeneity across all studies using the I2 test. I2 values below 25%, between 25% and 75%, and above 75% correspond to low, moderate, and high levels of heterogeneity, respectively. We conducted a meta-analysis to combine the results if I2 was less than 75%. We calculated the meta-analyzed prevalence ratio estimates for kidney stones using a random effects model and Cochrane Review Manager 5.3.
Results
Study Selection
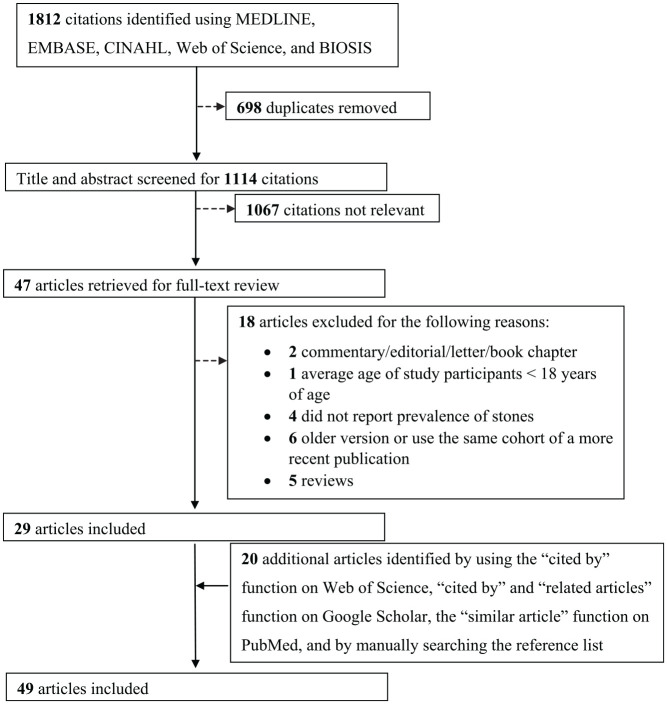
A schematic diagram of the study selection process is presented in Figure 1. Our search yielded 1812 citations, and we identified 29 eligible articles that met our eligibility criteria. We identified an additional 20 eligible articles through our further search strategy described above, which resulted in a total of 49 eligible articles (a total of 9396 patients with ADPKD).7-12,14,17-58 The chance-corrected agreement between 2 independent reviewers for full-text eligibility was excellent (κ = 0.86).
Figure 1.
Study selection.
Description of Included Studies
The characteristics of included studies are summarized in Table 1. The 49 eligible studies were published between 1977 and 2019, and the majority of the studies were conducted in Turkey (7 studies) followed by the United States (6 studies), Albania (5 studies), Brazil (3 studies), India (3 studies), Spain (3 studies), Canada (2 studies), Italy (2 studies), and Japan (2 studies). A single study was conducted in Bulgaria, China, Cyprus, Greece, Ireland, Korea, Pakistan, Philippines, Republic of Macedonia, Saudi Arabia, Senegal, Taiwan, Tunisia, and the United Kingdom, and one was a multinational study. The country where the study was conducted was unknown for one study. The number of centers participating in a study was unclear in 19 of 49 studies; of the remainder, 21 studies were single center and 9 were multicenter. Among the 49 included studies, 12 were cohort studies, 33 were cross-sectional studies, and the study design was unclear for 4 studies.
Table 1.
Study Characteristics.
| Author (year), country | No. of centers | Eligibility criteria | Recruitment period | Mean (SD) follow-up | ADPKD sample size | ADPKD case definition (imaging modality) | Control population (sample size) | Quality score a |
|---|---|---|---|---|---|---|---|---|
| Cross-sectional studies | ||||||||
| Al-Muhanna et al, 25 Saudi | 1 | ADPKD | NR | N/A | 30 | 1. 5+ renal cysts distributed between both kidneys (U/S, intravenous pyelogram, or CT) | None | 4 |
| Baishya et al, 17 India | Unclear | ADPKD | Since 1992 | N/A | 452 | NR (NR) | None | 6 |
| Bajrami et al, 20 Albania | Unclear | ADPKD | 2011 to 2014 | N/A | 100 | Ravine criteria (x-ray or U/S) | None | 9 |
| Chang et al, 44 Taiwan | 1 | ADPKD | October 2008 to May 2011 | N/A | 46 | 1. Ravine criteria; OR
2. No fam hx + bilateral kidney enlargement + at least 10 cysts in each kidney (U/S) |
None | 9 |
| Corradi et al, 27 Italy | Multicenter (unclear) | ADPKD | Since April 2007 | N/A | 100 | Ravine criteria (U/S) | None | 12 |
| Demetriou et al, 7 Cyprus | 1 | 1. Alive 2. Has an affected family member with a PKD2 mutation |
Up to August 1998 | N/A | 106 | 1. 1+ cyst in one kidney for patients aged 5 to 14 years; 2. 2+ unilateral cysts or one in each kidney for patients aged 15 to 19 years; 3. 3+ cysts in both kidneys combined for patients aged 20 to 29 years; 4. 2+ cysts in each kidney for patients aged 30 to 59 years; AND 5. 4+ cysts in each kidney for patients aged 60 years or above (U/S) |
Unaffected family members (105) | 11 |
| Duli et al, 36 Albania | Unclear | ADPKD | NR | N/A | 180 | Unclear (NR) | None | 7 |
| Ekin et al, 45 Turkey | 1 | ADPKD | 1995 to 2014 | N/A | 144 | 1. 5+ renal cysts in both kidneys (NR) | None | 9 |
| Cornec-Le Gall et al, 35 France | 22 | 1. Genkyst study participants 2. 18+ years old 3. Mutation in PKD2 gene |
January 2010 to March 2016 | N/A | 293 | 1. Pei criteria; OR
2. 10+ cysts in both kidneys combined + no fam hx (NR) |
None | 10 |
| Galliani et al, 47 Italy | 28 | ADPKD | February 2013 to April 2014 | N/A | 462 | NR (NR) | None | 2 |
| Gonzalo et al, 8 Spain | Unclear | 1. At risk of ADPKD 2. Asymptomatic 3. 13+ years old |
June 1993 to December 1994 | N/A | 65 | 1. 1+ cysts in each kidney; OR
2. 2+ cysts in one kidney (U/S) |
unaffected family members (60) | 13 |
| Grampsas et al, 23 United States | 1 | 1. ADPKD 2. Part of The University of Colorado Health Sciences Center’s Research Study Group database |
NR | N/A | 48 | NR (NR) |
None | 7 |
| Ishibashi, 49 Japan | 1 | ADPKD | May 1972 to September 1980 | N/A | 118 | NR (U/S or CT) |
None | 3 |
| Fary Ka et al, 39 Senegal | 1 | 1. ADPKD 2. Black 3. 16+ years 4. Without acquired simple cyst, angiomyolipoma, tuberous sclerosis, cyst calcification, any alterations suggestive of malignancy |
January 1, 1995 to December 31, 2005 | N/A | 53 | Ravine criteria (U/S) | None | 5 |
| Kaygısız et al, 40 Turkey | 1 | 1. Referred and diagnosed with ADPKD at a tertiary care center 2. Not on dialysis 3. eGFR >30 mL/min |
2010 to 2016 | N/A | 118 | Pei criteria (U/S) | None | 11 |
| Kazancioglu et al 28 Turkey | 12 | ADPKD | January 2003 to December 2009 | N/A | 1139 | 5+ cysts distributed between both kidneys (NR) | None | 11 |
| Kim et al, 43 Korea | 9 | 1. Korean 2. ADPKD and CKD 3. Pre-dialysis 4. Part of Korean Cohort Study for Outcomes in Patients with Chronic Kidney Disease cohort 5. Provided written consent 6. Not a transplant recipient 7. Without heart failure, liver cirrhosis, or current or past history of cancer 8. Not pregnant 9. No single kidney due to trauma or kidney donation |
April 2011 to February 2016 | N/A | 364 | Pei criteria (U/S) | None | 11 |
| Kumar et al, 41 India | 1 | ADPKD | November 2011 to October 2012 | N/A | 41 | Unclear (U/S, intravenous pyelogram, CT) | None | 7 |
| Memili et al, 29 Turkey | 1 | 1. ADPKD 2. Referred to nephrology outpatient clinic |
