Abstract
We propose a theory known as the Hyland model to help conceptualise Fibromyalgia within a complex adaptive control system. A fundamental assumption is that symptom generating mechanisms are causally connected, forming a network that has emergent properties. An illness narrative has been developed which has a ‘goodness of fit’ with the lived experience of those with Fibromyalgia. The theory guides management within the clinical setting and incorporates current evidence-based therapeutic strategies, within a multi-modal intervention described as ‘Body Reprogramming’. This intervention focuses on non-pharmacological and lifestyle-based considerations. The theoretical framework also helps explain why modest therapeutic effects are gained from current pharmacological options.
Keywords: Fibromyalgia, functional disorder, central sensitivity syndrome, adaptive network system, Hyland model, Body Reprogramming, stop signals
Introduction
“Why is it hard for some healthcare professionals to believe that Fibromyalgia is real?” This patient generated question was asked after protracted investigation, reluctant clinical diagnosis, and disappointment with ineffective therapeutic plans. The enquiry encapsulates the ongoing difficulty with adopting an epistemological framework, uniformly accepted by both patients and clinicians.
Fibromyalgia (FMS) presents a complex set of scientific and clinical challenges in definition, aetiology and diagnostic criteria, which result in a lack of consensus regarding management strategies. Clinical uncertainty is no more evident than in the repeated expert-led changes made to diagnostic criteria over the past decade (Wolfe et al., 1990, 2010, 2011, 2016). Controversy also continues regarding the legitimacy of symptoms, identity with psychiatric conditions and the extension beyond a pain specific disease, as classified within the latest International Classification of Diseases (ICD-11) (Hauser and Fitzcharles, 2018; Henningsen et al., 2019; Nicholas et al., 2019; Sarzi-Puttini et al., 2018; Treede et al., 2015; Wolfe et al., 2019).
FMS is currently considered by some clinicians and researchers as a “functional disorder” in a similar way to chronic fatigue syndrome and irritable bowel syndrome. There is a substantial overlap between functional somatic symptoms, challenging the acceptance of distinct syndromes as defined in the medical literature (Steingrímsdóttir et al., 2017; Wessely and White, 2004). As a result, these are increasingly referenced under the umbrella term of Central Sensitivity Syndromes (Yunus, 2007a). Whilst specific biomedical diseases are defined by unique pathophysiological fingerprints, functional disorders are identified by perceived patterns or clusters of symptoms. (Ceko et al., 2012; Kumbhare et al., 2018). Since diagnosis is viewed as a cornerstone of western medical practice, considerable uncertainty is introduced when it is based on the presence of a variable pattern of multiple symptoms (Srinivasan et al., 2019).
Further to this, the biomedical model of disease places emphasis on obtaining objective independent evidence to corroborate symptoms through clinical examination, imaging and laboratory investigations, all of which are typically negative in functional disorders. The lack of pathological changes can result in clinical ambivalence, communication related confusion and therapeutic dissonance at various stages on the patient journey (Arnold et al., 2008). It is little wonder that there is diagnostic turbulence from both clinical provider and recipient perspectives. We therefore propose that a paradigm shift is required to provide a more acceptable epistemological framework for phenomena such as FMS.
Diagnostic labelling
The provision of a diagnostic label, in itself, often helps legitimise a symptom complex, particularly when there has been prolonged clinician and patient uncertainty. Delay in this labelling or naming is common in FMS, with a mean time in excess of 2 years, and multiple clinicians often seen prior to diagnosis (Choy et al., 2010). A validated diagnosis of FMS can initially nurture satisfaction, reduce stigmatisation and potentially reduce healthcare utilisation (Asbring and Narvanen, 2002; Hughes et al., 2006; White et al., 2002).
Naming with a diagnostic label, is often seen as an important step in creating meaning; although this is far from being universally accepted by health-care professionals (Bidari et al., 2018; Undeland and Malterud, 2007). Whilst modern medical practice expects rigour from the labelling process, a prime function of diagnosis is to help the patient derive meaning regarding the illness experience itself (Madden and Sim, 2006). In functional disorders, patient enthusiasm can be undermined, as the negative aspects of the diagnosis or labelling, including lack of curative options, changed personal and occupational relationships along with poor prognosis, impact on the subsequent perception of their illness (Briones-Vosmediano et al., 2013). Relabelling FMS under a more utilitarian umbrella term such as Central Sensitivity Syndrome is unlikely to overcome this problem, as in most cases this does nothing to ameliorate these negative sequelae.
There can be a sense of medical illegitimacy with diagnoses such as FMS and Chronic Fatigue Syndrome/ME, particularly amongst other medical specialities (Madden and Sim, 2006). However, new diagnostic criteria for FMS were introduced in 2010 by the American College of Rheumatology (ACR), but these were modified in 2011 with further modifications again in 2016 (Wolfe et al., 2010, 2011, 2016). There is still ongoing debate regarding their utility, particularly with substantial clinical heterogeneity within the diagnosed population (Kumbhare et al., 2018). Further to this, non-specialists can have a suboptimal understanding of the condition and clinicians are variable in their compliance with applying diagnostic criteria: some preferring to rely on clinical acumen and professional judgement for diagnosis (Hayes et al., 2010; Perrot et al., 2008, 2012; Walitt et al., 2016).
In generic terms, diagnosis offered within a biomedical framework can help patients begin to develop an understanding of the illness experience. However, Madden and Sim (2006) suggests that this requires a ‘goodness of fit’ between diagnosis and the illness experience. If there is a mismatch between the conceptual meaning of the diagnosis and the experience of the condition, then the diagnosis may not be accepted, resulting in ongoing confusion and uncertainty. Adamson (1997) also suggests that receiving a diagnosis of an illness is not a specific finite event, but rather a process of discovery that evolves over time.
In FMS, quantitative research cannot provide a clear account of patients’ personal illness narratives and perceptions (Furness et al., 2018; Sturge-Jacobs 2002). In contrast, a meta-synthesis of the qualitative literature on the subjective experience of FMS identifies four central themes: experience of symptoms, search for diagnosis, legitimacy and coping (Sim and Madden, 2008). This reflects the ambiguous and heterogeneous nature of FMS symptoms and is therefore likely to challenge the patient’s understanding.
We propose that the clinical narrative provided to explain the patient experience during the diagnostic journey is central to cementing therapeutic engagement and fostering hope for the future. The narrative will help give meaning to the diagnosis (Hill, 2019; Hyland, 2017).
Biological and psychological paradigms
Numerous theories exist to help health care professionals explain the symptoms and underlying aetiology of FMS, with the most plausible suggesting the involvement of several interplaying mechanisms. These include blunting of inhibitory pain pathways, central sensitisation, alterations in neurotransmitters and mental health comorbidity (Abeles et al., 2007). Since 1990, studies have attempted to delineate clinical sub-groups using the initial FMS classification criteria, in the search for specific pathophysiological mechanisms, but without success (De Souza et al., 2009; Giesecke et al., 2003; Loevinger et al., 2012; Turk et al., 1998).
The dominant, biomedical pathological framework, is frequently applied to functional disorders, but whether viewed from a biological or a psychological perspective, neither adequately explains the aetiology nor subsequent symptomology displayed in these disorders (Hyland, 2017). Biological approaches include theories explaining functional disorders in terms of endocrine, immune, autonomic or neurological abnormalities, including central sensitisation and dysfunction (Kolacz and Porges, 2018; Nielsen and Henrikkson, 2007; Tanriverdi et al., 2007; Yunus, 2007b). However, the symptom profile is inconsistent between patients diagnosed and these theories have only opened modest therapeutic avenues.
The psychological paradigm has nurtured theories often based on the concept of psychological distress, directly or indirectly linked through atypical behaviours, resulting in the emergence of multiple somatic symptoms (Barsky and Borus, 1999; Fietta, 2007; Henningsen et al., 2018; Malin and Littlejohn, 2012; Nolen-Hoeksema, 2014). These latter explanations typically put forward the psycho-analytic notion that mental distress has evolved into body symptoms. In addition to being scientifically untestable, this approach has resulted in fractious clinical consultations and often hostile rejection by patients and support groups (Bekhuis et al., 2020; Rief and Broadbent, 2007).
Clinical evidence does support the notion that both biological and psychological factors impact on the development of functional disorders (Fitzcharles and Yunus, 2012; Gupta and Silman, 2004; Khalil et al., 2016; Lyon et al., 2011; Stanford and King, 2009). Developing an intermediary based understanding that incorporates both biological and psychological events could be useful for researchers, clinicians and patients. This is possible without leaving the current paradigm, but merely extending its scope and we would argue that it is therefore unlikely to provide the paradigm shift needed to foster a more mutually acceptable narrative and epistemological framework.
New conceptual model: Hyland model
A conceptual model favoured by the authors for understanding FMS is the Hyland model (Hyland, 2017). This has its scientific origin in Adaptive Network Theory, a form of Complexity theory. It builds on previous literature, recommending the development of integrated approaches based on complexity and network dynamics, for understanding physiological adaptation in health and disease (Baffy and Loscalzo, 2014). Previous authors concur that FMS cannot be understood through the prevailing linear-reductionist biomedical model and have utilised Complexity theory in FMS, conceptualising the condition as a failed attempt of our autonomic system to adjust to a hostile environment (Martinez-Lavin et al., 2008, Martinez-Lavin and Vargas, 2009).
The Hyland model considers a more comprehensive complex adaptive framework, within a network system. The fundamental assumption made is that symptom generating mechanisms are causally connected, forming a network that has emergent properties (Melidis et al., 2018). It is well established that individual body systems adapt to stressors (McEwen, 2008). For instance, the immunological system will develop a more effective response following repeated exposure to infective organisms and will also respond to psychological stressors (Segerstrom and Miller, 2004). Haematological adaptation will occur at high altitude and musculoskeletal development will follow exercise.