January 2003 to December 2006 | N/A | 136 | NR (NR) | None | 8 |
| Meng et al, 33 China | 1 | 1. ADPKD 2. Inpatient 3. Complete medical records |
January 2012 to December 2016 | N/A | 167 | Japanese criteria for patients with unknown genotype (NR) | None | 10 |
| Milutinovic et al, 12 United States | Unclear | At risk of ADPKD | NR | N/A | 140 | 1. Fam hx + multiple bilateral cysts (Unclear) | Unaffected family members (119) | 12 |
| Milutinovic et al, 11 United States | Unclear | 1. Fam hx of ADPKD 2. 50+ years old |
NR | N/A | 32 | 1. Bilateral renal cysts + fam hx (Unclear) | Unaffected family members (25) | 12 |
| Nikolov et al, 31 Unclear | 1 | ADPKD referred to center | 1998 to 2008 | N/A | 208 | NR (NR) | None | 4 |
| Nishiura et al, 24 Brazil | 1 | 1. Referred to PKD unit due to the presence of affected progenitor/sibling with ADPKD 2. ADPKD confirmed using U/S |
NR | N/A | 125 | Ravine criteria (U/S or CT) | None | 14 |
| Parfrey et al, 10 Canada | NR | Family members of index ADPKD cases | NR | N/A | Unclear | 1. Reported on autopsy report, surgical report or of a death due to CKD with an ADPKD diagnosis; 2. 1+ in each kidney; OR 3. 1+ in one kidney (excretory urography, CT, U/S) |
Unaffected family members (Unclear) | 12 |
| Romão et al, 55 Brazil | 1 | ADPKD | January 1985 to December 2003 | N/A | 92 | 1. Ravine criteria; OR
2. Fam hx + hepatic cyst (U/S) |
None | 9 |
| Roscoe et al,42,b Canada | Unclear | ADPKD | NR | N/A | 80 | NR (NR) | None | 9 |
| Segal et al, 56 United States | 2 | ADPKD | NR | N/A | 100 | NR (NR) | None | 3 |
| Strakosha et al, 48 Albania | NR | ADPKD | NR | N/A | 180 | NR (NR) | None | 5 |
| Torra et al, 9 Spain | Unclear | ADPKD or at-risk of ADPKD | NR | N/A | PKD1: 146;PKD2: 20; All: 166 | Ravine criteria (U/S) | Unaffected family members (150) | 13 |
| Torres et al, 18 United States | 1 | 1. ADPKD 2. Without any cyst wall calcification, or with poorly localized parenchymal calcification |
1976 to 1986 | N/A | 751 | 1. Bilateral polycystic kidneys + fam hx; OR
2. No fam hx + bilaterally enlarged and polycystic kidneys + exclusion of other disorders associated with renal cysts (NR) |
None | 10 |
| Vikrant and Parashar 32 India | 1 | 1. ADPKD 2. Attending renal clinic |
April 2009 to March 2015 | N/A | 208 | 1. Pei criteria; OR
2. Fam hx + hepatic cyst (U/S) |
None | 13 |
| Yildiz et al 46 Turkey | Unclear | 1. ADPKD 2. Not on renal replacement therapy 3. eGFR > 30mL/min 4. In the Turkish Nephrology Society Cystic Kidney Disease Working Group online database |
NR | N/A | 93 | NR (NR) | None | 3 |
| Cohort Study | ||||||||
| Gonzalo et al, 58 Spain | 1 | ADPKD | June 1977 to June 1988 | 6 years 3 months (NR) | 107 | 1. 3+ cysts in each kidney + fam hx (excretory urography or U/S) | None | |
| Hajji et al 53 Tunisia | Multicenter (unclear) | ADPKD | 1969 to 2016 | NR | 569 | NR (NR) | None | 10 |
| Hateboer et al
30
The Netherlands, Spain, Bulgaria, and the United Kingdom |
7 | ADPKD | NR | NR | 624 | 1. Ravine criteria; 2. Deoxyribonucleic acid linkage test; OR 3. Report of ADPKD on medical records (U/S) |
None | 14 |
| Idrizi et al, 37 Albania | Unclear | ADPKD | NR | NR | 180 | NR (NR) |
None | 10 |
| Ozkok et al, 14 Turkey | 1 | ADPKD | January 2000 to January 2012 | 100 (38) months | 323 | Pei criteria (U/S) |
None | 13 |
| Papadopoulou et al, 66 Greece | Unclear | At-risk of ADPKD | NR | NR | 85 | 1. 2+ cysts in one kidney and one cyst in the other kidney+ fam hx (U/S) |
None | 10 |
| Rabbani et al, 67 Pakistan | 1 | ADPKD | January 1997 to December 2003 | 7.6 (4.2) years | 56 | 1. Fam hx + 2+ cysts in either kidney + hypertension or renal insufficiency; 2. Bilateral cysts + no fam hx; OR 3. Unilateral polycystic kidney + liver cyst, berry aneurysm, arterio-venous malformation or evidence of prior cerebrovascular accident on MRI/MRA (U/S) |
None | 9 |
| Ristovska et al, 34 Republic of Macedonia | Unclear | ADPKD | NR | 3 (NR) years | 60 | Unclear (echosonography or CT) | None | 5 |
| Senel et al, 54 Turkey | Unclear | ADPKD | January 1990 to January 2015 | NR | 300 | NR (NR) | None | 6 |
| Tantoco and Alano, 68 Philippines | 1 | ADPKD | May 1973 to January 1986 | 3 (NR) years | 60 | 1. Signs and symptoms + fam hx + imaging (intravenous pyelogram, infusion intravenous pyelogram with tomogram, U/S or CT) | None | 3 |
| Thong and Ong,38,b United Kingdom | Unclear | 1. ADPKD 2. In research database 3. Have at least 5 years of renal function tests at the time of analysis |
1978 to 2012 | 11.3 (5.5) years | 210 | NR (NR) |
None | 8 |
| Wright et al, 50 Ireland | Unclear | Belonging to PKD1 family | NR | NR | PKD1: 49; non-PKD1: 17; All: 66 | ADPKD documented the following ways: (1) by post-mortem examination; (2) by report of a death due to chronic renal failure with a clinical diagnosis of ADPKD; (3) by operative report during abdominal surgery; (4) by excretory urography or CT scan; (5) by unequivocal findings on ultrasonography; OR (6) 1+ cyst in at least one kidney (diagnostic data files or ultrasound) |
None | 10 |
| Study design unclear | ||||||||
| Delaney et al, 26 United States | 1 | Symptomatic ADPKD | 1947 to 1980 | 12 (NR) years | 53 | 1. History and physical examination; OR 2. Diagnosis confirmed with imaging or autopsy (intravenous pyelogram with tomograms, sonography, CT with contrast, arteriography, laparotomy) | None | 4 |
| Dimitrakov and Simeonov, 22 Bulgaria | Unclear | ADPKD | NR | N/A | 82 | Unclear (echography, venous urography, or CT) | None | 5 |
| Higashihara et al, 22 Japan | 38 | ADPKD | January 1988 to December 1988 | N/A | 316 | NR(U/S or CT) | None | 11 |
| Idrizi et al, 21 Albania | Unclear | ADPKD | 2002 to 2009 | N/A | 200 | Ravine criteria (U/S) | None | 7 |
Note. ADPKD = autosomal dominant polycystic kidney disease; NR = not reported; N/A = not applicable; U/S = ultrasound; CT = computed tomography; Fam Hx = family history; PKD = polycystic kidney disease.
A modified Downs and Black checklist was used to assess the methodological quality of each included study. The methods quality score ranged between 0 and 22 with higher scores indicating higher quality.
Data were abstracted and methodological quality was assessed for the portion of the multicomponent study that reported the prevalence of stones.
Patient Population
The sample size of patients with ADPKD ranged from 30 to 1139 (Table 2). The mean age of patients with ADPKD ranged from 26 to 61 years, 35% to 71% of the patients with ADPKD were male, up to 51% developed end-stage renal disease (ESRD), 5% to 88% were hypertensive, and 1% to 73% experienced at least one prior urinary tract infection (UTI; Table 2).
Table 2.
Patient Characteristics.
| Author (year), country | Mean age (standard deviation) (years) | No. of male (%) | No. of patients on dialysis (%) | No. of transplant recipient (%) | No. of patients who had ESRD (%) | No. of hypertensive patients (%) | No. of patients with UTI (%) | Serum creatinine (µmol/L) |
|---|---|---|---|---|---|---|---|---|
| Al-Muhanna et al, 25 Saudi | 45 (10) | 13 (43) | 2 (7) | 2 (7) | 4 (13) | 17 (57) | 22 (73) | NR |
| Baishya et al, 17 India | NR | NR | NR | NR | NR | NR | NR | NR |
| Bajrami et al, 20 Albania | NR | 42 (42) | NR | NR | NR | NR | NR | NR |
| Chang et al, 44 Taiwan | 48 (13) | 24 (52) | NR | NR | NR | 31 (67) | 17 (37) | NR |
| Corradi et al, 27 Italy | 48 (NR) | 58 (58) | NR | 6 (6) | 29 (29) | 75 (75) | NR | NR |
| Demetriou et al, 7 Cyprus | ADPKD: 38 (NR) CONTROL: NR (NR) | NR | ADPKD: 0 (0) CONTROL: NR (NR) | ADPKD: 1 (1) CONTROL: NR (NR) | NR | ADPKD: 24 (23) CONTROL: 4 (4) | ADPKD: 24 (23) CONTROL: 12 (11) | NR |
| Duli et al, 36 Albania | NR | NR | NR | NR | NR | NR | NR | NR |
| Ekin et al, 45 Turkey | 45 (NR) | 61 (42) | NR (11) | NR | NR (11) | 117 (82) | 14 (2) a | 168 (186) |
| Cornec-Le Gall et al, 35 France | 61 (NR) | 123 (42) | NR | NR | Unclear | 221 (75) | NR | NR |
| Galliani et al, 47 Italy | NR | 194 (42) | NR | NR | NR | NR (60) | NR (28) | NR |
| Gonzalo et al, 8 Spain | ADPKD: 33 (NR) CONTROL: NR (NR) | ADPKD: 26 (40) CONTROL: 28 (47) | NR | NR | NR | ADPKD: 19 (29) CONTROL: 3 (5) | ADPKD: 4 (6) CONTROL: 1 (2) | NR |
| Grampsas et al, 23 United States | NR | 17 (35) | NR | NR | NR | 23 (48) | NR | NR |
| Ishibashi, 49 Japan | 44 (NR) | 54 (46) | NR | NR | NR | NR | 57 (54) a | NR |
| Fary Ka et al, 39 Senegal | 47 (5) | 30 (57) | 10 (19) | NR | 27 (51) | 36 (68) | 7 (13) | NR |
| Kaygısız et al, 40 Bursa | NR | 54 (46) | 0 (0) | NR | 0 (0) | 72 (61) | 29 (25) | NR |
| Kazancioglu et al, 28 Turkey | NR | 548 (48) | 108 (11) | 8 (1) | NR | 828 (73) | 228 (23) a | 194 (194) |
| Kim et al, 43 Korea | 47 (11) | 184 (51) | 0 (0) | 0 (0) | NR | 319 (88) | 8 (2) | 119 (79) |
| Kumar et al, 41 India | NR | 29 (71) | NR | NR | 13 (32) | 27 (66) | 6 (40) | 398 (283) |
| Memili et al, 29 Turkey | 47 (16) | 65 (48) | 16 (12) | 1 (1) | NR | 98 (72) | 22 (16) | NR |
| Meng et al, 33 China | 49 (NR) | 72 (43) | NR | NR | NR | 84 (50) | 41 (25) | 309 (290) |
| Milutinovic et al, 12 United States | ADPKD: 37 (14) CONTROL: 35 (16) | ADPKD: 64 (46) CONTROL: NR (NR) | ADPKD: 25 (18) CONTROLS: 0 (0) | NR | ADPKD: 28 (20) CONTROL: 0 (0) | ADPKD: 73 (52) CONTROLS: 13 (11) | ADPKD: 64 (46) CONTROLS: 33 (28) | NR |
| Milutinovic et al, 11 United States | ADPKD: 58 (7) CONTROL: 60 (7) | ADPKD: 15 (47) CONTROL: 9 (36) | NR | NR | ADPKD: 15 (47) CONTROL: 0 (0) | ADPKD: 22 (69) CONTROL: NR (36) | ADPKD: 13 (41) CONTROL: NR (36) | NR |
| Nikolov et al, 31 Unclear | NR | NR | NR | NR | NR | NR | NR | NR |
| Nishiura et al, 24 Brazil | NR | 45 (36) | NR | NR | NR | 59 (47) | 4 (3) | NR |
| Parfrey et al, 10 Canada | NR | NR | NR | NR | NR | ADPKD: 118 (36) CONTROL: 238 (16) | ADPKD: 24 (22) a CONTROL: 35 (17) a | NR |
| Romão et al, 55 Brazil | 35 (15) | 34 (37) | NR | NR | 27 (29) | 61 (63) | 33 (36) | 212 (247) |
| Roscoe et al,42,b Canada | NR | NR | NR | NR | 22 (28) | NR | NR | NR |
| Segal et al, 56 United States | NR | NR | NR | NR | NR | NR | NR | NR |
| Strakosha et al, 48 Albania | NR | NR | NR | NR | NR | NR | NR | NR |
| Torra et al, 9 Spain | NR | ADPKD: 72 (43) CONTROL: 72 (48) | NR | NR | ADPKD: 42 (25) CONTROL: NR (NR) | ADPKD: 76 (46) CONTROL: 23 (15) | ADPKD: 57 (34) a CONTROL: 26 (17) | NR |
| Torres et al, 18 United States | NR | 393 (52) | NR | NR | NR | NR | NR | NR |
| Vikrant and Parashar, 32 India | 46 (15) | 126 (61) | 5 (2) | NR | 20 (10) | 145 (70) | 81 (39) | 292 (318) |
| Yildiz et al, 46 Turkey | 41 (13) | 49 (53) | 0 (0) | 0 (0) | 0 (0) | NR (72) | NR | NR |
| Gonzalo et al, 58 Spain | 46 (14) | 58 (54) | NR | NR | NR | 73 (68) a | 33 (31) a | NR |
| Hajji et al, 53 Tunisia | 49 (14) | 297 (52) | 298 (52) | 13 (2) | NR | 321 (59) | NR (24) | 459 (NR) |
| Hateboer et al, 30 The Netherlands, Spain, Bulgaria, and the United Kingdom | NR | 308 (49) | NR | NR | NR | 227 (50) a | 119 (28) a | NR |
| Idrizi et al, 21 Albania | NR | 97 (49) | NR | NR | NR | NR | 108 (54) | NR |
| Ozkok et al, 14 Turkey | 53 (15) | 149 (46) | 46 (14) | NR | 48 (14) | 255 (79) a | 64 (21) a | NR |
| Papadopoulou et al, 66 Greece | 26 (12) | 44 (52) | NR | NR | NR | ADPKD: 4 (5) | ADPKD: 1 (1) | NR |
| Rabbani et al, 67 Pakistan | NR | 40 (71) | NR | NR | 7 (13) | 38 (68) | NR | 398 (282) |
| Ristovska et al, 34 Republic of Macedonia | 43 (13) | NR | NR | NR | NR | NR | NR | NR |
| Senel et al, 54 Turkey | NR | 143 (48) | NR | NR | NR | 231 (83) a | 52 (19) a | 203 (221) |
| Tantoco and Alano, 68 Philippines | 44 (NR) | 30 (50) | NR | NR | 17 (28) | 40 (67) | 17 (28) | NR |
| Thong and Ong,38, b United Kingdom | 46 (16) | 102 (49) | NR | NR | NR | 147 (70) | 57 (27.2) | NR |
| Wright et al, 50 Ireland | NR | NR | NR | NR | 12 (18) | 16 (24) | 5 (8) | NR |
| Delaney et al, 26 United States | NR | 21 (40) | 9 (17) | NR | NR | 11 (21) | 10 (19) | NR |
| Dimitrakov and Simeonov, 22 Bulgaria | NR | 34 (41) | NR | NR | NR | NR | NR | NR |
| Idrizi et al, 37 Albania | NR | NR | NR | NR | NR | NR | 108 (60) | NR |
| Higashihara et al, 22 Japan | 51 (13) | 167 (53) | 72 (23) | NR | 72 (23) | 201 (64) a | NR | 354 (380) |
Note. UTI = urinary tract infection; NR = not reported; ADPKD = autosomal dominant polycystic kidney disease; ESRD = end-stage renal disease.