The adaptive network theory however goes beyond simple, single system adaptation to propose a more complex set of processes, involving a combination of events (Hyland, 2017). We would suggest that interrogation of this dynamic complex process, within a network-based system, may help gain novel insights into the changes noted in the widespread symptomology displayed by people with FMS.
The body can be considered as an intelligent system, with control systems operating at both biological and psychological levels. The former has multiple systems including immunological, neurological, haematological, autonomic, endocrine and metabolic systems. The psychological control systems will incorporate those relating to threat, drive and ‘soothing’ including behaviour orientated to goals (Gilbert, 2014). The Hyland model is based on the proposal that biological and psychological factors form part of the same complex adaptive control system: a control system that has both biological and psychological mediated inputs, as well as biological and psychological outputs (Hyland, 2011, 2017; Melidis et al., 2018).
The model considers the functioning of control feedback loops, when they are repeatedly challenged by external inputs: physiologically described as the allostatic load (Ramsay and Woods, 2014). This may result in an allostatic state: defined as a chronic deviation of regulatory systems away from their normal state of operations, to establish a new set point (Koob and Le Moal, 2001; Ullmann et al., 2019). Sustained stressors can result in allostatic overload, which can have an adverse impact on the maintenance of stability within the system. Continued stressors will influence the parameters of the control system, including modification of the reference criterion (set target or goal) or potential change to the gain (amplification factor) within the system. An adaptive example of resetting the reference criterion would be lowering of basal heart rate following regular exercise. In FMS aberrant responses to psychological and physical stressors are often noted, with higher sustained heart rate and lower variability (Galvez-Sanchez et al., 2019; Reyes del Paso et al., 2011). This maladaptive change can frequently result in a secondary biomedical diagnosis of Postural Orthostatic Tachycardia Syndrome (POTS) (Staud, 2008).
An example of maladaptation in gain is seen in the bowel symptomology frequently noted in functional disorders, with its amplified cyclical pattern of diarrhoea and constipation. Another is in temperature dysregulation with patients fluctuating from feeling too hot to too cold. The resetting of the control loop to the sustained allostatic load will also influence the interconnecting network of control systems, as the organism attempts to maintain overall stability. Conceptually, in the Hyland model (2017), this interconnecting network functions as an intermediary controlling mechanism.
The symptom clusters displayed within individual control systems can be represented as an interconnecting network of ‘nodes’ within the body. As the level of activity in one node increases, it will also modulate the activation levels of other nodes to which it is connected, as well as resulting in the modulatory connections between them becoming more active (see Figure 1). As a consequence, differentiation between symptom clusters may become less obvious and trigger more widespread symptomology. Networks thus can adapt through alterations in the strength of the causal connections between the different nodes. Instability within the system, is more likely to occur when the network has less time for instigating change.
Figure 1.
Evolution of functional disorder symptom clusters utilising adaptive network theory. (a) Each node represents a symptom cluster relating to an individual control system. (b) Increased level of adaptive activity in a specific node when the control system is challenged. (c) Represents modulation of activation level of other control systems (nodes) via connective pathways. (d) Increased strength in casual connections between nodes with chronic activation. (e) Increased activation of secondary nodes further activate other nodal connectivity. (f) Continued activation results in reduced differentiation between symptom clusters (nodes) resulting in more widespread symptomology.
Melidis et al. (2018) describes a network created during a machine learning analysis, whilst testing the adaptive network explanation of functional disorders, whilst their data is consistent with two types of networks; namely symptom networks and adaptive networks, the former cannot explain the covariation of all the symptoms assessed in the study. Their findings suggest that whatever the underlying mechanisms (depicted as nodes), the mechanisms causing the different symptoms are not independent but are causally related to each other. The algorithm from their recent study produced 11 clusters of symptoms (nodes). Nine of these clusters mirror clinically meaningful groups of symptoms. The suggested cluster labels include; hypothalamic-pituitary-adrenal, limbic, atopic, central sensitisation, gastric, fatigue and cognitive, gastric, mood, micro-capillary and small nerve fibre related (see Figure 2).
Figure 2.

Reprinted from Melidis et al. (2018), with permission from Elsevier. Creation of an adaptive network algorithm for functional disorders from a machine learning analysis of symptoms. Each node in the graph corresponds to a cluster with the size of the nodes scaling according to the number of symptoms contained in each cluster. The edges connecting the nodes represent the connections between the clusters. Their size varies with respect to the strength of each connection, showing the value difference between in-coming and out-going connection for each edge. (1) Fatigue/cognitive – 9 symptoms. (2) Hypothalamic-pituitary-adrenal – 11 symptoms. (3) Limbic system – 7 symptoms. (4) Atopy (IgE) – 5 symptoms. (5) Central sensitisation – 8 symptoms. (6) Gastric – 6 symptoms. (7) Frequent urination – 1 symptom. (8) Mood – 4 symptoms. (9) Micro-capillary – 2 symptoms. (10) Tinnitus – 1 symptom. (11) Small nerve fibre – 7 symptoms.
This modelling also demonstrated that the ability to differentiate between the diagnostic categories FMS, CFS/ME and IBS becomes significantly less in the high, compared with the low symptom severity groups, which is consistent with the adaptive network theory. This theory may help explain the development of more widespread and unpredictable symptoms, as well as the increased severity noted when an individual with FMS deals with other sustained stressors such as infection, physical deconditioning or emotional trauma. Thus, symptom patterns appear to coalesce into similar manifestations with increasing severity. This suggests that a patient who has been labelled with FMS, CFS/ME and IBS should perhaps be considered as having a unified presentation, rather than having three separate medical syndromes.
This concept of a more systemic paradigm has been suggested by other authors in trying to account for such widespread symptomatology. For example, Hill (2019) suggested that such phenomena should be considered, not as a result of structural, pathological, psychological or autoimmune abnormalities, but as the product of a catastrophically dysregulated neurological protection system, that over-reacts to normal input, which is regarded as a potential threat.
The Hyland model (2017) introduces the theoretical constructs of ‘stop signals’ and ‘stop programs’ developing within the framework of this integrated mechanism. Stop signals are considered as adaptive symptom clusters, triggered by either biological or psychological events, designed to change and generally inhibit behaviour. The former will include responses to infection and tissue damage, triggered in part by inflammatory mediators. Psychological events include responses to a perceived failure to achieve life goals, repetition of goal orientated behaviour (reactive inhibition) and emotional difficulties (such as anxiety or threat responses). Thus, pain is regarded as a stop signal to reduce movement, in order to minimise further trauma and fatigue as a signal that aims to reduce over-activity.
The model suggests that if these symptom clusters or ‘stop signals’ consistently fail to modify behaviour and are overridden, suppressed or ignored, the control system is likely to potentiate these signals and so change the parameters, including the reference criterion (default position or goal) as well as having persistent amplification (gain) within the control system. These ‘stop signals’ now become ’stop programs’ as the network control systems fail to respond appropriately and adaptation continues, even after elimination of the initiating event or events (see Figure 3). In physiological terms this has become a maladaptive allostatic state.
Figure 3.

Development of biological and psychological stop signals into stop programs.
Hyland suggests that conditions such as FMS occur when there is persistence in goal orientated activity, despite the manifestation of ongoing ‘stop signals’ (see Figure 4). Three main reasons are proposed as driving this process. Social or family obligations driving a superordinate goal, secondly continuation whilst experiencing ongoing physical or psychological trauma, where there are few alternatives available to the individual. The third reason is one not typically considered by other theories, namely that the person is engaged in an activity which is so absorbing or interesting, that they fail to consciously register the ‘stop signals’ or are inhibited until the symptom complex overwhelms. To put it another way; the mind continues to write cheques that the body cannot cash. The body, by way of upregulation of causal network connections, “shouts louder” and by way of maladaptation of the control systems, ‘stop programs’ are developed.
Figure 4.

Pictorial representation of the evolution of stop signals to facilitate an explanation of the Hyland model.
The Hyland model narrative
We have established that diagnosis performs a key role in medicine. Jutel (2010) proposes that medical diagnosis explains, legitimises and normalises. Diagnosis is thus central to medicine, but it has been further suggested that a medical condition is only as real as its definition (Bell, 2014). A social model of health would emphasise that a diagnosis needs to be contextualised: as well as being shaped by the biomedical evidence, it needs to be shared in a way that provides meaning for both clinician and patient.
Any illness narrative should be accurate, but understandable to patients and carers, and crucially, have ‘goodness of fit’ with their lived experience. Only then will the sharing of a diagnosis allow personal control that facilitates, rather than inhibits, the patient’s active engagement with their therapeutic journey. In utilising the adaptive network theory, this requires describing symptom clusters and problematic control networks in a way that resonates for both patient and clinician.
In the Hyland model narrative, the human body is described through the analogy of a sophisticated computer (Hyland et al., 2016). This narrative was developed together with patients suffering from FMS, perhaps a key factor, since “the chief criterion of the truth of any psychotherapeutic formulation is its plausibility” (Frank, 1986). Thus, the narrative relating to why patients are ill should not only be plausible for the clinicians, but also crucially for patients in order to have a positive effect (Locher et al., 2019).
Both body and computer can develop hardware and software problems. The hardware relates to the structural components, including the specific physiological systems within the body. Components can be broken, such as fractured limbs, or require component replacement, such as ‘worn-out’ joints needing prosthetic surgery or considered as an under or overpowered system, such as in hypothyroidism/ hyperthyroidism.