Denominator includes a subset of the population.
Data were abstracted for the portion of the multicomponent study that reported the prevalence of stones.
Six studies compared the prevalence of stones in patients with ADPKD to unaffected family members as controls.7-12 The mean age of controls ranged from 35 to 60 years, 36% to 48% of the controls were male, 4% to 36% were hypertensive, and 2% to 36% experienced a prior UTI (Table 2).
Quality Assessment of Studies
The methodological quality of the studies was limited as the methods quality score ranged from 2 to 14 out of 22 (where higher scores indicate higher methodological quality).
The internal validity of studies’ results is affected by the definition of the exposure being investigated and the outcome of interest. Of the 49 studies, 29 specified the definition for ADPKD. Patients with ADPKD were identified using Ravine criteria in 6 studies, Ravine criteria or another additional criterion such as family history and liver cysts in 3 studies, Pei criteria in 3 studies, Pei criteria and an additional criterion in 2 studies, at least 5 cysts in each kidney in 3 studies, and other criteria in the remaining 13 studies; the definition for ADPKD was unclear or not reported in the remaining 19 studies. Ravine and Pei criteria to diagnose ADPKD are summarized in Supplementary Table S5 and Table S6, respectively.59,60 Some studies used a definition different from the most accepted diagnostic criteria at the time the study was published. For example, Ekin et al 45 and Kazancioglu et al 28 defined patients with at least 5 cysts in each kidney as patients with ADPKD, although Pei criteria were the most commonly used diagnostic criteria for ADPKD during the time period in which the studies were conducted.28,45
Thirty of the 49 studies described how they identified patients with stones, while the remaining 19 studies did not. Among the 30 studies that specified how the stones were detected, 3 studies relied on patient self-report of a history of stones, 14 solely relied on radiological evidence of stone, and 13 studies relied on combination of radiological evidence of stone and at least one other criterion (ie, stone passage and recovery, surgical removal of stone and self-report of stone). Among the 27 of the 30 studies that used radiological evidence of stones as one of their diagnostic criteria, 9 reviewed historic imaging, 10 reviewed recent imaging, and the nature of considered imaging was unclear in 8 studies. Eight of the 27 studies thoroughly described what they were looking for on the radiological image to identify stones. Among the 5 studies that reported asymptomatic stones, the percentage of patients ranged between 1% and 68%.17,18,21,37,48
The setting and source population from which the samples are recruited affects the study generalizability. For 21 of the studies, the setting or population from which the sample was recruited from was unclear or not reported. Patients were recruited from hospitals in 18 studies, outpatient clinics in 7 studies, solely from an inpatient setting in 1 study, an outpatient ADPKD speciality clinic in 1 study, and from both an inpatient and outpatient setting for 1 study. It is unclear if patients were recruited from an inpatient or outpatient setting for 20 studies and setting was not reported for one study.
Six of the 49 studies compared the prevalence of stones in patients with ADPKD to controls, which were unaffected family members. All of these studies were cross-sectional. Only 2 of the 6 studies statistically compared the prevalence of stones in patients with ADPKD to controls. Both of these studies used univariate analyses and did not adjust for any confounders.
Prevalence and Characteristics of Stones and Prevalence of Stone Intervention
In patients with ADPKD, the prevalence of stones ranged between 3% and 59% (Table 3). Of those patients with stones, 2% to 47% underwent at least one stone intervention. Urinary tract infections and flank pain were the predominant precursor to diagnosis of stones in patients with ADPKD.17,21,24,37,40,48 In most patients, stones were solely located in the renal calyces.17,18 Most stones were composed of uric acid according to 6 studies7,18,20,21,37,48 and oxalate according to 2 studies (Table 4).22,26
Table 3.
Prevalence of Stones and Stone Intervention in Patients With ADPKD and Controls.
| Author (year), country | Stone definition (modality) | No. of unique patients with stones (%) | No. of unique patients who underwent stone intervention (%) |
|---|---|---|---|
| Al-Muhanna et al, 25 Saudi | NR (Unclear) | 5 (17) | NR |
| Baishya et al, 17 India | NR (NR) | 19 (4) | 9 (2) |
| Bajrami et al, 20 Albania | Echogenic focus with posterior acoustic shadowing within the kidney
a
(U/S; or plain abdominal KUB film, intravenous pyelography and noncontrast helical CT in cases where stones were not observed on U/S or KUB film) |
58 (58) | NR |
| Chang et al, 44 Taiwan | NR (NR) | 19 (41) | NR |
| Corradi et al, 27 Italy | NR (NR) | 24 (24) | NR |
| Demetriou et al, 7 Cyprus | Passage of stone or presence of stone on a plain KUB film or U/S b (Plain KUB film or U/S) | ADPKD: 21 (20) CONTROL: 4 (4) |
NR |
| Duli et al, 36 Albania | Image of stone within the urinary collecting system a (U/S, renal radiography, CT) | 106 (59) | NR |
| Ekin et al, 45 Turkey | Presence and absence of stone on U/S b and/or history of passing stone (U/S) | 24 (17) | NR |
| Cornec-Le Gall et al, 35 France | NR (NR) | 57 (20) | NR |
| Galliani et al, 47 Italy | NR (NR) | 102 (22) | NR |
| Gonzalo et al, 8 Spain | Hyperechogenic image with posterior shadowing a (U/S or plain roentgenogram with tomograms) | ADPKD: 7 (11) CONTROL: 2 (3) |
NR |
| Grampsas et al, 23 United States | Echogenic focus with posterior acoustic shadowing within the kidney but outside an identifiable cyst a + with or without a clinical history of stone (U/S) | 15 (31) | NR |
| Ishibashi, 49 Japan | NR (NR) | 10 (13) | NR |
| Fary Ka et al, 39 Senegal | NR (NR) | 6 (11) | NR |
| Kaygısız et al, 40 Bursa | History of stone or positive imaging a (U/S, noncontrast CT) | 28 (24) | 10 (8) |
| Kazancioglu et al, 28 Turkey | Presence or absence of urinary tract stones on U/S c and/or history of passing stone (U/S) | 278 (27) d | NR |
| Kim et al, 43 Korea | NR (NR) | 92 (29) d | NR |
| Kumar et al, 41 India | NR (NR) | 6(15) | NR |
| Memili et al, 29 Turkey | Presence and absence of kidney stone b (U/S) | 39 (29) | NR |
| Meng et al, 33 China | NR (NR) | 65 (39) | NR |
| Milutinovic et al, 12 United States | Stones apparent on radiogram c or passed in urine (radiogram) | ADPKD: 16 (11) CONTROL: 5 (4) |