The software relates to the operating system, incorporating information processing within the body, and directing hardware function. Using this metaphorical narrative, a guide authored by Hyland, suggests that the software consists of instructions that are sent throughout the body (Body Reprogramming: Patient Guide, 2017). An important distinction within this model, is that it does not consider software and hardware in terms of a dualistic Cartesian framework of ‘mind and body’. All too often clinicians will utilise the term ‘software problem’ as a euphemism for mental health or psychiatrically focused explanations. The Hyland model, however, considers the software as distributed throughout the body and integral to its function. This includes the information processing between the neurological (including brain), autonomic, endocrine, metabolic and immune systems. These all incorporate symptom clusters, represented by the aforementioned interconnecting adaptive network, as control system nodes.
FMS and other central sensitivity syndromes can be described as software issues in this narrative. The Hyland model specifically describes them as ‘stop programs’. The narrative can be helped by initially describing the symptoms experienced in response to a significant stressor; such as when a person suffers from an influenza infection. These symptoms include overwhelming fatigue with post-exertional malaise, widespread discomfort (including joint, bone and muscle ache) often with cutaneous hyperalgesia, a non-refreshing sleep pattern, perceptual sensitivity to light, noise and smell, temperature dysregulation, postural dizziness and nausea. Rather than conceptualising these symptoms as directly caused by the infection, these can be considered as powerful ‘stop’ signals generated by the body, to ensure that its resources are prioritised to deal with this potential threat to the existence of the individual. The symptoms manifested will also include mental fatigue to ensure that cognitive activity is also minimised. These stop signals thus inhibit the individual from undertaking more routine activities, whilst it deals with this priority threat.
The model suggests that the feedback loops which result from a failure to respond to the stop signals due to ongoing physical, cognitive or emotional stressors, will result in sustained control system changes, in the form of amplified stop signals, generating greater symptomology (see Figure 4). In essence, the ‘body shouts louder’. The idiosyncratic nature of these signals suggests an interplay of genetic, epigenetic and environmental factors is likely to be influencing the development and maintenance of the proposed stop program in FMS.
Therapeutic application: Body Reprogramming
Whilst provision of a diagnosis, coupled with a well-constructed and plausible narrative, can be beneficial in providing meaning, it should also provide guidance for therapeutic direction. Hyland (2017) suggests that the narrative should provide all the stakeholders with an information map about the route out of the condition, as well as helping understand the individual’s route in.
In adopting this model, the focus of a therapeutic strategy is to attempt to ameliorate the ’stop program’ and return the control systems closer to their original homeostatic state. The computer analogy can be useful again, with the focus on how to resolve a ‘software’ problem and how this differs from ‘hardware’ malfunction. The former requires focus on the information processing within the system, rather than replacing, modifying or repairing specific components. This conceptualisation offers the individual an explanation as to why current specific biomedical therapeutic options are less likely to offer a definitive cure for the widespread symptomology noted in FMS.
The 2017 European League Against Rheumatism (EULAR) revised guidelines for FMS (Macfarlane et al., 2017) recommends a number of individual non-pharmacological options, including exercise, meditative movement therapies or mindfulness-based stress reduction; although, only exercise was given a strong recommendation in this document. A limited number of pharmacological therapies were given ‘weak’ recommendations in the presence of disabling pain (duloxetine, pregabalin, tramadol) or sleep disturbance (amitriptyline, cyclobenzaprine, pregabalin). It suggests that psychological therapies, should be considered for those with mood disorders or unhelpful coping strategies. In the case of lack of effect, individualised treatment should be considered, according to patient need. Since the size of effect for most treatments in FMS is relatively modest, EULAR propose research priorities to clarify who might benefit from specific interventions, effect of options in combination and the organisation of healthcare systems to optimise outcome.
The Body Reprogramming therapeutic approach, based on the Hyland model, mirrors non-pharmacological evidence-based modalities recommended within EULAR guidelines (Body Reprogramming: Patient Guide, 2017). The underlying rationale is to encourage patients to adopt strategies that do not create stop signals and ameliorate stop programs. A key message is that doing ‘nothing’, by passively accepting stop programs, will not help reset these problematic control systems.
Principles incorporated within Body Reprogramming
There are three main principles underpinning Body Reprogramming, which are designed to help support the body systems and importantly reduce the sense of threat by providing it with new information that can be positively assimilated within its control networks or software. The three principles are:
(1) Avoid creating new stop signals.
(2) Ameliorate the stop programs
(3) Support the body’s hardware and software longer term.
Within the Body Reprogramming intervention, these principles are developed through a series of lifestyle management considerations.
Changing activity
Three activity patterns often scrutinised in chronic pain are relevant for consideration: avoidance, overdoing and pacing (Cane et al., 2013). Avoidance and overdoing are typically associated with negative outcomes, whereas, pacing is associated with positive outcomes. The avoidance pattern appears the strongest predictor of poor psychological and physical function, as well as greater pain interference, however, this may also be a correlate of severity of presentation. High level of action proneness and “overactive” lifestyle have been suggested as predisposing risk factors and perpetuating factors for functional syndromes and counters the deconditioning hypothesis (Van Houdenhove et al., 2001). Pacing is associated with less pain interference and better psychological function, but there is marked variability in approach and response (Antcliff et al., 2019; Murphy and Kratz, 2014; Racine et al., 2018, 2020).
There are two main theoretical models guiding pacing treatment: namely Operant Theory and Energy Conservation (Nielson et al., 2013; Racine et al., 2019). The former informs adaptive pacing behaviours that aim to limit the extent to which somatic symptoms control activity goals, whilst the latter alternatively encourages interventions which preserve activity, to help reduce pain and fatigue (Macfarlane et al., 2017). Considerations such as naturalistic and programmatic pacing, as well as symptom-contingent versus ‘time-based’ activity pacing, add conceptual complexity. The difficulty in assessing the impact of ‘pacing’ in functional disorders is highlighted in the PACE trial, where adaptive pacing therapy is compared with cognitive behavioural therapy and graded exercise therapy (White et al., 2011). Debate on the study findings rages on years later and continues to divide patient and professional opinion (Sharpe et al., 2019; Wilshire et al., 2018).
The Body Reprogramming approach focuses on considering ‘changing activity’, rather than using the term pacing as a core therapeutic strategy. Activity is monitored primarily on a temporal basis. Hyland uses the concept of ‘reactive inhibition’ to underpin the need to encourage change of activity on a regular basis (Hull, 1943; Török et al., 2017). The ‘Pomodoro technique’ framework (Cirillo, 2007) is utilised for developing this strategy, with the recommendation that no routine activity is undertaken continuously for longer than 25 minutes without a break. For people with more severe symptoms, this timeframe will be considerably less.
Cognitive based activity, as well as physical exercise should be incorporated. More recent research on human factors has countered the belief that vigilance tasks are undemanding assignments, requiring little mental effort. Indeed, converging evidence using behavioural, neural, and subjective measures, shows that utilising active cognitive control systems, particularly involving attentional processing, requires hard mental work and is stressful (Ariga and Lleras, 2011; Thomson et al., 2015; Warm et al., 2008).
Prolonged cognitive tasks, as well as physically based ones, are therefore just as likely to promote the creation of further ‘stop signals’. The concept of ‘changing activity’ is modelled within the Body Reprogramming group setting and patients are encouraged to experiment with ways in which they may adapt and ‘change’ how they are approaching activity.
Relaxation and stress reduction
Whilst stress has been described as; “a fact of life” (Niazi and Niazi, 2011), the relationship between stress and illness remains complex. Short term stressors can have a positive developmental impact on immune control systems, but chronic stressors are often detrimental, particularly when severe and protracted (Segerstrom and Miller, 2004). The impact of chronic stressors will emerge as a function of the timing and the duration of the exposure, as well as interaction between gene effects and previous exposure to environmental adversity (Lupien et al., 2009). One of the Body Reprogramming strategies is to desensitise the systems to threat and recalibrate the sense of risk, to the extent that there can be ‘safeness’ in the world and that the body can be taught this.
This is likely to become a particularly challenging strategy following the recent pandemic crisis with its health and economic ramifications. An increased incidence of long-term fatigue and pain was noted after the Severe Acute Respiratory (SARS) outbreak in 2003 (Lam et al., 2009; Moldofsky and Patcai, 2011). The emergence of what is being called ‘Long Covid’ (Greenhalgh et al., 2020; Nabavi, 2020) where symptoms persist for some months after the acute episode, would be predicted by the Hyland model. The model would suggest that we are likely to note a disproportionate increase in sustained symptom clusters due to a range of prolonged stressors resulting from the COVID-19 pandemic, such as social isolation and financial concerns. This exposure to additional and chronic stressful situations is likely to increase hypervigilance, exacerbating symptom clusters relating to anxiety, as well as threat-based cognitions. It may also undermine the body’s ability to down regulate and facilitate recovery.
Stress reduction and managing the threat response, both physically and psychologically, are core considerations in avoiding further stop signals. ‘Stop programs’ typically drive higher arousal and threat levels and so people with FMS will find it extremely difficult to perform relaxation tasks, as the body remains primed for its next challenge. It is key that people are aware of this difficulty in undertaking relaxation strategies or mindful activity, to avoid counterproductive self-criticism or even abandonment of this therapeutic arm of the programme. The Body Reprogramming approach should be individualised, introducing different stress reduction options which can then be self-selected.
In order to enable the body to reduce its level of perceived threat or alertness, the theoretical underpinnings dovetail well with the third wave cognitive-behavioural approaches including Mindfulness based Cognitive Behavioural Therapy, Acceptance and Commitment (ACT) based therapy, as well as Compassion Focused Therapy (CFT and Attachment-based compassion therapy [ABCT]) (Feliu-Soler et al., 2018; Hayes et al., 1999; Hyland, 2011; Montero-Marin et al., 2018; Ruiz, 2010). Within a compassion framework, patients gain insight about the ‘threat’, ‘drive’ and ‘soothe’ systems. This more systemic evolution of psychologically based therapy, incorporating emotional elements, is more promising, as relaxation therapy as a unimodal treatment option has not proven to be effective in FMS (Thieme and Gracely, 2009).
The primary method for helping patients to do things that ‘fly under the radar’ of their body’s stop signals, is to help them learn how to pay attention to their bodies in more intuitive and compassionate way. A key component of the Body Reprogramming approach is the targeting of premorbid hyperactivity and self-orientated perfectionism, particularly relating to ongoing heavy demands and high self-imposed expectations which often result in neglect of bodily needs (Grisart et al., 2018).
Level 1 evidence exists for the effectiveness of mindfulness-based stress reduction in FMS, although the EULAR conclusion is for weak positive recommendation. Improvements in pain were noted immediately post treatment, compared with usual care and active control interventions, however, EULAR suggests that the effects were not robust against research bias (Lauche et al., 2013; Macfarlane et al., 2017). More recently, evidence of cost-effectiveness of ACT and Mindfulness-Based Stress Reduction in FMS has emerged, when compared against pharmacological options, multicomponent intervention and usual care (Luciano et al., 2017; Pérez-Aranda et al., 2019).
Mindful activity
Controlled trials have supported the benefit of exercise in both CFS/ME (White et al., 2011) and FMS (Macfarlane et al., 2017) with EULAR suggesting exercise as the gold standard intervention in the latter. In FMS, the EULAR review indicates consistency of benefit with regard to aerobic and strengthening exercises, although there is insufficient evidence to suggest superiority of one over the other, with both land and aquatic exercise appearing equally effective. A recent Cochrane review indicated an improvement in aerobic exercise of between 6% and 11% in pain, fatigue, stiffness, physical function and health related quality of life, over a 6-month period (Bidonde et al., 2017). Perceived benefit from exercise however is not universally reported by patients or support groups (Jones and Liptan, 2009; Sharpe et al., 2019; Wilshire et al., 2018).
The Hyland model may help explain the differing perceptions between health care professionals and recipients. A suitable analogy would be that you should avoid accelerating in a vehicle whilst the brakes are still applied. Thus, during ‘stop programs’, the person is encouraged to undertake activity which stimulates reduction rather than exacerbation of the stop signals.
The Hyland model supports a more mindful approach to activity, rather than poorly controlled or aggressive exercise regimens, particularly during symptom flares. It encourages optimal movement that reconditions the body to the idea that movement does not have to be experienced as threatening or dangerous. A gradual intensity progression in activity can be nurtured as symptoms ameliorate. As previously mentioned, ‘doing nothing’ will not reduce or eliminate stop signals, but counterproductively stimulate them further as the body deconditions.
Since the publication of the latest EULAR guidelines there has been a further well-designed randomised controlled trial comparing aerobic exercise with mindful activity, in the form of Tai Chi (Wang et al., 2018a). Tai Chi compared favourably with aerobic exercise, with a clinically significant difference observed between the higher intensity Tai Chi programme (twice weekly for 24 weeks) versus aerobic exercise. The drop-out rate for aerobic exercise also appeared higher than the Tai Chi groups, perhaps indicating greater difficulty adhering to an aerobic exercise programme. The authors suggest that it may be time to rethink the type of exercise that may be most effective and in particular what types or combinations of activity that patients may embrace longer-term (Wang, 2018b).
A further development which would sit well within the framework of the Hyland model is controlled graded activity, within a virtual reality (VR) programme. This may prove particularly appealing in a more socially distancing culture. It has emerged as an effective form of therapy within a variety of patient populations (Jansen-Kosterink et al., 2013; Park et al., 2015). A non-immersive version of VR, involving physical activity called ‘exergames,’ has shown positive physical improvements as well as increased cerebral blood flow, particularly in people with FMS diagnosed more recently (Collado-Mateo et al., 2017; Villafaina et al., 2019). If used within the Body Reprogramming approach, the duration of all sessions should be optimised to reinforce the aforementioned principle of changing activity regularly.
Positive psychology and well-being
A pioneering proponent of positive psychology, Seligman et al. (2005), describes it as; “the scientific study of optimal human functioning, that aims to discover and promote the factors that allow individuals and communities to thrive”. This was a radical change in promoting attention onto the positive aspects of human life, rather than the negative and pathology orientated understandings (Kim et al., 2012; Wood and Tarrier, 2010). If we return to our computer analogy, it is worth considering how software problems are usually overcome. New information by means of an updated software program is typically downloaded and in a similar fashion, the Hyland model suggests a forward-looking therapeutic approach, focusing on personal well-being and growth.
Providing patients with an appropriate narrative, which also validates their experiences, is a key component of the Body Reprogramming approach. As well as reducing fear and frustration, this narrative also can improve the subsequent clinician-patient relationship and foster positive active engagement and expectations (Ashe et al., 2017). Well-being is subsequently explored once this explanatory narrative is agreed, through a focus on choices, attitudes and behaviours. Positive emotions are reinforced as well as nurturing personal values. The Hyland model encourages; “doing things that you enjoy” whilst reducing self-critical motivations, as an aspect of reducing stop signals. Again 3rd wave CBT approaches (ACT and CFT) as well as Gratitude therapy, provide suitable vehicles for structuring this component of the group-based Body Reprogramming course (Peters et al., 2017; Toussaint et al., 2017; Wood et al., 2010).
Role of medication
EULAR guidelines (Macfarlane et al., 2017) mirror previous guidance, that non-pharmacological therapies should be the mainstay of management in FMS and that these are based on availability, cost, safety and patient preference (Fitzcharles et al., 2013). There also appears to be acknowledgement that pharmacological options typically provide modest symptom relief and are of questionable clinical benefit (Nüesch et al., 2013). Recent guidelines are clear that strong opioids have no role to play in the long-term management of FMS (Goldenberg et al., 2016; Littlejohn et al., 2016; Painter and Crofford, 2013). Pharmacological options with weak EULAR recommendations include only; low dose amitriptyline, pregabalin, cyclobenzaprine, duloxetine, milnacipran and tramadol whilst all other medications have no positive recommendation for use outside research considerations. It should be noted that these pharmacological studies assess relatively short-term usage of the medication, (6–12 weeks) with few studies confirming sustained benefit with long-term usage.
The Body Reprogramming approach considers the use of medication, particularly polypharmacy for pain, through a session facilitated by a health care prescriber. Adaptation typically occurs to analgesics with a central mode of action and patient feedback mirrors this perceived reduction in efficacy over time. The Hyland model suggests that the lack of appropriate response to ‘stop signals’ results in the body ‘shouting louder’. It makes little sense to use medication to subdue ‘stop signals’, whilst the patient also continues to maintain lifestyle management strategies which have been contributing to the severity of the presentation. Prescribing analgesics may inadvertently result in potentiation of stop signals, by rendering the intolerable, falsely tolerable. The long-term role of medication is limited and may explain the weak research findings for pharmaceutical agents overall. The model would suggest that any minor role for medication, specifically for FMS symptomology should be restricted to a time limited, supportive measure, to facilitate a change in lifestyle and adoption of other non-pharmacological options. To continue to merely add analgesic medication as a sole therapeutic strategy may well cause iatrogenic harm.
Nutrition aspects
The gut is often described as the second brain and is closely linked, via the limbic system, to the brain itself (Young, 2012). The gut microbiota has a role in this two-way interaction between the gut and the brain (Ridaura and Belkaid, 2015). The gut-brain interaction provides an important framework for understanding health complaints (Gershon, 1999).
Practical implications of the brain-gut relationship are exploited in the Hyland model in several ways. Eating when stressed has a number of pathogenic consequences, not limited to obesity, so patients are advised to avoid eating whilst stressed and provided with lifestyle advice as to how this can be avoided (Canetti et al., 2002). Time of eating is important, as the gut is governed by a circadian rhythm, as well as linked memories for time of day when eating takes place (Voigt et al., 2016). Patients are provided with information about regularity of eating and the way in which the body prepares or does not prepare for food. The effect of happiness whilst eating is discussed, in relation to creating a link between eating and positive experience (Zhou and Foster, 2015).
Nutritional advice is consistent with contemporary guidance, but with an emphasis on creating a healthy gut microbiome (Singh et al., 2017). The effect of insulin and insulin resistance on inflammation is considered in relation to dietary habits (Shimobayashi et al., 2018). We would like to emphasise that an important part of this section of the intervention is to steer patients away from untested and difficult to follow diets.
Implementation of the Body Reprogramming intervention
Behavioural change interventions typically incorporate three broad groups of interconnected components. This includes the theory, through which the intervention can influence change, the content and the form of delivery. Once theory and content are established, form of delivery should be considered a crucial ‘active ingredient’ in determining intervention effectiveness (Dombrowski et al., 2016). It should perhaps be noted that it is generally difficult to test an underlying treatment theory due to its unfalsifiable aspects (Gaab et al., 2016; Roth et al., 2005).
Delivery elements will include provider characteristics and training, mode of delivery, intervention intensity, venue, materials, tailoring and style. Hoffmann provides a suitable itemised checklist for the form of delivery of behaviour change interventions (Hoffmann et al., 2014). These components will impact on patient engagement, adherence and longer-term adoption of intervention content.
The Body Reprogramming approach can be offered to patients in a variety of ways, both online and face-to-face, as well as being considered either individually or as a group-based intervention. The Body Reprogramming patient guide was constructed with active patient collaboration, helping to ensure clarity and relevance. This literature allows reinforcement of the Hyland model narrative, in both primary and secondary care settings.