NR |
| Milutinovic et al, 11 United States | Stone apparent on radiograms a or were found in urine (radiogram) | ADPKD: 5 (17) CONTROL: 3 (12) |
NR |
| Nikolov et al, 31 Unclear | NR (NR) | 29 (14) | NR |
| Nishiura et al, 24 Brazil | Image of stone within the renal collection system a (U/S and CT) | 35 (28) | NR |
| Parfrey et al, 10 Canada | Self-report history of kidney stones during interview (NR) | ADPKD: 16 (15)
d
CONTROL: 20 (10) d |
NR |
| Romão et al, 55 Brazil | NR (NR) | 15 (16) | NR |
| Roscoe et al,42,e Canada | Acoustic shadowing on radiologic imaging b (NR) | 8 (10) | NR |
| Segal et al, 56 United States | NR (NR) | 20 (20) | NR |
| Strakosha et al, 48 Albania | Presence on imaging a (ultrasound or abdominal x-ray) | 81 (45) | 2 (1) |
| Torra et al, 9 Spain | Passage of stone with recovery of stone or evidence of stone within the collecting system as reported by the radiologist b (unclear) | ADPKD: 29 (18) CONTROL: 15 (10) d |
NR |
| Torres et al, 18 United States | Historical evidence of passage, recovery, surgical removal of stone, evidence of stone within the collecting system, or renal papillary tips as reported by radiologist b (excretory urogram for a subset [79 patients]; unclear for remaining patients) | 151 (20) | 31 (4) |
| Vikrant and Parashar, 32 India | History of stone passage, removal of stone or calcific foci/nephrocalcinosis seen on imaging b (unclear) | 81 (39) | NR |
| Yildiz et al, 46 Turkey | Self-reported history of stone (NR) | 23 (25) | NR |
| Gonzalo et al, 58 Spain | Passage or surgical removal of stones or presence of radio-opaque deposits on X-ray c (X-ray) | 32 (30) f | NR |
| Hajji et al, 53 Tunisia | NR (NR) | 28 (5) f | NR |
| Hateboer et al, 30 The Netherlands, Spain, Bulgaria, and the United Kingdom | Radiological evidence of kidney stone c (U/S, plain radiographs, intravenous pyelograms, CT) | 42 (10)d, g | NR |
| Idrizi et al, 37 Albania | An echogenic focus with posterior acoustic shadowing within the kidney but outside an identifiable cyst and with or without clinical history of stone a (U/S and X-ray) | 76 (42) h | 2 (1) |
| Ozkok et al, 14 Turkey | Self-reported hx of passing stone or presence or absence of kidney stone on ultrasound b (U/S) | 101 (33) h | NR |
| Papadopoulou et al, 66 Greece | Self-reported history of stone during interview (NR) | 3 (4) h | NR |
| Rabbani et al, 67 Pakistan | Presentation on imaging b (NR) | 6 (11) h | NR |
| Ristovska et al, 34 Republic of Macedonia | Evidence on imaging a (echosonography and CT scan) | 22 (37) h | NR |
| Senel et al, 54 Turkey | NR (NR) | 68 (28)d,h | NR |
| Tantoco and Alano, 68 Philippines | Presence of radiopaque stone on radiographic ultrasound c (radiograph or U/S) | 18 (30) f | NR |
| Thong and Ong,38,e United Kingdom | NR (NR) | 16 (8) h | NR |
| Wright et al, 50 Ireland | NR (NR) | 2 (3) h | NR |
| Delaney et al, 26 United States | Passage of stone or surgical removal of stones from urinary tract or presence of radio-opaque deposits on X-ray c (X-ray) | 18 (34) | 1 (2) |
| Dimitrakov and Simeonov, 22 Bulgaria | Presence or absence of kidney stone on imaging c (echography, venous urography, CT) | 23 (28) | NR |
| Higashihara et al, 22 Japan | NR (NR) | 53 (18)d | NR |
| Idrizi et al, 21 Albania | Echogenic focus with posterior acoustic shadowing within the kidney c (U/S; or plain abdominal KUB film, intravenous pyelography and noncontrast helical CT in cases where stones were not observed on U/S or KUB film) | 116 (58) | 4 (2) |
Note. NR = not reported; U/S = ultrasound; KUB = kidney, ureter, bladder; CT = computed tomography scan; ADPKD = autosomal dominant polycystic kidney disease.
Patients underwent prospective abdominal imaging.
Authors reviewed historic images to ascertain stone event.
Unclear whether investigators prospectively imaged abdomen or reviewed past abdominal images or imaging report to identify stone event.
The denominator only includes a subset of the study population.
Data were abstracted for the portion of the multicomponent study that reported the prevalence of stones.
Unclear whether stone event was ascertained at baseline or during follow-up; therefore, unknown whether the reported percentage was a prevalence or incidence estimate.
Stone was ascertained at baseline and during follow-up; therefore, the percentage is a prevalence estimate.
Stone event was ascertained at baseline; therefore, the percentage is a prevalence estimate.
Table 4.
Symptoms and Characteristics of Stones.
| Author (year), country | Symptoms | Location | Composition |
|---|---|---|---|
| Baishya et al, 17 India | • Anorexia: 3 (16%) • Fever: 1 (5%) • Fluid Overload: 2 (11%) • Hematuria: 5 (26%) • Pain: 6 (32%) • Vomiting: 3 (16%) • Weakness: 2 (11%) |
Location of stones in the 23 kidneys with stones among 19 patients (denominator is 23): • Renal calyces: 10 (28%) • Renal pelvis: 2 (9%) • Both renal pelvis and calyces: 5 (22%) • Ureter: 5 (22%) • Staghorn: 1 (4%) |
NR |
| Bajrami et al, 20 Albania | NR | NR | • Calcium oxalate: NR (39%) • Urate: NR (47%) • Other compounds: NR (14%) |
| Demetriou et al, 7 Cyprus | NR | NR | Majority were uric acid |
| Kaygısız et al, 40 Bursa | Lower back pain: 10 (36%) | NR | NR |
| Nishiura et al, 24 Brazil | Low back pain | NR | NR |
| Strakosha et al, 48 Albania | • 40% of patients with stone associated with a history of UTI and flank pain | NR | • Calcium oxalate: NR (39%) • Urate: NR (47%) • Other Compounds: NR (14%) |
| Torres et al, 18 United States | NR | Among the 71 patients where details about stone location is available: • Only renal calyces: 63 (89%) • Renal pelvis/Staghorn: 4 (6%) • Ureter: 4 (6%) |
Composition examined in 30 patients: • Calcium carbonate: 3 (10%) • Calcium oxalate: 14 (47%) • Calcium phosphate: 6 (20%) • Struvite: 3 (10%) • Uric acid: 17 (57%) |
| Idrizi et al, 37 Albania | History of UTI and flank pain: NR (40%) | NR | • Calcium oxalate: NR (39%) • Urate: NR (47%) • Other compounds: NR (14%) |
| Idrizi et al, 21 Albania | • UTI and Flank pain: 70 (60%) • Gross Hematuria: 65 (56%) |
NR | Among the 63 patients with information on stone composition: • Calcium oxalate: 25 (39%) • Uric acid: 30 (47%) • Other compounds: 8 (14%) |
| Delaney et al, 26 United States | NR | NR | • Calcium oxalate: 3 (50%) • Uric acid stones: 1 (17%) • Calcium oxalate stones in one occasion and uric acid or calcium phosphate stones on the other occasion: 2 (33%) |
| Dimitrakov and Simeonov, 22 Bulgaria | NR | NR | • Oxalate: 12 (52%) • Urate: 6 (26%) • Mixed composition: 5 (22%) |
Note. NR = not reported; UTI = urinary tract infection.