Finite health care resources require that implementation of any intervention is delivered in a manner seen as acceptable to service commissioners, providers and patients. Form of delivery is also important in the context of health inequalities and needs to ensure that it does not disproportionately exclude those from more disadvantaged backgrounds (Hoffmann et al., 2014). Restricting access exclusively to digital media platforms may have an adverse impact on the elderly and those from deprived backgrounds, although there is evidence of therapeutic benefit (Macfarlane et al., 2016; NHS Digital Report, 2019; Peters et al., 2017; Swancutt et al., 2019).
Group based programmes have been adopted to support delivery of many health-care interventions in chronic conditions, including pain and obesity management. From an economic perspective they allow for efficient use of staff time and so may impact favourably on waiting times for treatment (Swancutt et al., 2019). They also may confer health benefits beyond efficiency considerations, by altering members’ perceptions, beliefs, expectations, and behaviour patterns (Borek and Abraham, 2018). The shared social identity may help structure patients’ engagement with the intervention content and initiate changes in behaviour. However, there is an incomplete understanding of the components and course characteristics that optimise behaviour change from these complex interventions.
Group based interventions to support the self-management of chronic musculoskeletal pain, suggest more favourable outcomes when there is health professional input (Carnes et al., 2013). Those incorporating a psychological component show more consistent beneficial effects over the follow-up period (Taylor et al., 2016). Longer-term outcomes, however, are infrequently reported for group programmes and those that do report, are again mainly restricted to chronic musculoskeletal pain, rather than chronic widespread pain (Volker et al., 2017). Longer and more individually tailored interventions (>8 weeks) are likely to produce better longer-term outcomes, but their cost-effectiveness needs to be considered (Monticone et al., 2017; Rasmussen et al., 2017; Wertli et al., 2017).
Influencing change management
Implementation of the Hyland model within a therapeutic programme for FMS should incorporate more than the provision of a specific cognitive or behavioural skill set. It provides healthcare professionals with a rounded integrative approach to facilitate clinical improvement.
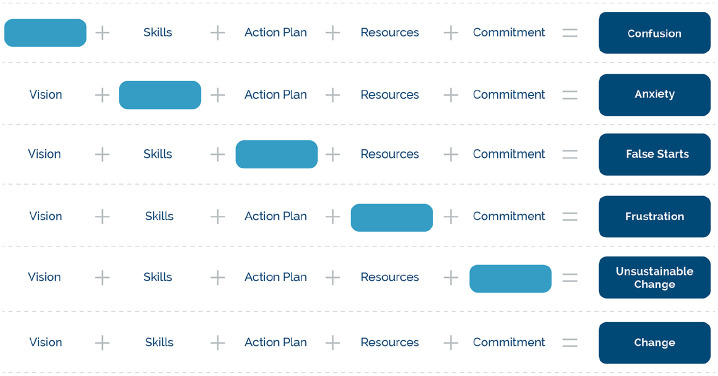
To operationalise this paradigm shift successfully in a healthcare system, it is helpful to utilise a theory for complex change management (Mitchell, 2013). There are many theoretical frameworks that have been applied to healthcare systems. One of the original theorists Kurt Lewin, influenced others such as Lippitt who developed a model for influencing complex change processes, incorporating five key elements: Vision, Provision of Skill Set, Incentives (Commitment), Action Plan and Resources (Lippitt, 1987; Shirey, 2013). This model provides a framework that can be applied both at the level of individual and a systems’ change perspective (see Figure 5).
Figure 5.
Adaptation of Lippitt model for influencing complex change processes.
The first component in this model necessary for successful change is the ‘vision’, which can be understood in the current context as an acceptable narrative, for both patient and clinician. Without this narrative there is no guiding force to trigger change and confusion may ensue when new skills are suggested by healthcare providers. The second step requires the development of a skill set to nurture the change process. Here, the skill sets include mindful activity and other well-being strategies. Without the development of appropriate skill sets, anxiety is likely to be heightened, potentially interrupting the change process.
The incentives component, when applied to self-management support programs, should perhaps be reconceptualised as the incentives to maintain the new self-management behaviours, once the programme has been completed (Finlay and Elander, 2016). Without this component, change can stagnate and will be unsustainable. Indeed, one of the commonest challenges to consider is the sense of abandonment experienced by some recipients at the end of a therapeutic process. A challenge facing all self-management support programs is how to ensure long term support, without encouraging passivity and dependency. Resources can include time, perhaps social prescribing options and most importantly peer support for maintaining the new behaviours, rather than resorting to previous patterns, which may retrigger the ‘stop’ programs. This component is integral to encouraging change, without which frustration will ensue. Finally, an action plan helps define the individual’s strategic direction, without which false starts can prevail. An action plan can also direct both patient and primary care provider, following completion of a specialist programme.
Instigating and maintaining complex change, such as that suggested by the Body Reprogramming approach, requires the facilitator to address all five of these integral components. This will be necessary whether therapeutic input is undertaken on an individual or group basis. The latter, in particular, will require a high level of professional understanding, particularly in tackling the dynamics of group programmes.
This framework is also helpful when considering how a healthcare approach might be organised. For example, one potential strategy is to introduce a tiered approach to group programmes, with the provision of a community programme for those less severely impacted by the condition and a more comprehensive specialist setting for people with more complex needs. Developing such a strategy involving multiple stakeholders requires attention to the same five components advocated by Lippitt.
Discussion
Translating a theoretical model into a successful therapeutic approach should provide meaning to both provider and recipient, support evidence-based therapeutic avenues and ultimately improve prognosis. Currently, long-term prognosis is poor in FMS. Walitt undertook a large scale longitudinal study, demonstrating that 5-year outcomes for patients receiving expert care produced no average clinically meaningful improvement in symptom severity overall (Walitt et al., 2011). Whilst there has been variable outcome data in smaller studies, this study typically mirrors feedback from patients, patient support groups and clinicians working in this field (Henriksson, 1994; Kennedy and Felson, 1996; Schaefer et al., 2016).
The Body Reprogramming approach has been translated from the Hyland theoretical model and appears to provide a robust framework for therapeutic management. The components dovetail with EULAR recommendations and indeed the underlying model was also recently predictive of the relative benefits of mindful activity versus aerobic exercise (Pérez-Aranda et al., 2019). Early evaluation appears to demonstrate proof of concept for the Body Reprogramming approach and service improvement data relating to this is also highly encouraging (Dee et al., 2019). Further evaluation will help clarify its position as a suitable alternative epistemological framework.
Conclusion
Patient engagement, including active participation in therapeutic interventions, typically requires the provision of a healthcare approach that reflects the illness experience. This can be challenging in conditions such as FMS, where current explanations often fail to provide this necessary legitimisation. The Hyland model offers a theory-driven and plausible narrative, which appears to align itself as an integrated paradigm, allowing incorporation of biological, psychological, emotional and social considerations. Crucially, this narrative and its application does appear to resonate with patients.
Footnotes
Authors’ note: Permission to reuse and copyright: Permission gained for use of Figure 2 (Melidis) from Elsevier on May 4th 2020. Licence number 4847020299093.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. The article processing charge was provided by the first author’s health-care institution.
ORCID iDs: Anthony Fitzdonald Davies  https://orcid.org/0000-0002-0818-441X
https://orcid.org/0000-0002-0818-441X
Patrick Hill  https://orcid.org/0000-0002-3296-8281
https://orcid.org/0000-0002-3296-8281
References
- Abeles AM, Pillinger MH, Solitar BM, et al. (2007) Narrative review: The pathophysiology of fibromyalgia. Annals of Internal Medicine 146: 726–734. [DOI] [PubMed] [Google Scholar]
- Adamson C. (1997) Existential and clinical uncertainty in the medical encounter: An idiographic account of an illness trajectory defined by inflammatory bowel disease and avascular necrosis. Sociology of Health and Illness 19(2): 133–159. [Google Scholar]
- Antcliff D, Keenan AM, Keeley P, et al. (2019) Survey of activity pacing across healthcare professionals informs a new activity pacing framework for chronic pain/fatigue. Musculoskeletal Care 17(4): 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga A, Lleras A. (2011) Brief and rare mental “breaks” keep you focused: Deactivation and reactivation of task goals preempt vigilance decrements. Cognition 118(3): 439–443. [DOI] [PubMed] [Google Scholar]
- Arnold LM, Crofford LJ, Mease PJ, et al. (2008) Patient perspectives on the impact of fibromyalgia. Patient Education and Counselling 73(1): 114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asbring P, Narvanen AL. (2002) Women’s experiences of stigma in relation to chronic fatigue syndrome and fibromyalgia. Qualitative Health Research 12: 148–160. [DOI] [PubMed] [Google Scholar]
- Ashe SC, Furness PJ, Taylor SJ, et al. (2017) A qualitative exploration of the experiences of living with and being treated for fibromyalgia. Health Psychology Open 4(2): 2055102917724336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffy G, Loscalzo J. (2014) Complexity and network dynamics in physiological adaptation: An integrated view. Physiology and Behavior 131: 49–56. [DOI] [PubMed] [Google Scholar]
- Barsky AJ, Borus JF. (1999) Functional somatic syndromes. Annals of Internal Medicine 130: 910–921. [DOI] [PubMed] [Google Scholar]
- Bekhuis E, Gol J, Burton C. (2020) Patients’ descriptions of the relation between physical symptoms and negative emotions: A qualitative analysis of primary care consultations. British Journal of General Practice 70(691): e78–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AV. (2014) Diagnostic diversity: The role of social class in diagnostic experiences of infertility. Sociology of Health and Illness 36(4): 516–530. [DOI] [PubMed] [Google Scholar]
- Bidari A, Parsa BG, Ghalehbaghi B. (2018) Challenges in fibromyalgia diagnosis: From meaning of symptoms to fibromyalgia labelling. The Korean Journal of Pain 31(3): 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidonde J, Busch AJ, Schachter CL, et al. (2017) Aerobic exercise training for adults with fibromyalgia. Cochrane Database Systematic Review 6(6): CD012700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body Reprogramming: Patient Guide (2017) Available at: www.bodyreprogramming.org (accessed 18 April 2020).
- Borek AJ, Abraham C. (2018) How do small groups promote behaviour change? An integrative conceptual review of explanatory mechanisms. Health and Well-being 10(1): 30–61. [DOI] [PubMed] [Google Scholar]
- Briones-Vosmediano E, Vives-Cases C, Ronda-Perez E, et al. (2013) Patients’ and professionals’ views on managing fibromyalgia. Pain Research Management 18(1): 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cane D, Nielson WR, McCarthy M, et al. (2013) Pain-related activity patterns: Measurement, interrelationships and associations with psychosocial function. Clinical Journal of Pain 29(5): 435–442. [DOI] [PubMed] [Google Scholar]
- Canetti L, Bachar E, Berry EM. (2002) Food and emotion. Behavioural Processes 60(2): 157–164. [DOI] [PubMed] [Google Scholar]
- Carnes D, Homer K, Underwood M, et al. (2013) Pain management for chronic musculoskeletal conditions: The development of an evidence-based and theory-informed pain self-management course. BMJ Open 3: e003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceko M, Bushnell C, Gracely RH. (2012) Neurobiology underlying fibromyalgia symptoms. Pain Research and Treatment 2012: 585419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E, Perrot S, Leon T, et al. (2010) A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Services Research 10: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo F. (2007) The Pomodoro Technique (The Pomodoro) Publication v1.3 June 2007. Available at: https://lasolutionestenvous.com/wp-content/uploads/2014/04/ThePomodoroTechnique_v1-3.pdf (accessed 15 February 2020).
- Collado-Mateo D, Dominguez-Munoz FJ, Adsuar JC, et al. (2017) Effects of exergames on quality of life, pain, and disease effect in women with fibromyalgia: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 98: 1725–1731. [DOI] [PubMed] [Google Scholar]
- De Souza JB, Goffaux P, Julien N, et al. (2009) Fibromyalgia subgroups: Profiling distinct subgroups using the fibromyalgia impact questionnaire. A preliminary study. Rheumatology International 29: 509–515. [DOI] [PubMed] [Google Scholar]
- Dee A, Locher C, Bothma C, et al. (2019) From old chestnut to new acorn. Body reprogramming as a reconceptualised approach to fibromyalgia. In: 17th biennial national conference of the pain management programmes, British Pain Society, Bristol, UK, 11–12 September. [Google Scholar]
- Dombrowski SU, O’Carroll RE, Williams B. (2016) Form of delivery as a key ‘active ingredient’ in behaviour change interventions. British Journal of Health Psychology 21(4): 733–740. [DOI] [PubMed] [Google Scholar]
- Feliu-Soler A, Montesinos S, Gutierrez-Martinez O, et al. (2018) Current status of acceptance and commitment therapy for chronic pain: A narrative review. Journal of Pain Research 11: 2145–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietta P, Fietta P, Manganelli P. (2007) Fibromyalgia and psychiatric disorders. Acta Bio-Medica: Atenei Parmensis 78(2): 88–95. [PubMed] [Google Scholar]
- Finlay K, Elander J. (2016) Reflecting the transition from pain management services to chronic pain support group attendance: An interpretative phenomenological analysis. British Journal of Health Psychology 21(3): 660–676. [DOI] [PubMed] [Google Scholar]
- Fitzcharles MA, Yunus MB. (2012) The clinical concept of fibromyalgia as a changing paradigm over the past 20 years. Pain Research and Treatment 2012: 184835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzcharles MA, Ste-Marie PA, Goldenberg DL, et al. (2013) 2012 Canadian guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Research Management 18(3): 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JD. (1986) Psychotherapy - the transformation of meanings: Discussion paper. Journal of Royal Society of Medicine 79: 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness PJ, Vogt K, Ashe S, et al. (2018) What causes fibromyalgia. An online survey of patient perspectives. Health Psychology Open 5(2): 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaab J, Blease C, Locher C, et al. (2016) Go open: A plea for transparency in psychotherapy. Psychology of Consciousness: Theory, Research, and Practice 3(2): 175. [Google Scholar]
- Galvez-Sanchez CM, Duschek S, Reyes del Paso GA. (2019) Psychological impact of fibromyalgia: Current perspectives. Psychology Research and Behaviour Management 12: 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. (1999) The Second Brain: A Ground Breaking New Understanding of Nervous Disorders of the Stomach and Intestine. New York, NY: HarperCollins. [Google Scholar]
- Giesecke T, Williams DA, Harris RE, et al. (2003) Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis and Rheumatology 48: 2916–2922. [DOI] [PubMed] [Google Scholar]
- Gilbert P. (2014) The origins and nature of compassion focused therapy. British Journal of Clinical Psychology 53: 6–41. [DOI] [PubMed] [Google Scholar]
- Goldenberg DL, Clauw DJ, Palmer RE, et al. (2016) Opioid use in fibromyalgia: A cautionary tale. Mayo Clinic Proceedings 91(5): 640–648. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T, Knight M, A’Court C, et al. (2020) Management of post-acute covid-19 in primary care. British Medical Journal 370: m3026. [DOI] [PubMed] [Google Scholar]
- Grisart J, Scaillet N, Michaux M, et al. (2018) Determinants of representational and behavioral hyperactivity in patients with fibromyalgia syndrome. Journal of Health Psychology: An Interdisciplinary, International Journal 25(8): 1128–1137. [DOI] [PubMed] [Google Scholar]
- Gupta A, Silman AJ. (2004) Psychological stress and fibromyalgia: A review of the evidence suggesting a neuroendocrine link. Arthritis Research and Therapy 6(3): 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser W, Fitzcharles MA. (2018) Facts and myths pertaining to fibromyalgia. Dialogues in Clinical Neuroscience 20(1): 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. (1999) Acceptance and Commitment Therapy: An Experiential Approach to Behaviour Change. New York, NY: Guilford Press. [Google Scholar]
- Hayes SM, Myhal GC, Thornton JF, et al. (2010) Fibromyalgia and the therapeutic relationship: Where uncertainty meets attitude. Pain Research Management 15(6): 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningsen P, Gundel H, Kop WJ, et al. (2018) Persistent physical symptoms as perceptual dysregulation: A neuropsychobehavioral model and its clinical implications. Psychosomatic Medicine 80(5): 422–431. [DOI] [PubMed] [Google Scholar]
- Henningsen P, Layer P, Fink P, et al. (2019) Chronic primary pain: A pain centred view of the world is too narrow. Pain 160(7): 1683. [DOI] [PubMed] [Google Scholar]
- Henriksson CM. (1994) Longterm effects of fibromyalgia on everyday life. A study of 56 patients. Scandinavian Journal of Rheumatology 23(1): 36–41. [DOI] [PubMed] [Google Scholar]
- Hill P. (2019) Chronic pain: A consequence of dysregulated protective action. British Journal of Pain 13(1): 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TC, Glasziou PP, Boutron I, et al. (2014) Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal 348: g1687. [DOI] [PubMed] [Google Scholar]
- Hughes G, Martinez C, Myon E, et al. (2006) The impact of a diagnosis of Fibromyalgia on health care resource use by primary care patients in the UK: An observational study based on clinical practice. Arthritis and Rheumatology 54(1): 177–183. [DOI] [PubMed] [Google Scholar]
- Hull CL. (1943) Principles of Behavior: An Introduction to Behavior Theory. New York, NY: Appleton-Century-Crofts, pp.1–20. [Google Scholar]
- Hyland ME, Hinton C, Hill C, et al. (2016) Explaining unexplained pain to fibromyalgia patients. Finding a narrative that is acceptable to patients and provides a rationale for evidence-based interventions. British Journal of Pain 10(3): 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland ME. (2011) The Origins of Health and Disease. Cambridge: Cambridge University Press. [Google Scholar]
- Hyland ME. (2017) A new paradigm to explain functional disorders and the adaptive network theory of chronic fatigue syndrome and fibromyalgia syndrome. In: Sullivan GB, Cresswell J, Ellis B, et al. (eds) Resistance and Renewal in Theoretical Psychology. Concord, ON: Captus University Publications. [Google Scholar]
- Jansen-Kosterink SM, Huis In’t Veld RM, Schonauer C, et al. (2013) A serious exergame for patients suffering from chronic musculoskeletal back and neck pain: A pilot study. Games for Health Journal 2: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KD, Liptan GL. (2009) Exercise interventions in fibromyalgia: Clinical applications from the evidence. Rheumatic Diseases Clinics of North America 35(2): 373–391. [DOI] [PubMed] [Google Scholar]
- Jutel A. (2010) Medically unexplained symptoms and the disease label. Social Theory and Health 8(3): 229–245. [Google Scholar]
- Kennedy M, Felson DT. (1996) A prospective long-term study of fibromyalgia syndrome. Arthritis and Rheumatology 39(4): 682–685. [DOI] [PubMed] [Google Scholar]
- Khalil RB, Khoury E, Richa S. (2016) Do fibromyalgia flares have a neurobiological substrate. Pain Medicine 17(3): 469–475. [DOI] [PubMed] [Google Scholar]
- Kim JH, Keck P, Miller D, et al. (2012) Introduction to positive psychology: Overview and controversies. Journal of Asia Pacific Counseling 2(1): 45–60. [Google Scholar]
- Kolacz J, Porges SW. (2018) Chronic diffuse pain and functional gastrointestinal disorders after traumatic stress: Pathophysiology through a polyvagal perspective. Frontiers in Medicine (Lausanne) 5: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129. [DOI] [PubMed] [Google Scholar]