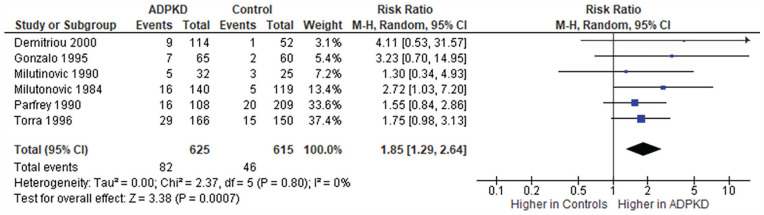
The prevalence of stones ranged from 3% to 12% in family members confirmed not to be affected with ADPKD (Table 3). None of the studies described the characteristics of stones in unaffected family members. All 6 studies that compared the prevalence of stones in patients with and without ADPKD reported stones were more prevalent in patients with ADPKD; however, 4 studies did not statistically analyze the prevalence of stones between the 2 groups, and the remaining 2 studies found no statistical difference. When we statistically compared the prevalence of stones in patients with ADPKD to unaffected family members in the 4 studies that did not conduct any statistical analyses, we found that only one out of the 4 studies found a significant difference. Meta-analysis of the calculated prevalence ratios across 6 cross-sectional studies show that patients with ADPKD had a higher prevalence of kidney stones compared to unaffected family members (unadjusted prevalence ratio: 1.8, 95% confidence interval: 1.3 to 2.6, P = .0007; test for heterogeneity: I2 = 0%, P = .8; Figure 2).
Figure 2.
Calculated unadjusted prevalence ratio of stones in patients with autosomal dominant polycystic kidney disease compared to unaffected family members.
Note. The prevalence ratios were calculated using prevalence estimates obtained from studies and Cochrane Review Manager 5.3. CI = confidence interval.
Six studies reported the prevalence of stone intervention in patients with ADPKD, which ranged between 1% and 8% (Table 3). None of the studies with controls reported the prevalence of stone intervention in unaffected family members.
Stone Incidence
No study clearly reported the incidence of kidney stones and the incidence of stone intervention in patients with ADPKD. Most cohort studies included in this review assessed kidney stones at cohort entry and not during follow-up. Whether the reported percentage was a prevalence or incidence estimate was unclear for 3 of the included cohort studies.
Discussion
Many popular educational materials and clinical practice guidelines state that kidney stones are common in patients with ADPKD, and its prevalence may be 5 to 10 times higher than the general population.61,62 This make clinical sense based on our knowledge of the pathophysiology of ADPKD; the kidney cysts in patients with ADPKD lead to urinary stasis which promotes stone formation. 23 Our review of the literature, however, indicates that the evidence to support these assertions is weak and illuminates several knowledge gaps about the clinical epidemiology of stones in ADPKD. No study has clearly reported the incidence of stones in ADPKD. Prevalence estimates in ADPKD varied widely ranging from 3% to 59% for kidney stones and from 1% to 8% for stone interventions. Urinary tract infections and flank pain were the predominant precursors to diagnosis of stones; however, UTI and flank pain are not specific to stones and are also manifestations of ADPKD independent of stones. It is likely that UTI and flank pain were associated with ADPKD itself rather than stone because most of the stones in ADPKD were located in the renal calyces where they would be less likely to be symptomatic. Uric acid stones are the most prevalent stone composition in patients with ADPKD. The wide-ranging prevalence estimates along with the discovery that no published studies clearly reported stone incidence confirm that how often patients with ADPKD develop kidney stones remains uncertain.
There are several reasons why prevalence estimates of stones varied drastically across studies. These include inconsistent stone definitions, different distributions of stone risk factors, potential recall bias in studies that relied on patient self-report to identify stone events, and relying on past imaging reports done for reasons other than stone identification. Self-report is particularly problematic because the symptoms of flank pain and hematuria are common with ADPKD in the absence of stone disease. Patients with ADPKD may be more likely to undergo renal imaging, which would lead to over-detection of potentially clinically insignificant stones which may also exist undetected in the general population. The variability in imaging modalities used across studies and even between patients in the same study may also explain the variable prevalence estimates across studies. For example, computed tomography (CT) is a more sensitive method of stone detection than ultrasound and would provide a more accurate estimate of stone prevalence.63,64 There are many in the current literature. Most of the studies published to date on stones in ADPKD were conducted in a single center and are of poor methodological quality. Additionally, only 6 studies compared the prevalence of stones in patients with ADPKD to controls.7-12 Among these 6 studies, only 2 statistically compared the prevalence of stones between the 2 groups,9,10 and none of these studies adjusted for confounders.7-12 Additionally, not all patients with ADPKD were hospitalized; as a result, prevalence estimates obtained from patients recruited from an inpatient setting must be generalized to the broader ADPKD population with caution. Similarly, the prevalence estimates obtained from patients recruited from an outpatient speciality clinic must also be generalized to the broader ADPKD population with caution due to increased surveillance. Also, only 8 of 49 of the included studies described the composition of stones in patients with ADPKD; none of the 8 studies compared the composition of stones in patients with ADPKD to patients without ADPKD.
This review serves as a call to action for better research in this field. We recommend conducting large, multicenter studies that compare the risk of stones and risk of stone intervention between a representative population of ADPKD and controls to better characterize the magnitude of kidney stone and stone intervention risk in patients with ADPKD. We also recommend that such studies adjust for important confounders, such as hypertension, to better characterize the true association between ADPKD and kidney stones and stone intervention. Imaging tests are much more advanced, widespread, and frequent over time; this may lead to the possibility of detecting stones in ADPKD that may not be clinically relevant. Examining risk of kidney stone diagnosis and kidney stones that require intervention separately would provide insight into whether there is a potentially higher burden of asymptomatic stone that were detected incidentally on imaging. More reliable estimates of the magnitude of risk of stones and stone intervention would provide insight into clinical management practices and help patients with ADPKD and their physicians better prognosticate. If patients with ADPKD are truly at higher risk for kidney stones, then nephrologists may want to consider preventative measures for kidney stones. For example, if patients with ADPKD are at higher risk of kidney stones and hypocitraturia, then nephrologists may want to screen for hypocitraturia and treat patients with potassium citrate. Nephrologists may also want to consider treating large cysts that obstruct the urinary system and cause urinary stasis. Preventing stone formation would alleviate pain due to kidney stones and potentially slow down disease progression in patients with ADPKD. We also recommend comparing the composition of stones observed in patients with ADPKD compared to patients without ADPKD. New medications used in ADPKD, such as vasopressin receptor 2 antagonists, may alter the urine composition and change the types of renal stones that these patients get. Future ADPKD-specific risk factors, such as mutation type, of kidney stone studies may help identify patients at high risk for stones and provide further insight into the pathophysiology of kidney stones in patients with ADPKD.
Our study is the first to systematically review and summarize the prevalence of stones in patients with ADPKD. Unlike past narrative reviews, we used a comprehensive search strategy across 6 different databases, and 2 reviewers independently screened all citations retrieved from the search strategy to identify all relevant articles. We also conducted this review in accordance with an a priori protocol and published guidelines for systematic reviews. Two independent reviewers abstracted the data to minimize human error and bias.
There are some limitations inherent in our systematic review. First, we only included original journal articles and conference proceedings published in English. However, studies show that language-restricted meta-analysis does not lead to biased estimates. 65 Second, the definitions for ADPKD and stones varied across studies; therefore, the pooled estimate must be interpreted with caution.
Conclusions
Our systematic review highlights that there is poor consensus on the prevalence of stones in patients with ADPKD. A more methodologically robust study is needed to better characterize and understand the magnitude of risk of stones and stone intervention in patients with ADPKD. This information can help patients with ADPKD and physicians with their prognostication and might inform the use of interventions to reduce the risk of stones.
Supplemental Material
Supplemental material, SupplementaryMaterials_PrevalenceOfStone_CJKHD for Stone Prevalence in Autosomal Dominant Polycystic Kidney Disease: A Systematic Review and Meta-Analysis by Vinusha Kalatharan, Gary Grewal, Danielle M Nash, Blayne Welk, Sisira Sarma, York Pei and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
We thank John Costello for reviewing the search strategy developed by V.K.