- Kumbhare D, Ahmed S, Watter S. (2018) A narrative review on the difficulties associated with Fibromyalgia diagnosis. Therapeutic Advances in Musculoskeletal Diseases 10(1): 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MH, Wing YK, Yu MW, et al. (2009) Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: Long-term follow-up. Archives of Internal Medicine 169(22): 2142–2147. [DOI] [PubMed] [Google Scholar]
- Lauche R, Cramer H, Dobos G, et al. (2013) A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. Journal of Psychosomatic Research 75: 500–510. [DOI] [PubMed] [Google Scholar]
- Lippitt M. (1987) Managing complex change model sourced in chapter: Knoster T, Villa R, Thousand J. A framework for thinking about systems change. In: Villa R, Thousand J. (eds) Restructuring for Caring and Effective Education: Piecing the Puzzle Together, 2nd edn. Baltimore, MD; London: Paul H. Brookes, pp.93–128. 1999. [Google Scholar]
- Littlejohn GO, Guymer EK, Ngian GS. (2016) Is there a role for opioids in the treatment of fibromyalgia? Pain Management 6(4): 347–355. [DOI] [PubMed] [Google Scholar]
- Locher C, Meier S, Gaab J. (2019) Psychotherapy: A world of meanings. Frontiers in Psychology 10: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loevinger BL, Shirtcliff EA, Muller D, et al. (2012) Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clinical Rheumatology 31: 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano JV, D’Amico F, Feliu-Soler A, et al. (2017) Cost-utility of group acceptance and commitment therapy for fibromyalgia versus recommended drugs: An economic analysis alongside a 6-month randomised controlled trial conducted in Spain (EFFIGACT Study). Journal of Pain 18(7): 868–880. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, et al. (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Natural Review Neuroscience 10: 434–445. [DOI] [PubMed] [Google Scholar]
- Lyon P, Cohen M, Quintner J. (2011) An evolutionary stress response hypothesis for chronic widespread pain (Fibromyalgia). Pain Medicine 12(8): 1167–1178. [DOI] [PubMed] [Google Scholar]
- Macfarlane GJ, Beasley M, Prescott G. (2016) The Maintaining Musculoskeletal Health (MAmMOTH) Study: Protocol for a randomised trial of cognitive behavioural therapy versus usual care for the prevention of chronic widespread pain. BMC Musculoskeletal Disorders 17: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane GJ, Kronisch C, Dean LE, et al. (2017) EULAR revised recommendations for the management of fibromyalgia. Annals of Rheumatological Diseases 76: 318–328. [DOI] [PubMed] [Google Scholar]
- Madden S, Sim J. (2006) Creating meaning in fibromyalgia syndrome. Social Science and Medicine 63: 2962–2973. [DOI] [PubMed] [Google Scholar]
- Malin K, Littlejohn GO. (2012) Personality and fibromyalgia syndrome. The Open Rheumatology Journal 6: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lavin M, Vargas A. (2009) Complex adaptive systems allostasis in fibromyalgia. Rheumatological Diseases Clinics North America 35(2): 285–198. [DOI] [PubMed] [Google Scholar]
- Martinez-Lavin M, Infante O, Lerma C. (2008) Hypothesis: The chaos and complexity theory may help our understanding of fibromyalgia and similar maladies. Seminars in Arthritis and Rheumatism 37(4): 260–264. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2008) Central effects of stress hormones in health and disease. European Journal of Pharmacology 583(2–3): 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melidis C, Denham SL, Hyland ME. (2018) A test of the adaptive network explanation of functional disorders using a machine learning analysis of symptoms. Biosystems 165: 22–30. [DOI] [PubMed] [Google Scholar]
- Mitchell G. (2013) Selecting the best theory to implement planned change. Nursing Management (Harrow) 20(1): 32–37. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Patcai J. (2011) Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurology 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Marin J, Navarro-Gil M, Puebla-Guedea M, et al. (2018) Efficacy of “attachment-based compassion therapy” in the treatment of fibromyalgia: A randomized controlled trial. Frontiers in Psychiatry 8: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticone M, Ambrosini E, Rocca B, et al. (2017) Group-based multimodal exercises integrated with cognitive-behavioural therapy improve disability, pain and quality of life of subjects with chronic neck pain: A randomized controlled trial with one-year follow-up. Clinical Rehabilitation 31(6): 742–752. [DOI] [PubMed] [Google Scholar]
- Murphy SL, Kratz AL. (2014) Activity pacing in daily life: A within day analysis. Pain 155(12): 2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N. (2020) Long Covid: How to define it and how to manage it. BMJ 370: m3489. [DOI] [PubMed] [Google Scholar]
- NHS Digital Report (2019) Digital Inclusion for Health Care. July 2019. UK. Available at: https://digital.nhs.uk/about-nhs-digital/our-work/digital-inclusion (accessed 28 April 2020).
- Niazi AK, Niazi SK. (2011) Mindfulness-based stress reduction: A non-pharmacological approach for chronic illnesses. North American Journal of Medical Sciences 3: 20–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas M, Vlaeyen JWS, Rief W, et al. (2019) The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 160(1): 28–37. [DOI] [PubMed] [Google Scholar]
- Nielsen LA, Henrikkson KG. (2007) Pathophysiological mechanisms in chronic musculoskeletal (Fibromyalgia): The role of central and peripheral sensitization and pain disinhibition. Best Practice and Research Clinical Rheumatology 21(3): 465–480. [DOI] [PubMed] [Google Scholar]
- Nielson WR, Jensen MP, Karsdorp PA, et al. (2013) Activity pacing in chronic pain: Concepts, evidence, and future directions. Clinical Journal of Pain 29: 461–468. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (2014) Abnormal Psychology, 6th edn. New York, NY: McGraw-Hill, pp.150–157. [Google Scholar]
- Nüesch E, Häuser W, Bernardy K, et al. (2013) Comparative efficacy of pharmacological and non-pharmacological interventions in fibromyalgia syndrome: Network meta-analysis. Annals of Rheum Diseases 72(6): 955–962. [DOI] [PubMed] [Google Scholar]
- Painter JT, Crofford LJ. (2013) Chronic opioid use in fibromyalgia syndrome: A clinical review. Journal of Clinical Rheumatology 19(2): 72–77. [DOI] [PubMed] [Google Scholar]
- Park EC, Kim SG, Lee CW. (2015) The effects of virtual reality game exercise on balance and gait of the elderly. Journal of Physical Therapy Science 27: 1157–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Aranda A, D’Amico F, Feliu-Soler A, et al. (2019) Cost-utility of mindfulness-based stress reduction for fibromyalgia versus a multicomponent intervention and usual care: A 12-month randomized controlled trial (EUDAIMON Study). Journal of Clinical Medicine 8(7): E1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot S, Choy E, Petersel D, et al. (2012) Survey of the physician experiences and perceptions about the diagnosis and treatment of Fibromyalgia. BMC Health Service Research 2012; 12: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot S, Dickenson AH, Bennett RM. (2008) Fibromyalgia: Harmonizing science with clinical practice considerations. Pain Practice 8: 177–189. [DOI] [PubMed] [Google Scholar]
- Peters ML, Smeets E, Feijge M, et al. (2017) Happy despite pain. A randomized controlled trial of an 8-week internet-delivered positive psychology intervention for enhancing well-being in patients with chronic pain. Clinical Journal of Pain 33(11): 962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine M, Galan S, de la Vega R, et al. (2018) Pain-related activity management patterns and function in patients with fibromyalgia syndrome. Clinical Journal of Pain 34(2): 122–129. [DOI] [PubMed] [Google Scholar]
- Racine M, Jensen MP, Harth M, et al. (2019) Operant learning versus energy conservation activity pacing treatments in a sample of patients with fibromyalgia syndrome: A pilot randomized controlled trial. Journal of Pain 20(4): 420–439. [DOI] [PubMed] [Google Scholar]
- Racine M, Sánchez-Rodríguez E, de la Vega R, et al. (2020) Pain-related activity management patterns as predictors of treatment outcomes in patients with fibromyalgia syndrome. Pain Medicine 21(2): e191–e200. [DOI] [PubMed] [Google Scholar]
- Ramsay DS, Woods SC. (2014) Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychological Review 121(2): 225–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen MU, Amris K, Rydahl-Hansen S. (2017) How can group-based multidisciplinary rehabilitation for patients with fibromyalgia influence patients’ self-efficacy and ability to cope with their illness: A grounded theory approach. Journal of Clinical Nursing 26(7–8): 931–945. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Garrido S, Pulgar Á, et al. (2011) Autonomic cardiovascular control and responses to experimental pain stimulation in fibromyalgia syndrome. Journal of Psychosomatic Research 70(2): 125–134. [DOI] [PubMed] [Google Scholar]
- Ridaura V, Belkaid Y. (2015) Gut microbiota: The link to your second brain. Cell 161(2): 193–194. [DOI] [PubMed] [Google Scholar]
- Rief W, Broadbent E. (2007) Explaining medically unexplained symptoms- models and mechanisms. Clinical Psychology Review 27(7): 821–841. [DOI] [PubMed] [Google Scholar]
- Roth WT, Wilhelm FH, Pettit D. (2005) Are current theories of panic falsifiable? Psychological Bulletin 131: 171–192. [DOI] [PubMed] [Google Scholar]
- Ruiz FJ. (2010) A review of Acceptance and Commitment Therapy (ACT) empirical evidence: Correlational, experimental psychopathology, component and outcome studies. International Journal of Psychology and Psychological Therapy 10: 125–162. [Google Scholar]
- Sarzi-Puttini P, Atzeni F, Masala IF, et al. (2018) Are the ACR 2010 diagnostic criteria for fibromyalgia better than the 1990 criteria. Autoimmunity Reviews 17(1): 33–35. [DOI] [PubMed] [Google Scholar]