Footnotes
Ethics Approval and Consent to Participate: Since this systematic review and meta-analysis did not involve human investigation, ethics approval was not required. Consent to participate was not required as our study did not rely on human subjects and reviewed the existing literature.
Consent for Publication: All authors have consented for publication.
Availability of Data and Materials: All data is presented in the original article.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. York Pei served as an expert consultant on drug development (Otsuka, Pfizer, and Genzyme/Sanofi) related to autosomal dominant polycystic kidney disease. All other authors declare no competing interests.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The ICES Kidney, Dialysis, and Transplantation Program provided funding for this study. Vinusha Kalatharan’s training was supported by the Canadian Institutes of Health Research Doctoral Scholarship and the Doctoral Scholarship from the KRESCENT Program (a national kidney research training partnership of the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institutes of Health Research). Dr. Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a Clinician Investigator Award from the Canadian Institutes of Health Research.
ORCID iDs: Vinusha Kalatharan  https://orcid.org/0000-0001-7431-8087
https://orcid.org/0000-0001-7431-8087
Amit X. Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol. 2006;1(1):148-157. [DOI] [PubMed] [Google Scholar]
- 3. Wong H, Vivian L, Weiler G, Filler G. Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis. 2004;43(4):624-628. [DOI] [PubMed] [Google Scholar]
- 4. Grantham JJ, Torres VE. The importance of total kidney volume in evaluating progression of polycystic kidney disease. Nat Rev Nephrol. 2016;12(11):667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McEwan P, Bennett Wilton H, et al. A model to predict disease progression in patients with autosomal dominant polycystic kidney disease (ADPKD): the ADPKD Outcomes Model. BMC Nephrol. 2018;1319(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres VE, Wilson DM, Hattery RR, Segura JW. Renal stone disease in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1993;22(4):513-519. [DOI] [PubMed] [Google Scholar]
- 7. Demetriou K, Tziakouri C, Anninuo K, et al. Autosomal dominant polycystic kidney disease-Type 2. Ultrasound, genetic and clinical correlations. Nephrol Dial Transplant. 2000;15(2):205-211. [DOI] [PubMed] [Google Scholar]
- 8. Gonzalo A, Gallego A, Orte L, et al. Asymptomatic complications of autosomal dominant polycystic kidney disease. J Nephrol. 1995;8(4):202-205. [Google Scholar]
- 9. Torra R, Badenas C, Darnell A, et al. Linkage, clinical features, and prognosis of autosomal dominant polycystic kidney disease types 1 and 2. J Am Soc Nephrol. 1996;7(10):2142-2151. [DOI] [PubMed] [Google Scholar]
- 10. Parfrey PS, Bear JC, Morgan J, et al. The Diagnosis and prognosis of autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1085-1090. [DOI] [PubMed] [Google Scholar]
- 11. Milutinovic J, Fialkow PJ, Agodoa LY, Phillips LA, Rudd TG, Sutherland S. Clinical manifestations of autosomal dominant polycystic kidney disease in patients older than 50 years. Am J Kidney Dis. 1990;15(3):237-243. [DOI] [PubMed] [Google Scholar]
- 12. Milutinovic J, Fialkow PJ, Agodoa LY, Phillips LA, Rudd TG, Bryant JI. Autosomal dominant polycystic kidney disease: symptoms and clinical findings. Q J Med. 1984;53(212):511-522. [PubMed] [Google Scholar]
- 13. Nishiura JL, Eloi SRM, Heilberg IP. Pain determinants of pain in autosomal dominant polycystic kidney disease. J Bras Nefrol. 2013;35(3):242-243. [DOI] [PubMed] [Google Scholar]
- 14. Ozkok A, Akpinar TS, Tufan F, et al. Clinical characteristics and predictors of progression of chronic kidney disease in autosomal dominant polycystic kidney disease: a single center experience. Clin Exp Nephrol. 2013;17(3):345-351. [DOI] [PubMed] [Google Scholar]
- 15. Moher D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. [DOI] [PubMed] [Google Scholar]
- 16. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baishya R, Dhawan DR, Kurien A, Ganpule A, Sabnis RB, Desai MR. Management of nephrolithiasis in autosomal dominant polycystic kidney disease—a single center experience. Urol Ann. 2012;4(1):29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres VE, Erickson SB, Smith LH, Wilson DM, Hattery RR, Segura JW. The association of nephrolithiasis and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1988;11(4):318-325. [DOI] [PubMed] [Google Scholar]
- 19. Higashihara E, Aso Y, Shimazaki J, Ito H, Koiso K, Sakai O. Clinical aspects of polycystic kidney disease. J Urol. 1992;147(2):329-332. [DOI] [PubMed] [Google Scholar]
- 20. Bajrami V, Idrizi A, Roshi E, Barbullushi M. Association between nephrolithiasis, hypertension and obesity in polycystic kidney disease. Open Access Maced J Med Sci. 2016;4(1):43-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idrizi A, Barbullushi M., Gjata M., et al. Prevalence of nephrolithiasis in polycystic kidney disease. Cent Eur J Med. 2011;6(4):497-501. [Google Scholar]
- 22. Dimitrakov D, Simeonov S. Studies on nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Folia Med (Plovdiv). 1994;36(3):27-30. [PubMed] [Google Scholar]
- 23. Grampsas SA, Chandhoke PS, Fan J, et al. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;36(1):53-57. [DOI] [PubMed] [Google Scholar]
- 24. Nishiura JL, Neves Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(4):838-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Al-Muhanna FA, Malhotra KK, Saeed I, Al-Mueilo S. Autosomal dominant polycystic kidney disease: observations from a university hospital in Saudi Arabia. Saudi J Kidney Dis Transpl. 1995;6(1):28-31. [PubMed] [Google Scholar]
- 26. Delaney VB, Adler S, Bruns FJ, Licinia M, Segel DP, Fraley DS. Autosomal dominant polycystic kidney disease: presentation, complications, and prognosis. Am J Kidney Dis. 1985;5(2):104-111. [DOI] [PubMed] [Google Scholar]
- 27. Corradi V, Gastaldon F, Virzì GM, et al. Clinical pattern of adult polycystic kidney disease in a northeastern region of Italy. Clin Nephrol. 2009;72(4):259-267. [DOI] [PubMed] [Google Scholar]
- 28. Kazancioglu R, Ecder T, Altintepe L, et al. Demographic and clinical characteristics of patients with autosomal dominant polycystic kidney disease: a multicenter experience. Nephron Clin Pract. 2011;117(3):c270-c275. [DOI] [PubMed] [Google Scholar]
- 29. Memili VK, Kutlu C, Sar F, Kazancioglu R. Demographic analysis of polycystic kidney disease patients: a single center experience. BANTOA J. 2007;5(1):6-9. [Google Scholar]
- 30. Hateboer N, van Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. Lancet. 1999;353(9147):103-107. [DOI] [PubMed] [Google Scholar]
- 31. Nikolov I, Ivanovski O, Daudon M, et al. Uric acid is the main component of kidney stones in patients with autosomal dominant polycystic kidney disease (ADPKD)—a study based on stone composition, morphology and infrared spectophotometry analysis. Eur Urol. 2012; 11(1):e860. [Google Scholar]
- 32. Vikrant S, Parashar A. Autosomal dominant polycystic kidney disease: study of clinical characteristics in an Indian population. Saudi J Kidney Dis Transpl. 2017;28(1):115-124. [DOI] [PubMed] [Google Scholar]