- Schaefer CP, Adams EH, Udall M, et al. (2016) Fibromyalgia outcomes over time: Results from a prospective observational study in the United States. The Open Rheumatology Journal 10: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. (2004) Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin 130(4): 601–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Steen TA, Park N, et al. (2005) Positive psychology progress: Empirical validation of interventions. American Psychologist 60(5): 410–421. [DOI] [PubMed] [Google Scholar]
- Sharpe M, Goldsmith K, Chalder T. (2019) The PACE trial of treatments for chronic fatigue syndrome: A response to WILSHIRE et al. BMC Psychology 7: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimobayashi M, Albert V, Woelnerhanssen B, et al. (2018) Insulin resistance causes inflammation in adipose tissue. Journal of Clinical Investment 128(4): 1538–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey MR. (2013) Lewin’s theory of planned change as a strategic resource. Journal of Nursing Administration 43(2): 69–72. [DOI] [PubMed] [Google Scholar]
- Sim J, Madden S. (2008) Illness experience in fibromyalgia syndrome: A metasynthesis of qualitative studies. Social Science and Medicine 67: 57–67. [DOI] [PubMed] [Google Scholar]
- Singh RK, Chang HW, Yan D, et al. (2017) Influence of diet on the gut microbiome and implications for human health. Journal of Translational Medicine 15(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S, Maloney E, Wright B, et al. (2019) The problematic nature of fibromyalgia diagnosis in the community. ACR Open Rheumatology 1(1): 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SB, King AS. (2009) Is fibromyalgia a pain disorder? Current Psychiatry Reports 8(5): 9. [Google Scholar]
- Staud R. (2008) Autonomic dysfunction in fibromyalgia syndrome: Postural orthostatic tachycardia. Current Rheumatology Reports 10(6): 463–466. [DOI] [PubMed] [Google Scholar]
- Steingrímsdóttir ÓA, Landmark T, Macfarlane GJ, et al. (2017) Defining chronic pain in epidemiological studies: A systematic review and meta-analysis. Pain 158(11): 2092–2107. [DOI] [PubMed] [Google Scholar]
- Sturge-Jacobs M. (2002) The experience of living with fibromyalgia: Confronting an invisible disability. Research and Theory for Nursing Practice 16(1): 19–31. [DOI] [PubMed] [Google Scholar]
- Swancutt D, Tarrant M, Pinkney J. (2019) How group-based interventions can improve services for people with severe obesity. Current Obesity Reports 8(3): 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi F, Karaca Z, Unluhizarci K, et al. (2007) The hypothalamopituitary-adrenal axis in chronic fatigue syndrome and fibromyalgia syndrome. Stress 10(1): 13–25. [DOI] [PubMed] [Google Scholar]
- Taylor SJC, Carnes D, Homer K, et al. (2016) Improving the self-management of chronic pain: COping with persistent Pain, Effectiveness Research in Self-management (COPERS). Programme Grants for Applied Research: No 4. 14. Southampton: NIHR Journals Library. [PubMed] [Google Scholar]
- Thieme K, Gracely RH. (2009) Are psychological treatments effective for fibromyalgia pain? Current Rheumatology Reports 11(6): 443–450. [DOI] [PubMed] [Google Scholar]
- Thomson DR, Besner D, Smilek D. (2015) A resource-control account of sustained attention: Evidence from mind-wandering and vigilance paradigms. Perspectives on Psychological Science 10(1): 82–96. [DOI] [PubMed] [Google Scholar]
- Török B, Janacsek K, Nagy DG, et al. (2017) Measuring and filtering reactive inhibition is essential for assessing serial decision making and learning. Journal of Experimental Psychology 146(4): 529–542. [DOI] [PubMed] [Google Scholar]
- Toussaint L, Sirois F, Hirsch J, et al. (2017) Gratitude mediates quality of life differences between fibromyalgia patients and healthy controls. Quality of Life Research 26(9): 2449–2457. [DOI] [PubMed] [Google Scholar]
- Treede RD, Rief W, Barke A, et al. (2015) A classification of chronic pain for ICD-11. Pain 156(6): 1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Sinclair JD, et al. (1998) Differential responses by psychosocial subgroups of fibromyalgia syndrome patients to an interdisciplinary treatment. Arthritis Care and Research 11: 397–404. [DOI] [PubMed] [Google Scholar]
- Ullmann E, Perry SW, Licinio J. (2019) From allostatic load to allostatic state-an endogenous sympathetic strategy to deal with chronic anxiety and stress. Frontiers in Behavioural Neuroscience 13: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undeland M, Malterud K. (2007) The fibromyalgia diagnosis- hardly helpful for the patients? Scandinavian Journal of Primary Health Care 25: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houdenhove B, Neerinckx E, Onghena P, et al. (2001) Premorbid “overactive” lifestyle in chronic fatigue syndrome and fibromyalgia: An etiological factor or proof of good citizenship? Journal of Psychosomatic Research 51(4): 571–576. [DOI] [PubMed] [Google Scholar]
- Villafaina S, Collado-Mateo D, Gusi N, et al. (2019) Effects of exergames on brain dynamics in women with fibromyalgia: A randomized controlled trial. Journal of Clinical Medicine 8(7): 1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, et al. (2016) Circadian rhythm and the gut microbiome. International Review of Neurobiology 131: 193–205. [DOI] [PubMed] [Google Scholar]
- Volker G, van Vree F, Wolterbeek R, et al. (2017) Long-term outcomes of multidisciplinary rehabilitation for chronic musculoskeletal pain. Musculoskeletal Care 15(1): 59–68. [DOI] [PubMed] [Google Scholar]
- Walitt B, Fitzcharles MA, Hassett AL, et al. (2011) The longitudinal outcome of Fibromyalgia: A study of 1555 patients. Journal of Rheumatology 38(10): 2238–2246. [DOI] [PubMed] [Google Scholar]
- Walitt B, Katz RS, Bergman MJ, et al. (2016) Three-quarters of persons in the US population reporting a clinical diagnosis of Fibromyalgia do not satisfy Fibromyalgia criteria: The 2012 National health interview survey. PLoS One 11(6): e0157235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Schmid CH, Fielding RA, et al. (2018. a) Effect of tai chi versus aerobic exercise for fibromyalgia: Comparative effectiveness randomised controlled trial. British Medical Journal 360: k851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. (2018. b) Time to Rethink Exercise for Fibromyalgia Care. The BMJ opinion March 21st Available at: https://blogs.bmj.com/bmj/2018/03/21/chenchen-wang-time-to-rethink-exercise-for-Fibromyalgia-care/ (accessed 17 February 2020).
- Warm JS, Parasuraman R, Matthews G. (2008) Vigilance requires hard mental work and is stressful. Human Factors 50(3): 433–441. [DOI] [PubMed] [Google Scholar]
- Wertli MM, Reich O, Signorell A, et al. (2017) Changes over time in prescription practices of pain medications in Switzerland between 2006 and 2013: An analysis of insurance claims. BMC Health Services Research 17(1): 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely S, White PD. (2004) There is only one functional somatic syndrome. British Journal of Psychiatry 185(2): 95–96. [DOI] [PubMed] [Google Scholar]
- White KP, Nielson WR, Harth M, et al. (2002) Chronic widespread musculoskeletal pain with or without fibromyalgia: Psychological distress in a representative community adult sample. Journal of Rheumatology 29(3): 588–594. [PubMed] [Google Scholar]
- White PD, Goldsmith KA, Johnson AL, et al. (2011) Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue syndrome (PACE): A randomised trial. Lancet 377: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilshire CE, Kindlon T, Courtney R, et al. (2018) Rethinking the treatment of chronic fatigue syndrome—A reanalysis and evaluation of findings from a recent major trial of graded exercise and CBT. BMC Psychology 6(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA. (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Research 62: 600–610. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. (2016) Revisions to the 2010/2011 Fibromyalgia diagnostic criteria. Seminars in Arthritis and Rheumatism 46: 319–329. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for Fibromyalgia. Journal of Rheumatology 38: 1113–1122. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Schmukler J, Jamal S, et al. (2019) Diagnosis of fibromyalgia: Disagreement between fibromyalgia criteria and clinician-based fibromyalgia diagnosis in a university clinic. Arthritis Care Research (Hoboken) 71(3): 343–351. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of Fibromyalgia. Report of the multicenter criteria committee. Arthritis and Rheumatology 33(2): 160–172. [DOI] [PubMed] [Google Scholar]
- Wood AM, Tarrier N. (2010) Positive clinical psychology: A new vision and strategy for integrated research and practice. Clinical Psychology Review 30(7): 819–829. [DOI] [PubMed] [Google Scholar]
- Wood AM, Froh JJ, Geraghty AW. (2010) Gratitude and well-being: A review and theoretical integration. Clinical Psychology Review 30(7): 890–905. [DOI] [PubMed] [Google Scholar]
- Young E. (2012) Gut instincts: The secrets of your second brain. New Scientist 216(2895): 38–42. [Google Scholar]
- Yunus MB. (2007. a) Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Seminars in Arthritis and Rheumatism 36: 339–356. [DOI] [PubMed] [Google Scholar]
- Yunus MB. (2007. b) Role of central sensitization in symptoms beyond muscle pain, and the evaluation of a patient with widespread pain. Best Practice Research Clinical Rheumatology 21(3): 481–497. [DOI] [PubMed] [Google Scholar]
- Zhou L, Foster JA. (2015) Psychobiotics and the gut–brain axis: In the pursuit of happiness. Neuropsychiatric Disease and Treatment 11: 715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]