- 33. Meng J, Xu Y, Li A, et al. Clinical features of 167 inpatients with autosomal dominant polycystic kidney disease at a single center in China. Med Sci Monit. 2018;24:6498-6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ristovska V, Grcevska L. Nephrolithiasis and urinary tract infections increase the progression of renal failure in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29(suppl 3):378. [Google Scholar]
- 35. Cornec-Le Gall E, Audrezet MP, Renaudineau E, et al. PKD2-related autosomal dominant polycystic kidney disease: prevalence, clinical presentation, mutation spectrum, and prognosis. Am J Kidney Dis. 2017;70(4):476-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duli M, Idrizi A, Barbullushi M, et al. Role of imaging in detection of nephrolithiasis in autosomal dominant polycystic kidney disease. Eur Urol Suppl. 2013;12(3):81-82. [Google Scholar]
- 37. Idrizi A, Barbullushi M, Petrela E, Kodra S, Koroshi A, Thereska N. The influence of renal manifestations to the progression of autosomal dominant polycystic kidney disease. Hippokratia. 2009;13(3):161-164. [PMC free article] [PubMed] [Google Scholar]
- 38. Thong KM, Ong ACM. The natural history of autosomal dominant polycystic kidney disease: 30-year experience from a single centre. QJM. 2013;106(7):639-646. [DOI] [PubMed] [Google Scholar]
- 39. Fary Ka E, Seck SM, Niang A, Cisse MM, Diouf B. Patterns of autosomal dominant polycystic kidney diseases in black Africans. Saudi J Kidney Dis Transpl. 2010;21(1):81-86. [PubMed] [Google Scholar]
- 40. Kaygısız O, Coşkun B, Oruç A, et al. Evaluation of nephrolithiasis risk factors in Autosomal Dominant Polycystic Kidney Disease (ADPKD): a single center experience. Okmeydanu Tip Dergisi. 2018;34(2):87-91. [Google Scholar]
- 41. Kumar A, Kawoosa Z, Hamid S, et al. A prospective study on clinical profile of Autosomal Dominant Polycystic Kidney Disease (ADPKD) in Jammu for a period of 1 year. J Nephrol. 2012;2:123-135. [Google Scholar]
- 42. Roscoe JM, Brissenden JE, Williams EA, Chery AL, Silverman M. Autosomal dominant polycystic kidney disease in Toronto. Kidney Int. 1993;44(5):1101-1108. [DOI] [PubMed] [Google Scholar]
- 43. Kim H, Koh J, Park SK, et al. Baseline characteristics of the autosomal dominant polycystic kidney disease subcohort of the KoreaN Cohort Study for Outcomes in Patients with Chronic Kidney Disease (KNOW-CKD). Nephrol. 2019;24)4):422-429. [DOI] [PubMed] [Google Scholar]
- 44. Chang MY, Chen HM, Jenq CC, et al. Novel PKD1 and PKD2 mutations in Taiwanese patients with autosomal dominant polycystic kidney disease. J Hum Genet. 2013;58(11):720-727. [DOI] [PubMed] [Google Scholar]
- 45. Ekin B, Çörekçioğlu B, Çiftkaya A, et al. Demographic and clinical characteristics of patients with autosomal dominant polycystic kidney disease in Istanbul faculty of medicine. Turk Med Stud J. 2014;2(1):15-8. [Google Scholar]
- 46. Yildiz A, Sag S, Oruc A, et al. Demographic and clinical characteristics of patients with autosomal dominant polycystic kidney disease: a single center experience. Turk Nephrol Dial Transplant J. 2016;25(1):100-103. [Google Scholar]
- 47. Galliani M, Chicca S, Vitaliano E, et al. Clinical phenotype of ADPKD patients at the time of referral to nephrologist: a multicenter survey in Italy. Nephrol Dial Transplant. 2015;30(suppl 3):SP366. [Google Scholar]
- 48. Strakosha A, Idrizi A, Barbullushi M, et al. Lithiasic complication in autosomal dominant polycystic kidney disease: an experience of 15 years. Nephrol Dial Transplant. 2006;21(suppl 4):355.16249196 [Google Scholar]
- 49. Ishibashi A. Renal imagings in the diagnosis of polycystic kidney disease. Nihon Jinzo Gakkai Shi. 1981;23(7):1003-1013. [PubMed] [Google Scholar]
- 50. Wright GD, Hughes AE, Larkin KA, Doherty CC, Nevin NC. Genetic linkage analysis, clinical features and prognosis of autosomal dominant polycystic kidney disease in Northern Ireland. Q J Med. 1993;86(7):459-463. [PubMed] [Google Scholar]
- 51. Rall JE, Odel HM. Congenital polycystic disease of the kidney; review of the literature and data on 207 cases. Am J Med Sci. 1949;218(4):399-407. [DOI] [PubMed] [Google Scholar]
- 52. Merta M, Stekrovã J, Zidovskã J, et al. DNA diagnosis and clinical manifestations of autosomal dominant polycystic kidney disease. Folia Biol (Praha). 1997;43(5):201-204. [PubMed] [Google Scholar]
- 53. Hajji M, Barbouch S, Harzallah A, et al. Clinical study on autosomal dominant polycystic kidney disease among North Tunisians. Saudi J Kidney Dis Transpl. 2019;30(1):175-184. [PubMed] [Google Scholar]
- 54. Senel TE, Trabulus S, Yalin SF, Seyahi N, Altiparmak MR. Renal survival and associated factors in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2016;31(suppl. 1):93-94. [Google Scholar]
- 55. Romão EA, Moysés Neto M, Teixeira SR, Muglia VF, Vieira-Neto OM, Dantas M. Renal and extrarenal manifestations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006;39(4):533-538. [DOI] [PubMed] [Google Scholar]
- 56. Segal AJ, Spataro RF, Barbaric ZL. Adult polycystic kidney disease: a review of 100 cases. J Urol. 1977;118(5):711-713. [DOI] [PubMed] [Google Scholar]
- 57. Tantoco ML, Alano FA. Adult polycystic kidney disease. Philipp J Nephrol. 2017;1(1):5. [Google Scholar]
- 58. Gonzalo A, Rivera M, Quereda C, Ortuño J. Clinical features and prognosis of adult polycystic kidney disease. Am J Nephrol. 1990;10(6):470-474. [DOI] [PubMed] [Google Scholar]
- 59. Ravine D, Gibson RN, Walker RG, Kincaid-Smith P. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343(8901):824-827. [DOI] [PubMed] [Google Scholar]
- 60. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chapman AB, Devuyst O, Eckardt KU, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mallett A, Patel M, Tunnicliffe DJ, Rangan GK. KHA-CARI autosomal dominant polycystic kidney disease guideline: management of renal stone disease. Semin Nephrol. 2015;35(6):603-606.e3 [DOI] [PubMed] [Google Scholar]
- 63. Levine E, Jared JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease clinical and CT study in 84 patients. AJR Am J Roentgenol. 1992;159(1):77-81. [DOI] [PubMed] [Google Scholar]
- 64. Brisbane W, Bailey MR, Sorensen MD. An overview of kidney stone imaging techniques. Nat Rev Urol. 2016;13(11):654-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses. J Clin Epidemiol. 2000;53(9):964-972. [DOI] [PubMed] [Google Scholar]
- 66. Papadopoulou D, Tsakiris D, Papadimitriou M. The use of ultrasonography and linkage studies for early diagnosis of autosomal dominant polycystic kidney disease (ADPKD). Ren Fail. 1999;21(1):67–84. [DOI] [PubMed] [Google Scholar]
- 67. Rabbani MA, Ali SS, Murtaza G, et al. Clinical presentation and outcome of autosomal dominant polycystic kidney disease in Pakistan: a single center experience. J Pak Med Assoc. 2008;58(6):305-309. [PubMed] [Google Scholar]
- 68. Tantoco ML, Alano FA. Adult polycystic kidney disease. Philippine Nephro Online. 1986;1(1):5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, SupplementaryMaterials_PrevalenceOfStone_CJKHD for Stone Prevalence in Autosomal Dominant Polycystic Kidney Disease: A Systematic Review and Meta-Analysis by Vinusha Kalatharan, Gary Grewal, Danielle M Nash, Blayne Welk, Sisira Sarma, York Pei and Amit X. Garg in Canadian Journal of Kidney Health and Disease